Abstract
Introduction
Mirasol Pathogen Reduction Technology® (PRT) treatment uses riboflavin and UV light to inactivate pathogens in blood components. Neutrophil [polymorphonuclear cells (PMN)] priming activity accumulates during routine storage of cellular blood components, and this activity has been implicated in transfusion-related acute lung injury (TRALI). We hypothesize that PRT-treatment of blood components affects the priming activity generated during storage of packed RBCs (PRBCs) or platelet concentrates (PCs), which can elicit ALI in vivo.
Methods
Plasma, PRBCs and PCs were isolated from healthy donor’s whole blood or by apheresis. Half of a collected unit was treated with PRT treatment and the remainder was left as an unmodified control. Supernatant was collected during storage of PCs and PRBCs and assayed for PMN priming activity and used as the second event in a two-event in vivo model of TRALI.
Results
PRT treatment did not induce priming activity in plasma or affect the priming activity generated during storage of PCs or PRBCs as compared with the unmodified controls. The supernatants from stored, but not fresh, PCs and PRBCs did cause ALI as the second event in a two-event animal model of TRALI, which was unaffected by PRT treatment. We conclude that the PRT® treatment does not induce priming activity in plasma nor does it affect the priming activity generated during storage of PCs or PRBCs or their ability to cause ALI as the second event in a two-event in vivo model of TRALI. Moreover, the amount of priming activity in TRIMA®-isolated PCs was significantly less than SPECTRA®-isolated PCs.
Keywords: neutrophils, oxidase, platelets, priming, red blood cells, storage
Introduction
Transfusion is an open portal for infection, but due to the many blood safety interventions introduced in developed countries, the risk of transfusion-transmitted disease is low. However, the arrival of emerging pathogens, such as West Nile virus, as well as transfusion in under-developed countries where widespread testing is limited, make pathogen reduction important to limit transfusion-transmitted infections [1]. Mirasol PRT (PRT) treatment uses riboflavin in the presence of UV light (280–360 nm) to inactivate pathogens and leucocytes in blood products. This PRT System was approved for platelet and plasma products in Europe, and the FDA approved an IDE for a feasibility clinical trial in the United States using the PRT® System for Whole Blood [1].
Transfusion-related acute lung injury (TRALI) is defined as ALI that is temporally linked (≤ 6 h) to transfusion of blood products and must satisfy all clinical criteria for this diagnosis [2-4]. Recently, both antibodies and lipids that have been implicated in TRALI were shown to rapidly prime the PMN oxidase [5-7]. Pro-inflammatory activation of neutrophils (PMNs) is vital for eradication of pathogens and may result in end-organ injury if it occurs out of physiological context, such that activation of the PMN microbicidal arsenal is responsible for acute lung injury (ALI) [8-10]. Priming is defined operationally as augmentation of the PMN oxidase but is part of PMN emigration in vivo from the vasculature to the tissues where PMNs destroy invading pathogens [11-15]. All priming agents are chemo-attractants and are involved in PMN chemotaxis to the nidus of infection to augment the release of the microbicidal arsenal to efficiently kill the pathogens [11-13, 16-18]. Priming also changes the PMN phenotype from non-adherent to adherent, resulting in firm adhesion to the vascular endothelium [11-13,16]. Furthermore, priming agents are aetiological as the second event in the two-event in vivo models of ALI and have been implicated in clinical TRALI [5,15,19-21]. We investigated the effects of PRT-treated plasma, PCs and PRBCs (derived from treated whole blood) on PMN priming activity in vitro and whether PRT-treated components could cause PMN-mediated ALI in a two event, in vivo model of TRALI. In addition, we compared the PMN priming activity generated during routine storage of PCs collected on TRIMA® with SPECTRA®-collected PCs.
Materials and methods
Materials
All chemicals were purchased from Sigma Chemical Company unless otherwise stated (St. Louis, MO). Solutions were made from sterile water for injection, United States Pharmacopeia (USP), from Baxter Healthcare Corp. (Deerfield, IL). All buffers were made from the following stock USP solutions: 10% CaCl2, 23·4% NaCl, 50% MgSO4 (American Reagent Laboratories, Inc., Shirley, NY); sodium phosphates (278 mg/ml monobasic and 142 mg/ml dibasic) and 50% dextrose (Abbott Laboratories, North Chicago, IL). Furthermore, all solutions were sterile-filtered with Nalgene™ MF75 series disposable sterilization filter units purchased from Fisher Scientific Corp. (Pittsburgh, PA). Ficoll–Paque was purchased from Amersham Biosciences (Piscataway, NJ). Plastic microplates, manufactured by Nunc were purchased from Life Sciences Products, Inc (Frederick, CO). PE50 tubing was purchased from Fisher Scientific (Pittsburgh, PA).
PMN isolation and priming of the oxidase
Heparinized whole blood was drawn from healthy human donors after obtaining informed consent employing a protocol approved by the Colorado Multiple Institutional Review Board. PMNs were isolated by standard techniques, including dextran sedimentation, ficoll–hypaque gradient centrifugation and hypotonic lysis of contaminating red blood cells [22]. PMN priming assays were completed as previously described using buffer (control) or 10% [vol:-vol]FINAL of the plasma fraction of the blood component as the priming agent [14,22]. Priming activity was calculated as the augmentation of the maximal rate of O2−) (nmol/min) as calculated by the superoxide dismutase inhibitable reduction of cytochrome c at 550 nm in response to 1 μm fMLP [14,22].
Mirasol PRT Treatment
Units of whole blood were treated with the PRT® System for Whole Blood; units of platelets and units of plasma were treated separately with the Mirasol PRT System for Platelets and Plasma. Whole blood units (n = 5, isolated from three males and two females, both of whom answered ‘never pregnant’ on their donor cards) were split into two units, one control (approximately 200 ml) and one treated (approximately 250 ml) unit. To keep the amount of riboflavin added consistent with the configuration of the PRT System for Whole Blood (35 ml riboflavin per 470 ml of whole blood) 19 ml of 500 μm Riboflavin was added to 250 ml of whole blood. Units were illuminated to 33 J/mlRBC. PRT-treated PRBCs were generated from the whole blood, leucoreduced using a Pall BPF4 filter and stored refrigerated in AS-5 for 42 days. Samples were taken on Day 1 of storage and weekly thereafter and analyzed for neutrophil priming. Apheresis plasma units (n = 2 from two disparate male donors) were split and one 200-ml unit from each pair was illuminated to 6·24 J/ml in the presence of 35 ml of 500 μm riboflavin. Double-apheresis platelet concentrates (PC) were collected from five antibody negative donors by the TRIMA® apheresis system (CaridianBCT Biotechnologies). Antibody-negative donors are defined as never transfused males or females that answered ‘never pregnant’ on their donor cards. The double platelet units were split, and one unit was treated using riboflavin solution (35 ml) and UV light and the other unit was left untreated. Units were stored for 7 days under standard blood banking conditions and samples were obtained via sterile couplers. Supernatants were isolated by centrifugation per AABB guidelines and aliquotted and stored at −80°C [23]. PCs (5) were also collected from five healthy, antibody-negative donors employing the SPECTRA® system (CaridianBCT Biotechnologies), and samples were obtained on identical days of storage as the TRIMA®-isolated PCs. All supernatants were assayed in vitro for PMN priming activity, but only TRIMA® collected samples were assayed for ALI.
A two-event in vivo TRALI model
This animal model was performed as previously described [5]. Male Sprague Dawley rats (Harlan, Indianapolis, IN) underwent ALI via a treatment protocol approved by the Animal Care and Use Committee, UCD. Briefly, rats were injected with 2 mg/kg lipopolysaccharide (LPS, Salmonella enteritides) or normal saline (NS) and observed for 2 h, anesthetized with 60 mg/kg pentobarbital and the femoral vessels were cannulated with PE50 tubing filled with 50/50 heparin:saline. The heart rate and the mean arterial blood pressure (MAP) were monitored via the arterial line. Five to ten percent of the total blood volume (total ml blood = kg of body weight × 60 [24]) was slowly removed (5–10 min) followed by infusion of an identical volume of normal saline (NS) or heat-treated (56°C for 30 min to obviate the effects of complement and fibrinogen) supernatants from untreated or PRT®-treated PRBCs (10% of blood volume) or PCs (5% of blood volume) at a rate of 4 ml/h (15–30 min) through the femoral vein. After these infusions, 30 mg/kg of Evans Blue Dye (EBD; 10 mg/ml) was injected (5 min), the rats were observed for 6 h and re-anesthetized. Blood (3 ml) was drawn and the rats were euthanized with an overdose of pentobarbital. A bronchoalveolar lavage (BAL) was performed with three washes of NS (5 ml), the BAL fluid (BALF) and blood were centrifuged, and the plasma/-cell-free fractions were aliquotted and frozen at −80°C. Lung leak was measured as the percentage of the plasma EBD which leaked into the BALF as previously described [23].
Statistics
Data are presented as the mean ± the standard deviation or the standard error of the mean (SEM). For the comparison of the SPECTRA®- vs. the TRIMA®-isolated PCS, the 95% confidence intervals (95% CI) are included. Statistical differences among groups were measured by paired or independent anovas followed by either a Bonferroni’s or Newman–Keuls post hoc test for multiple comparisons depending upon the equality of variance. Significance was determined at the P < 0·05 level.
Results
Priming activity during routine storage of stored blood components
As a necessary prerequisite for studying ALI in vivo, the priming activity of supernatants from PRBCs and PCs collected throughout storage was measured in vitro. The priming activity of supernatants from PRBC units was not different from buffer-primed controls on day 1 of storage but did significantly increase in both the untreated PRBCs and PRT-treated PRBCs on day 14 (Fig. 1). This priming activity reached a plateau by day 28 of storage. Importantly, there was no statistical difference in the supernatant priming activity between the PRT-treated PRBCs and the untreated controls throughout routine PRBC storage (Fig. 1).
Fig. 1.
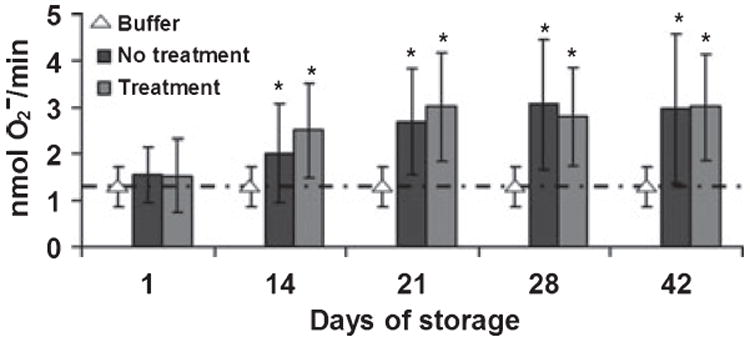
The accumulation of plasma priming activity during routine PRBC storage. Priming activity, the augmentation of the fMLP-activated respiratory burst, is depicted as a function of the storage time. The data are presented as the mean ± the standard deviation. Significant priming activity was present in both the untreated control (no treatment) and PRT-treated (treatment) PRBCs by day 14 of storage compared with supernatants from day 1 and buffer controls. Priming reached a relative maximum by day 28 of storage. PRT treatment did not increase the amount of priming activity that accumulates in the supernatant during routine PRBC storage. This figure represents the priming activity from 5 units of PRBCs from five different donors.
As PCs contain significant amounts of plasma, we tested whether or not PRT treatment of plasma units induces PMN priming. PRT-treated plasma did not increase priming of the PMN oxidase compared with buffer controls and samples of paired untreated plasma (buffer control: 1·6 ± 0·3, FFP: 1·8 ± 0·3, PRT treated-FFP: 2·0 ± 0·2). Moreover, supernatants neither from untreated controls nor from PRT-treated PCs collected on day 0 caused priming of the fMLP-activated respiratory burst compared with buffer-primed controls (Fig. 2). In untreated control PCs, significant priming activity was not present until day 5 of storage and reached a relative maximum by day 7. PRT treatment did not increase the priming activity throughout storage compared with the untreated control PCs. However, there was a significant increase in priming activity on day 3 of storage in the PRT-treated PCs as compared with the day 0 PRT--treated PCs, and similar to the untreated control PCs, there was a significant supernatant priming activity on day 5 of storage which persisted through day 7 of storage.
Fig. 2.
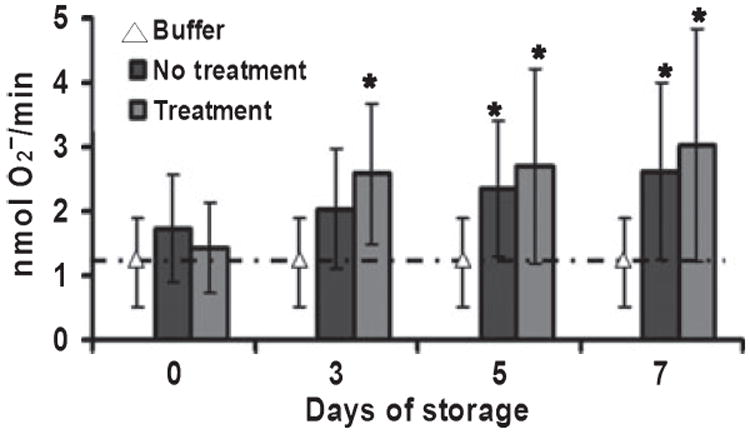
The accumulation of plasma priming activity during routine platelet storage. Priming activity, the augmentation of the fMLP-activated respiratory burst, is shown as a function of the days of platelet storage. The data are presented as the mean ± the standard deviation. The supernatant from day 0 of both untreated (no treatment) and PRT-treated (treatment) controls did not exhibit any priming activity as compared with the buffer-treated controls. Under routine storage, PRT-treated PCs demonstrated an increase in priming activity by day 3, which was not significantly different as compared to day 0, and priming reached a relative maximum by day 7. By contrast, supernatants from the untreated control PCs did not demonstrate significant priming activity compared to day 0 until day 5 and reached a relative maximum by day 7 of storage. Importantly, there were no statistical differences between the priming activity of the PRT-treated and the untreated control units. This figure represents the data obtained from 5 double units of apheresis platelets from five disparate donors.
The supernatants from five PCs collected from disparate antibody negative donors using the SPECTRA® apheresis collection system and stored over 5 days demonstrated significantly higher priming activity than TRIMA®-collected PCs (Table 1). SPECTRA® collected PCs demonstrated significant priming activity on day 3 of storage in contrast with TRIMA® PCs that did not exhibit an increase in priming activity until day 5 of storage (P < 0·05, n = 5). In addition, the priming activity on both day 3 and day 5 was significantly higher in SPECTRA®-isolated PCs vs. TRIMA®-isolated PCs (P < 0·05, n = 5).
Table 1.
Priming of the PMN oxidase by supernatants from stored PCs
| Days of storage | SPECTRA® | 95% CI | TRIMA® | 95% CI |
|---|---|---|---|---|
| Day 0 | 1·7 ± 0·2 | 0·9–2·6 | 1·7 ± 0·3 | 1·1–2·3 |
| Day 3 | 3·4 ± 0·3*,** | 2·7–4·1 | 2·0 ± 0·3 | 1·4–2·6 |
| Day 5 | 4·6 ± 0·6*,** | 3·3–5·9 | 2·4 ± 0·5* | 1·8–2·9 |
Data are expressed as the mean ± the standard error of the mean of the maximal rate of superoxide anion production (nmol O2−/min) and the 95% confidence intervals (CI) are shown for each measurement (n = 5).
P < 0·05 as compared to day 0.
P < 0·05 as compared to supernatant priming activity from TRIMA®-isolated PCs.
A two-event in vivo model of ALI
Pulmonary oedema was measured as the percentage of Evans Blue dye that leaked from the plasma into the bronchoalveolar lavage fluid [5]. An intraperitoneal (IP) injection of LPS was employed as the first event that causes pulmonary sequestration of PMNs and NS was used as the vehicle control. The first set of experiments used the supernatant of PRT-treated or -untreated PRBCs as the second event. In rats given NS, supernatants neither from day 1 nor from day 42 of untreated or PRT-treated PRBCs caused pulmonary oedema. In animals injected with LPS, no pulmonary oedema was demonstrated in animals infused with day 1 supernatants from untreated or PRT-treated PRBCs. However, in rats injected with LPS and supernatant from day 42, significant pulmonary oedema, synonymous to ALI, was observed (Fig. 3). It is of note that the amount of ALI induced by supernatants of day 42 PRBCs was not increased by the PRT-treatment.
Fig. 3.
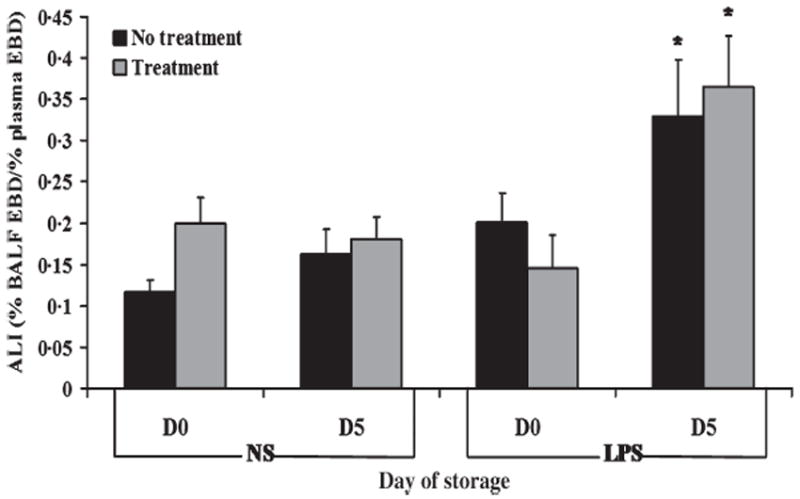
Acute lung injury (ALI) induced by stored PRBCs is not affected by PRT treatment. Evans Blue dye (EBD) leak, a measure of pulmonary oedema, is shown as a function of treatment group. The data are represented as the mean ± SEM. In rats that received normal saline (NS) as the first event, no plasma supernatants caused ALI. However, in animals that received endotoxin (LPS) as the first event, stored (day 42) but not fresh (day 1) supernatants (non-treated) induced ALI as demonstrated by significant EBD leak from the plasma into the bronchalveolar lavage fluid (BALF) (P < 0·05). PRT treatment (treatment) did not increase ALI as quantified by EBD leak. This figure consists of a sample size of 5 with all units being represented. *P < 0·05 compared to day 1.
The same experiments were carried out with supernatants of stored PCs. Rats injected with NS and infused with day 0 or day 5 supernatants from untreated or PRT-treated PCs did not demonstrate capillary leak. Furthermore, rats injected with LPS and then infused with day 0 supernatants also did not show evidence of EBD leak (Fig. 4). By contrast, rats injected with LPS and infused with the supernatants of day 5 untreated or PRT-treated PCs demonstrated EBD leak. As observed for PRT-treated PRBCs, PRT treatment of PCs did not augment the observed ALI compared with supernatants from untreated control PCs.
Fig. 4.
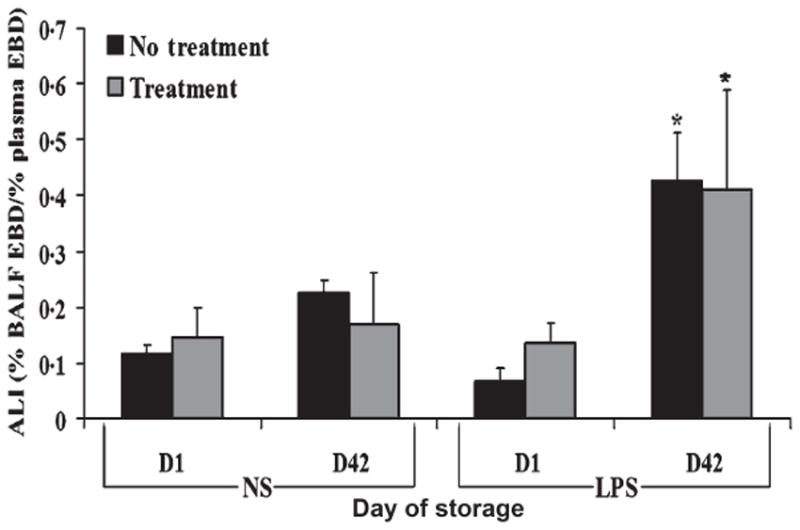
Acute lung injury (ALI) from stored PCs in vivo is unaffected by PRT treatment. Evans blue dye (EBD) leak, a measure of pulmonary oedema, is depicted as a function of treatment group. The data are represented as the mean ± SEM. In rats that received normal saline (NS) as the first event, no supernatants caused EBD leak from the plasma into the bronchoalveolar lavage fluid (BALF). By contrast, in endotoxin- (LPS) injected rats day 5 supernatant from untreated (no treatment) and PRT-treated (treatment) stored PCs caused significant EBD leak compared with rats injected with LPS and infused with the day 0 supernatants from identical PCs (P < 0·05). PRT treatment did not increase ALI as quantified by EBD leak. This figure consists of a sample size of 5 with all 5 units being represented. *P < 0·05 compared to day 0.
Discussion
PRT-treatment had no significant effects on the priming activity, which accumulates during the routine storage of PRBCs. In addition, PRT-treatment of plasma did not induce PMN priming and PRT treatment had little effect on the priming activity that accumulates in supernatants during routine storage of PCs as compared with the unmodified control PCs. PRT treatment also did not augment the ability of supernatants from stored PRBCs or PCs, as the second event in a two-event model, to cause pulmonary oedema in vivo. Importantly, PRT treatment did not inhibit TRALI in the in vivo model elicited by the supernatants from stored (day 42 or day 5) vs. fresh (day 1 or day 0) PRBCs or PCs respectively.
These data are congruous with previous work with stored PRBCs, which demonstrated the accumulation of significant priming activity by day 14, that further increased throughout routine storage for 42 days [22]. The supernatants of PRBCs also caused ALI as the second event in a two-event in vivo model, identical to previously published work [5]. By contrast, the accumulation of PMN priming activity during routine storage of PCs collected by the TRIMA® apheresis system differed from that seen for units collected by the SPECTRA® System. Significant priming activity was detected earlier when platelets were collected on the SPECTRA® vs. the TRIMA® [14]. In the experiments shown here, supernatants from TRIMA® collected PCs (unmodified) did not contain significant priming activity until day 5, the date of component outdate, and this activity modestly increased further by day 7 of storage. This data suggests that the collection method of platelets affects the generation of priming activity in supernatants throughout storage. Furthermore, no priming activity was present in the supernatants from PCs or PRBCs on the day of collection, indicating that the priming activity was due to the accumulation of lipids over routine storage. If antibodies were present in the antibody-negative donors, one would expect persistent priming in the supernatants irrespective of storage times identical to previous work with plasma, which contained HNA-3a and its ability to prime HNA-3a+ PMNs [25]. No such activity was present for any of the plasma or cellular products tested providing indirect evidence that none of the described activity was due to donor antibodies recognizing HLA or HNA antigens on the PMNs. Moreover, it is unlikely that cytokines, chemokines or soluble CD40 ligand are present in the PRBCs because of the prestorage leucoreduction that reduces the accumulation of these polypeptides during routine storage [26-29].
In addition, similar to previously published experiments with isolated perfused rat lungs, supernatants from stored but not fresh PCs caused ALI as the second event in an in vivo model of ALI [30]. The data reported here are the first to demonstrate that the supernatant from stored PCs has the ability to cause ALI in vivo. Supernatants from 7-day-old PCs were not tested because it is outside the current guidelines for platelet storage.
PRT treatment has been shown to inactivate leucocytes and a wide range of bacteria, viruses and parasites that may be present in blood products [31-33]. The presented data have demonstrated that the treatment of cellular components does not enhance priming of the PMN oxidase compared with paired untreated control blood products. The priming activity of antibodies and other biologic response modifiers including lipids have been correlated with the ability of such mediators to cause TRALI in vivo [5,6,21,25,30]. Collection of PCs using TRIMA® appears to reduce priming activity compared with supernatants from PCs collected on SPECTRA® equipment, which may imply that fewer antibody-negative cases of TRALI are to be expected; however, no such studies have demonstrated that this assertion has clinical validity. The decrease in priming activity during storage may likely be due to the decreased g-forces to which the platelets are subjected to in the TRIMA® system.
Acknowledgments
This work was supported by a grant from CaridianBCT Biotechnologies, LLC, Lakewood, CO.
References
- 1.Solheim BG. Pathogen reduction of blood components. Transfus Apher Sci. 2008;39:75–82. doi: 10.1016/j.transci.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R. Report of the American-European Consensus conference on acute respiratory distress syndrome: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Consensus Committee. J Crit Care. 1994;9:72–81. doi: 10.1016/0883-9441(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 3.Kleinman S, Caulfield T, Chan P, Davenport R, McFarland J, McPhedran S, Meade M, Morrison D, Pinsent T, Robillard P, Slinger P. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004;44:1774–1789. doi: 10.1111/j.0041-1132.2004.04347.x. [DOI] [PubMed] [Google Scholar]
- 4.Toy P, Popovsky MA, Abraham E, Ambruso DR, Holness LG, Kopko PM, McFarland JG, Nathens AB, Silliman CC, Stroncek D. Transfusion-related acute lung injury: definition and review. Crit Care Med. 2005;33:721–726. doi: 10.1097/01.ccm.0000159849.94750.51. [DOI] [PubMed] [Google Scholar]
- 5.Kelher MR, Masuno T, Moore EE, Damle S, Meng X, Song Y, Liang X, Niedzinski J, Geier SS, Khan SY, Gamboni-Robertson F, Silliman CC. Plasma from stored packed red blood cells and MHC class I antibodies causes acute lung injury in a 2-event in vivo rat model. Blood. 2009;113:2079–2087. doi: 10.1182/blood-2008-09-177857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silliman CC, Voelkel NF, Allard JD, Elzi DJ, Tuder RM, Johnson JL, Ambruso DR. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Invest. 1998;101:1458–1467. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silliman CC, Elzi DJ, Ambruso DR, Musters RJ, Hamiel C, Harbeck RJ, Paterson AJ, Bjornsen AJ, Wyman TH, Kelher M, England KM, McLaughlin-Malaxecheberria N, Barnett CC, Aiboshi J, Bannerjee A. Lysophosphatidylcholines prime the NADPH oxidase and stimulate multiple neutrophil functions through changes in cytosolic calcium. J Leukoc Biol. 2003;73:511–524. doi: 10.1189/jlb.0402179. [DOI] [PubMed] [Google Scholar]
- 8.Lee WL, Downey GP. Neutrophil activation and acute lung injury. Curr Opin Crit Care. 2001;7:1–7. doi: 10.1097/00075198-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Repine JE. Scientific perspectives on adult respiratory distress syndrome. Lancet. 1992;339:466–469. doi: 10.1016/0140-6736(92)91067-i. [DOI] [PubMed] [Google Scholar]
- 10.Reutershan J, Ley K. Bench-to-bedside review: acute respiratory distress syndrome – how neutrophils migrate into the lung. Crit Care. 2004;8:453–461. doi: 10.1186/cc2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB J. 1994;8:504–512. [PubMed] [Google Scholar]
- 12.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 13.Granger DN, Kubes P. The microcirculation and inflammation: modulation of leukocyte-endothelial cell adhesion. J Leukoc Biol. 1994;55:662–675. [PubMed] [Google Scholar]
- 14.Silliman CC, Dickey WO, Paterson AJ, Thurman GW, Clay KL, Johnson CA, Ambruso DR. Analysis of the priming activity of lipids generated during routine storage of platelet concentrates. Transfusion. 1996;36:133–139. doi: 10.1046/j.1537-2995.1996.36296181925.x. [DOI] [PubMed] [Google Scholar]
- 15.Silliman CC, Paterson AJ, Dickey WO, Stroneck DF, Popovsky MA, Caldwell SA, Ambruso DR. The association of biologically active lipids with the development of transfusion-related acute lung injury: a retrospective study. Transfusion. 1997;37:719–726. doi: 10.1046/j.1537-2995.1997.37797369448.x. [DOI] [PubMed] [Google Scholar]
- 16.Silliman CC, Ambruso DR, Boshkov LK. Transfusion-related acute lung injury. Blood. 2005;105:2266–2273. doi: 10.1182/blood-2004-07-2929. [DOI] [PubMed] [Google Scholar]
- 17.Silliman CC, McLaughlin NJ. Transfusion-related acute lung injury. Blood Rev. 2006;20:139–159. doi: 10.1016/j.blre.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Silliman CC. The two-event model of transfusion-related acute lung injury. Crit Care Med. 2006;34:S124–S131. doi: 10.1097/01.CCM.0000214292.62276.8E. [DOI] [PubMed] [Google Scholar]
- 19.Rabinovici R, Bugelski PJ, Esser KM, Hillegass LM, Vernick J, Feuerstein G. ARDS-like lung injury produced by endotoxin in platelet-activating factor-primed rats. J Appl Physiol. 1993;74:10791–10802. doi: 10.1152/jappl.1993.74.4.1791. [DOI] [PubMed] [Google Scholar]
- 20.Salzer WL, McCall CE. Primed stimulation of isolated perfused rabbit lung by endotoxin and platelet activating factor induces enhanced production of thromboxane and lung injury. J Clin Invest. 1990;85:1135–1143. doi: 10.1172/JCI114545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silliman CC, Boshkov LK, Mehdizadehkashi Z, Elzi DJ, Dickey WO, Podlosky L, Clarke G, Ambruso DR. Transfusion-related acute lung injury: epidemiology and a prospective analysis of etiologic factors. Blood. 2003;101:454–462. doi: 10.1182/blood-2002-03-0958. [DOI] [PubMed] [Google Scholar]
- 22.Silliman CC, Clay KL, Thurman GW, Johnson CA, Ambruso DR. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil NADPH oxidase. J Lab Clin Med. 1994;124:684–694. [PMC free article] [PubMed] [Google Scholar]
- 23.Song Y, Ao L, Raeburn CD, Calkins CM, Abraham E, Harken AH, Meng X. A low level of TNF-alpha mediates hemorrhage-induced acute lung injury via p55 TNF receptor. Am J Physiol Lung Cell Mol Physiol. 2001;281:L677–L684. doi: 10.1152/ajplung.2001.281.3.L677. [DOI] [PubMed] [Google Scholar]
- 24.Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med. 1985;26:72–76. [PubMed] [Google Scholar]
- 25.Silliman CC, Curtis BR, Kopko PM, Khan SY, Kelher MR, Schuller RM, Sannoh B, Ambruso DR. Donor antibodies to HNA-3a implicated in TRALI reactions prime neutrophils and cause PMN-mediated damage to human pulmonary microvascular endothelial cells in a two-event in vitro model. Blood. 2007;109:1752–1755. doi: 10.1182/blood-2006-05-025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan SY, Kelher MR, Heal JM, Blumberg N, Boshkov LK, Phipps R, Gettings KF, McLaughlin NJ, Silliman CC. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–2462. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristiansson M, Soop M, Saraste L, Sundqvist KG. Cytokines in stored red blood cell concentrates: promoters of systemic inflammation and simulators of acute transfusion reactions? Acta Anaesthesiol Scand. 1996;40:496–501. doi: 10.1111/j.1399-6576.1996.tb04475.x. [DOI] [PubMed] [Google Scholar]
- 28.Shanwell A, Kristiansson M, Remberger M, Ringden O. Generation of cytokines in red cell concentrates during storage is prevented by prestorage white cell reduction. Transfusion. 1997;37:678–684. doi: 10.1046/j.1537-2995.1997.37797369441.x. [DOI] [PubMed] [Google Scholar]
- 29.Stack G, Snyder EL. Cytokine generation in stored platelet concentrates. Transfusion. 1994;34:20–25. doi: 10.1046/j.1537-2995.1994.34194098597.x. [DOI] [PubMed] [Google Scholar]
- 30.Silliman CC, Bjornsen AJ, Wyman TH, Kelher M, Allard J, Bieber S, Voelkel NF. Plasma and lipids from stored platelets cause acute lung injury in an animal model. Transfusion. 2003;43:633–640. doi: 10.1046/j.1537-2995.2003.00385.x. [DOI] [PubMed] [Google Scholar]
- 31.Cardo LJ, Rentas FJ, Ketchum L, Salata J, Harman R, Melvin W, Weina PJ, Mendez J, Reddy H, Goodrich R. Pathogen inactivation of Leishmania donovani infantum in plasma and platelet concentrates using riboflavin and ultraviolet light. Vox Sang. 2006;90:85–91. doi: 10.1111/j.1423-0410.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- 32.Fast LD, DiLeone G, Li J, Goodrich R. Functional inactivation of white blood cells by Mirasol treatment. Transfusion. 2006;46:642–648. doi: 10.1111/j.1537-2995.2006.00777.x. [DOI] [PubMed] [Google Scholar]
- 33.Ruane PH, Edrich R, Gampp D, Keil SD, Leonard RL, Goodrich RP. Photochemical inactivation of selected viruses and bacteria in platelet concentrates using riboflavin and light. Transfusion. 2004;44:877–885. doi: 10.1111/j.1537-2995.2004.03355.x. [DOI] [PubMed] [Google Scholar]


