Abstract
Objective
Estimate the transmucosal CO2 and O2 rate-constants for adult middle ears (MEs).
Methods
Ten adults with healthy MEs had a unilateral myringotomy. A custom-fitted acrylic mold with a valved line to a Mass Spectrometer (MS) and central tube coupled to a 3-way valve and connected to a pressure transducer (the probe) was sealed with adhesive glue within the ipsilateral ear-canal. A second 3-way valve was attached to the probe valve, a flow-regulated tank gas source and paired syringes. Volumes of the ME and probe were measured. On sequential days, the probe+ME was washed for 15-minutes with 6% O2, Balance N2 and 25% O2, 6% CO2, Balance N2 to create transmucosal CO2 and O2 gradients, respectively. After washing, the probe+ME was isolated from the gas source, and baseline and 10-minute gas samples were obtained for MS analysis of gas partial-pressures. The rates of change in ME CO2 and O2 pressures were divided by their established transmucosal gradients to yield CO2 and O2 rate-constants.
Results
The average (±std) transmucosal CO2 and O2 rate-constants were 0.062±0.034 (N=10, range: 0.032-0.119) and 0.011±0.009 (N=8, range: 0.002-0.032) mmHg/min/mmHg, respectively. The average half-life for the CO2 and O2 gradient was 11.1 and 61.6 minutes. The average CO2:O2 rate-constant ratio was 8.1±4.0 (N=8, range 3.6-14.6).
Conclusions
For adult human MEs, transmucosal CO2 exchange is rapid and much faster than transmucosal O2 exchange. The estimated CO2/O2 rate-constant ratio for the human ME is not consistent with that predicted for diffusion-limited gas exchange across a water-based barrier.
Keywords: Gas Exchange, Middle Ear, Adults
INTRODUCTION
For normal middle ear (ME) function, the gas pressure in the ME cavity needs to be maintained at approximate atmospheric pressure levels. The gas pressure in the relatively fixed-volume ME cavity is affected by the volume of physiologic gases exchanged between the nasopharynx and ME during muscle-assisted, transient openings of the Eustachian tube and by the volume of those gases exchanged between the ME cavity and local blood across the ME mucosa (MEM) [1]. While gas exchange across the Eustachian tube has been well studied in humans and animals [2] and the rate of transMEM exchange of physiologic gases was estimated in animals [3-6], trans MEM exchange of physiologic gases is not well characterized for the human ME.
Indirect evidence from studies in humans supports the transMEM exchange of CO2 and O2 between blood and ME in response to established partial-pressure gradients. For example, the results of experiments documented a greater ME pressure upon waking (i.e. morning pressures) [7, 8], an increase in total ME pressure after partial evacuation of ME gas volume by sniffing or swallowing at relative ME underpressures [9, 10], and an increase in total ME pressure during hypo-ventilation and a decrease during hyper-ventilation [11, 12]. All of these conditions established transMEM CO2 and/or O2 pressure gradients with the subsequent change in total ME pressure attributable to gradient-driven exchange of those gases between blood and ME [8, 12, 13]. Other studies exposed the ME to atmospheric gas partial-pressures thereby creating significant blood to ME CO2 and ME to blood O2 transMEM gradients. Total ME pressure or, where measured, ME gas volume showed an initial, rapid increase to a plateau followed by a less rapid decrease for the remainder of the experiment [14-16]. Mathematical modeling of these curves suggested that the initial increase in ME pressure/volume could be attributed to the influx of CO2 into the ME while the subsequent decrease could be attributed to the efflux of O2 from the ME in response to the established gradients [16]. Of importance, the rate of these exchanges in the human ME was related to ME status with more rapid rates associated with healthy MEs [10, 12, 14, 15].
To date, no study has measured directly the rate of transMEM O2 and CO2 exchange for the human ME. In the present study, a unilateral myringotomy was performed on 10 adult subjects and, on separate days, the ME was washed with gas compositions chosen to establish a transMEM blood to ME CO2 gradient or a transMEM ME to blood O2 gradient while preserving the existing gradients for the other physiologic gases. The rates of change in ME CO2 or O2 pressures were measured over a 10-minute interval, divided by 10 to estimate pressure change/minute and divided by the respective driving gradient to estimate rate-constants for the transMEM exchange of those gases. The rate-constant is a normalized measure of the rate of exchange of gas between the ME and blood for a given ME and can be transformed to gradient half-lives using a log linear function or to transMEM conductance if the volume of the ME exchange compartment is known.
METHODS
Healthy, male and female subjects ≥18 years of age and of any race were recruited by advertisement. Respondents provided written informed consent and were screened for generally good health by history and physical examination, normal ME status by pneumatic otoscopy and tympanometry, normal hearing by audiometry, and, if female, pregnancy by a urine pregnancy test. They were also asked whether or not they had otitis media during childhood. Subjects were excluded if they were unable to comprehend the risks of the study and/or to provide written informed consent; or if they had any chronic health problem, a baseline hearing threshold >15 dB or a >10 dB air-bone gap at any of the speech frequencies, abnormal tympanic membrane (TM) mobility or ME disease at the time of presentation; a past history of sensitivity or allergic reaction to lidocaine or related compounds, or a positive pregnancy test. Twenty subjects were tested on consecutive days using a variety of Eustachian tube function tests to define those results for normal adult subjects (data presented in a separate report) and a subset of these subjects (N=10) was studied for transMEM O2 and CO2 exchange using the protocol described below. The protocols were approved by the University of Pittsburgh Institutional Review Board.
For qualifying subjects, the general protocol for the gas exchange experiments included: an ENT examination; preparation of a custom fitted ear mold; a unilateral myringotomy performed by an otolaryngologist; blood to ME transMEM CO2 exchange testing on that day (Experiment 1), and ME to blood transMEM O2 exchange testing on the next day (Experiment 2). At approximately weekly intervals until the TM had healed, the TM and ME were examined by pneumatic otoscopy and tympanometry. Audiologic testing was repeated when the perforation had healed.
Myringotomy
For myringotomy, the subject laid supine on an exam table with his/her head rotated to expose the test ear. The TM was visualized through a speculum with the aid of an operating microscope and 4% lidocaine (with epinephrine) was applied topically for 20 minutes. An approximately 3-4 mm radial incision was made unilaterally in the anterior-inferior quadrant of the TM using a myringotomy knife. A non-patent TM was confirmed by pneumatic otoscopy and tympanometry.
Construction of the Ear Canal Probe (ECP)
For each subject, an impression of one ear-canal (EC) was made using a two-part silicone impression material (Mega-sil, Microsonics Inc, Burnham, PA). These were sent to a manufacturing company (Microsonic Inc, Ambridge, PA 15003) for fabrication of acrylic ear plugs custom-fitted to the molded ECs. Each acrylic ear plug was drilled and fitted with a 23 mm length, polypropylene male slip luer tube (2 mm diameter, Cole Parmer, Vernon Hills, IL) and a 40 cm length of fused silica glass tubing (50 micron diameter, Scientific Glass Engineering model number 062463), both extending from the interior bore of the plug to its environmental surface. These were hermetically sealed within the ear plug using Epoxy Gel (The Original Super Glue, Pacer Technology, LLC, Rancho Cucamango, CA USA). The environmental projection of the male portion of the luer tube was inserted and sealed into one port of a 3-way valve (Baxter Healthcare Corporation, Deerfield, IL) and the silica tubing was connected to a pneumatic pinch valve. A differential micropressure sensor (Honeywell SDX01D4, Morris Township, NJ) whose output was routed to a microcomputer was fitted and sealed to the second port of the ECP valve to complete the ECP assembly. The volume of each ECP was measured using water displacement.
Experimental Protocol
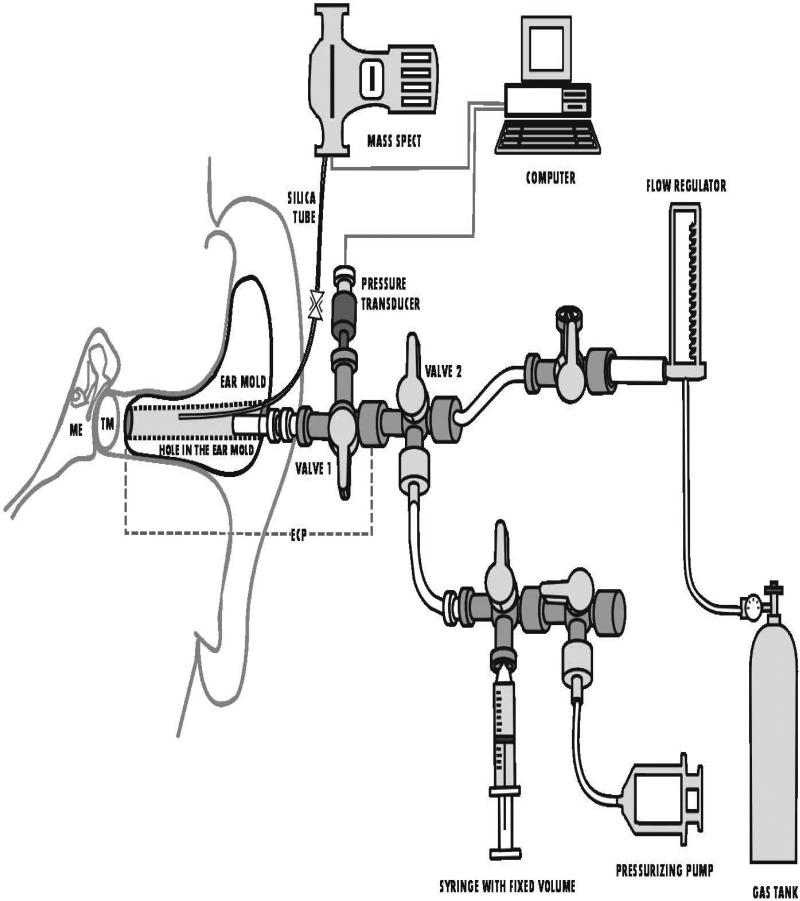
A schematic of the experimental setup is shown in Figure 1. For each experiment, the subject was seated in an ENT exam chair that was reclined to a comfortable position. Atmospheric pressure was recorded. The ECP surface that contacts the EC was covered with water-based, spirit gum glue (Kryolan, Water Soluble Spirit Gum, Berlin Kryolan Corp, SF, CA USA), then fitted and sealed within the ipsilateral EC and supported by a head-band. The silica microtubing from the ECP was attached via the pneumatic pinch valve to 100 cm of same diameter silica microtubing coupled to the inlet port of an Ametek Turbo-Molecular pump (TCP 121 Electronic Drive Unit and TSU 050 Pumping Unit) attached to a Dycor quadrapole (model#M100M) mass spectrometer (MS). MS output was routed to the serial port of the microcomputer for data display and storage. The remaining port of the ECP valve was attached to a second 3-way valve (Valve 2) with one port coupled via a valve to a fixed-volume syringe and to a second syringe for pressurizing the system with a valve that could be opened to the environment. The other port was fitted with plastic tubing (Saint Gobain; Tygon) that connected via a 3-way valve to a flow regulator (Gilmont, Barrington, IL) and then to one of two pressure-regulated tank gas sources of certified composition (Airgas Great Lakes, Cleveland, OH). The integrity of the ECP+ME to pressure leaks was tested by opening the ECP valve position to all 3 ports and Valve 2 to the pressure-regulating syringe and to the ECP. A pressure was applied to the system, the ECP valve was closed to Valve 2 and ECP/ME pressure was monitored for evidence of decay. Then, the system pressure was returned to ambient, Valve 2 was closed to the upstream volume and the valve to the fixed-volume syringe was closed. The pressure of the system was increased by compression of the pressure-regulating syringe. The valve to the pressure-regulating syringe was closed and that to the fixed-volume syringe opened. The change in system pressure was recorded and the volume of the ECP+ME+Valve 2+fixed-volume syringe with tubing was calculated using Boyle's law. The volume of this system was measured using a similar technique with the ECP valve closed to the upstream system. This volume was added to the ECP volume and subtracted from the system volume to estimate ME volume.
Figure 1.
Schematic diagram of the test system including the middle ear (ME), ear canal probe (ECP), Mass Spectrometer (Mass Spec), computer and related instrumentation. Not drawn to scale. See Methods section for details.
In preparation for each experiment the port of the ECP valve to Valve 2 was closed (i.e. the ECP was isolated) and the ports of Valve 2 were opened to the test gas delivery system and to the syringes, and the valve connecting the syringes to the environment was opened to atmosphere. A flow of the designated gas was applied through the flow meter to this open system for 2 minutes at a flow rate of 35 ml/min to “wash out” any non-test gases in the line upstream to the ECP. Then, the valve connecting the syringes to the atmosphere was closed, the ECP valve and Valve 2 were opened to all three ports and the test gas was washed through this system and the ECP and ME volume at a flow rate of 35 ml/min for approximately 3 minutes (See Figure 1). This caused the system pressure to increase, the Eustachian tube to open and the test gas to also wash through the ECP+ME volume. Toward the end of the washing period, the volume of the pressure-regulating syringe was oscillated to vary system pressure over approximately 10 cycles (pulsed system pressure) to promote gas mixing in the tympanum and mastoid volumes (see Figure 2 for an example). The ECP valve was closed to isolate the ECP+ME volume from Valve 2, the pinch valve to the MS was opened and a 30 second gas sample (approximately 10 μl) was taken for MS analysis of gas composition within the ECP+ME volume. This sequence was repeated 4 additional times for a total of 5 washes.
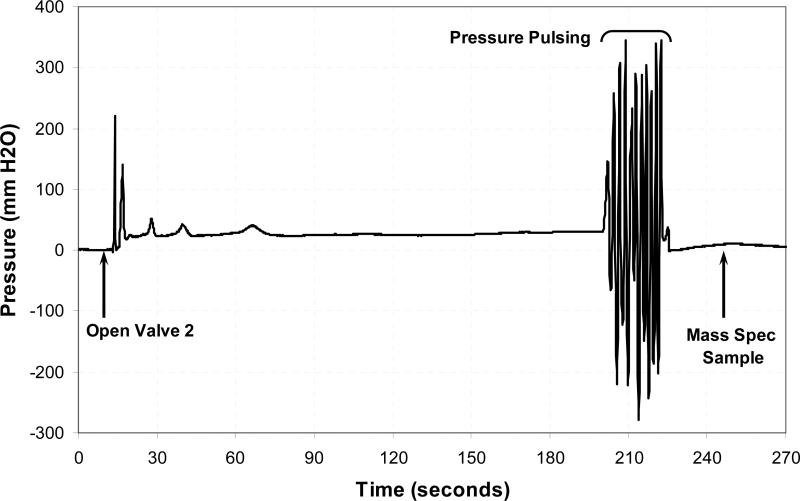
Figure 2.
System pressure as a function of time for one ME wash of approximately 3-minutes. The label, “Open Valve 2”, indicates the onset of ME exposure to the test gas. This is followed by an increase in pressure until the Eustachian tube opens which is followed by a stable equilibrium pressure. Toward the end of approximately 3-minute wash, the ME was subjected to a series of 10 pressure-pulses by varying the position of the volume syringe, and then the ME/ECP was isolated from the gas source. After that time a “Mass Spec sample” was obtained for gas analysis.
To measure the change in ECP/ME gas partial-pressures for each experiment, the final MS analysis from the washing period was used to calculate the baseline gas partial-pressures and a second MS sample was taken after a 10 minute test interval to calculate the post-test ECP/ME gas partial-pressures. During this 10-minute interval, the subject was instructed not to swallow. On the day of myringotomy, the test gas composition was 6%O2, Balance N2 (Experiment 1) and on the following day the test gas composition was 25%O2, 6%CO2, Balance N2 (Experiment 2).
Data Analysis
Past studies reported venous blood gas partial-pressures of approximately 50 mmHg for CO2, 40 mmHg for O2 and 570 mmHg for N2 [17], and these values are assumed in the following calculations. The exchange of gas (g) across a passive barrier (B) requires the existence of a transbarrier gas partial-pressure gradient to drive the exchange (GBg). Therefore, the composition of the two test gases was chosen to create a transMEM CO2 gradient of approximately 50 mmHg (Experiment 1) and a transMEM O2 gradient of approximately 150 mmHg (Experiment 2) while preserving the typical transMEM partial-pressure gradients for the other physiologic gases. The rate-constant for exchange of a gas, g across a barrier (KBg) is defined as the ratio of the rate of change in gas partial-pressure within a compartment (C1) due to transbarrier exchange (δPC1g/δt) divided by the average driving gradient between compartments, C1 and C2 (i.e. GC1-C2g) [3]. Therefore, the data analysis focused on estimating δPMECO2/δt and GMEMCO2 for Experiment 1 and δPMEO2/δt and GMEMO2 for Experiment 2 over the 10-minute test interval.
The data for analysis from each experiment were the atmospheric pressure, ECP/ME pressure, ME and ECP volumes and the initial (T=0 minutes) and final (T=10 minutes) MS ion currents at 18 (H2O), 28 (N2), 32 (O2), 40 (Ar) and 44 (CO2) Atomic Mass Units for the ECP+ME volume. At T=0 minutes, the partial-pressures of the physiologic gases were calculated by summing the ion currents for the 5 gas species and dividing the ion current for each gas species by the total ion current for the 5 gases to yield a fractional pressure for each gas. The fractional pressures were then multiplied by the ECP/ME pressure (calculated as the sum of atmospheric pressure and differential pressure measured by the online pressure transducer) to yield the respective gas partial-pressures. To determine the gas partial-pressures in the ECP+ME volume at T=10 minutes, the ion current for each gas at that time was divided by the respective ion current at T=0 minutes and that ratio multiplied by the calculated gas partial-pressure at T=0 minutes.
Estimates of δPMECO2/δt for Experiment 1 and δPMEO2/δt for Experiment 2 were calculated as the difference between the CO2 or O2 pressure at the end and beginning of the respective 10-minute test sessions divided by the duration of the test session in minutes (i.e. 10 minutes). The driving gradient for the interval was calculated as the difference between the expected blood gas partial-pressures for CO2 (50 mmHg) or O2 (40 mmHg) and the average of the CO2 or O2 pressures measured in the ECP volume at the beginning and end of the test interval. The rate-constants for transMEM CO2 and O2 exchange were calculated by dividing the rate of change/minute in ECP CO2 or O2 pressure by the average transMEM CO2 or O2 gradient for the 10-minute interval. For each experiment, gradient half-life for O2 and CO2 was calculated by iterating the following equation: GMEMg(t+1)=G MEMg(t)-G MEMg(t)*KMEMg over increasing values of “t” until GMEMg(t=x) was equal to 0.5*G MEMg(t=0), with the half-life defined as x in minutes.
The calculation of the transMEM rate-constants assumes that, during the 10-minute measurement interval, the only pathway for ME gas exchange is via the MEM. Gas leakage between the ECP and environment or gas exchange between the ME and nasopharynx via the Eustachian tube during the test period would bias the estimated rate-constants. For Experimental 1, such events will cause the measured O2 pressure within the ECP+ME volume to increase over the 10 minute test interval and, for Experiment 2, leakage would cause the CO2 pressure within the ECP+ME volume to decrease over the 10-minute interval as they approach atmospheric levels. Therefore, to determine if the estimates of the transMEM CO2 or O2 rate-constant are significantly biased, the percent change in O2 pressure for Experiment 1 and in CO2 for Experiment 2 within the ECP+ME volume between T=0 and T=10 minutes was calculated and compared to an expected value of 0%.
Comparisons of measured values between groups or between measured and expected values were evaluated for significance at alpha≤0.05 using a 2-tailed, two group or paired Student's t test, respectively. Relationships between variables were evaluated using the Pearson Product Moment Correlation Coefficient and by linear regression analysis. Throughout, the format average±standard deviation is used to summarize the data.
RESULTS
Ten subjects aged 22.1 to 47.7 (average=33.0±10.4) years were enrolled. There were 2 females and 8 males with a self-reported racial distribution of 5 White, 4 Black and 1 Asian; 2 subjects (#4 and #6) reported a history of otitis media during childhood. Six right ears and 4 left ears were studied. No adverse events were recorded and the pre- and post-myringotomy audiometric testing results were within 5 dB of the baseline values. The average duration of the TM perforation was 14±8 (range 1 to 25) days. Two subjects (#2 and #10) healed their TM perforation within 1 day of the myringotomy and could not complete Experiment 2 (transmucosal O2 Exchange).
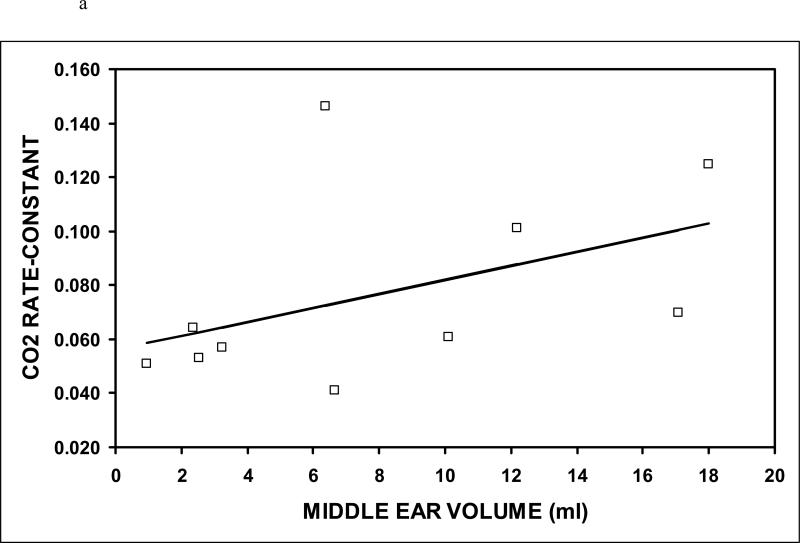
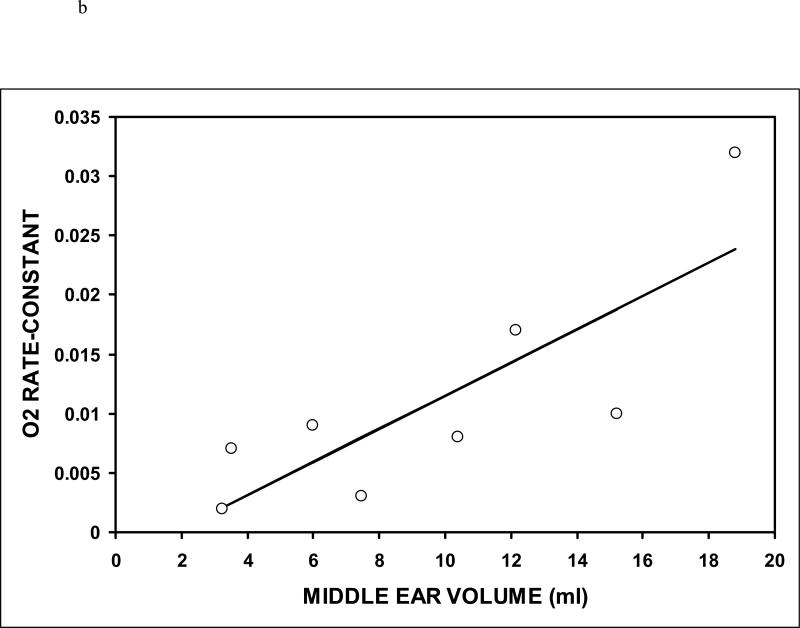
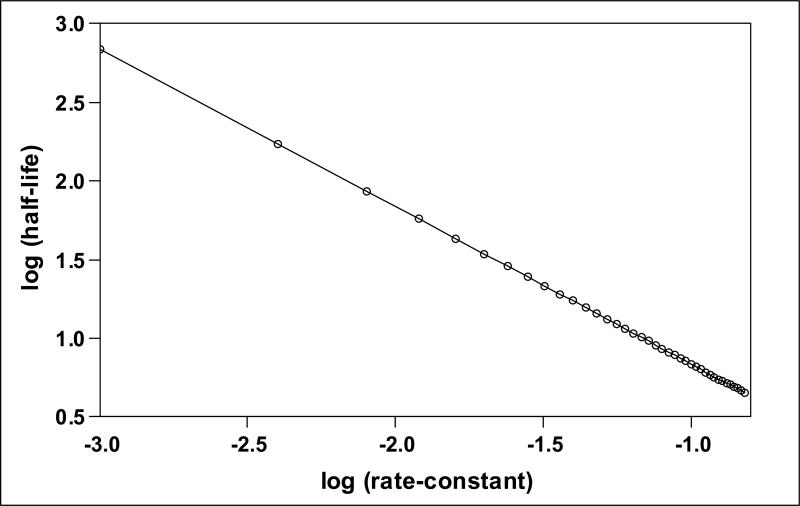
Table I summarizes the data for Experiment 1 and Table II summarizes the data for Experiment 2. The average estimated ME volume was variable across subjects for both Experiments, but was highly correlated for Experiments 1 and 2 (r=.99, p<0.001). The average estimated transmucosal CO2 and O2 rate-constants were 0.062±0.034 (N=10, range: 0.032-0.119) and 0.011±0.009 (N=8, range: 0.002-0.032) mmHg/min/mmHg, respectively. The O2 (t=3.22, P=0.015) and CO2 rate-constants (t=5.81, p<0.001) were significantly different from a value of 0 mmHg/min/mmHg. The CO2 rate-constant was significantly greater than the O2 rate-constant (t=4.13, p<0.001) and the average CO2:O2 rate-constant ratio for the 8 subjects with paired CO2 and O2 data was 8.1±4.0 (range 3.6-14.6). That ratio excludes a value of 20 which is approximately the value expected for diffusion-limited transMEM exchange across a primarily water barrier (t=-7.72, p<0.001), but does not exclude a value of 10 which is approximately the expected ratio for diffusion-limited transMEM exchange across a primarily lipid barrier (t=-1.33, p=0.225). There was a positive linear relationship between the CO2 (r=0.46) and the O2 (r=0.81) rate-constants and ME volume (see Figure 3), but only the relationship for O2 was statistically significant (CO2: p=0.182; O2: p=0.014). There was a log linear relationship (r=-1.00, p<0.001) between the measured rate-constant and the half-life of the established gradient (see Figure 4). For CO2, the average, minimum and maximum gradient half-life was 11.1, 5.9 and 21.6 minutes and for O2 those values were 61.6, 21.7 and 279.0 minutes.
TABLE I.
Values of Study Variables for Calculation of Transmucosal CO2 Rate-Constant (KMEMCO2) for each Subject, and the Average (AVG) and Standard Deviation (STD) of those Values
| PARAMETER/SUBJECT | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | AVG | STD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ECP Volume | 0.40 | 0.43 | 0.43 | 0.40 | 0.45 | 0.48 | 0.40 | 0.42 | 0.44 | 0.40 | 0.43 | 0.03 |
| ME Volume | 0.94 | 2.24 | 6.37 | 9.94 | 17.67 | 6.03 | 16.83 | 2.11 | 11.94 | 2.98 | 7.71 | 6.14 |
| ME Pressure, T=0 | 768.7 | 769.6 | 767.1 | 771.5 | 762.8 | 771.0 | 765.8 | 762.5 | 764.2 | 763.4 | 766.7 | 3.4 |
| PMECO2, T=0 | 5.2 | 6.8 | 6.6 | 6.7 | 15.4 | 4.5 | 12.5 | 6.0 | 14.0 | 6.3 | 8.4 | 4.0 |
| PMECO2, T=10 | 18.9 | 18.9 | 19.1 | 25.0 | 40.7 | 38.5 | 30.7 | 20.7 | 36.4 | 20.5 | 26.9 | 8.8 |
| δPMECO2/min | 1.4 | 1.2 | 1.3 | 1.8 | 2.5 | 3.4 | 1.8 | 1.5 | 2.2 | 1.4 | 1.9 | 0.7 |
| GMEMCO2 | 38.0 | 37.1 | 37.2 | 34.2 | 22.0 | 28.5 | 28.4 | 36.6 | 24.8 | 36.6 | 32.3 | 5.9 |
| KMEMCO2 | 0.036 | 0.032 | 0.034 | 0.053 | 0.115 | 0.119 | 0.064 | 0.040 | 0.090 | 0.039 | 0.062 | 0.034 |
| PMEO2, T=0 | 40.2 | 44.9 | 62.8 | 46.1 | 43.5 | 40.0 | 52.0 | 54.8 | 50.9 | 47.0 | 48.2 | 7.1 |
| PMEO2, T=10 | 41.7 | 47.0 | 62.1 | 46.4 | 45.4 | 40.9 | 48.5 | 50.0 | 60.4 | 44.1 | 48.6 | 7.2 |
| %change O2 | 3.7 | 4.5 | -1.2 | 0.6 | 4.3 | 2.1 | -6.7 | -8.8 | 18.7 | -6.2 | 1.1 | 7.9 |
Time measured in minutes, Volume measured in ml, Pressure in mmHg, Rate-Constant in mmHg/min/mmHg
TABLE II.
Values of Study Variables for Calculation of Transmucosal O2 Rate-Constant (KMEMO2) for each Subject, and the Average (AVG) and Standard Deviation (STD) of those Values
| PARAMETER/SUBJECT | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | AVG | STD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ECP Volume | 0.40 | ND | 0.43 | 0.40 | 0.45 | 0.48 | 0.40 | 0.42 | 0.44 | ND | 0.43 | 0.03 |
| ME Volume | 3.24 | 7.48 | 10.37 | 18.81 | 6.00 | 15.22 | 3.53 | 12.15 | 9.60 | 5.58 | ||
| ME Pressure, T=0 | 769.8 | 766.5 | 777.1 | 762.5 | 764.6 | 768.3 | 770.2 | 769.9 | 768.6 | 4.4 | ||
| PMEO2, T=0 | 158.9 | 153.0 | 163.5 | 145.6 | 158.6 | 158.5 | 159.6 | 145.1 | 155.4 | 6.8 | ||
| PMEO2, T=10 | 156.0 | 149.5 | 153.7 | 116.6 | 147.9 | 146.8 | 151.2 | 128.7 | 143.8 | 13.8 | ||
| δPMEO2/min | -0.3 | -0.3 | -1.0 | -2.9 | -1.1 | -1.2 | -0.8 | -1.6 | -1.2 | 0.8 | ||
| GMEMO2 | -117.4 | -111.2 | -118.6 | -91.1 | -113.2 | -112.6 | -115.4 | -96.9 | -109.6 | 10.0 | ||
| KMEMO2 | 0.002 | 0.003 | 0.008 | 0.032 | 0.009 | 0.010 | 0.007 | 0.017 | 0.011 | 0.009 | ||
| PMECO2, T=0 | 64.4 | 62.0 | 60.7 | 63.2 | 57.7 | 63.0 | 55.7 | 56.4 | 60.4 | 3.4 | ||
| PMECO2, T=10 | 65.6 | 63.7 | 61.0 | 76.7 | 64.6 | 68.0 | 57.5 | 72.3 | 66.2 | 6.1 | ||
| %change CO2 | 1.8 | 2.8 | 0.5 | 21.2 | 12.0 | 7.9 | 3.2 | 28.2 | 9.7 | 10.1 |
Time measured in minutes, Volume measured in ml, Pressure in mmHg, Rate-Constant in mmHg/min/mmHg
ND-Not Done due to closure of TM perforation
Figure 3.
The rate-constants for CO2 (a) and O2 (b) as a function of the measured ME volume. Open symbols indicate the individual data points and the solid lines represent the solutions to the respective regression equations.
Figure 4.
The linear relationship between the log of the gradient half-life and the log of the rate-constant. Open circles indicate individual points for each paired value and the solid line represents the solution to the regression equation.
For Experiment 1, only 1 subject (#9) evidenced a large percent loss in O2 pressure during the experimental period, but the difference between the average percent change in O2 pressure and a value of 0% was not significant (t=0.44, P=0.671). For Experiment 2, all subjects showed a percent change in CO2 pressure during the experimental period and this was significantly different from a value of 0% (t=2.71, P=0.030). In all cases, CO2 in the ECP+ME pressure increased as opposed to the expected decrease if the change was due to a system leak. These results rule out a significant effect of system leaks on the reported results.
DISCUSSION
Past studies used indirect methods to demonstrate transMEM exchange of CO2 and O2 in humans but did not quantify those exchange rates [7-12, 14, 15]. However, the rate of transMEM exchange as measured by the rate-constant for each of the physiologic gases defines the trajectory of ME pressure change during periods between functional Eustachian tube openings [4] and the purpose of the present study was to estimate the rate-constants for transMEM CO2 and O2 exchange in the adult human ME. To that end, the ME was washed with gas compositions specifically chosen to create significant Blood to ME CO2 and ME to Blood O2 driving gradients while preserving the approximate extant gradients for the other physiologic gases. Gas partial-pressures were measured at 0 and 10 minutes in a small volume ECP open to the ME via a TM perforation using an on-line MS. Rate-constants for the exchange of CO2 and O2 were estimated by dividing the rate of change in O2 and CO2 partial-pressures/minute by their estimated transMEM gradients calculated as the average difference between the expected blood gas partial-pressures [17] and the measured ECP/ME partial-pressures. As expected, the results showed that the rate-constants for both gases were significantly greater than 0 mmHg/min/mmHg (i.e. transMEM exchange of those gases occurred at measurable rates) and that the estimated CO2 rate-constant was significantly greater than that for O2. For both gases, the estimated rate-constant was a linear function of ME volume but the relationship was significant only for O2. Also, the log of the gradient half-life was significantly correlated with the log of the rate-constant.
For diffusion-limited gas exchange, the rate-constant is a function of the product of the diffusivity and solubility of the gas in the barrier (Krogh's coefficient), the surface area available for exchange and the inverse of the barrier thickness between exchange compartments [18]. For the ME, if the mucosal perfusion rate, MEM thickness and MEM surface area are unaffected by the experimental conditions, the ratio of rate-constants (or volume exchange rates) for paired, diffusion-limited gases should approximate the ratio of the Krogh's coefficients for those gases. Past studies of the transMEM exchange of physiologic gases in monkeys [3, 4] and mathematical modeling of the changes in ME gas volume after “washing” the human ME with air yielded an estimated CO2:O2 rate-constant ratio of approximately 20 which is not different from the predicted Krogh's coefficient ratio for diffusion-limited gas exchange across a water barrier. In contrast, the results of the present study yielded an average CO2:O2 rate-constant ratio of 8.1 which is more similar to Krogh's coefficient ratio for diffusion-limited gas exchange across a lipid barrier (≈10). One previous study in guinea pigs also measured a CO2:O2 rate-constant ratio more consistent with exchange across a lipid barrier [6, 19]. The possible reasons for these discrepant results are multiple and cannot be addressed using the data available from the present study, but it should be noted that only the present study and that conducted in guinea pigs directly measured the changes in gas partial-pressures using MS.
There are a number of possible factors that could have affected the measurement of the O2 and CO2 rate-constants in the current study. Some of these result from the fact that while gas exchange is limited to the volume of the ME, gas partial-pressures were measured in a non-exchange compartment, the ECP volume. Slow diffusive exchange between these compartments could bias the measured partial-pressures in the ECP but we discount this for two reasons. Diffusive gas exchange within the ME (i.e. tympanum+mastoid) in humans was measured to be very fast [20] and the same can be expected for gas exchange between the ME and ECP, especially after the washing protocol used. Also, the MS sampling from the ECP causes ME gas to be actively transferred to the ECP in response to the evacuation of gas from that structure. A related concern is that the measurement of change in partial-pressures for the ME+ECP volume underestimates the change in partial-pressures for the ME volume in isolation. However, corrections can be applied using Boyle's law to adjust for this effect by multiplying the rate-constant by the ratio of the ECP+ME volume to ME volume. However, on average this correction would only increase the CO2 rate-constant by a factor of 1.09±0.07 and the O2 rate-constant by a factor of 1.06±0.04, which we consider to be insignificant.
Also, while the 10-minute time interval and established CO2 gradient allowed for a rather large change in the average δPCO2/10 minutes of 19.0±7.0 mmHg/10 minutes, this was not true of for the established O2 gradient where the average δPO2/10 minutes was 11.6±8.3 mmHg/10 minutes. Expectedly, smaller pressure changes should be more susceptible to errors in measurements by the techniques used. Finally, the myringotomy exposed the MEM to non-physiologic, atmospheric gas partial-pressures and, similarly, the test gases were not physiologic for the ME. Exposure of the MEM to these altered gas partial-pressures may have changed the physiochemical properties of the MEM thereby affecting the rate-constants for the two gases.
Nonetheless, the methods outlined in the present study provide a protocol for directly measuring the transMEM exchange of physiologic gases for the human ME. A limitation of this approach is the need for an invasive procedure (myringotomy) to expose the ME to the measuring system. As expected, gradient-driven, transMEM exchange of CO2 and O2 was measurably different from a value of 0 mmHg/min/mmHg and the rate-constant for CO2 exchange was greater than that for O2 exchange. Future experiments should attempt to make more accurate measures of the O2 rate-constant by including a longer test interval for that gas, clarify if the MEM acts primarily as a lipid, water or mixed barrier to physiologic gas exchange and apply similar techniques to estimate the rate-constant for the other physiologic gas, N2.
ACKNOWLEDGEMENTS
This work was supported in part by a grant from the National Institutes of Health (P50 DC007667). The investigators thank Dennis Kitsko, DO, Richard Villardo, MD, Jennifer McLevy, MD, and Michael Cohen, MD for their assistance with various aspects of the study.
Supported in part by a grant from the National Institutes of Health (P50 DC007667)
Footnotes
Conflict of Interest Statement
None of the authors have any real or potential conflicts of interest.
REFERENCES
- 1.Kanick SC, Doyle WJ. Barotrauma during air travel: predictions of a mathematical model. J Appl Physiol. 2005;98(5):1592–1602. doi: 10.1152/japplphysiol.00974.2004. [DOI] [PubMed] [Google Scholar]
- 2.Bluestone CD, Doyle WJ. Anatomy and physiology of eustachian tube and middle ear related to otitis media. J Allergy Clin Immunol. 1988;81(5 Pt 2):997–1003. doi: 10.1016/0091-6749(88)90168-6. [DOI] [PubMed] [Google Scholar]
- 3.Doyle WJ, Seroky JT. Middle ear gas exchange in rhesus monkeys. Ann Otol Rhinol Laryngol. 1994;103(8 Pt 1):636–645. doi: 10.1177/000348949410300811. [DOI] [PubMed] [Google Scholar]
- 4.Doyle WJ, Seroky JT, Alper CM. Gas exchange across the middle ear mucosa in monkeys. Estimation of exchange rate. Arch Otolaryngol Head Neck Surg. 1995;121(8):887–892. doi: 10.1001/archotol.1995.01890080055011. [DOI] [PubMed] [Google Scholar]
- 5.Doyle WJ, Alper CM, Seroky JT. Trans-mucosal inert gas exchange constants for the monkey middle ear. Auris Nasus Larynx. 1999;26(1):5–12. doi: 10.1016/s0385-8146(98)00060-1. [DOI] [PubMed] [Google Scholar]
- 6.Mover-Lev H, Sade J, Ar A. Rate of gas exchange in the middle ear of guinea pigs. Ann Otol Rhinol Laryngol. 1998;107(3):194–198. doi: 10.1177/000348949810700302. [DOI] [PubMed] [Google Scholar]
- 7.Hergils L, Magnuson B. Morning pressure in the middle ear. Arch Otolaryngol. 1985;111(2):86–89. doi: 10.1001/archotol.1985.00800040050004. [DOI] [PubMed] [Google Scholar]
- 8.Shinkawa H, Okitsu T, Yusa T, Yamamuro M, Kaneko Y. Positive intratympanic pressure in the morning and its etiology. Acta Otolaryngol Suppl. 1987;435:107–111. doi: 10.3109/00016488709107358. [DOI] [PubMed] [Google Scholar]
- 9.Hergils L, Magnuson B. Regulation of negative middle ear pressure without tubal opening. Arch Otolaryngol Head Neck Surg. 1988;114(12):1442–1444. doi: 10.1001/archotol.1988.01860240092030. [DOI] [PubMed] [Google Scholar]
- 10.Miura M, Takahashi H, Honjo I, Hasebe S, Tanabe M. Influence of the gas exchange function through the middle ear mucosa on the development of sniff-induced middle ear diseases. Laryngoscope. 1998;108(5):683–686. doi: 10.1097/00005537-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Ikarashi F. The effect of respiratory mode on human middle ear pressure. Auris Nasus Larynx. 1998;25(4):349–354. doi: 10.1016/s0385-8146(98)00011-x. [DOI] [PubMed] [Google Scholar]
- 12.Ikarashi F, Tsuchiya A. Middle ear gas exchange via the mucosa: estimation by hyperventilation. Acta Otolaryngol. 2008;128(1):9–12. doi: 10.1080/00016480701200269. [DOI] [PubMed] [Google Scholar]
- 13.Doyle WJ. Increases in middle ear pressure resulting from counter-diffusion of oxygen and carbon dioxide into the middle ear of monkeys. Acta Otolaryngol. 1997;117(5):708–713. doi: 10.3109/00016489709113464. [DOI] [PubMed] [Google Scholar]
- 14.Aoki K, Mitani Y, Tuji T, Hamada Y, Utahashi H, Moriyama H. Relationship between middle ear pressure, mucosal lesion, and mastoid pneumatization. Laryngoscope. 1998;108(12):1840–1845. doi: 10.1097/00005537-199812000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Uchimizu H, Utahashi H, Hamada Y, Aoki K. Middle ear total pressure measurement as a useful parameter for outcome prediction in pediatric otitis media with effusion. Int J Pediatr Otorhinolaryngol. 2005;69(12):1659–1665. doi: 10.1016/j.ijporl.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 16.Fink N, Ar A, Sade J, Barnea O. Mathematical analysis of atelectasis formation in middle ears with sealed ventilation tubes. Acta Physiol Scand. 2003;177(4):493–505. doi: 10.1046/j.1365-201X.2003.01096.x. [DOI] [PubMed] [Google Scholar]
- 17.Hergils L, Magnuson B. Human middle ear gas composition studied by mass spectrometry. Acta Otolaryngol. 1990;110(1-2):92–99. doi: 10.3109/00016489009122520. [DOI] [PubMed] [Google Scholar]
- 18.Ranade A, Lambertsen CJ, Noordergraaf A. Inert gas exchange in the middle ear. Acta Otolaryngol Suppl. 1980;371:1–23. [PubMed] [Google Scholar]
- 19.Doyle WJ. Mathematical model explaining the sources of error in certain estimates of the gas exchange constants for the middle ear. Ann Otol Rhinol Laryngol. 2000;109(6):533–541. doi: 10.1177/000348940010900602. [DOI] [PubMed] [Google Scholar]
- 20.Raveh E, Sade J, Mover-Lev H, Guney S. Mastoid buffering properties: I. Gas partial pressures. Ann Otol Rhinol Laryngol. 1999;108(8):750–755. doi: 10.1177/000348949910800807. [DOI] [PubMed] [Google Scholar]