Abstract
Problem
Decidual macrophages are thought to promote pregnancy success, in part through interactions with invading trophoblast cells in hemochorial placentation. However, the factors that constitute this regulatory cross-talk are not well understood.
Method of Study
Rhesus monkey decidual and peripheral blood-derived macrophages were co-cultured with primary rhesus trophoblasts. Macrophage functions including cell surface marker expression, antigen uptake and processing, in vitro migration, and cytokine and chemokine secretion were evaluated.
Results
While most macrophage functions were unchanged by trophoblast co-culture, changes in the secretion of selected cytokines and the migration of trophoblasts were noted when decidual (but generally, not peripheral blood monocyte-derived) macrophages were cultured with trophoblasts. In addition, basal secretion differed significantly between peripheral blood-derived and decidual macrophages for a broad spectrum of cytokines. When trophoblasts were pre-treated with an anti Mamu-AG antibody, 25D3, there was no change in cytokine or chemokine secretion.
Conclusions
Macrophage cytokine expression can be modulated by trophoblast co-culture, but it remains unclear how Mamu-AG is involved.
Keywords: deciduas, macrophage, HLA-G, trophoblast, chemokine, cytokine
Introduction
The semi-allogenic embryo is able to avoid a detrimental immune response by the mother allowing it to implant and develop despite its expression of paternal molecules. The exact mechanisms contributing to maternal-fetal tolerance remain incompletely understood, however, it has been suggested that the expression of Human Leukocyte Antigen-G (HLA-G) on invading trophoblasts may contribute to successful pregnancy1, 2. HLA-G was first reported to be expressed on extravillous human placental trophoblasts3, and trophoblasts are considered the primary site of HLA-G expression4. HLA-G differs from the classical major histocompatibility complex (MHC) class I molecules HLA-A, - B, and –C, expressed on most somatic cells, in that HLA-G has limited polymorphism and restricted tissue distribution. In vitro studies have shown that HLA-G can modulate T cell, NK cell (see Carosella 20085 for a recent review), and macrophage function6, 7, therefore, HLA-G may be important for pregnancy success because of its ability to modulate decidual immune cell responses and establish an appropriate environment for implantation and placental development1.
The rhesus monkey molecule Mamu-AG (designated Mamu for Macaca mulatta) is considered the functional homolog of HLA-G and shares many characteristics including restricted tissue distribution and limited polymorphism. Mamu-AG is most highly expressed in the placenta and is found on both invading extravillous trophoblasts and villous syncytiotrophoblasts8. Previously, our lab has passively immunized pregnant monkeys against Mamu-AG in the second and third weeks of gestation9. Many effects were noted, including a delay in placental development and villous blood vessel formation and decreased remodeling of maternal spiral arterioles by invading trophoblasts in the decidua. Changes in the decidua also included a failure to initiate DC-SIGN expression in a subset of decidual macrophages, an expected response to embryo implantation in rhesus monkeys10. This suggests that Mamu-AG is important for the establishment of a successful pregnancy, and the effect of anti-Mamu-AG treatment on DC-SIGN expression indicates macrophages may be involved in the Mamu-AG response9.
The primate uterus contains numerous leukocytes, primarily natural killer cells (NK cells) and macrophages, with relatively few T or B cells11. During early human pregnancy, NK cells make up 30–40% of the total cells in the uterine decidua and macrophages account for 10–15% of the total cells11. Likewise, NK cells and macrophages are also observed at high density in the rhesus monkey and represent up to one-third of the total decidual cells12. After implantation, macrophages congregate at the implantation site, around blood vessels, and close to invading trophoblasts of the placenta12, 13. These cells are thought to play an important role in the maternal-fetal immune response. Specifically, the balance of cytokines, growth factors, and chemokines present at the maternal-fetal interface may provide important communication between the invading placenta cells and immune cells in the uterus14–16. This communication may also be important in the response to infection or pathologies of placentation (reviewed in Koga and Mor 201017).
Study of the maternal-fetal interface in human implantation is difficult because of the lack of access to the early stages of implantation and limited availability to conduct experiments in early human pregnancy. In addition, due to the unique MHC class I expression profile of trophoblasts2,3,4,45 of the primate placenta and their recognition by decidual NK cells and macrophages, it is difficult to extrapolate data from non-primate species lacking homologous MHC class 1 expression to human implantation. We have used a rhesus monkey model to study the effects of trophoblasts on macrophages to determine if the implanting trophoblasts cells direct cytokine responses from the maternal macrophages (or vice versa) to promote successful implantation. We found that decidual macrophages had a significantly different secretory profile from peripheral blood monocyte-derived macrophages. In addition, changes in the secretion of multiple cytokines when macrophages were co-cultured with trophoblasts were noted, suggesting that trophoblasts can alter the maternal immune response and optimize the uterine environment for implantation.
Materials and Methods
Animals
Rhesus monkeys (Macaca mulatta) were from the colony maintained at the Wisconsin National Primate Research Center. All surgical procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and under the approval of the University of Wisconsin Graduate School Animal Care and Use Committee. A total of 14 monkeys were used for surgical fetectomy.
Cell culture
Rhesus monkey decidual macrophages were isolated from the decidual tissue on Days 36 or 37 of pregnancy (equivalent to week 8 in human pregnancy) by enzymatic digestion with DNAse and collagenase IV (Sigma, St. Louis, MO) and density gradient centrifugation as previously described 18. Briefly, cells were suspended in 30 mL RPMI-1640 then slowly layered over 15 mL of Ficoll-Paque (GE Healthcare, Piscataway, NJ), and centrifuged at 800 × g for 30 minutes at room temperature. Mononuclear cells were collected, washed in RPMI-1640 (Gibco, Grand Island, NY), labeled with nonhuman primate anti-CD14 microbeads (Miltenyi Biotec Auburn, CA) and sorted by MACS on an LS column (Miltenyi Biotec). The resulting CD14-positive cells were plated according to the experimental design.
Peripheral blood was obtained from female non-pregnant monkeys of reproductive age. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by Ficoll gradient centrifugation and monocytes were separated by adhesion for 24 hours. The cells were then washed twice with RPMI and cultured for five days in RPMI-1640 supplemented with 1% human serum, 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 0.1 mM sodium pyruvate, 1% non-essential amino acids, 5 × 10−5 M 2-mercaptoethanol, 20 ng/mL M-CSF, and 10 ng/mL IL-1β19. The differentiated macrophages were washed, scraped, counted, and re-plated according to the experimental design.
Villous cytotrophoblasts were isolated from first trimester placentas by trypsin/DNAse (Sigma) enzymatic digestion and Percoll (Kabi Pharmacia AB, Uppsala, Sweden) gradient centrifugation as previously described18.
To study the effect of Mamu-AG on decidual macrophages, we used the MHC null human B lymphoblastoma cell line 721.221 20 (control), and 721.221 cells stably transfected with an expression vector for Mamu-AG21.
During these experiments all cells were cultured in RPMI-1640 supplemented with 2 mM L-glutamine (Gibco), 100 U/ml penicillin (Gibco), 100 µg/ml streptomycin (Gibco), 0.1 mM sodium pyruvate (Gibco), 1% non-essential amino acids (Gibco), 50 µM 2-mercaptoethanol (Gibco), 1% heat-inactivated human AB serum (Sigma, St. Louis, MO), 20 ng/mL M-CSF (Peprotech, Rocky Hill, NJ), and 10 ng/mL IL-1β (Peprotech). Cells were cultured in 96-well culture dishes at a density of 50,000 of each cell type per well in 150 uL of media.
Flow Cytometric analysis of cell surface marker expression
Macrophages were incubated with saturating concentrations of monoclonal antibodies for 25 minutes at 4°C in PBS supplemented with 2% fetal calf serum (FCS) (Atlanta Biological, Lawrenceville, CA). Antibodies included FITC conjugated anti-CD64 or anti-CD206, PE conjugated anti-CD16, anti-CD86, or anti-CD83, PerCP-Cy5.5 conjugated anti-HLA-DR, or APC conjugated anti-CD14 (BD Bioscience, Bedford, MA).
Isotype-matched non specific antibodies at the same concentration were used as the controls. Cells were washed with PBS supplemented with 2% FCS and resuspended in 2% paraformaldehyde. Flow cytometry data collection was done on a FACSCalibur flow cytometer (BD Biosciences) with Cell Quest software (BD Biosciences). The data were analyzed using Flow Jo software (Tree Star). Macrophages were separated from trophoblasts for analysis based on CD14 positive staining.
Cytokine analysis
Culture media were collected at 24 and 48 hours after culture initiation. The levels of CSF3 (G-CSF), CSF2 (GM-CSF), CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β), TNF, VEGF, IL-1ra, IL-6, CXCL8 (IL-8), and IL-18 in the culture media were determined using a Non-Human Primate Cytokine Milliplex Map Kit (Millipore, Billerica, MA) analyzed with a Luminex 100 instrument (HLA/Molecular Diagnostics Lab, UW-Madison Hospital and Clinics). The levels of IL-10 and TGF- β1 in the culture media were determined using a rhesus monkey IL-10 ELISA (U-CyTech biosciences, CM Utrecht, The Netherlands) and multispecies TGF-β1 ELISA (Invitrogen, Camarillo, CA), respectively.
Antigen uptake and processing
To determine the phagocytic and antigen processing ability of peripheral blood monocyte-derived macrophages, macrophages were incubated in culture medium with 100 ug/mL FITC labeled DQ-ovalbumin (Invitrogen, Carlsbad, CA) for 1 hour at 4°C or 37°C. Cells were washed with PBS supplemented with 2% FCS and mean fluorescence intensity was determined on a flow cytometer and analyzed as described above.
Migration Assay
To determine if the migration of trophoblasts or macrophages was altered during co-culture cells were plated in FluoroBlok multiwell inserts (BD Falcon, Bedford, MA) and cultured for 48 hours. Cells in the insert were stained with 16.6 ug/mL CellTracker Green CMFDA (Invitrogen) for 45 minutes at 37°C prior to culture. Photomicrographs were taken for quantification of the cells that had passed through the insert.
Antibody treatment
To determine if Mamu-AG was involved in the macrophage response to trophoblasts, trophoblasts were incubated with 25D3 F(ab′)2, a Mamu-AG-specific antibody, for 30 minutes prior to co-culture with macrophages. F(ab′)2 fragments were used to remove the potential confounder of Fc binding to immune cells causing indirect activation.
Statistics
Wilcoxon matched pairs test (macrophage trophoblast co-cultures) or Student’s t test (surface marker expression) were done to define statistically significant differences using Prism Graph Pad software. Differences were considered significant when P < 0.05.
Results
Surface marker expression
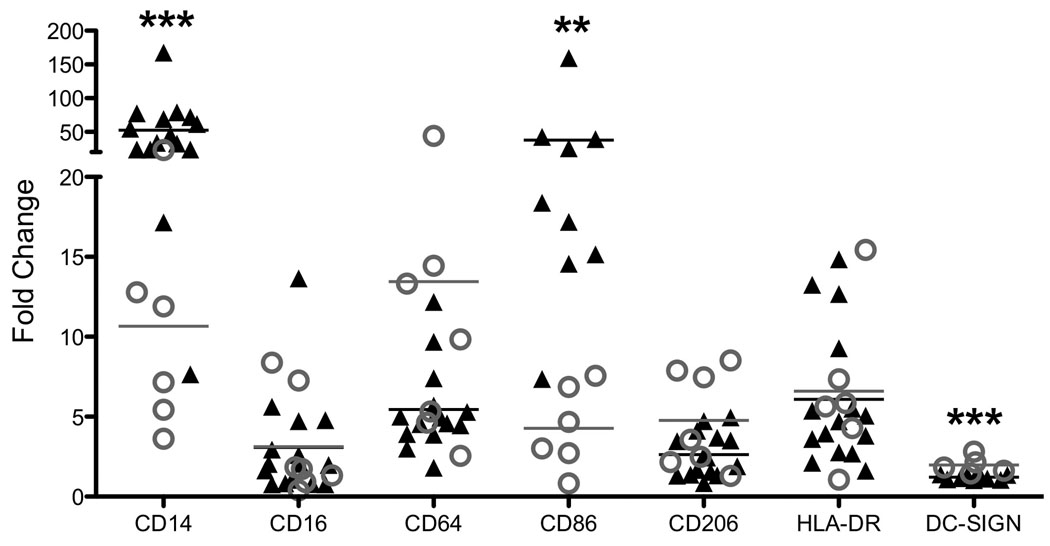
We have previously described the surface marker phenotype of peripheral monocyte-derived 19 and decidual10 macrophages. Briefly, the overall phenotype varies somewhat from monkey to monkey but CD14, CD16, CD64, CD86, CD206, and HLA-DR are expressed on both peripheral derived and decidual macrophages. The overall expression of CD14 and CD86 are significantly lower in decidual macrophages than peripheral-derived macrophages, and while DC-SIGN is expressed in a subset of decidual macrophages (10–20%), there is essentially no DC-SIGN expression in peripheral derived macrophages (Fig. 1).
Figure 1.
Flow cytometric analysis of decidual (gray open circle) and peripheral monocyte-derived (black triangle) macrophages. The data from peripheral derived macrophages has been previously published and is included for comparison with decidual cells19. Data are presented as fold increase over the background fluorescent intensity (MFI) of the Isotype control; each data point represents results from an individual animal. Statistical analysis was done to compare decidual to peripheral blood-derived macrophages. ** p-value < 0.01, *** p-value < 0.001
Cytokine production in response to trophoblasts
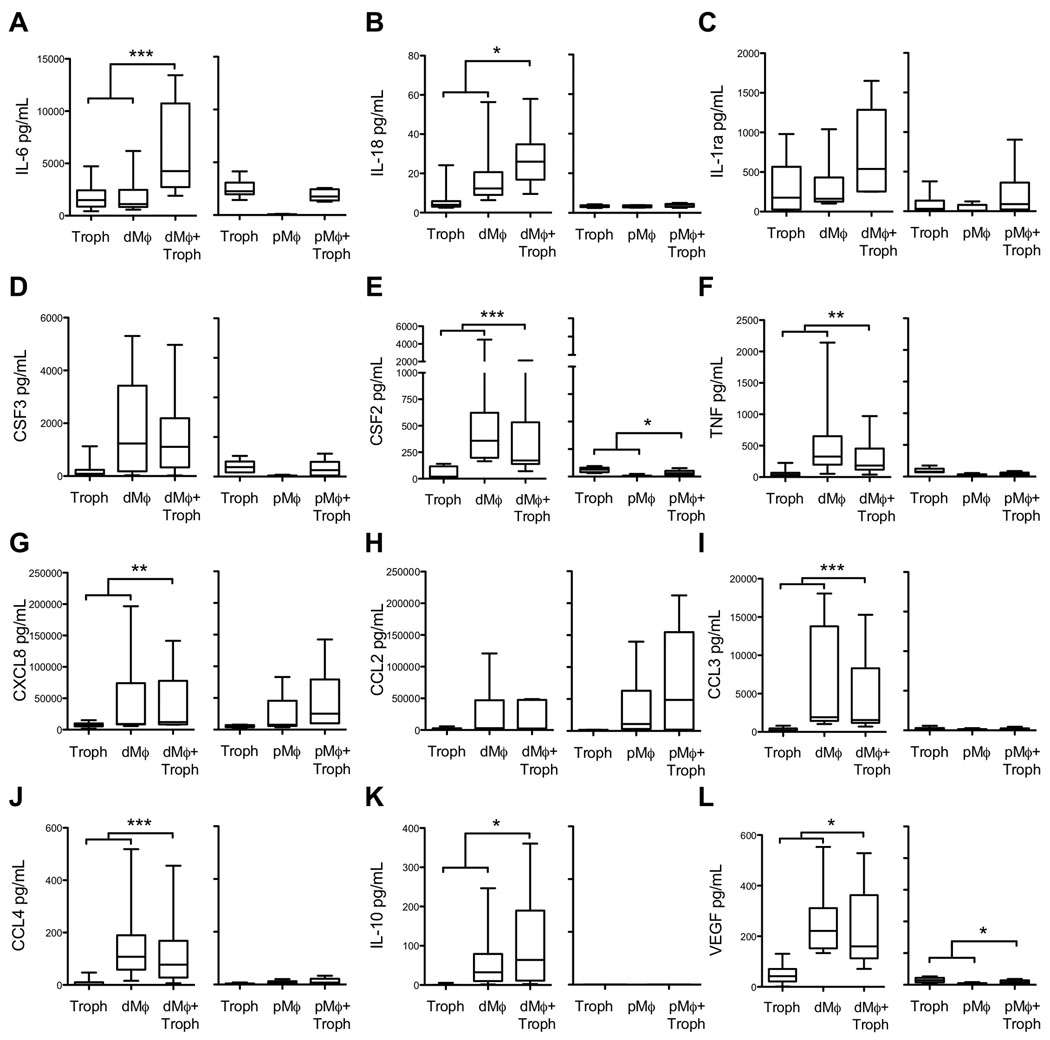
The local cytokine profile of the decidua affects the function of macrophages, NK cells, and T cells as well as the surrounding tissue cells including invasive trophoblasts 14–16. We wanted to determine if trophoblasts could change the profile of the cytokines produced by macrophages. To do this, trophoblasts were co-cultured with autologous decidual and peripheral blood-derived macrophages and media was collected at 24 and 48 hours for cytokine analysis. Figure 2 presents the 24hr results of these experiments, with decidual and peripheral-monocyte derived cell co-cultures presented side by side for each cytokine or chemokine. Due to the timing of surgery for collection of decidual macrophages and trophoblasts, peripheral blood-derived macrophage experiments were done separately from decidual macrophage co-culture experiments using autologous frozen trophoblasts. Fresh vs. frozen trophoblast cytokine secretion was not significantly different, suggesting that it was appropriate to use trophoblasts previously frozen (compare the two trophoblast bars within panels A-L of Fig. 2).
Figure 2.
Cytokine measurement in media from trophoblasts, macrophages, and macrophages co-cultured with trophoblasts for decidual macrophages (dMϕ) and peripheral derived macrophages (pMϕ) cultured for 24 hours. Data represents 14 separate experiments. Statistical significance was determined by Wilcoxon matched pairs test, comparing the sum of trophoblasts and macrophages cultured individually to macrophage+trophoblast co-cultures to account for the additive effect (Table 1). *p-value < 0.05, ** p-value < 0.01, *** p-value < 0.001
Decidual macrophages secrete higher levels of all the measured cytokines than peripheral blood-derived macrophages (compare decidual with peripheral derived macrophages within panels A-L of Fig. 2), with the exception of CXCL8 and CCL2, which were found in similar concentration (Fig. 2G and 2H, respectively). In several instances, measurement of cytokine and chemokine levels in the co-culture media indicated there might be synergistic interactions between trophoblasts and decidual macrophages. In order to test this directly, the secretion of cytokines and chemokines in co-culture (Fig. 2) was further compared with the sum of individual secretion by trophoblasts and macrophages (Table 1). Significant positive synergistic interactions were noted for the secretion of IL-6, IL-18, and IL-10. On the other hand, statistically significant decreases were noted for the secretion of CSF2, TNF, CXCL8, CCL3, CCL4, and VEGF. There were no statistically significant differences for IL-1ra, CSF3, or CCL2, and therefore, these will not be evaluated in subsequent figures. Similar analysis was done for the peripheral blood-derived macrophages, however, only CSF2 and VEGF were significantly decreased in peripheral blood-derived macrophage-trophoblast co-cultures (Fig. 2 and Table 1). Cytokine concentrations in media collected at 24 hours is shown and similar results were seen at 48 hours. Finally, the following cytokines were below the detectable limit of the assay: TGFα, TGFβ, IL-2, IL-5, IL-15, and IL-12(p40 and p70).
Table 1.
Cytokine measurements in media (pg/mL ± SEM) of macrophage and trophoblast cultures. The trophoblast, macrophage, and co-culture data are the same data presented in Figure 2. To investigate the synergistic effect of the two cell types in coculture, the p value was determined by comparing the sum of individually cultured trophoblasts plus individually cultured macrophages (column labeled Sum of Individual Troph and Macro) to macrophages co-cultured with trophoblasts (column labeled Coculture Macro-Troph).
| Cytokine | Trophoblast | Macrophage | Sum of Individual Troph and Macro |
Co-culture Macro-Troph |
p-value |
|---|---|---|---|---|---|
| Decidual Macrophage Experiments | |||||
| IL-6 | 1740.2 ± 331.8 | 1983.5 ± 472.2 | 3723.7 | 6268.2 ± 1136.2 | 0.0005 |
| IL-18 | 5.7 ± 1.5 | 17.2 ± 3.5 | 22.9 | 26.8 ± 3.5 | 0.0327 |
| IL-10 | 0 | 55.7 ± 18.2 | 55.7 | 107.4 ± 30.9 | 0.0215 |
| CSF2 | 53.1 ± 13.9 | 911.1 ± 377.2 | 964.2 | 440.2 ± 154.6 | 0.0002 |
| TNF | 53.7 ± 20.2 | 562.4 ± 207.9 | 616.1 | 303.0 ± 98.2 | 0.002 |
| CXCL8 | 7803.5 ± 1013.3 | 47806.0 ± 14954.3 | 55609.5 | 44374.0 ± 11694.2 | 0.0034 |
| CCL3 | 268.2 ± 69.6 | 6825.0 ± 1788.2 | 7093.2 | 4469.5 ± 1289.1 | 0.0002 |
| CCL4 | 7.8 ± 3.8 | 151.6 ± 38.9 | 159.4 | 120.4 ± 35.9 | 0.0002 |
| VEGF | 51.1 ± 10.6 | 252.2 ± 33.3 | 303.3 | 225.8 ± 43.4 | 0.0134 |
| CCL2 | 1962.8 ± 409.5 | 31210.0 ± 10696.1 | 33172.8 | 21711.2 ± 5951.4 | 0.0681 |
| IL-1ra | 301.6 ± 99.0 | 305.4 ± 97 | 607 | 733.1 ± 155.6 | 0.3125 |
| CSF3 | 248.7 ± 106.4 | 1740.2 ± 476.9 | 1988.9 | 1522.8 ± 401.9 | 0.0674 |
| Peripheral Blood Derived Macrophage Experiments | |||||
| CSF2 | 62.5 ± 9.6 | 5.6 ± 3.7 | 68.1 | 34.6 ± 9.9 | 0.0313 |
| VEGF | 74.2 ± 16.3 | 14.6 ± 6.7 | 88.8 | 42.3 ± 13.2 | 0.0313 |
Antigen uptake and processing in response to trophoblasts
Antigen uptake and processing is an important macrophage function. We investigated if trophoblasts could modulate the maternal immune system by altering the antigen uptake and processing ability of macrophages thus modulating a T cell response to the invading placenta. Cells were tested for their functional ability to internalize and process DQ-ovalbumin. DQ-ovalbumin FITC becomes highly fluorescent upon proteolytic processing (denaturation) and becomes detectable by flow cytometry. Macrophages cultured at 4°C to inhibit uptake and served as the negative control. Essentially all decidual macrophages processed the ovalbumin at 37°C, regardless of the presence of trophoblasts (Fig. 3).
Figure 3.
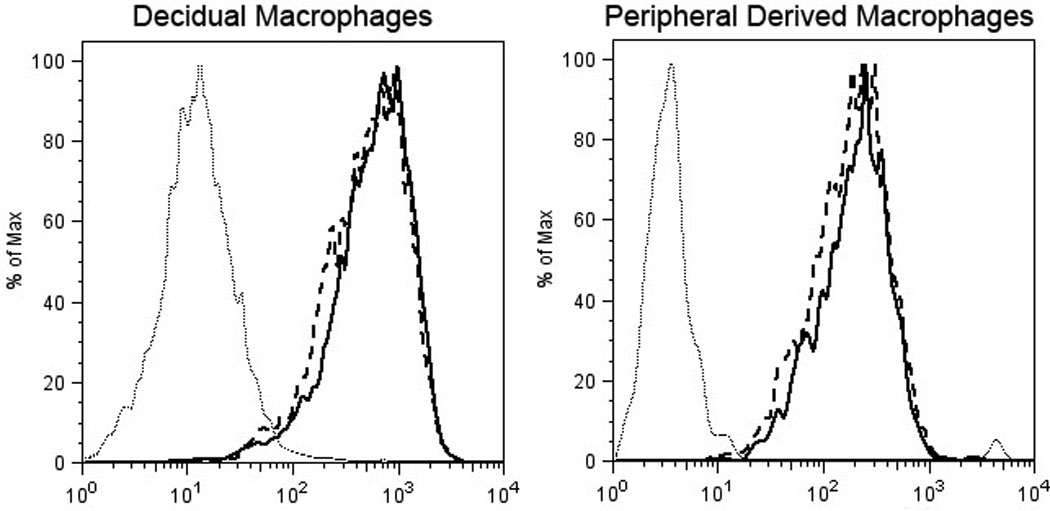
Representative histogram showing antigen processing of ovalbumin by macrophages at 37°C (solid line), macrophages after co-culture with trophoblasts for 24 hours at 37°C (dashed line), and macrophages cultured at 4°C (dotted line).
Macrophage surface marker phenotype in response to trophoblasts
Since we have previously shown that in vivo treatment with the anti-Mamu-AG antibody 25D3 decreased the number of DC-SIGN positive macrophages, we wanted to see if co-culture with trophoblasts affected cell surface marker expression on macrophages in vitro. We evaluated the surface markers of CD14 positive decidual and peripheral blood-derived macrophages 7 days after co-culture with trophoblasts. No dramatic differences of surface markers were noted (Fig. 4).
Figure 4.
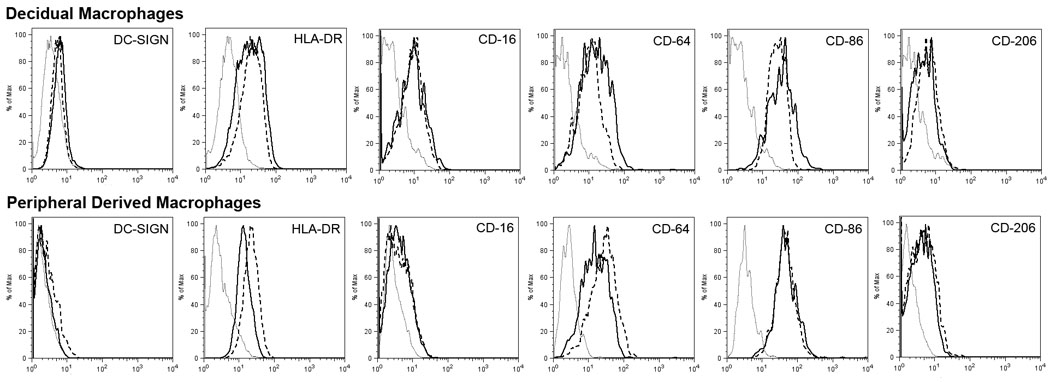
Representative histograms of FACS cytometric analysis: macrophages (solid line), macrophages after co-culture with trophoblasts for 7 days (dashed line), and matched isotype (dotted line).
Migration of macrophages and trophoblasts
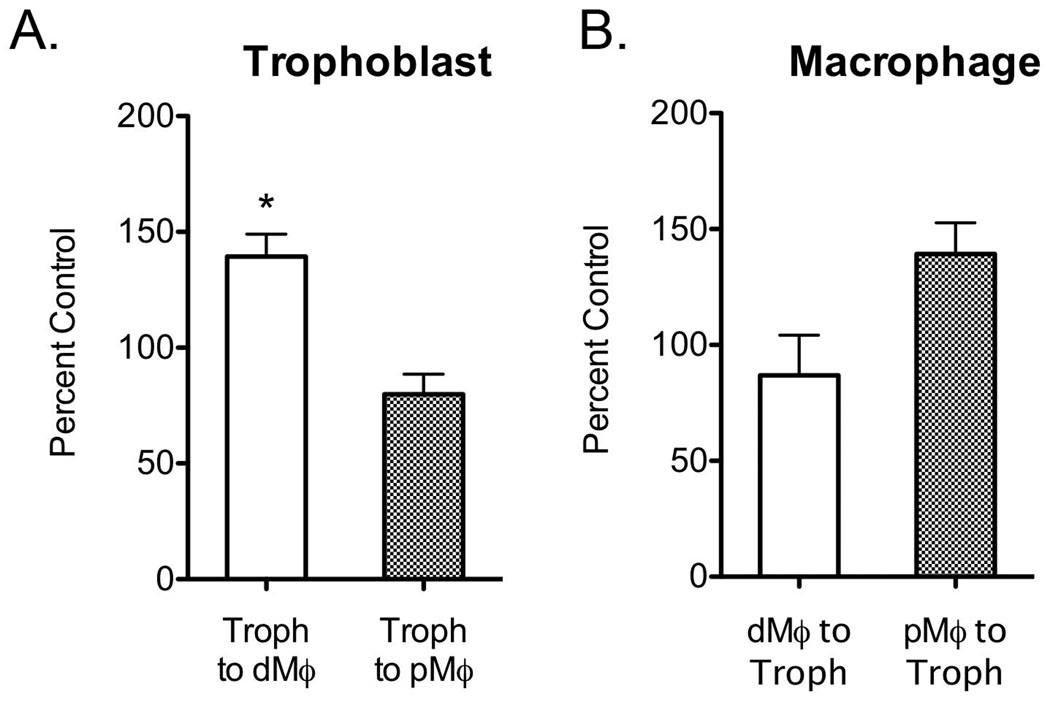
Chemotaxis is thought to be an important component of trophoblast-leukocyte interactions at the implantation site22. Migration of macrophages toward trophoblasts and trophoblasts toward macrophages was evaluated. Cells were cultured in a transwell system with one cell type in the upper chamber and the other in the reservoir. Trophoblasts, decidual, and peripheral blood-derived macrophages all migrated through the membrane to some extent with no additional stimulus. Trophoblast migration was significantly increased when cultured with decidual macrophages, but statistically unchanged in response to peripheral derived macrophages (Fig. 5A). Decidual macrophage migration was unchanged in response to trophoblasts (Fig. 5B). Although peripheral derived macrophages show a quantitatively increased migration toward trophoblasts, it was not a statistically significant change (Fig. 5B).
Figure 5.
Migration of trophoblasts (Panel A) toward macrophages and migration of macrophages toward trophoblasts (Panel B). Data from decidual macrophages are shown as a white bar and peripheral derived macrophages are shown as a checkered bar. Data are shown as percent control, the control being the number of cells that migrated in media with no co-cultured cells, as the mean ± SE of four separate experiments. *p-value < 0.05, ** p-value < 0.01, *** p-value < 0.001
Cytokine production in response to Mamu-AG expressing cells
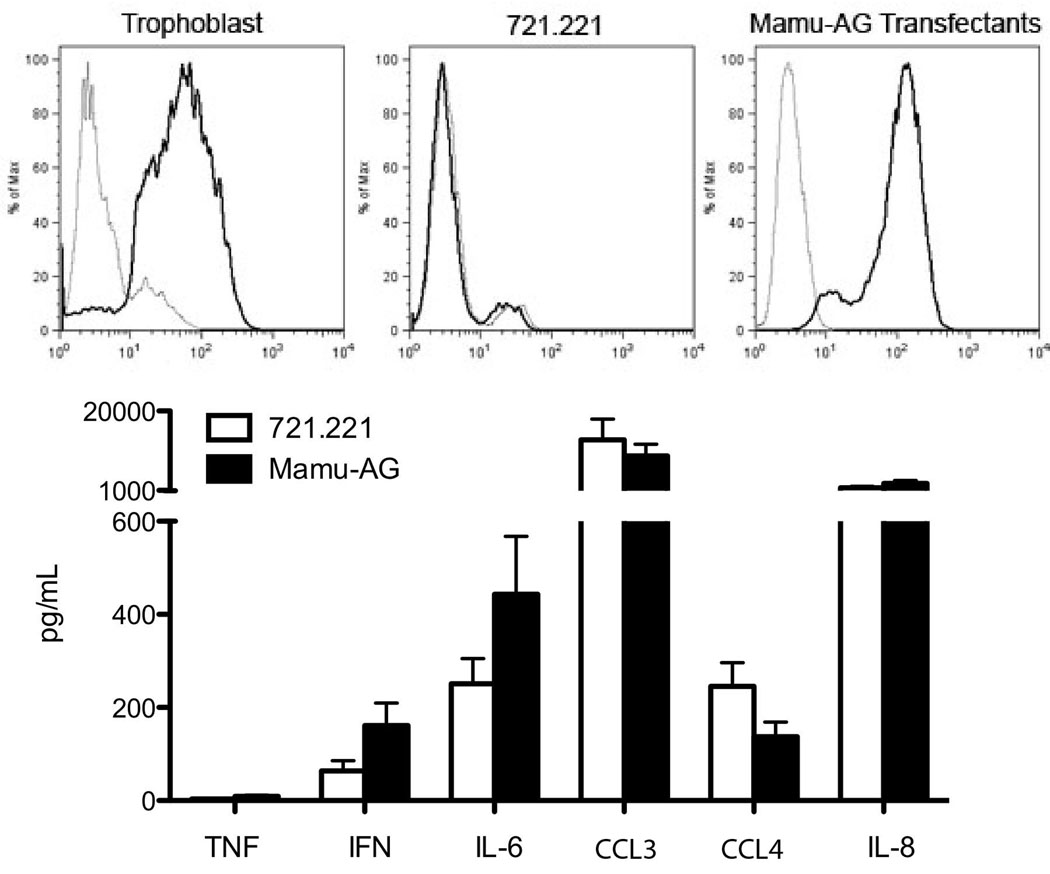
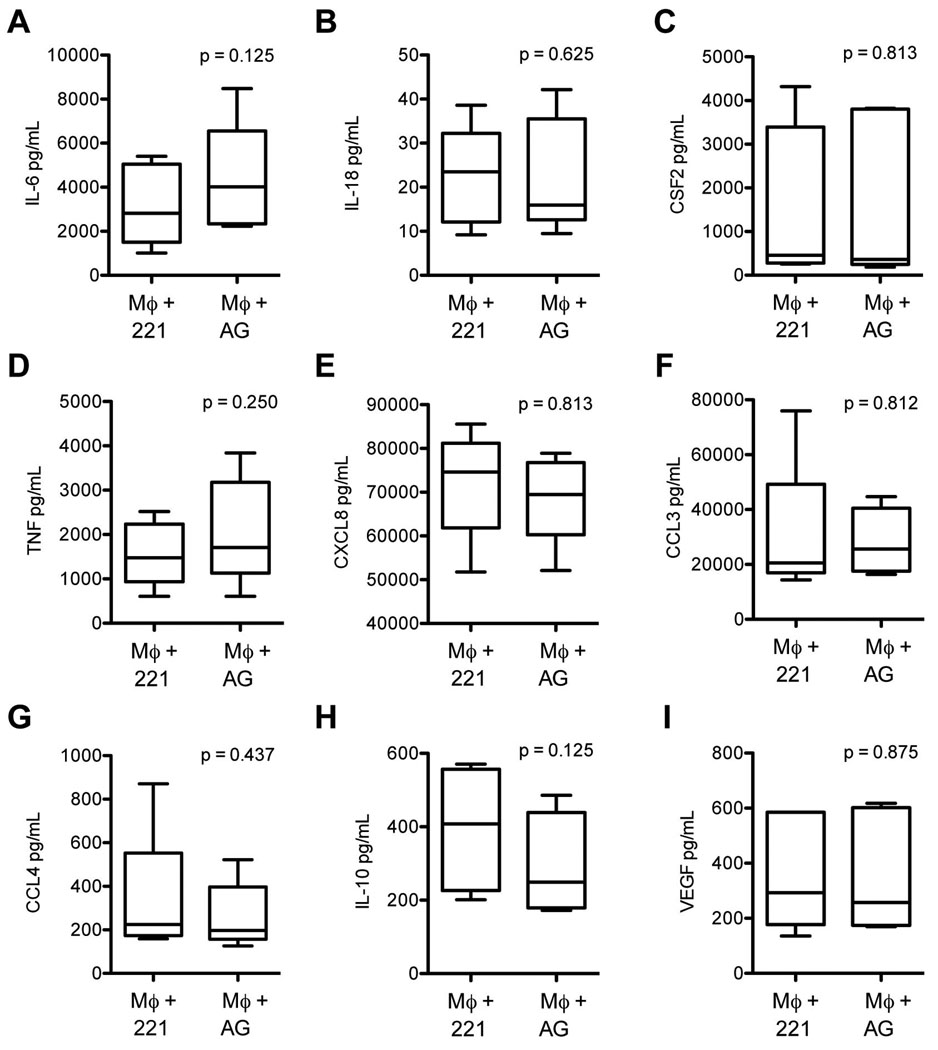
Mamu-AG is present on trophoblasts along with another non-classical MHC molecule, Mamu-E. To determine if Mamu-AG was responsible for the changes in the cytokine profile seen when macrophages were co-cultured with trophoblast cells, we used an MHC null B-lymphoblastoma cell line (BLCL), 721.221, which was stably transfected with Mamu-AG21. Untransfected 721.221 cells were used as controls. When these cells were incubated with a Mamu-AG specific antibody, 25D3, and analyzed by flow cytometry we confirmed that trophoblasts and Mamu-AG transfected cells express Mamu-AG while the untransfected 721.221 cells do not (Fig. 6). The control 721.221 cells and 721.221 cells transfected with Mamu-AG both produce cytokines at varying levels, with no apparent significant differences between the cell lines (Fig. 6).
Figure 6.
Histograms (upper panels) showing Mamu-AG expression (solid line) on trophoblasts, control 721.221 cells, and Mamu-AG transfected 721.221 cells. Isotype controls are shown as dotted lines. Cytokine measurement (lower panel) in the media of 721.221 cells (white) and Mamu-AG transfected 721.221 cells (black).
Because of the overall lack of response of peripheral derived macrophages to trophoblasts, we only evaluated the decidual cell response to Mamu-AG expression in BLCLs. No significant differences were noted between cytokines produced by macrophages co-cultured with control 721.221 cells or Mamu-AG expressing cells (Fig. 7). However, secretion of CSF3, CSF2, TNF, and CCL3 were increased in co-cultures of decidual macrophages with both control and Mamu-AG expressing cells compared to decidual macrophages alone (data not shown), possibly obscuring subtle Mamu-AG effects.
Figure 7.
Cytokine levels in media from decidual macrophages co-cultured with control 721.221 cells (221) and Mamu-AG transfected cells (AG). Data represents ten separate experiments.
Blocking cytokine production in response to trophoblasts
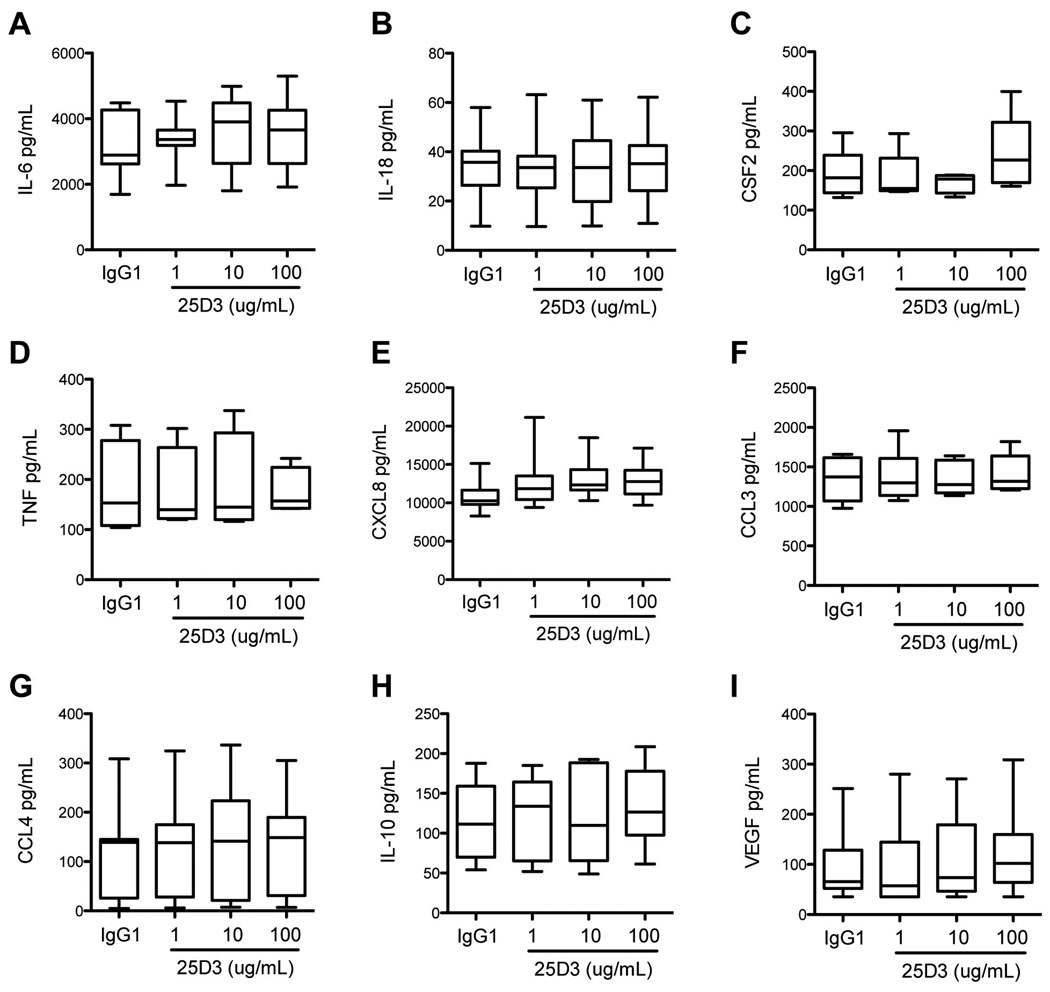
It was possible that Mamu-AG expression in the context of the trophoblast was necessary for recognition by macrophages. An antibody specific to Mamu-AG, 25D3, was used to determine whether Mamu-AG could be shown to influence macrophage cytokine secretion. No differences were noted for the cytokines measured when 25D3 was added to the culture media of decidual macrophage and trophoblast co-cultures (Fig. 8).
Figure 8.
Cytokine levels in media from decidual macrophages co-cultured with trophoblast treated with 1, 10, or 100 ug/mL 25D3, a Mamu-AG specific antibody, or 100 ug/mL nonspecific IgG1 isotype control. Data shown represents seven separate experiments.
Discussion
This study was conducted to define rhesus monkey trophoblast-macrophage interactions. While no significant effect on macrophage cell-surface marker expression or antigen processing were noted, there were significant changes in the secretion of a number of chemokines and cytokines in co-culture, as well as enhanced trophoblast migration in response to decidual but not peripheral blood derived macrophages. These results are consistent with the hypothesis that trophoblasts alter macrophage interaction to promote a suitable environment for pregnancy.
While there was positive synergism between macrophages and trophoblasts in IL-6, IL-18, and IL-10 secretion when decidual macrophages were co-cultured with trophoblasts, most synergistic interactions were actually inhibitory. For CSF2 (GM-CSF), TNF, CXCL8 (IL-8), CCL2 (MCP-1), CCL3 (MIP-1α, and CCL4 (MIP-1β trophoblast secretion was quite low, whereas there was substantial secretion by decidual macrophages, thus, it is reasonably clear that in these cases trophoblasts inhibit the secretion of these factors by macrophages in co-culture. No common regulatory pathway or promoter region was found among the cytokines that were up-regulated or those that were down-regulated (Ingenuity Systems Pathway Analysis). It was interesting that decidual macrophages were shown to secrete high (> 40 ng/mL) CCL2. This would assure that their own receptors would be exposed in situ in the decidua to CCL2. In addition, secretion of CCL2 would serve to recruit the migration of additional macrophages to the decidua23.
These data suggest that either macrophages or trophoblasts increased production of cytokines in response to the other cell type. IL-6 has been shown to stimulate trophoblast migration, invasion, and growth24–26 and stimulates chorionic gonadatropin (CG) secretion24. An increase in IL-6 would thus be considered beneficial for implantation and maintenance of pregnancy and thus may contribute to the enhanced trophoblast migration in invasion chambers noted in our study. The effect of IL-18 can be complex depending on the presence of IL-15 and IL-12. When no or low levels of IL-12 are available, as is the case in these experiments, IL-18 increases NK cell production of angiopoietin 2, a molecule beneficial for vascular transformation and spiral artery remodeling27 and induces a Th2 response28. IL-18 alone would thus be beneficial for implantation by aiding in spiral artery remodeling, necessary for proper blood flow to the developing implantation site. Furthermore, IL-10 has been shown to modulate trophoblast differentiation and growth29, and inhibit proinflammatory cytokine secretion including TNF, IL-1, and CCL229, 30. Therefore, an increase in IL-10 would also be considered beneficial for implantation.
Decreased expression of CSF2, TNF, CCL3, CCL4, CXCL8, and VEGF was observed when decidual macrophages were co-cultured with trophoblasts. These cytokines have both beneficial and detrimental actions at the maternal fetal interface. CSF2 and TNF are both thought to protect against infection, therefore benefiting pregnancy overall31, 32. However, CSF2 also inhibits angiogenesis33 and stimulates trophoblast syncium formation,34 potentially limiting trophoblast invasion. TNF has been shown to inhibit trophoblast migration and invasion16 and induce apoptosis31, although, TNF also increases VEGF production and thus angiogenesis35 and inhibits syncium differentiation 36. CCL3 and CCL4 are chemokines that have similar functions during pregnancy. Both have been shown to recruit macrophages, monocytes, dendritic cells, and NK cells to the decidua30, 37, 38. They also promote trophoblast and endothelial migration37, 39. Not only are CCL3 and CCL4 chemoattractants, they also promote Th1 differentiation38 increase NK cell cytolytic activity30, and stimulate macrophage proliferation and production of TNF and IL-640. It has been suggested that excess macrophages in the decidua, which could be due to excessive monocyte recruitment to the decidua, may contribute to improper implantation and possibly preeclampsia or spontaneous abortion41. Therefore, a decrease in monocyte/macrophage chemoattractants like CCL3 and CCL4 upon trophoblast-macrophage interaction could be beneficial for maintaining an appropriate progestational environment at the maternal-fetal interface.
CXCL8 has been suggested to increase trophoblast invasion23, 42 and therefore may be beneficial during implantation. However, this was not seen in the migration assay where trophoblasts increased migration in response to decidual but not peripheral derived macrophages, even though both cell types secreted similar levels of CXCL8. CXCL8 also is a chemoattractant for neutrophils35, and an influx of neutrophils could contribute to placental abruptions and subsequent loss of pregnancy43. Although a decrease in IL-8 was observed, CXCL8 was still found in high concentrations, suggesting that the decrease may not be physiologically relevant. The decrease in VEGF was unexpected because VEGF increases trophoblast proliferation35, is important for tissue remodeling16, and is well known to be necessary for pregnancy44. Both decidual macrophages and trophoblasts secrete soluble FLT-1 (data not shown), a soluble receptor for VEGF. An increase in soluble FLT-1 would lead to decreases in measurable VEGF in the media. Alternatively, one possible role of macrophages is to prevent an over invasion of the placenta12 and this may explain why the interaction between macrophages and trophoblasts would decrease VEGF secretion.
Mamu-AG is a nonclassical MHC class I gene that is thought to be the rhesus monkey homolog of HLA-G45. It is highly expressed on endovascular, extravillous and syncytial trophoblasts at the rhesus implantation site. HLA-G has been shown to alter cytokine and chemokine secretion of macrophages and monocytes6, 46. Therefore, we evaluated the effects of Mamu-AG on macrophage function by co-culture with Mamu-AG-expressing cells. Surprisingly, Mamu-AG-expressing BLCLs did not have the same effect on cytokine production as trophoblasts. However, both the 721.221 cells and 721.221 cells transfected with Mamu-AG produced an array of cytokines at varied levels. This included INFγ known macrophage stimulator, making it difficult to isolate the action of Mamu-AG on macrophages using this culture system. For example, IFNγ may alter macrophage cytokine production so that significant effects of Mamu-AG may be obscured.
To further define the effects of Mamu-AG in the context of the trophoblast, an anti-Mamu-AG antibody, 25D3, was added to the media in an effort to block any Mamu-AG effect in the trophoblast-macrophage co-culture. Based on the in vivo observation of decreased DC-SIGN expression on macrophages following passive immunization with this antibody, we expected to block the changes in cytokines observed in response to trophoblasts. However, this was not the case, suggesting that trophoblasts may not be altering macrophage function directly through Mamu-AG, but rather using an alternate mechanism. HLA-G is a ligand for LILR-1A and LILR-1B47, and rhesus monkeys express homologous LILR molecules48. However, it remains possible that Mamu-AG is not recognized by macrophages, or that 25D3 is not a blocking antibody for Mamu-AG interactions with its macrophage receptor. Further studies will be required to explore these alternative interpretations.
Peripheral monocyte-derived macrophages were also evaluated for cytokine production, antigen processing, and surface marker expression in response to co-culture with trophoblasts. Peripheral derived macrophages are similar to decidual macrophages but have higher expression of CD14 and CD86 and no expression of DC-SIGN. Defining phenotypic differences is important to determine the appropriateness of using peripheral-derived macrophage as a model for decidual macrophage responses. The unstimulated peripheral derived macrophages produce lower levels of all cytokines measured compared to decidual macrophages. However, when stimulated with LPS for 24 hours peripheral derived macrophages produce these cytokines at similar levels to decidual macrophages19. This suggests the decidual macrophages are in an activated state while the peripheral derived macrophages are in a resting state and the presence of the trophoblasts does not alter their activation state. Macrophages can be classified as classically activated, M1, or alternatively activated, M2. M1 cells respond to inflammatory stimuli to increase innate immune responses49. M2 cells respond to anti-inflammatory stimuli to increase tissue change reconfiguration and regeneration. It has been reported that decidual macrophages shift toward the M2 phenotype49. In the current study unstimulated cells were used to determine the direct effect of trophoblasts on macrophages since typical agents used in the laboratory, such as LPS, are not present in the normal decidua and direct the macrophage to an M1 phenotype. Similar to decidual macrophages, no changes were seen in antigen processing or surface marker expression when peripheral derived macrophages were co-cultured with trophoblasts. Decreases in CSF2 and VEGF production, but no other cytokines, were noted when peripheral derived macrophages were cultured with trophoblasts.
This difference in cytokine production by decidual versus peripheral derived macrophages in response to trophoblasts may be due to the lower basal cytokine production of peripheral derived macrophages or the fact that decidual macrophages had been already exposed to Mamu-AG, and other pregnancy factors including placental as well as decidual factors, in vivo. These differences underscore the importance of evaluating decidual immune cells in addition to peripheral immune cell responses. The rhesus monkey animal model will allow us to design in vivo experiments to test the role of macrophage and trophoblast cytokines and chemokines at the maternal fetal interface.
Acknowledgements
We extend our appreciation to the Animal Care Staff at the Wisconsin National Primate Research Center for doing the blood draws and surgeries, Kim Weisgrau for assistance with the FACSCalibur, and David Lorentzen for the use of the Luminex at the HLA/Molecular Diagnostics Lab, UW-Madison Hospital and Clinics. 721.221 cells were kindly provided by R. DeMars, and 721.221-Mamu-AG cells were provided by D. Watkins, both at the University of Wisconsin-Madison. This research was supported by NIH grants HD37120, HD053925, AI076734, and RR021876 to T.G.G. and RR000167 to the Wisconsin National Primate Research Center, University of Wisconsin-Madison. This research was conducted in part at a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01. This publication's contents are solely the responsibility of the authors and does not necessarily represent the official views of NCRR or NIH.
References
- 1.Roussev RG, Coulam CB. HLA-G and its role in implantation (review) J Assist Reprod Genet. 2007;24:288–295. doi: 10.1007/s10815-007-9148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McIntire RH, Hunt JS. Antigen presenting cells and HLA-G--a review. Placenta. 2005;26 Suppl A:S104–S109. doi: 10.1016/j.placenta.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248:220–223. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- 4.Apps R, Gardner L, Moffett A. A critical look at HLA-G. Trends Immunol. 2008;29:313–321. doi: 10.1016/j.it.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Carosella ED, Moreau P, Lemaoult J, Rouas-Freiss N. HLA-G: from biology to clinical benefits. Trends Immunol. 2008;29:125–132. doi: 10.1016/j.it.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 6.McIntire RH, Morales PJ, Petroff MG, Colonna M, Hunt JS. Recombinant HLA-G5 and -G6 drive U937 myelomonocytic cell production of TGF-beta1. J Leukoc Biol. 2004;76:1220–1228. doi: 10.1189/jlb.0604337. [DOI] [PubMed] [Google Scholar]
- 7.Apps R, Gardner L, Sharkey AM, Holmes N, Moffett A. A homodimeric complex of HLA-G on normal trophoblast cells modulates antigen-presenting cells via LILRB1. Eur J Immunol. 2007;37:1924–1937. doi: 10.1002/eji.200737089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slukvin II, Watkins DI, Golos TG. Phenotypic and functional characterization of rhesus monkey decidual lymphocytes: rhesus decidual large granular lymphocytes express CD56 and have cytolytic activity. J Reprod Immunol. 2001;50:57–79. doi: 10.1016/s0165-0378(00)00090-5. [DOI] [PubMed] [Google Scholar]
- 9.Bondarenko GI, Burleigh DW, Durning M, Breburda EE, Grendell RL, Golos TG. Passive immunization against the MHC class I molecule Mamu-AG disrupts rhesus placental development and endometrial responses. J Immunol. 2007;179:8042–8050. doi: 10.4049/jimmunol.179.12.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breburda EE, Dambaeva SV, Slukvin II, Golos TG. Selective distribution and pregnancy-specific expression of DC-SIGN at the maternal-fetal interface in the rhesus macaque: DC-SIGN is a putative marker of the recognition of pregnancy. Placenta. 2006;27:11–21. doi: 10.1016/j.placenta.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Hunt JS, Petroff MG, Burnett TG. Uterine leukocytes: key players in pregnancy. Semin Cell Dev Biol. 2000;11:127–137. doi: 10.1006/scdb.2000.0158. [DOI] [PubMed] [Google Scholar]
- 12.Slukvin II, Breburda EE, Golos TG. Dynamic changes in primate endometrial leukocyte populations: differential distribution of macrophages and natural killer cells at the rhesus monkey implantation site and in early pregnancy. Placenta. 2004;25:297–307. doi: 10.1016/j.placenta.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Trundley A, Moffett A. Human uterine leukocytes and pregnancy. Tissue Antigens. 2004;63:1–12. doi: 10.1111/j.1399-0039.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- 14.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 15.Vince GS, Johnson PM. Leucocyte populations and cytokine regulation in human uteroplacental tissues. Biochem Soc Trans. 2000;28:191–195. doi: 10.1042/bst0280191. [DOI] [PubMed] [Google Scholar]
- 16.Orsi NM, Tribe RM. Cytokine networks and the regulation of uterine function in pregnancy and parturition. J Neuroendocrinol. 2008;20:462–469. doi: 10.1111/j.1365-2826.2008.01668.x. [DOI] [PubMed] [Google Scholar]
- 17.Koga K, Mor G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol. 2010;63:587–600. doi: 10.1111/j.1600-0897.2010.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golos TG, Bondarenko GI, Breburda EE, Dambaeva SV, Durning M, Slukvin II. Immune and trophoblast cells at the rhesus monkey maternal-fetal interface. Methods Mol Med. 2006;122:93–108. doi: 10.1385/1-59259-989-3:93. [DOI] [PubMed] [Google Scholar]
- 19.Rozner AE, Dambaeva SV, Drenzek JG, Durning M, Golos TG. Generation of macrophages from peripheral blood monocytes in the rhesus monkey. J Immunol Methods. 2009;351:36–40. doi: 10.1016/j.jim.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimizu Y, Geraghty DE, Koller BH, Orr HT, DeMars R. Transfer and expression of three cloned human non-HLA-A,B,C class I major histocompatibility complex genes in mutant lymphoblastoid cells. Proc Natl Acad Sci U S A. 1988;85:227–231. doi: 10.1073/pnas.85.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyson JE, Iwanaga KK, Golos TG, Watkins DI. Identification of a novel MHC class I gene, Mamu-AG, expressed in the placenta of a primate with an inactivated G locus. J Immunol. 1997;159:3311–3321. [PubMed] [Google Scholar]
- 22.Hannan NJ, Salamonsen LA. Role of chemokines in the endometrium and in embryo implantation. Curr Opin Obstet Gynecol. 2007;19:266–272. doi: 10.1097/GCO.0b013e328133885f. [DOI] [PubMed] [Google Scholar]
- 23.Kayisli UA, Mahutte NG, Arici A. Uterine chemokines in reproductive physiology and pathology. Am J Reprod Immunol. 2002;47:213–221. doi: 10.1034/j.1600-0897.2002.01075.x. [DOI] [PubMed] [Google Scholar]
- 24.Nishino E, Matsuzaki N, Masuhiro K, Kameda T, Taniguchi T, Takagi T, Saji F, Tanizawa O. Trophoblast-derived interleukin-6 (IL-6) regulates human chorionic gonadotropin release through IL-6 receptor on human trophoblasts. J Clin Endocrinol Metab. 1990;71:436–441. doi: 10.1210/jcem-71-2-436. [DOI] [PubMed] [Google Scholar]
- 25.Jovanovic M, Vicovac L. Interleukin-6 stimulates cell migration, invasion and integrin expression in HTR-8/SVneo cell line. Placenta. 2009;30:320–328. doi: 10.1016/j.placenta.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Dominguez F, Martinez S, Quinonero A, Loro F, Horcajadas JA, Pellicer A, Simon C. CXCL10 and IL-6 induce chemotaxis in human trophoblast cell lines. Mol Hum Reprod. 2008;14:423–430. doi: 10.1093/molehr/gan032. [DOI] [PubMed] [Google Scholar]
- 27.Ledee N, Dubanchet S, Lombroso R, Ville Y, Chaouat G. Downregulation of human endometrial IL-18 by exogenous ovarian steroids. Am J Reprod Immunol. 2006;56:119–123. doi: 10.1111/j.1600-0897.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 28.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 29.White CA, Johansson M, Roberts CT, Ramsay AJ, Robertson SA. Effect of interleukin-10 null mutation on maternal immune response and reproductive outcome in mice. Biol Reprod. 2004;70:123–131. doi: 10.1095/biolreprod.103.018754. [DOI] [PubMed] [Google Scholar]
- 30.Menten P, Wuyts A, Van Damme J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002;13:455–481. doi: 10.1016/s1359-6101(02)00045-x. [DOI] [PubMed] [Google Scholar]
- 31.Toder V, Fein A, Carp H, Torchinsky A. TNF-alpha in pregnancy loss and embryo maldevelopment: a mediator of detrimental stimuli or a protector of the fetoplacental unit? J Assist Reprod Genet. 2003;20:73–81. doi: 10.1023/A:1021740108284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robertson SA. GM-CSF regulation of embryo development and pregnancy. Cytokine Growth Factor Rev. 2007;18:287–298. doi: 10.1016/j.cytogfr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Eubank TD, Roberts R, Galloway M, Wang Y, Cohn DE, Marsh CB. GM-CSF induces expression of soluble VEGF receptor-1 from human monocytes and inhibits angiogenesis in mice. Immunity. 2004;21:831–842. doi: 10.1016/j.immuni.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Lloret MI, Morrish DW, Wegmann TG, Honore L, Turner AR, Guilbert LJ. Demonstration of functional cytokine-placental interactions: CSF-1 and GM-CSF stimulate human cytotrophoblast differentiation and peptide hormone secretion. Exp Cell Res. 1994;214:46–54. doi: 10.1006/excr.1994.1232. [DOI] [PubMed] [Google Scholar]
- 35.Bowen JM, Chamley L, Mitchell MD, Keelan JA. Cytokines of the placenta and extra-placental membranes: biosynthesis, secretion and roles in establishment of pregnancy in women. Placenta. 2002;23:239–256. doi: 10.1053/plac.2001.0781. [DOI] [PubMed] [Google Scholar]
- 36.Haider S, Knofler M. Human tumour necrosis factor: physiological and pathological roles in placenta and endometrium. Placenta. 2009;30:111–123. doi: 10.1016/j.placenta.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salamonsen LA, Hannan NJ, Dimitriadis E. Cytokines and chemokines during human embryo implantation: roles in implantation and early placentation. Semin Reprod Med. 2007;25:437–444. doi: 10.1055/s-2007-991041. [DOI] [PubMed] [Google Scholar]
- 38.Maurer M, von Stebut E. Macrophage inflammatory protein-1. Int J Biochem Cell Biol. 2004;36:1882–1886. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 39.Berger O, Gan X, Gujuluva C, Burns AR, Sulur G, Stins M, Way D, Witte M, Weinand M, Said J, Kim KS, Taub D, Graves MC, Fiala M. CXC and CC chemokine receptors on coronary and brain endothelia. Mol Med. 1999;5:795–805. [PMC free article] [PubMed] [Google Scholar]
- 40.Fahey TJ, 3rd, Tracey KJ, Tekamp-Olson P, Cousens LS, Jones WG, Shires GT, Cerami A, Sherry B. Macrophage inflammatory protein 1 modulates macrophage function. J Immunol. 1992;148:2764–2769. [PubMed] [Google Scholar]
- 41.Lockwood CJ, Matta P, Krikun G, Koopman LA, Masch R, Toti P, Arcuri F, Huang ST, Funai EF, Schatz F. Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha and interleukin-1beta in first trimester human decidual cells: implications for preeclampsia. Am J Pathol. 2006;168:445–452. doi: 10.2353/ajpath.2006.050082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Oliveira LG, Lash GE, Murray-Dunning C, Bulmer JN, Innes BA, Searle RF, Sass N, Robson SC. Role of interleukin 8 in uterine natural killer cell regulation of extravillous trophoblast cell invasion. Placenta. 2010;31:595–601. doi: 10.1016/j.placenta.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Lockwood CJ, Paidas M, Murk WK, Kayisli UA, Gopinath A, Huang SJ, Krikun G, Schatz F. Involvement of human decidual cell-expressed tissue factor in uterine hemostasis and abruption. Thromb Res. 2009;124:516–520. doi: 10.1016/j.thromres.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krussel JS, Bielfeld P, Polan ML, Simon C. Regulation of embryonic implantation. Eur J Obstet Gynecol Reprod Biol. 2003;110 Suppl 1:S2–S9. doi: 10.1016/s0301-2115(03)00167-2. [DOI] [PubMed] [Google Scholar]
- 45.Golos TG, Bondarenko GI, Dambaeva SV, Breburda EE, Durning M. On the role of placental Major Histocompatibility Complex and decidual leukocytes in implantation and pregnancy success using non-human primate models. Int J Dev Biol. 2010;54:431–443. doi: 10.1387/ijdb.082797tg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li C, Houser BL, Nicotra ML, Strominger JL. HLA-G homodimer-induced cytokine secretion through HLA-G receptors on human decidual macrophages and natural killer cells. Proc Natl Acad Sci U S A. 2009;106:5767–5772. doi: 10.1073/pnas.0901173106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hunt JS, Langat DK, McIntire RH, Morales PJ. The role of HLA-G in human pregnancy. Reprod Biol Endocrinol. 2006;4 Suppl 1:S10. doi: 10.1186/1477-7827-4-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slukvin II, Grendell RL, Rao DS, Hughes AL, Golos TG. Cloning of rhesus monkey LILRs. Tissue Antigens. 2006;67:331–337. doi: 10.1111/j.1399-0039.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- 49.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. TRENDS in Immunology. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 50.Gustafsson C, Mjosberg J, Matussek A, Geffers R, Matthiesen L, Berg G, Sharma S, Buer J, Ernerudh J. PLoS ONE. 2008;3(4):e2078. doi: 10.1371/journal.pone.0002078. [DOI] [PMC free article] [PubMed] [Google Scholar]