Abstract
BACKGROUND
Hip arthroplasty frequently requires potent postoperative analgesia, often provided with an epidural or posterior lumbar plexus local anesthetic infusion. However, American Society of Regional Anesthesia guidelines now recommend against epidural and continuous posterior lumbar plexus blocks during administration of various perioperative anticoagulants often administered after hip arthroplasty. A continuous femoral nerve block is a possible analgesic alternative, but whether it provides comparable analgesia to a continuous posterior lumbar plexus block after hip arthroplasty remains unclear. We therefore tested the hypothesis that differing the catheter insertion site (femoral versus posterior lumbar plexus) after hip arthroplasty has no impact on postoperative analgesia.
METHODS
Preoperatively, subjects undergoing hip arthroplasty were randomly assigned to receive either a femoral or posterior lumbar plexus stimulating catheter inserted 5 to 15 cm or 0 to 1 cm past the needletip, respectively. Postoperatively, patients received perineural ropivacaine, 0.2% (basal 6 mL/hour, bolus 4 mL, 30 min lockout) for at least two days. The primary end point was the average daily pain scores as measured with a numeric rating scale (0–10) recorded in the 24-h period beginning at 07:30 the morning after surgery, excluding twice-daily physical therapy sessions. Secondary end points included pain during physical therapy, ambulatory distance, and supplemental analgesic requirements during the same 24-h period, as well as satisfaction with analgesia during hospitalization.
RESULTS
The mean (SD) pain scores for subjects receiving a femoral infusion (n = 25) were 3.6 (1.8) versus 3.5 (1.8) for patients receiving a posterior lumbar plexus infusion (n = 22) resulting in a group difference of 0.1 (95% confidence interval −0.9 to 1.2; P = 0.78). Because the confidence interval was within a prespecified −1.6 to 1.6 range, we conclude that the effect of the two analgesic techniques on postoperative pain was equivalent. Similarly, we detected no differences between the two treatments with respect to the secondary end points, with one exception: subjects with a femoral catheter ambulated a median (10th–90th percentiles) 2 (0–17) m the morning after surgery, compared with 11 (0–31) m for subjects with a posterior lumbar plexus catheter (data nonparametric; P = 0.02).
CONCLUSIONS
After hip arthroplasty, a continuous femoral nerve block is an acceptable analgesic alternative to a continuous posterior lumbar plexus block when using a stimulating perineural catheter. However, early ambulatory ability suffers with a femoral infusion.
More than 350,000 hip arthroplasties are performed every year in the United States alone, often resulting in significant postoperative pain. Reports of the use of posterior lumbar plexus local anesthetic infusions to provide analgesia after hip arthroplasty have increased dramatically within the last decade,1–7 particularly after they were demonstrated to provide comparable analgesia to epidural infusions.7 However, the recently published American Society of Regional Anesthesia (ASRA) guidelines now explicitly recommend the same precautions as neuraxial techniques be exercised for deep procedures such as posterior lumbar plexus blocks/catheters, specifically that any catheter be removed before administration of various anticoagulants at certain doses (e.g., enoxaparin 30 mg twice daily).8 Therefore, many patients are now precluded from receiving either a postoperative epidural or a continuous posterior lumbar plexus block.
One possible analgesic option is a continuous femoral nerve block in which the perineural catheter is inserted at the inguinal crease.9 Unlike epidural or posterior lumbar plexus techniques, current ASRA guidelines allow for simultaneous administration of nearly any perioperative anticoagulant and a continuous femoral nerve block.8 However, there is reason to question whether a perineural catheter inserted at the level of the inguinal crease will provide adequate analgesia after hip arthroplasty.10,11 Yet, if validated against the posterior lumbar plexus approach, the anterior femoral technique could be a viable option within ASRA guidelines for many patients undergoing hip arthroplasty. Data from two randomized trials comparing the continuous femoral and posterior lumbar plexus techniques failed to find one superior after knee arthroplasty,12,13 but it remains unclear if a continuous femoral nerve block provides equivalent analgesia to the posterior option for hip arthroplasty.
We therefore tested the hypothesis that differing the catheter location (femoral versus posterior lumbar plexus) after hip arthroplasty has no impact on postoperative analgesia. The primary end point was the difference in mean pain scores during the 24-hour period beginning at 07:30 the morning after surgery, excluding twice-daily physical therapy sessions.
MATERIALS AND METHODS
Enrollment
The local IRB (University of California San Diego, San Diego, CA) approved all study procedures and all participants provided written, informed consent. The trial was prospectively registered at clinicaltrials.gov (NCT00967980). Patients offered enrollment included adults (≥18 yr) scheduled for primary, unilateral hip arthroplasty via a 15 to 25 cm curvilinear lateral skin incision centered over the greater trochanter. Exclusion criteria included a history of opioid dependence, abuse, or current chronic analgesic therapy (daily use [mt]20 mg oxycodone-equivalent opioid use within the 2 wk before surgery and duration of use [mt]4 wk); allergy to study medications; any neuromuscular deficit of the ipsilateral femoral nerve and/or quadriceps femoris muscle (including diabetic peripheral neuropathy); body mass index [mt]40 kg/m2; pregnancy; or incarceration.
Randomization
Subjects were randomized to one of two treatment groups, a femoral or posterior lumbar plexus perineural catheter, using a computer-generated randomization table in blocks of four, stratified by surgeon and surgical procedure (total or resurfacing), and based in a secure, password-protected, encrypted central server (www.PAINfRE.com). Subjects had their perineural catheter inserted before entering the operating room.
Ultrasound was used to scan the block area before needle insertion using a linear array transducer providing a frequency of 6 to 13 MHz (HFL38, SonoSite M-Turbo, Bothell, WA). Time for catheter placement started when the catheter-placement needle first touched the patient and ended when this needle was removed after catheter insertion for the posterior lumbar plexus group and catheter tunneling for the femoral group. Catheters were inserted using techniques described previously,14–16 with the following revisions. An 8.9 or 15.0 cm, 17 gauge, insulated needle (StimuCath, Teleflex Medical, Research Triangle Park, North Carolina) was inserted for both groups, and 5 mL of a solution of 5% dextrose in water was injected via the needle.
Femoral catheter insertion
Once the catheter was successfully inserted 5 cm past the needletip, we continued to advance it up to 10 cm further. If the evoked motor response began to decrease, the catheter was withdrawn 1 cm to regain the original evoked motor response. The catheter was tunneled subcutaneously 6 to 8 cm medial and slightly cephalad.
Posterior lumbar plexus catheter insertion
The catheter was advanced 0 to 1 cm past the needletip. If the evoked motor response decreased as the stimulating catheter was advanced, the catheter was withdrawn into the needle, the needle redirected, and the catheter then readvanced. The catheter was secured caudad and anterior to the ipsilateral axilla.
For both treatment groups, 20 mL of 1.5% mepivacaine (with 2.5 µg/mL of epinephrine) was injected via the perineural catheter. Catheter placement was considered successful if, within 15 min, the patient experienced a decreased sensation to light touch over the ipsilateral distal thigh and weakness upon knee extension relative to the contralateral limb. Patients without a successful catheter insertion had their catheters replaced or were withdrawn from the study.
Intraoperative management
Patients were administered a standardized general anesthetic using inhaled sevoflurane, nitrous oxide, and oxygen via an endotracheal tube during surgery. A 0.2% ropivacaine infusion (6 mL/h basal; 4 mL bolus; 30 min lockout) was initiated via the perineural catheter before the end of surgery via a portable infusion pump (PainPump2, Stryker Instruments, Kalamazoo, MI). Intravenous fentanyl was administered as needed during surgery, and IV hydromorphone was titrated to a respiratory rate of 12 to 14 just before emergence.
Postoperative management
All patients received oral acetaminophen (975 mg every 6 h), celecoxib (200 mg every 12 h), and sustained-release oxycodone (Oxycontin, 10 mg every 12 h). For breakthrough pain, patients depressed the infusion pump bolus button. When necessary, rescue opioid and route of administration were titrated to pain severity using a numeric rating scale (NRS) of 0 to 1017: mild pain (NRS[lt]4): oral oxycodone 5 mg; moderate pain (NRS 4 to 7): oral oxycodone, 10 mg; and severe pain (NRS [mt]7), IV hydromorphone, 0.5 mg. Patients underwent physical therapy twice daily beginning the morning after surgery at approximately 08:00 and 13:00. If the physical therapist believed subject ambulation was unacceptably limited due to quadriceps femoris weakness, the perineural infusion was stopped for one hour and then restarted with a new portable infusion pump at half the basal rate (3 mL/h) and bolus dose volume (2 mL). Subjects received deep vein thrombosis prophylaxis with 40 mg subcutaneous enoxaparin sodium once daily beginning the morning after surgery. This specific anticoagulant and dosing regimen may Be used concurrently with a continuous posterior lumbar plexus block within the current ASRA guidelines.8
Outcome measurements
All end points were recorded by nursing and physical therapy staff, as well as a research coordinator. The primary end point was the average of all pain scores recorded in the 24-h period beginning at 07:30 the morning after surgery, excluding the periods of physical therapy. Pain measurements were evaluated using the 10-point NRS17 and recorded every 4 h (excluding periods of sleep), as well as immediately after physical therapy and upon analgesic request. Seven days after surgery, subjects were contacted by phone and asked about their satisfaction with postoperative analgesia before hospital discharge (0 to 10; 0 = very unsatisfied and 10 = very satisfied).
Statistical Analysis
Sample size estimation was based on our primary hypothesis that differing the catheter location (femoral versus posterior lumbar plexus) after hip arthroplasty has no impact on postoperative analgesia. To this end, we chose the mean NRS during the 24-h period beginning at 07:30 the morning after surgery as the primary outcome variable to estimate a probable sample size. Since the aim of the study was to evaluate equivalency (due to a proposed hypothesis of no group effect), standard inferential statistics used to demonstrate statistically significant nonzero effects Do not strictly apply. Instead, we used the method described by Armitage et al. for equivalency trials whereby we concluded equivalence if the 95% confidence interval for the difference was within a tolerated interval.18 We considered a difference of 1.6 on the NRS (one SD) to Be clinically relevant.2 If the confidence interval was within −1.6 to 1.6 range, we concluded the effect of the two treatments was equivalent. To calculate power, we simulated 10,000 trials with a total enrolled sample size of 50, assuming a SD in each group of 1.6 and 10% dropout.2 This sample size provided 82% power to correctly conclude equivalence, while the probability of falsely concluding equivalence in the presence of a simulated group difference of 1.6 was 2.5% (“α”).
These analyses were executed using R version 2.11 (2010).† The normality assumption was assessed using the Kolmogorov-Smirnov test with Lilliefors correction (Sigma Stat 2.03). Additional analyses included the Fisher’s exact test for categorical variables, and the Mann-Whitney U for comparisons of nonparametric continuous variables (GraphPad InStat, GraphPad Software, San Diego, California). Subject data were included only during perineural infusion.
† For power simulations and data analysis, confidence intervals were estimated using t.test() in the R package “stat.” http://finzi.psych.upenn.edu/R/library/stats/html/t.test.html. R Software Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria. Available at: http://www.r-project.org. Accessed May 17, 2010.
RESULTS
Fifty subjects were enrolled during a 10-mo period beginning October 2009. Three subjects were withdrawn from the study before catheter insertion (exclusion criteria identified or the case cancelled after randomization). Of the remaining 47 subjects, 25 were randomized to receive a femoral perineural catheter while 22 received a posterior lumbar plexus catheter, and these two groups were similar in demographic and surgical characteristics (Table 1). All 47 subjects exhibited a sensory and motor block within 15 min after a local anesthetic bolus was administered via the catheter (Table 2).
Table 1.
Demographic and pre-randomization surgical characteristics of the study subjects
| Femoral Infusion (n=25) |
Posterior Lumbar Plexus Infusion (n=22) |
|
|---|---|---|
| Age (yr) | 55 (13) | 57 (10) |
| Sex (female) | 10 | 11 |
| Height (cm) | 170 (8) | 172 (11) |
| Weight (kg) | 85 (18) | 85 (17) |
| Body mass index (kg/m2) | 30 (6) | 29 (4) |
| Surgical procedure (hip resurfacing) | 3 | 3 |
| Procedure side (right) | 12 | 11 |
Values are reported as mean (SD) or number of subjects.
Table 2.
Post-randomization catheter insertion and surgical characteristics
| Femoral Infusion (n=25) |
Posterior Lumbar Plexus Infusion (n=22) |
p-Value | |
|---|---|---|---|
| Catheter insertion time (min) | 7.0 (4.0–17.2) | 12.5 (6.2–19.7) | 0.03 |
| Catheter inserted past needletip (cm) | 11.0 (5.0–13.0) | 0.5 (0.0–1.0) | N/A |
| Minimum current via needle (mA) | 0.46 (0.33–0.56) | 0.52 (0.27–0.72) | 0.09 |
| Minimum current via catheter (mA) | 0.48 (0.24–0.65) | 0.40 (0.20–0.80) | 0.82 |
| OR hydromorphone equivalents (mg) | 0.5 (0.4–0.9) | 0.7 (0.4–2.3) | 0.10 |
| OR fentanyl (µg) | 350 (180–500) | 350 (250–500) | 0.66 |
| Time of incision (time) | 11:00 (8:00–13:42) | 10:30 (7:06–14:00) | 0.93 |
| Surgery duration (min) | 134 (107–207) | 138 (100–177) | 0.74 |
Values are reported as median (10th–90th percentiles). P-values were derived from two-sided, two-sample t-tests assuming unequal variances.
N/A: not applicable
OR: operating room.
Primary end point
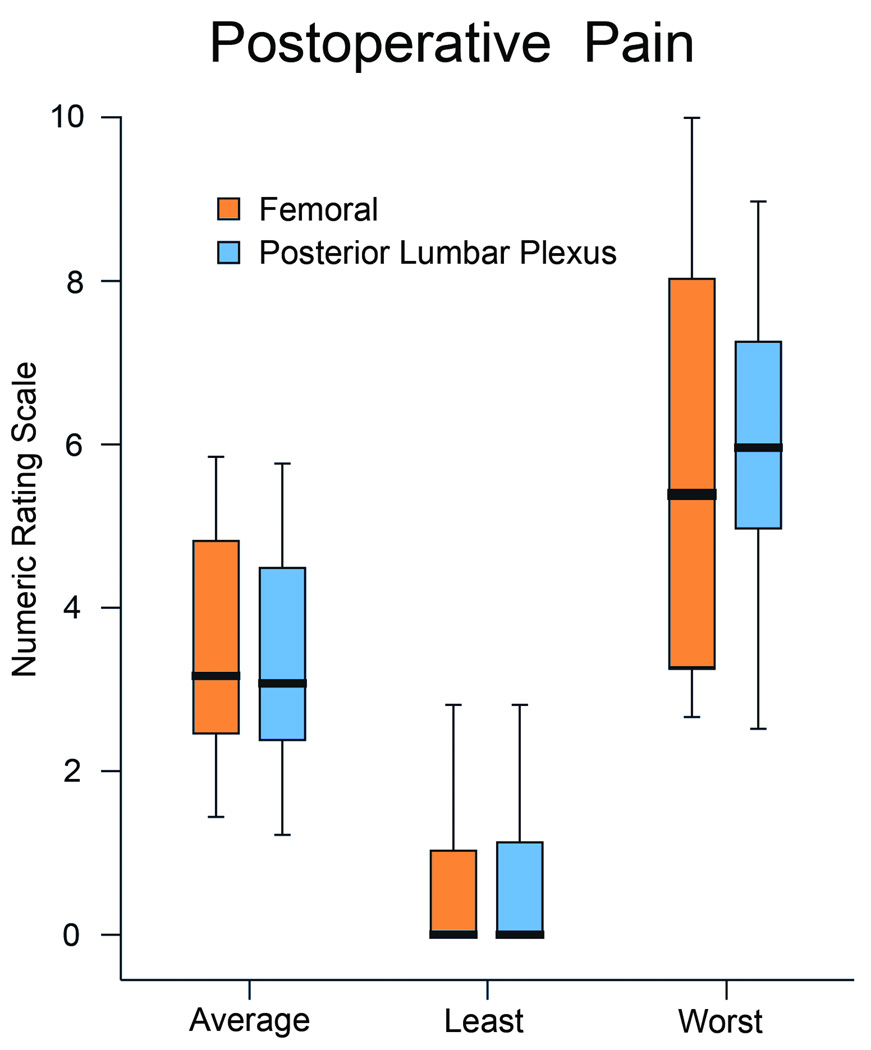
The mean (SD) pain scores for subjects receiving a femoral infusion (n = 25) were 3.6 (1.8) versus 3.5 (1.8) for patients receiving a posterior lumbar plexus infusion (n = 22) resulting in a group difference of 0.1 (95% confidence interval −0.9 to 1.2; P = 0.78; Fig. 1). Because the confidence interval was within the prespecified −1.6 to 1.6 range, we conclude that the effect of the two treatments on postoperative pain were equivalent.
Figure 1.
Perineural catheter location, either a femoral or posterior lumbar plexus, effect on postoperative pain after hip arthroplasty with a ropivacaine 0.2% perineural infusion. Pain severity indicated using a numeric rating scale of 0 to 10, with 0 equal to no pain and 10 being the worst imaginable pain. Data include the 24 h period beginning at 07:30 the morning after surgery. Data are expressed as median (horizontal bar) with 25th–75th (box) and 10th–90th (whiskers). The 95% confidence intervals for the estimated group differences were within prespecified tolerances and were therefore deemed equivalent.
The prespecified primary end point was mean pain score over 24 h. However, because pain scores were recorded every four hours during waking hours and on request of supplemental analgesics, the number of observations varied among subjects. Given this variability, it is not appropriate to assume the mean pain score from each individual arises from the same distribution. A more appropriate way to estimate the mean pain score over the 24-h period is with a mixed-effects model of the repeated hourly pain scores.19 We fit such a model with a subject-specific random intercept, and fixed effects for the pain score at each hour and the difference in pain scores between the infusion groups. The estimated group difference from this model was 0.2 (95% confidence interval −0.9 to 1.3; P = 0.77), which confirms the conclusions of the prespecified analysis.
Secondary end points
Similarly, we detected no differences between the two treatments with respect to the secondary end points, with one exception: subjects with a femoral catheter ambulated a median (10th–90th percentiles) 2(0–17) m the morning after surgery, compared with 11 0–31 m within the posterior lumbar plexus group (P = 0.02; Table 3). However, pain scores between groups differed little during physical therapy: the means (SD) were 4.3 (2.9) versus 3.9 (2.7) in the morning for the femoral and posterior lumbar plexus groups, respectively (group difference 0.4; 95% confidence interval −1.2 to 2.1; P = 0.65); and 3.4 (2.6) versus 3.3 (2.8) in the afternoon (group difference 0.1; 05% confidence interval −1.5 to 1.7; P = 0.90). There were no patient falls in either treatment group. Satisfaction (0 to 10, 10: very satisfied) with analgesia during hospitalization was rated a median (10th–90th percentiles) of 8.0 (7.0 to 10.0) for subjects with a femoral infusion, compared with 10.0 (7.0 to 10.0) for those with a posterior lumbar plexus infusion (P = 0.11). However, the average differences between groups were minimal, with the femoral and posterior lumbar plexus groups reporting a mean (SD) of 8.5 (1.2) and 9.2 (1.4), respectively (group difference −0.7; 95% confidence interval −1.5 to 0.1; P = 0.10).
Table 3.
Secondary end points (24 h period beginning at 07:30 the morning after surgery)
| Femoral Infusion (n=25) |
Posterior Lumbar Plexus Infusion (n=22) |
p-value | |
|---|---|---|---|
| Hydromorphone equivalents (mg) | 2.8 (1.0–7.4) | 2.2 (1.2–7.6) | 0.82 |
| Total ambulation, morning (m) | 2 (0–17) | 11 (0–31) | 0.01 |
| Total ambulation, afternoon (m) | 10 (3–50) | 33 (2–67) | 0.16 |
| Infusion basal rate decreased (#) | 3 | 1 | 0.61 |
| Catheter inadvertently dislodged (#) | 0 | 0 | |
| Local anesthetic infusion duration (h) | 64 (47–76) | 51 (44–72) | 0.27 |
| Hospitalization duration (days) | 3.0 (2.0–4.0) | 3.0 (2.0–3.0) | 0.32 |
Values are reported as median (10th–90th percentiles)
NRS: Numeric Rating Scale (0–10: 0=no pain, 10=worst imaginable pain).
DISCUSSION
This investigation provides evidence that continuous femoral and posterior lumbar plexus blocks provide similar analgesia after hip arthroplasty. However, a posterior lumbar plexus perineural infusion appears to weaken the quadriceps femoris muscle to a lesser extent than a femoral infusion.20 While we did not directly measure quadriceps femoris strength,21 ambulation distance the morning after surgery was greatly decreased with a femoral infusion; an observation reflected in one previous controlled study,20 but not two others.12,13 In our study three patients with femoral catheters had their basal infusion decreased the morning after surgery due to weak quadriceps inhibiting ambulation per the physical therapist, compared with only one subject with a posterior lumbar plexus catheter (P = 0.61). Because quadriceps femoris weakness is associated with significant functional disability22 and an increased risk of falls in elderly patients,23 it is postulated that any nerve block-induced muscular weakness is best minimized during perineural local anesthetic infusion. Both continuous femoral and posterior lumbar plexus blocks are associated with an increased risk of falling after knee and hip arthroplasty, respectively.24
Previously published data
A similar study to ours was recently published, with somewhat different results.20 The previous study involving unilateral hip arthroplasty found that, compared with a continuous femoral nerve block, a continuous posterior lumbar plexus block provides improved analgesia specifically during physical therapy the day after surgery (mean of 3.5 and 5.8 on a visual analog scale; P [lt] 0.05). In this previous study, the femoral catheter failed in providing dynamic analgesia to such a large degree that there was little difference between the group receiving a femoral perineural infusion and a third treatment group receiving only IV opioids (mean of 5.8 and 6.3; P value reported as [mt]0.05). We believe that the differences between the previously published study and our current investigation may Be explained with four differences in the study protocols.
First, in the previous study, an insulated needle was used to locate the femoral nerve, and a nonstimulating catheter advanced a full 10 cm past the needletip for the femoral technique.20 However, nonstimulating catheters advanced past the needletip below the fascia iliaca do not reliably remain adjacent to the femoral nerve,11 possibly accounting for the relatively poor dynamic analgesia in the femoral infusion group.20 It was for this reason that we chose to use stimulating catheters in our study, because there are data suggesting that stimulating catheters may be placed, on average, closer to the target nerve/plexus compared with nonstimulating devices.25–27 For hip arthroplasty, it is theoretically important to insert the catheter tip further cephalad in an attempt to deliver local anesthetic to the obturator and lateral femoral cutaneous nerves28; therefore, we advanced the stimulating catheter 15 cm beyond the needletip. We do not mean to suggest that the technique we used for our study reliably delivers the catheter tip to the lumbar plexus. However, published data do suggest that using a stimulating versus a nonstimulating catheter permitted an increased degree of catheter insertion (up to 15 cm) while keeping the catheter tip adjacent to the femoral nerve.11;21;25–27;29–31
A second possible explanation for our differing results is that unlike the previously published study,20 our protocol included a scheduled long-acting oral opioid (Oxycontin 10 mg twice daily). This analgesic probably decreased any differences in dynamic analgesia between the two treatment groups (as well as increased possible opioid-related side effects).20 In addition, the previous study used a higher basal infusion rate (10.5 vs 6.0 mL/h for a 70 kg subject) which may have increased the effectiveness of the posterior lumbar plexus, but not femoral infusions. We chose a 6 mL/h basal rate since two previous investigations reported 42%–43% of patients receiving femoral or posterior lumbar plexus perineural ropivacaine 0.2% at 8 mL/h resulted in quadriceps femoris weakness requiring a decrease in the basal infusion rate,2,32 and a possible increase in patient falls.24
Lastly, while the previous study recorded pain scores specifically at rest,20 we collected pain scores at all times other than physical therapy sessions (every 4 hours and upon analgesic request). This protocol results in a more-global reflection of subjects’ experiences in the postoperative period, not simply “at rest.”
Study limitations
Subjects, clinical personnel, and investigators were not masked to treatment group. In addition, the current findings involved patients undergoing unilateral hip arthroplasty, and may not Be applicable to other surgical procedures of the hip joint. Lastly, the results of this study are specific to the catheter-insertion and subsequent perineural infusion protocol described above. Whether our findings remain valid for alternative protocols (e.g., in-plane ultrasound-guided catheter insertion, bupivacaine infusion, etc.) remains unknown.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the invaluable assistance of Eliza Ferguson, BS, Research Coordinator, Department of Anesthesiology, University of California San Diego (San Diego, CA); Beverly Morris, RN, CNP, MBA, Educator, Department of Nursing, University of California San Diego (San Diego, CA); and the entire Orthopedic Ward staffs at Thornton (La Jolla, CA) and Hillcrest (San Diego, CA) Hospitals.
Funding: Funding for this project provided by National Institutes of Health grant GM077026 (P.I.: Dr. Ilfeld) from the National Institute of General Medical Sciences (Bethesda, MD); National Institutes of Health grant RR000827 from the National Center for Research Resources (Bethesda, MD); the University of California San Diego Department of Anesthesiology (San Diego, CA), Teleflex Medical (Research Triangle Park, NC), and Stryker Instruments (Kalamazoo, MI). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of these entities. Teleflex Medical and Stryker Instruments had no input into any aspect of the present study conceptualization, design, and implementation; data collection, analysis and interpretation; or manuscript preparation. Drs. Mariano and Kim are currently affiliated with Stanford University School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: See Disclosures at the end of the article
Reprints will not be available from the authors.
DISCLOSURES: Name: Brian M. Ilfeld, MD, MS, Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript., Attestation: Brian M. Ilfeld has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files., Conflicts of Interest: Brian M. Ilfeld received research funding from Stryker Instruments and received research funding from Teleflex/Arrow The authors listed above have received funding for other research investigations from Teleflex/Arrow International and Stryker Instruments. These companies had no input into any aspect of the present study conceptualization, design, and implementation; data collection, analysis and interpretation; or manuscript preparation.; Name: Edward R. Mariano, MD, MAS (Clinical Research), Contribution: This author helped design the study, conduct the study, and write the manuscript., Attestation: Edward R. Mariano approved the final manuscript.; Conflicts of Interest: Edward R. Mariano received honoraria from Stryker Instruments, received research funding from Stryker Instruments, and received research funding from Teleflex/Arrow Drs. Mariano, and Loland have received funding for other research investigations from Teleflex/Arrow International and Stryker Instruments. These companies had no input into any aspect of the present study conceptualization, design, and implementation; data collection, analysis and interpretation; or manuscript preparation. Dr. Mariano previously conducted continuous peripheral nerve block workshops for Stryker Instruments.; Name: Sarah J. Madison, MD, Contribution: This author helped conduct the study and write the manuscript., Attestation: Sarah J. Madison approved the final manuscript., Conflicts of Interest: Sarah J. Madison received research funding from Stryker Instruments and received research funding from Teleflex/Arrow The author listed above have received funding for other research investigations from Teleflex/Arrow International and Stryker Instruments. These companies had no input into any aspect of the present study conceptualization, design, and implementation; data collection, analysis and interpretation; or manuscript preparation.; Name: Vanessa J. Loland, MD, Contribution: This author helped design the study, conduct the study, and write the manuscript., Attestation: Vanessa J. Loland approved the final manuscript., Conflicts of Interest: Vanessa J. Loland received research funding from Stryker Instruments and received research funding from Teleflex/Arrow The author listed above has received funding for other research investigations from Teleflex/Arrow International and Stryker Instruments. These companies had no input into any aspect of the present study conceptualization, design, and implementation; data collection, analysis and interpretation; or manuscript preparation.; Name: NavParkash S. Sandhu, MD, Contribution: This author helped conduct the study and write the manuscript., Attestation: NavParkash S. Sandhu approved the final manuscript., Conflicts of Interest: NavParkash S. Sandhu received research funding from Stryker Instruments and received research funding from Teleflex/Arrow The authors listed above has received funding for other research investigations from Teleflex/Arrw International and Stryker Instruments. These companies had no input into any aspect of the present study conceptualization, design, and implementation; data collection, analysis and interpretation; or manuscript preparation.; Name: Preetham J. Suresh, MD, Contribution: This author helped conduct the study and write the manuscript., Attestation: Preetham J. Suresh approved the final manuscript., Conflicts of Interest: Preetham J. Suresh reported no conflicts of interest.; Name: Michael L. Bishop, MD, Contribution: This author helped conduct the study and write the manuscript., Attestation: Michael L. Bishop approved the final manuscript., Conflicts of Interest: Michael L. Bishop received research funding from Stryker Instruments and received research funding from Teleflex/Arrow The author listed above has received funding for other research investigations from Teleflex/Arrow International and Stryker Instruments. These companies had no input into any aspect of the present study conceptualization, design, and implementation; data collection, analysis and interpretation; or manuscript preparation.; Name: T. Edward Kim, MD, Contribution: This author helped design the study, conduct the study, and write the manuscript., Attestation: T. Edward Kim approved the final manuscript., Conflicts of Interest: T. Edward Kim reported no conflicts of interest.; Name: Michael C. Donohue, PhD, Contribution: This author helped design the study, analyze the data, and write the manuscript., Attestation: Michael C. Donohue has seen the original study data, reviewed the analysis of the data, and approved the final manuscript., Conflicts of Interest: Michael C. Donohue received research funding from Stryker Instruments and received research funding from Teleflex/Arrow The author listed above has received funding for other research investigations from Teleflex/Arrow International and Stryker Instruments. These companies had no input into any aspect of the present study conceptualization, design, and implementation; data collection, analysis and interpretation; or manuscript preparation.; Name: Anna A. Kulidjian, MD, Contribution: This author helped design the study, conduct the study, and write the manuscript., Attestation: Anna A. Kulidjian approved the final manuscript., Conflicts of Interest: Anna A. Kulidjian reported no conflicts of interest.; Name: Scott T. Ball, MD, Contribution: This author helped design the study, conduct the study, and write the manuscript., Attestation: Scott T. Ball approved the final manuscript., Conflicts of Interest: Scott T. Ball received research funding from Stryker Instruments and received research funding from Teleflex/Arrow. The author listed above has received funding for other research investigations from Teleflex/Arrow International and Stryker Instruments. These companies had no input into any aspect of the present study conceptualization, design, and implementation; data collection, analysis and interpretation; or manuscript preparation.
Contributor Information
Brian M. Ilfeld, Department of Anesthesiology, University of California San Diego.
Edward R. Mariano, Department of Anesthesiology, University of California San Diego (current affiliation: Stanford University).
Sarah J. Madison, Department of Anesthesiology, University of California San Diego.
Vanessa J. Loland, Department of Anesthesiology, University of California San Diego.
NavParkash S. Sandhu, Department of Anesthesiology, University of California San Diego.
Preetham J. Suresh, Department of Anesthesiology, University of California San Diego.
Michael L. Bishop, Department of Anesthesiology, University of California San Diego.
T. Edward Kim, Department of Anesthesiology, University of California San Diego (current affiliation: Stanford University).
Michael C. Donohue, Department of Family and Preventive Medicine, University of California San Diego.
Anna A. Kulidjian, Department of Orthopedic Surgery, University of California San Diego.
Scott T. Ball, Department of Orthopedic Surgery, University of California San Diego.
REFERENCES
- 1.Buckenmaier CC, III, Xenos JS, Nilsen SM. Lumbar plexus block with perineural catheter and sciatic nerve block for total hip arthroplasty. J Arthroplasty. 2002;17:499–502. doi: 10.1054/arth.2002.32176. [DOI] [PubMed] [Google Scholar]
- 2.Ilfeld BM, Ball ST, Gearen PF, Le LT, Mariano ER, Vandenborne K, Duncan PW, Sessler DI, Enneking FK, Shuster JJ, Theriaque DW, Meyer RS. Ambulatory continuous posterior lumbar plexus nerve blocks after hip arthroplasty: a dual-center, randomized, triple-masked, placebo-controlled trial. Anesthesiology. 2008;109:491–501. doi: 10.1097/ALN.0b013e318182a4a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ilfeld BM, Moeller LK, Mariano ER, Loland VJ, Stevens-Lapsley JE, Fleisher AS, Girard PJ, Donohue MC, Ferguson EJ, Ball ST. Continuous Peripheral Nerve Blocks: Is Local Anesthetic Dose the Only factor, or Do Concentration and volume Influence Infusion Effects as Well? Anesthesiology. 2010;112:347–354. doi: 10.1097/ALN.0b013e3181ca4e5d. [DOI] [PubMed] [Google Scholar]
- 4.Capdevila X, Macaire P, Dadure C, Choquet O, Biboulet P, Ryckwaert Y, d’Athis F. Continuous psoas compartment block for postoperative analgesia after total hip arthroplasty: New landmarks, technical guidelines, and clinical evaluation. Anesth Analg. 2002;94:1606–1613. doi: 10.1097/00000539-200206000-00045. [DOI] [PubMed] [Google Scholar]
- 5.Siddiqui ZI, Cepeda MS, Denman W, Schumann R, Carr DB. Continuous lumbar plexus block provides improved analgesia with fewer side effects compared with systemic opioids after hip arthroplasty: a randomized controlled trial. Reg Anesth Pain Med. 2007;32:393–398. doi: 10.1016/j.rapm.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Hebl JR, Dilger JA, Byer DE, Kopp SL, Stevens SR, Pagnano MW, Hanssen AD, Horlocker TT. A pre-emptive multimodal pathway featuring peripheral nerve block improves perioperative outcomes after major orthopedic surgery. Reg Anesth Pain Med. 2008;33:510–517. [PubMed] [Google Scholar]
- 7.Turker G, Uckunkaya N, Yavascaoglu B, Yilmazlar A, Ozcelik S. Comparison of the catheter-technique psoas compartment block and the epidural block for analgesia in partial hip replacement surgery. Acta Anaesthesiol Scand. 2003;47:30–36. doi: 10.1034/j.1399-6576.2003.470106.x. [DOI] [PubMed] [Google Scholar]
- 8.Horlocker TT, Wedel DJ, Rowlingson JC, Enneking FK, Kopp SL, Benzon HT, Brown DL, Heit JA, Mulroy MF, Rosenquist RW, Tryba M, Yuan CS. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: Am Society of Regional Anesthesia and Pain Med Evidence-Based Guidelines (Third edition) Reg Anesth Pain Med. 2010;35:64–101. doi: 10.1097/aap.0b013e3181c15c70. [DOI] [PubMed] [Google Scholar]
- 9.Capdevila X, Pirat P, Bringuier S, Gaertner E, Singelyn F, Bernard N, Choquet O, Bouaziz H, Bonnet F. Continuous Peripheral Nerve Blocks in Hospital Wards after Orthopedic Surgery: A Multicenter Prospective Analysis of the Quality of Postoperative Analgesia and Complications in 1,416 Patients. Anesthesiology. 2005;103:1035–1045. doi: 10.1097/00000542-200511000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Bouaziz H, Vial F, Jochum D, Macalou D, Heck M, Meuret P, Braun M, Laxenaire MC. An evaluation of the cutaneous distribution after obturator nerve block. Anesth Analg. 2002;94:445–449. doi: 10.1097/00000539-200202000-00041. [DOI] [PubMed] [Google Scholar]
- 11.Capdevila X, Biboulet P, Morau D, Bernard N, Deschodt J, Lopez S, d’Athis F. Continuous three-in-one block for postoperative pain after lower limb orthopedic surgery: where do the catheters go? Anesth Analg. 2002;94:1001–1006. doi: 10.1097/00000539-200204000-00042. [DOI] [PubMed] [Google Scholar]
- 12.Morin AM, Kratz CD, Eberhart LH, Dinges G, Heider E, Schwarz N, Eisenhardt G, Geldner G, Wulf H. Postoperative analgesia and functional recovery after total-knee replacement: comparison of a continuous posterior lumbar plexus (psoas compartment) block, a continuous femoral nerve block, and the combination of a continuous femoral and sciatic nerve block. Reg Anesth Pain Med. 2005;30:434–445. doi: 10.1016/j.rapm.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Kaloul I, Guay J, Cote C, Fallaha M. The posterior lumbar plexus (psoas compartment) block and the three-in-one femoral nerve block provide similar postoperative analgesia after total knee replacement: [lsqb]Le bloc du plexus lombaire par voie posterieure (loge du psoas) et le bloc du nerf femoral trois-en-un produisent une analgesie similaire apres une arthroscopie totale du genou[rsqb] Can J Anaesth. 2004;51:45–51. doi: 10.1007/BF03018546. [DOI] [PubMed] [Google Scholar]
- 14.Ilfeld BM, Mariano ER, Girard PJ, Loland VJ, Meyer RS, Donovan JF, Pugh GA, Le LT, Sessler DI, Shuster JJ, Theriaque DW, Ball ST. A multicenter, randomized, triple-masked, placebo-controlled trial of the effect of ambulatory continuous femoral nerve blocks on discharge-readiness following total knee arthroplasty in patients on general orthopaedic wards. Pain. 2010;150:477–484. doi: 10.1016/j.pain.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilfeld BM, Thannikary LJ, Morey TE, Vander Griend RA, Enneking FK. Popliteal sciatic perineural local anesthetic infusion: a comparison of three dosing regimens for postoperative analgesia. Anesthesiology. 2004;101:970–977. doi: 10.1097/00000542-200410000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Ilfeld BM, Loland VJ, Mariano ER. Prepuncture ultrasound imaging to predict transverse process and lumbar plexus depth for psoas compartment block and perineural catheter insertion: a prospective, observational study. Anesth Analg. 2010;110:1725–1728. doi: 10.1213/ANE.0b013e3181db7ad3. [DOI] [PubMed] [Google Scholar]
- 17.Van Tubergen A, Debats I, Ryser L, Londono J, Burgos-Vargas R, Cardiel MH, Landewe R, Stucki G, Van Der HD. Use of a numerical rating scale as an answer modality in ankylosing spondylitis-specific questionnaires. Arthritis Rheum. 2002;47:242–248. doi: 10.1002/art.10397. [DOI] [PubMed] [Google Scholar]
- 18.Armitage P, Berry G, Matthews JNS. Statistical methods in medical research. Oxford: Blackwell Science; 2002. [Google Scholar]
- 19.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 20.Marino J, Russo J, Kenny M, Herenstein R, Livote E, Chelly JE. Continuous lumbar plexus block for postoperative pain control after total hip arthroplasty. A randomized controlled trial. J Bone Joint Surg Am. 2009;91:29–37. doi: 10.2106/JBJS.H.00079. [DOI] [PubMed] [Google Scholar]
- 21.Morin AM, Eberhart LH, Behnke HK, Wagner S, Koch T, Wolf U, Nau W, Kill C, Geldner G, Wulf H. Does femoral nerve catheter placement with stimulating catheters improve effective placement? A randomized, controlled, and observer-blinded trial. Anesth Analg. 2005;100:1503–1510. doi: 10.1213/01.ANE.0000151160.93288.0A. [DOI] [PubMed] [Google Scholar]
- 22.Mizner RL, Snyder-Mackler L. Altered loading during walking and sit-to-stand is affected by quadriceps weakness after total knee arthroplasty. J Orthop Res. 2005;23:1083–1090. doi: 10.1016/j.orthres.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Stevens JE, Mizner RL, Snyder-Mackler L. Quadriceps strength and volitional activation before and after total knee arthroplasty for osteoarthritis. J Orthop.Res. 2003;21:775–779. doi: 10.1016/S0736-0266(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 24.Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111:1552–1554. doi: 10.1213/ANE.0b013e3181fb9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salinas FV, Neal JM, Sueda LA, Kopacz DJ, Liu SS. Prospective comparison of continuous femoral nerve block with nonstimulating catheter placement versus stimulating catheter-guided perineural placement in volunteers. Reg Anesth Pain Med. 2004;29:212–220. doi: 10.1016/j.rapm.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Casati A, Fanelli G, Koscielniak-Nielsen Z, Cappelleri G, Aldegheri G, Danelli G, Fuzier R, Singelyn F. Using stimulating catheters for continuous sciatic nerve block shortens onset time of surgical block and minimizes postoperative consumption of pain medication after halux valgus repair as compared with conventional nonstimulating catheters. Anesth Analg. 2005;101:1192–1197. doi: 10.1213/01.ane.0000167232.10305.cd. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez J, Taboada M, Carceller J, Lagunilla J, Barcena M, Alvarez J. Stimulating popliteal catheters for postoperative analgesia after hallux valgus repair. Anesth Analg. 2006;102:258–262. doi: 10.1213/01.ane.0000189219.00096.0c. [DOI] [PubMed] [Google Scholar]
- 28.Birnbaum K, Prescher A, Hessler S, Heller KD. The sensory innervation of the hip joint–an anatomical study. Surg Radiol Anat. 1997;19:371–375. doi: 10.1007/BF01628504. [DOI] [PubMed] [Google Scholar]
- 29.Stevens MF, Werdehausen R, Golla E, Braun S, Hermanns H, Ilg A, Willers R, Lipfert P. Does interscalene catheter placement with stimulating catheters improve postoperative pain or functional outcome after shoulder surgery? A prospective, randomized and double-blinded trial. Anesth Analg. 2007;104:442–447. doi: 10.1213/01.ane.0000253513.15336.25. [DOI] [PubMed] [Google Scholar]
- 30.Hayek SM, Ritchey RM, Sessler D, Helfand R, Samuel S, Xu M, Beven M, Bourdakos D, Barsoum W, Brooks P. Continuous femoral nerve analgesia after unilateral total knee arthroplasty: stimulating versus nonstimulating catheters. Anesth Analg. 2006;103:1565–1570. doi: 10.1213/01.ane.0000244476.38588.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrington MJ, Olive DJ, McCutcheon CA, Scarff C, Said S, Kluger R, Gillett N, Choong P. Stimulating catheters for continuous femoral nerve blockade after total knee arthroplasty: a randomized, controlled, double-blinded trial. Anesth Analg. 2008;106:1316–1321. doi: 10.1213/ane.0b013e318164efd1. [DOI] [PubMed] [Google Scholar]
- 32.Ilfeld BM, Le LT, Meyer RS, Mariano ER, Vandenborne K, Duncan PW, Sessler DI, Enneking FK, Shuster JJ, Theriaque DW, Berry LF, Spadoni EH, Gearen PF. Ambulatory continuous femoral nerve blocks decrease time to discharge readiness after tricompartment total knee arthroplasty: a randomized, triple-masked, placebo-controlled study. Anesthesiology. 2008;108:703–713. doi: 10.1097/ALN.0b013e318167af46. [DOI] [PMC free article] [PubMed] [Google Scholar]