Abstract
Focal adhesion kinase (FAK) is a cytoplasmic tyrosine kinase that plays critical roles in integrin-mediated signal transductions and also participates in signaling by other cell surface receptors. In integrin-mediated cell adhesion, FAK is activated via disruption of an auto-inhibitory intra-molecular interaction between its amino terminal FERM domain and the central kinase domain. The activated FAK forms a complex with Src family kinases, which initiates multiple downstream signaling pathways through phosphorylation of other proteins to regulate different cellular functions. Multiple downstream signaling pathways are identified to mediate FAK regulation of migration of various normal and cancer cells. Extensive studies in cultured cells as well as conditional FAK knockout mouse models indicated a critical role of FAK in angiogenesis during embryonic development and cancer progression. More recent studies also revealed kinase-independent functions for FAK in endothelial cells and fibroblasts. Consistent with its roles in cell migration and angiogenesis, increased expression and/or activation of FAK are found in a variety of human cancers. Therefore, small molecular inhibitors for FAK kinase activity as well as future development of novel therapies targeting the potentially kinase-independent functions of FAK are promising treatments for metastatic cancer as well as other diseases.
1. Introduction
Cell migration plays crucial roles not only in a various biological processes such as embryonic development, but also in different diseases including cancer and cardiovascular disorders [1, 2]. Cell migration is a dynamic and multistep process of leading edge protrusion, turnover of focal adhesions, generation of tractional forces, and tail retraction and detachment [3, 4]. As the major cellular receptors for extracellular matrix (ECM), integrin family cell adhesion receptors are essential for each of these steps in cell migration [5]. Although they have relatively short cytoplasmic domains, integrins regulate cell migration as well as other cellular functions through their coupling to multiple cytoskeletal and signaling molecules, many of which co-cluster with integrins in focal adhesions in adherent cells. Focal adhesion kinase (FAK), a non-receptor tyrosine kinase, is the earliest identified and one of the most prominent signaling molecules among these proteins [6–10].
FAK was discovered by two converging lines of research in the early ‘90s. In the first, an approximately 120 kDa protein was found as a major integrin-dependent tyrosine phosphorylated protein localized in focal adhesions [11, 12]. In another set of studies, several potential substrates of the v-Src oncogenes were described based on their high tyrosine phosphorylation in transformed cells by v-Src [13]. One of these substrates was soon found to be a tyrosine kinase itself and identical to the 120 kDa protein whose phosphorylation was triggered by integrin-mediated cell adhesion [14, 15]. This protein was named as FAK based on its prominent localization in the focal adhesions [14]. Since these early reports implicating FAK in anchorage-independent growth of cancer cells [15], numerous studies in the last 20 years have established FAK as a central mediator of integrin signaling as well as important components of signaling by other cell surface receptors. In particular, regulation of cell migration by integrin signaling through FAK is well established in many cell types which contribute to pathogenesis of cancer and other diseases [16, 17]. As such, FAK is considered a promising therapeutic target for treatment of cancer and cardiovascular diseases as well as potentially other diseases. Indeed several small molecule inhibitors for FAK have already been developed by different pharmaceutical companies for clinical trials [18–20]. In this review, we will focus on the roles of FAK and its downstream signaling pathways in cell migration and angiogenesis, as well as recent findings of the kinase-independent functions of FAK which will be important in future considerations to develop therapies targeting FAK. For more comprehensive perspective on FAK signaling in other cellular functions and developmental and disease processes, the readers are referred to a number of other excellent review articles [6–10].
2. Structural features of FAK and its activation by integrins
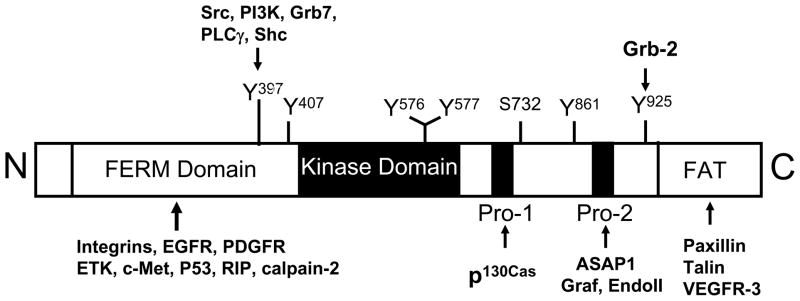
The FAK gene is highly conserved with over 90% sequence identity across different species including human, mouse, chicken and Xenopus [14, 21–23]. It has been mapped to human chromosome 8 and mouse chromosome 15, respectively [24]. FAK is composed of a central kinase domain flanked by a N-terminal FERM (Band 4.1, ezrin-radixin-moesin) domain and a C-terminal domain that includes the focal adhesion targeting (FAT) sequence (Fig. 1). The FERM domain of FAK share similar structure with that of cytoskeletal proteins such as talin and the ezrin-radixin-moesin (ERM) family of proteins, as well as signaling molecules such as the JAK family of tyrosine kinases and several tyrosine phosphotases [25, 26]. It has been proposed to mediate FAK interactions with integrins, growth factor receptors and a few other proteins [27–31] as well as an intra-molecular interaction with the kinase domain of FAK [32–34]. In addition to the FAT sequence, the C-terminal domain contains two proline-rich sequences as docking sites for SH3 domain-containing proteins, such as p130cas [35], endophilin A2 [36], Graf ([37] and ASAP ([38], some of these (e.g. p130cas) act to recruit additional signaling proteins [39, 40]. Several sites of tyrosine phosphorylation have been identified in FAK which serve to modulate FAK kinase activity or mediate FAK interaction with SH2-domain containing proteins, including the major autophosphorylation site Tyr 397 essential for the majority of FAK functions.
Fig. 1. FAK structural features and interaction proteins.
FAK is composed of a central kinase domain flanked by a N-terminal FERM domain and a C-terminal region containing two proline-rich (PR1 and PR2) motifs and a FAT domain. Several known phosphorylation sites as well as residues or regions of FAK mediating association with some of its interacting-proteins are indicated in the diagram.
The crystal structure of FAK in both active and inactive states have been solved recently, which, together with the earlier mutational analysis, provide a model for FAK activation by integrins [32–34, 41]. In its inactive state, FAK is in a closed conformation with its FERM domain binding to the kinase domain. There are two major points of regulatory contact: the F1 FERM lobe binding to a linker segment containing Y397 and a hydrophobic pocket within the F2 FERM lobe to F596 within the FAK kinase domain. FAK FERM-mediated inhibition of FAK kinase activity therefore results from steric inhibition of target protein access to the catalytic cleft and to the Y397 autophosphorylation site. Upon integrin-mediated cell adhesion to ECM, FERM domain is displaced by an activating protein (e.g. integrin β cytoplasmic domain which can interact with FERM domain [30] or other activators). This will induce a conformational change to allow for FAK autophosphorylation at Y397 and its exposure for binding Src family kinases, which phosphorylates additional sites on FAK leading to its full activation. FAK binding to the SH2 domain of Src also displaces its intra-molecular association with residue Y527, relieving the auto-inhibitory interaction and leading to activation of Src. The mutually activated FAK/Src complex then initiates multiple downstream signaling pathways through phosphorylation of other proteins to regulate different cellular functions.
3. Regulation of cell migration by FAK signaling pathways
Integrin signaling through FAK has been shown to promote cell migration in numerous studies. Initial suggestion for a role for FAK in cell migration was based on correlative observations of increased expression or activation of FAK in the migrating keratinocytes in epidermal wound healing or ECs migrating into the wounded monolayer in vitro, respectively [42, 43]. Increased levels of FAK expression have also been correlated with the invasive and metastatic potential of several human tumors [44, 45]. FAK knockout studies showed an early embryonic lethal phenotype with extensive mesodermal deficiency and FAK−/− embryonic fibroblasts from these mice exhibited a profound defects in migration, providing more direct evidence for a role of FAK in promoting migration [46]. Consistent with these observations in vivo, microinjection of the FAK C-terminal recombinant protein (i.e. FRNK) inhibited FAK activation and reduced migration of both fibroblasts and ECs [47]. Conversely, overexpression of FAK in various cells including the FAK−/− MEFs promotes their migration on FN [48–50].
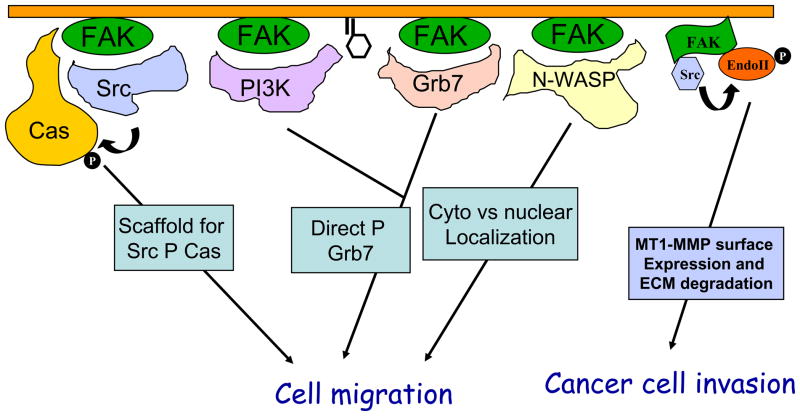
Several FAK downstream signaling pathways have been implicated in mediating FAK promotion of cell migration (Fig. 2). One particularly well characterized pathway is through the association and phosphorylation of p130cas by the FAK/Src complex [39, 48–51]. Disruption of FAK binding to either Src or p130cas prevents p130cas phosphorylation at multiple sites and reduces cell migration [48, 50, 51]. Tyrosine phosphorylated p130cas associates with several SH2-containing proteins including Crk. The Cas/Crk complex formation plays a key role to regulate membrane ruffling and cell migration through DOCK180 and Rac [52, 53]. Another cytoskeletal and adaptor protein paxillin is also a major substrate of FAK/Src kinase complex and its phosphorylation at Y31 and Y118 residues could function to recruit Crk in a similar manner as Cas [54]. Paxillin is also found in a multi-protein complex containing another adaptor molecule PKL, a guanine nucleotide exchange factor Pix/Cool whose phosphorylation is also dependent on FAK, and the Cdc42/Rac target-effector PAK. This complex may link Cdc42 and Rac to PAK and its downstream targets, such as LIMK and MLCK to regulate cell migration [55] [56].
Fig. 2. Regulation of cell migration and invasion by FAK through multiple signaling pathways.
Several mechanisms by which FAK regulates cell migration and invasion are depicted here. These include serving as a scaffold for Src phosphorylation of p130cas, direct phosphorylation of Grb7 in a PI3K dependent manner and phosphorylation of N-WASP to regulate its cytoplasmic retention to control cell migration in a variety of cells, and mediating phosphorylation of endophilin A2 by Src to regulate surface expression of MT1-MMP in cancer cells.
A second pathway mediating FAK promotion of cell migration involves its interactions with PI3K and an adaptor molecule Grb7 [57, 58]. This was first suggested by the observation that a FAK mutant that selectively disrupted its binding to PI3K and Grb7, but retained binding to Src and induction of Cas phosphorylation, failed to promote cell migration [59]. The activated PI3K could stimulate cell migration through its downstream effector Rac, which is a key regulator of cortical actin and lamellipodia in cell migration [60]. Moreover, the increased D3-phosphoinositides facilitate Grb7 association with phosphatidylinositol phosphates in the plasma membrane through its PH domain. The phosphatidylinositol phosphates binding to Grb7 also induces a conformational change in Grb7, leading to increased phosphorylation of Grb7 by FAK [61]. Mutational analyses showed that both FAK binding to and phosphorylation of Grb7 are important for Grb7 stimulation of cell migration [58]. Although FAK independently binds to PI3K and Grb7, the FAK/PI3K and FAK/Grb7 complexes mediate FAK promotion of cell migration in a cooperative manner.
FAK also regulates cell migration through its effects on the Rho subfamily of small GTPases and the assembly and disassembly of actin cytoskeleton. FAK deletion in fibroblasts led to increased RhoA activity as well as increased cell spreading and reduced cell migration, which can be rescued by re-expression of FAK in these cells [62–64]. More importantly, inhibition of ROCK, a downstream effecter of RhoA, also rescued the migration defect of FAK-null cells [64]. Interestingly, FAK has also been shown to stimulate RhoA activation by induction of phosphorylation and activation of p190RhoGEF in other studies [65]. More recent studies suggest that compensatory expression of Pyk2 in FAK-null cells may be responsible for stimulation of RhoA in these cells [66]. FAK also associates with regulators of other small GTPases such as GRAF containing GAP activity for RhoA and Cdc42 [37] and ASAP1, a GAP protein for Arf subfamily small GTPases [38]. These interactions could also contribute to FAK regulation of actin cytoskeleton in cell migration. Lastly, FAK can phosphorylate N-WASP, a key Cdc42 downstream effector, to increase its cytoplasmic localization, which will facilitate its activation of the Arp2/3 complex for actin polymerization in the leading edge of migrating cells [67].
FAK signaling has been shown to promote invasion of both normal and transformed cells in addition to cell migration. In v-Src transformed cells, FAK was found to mediate Src phosphorylation of endophilin A2 to decrease its interaction with dynamin, which is important in the regulation of cell surface matrix metalloprotease MT1-MMP via endocytosis. The reduced internalization of MT1-MMP leads to its accumulation on the cell surface to stimulate the invasive activity of v-Src transformed cells [36]. In addition, FAK also promotes both the expression of MMP2 and MMP9 through the v-Src-Cas-Crk-Dock180 signaling cascade and activation of Rac1 and JNK as well as their secretion into the matrix in cancer cells [63, 68].
4. Critical roles of FAK signaling in angiogenesis
Early observations that FAK regulates EC migration suggested its potential role in angiogenesis, as migration and invasion of ECs are integral to the process of angiogenesis. This is further supported by the patterns of FAK expression in the developing vasculature during embryogenesis [69]. In consistent with its expression pattern, FAK gene knockout in mouse caused early embryonic lethality with extensive cardiovascular defects [46]. Additional support for a role of FAK in angiogenesis includes data suggesting regulation of FAK by several angiogenic growth factor receptors. FAK association with PI3K is increased upon stimulation of VEGF receptor-2 by VEGF-A, which is shown to promote migration of porcine aortic ECs [70]. FAK phosphorylation is also stimulated by angiopoietin-1, another pro-angiogenesis growth factor for ECs [71]. Several studies demonstrating important functions of integrins in tumor angiogenesis also implicate a role for FAK, as integrins are major upstream activators of FAK. Blockade of integrins αvβ3 with monoclonal antibodies or small molecules significantly reduced tumor angiogenesis in a variety of animal models [72–74]. Interestingly, FAK was found to form a signaling complex containing integrin αvβ5 in a Src-dependent manner, which is essential for VEGF stimulated angiogenesis in a mouse model [75].
Several recent studies using transgenic and knockout mouse genetic approaches provided direct evidence supporting a role of FAK in angiogenesis in vivo. Transgenic expression of ectopic FAK in ECs under the control of the Tie2 promotor and enhancer increased angiogenesis in both hind limb ischemia and wound-induced angiogenesis models [76]. Conversely, EC-specific FAK knockout mice were generated by two groups, both showing that FAK is required for embryonic angiogenesis in vivo [77, 78]. Moreover, both studies showed that FAK deletion increased apoptosis of ECs, which contributed to the defective vascular phenotype in the developing embryos. One study also showed that deletion of FAK in ECs reduced their migration in response to FN and VEGF in wound closure assays, which can be rescued by re-expression of FAK [77]. Surprisingly, however, the other report did not detect any decrease in migration of mutant ECs by tracking individual cells using time-lapse microscopy [78]. Future studies will be necessary to resolve the different findings, which could be due to the different methods and/or experimental conditions. However, it is likely that defective EC migration also contributes to the defective embryonic angiogenesis, given many studies in vitro supporting such a role for FAK.
Recently, FAK mutant knockin mouse models were also generated by a number of groups to explore the roles of FAK signaling pathways in development and function in vivo. In one study, the mutant FAK allele deleted exon 15 which contains the major autophosphorylation site Y397. This mutant FAK (designated as FAKΔ) is expressed at a normal level and acts as an active kinase. However, FAKΔ/Δ embryos displayed various defects including hemorrhages, edema, delayed artery formation, vascular remodeling deficiency, multiple organ abnormalities, and overall developmental retardation at E13.5–14.5, and died thereafter [79]. A second mouse model contains FAK mutant allele with the key K454 mutated to R in the catalytic domain that abolished the kinase activity of FAK. Homozygous mice for this mutant allele also exhibited embryonic lethality showing extensive defects in blood vessel formation such as lack of yolk sac primary capillary plexus formation and disorganized EC patterning in embryos [80]. These results suggested that FAK kinase activity and its autophosphorylation at Y397 are necessary for normal vascular development. Nevertheless, they do not establish a direct role of FAK kinase or Y397 in ECs during angiogenesis, as the mutant alleles were in all cells and tissues rather than introduced specifically for ECs only.
Besides a direct effect on angiogenesis by FAK in ECs, the increased expression of FAK in cancer cells have been suggested to play a role in the tumor angiogenic switch to promote aggressive tumor progression and metastasis [81]. For example, Mitra et al. showed that inhibiting FAK activity via stable FRNK expression in 4T1 breast carcinoma cells resulted in small avascular tumors in mouse xenograft models [82]. In these carcinoma cells, FRNK inhibited a FAK-Grb2-MAPK signaling linkage regulating VEGF expression, hence tumor angiogenesis, without interfering cell proliferation or anchorage-independent cell survival. Inhibition of FAK expression in neuroblastoma and breast, prostate carcinoma cells also resulted in reduced VEGF expression [82].
5. FAK possesses both kinase-dependent and –independent functions
Although a few direct substrates have been identified for FAK, the majority of studies so far indicate an important role of FAK is to mediate tyrosine phosphorylation of proteins by Src family kinases in FAK/Src complex. In another word, FAK was found to function as a scaffold in various signaling events regulating different cellular functions [18, 83–85]. Nevertheless, since the binding motif for Src is dependent on Y397, which is an autophosphorylation site for FAK, it can be inferred that FAK kinase activity should be indispensable for FAK functions. Indeed, abundant evidence indicates the key roles of the kinase activity and autophosphorylation at Y397 for FAK functions in various cellular and developmental processes, as discussed above. Interestingly, however, several recent studies provided evidence that FAK can also regulate certain cellular functions in a kinase-independent manner.
Early mutational analysis of FAK observed that kinase-defective FAK promoted migration of CHO cells as effectively as the wild type FAK [86]. However, this kinase-defective FAK mutant was found to be phosphorylated at Y397, possibly due to trans-phosphorylation by the endogenous FAK in CHO cells, to allow it to bind Src and trigger the downstream pathways. This potential complication is eliminated in a more recent study where FAK mutants were expressed in ECs and MEFs after deletion of the endogenous FAK gene [77]. These analyses showed that while it was unable to promote EC migration in response to VEGF, kinase-defective FAK stimulated migration of ECs or MEFs on FN as effectively as wild type FAK. These results provided the first evidence that FAK may function to promote cell migration on FN in a kinase-independent manner.
Kinase-independent function of FAK has been suggested to regulate other cellular activities also. It was found recently that FAK may function in the nucleus to facilitate p53 interaction with Mdm2 and its subsequent ubiquitination and degradation [87]. Deletion of FAK resulted in the increased expression of p53 in a number of cells, which can be rescued by re-expression of FAK. The FERM domain of FAK was found to bind to both p53 and Mdm2 in separate lobes as well as to mediate nuclear localization of FAK. Indeed, re-expression of the FERM domain alone reduced p53 level as effectively as the full length FAK, suggesting that the nuclear FAK can regulate p53 and cell survival through a kinase-independent mechanism. A potential kinase-independent function of FAK in cell proliferation was also suggested in a recent study examining MEFs isolated from mouse embryos with homozygous kinase-defective mutant FAK alleles [80]. In contrast to the inability of FAK-null MEFs to proliferation ex vivo, primary MEFs containing kinase-defective FAK were established and exhibited no defects in cell growth.
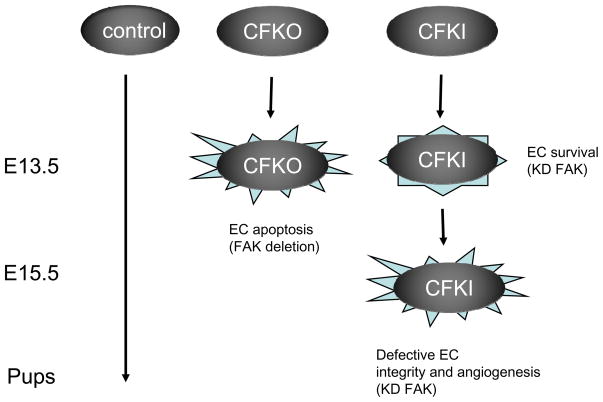
In addition to the above studies in vitro, the differential roles of FAK kinase-dependent and -independent functions in EC survival and functions were assessed directly in vivo in an EC-specific FAK kinase-defective mutant knockin mouse model [88]. It was found that these mice embryos with only kinase-deficient FAK in their vasculature survived longer than animals without any FAK in their ECs. FAK-null ECs exhibit higher apoptosis compared to wild type cells as observed previously, but the kinase-defective FAK rescued this deficiency by suppressing the cyclin-dependent kinase inhibitor p21. On the other hand, however, FAK’s kinase activity was found to be required for the maintenance of normal integrity of blood vessels and their angiogenesis in development. Deletion of FAK caused mislocalization and reduced phosphorylation of VE-cadherin, which was not rescued by kinase-defective FAK. These results led to our proposal of a working model for the role of both kinase-independent and dependent functions of FAK in vascular development (Fig. 3). Together, these recent data from multiple studies strongly suggest that FAK has both kinase-dependent and -independent functions to regulate various cellular activities in various cells.
Fig. 3. A working model of kinase-dependent and -independent functions of FAK in vascular development.
In control embryos, normal FAK functions in ECs allow completion of embryonic development. In FAK conditional knockout (CFKO) embryos, absence of FAK leads to EC apoptosis and embryonic lethality at E13.5. In EC-specific FAK kinase-defective mutant knockin (CFKI) embryos, the kinase-independent functions of FAK support EC survival to allow development beyond E13.5. However, lack of FAK kinase activity results in other defects in EC junctions and angiogenesis, leading to embryonic lethality at E15.5.
6. Concluding remarks
As an important regulator of cell migration and angiogenesis, FAK is likely a suitable target for a variety of diseases that are highly dependent on these biological processes. A particularly good case can be made for cancer metastasis, which is driven by both of these processes. It has been well established that solid tumor growth and survival requires a well-functioning vascular network, which enables rapid proliferation of tumor cells by the delivery of oxygen and other nutrients and facilitating the development of metastatic disease. After the early phase of solid tumor growth, every increase in tumor cell population must be preceded by an increase in new capillaries converging on the tumor, and the sprouting of local blood vessels, i.e. angiogenesis, contributes significantly to the vasculature formation of tumors. The new vessels are often irregular and leaky due to the loss of adherence between endothelial junctions, as well as lack of the pericyte cover. As a result, tumor cells can penetrate them more easily, and neovascularization permits the shedding of cells from the primary tumor. Therefore, the onset of angiogenesis also contributes to metastasis, though the capacity of tumor cells to induce angiogenesis does not always correlate with their degree of malignancy, and decreased angiogenesis is associated with a decreased rate of metastasis [89]. Indeed, enhanced tumor angiogenesis during cancer progression has been associated with poor clinical prognosis and unsuccessful treatment [90]. Therefore, targeting FAK in metastatic cancers could inhibit the disease progression through regulation of cancer cell migration and invasion, angiogenic switch as well as ECs in tumor angiogenesis.
Several small molecule FAK inhibitors have already been developed and evaluated in both pre-clinical models and human clinical trials [20, 91–93]. TAE226, one of FAK inhibitors, was used in therapy experiments and FAK inhibition by it significantly reduced tumor burden and prolonged survival in tumor-bearing mice [94]. The therapeutic efficacy was related to the reduced pericyte coverage, induction of apoptosis of tumor-associated endothelial cells and reduced microvessel density. Some of the small molecule inhibitors have also shown potential efficacy responses in tumor regression and disease stabilization in phase I clinical trials [20]. It is worth noting, however, that these inhibitors are functioning through targeting and blocking the catalytic activity of FAK [20, 93]. Because the kinase-independent functions of FAK observed in ECs and MEFs may also be true in cancer cells, drugs that only target FAK’s catalytic activity may not be sufficient to block its promotion of cancer metastasis. Other strategies such as the use of siRNAs to known-down FAK expression or dominant-negative variants (e.g. FRNK) could be effective in blocking both kinase-dependent and –independent functions of FAK. Nevertheless, small molecule inhibitors are preferred for many advantages in drug development. In this regard, screening for or rationale design of small molecule compounds that inhibit kinase-independent functions of FAK by disruption of specific interactions of FAK with other molecules will be important. These compounds could be used in combination with FAK kinase inhibitors to improve the effectiveness of cancer therapy. Likewise, the continued investigation of FAK kinase-dependent and –independent functions in cell culture as well as mouse models will be a fertile area of future research that could guide the design of such new drugs that target FAK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Erickson CA. Cell migration in the embryo and adult organism. Curr Opin Cell Biol. 1990;2(1):67–74. doi: 10.1016/s0955-0674(05)80033-x. [DOI] [PubMed] [Google Scholar]
- 2.Stoker M, Gherardi E. Regulation of cell movement: the motogenic cytokines. Biochim Biophys Acta. 1991;1072(1):81–102. doi: 10.1016/0304-419x(91)90008-9. [DOI] [PubMed] [Google Scholar]
- 3.Ridley AJ, et al. Cell migration: integrating signals from front to back. Science. 2003;302(5651):1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 4.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84(3):359–69. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 5.Hynes R. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 6.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116(Pt 8):1409–16. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 7.Schlaepfer DD, Mitra SK. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev. 2004;14(1):92–101. doi: 10.1016/j.gde.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Siesser PM, Hanks SK. The signaling and biological implications of FAK overexpression in cancer. Clin Cancer Res. 2006;12(11 Pt 1):3233–7. doi: 10.1158/1078-0432.CCR-06-0456. [DOI] [PubMed] [Google Scholar]
- 9.Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta. 2001;1540(1):1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- 10.Cohen LA, Guan JL. Mechanisms of focal adhesion kinase regulation. Curr Cancer Drug Targets. 2005;5(8):629–43. doi: 10.2174/156800905774932798. [DOI] [PubMed] [Google Scholar]
- 11.Guan JL, Trevithick JE, Hynes RO. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991;2(11):951–64. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kornberg LJ, et al. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc Natl Acad Sci U S A. 1991;88(19):8392–6. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanner SB, et al. Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc Natl Acad Sci U S A. 1990;87(9):3328–32. doi: 10.1073/pnas.87.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaller MD, et al. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992;89(11):5192–6. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan JL, Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992;358(6388):690–2. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- 16.Golubovskaya VM, Cance WG. Focal adhesion kinase and p53 signaling in cancer cells. Int Rev Cytol. 2007;263:103–53. doi: 10.1016/S0074-7696(07)63003-4. [DOI] [PubMed] [Google Scholar]
- 17.McLean GW, et al. The role of focal-adhesion kinase in cancer - a new therapeutic opportunity. Nat Rev Cancer. 2005;5(7):505–15. doi: 10.1038/nrc1647. [DOI] [PubMed] [Google Scholar]
- 18.Zhao J, Guan JL. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009;28(1–2):35–49. doi: 10.1007/s10555-008-9165-4. [DOI] [PubMed] [Google Scholar]
- 19.Lim ST, et al. FERM control of FAK function: implications for cancer therapy. Cell Cycle. 2008;7(15):2306–14. doi: 10.4161/cc.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parsons JT, et al. Focal adhesion kinase: targeting adhesion signaling pathways for therapeutic intervention. Clin Cancer Res. 2008;14(3):627–32. doi: 10.1158/1078-0432.CCR-07-2220. [DOI] [PubMed] [Google Scholar]
- 21.Hanks SK, et al. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci U S A. 1992;89(18):8487–91. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andre E, Becker-Andre M. Expression of an N-terminally truncated form of human focal adhesion kinase in brain. Biochem Biophys Res Commun. 1993;190(1):140–7. doi: 10.1006/bbrc.1993.1022. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Wright CV, Hanks SK. Cloning of a Xenopus laevis cDNA encoding focal adhesion kinase (FAK) and expression during early development. Gene. 1995;160(2):219–22. doi: 10.1016/0378-1119(95)00153-w. [DOI] [PubMed] [Google Scholar]
- 24.Fiedorek FT, Jr, Kay ES. Mapping of the focal adhesion kinase (Fadk) gene to mouse chromosome 15 and human chromosome 8. Mamm Genome. 1995;6(2):123–6. doi: 10.1007/BF00303256. [DOI] [PubMed] [Google Scholar]
- 25.Chishti AH, et al. The FERM domain: a unique module involved in the linkage of cytoplasmic proteins to the membrane. Trends Biochem Sci. 1998;23(8):281–2. doi: 10.1016/s0968-0004(98)01237-7. [DOI] [PubMed] [Google Scholar]
- 26.Girault JA, et al. The N-termini of FAK and JAKs contain divergent band 4.1 domains. Trends Biochem Sci. 1999;24(2):54–7. doi: 10.1016/s0968-0004(98)01331-0. [DOI] [PubMed] [Google Scholar]
- 27.Chen R, et al. Regulation of the PH-domain-containing tyrosine kinase Etk by focal adhesion kinase through the FERM domain. Nat Cell Biol. 2001;3(5):439–44. doi: 10.1038/35074500. [DOI] [PubMed] [Google Scholar]
- 28.Golubovskaya V, et al. Dual inhibition of focal adhesion kinase and epidermal growth factor receptor pathways cooperatively induces death receptor-mediated apoptosis in human breast cancer cells. J Biol Chem. 2002;277(41):38978–87. doi: 10.1074/jbc.M205002200. [DOI] [PubMed] [Google Scholar]
- 29.Poullet P, et al. Ezrin interacts with focal adhesion kinase and induces its activation independently of cell-matrix adhesion. J Biol Chem. 2001;276(40):37686–91. doi: 10.1074/jbc.M106175200. [DOI] [PubMed] [Google Scholar]
- 30.Schaller MD, et al. Focal adhesion kinase and paxillin bind to peptides mimicking beta integrin cytoplasmic domains. J Cell Biol. 1995;130(5):1181–7. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sieg DJ, et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2(5):249–56. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 32.Cooper LA, Shen TL, Guan JL. Regulation of focal adhesion kinase by its amino-terminal domain through an autoinhibitory interaction. Mol Cell Biol. 2003;23(22):8030–41. doi: 10.1128/MCB.23.22.8030-8041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen LA, Guan JL. Residues within the first subdomain of the FERM-like domain in focal adhesion kinase are important in its regulation. J Biol Chem. 2005;280(9):8197–207. doi: 10.1074/jbc.M412021200. [DOI] [PubMed] [Google Scholar]
- 34.Lietha D, et al. Structural basis for the autoinhibition of focal adhesion kinase. Cell. 2007;129(6):1177–87. doi: 10.1016/j.cell.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polte TR, Hanks SK. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas. Proc Natl Acad Sci U S A. 1995;92(23):10678–82. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu X, et al. FAK-mediated src phosphorylation of endophilin A2 inhibits endocytosis of MT1-MMP and promotes ECM degradation. Dev Cell. 2005;9(2):185–96. doi: 10.1016/j.devcel.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Hildebrand JD, Taylor JM, Parsons JT. An SH3 domain-containing GTPase-activating protein for Rho and Cdc42 associates with focal adhesion kinase. Mol Cell Biol. 1996;16(6):3169–78. doi: 10.1128/mcb.16.6.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, et al. The association of ASAP1, an ADP ribosylation factor-GTPase activating protein, with focal adhesion kinase contributes to the process of focal adhesion assembly. Mol Biol Cell. 2002;13(6):2147–56. doi: 10.1091/mbc.E02-01-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klemke RL, et al. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J Cell Biol. 1998;140(4):961–72. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, et al. Mislocalization or reduced expression of Arf GTPase-activating protein ASAP1 inhibits cell spreading and migration by influencing Arf1 GTPase cycling. J Biol Chem. 2005;280(10):8884–92. doi: 10.1074/jbc.M412200200. [DOI] [PubMed] [Google Scholar]
- 41.Dunty JM, et al. FERM domain interaction promotes FAK signaling. Mol Cell Biol. 2004;24(12):5353–68. doi: 10.1128/MCB.24.12.5353-5368.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romer LH, et al. Tyrosine kinase activity, cytoskeletal organization, and motility in human vascular endothelial cells. Mol Biol Cell. 1994;5(3):349–61. doi: 10.1091/mbc.5.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gates RE, et al. Potential role for focal adhesion kinase in migrating and proliferating keratinocytes near epidermal wounds and in culture. Cell Growth Differ. 1994;5(8):891–9. [PubMed] [Google Scholar]
- 44.Weiner TM, et al. Expression of focal adhesion kinase gene and invasive cancer. Lancet. 1993;342(8878):1024–5. doi: 10.1016/0140-6736(93)92881-s. [DOI] [PubMed] [Google Scholar]
- 45.Owens LV, et al. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55(13):2752–5. [PubMed] [Google Scholar]
- 46.Ilic D, et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377(6549):539–44. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 47.Gilmore AP, Romer LH. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol Biol Cell. 1996;7(8):1209–24. doi: 10.1091/mbc.7.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cary LA, Chang JF, Guan JL. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J Cell Sci. 1996;109(Pt 7):1787–94. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- 49.Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112(Pt 16):2677–91. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- 50.Owen JD, et al. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol Cell Biol. 1999;19(7):4806–18. doi: 10.1128/mcb.19.7.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cary LA, et al. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J Cell Biol. 1998;140(1):211–21. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cho SY, Klemke RL. Extracellular-regulated kinase activation and CAS/Crk coupling regulate cell migration and suppress apoptosis during invasion of the extracellular matrix. J Cell Biol. 2000;149(1):223–36. doi: 10.1083/jcb.149.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheresh DA, Leng J, Klemke RL. Regulation of cell contraction and membrane ruffling by distinct signals in migratory cells. J Cell Biol. 1999;146(5):1107–16. doi: 10.1083/jcb.146.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turner CE. Paxillin interactions. J Cell Sci. 2000;113(Pt 23):4139–40. doi: 10.1242/jcs.113.23.4139. [DOI] [PubMed] [Google Scholar]
- 55.Turner CE, et al. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: A role in cytoskeletal remodeling. J Cell Biol. 1999;145(4):851–63. doi: 10.1083/jcb.145.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.West KA, et al. The LD4 motif of paxillin regulates cell spreading and motility through an interaction with paxillin kinase linker (PKL) J Cell Biol. 2001;154(1):161–76. doi: 10.1083/jcb.200101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han DC, Guan JL. Association of focal adhesion kinase with Grb7 and its role in cell migration. J Biol Chem. 1999;274(34):24425–30. doi: 10.1074/jbc.274.34.24425. [DOI] [PubMed] [Google Scholar]
- 58.Han DC, Shen TL, Guan JL. Role of Grb7 targeting to focal contacts and its phosphorylation by focal adhesion kinase in regulation of cell migration. J Biol Chem. 2000;275(37):28911–7. doi: 10.1074/jbc.M001997200. [DOI] [PubMed] [Google Scholar]
- 59.Reiske HR, et al. Requirement of phosphatidylinositol 3-kinase in focal adhesion kinase-promoted cell migration. J Biol Chem. 1999;274(18):12361–6. doi: 10.1074/jbc.274.18.12361. [DOI] [PubMed] [Google Scholar]
- 60.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6(1):56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 61.Shen TL, Han DC, Guan JL. Association of grb7 with phosphoinositides and its role in the regulation of cell migration. J Biol Chem. 2002;277(32):29069–77. doi: 10.1074/jbc.M203085200. [DOI] [PubMed] [Google Scholar]
- 62.Ren XD, et al. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J Cell Sci. 2000;113(Pt 20):3673–8. doi: 10.1242/jcs.113.20.3673. [DOI] [PubMed] [Google Scholar]
- 63.Hsia DA, et al. Differential regulation of cell motility and invasion by FAK. J Cell Biol. 2003;160(5):753–67. doi: 10.1083/jcb.200212114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen BH, et al. Roles of Rho-associated kinase and myosin light chain kinase in morphological and migratory defects of focal adhesion kinase-null cells. J Biol Chem. 2002;277(37):33857–63. doi: 10.1074/jbc.M204429200. [DOI] [PubMed] [Google Scholar]
- 65.Zhai J, et al. Direct interaction of focal adhesion kinase with p190RhoGEF. J Biol Chem. 2003;278(27):24865–73. doi: 10.1074/jbc.M302381200. [DOI] [PubMed] [Google Scholar]
- 66.Lim Y, et al. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J Cell Biol. 2008;180(1):187–203. doi: 10.1083/jcb.200708194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu X, et al. Focal adhesion kinase regulation of N-WASP subcellular localization and function. J Biol Chem. 2004;279(10):9565–76. doi: 10.1074/jbc.M310739200. [DOI] [PubMed] [Google Scholar]
- 68.Shibata K, et al. Both focal adhesion kinase and c-Ras are required for the enhanced matrix metalloproteinase 9 secretion by fibronectin in ovarian cancer cells. Cancer Res. 1998;58(5):900–3. [PubMed] [Google Scholar]
- 69.Polte TR, Naftilan AJ, Hanks SK. Focal adhesion kinase is abundant in developing blood vessels and elevation of its phosphotyrosine content in vascular smooth muscle cells is a rapid response to angiotensin II. J Cell Biochem. 1994;55(1):106–19. doi: 10.1002/jcb.240550113. [DOI] [PubMed] [Google Scholar]
- 70.Qi JH, Claesson-Welsh L. VEGF-induced activation of phosphoinositide 3-kinase is dependent on focal adhesion kinase. Exp Cell Res. 2001;263(1):173–82. doi: 10.1006/excr.2000.5102. [DOI] [PubMed] [Google Scholar]
- 71.Kim I, et al. Angiopoietin-1 induces endothelial cell sprouting through the activation of focal adhesion kinase and plasmin secretion. Circ Res. 2000;86(9):952–9. doi: 10.1161/01.res.86.9.952. [DOI] [PubMed] [Google Scholar]
- 72.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264(5158):569–71. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 73.Brooks PC, et al. Antiintegrin alpha v beta 3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest. 1995;96(4):1815–22. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eliceiri BP, Cheresh DA. The role of alphav integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Invest. 1999;103(9):1227–30. doi: 10.1172/JCI6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eliceiri BP, et al. Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J Cell Biol. 2002;157(1):149–60. doi: 10.1083/jcb.200109079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peng X, et al. Overexpression of focal adhesion kinase in vascular endothelial cells promotes angiogenesis in transgenic mice. Cardiovascular Research. 2004;64(3):421–430. doi: 10.1016/j.cardiores.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 77.Shen TL, et al. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J Cell Biol. 2005;169(6):941–52. doi: 10.1083/jcb.200411155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Braren R, et al. Endothelial FAK is essential for vascular network stability, cell survival, and lamellipodial formation. J Cell Biol. 2006;172(1):151–62. doi: 10.1083/jcb.200506184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Corsi JM, et al. Autophosphorylation-independent and -dependent functions of focal adhesion kinase during development. J Biol Chem. 2009;284(50):34769–76. doi: 10.1074/jbc.M109.067280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lim ST, et al. Knock-in mutation reveals an essential role for focal adhesion kinase activity in blood vessel morphogenesis and cell motility-polarity but not cell proliferation. J Biol Chem. 285(28):21526–36. doi: 10.1074/jbc.M110.129999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gabarra-Niecko V, Schaller MD, Dunty JM. FAK regulates biological processes important for the pathogenesis of cancer. Cancer Metastasis Rev. 2003;22(4):359–74. doi: 10.1023/a:1023725029589. [DOI] [PubMed] [Google Scholar]
- 82.Mitra SK, et al. Intrinsic FAK activity and Y925 phosphorylation facilitate an angiogenic switch in tumors. Oncogene. 2006;25(44):5969–84. doi: 10.1038/sj.onc.1209588. [DOI] [PubMed] [Google Scholar]
- 83.Hauck CR, Hsia DA, Schlaepfer DD. The focal adhesion kinase--a regulator of cell migration and invasion. IUBMB Life. 2002;53(2):115–9. doi: 10.1080/15216540211470. [DOI] [PubMed] [Google Scholar]
- 84.Hanks SK, et al. Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front Biosci. 2003;8:d982–96. doi: 10.2741/1114. [DOI] [PubMed] [Google Scholar]
- 85.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18(5):516–23. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 86.Cary LA, Chang JF, Guan JL. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J Cell Sci. 1996;109(Pt 7):1787–94. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- 87.Lim ST, et al. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008;29(1):9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao X, et al. Role of kinase-independent and -dependent functions of FAK in endothelial cell survival and barrier function during embryonic development. J Cell Biol. 189(6):955–65. doi: 10.1083/jcb.200912094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liotta LA, Kleinerman J, Saidel GM. Quantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantation. Cancer Res. 1974;34(5):997–1004. [PubMed] [Google Scholar]
- 90.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2(8):563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 91.Roberts WG, et al. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 2008;68(6):1935–44. doi: 10.1158/0008-5472.CAN-07-5155. [DOI] [PubMed] [Google Scholar]
- 92.Shi Q, et al. A novel low-molecular weight inhibitor of focal adhesion kinase, TAE226, inhibits glioma growth. Mol Carcinog. 2007;46(6):488–96. doi: 10.1002/mc.20297. [DOI] [PubMed] [Google Scholar]
- 93.Slack-Davis JK, et al. Cellular characterization of a novel focal adhesion kinase inhibitor. J Biol Chem. 2007;282(20):14845–52. doi: 10.1074/jbc.M606695200. [DOI] [PubMed] [Google Scholar]
- 94.Halder J, et al. Therapeutic efficacy of a novel focal adhesion kinase inhibitor TAE226 in ovarian carcinoma. Cancer Res. 2007;67(22):10976–83. doi: 10.1158/0008-5472.CAN-07-2667. [DOI] [PubMed] [Google Scholar]