Abstract
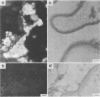
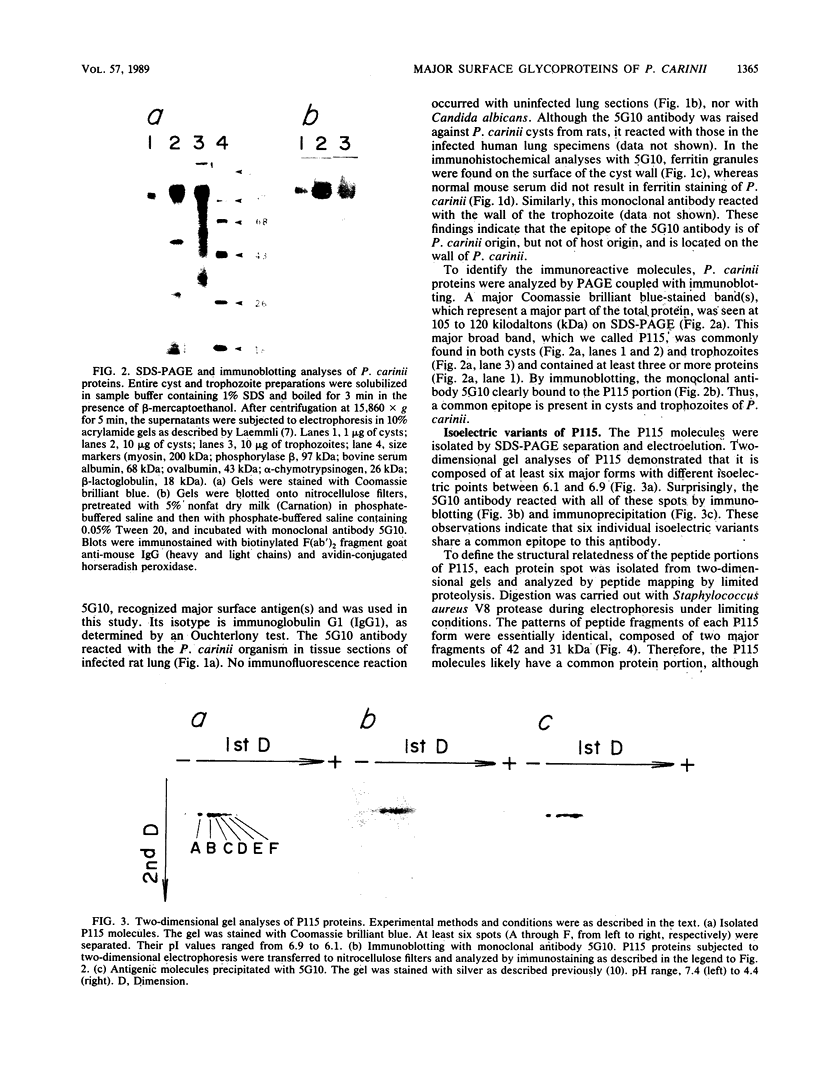
Pneumocystis carinii is a pathogen which causes fatal pneumonia in patients with acquired immune deficiency syndrome. To facilitate the basic study of P. carinii, we analyzed the major surface proteins by immunochemical and biochemical methods. The major protein components of both cysts (resting form) and trophozoites (vegetative form) are part of a group of proteins called P115 with apparent masses of 105 to 120 kilodaltons. They represent an unusually large portion of the total proteins of this organism. The purified proteins exhibited six isoelectric variants when analyzed by two-dimensional gel electrophoresis. A monoclonal antibody raised against cysts recognized all six variants and reacted with epitopes that were located in the cell wall, thereby indicating that P115 is an immunoreactive surface component. Data are presented that the isoelectric variants contain identical or closely related protein components and that they are mannose-rich glycoproteins. Deglycosylated P115 migrates primarily as a single more acidic protein in two-dimensional gels, suggesting that the isoelectric variants may be due primarily to differences in glycosylation. The majority of sera tested from humans with diagnosed pneumocystosis reacted strongly with the P115 proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Edman J. C., Kovacs J. A., Masur H., Santi D. V., Elwood H. J., Sogin M. L. Ribosomal RNA sequence shows Pneumocystis carinii to be a member of the fungi. Nature. 1988 Aug 11;334(6182):519–522. doi: 10.1038/334519a0. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliotti F., Hughes W. T. Passive immunoprophylaxis with specific monoclonal antibody confers partial protection against Pneumocystis carinii pneumonitis in animal models. J Clin Invest. 1988 Jun;81(6):1666–1668. doi: 10.1172/JCI113503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliotti F., Stokes D. C., Cheatham A. B., Davis D. S., Hughes W. T. Development of murine monoclonal antibodies to Pneumocystis carinii. J Infect Dis. 1986 Aug;154(2):315–322. doi: 10.1093/infdis/154.2.315. [DOI] [PubMed] [Google Scholar]
- Graves D. C., McNabb S. J., Ivey M. H., Worley M. A. Development and characterization of monoclonal antibodies to Pneumocystis carinii. Infect Immun. 1986 Jan;51(1):125–133. doi: 10.1128/iai.51.1.125-133.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeBlanc G. A., Cochrane B. J. A rapid method for staining proteins in acrylamide gels. Anal Biochem. 1987 Feb 15;161(1):172–175. doi: 10.1016/0003-2697(87)90668-3. [DOI] [PubMed] [Google Scholar]
- Lee C. H., Bolinger C. D., Bartlett M. S., Kohler R. B., Wilde C. E., 3rd, Smith J. W. Production of monoclonal antibody against Pneumocystis carinii by using a hybrid of rat spleen and mouse myeloma cells. J Clin Microbiol. 1986 Mar;23(3):505–508. doi: 10.1128/jcm.23.3.505-508.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Murray J. F., Felton C. P., Garay S. M., Gottlieb M. S., Hopewell P. C., Stover D. E., Teirstein A. S. Pulmonary complications of the acquired immunodeficiency syndrome. Report of a National Heart, Lung, and Blood Institute workshop. N Engl J Med. 1984 Jun 21;310(25):1682–1688. doi: 10.1056/NEJM198406213102529. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Ballou C. E. Structure of the linkage region between the polysaccharide and protein parts of Saccharomyces cerevisiae mannan. J Biol Chem. 1974 Dec 10;249(23):7685–7694. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P., Schlesinger P. H., Sigardson E., Rodman J. S., Lee Y. C. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Cell. 1980 Jan;19(1):207–215. doi: 10.1016/0092-8674(80)90402-x. [DOI] [PubMed] [Google Scholar]
- Sung S. S., Nelson R. S., Silverstein S. C. Yeast mannans inhibit binding and phagocytosis of zymosan by mouse peritoneal macrophages. J Cell Biol. 1983 Jan;96(1):160–166. doi: 10.1083/jcb.96.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M., Takasaki S., Inoue N., Kobata A. Sensitive method for carbohydrate composition analysis of glycoproteins by high-performance liquid chromatography. J Chromatogr. 1987 Jul 29;400:207–213. doi: 10.1016/s0021-9673(01)81613-7. [DOI] [PubMed] [Google Scholar]
- Tanabe K., Fuchimoto M., Egawa K., Nakamura Y. Use of Pneumocystis carinii genomic DNA clones for DNA hybridization analysis of infected human lungs. J Infect Dis. 1988 Mar;157(3):593–596. doi: 10.1093/infdis/157.3.593. [DOI] [PubMed] [Google Scholar]
- Watanabe J., Hori H., Tanabe K., Nakamura Y. Phylogenetic association of Pneumocystis carinii with the 'Rhizopoda/Myxomycota/Zygomycota group' indicated by comparison of 5S ribosomal RNA sequences. Mol Biochem Parasitol. 1989 Jan 15;32(2-3):163–167. doi: 10.1016/0166-6851(89)90067-4. [DOI] [PubMed] [Google Scholar]
- Watanabe J., Tanabe K., Shimada K. Separation of trophozoites of Pneumocystis carinii from lung of the inoculated nude rat. Jpn J Exp Med. 1987 Oct;57(5):295–297. [PubMed] [Google Scholar]