Abstract
Purpose
To evaluate the long term effectiveness of transrectal ultrasound (TRUS)-guided permanent radioactive I125 implantation of the prostate for organ confined adenocarcinoma of the prostate compared with historical data of prostatectomy and external beam radiotherapy (EBRT) within a cooperative group setting.
Methods and Materials
Patients accrued to this study had histologically confirmed, locally confined, adenocarcinoma of the prostate clinical stage T1b, T1c, or T2a, no nodal or metastatic disease, prostate specific antigen (PSA) level of ≤ 10 ng/ml and a Gleason score of ≤ 6. All patients underwent TRUS-guided radioactive I125 seed implantation into the prostate. The prescribed dose was 145 Gy to the prostate planning target volume (PTV).
Results
A total of 101 patients from 27 institutions were accrued to this protocol; by design no single institution accrued more than 8 patients. There were 94 eligible patients. The median follow up was 8.1 years (range 0.1 to 9.2 years). After 8 years, 8 patients had protocol-defined biochemical (PSA) failure (cumulative incidence 8.0%), 5 patients had local failure (cumulative incidence 5.5%), and 1 patient had distant failure (cumulative incidence 1.1%; this patient also had biochemical failure and non-prostate cancer related death). The 8-year overall survival (OS) rate was 88%. At last follow up, no patient died of prostate cancer or related toxicities. Three patients had maximum late toxicity of Grade 3 and all were genitourinary (GU). There were no Grade 4 or 5 toxicities observed.
Conclusion
The long term results of this clinical trial have demonstrated that this kind of trial can be successfully completed through the RTOG and that results in terms of biochemical failure and toxicity compare very favorably with other brachytherapy published series as well as surgical and external beam radiotherapy series. Additionally, the prospective, multi-centered design highlights the probably generalizability of the outcomes.
Keywords: Prostate, brachytherapy, low dose rate (LDR)
INTRODUCTION
The goal of Radiation Therapy Oncology Group (RTOG) protocol 98-05, which was written in 1998, was to evaluate the state of the art brachytherapy technique (the preplanned I-125 transperineal approach) in a multi-center protocol. This approach offered an outpatient, one-time treatment whose cost and morbidity appeared to be reasonably low at least from the perspective of single institutions. It was the multi-center approach that had not been tested. Given the rate at which prostate brachytherapy was being utilized across the United States, it was imperative that the multi-institutional trial be attempted to try to understand whether the single institution results could be extended to multiple institutions.
The first report of this trial(1) was at a median follow up of 5.3 years. At that time, 6% of the patients had biochemical failure and 5 patients had local failure with 1 having evidence of distant failure. The 5-year overall survival (OS) rate was 96.7%. No patient had died of prostate cancer or related toxicities and the maximum acute toxicity level was Grade 3 with no patient experience Grade 4 or 5 acute toxicity. Late toxicity reported at that time showed 2 patients with a maximum Grade 3 toxicity both related to genitourinary (GU) issues and no patient experienced Grade 4 or 5 toxicity. The initial report of the study suggested that this type of multi-institutional trial was successful, but the long term results were not available.
The primary aim of this report is to evaluate the long term effectiveness of TRUS-guided permanent implantation of the prostate for organ confined adenocarcinoma of the prostate compared with historical data of prostatectomy or EBRT within a cooperative group setting. In addition, the long term toxicity is an important aspect of this report because it allows the RTOG to prove its ability to safely and effectively evaluate brachytherapy protocols.
Methods and Materials
Patient Eligibility
Patients accrued to this trial had histologically confirmed locally confined adenocarcinoma of the prostate, clinical stage T1b, T1c, T2a, NX,N0 disease. They had a Karnofsky status of ≥ 70 and a prostate specific antigen (PSA) that was ≤ 10 ng/ml. No patient had prior pelvic radiation, chemotherapy, or any hormonal therapy including 5-alpha reductase inhibitors. Maximum prostatic volume on TRUS was 45cc. American Urological Association (AUA) obstructive sympton scoring was done and no patient was accrued to the study unless their voiding score was ≤ 18. A PSA and serum testosterone had to be done within 30 days prior to registration. The combined Gleason score for each patient accrued was ≤ 6. Patients were required to sign a study specific consent form prior to registration. Patients who had radical surgery for their prostatic adenocarcinoma or a hip prosthesis were ineligible. Patients who had previous or concurrent cancers other than basal or squamous cell skin cancers were also ineligible unless disease free for ≥ 5 years. Patients with major medical or psychiatric illness, which in the investigators opinion would prevent completion of the treatment or would interfere with follow-up, were also ineligible.
No institution was allowed to enter more than 8 patients to this trial to ensure that the multi-institutional portion of the primary endpoint is satisfied. This part states that the effectiveness of TRUS-guided implantation of the prostate will be tested in the cooperative group setting, with data collection from multiple institutions to test dosimetric evaluation approaches and standard definitions and to establish quality assurance standards for future protocols.
Preimplant Ultrasound
Patients were to be placed in the dorsal lithotomy position with care taken to insure that the patient’s spine was centered on the table and that the elevation of the legs was symmetric. TRUS was performed on each patient with a probe and stepping apparatus stabilized to the floor or to the table and the probe inserted into the rectum such that the bottom row of the perineal template grid markers line up 1–2 mm inside the posterior prostate capsule. The probe was to be advanced until the base of the gland was visualized and this was designated the zero plane. Serial images of the prostate at 0.5 cm increments were to be obtained and the prostate capsule outlined on each. On each image the grid position was to be evaluated to meet the following criteria: the grid pattern must bisect the prostate into equal right and left halves, the first row of the template position 1–2 mm inside the prostatic capsule at the mid gland, and the bottom row of the grid is outside of the rectal wall at all levels. Total gland volume was to be calculated automatically and displayed on the monitor. After serial imaging of the prostate, the pubic arch study was to be performed by moving the probe caudally until the pubic arch shadowing was visualized. The prostate was then traced from the image with the widest dimensions superimposed over the pubic arch image and grid lined up. The intersection of the pubic arch and the prostate cross section was then determined and the amount of gland area that would be blocked by the pubic arch estimated. Serial prostate images were to be mounted and delivered to the radiation physicist for dosimetric calculations.
Flexible cystoscopy if advised by the urologist was to be performed to check for urethral strictures or bladder pathology. Tumors had to be graded and a Gleason score provided as well as the results of pretreatment PSA and serum testosterone testing. A CT scan of the pelvis was required. Lymph node evaluation was required by at least one of the following: CT or MR of the pelvis, exploratory laparotomy, or laparoscopy with lymph node sampling. Only those lymph nodes evaluated by surgical sampling could be classified as N0, those evaluated by imaging were classified as NX. An AUA symptom score was performed on each patient and quality of life questionnaires had to be filled out prior to the implant. The questionnaires used for this trial were FACT-P and SAQ, and these results were described previously.(2)
Radiation Therapy
Target volume definitions were based on the ICRU report 58, dose and volume specification for reporting interstitial therapy.(3) The clinical target volume (CTV) was defined as the pre-implant TRUS definition of the prostate. The planning target volume (PTV) was attained by enlargement of the CTV as follows:
Expand the TRUS definition of the prostate by 2–3 mm in the lateral dimension for each TRUS axial image. Thus the lateral dimensions of the prostate will be increased by approximately 5 mm.
Expand the transrectal definition of the prostate by 2–3 mm in the anterior dimension for each TRUS axial image.
Maintain the same posterior border of the prostate as defined by the TRUS.
Project and expand the most cephalad axial definition of the prostate to a plane 5 mm cephalad to the most cephalad TRUS plane.
Project and expand the most caudal axial definition of the prostate on ultrasound to a plane 5 mm caudal to the most caudad TRUS plane. Thus the PTV is approximately 10 mm longer in the cephalad caudad dimension than the CTV.
Evaluation Target Volumes (ETV)
The ETV is defined as the post implant CT definition of the prostate (the ETV concept is not found in the ICRU report.)
Seed Calibration Handling
Only I125, model 6711, seeds were to be used. The seeds were to be received and inventoried according to each institution’s policies and procedures in a manner consistent with federal or state regulations. A random sampling of at least 10% of the seeds was to be calibrated in a manner such that there was a direct traceability to either the NIST (National Institute of Standards and Technology) or AAPM (American Association of Physicists in Medicine) ADCL (Accredited Dosimetry Calibration Laboratory) for the I-125 seeds, as described by AAPM report TG40 (Task Group 40 of the AAPM), paragraph V.A.2. The measured activity was then compared against the vender’s statement of activity. If seeds in sterile absorbable material were used then one seed from every 5 packets was to be removed and calibrated. The expected activity for I-125 seeds for this protocol was 0.508 units per seed (0.4 mCi/seed).
Dosimetry
The dosimetry of the I125 seeds was based on the information that is contained in the AAPM report TG43 for I125 seeds.(4)
-
Prescribed dose:
The prescribed dose was the dose that the oncologist intends to deliver and was the dose entered into the treatment record. For the purposes of this protocol the prescribed dose to the PTV was 145 Gy (TG-43 dosimetry).
-
Minimum target dose:
ICRU 58 defines the minimum target dose as the minimum dose to the periphery of the CTV. For the purposes of this protocol the minimum target dose was defined as the minimum dose to the ETV. This can be determined by an evaluation of the dose distribution on each CT image containing the prostate.
-
High dose volume:
For the purposes of this protocol the high dose volume was defined as the volume enclosed 200% of the prescribed dose. The maximum dimensionsof the high dose volume in all axial planes was to be reported on the appropriate data form.
-
Low dose volume:
ICRU 58 defines the low dose volume as the volume within the clinical target volume, encompassed by an isodose corresponding to 90% of the prescribed dose which for this protocol was 130 Gy (TG 43 dosimetry). For the purposes of this protocol the low dose volume was defined in terms of the ETV. The maximum dimensions of the low dose volume in any plane that contains the ETV was to be reported on the appropriate form.
-
Dose volume histograms (DVH):
The size of the grid and the voxels used in these calculations was to be stated.
A DVH for the ETV was calculated in 10 Gy increments and presented in tabular form.
A DVH for the rectum, as defined in the region of the prostate such that the high dose volume of the implant is included was calculated in 10 Gy increments and presented in tabular form.
A DVH for the bladder is defined in the region of the prostate such that the high dose volume of the implant is included, was calculated in 10 Gy increments and presented in tabular form.
Post Implant Confirmation
Following implantation cystoscopy was to be performed to retrieve seeds that had been extruded into the bladder or lodged in the urethral wall. Fluoroscopy or anterior radiograph and ultrasound were used to confirm uniform seed distribution. Extra seeds were to be implanted into any qualitatively identified cold spots, at the discretion of the treating physician.
Postoperative Care
A Foley catheter was allowed to be inserted at the end of the implant procedure and left in dwelling until the patient fully recovered from spinal or general anesthesia. If a patient had significant obstructive voiding symptoms the catheter could be left in dwelling for several days as needed. The patient was to complete a four-day course of antibiotics.
Postoperative Evaluation
The post implant CT was to be taken 3–5 weeks after the implant. By this time any swelling caused by the procedure would be significantly reduced. The patient was to be positioned in the supine position. Contrast was not to be used. Axial 5 mm thick slices or less were to be acquired from at least 20 mm cephalad to the base of the prostate to at least 20 mm caudad to the apex of the prostate. Images were to be filmed in such a way that there were four CT images on one 14 × 7 in film. As defined above the post implant CT definition of the prostate is the ETV. As a minimum, dose distributions were to be calculated on each image on which the ETV was defined. The post implant dosimetry data form was to be completed. This form required the determination of minimum dose of the ETV for each axial image on which the ETV was defined, the dimensions of the high dose area on each axial image on which the ETV was defined, the dimensions of the low dose area on each axial image on which the ETV was defined and tabular DVHs for the prostate and high dose regions of rectum and bladder in 10 Gy increments.
Evaluation Criteria
An implant was considered per protocol if ≥ 80% of the ETV received at least 90% of the prescription dose. An acceptable variation was defined as ≥ 50% of the ETV receives at least 90% of the prescription dose. An unacceptable deviation was defined as ≥ 50% of the ETV receives < 90% of the prescription dose. Data submitted to RTOG for each patient included:
Copies of the pre implant TRUS images.
Drawing of the PTV which also displays the CTV (the TRUS images) and includes the projection of the PTV 5mm from the cephalad and caudad directions.
A preimplant form that described the actual prostate seed loading pattern was to be attached to the above material.
Film copies of the post implant CT in a format which displays four images on a 14 × 7 in film was to be provided. Films to be provided included the entire prostate and any other axial level that contains seeds. Two separate sets of films were to be provided. The first set was not to contain any annotations and the second set to be annotated to display the definition of prostate, rectum, and bladder.
A post implant form that described the volume, the dose distribution, and the dose volume histograms was to be sent with the above material.
Toxicity
Acute toxicity reporting was to be done utilizing the NCI common terminology criteria version 2 for toxicity and adverse event reporting.(5) Late toxicity was reported using the RTOG late toxicity scoring criteria.(6) Acute toxicity was defined as toxicity occurring < 9 months after implant and late toxicity ≥ 9 months.
Pathology
Central pathologic review of the diagnostic material as well as two year biopsies were done for this study. Hematoxylin and eosin (H & E) stained slides and a representative tissue block of all pathologic material, the pathology report and pathology submission form was to be submitted for central review. All pretreatment biopsies were to be assessed for the presence of tumor and graded according to Gleason scoring. DNA content and proliferation rate was to be assessed in all cases by imaging analysis (Feulgen staining) and immunohistochemistry (MIB-1 antibody). Post treatment biopsies were to be assessed for the presence of persistent tumor. All positive biopsies were to be histologically graded according to Gleason and the degree of therapy effect in the tumor cells was to be graded according to Dhom and Degro.7) In all cases where there was difficulty in diagnosis, immunohistochemical staining for high molecular weight cytokeratin was to be performed to aid in the distinction of atypical benign glands from carcinoma. The patient consent for pathologic review was required.
Follow Up Schedule
An initial follow up visit was to be performed within one month (i.e., 4 weeks of the implant) and then at 3, 6, 9, and 12 months post implant. Subsequently follow up was every six months for two years and then annually after the third year for the remainder of the patient’s life. Bone scans were to be performed on any patient who presented with complaints of bony pain that could not be attributed to other inter-current disease. Prostate biopsy was required at 2 years from the implant date and prior to any further intervention for prostate cancer such as hormone therapy. Discretionary plain films were recommended to evaluate lesions seen on bone scan or to confirm the diagnosis of metastatic disease. The treating physician also asked the patient whether he was able to achieve and maintain an erection capable of vaginal intercourse. This assessment was to be done prior to the implant procedure and at each follow up visit.
Measurement of Effect/Response/Outcome
Prostate tumor dimensions in centimeters were calculated from the physical exam and recorded at the time of study entry. Definitions of response and outcome were as follows:
No Evidence of Disease (NED): No clinical evidence of disease on digital rectal examination and no PSA failure.
-
Equivocal Disease (ED): This rating was to be assigned under the following circumstances:
If abnormalities are present on the prostate digital rectal examination, but are thought to be abnormal due to treatment and felt not to represent tumor.
If clinical evidence of residual tumor is present, but this has regressed from a previous examination (initial registration)
PSA 2.1 – 4.0 ng/ml.
Rebiopsy was required before starting hormonal manipulation or other salvage treatment in any patient with PSA failure, but with negative bone scan and CT scan. If the biopsy was negative patients were to be scored as NED.
Locally Progressive Disease (PD): This rating was to be assigned when there was clinical evidence in the prostate gland of disease progression or recurrence. Only those patients with progressive disease on digital rectal exam were to be scored as digital rectal examination failure. The time of failure was to be back dated to the first occurrence of equivocal disease after a prior normal exam or to the date of implantation if a normal digital rectal exam was never achieved. Rebiopsy of the suspicious area was to be done to document disease.
Disease-Free Interval: The disease-free interval was to be measured from the date of accession to the date of documentation of progression or until the date of death (from other causes).
Time to Complete Response (CR): Time in months from accession to documentation of no evidence of disease (NED).
Time to Biochemical (PSA) Failure: Measured from the date of accession to date of the first of at least two consecutive rises in PSA above the nadir. The rise in PSA must have exceeded 1 ng/ml above the nadir. In patients who have been declared a PSA failure, every effort should be made to withhold further treatment until clinical relapse was evident. When this was impossible the site of failure was to be ascertained before instituting further treatment. This necessitated a bone scan, CT, and prostate re biopsy. In addition three other definitions of failure were evaluated: (1) RTOG Phoenix which is defined(8) as a PSA value ≥ 2 ng/ml above the nadir value without backdating, (2)ASTRO definition which is defined(9) as three consecutive PSA rises above the nadir or PSA > 4 ng/ml with backdating, and (3) Blasko definition which is defined as two consecutive increases in PSA values or first PSA post implant ≥ 4 ng/ml in patients whose pre implant PSA value was > 4 or PSA value greater than the pre implant PSA value in patients who had PSA pre implant < 4 ng/ml. However, only the protocol definition of PSA failure and the ASTRO-RTOG Phoenix definition will be used in this report.
Time to Local Progression: Measured from the date of accession to the date of documented local progression as determined by clinical exam.
Time to Distant Failure: Measured from the date of accession to the date of documented metastatic disease.
Overall Survival (OS) Time: Measured from the time of accession to the date of death. All patients were followed for survival. Every effort was to be made to document the cause of death. Post mortem examination was to be carried out when feasible and a copy of the final autopsy report sent to RTOG.
Disease-Specific Survival (DSS) Time: Measured from the date of accession to the date of death certified as due to prostate cancer, death from other causes with active malignancy (clinical or biochemical progression), or death due to complications of treatment (irrespective of malignancy status).
Statistical Considerations
Death from other causes with previously documented relapse (either clinical or biochemical), but inactive at the time of death, was not to be considered in DSS, but analyzed separately. DSS is not reported as there were too few events. Estimates of OS were calculated using Kaplan-Meier (10) methods and the cumulative incidence method(11) was used to estimate treatment, local and biochemical failure rates.
Results
Twenty seven institutions accrued 101 patients with no institution accruing more than 8 patients. Seven patients were ineligible and the ineligibility resulted from PSA determination > 30 days before registration in 3 patients, hip prosthesis in 1 patient, another malignancy in 1 patient, serum testosterone measurement > 30 days before registration in 1 patient, and withdrawal of consent in 1 patient. Thus a total of 94 patients were properly entered and eligible, 93 of whom had radiotherapy data and late toxicity data and follow up, and 94 of whom had acute toxicity data. For all eligible patients, the median follow-uptime was 8.1 years (range, 0.1 – 9.2 years). Median follow-up of those patients still living is 8.2 years.
Table 1 outlines the pretreatment characteristics. The median age was 67 years (range, 46–81 years), 61% had clinical stage T1c disease while 39% had clinical T2a disease. The median PSA was 5.9 ng/ml (range, 0.9 – 10.0) and 88% of the patients had Gleason score 6 disease. Ninety-nine percent of the patients had a Karnofsky performance score of 90–100. The median gland size was 33.3 gm (range 3.5–45.0). Also, 87 out of 94 eligible patients (93%) patients were white. Ninety-one out of 94 (97%) of the patients had implants done that were either per the protocol or had an acceptable variation by central review. Three implants were not acceptable: in one implant the deviation was unacceptable, one implant was never done, and one implant was not evaluable.
Table 1.
Pretreatment Characteristics (n=94)
| Characteristic | n(%) |
|---|---|
| Age | |
| <60 | 10 (11%) |
| 60–69 | 52 (55%) |
| ≥70 | 32 (34%) |
| Median (Range) | 67 (46–81) |
| T-Stage | |
| T1c | 57 (61%) |
| T2a | 37 (39%) |
| PSA | |
| ≤4 | 23 (24%) |
| 4–10 | 71 (76%) |
| Median (Range) | 5.93 ng/mL (0.9 – 10.0) |
| Gleason Score (Institutional) | |
| 2–4 | 2 ( 2%) |
| 5 | 9 ( 10%) |
| 6 | 83 (88%) |
| KPS | |
| 80 | 1 (1%) |
| 90 | 25 (27%) |
| 100 | 68 (72%) |
| Volume of Gland by TRUS (grams) | |
| Median (Range) | 33.3 (3.5 – 45.0) |
Abbreviations: PSA=prostate-specific antigen; KPS=Karnofsky performance status; TRUS=transrectal ultrasound
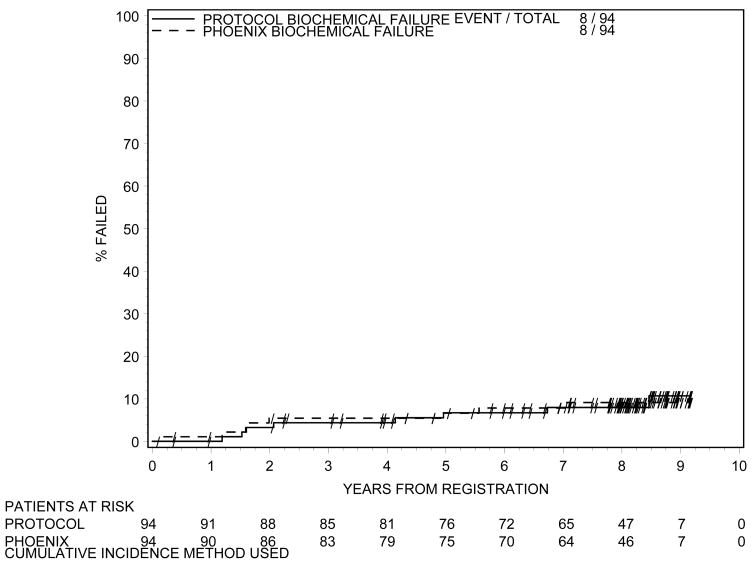
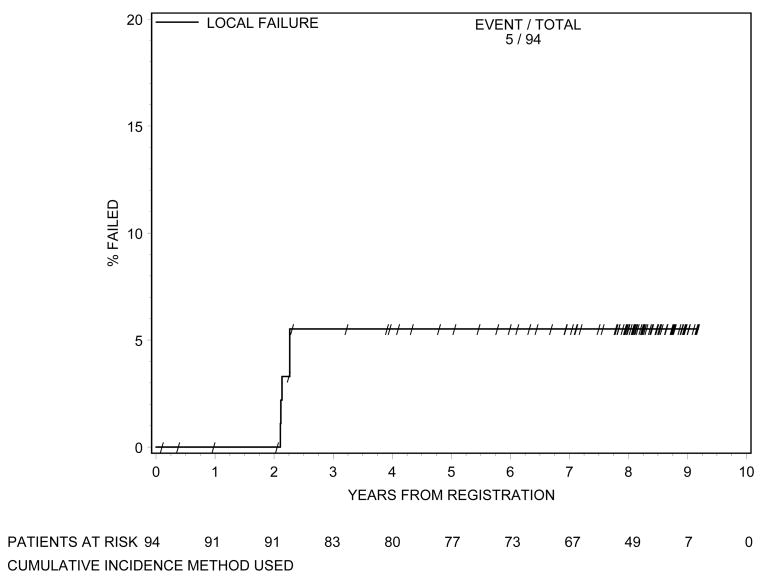
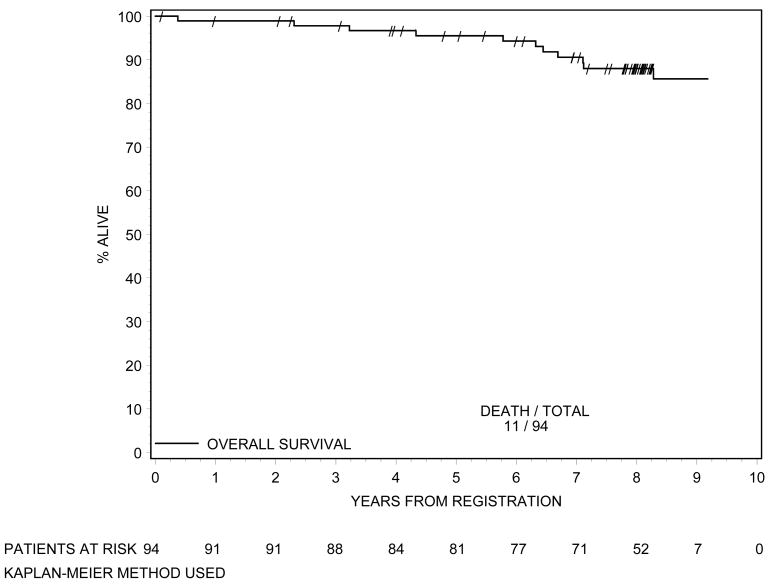
Biochemical failure is shown in Figure 1 using the protocol definition and the RTOG Phoenix definition. According to both definitions 8 patients had biochemical failure. Local failure is shown in Figure 2. At 8 years of follow-up, cumulative incidence of local failure is 5.5%, with a total of 5 patients having had local failure. This is exactly the same as the incidence of local failure at 5 years.(1) One patient developed distant metastasis at 8 years (cumulative incidence=1.1%), again the same level as at 5 years.(1) The OS curve is shown in Figure 3. At 8 years the OS rate was 88%. At last follow-up, no patient died of prostate cancer or related toxicities.
Figure 1.
Biochemical Failure According to Protocol Definition and RTOG Phoenix Definition
Figure 2.
Time to Local Failure
Figure 3.
Overall Survival
Acute toxicity data is shown in Table 2. Eight patients had a maximal acute toxicity level of Grade 3, the majority of which were GU toxicity or hemorrhage that was genitourinary in nature. Approximately one half of the patients had a maximal acute toxicity of Grade 2 (n=48). There were no Grade 4 or 5 acute toxicities.
Table 2.
Acute Toxicity (n=94)
| Toxicity | Grade |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Auditory/hearing | 1 | 0 | 0 | 0 | 0 |
| Cardiovascular – (General) | |||||
| Edema NOS | 2 | 0 | 0 | 0 | 0 |
| Constitutional symptoms | 6 | 0 | 0 | 0 | 0 |
| Dermatology/skin | 4 | 0 | 0 | 0 | 0 |
| Endocrine | 1 | 0 | 0 | 0 | 0 |
| Gastrointestinal | 17 | 9 | 0 | 0 | 0 |
| Hemorrhage | 4 | 4 | 3 | 0 | 0 |
| Infection Febrile | 0 | 0 | 1 | 0 | 0 |
| Neutropenia | |||||
| Musculoskeletal | 0 | 1 | 0 | 0 | 0 |
| Neurology | 2 | 0 | 0 | 0 | 0 |
| Pain | 7 | 3 | 0 | 0 | 0 |
| Renal/Genitourinary | 35 | 42 | 4 | 0 | 0 |
| Sexual/Reproductive | 10 | 7 | 2 | 0 | 0 |
| Function | |||||
| Syndromes | 0 | 1 | 0 | 0 | 0 |
| Worst Overall | 28 | 48 | 8 | 0 | 0 |
Abbreviations: NOS=not otherwise specified
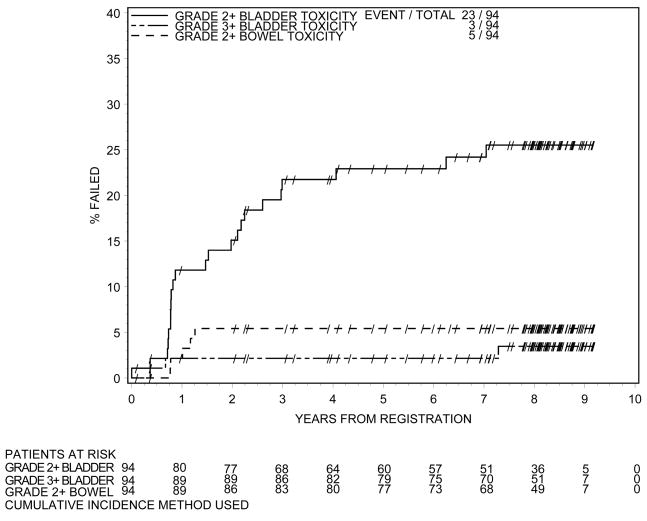
Late toxicity is shown in Table 3. Three patients had maximal toxicity of Grade 3, all of which were GU related. One patient had urinary obstruction, one patient urinary retention, and one patient had stricture. No patient experienced Grade 4 or 5 toxicity in follow up. Twenty- three patients experienced a maximal toxicity of Grade 2 during follow-up, with 20 patients having GU related toxicity and 5 patients experiencing GI or bowel related toxicity. Timing and severity of late toxicity is shown in Figure 4. Of note in Figure 4 is that the Grade 3 bladder toxicity level is fairly stable after year one. Nine patients reported moderate or severe impotence.
Table 3.
Toxicity in Follow-up (n=93)
| Grade* |
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Bladder | 23 | 20 | 3 | 0 | 0 |
| Bowel | 11 | 5 | 0 | 0 | 0 |
| Dyspnea | 1 | 0 | 0 | 0 | 0 |
| Headache | 1 | 0 | 0 | 0 | 0 |
| Liver | 1 | 0 | 0 | 0 | 0 |
| Skin | 1 | 0 | 0 | 0 | 0 |
| Worst Overall | 22 | 23 | 3 | 0 | 0 |
| Mild† | Moderate | Severe | Fatal | |
|---|---|---|---|---|
| Impotence | 3 | 6 | 3 | 0 |
| Other | 3 | 2 | 0 | 0 |
| Pain | 2 | 1 | 0 | 0 |
| Worst Overall | 7 | 8 | 3 | 0 |
RTOG late scoring criteria
Non-RTOG late scoring criteria
Figure 4.
Time to Late Toxicity
Of the 8 patients with biochemical failure according to the protocol definition of failure all had an implant that was according to protocol with no variations. Thirty patients underwent biopsy at ≥ 2 years after implantation for a compliance rate of 32%. Of these biopsies 5 were positive for malignancy. All positive biopsies were associated with local failures.
One of the objectives of the study was to evaluate the effectiveness of TRUS-guided permanent implantation of the prostate for organ-confined adenocarcinoma of the prostate compared with historical data, including prostatectomy and EBRT findings within a cooperative group setting.
It was important to consider the results of this trial compared with other data sets of prostatectomy and EBRT. Data from brachytherapy at multiple single institutions has shown that our data with a biochemical free survival rate of approximately 91% at 8 years is comparable.(12,13) Comparing the results of this study with those of EBRT or surgery data sets show that our data are comparable with both surgical and EBRT series.(14–16)
Quality assessment data published subsequent to the development of this trial have suggested that DVH’s describing the minimum dose delivered to 90% of the prostate (D90) may correlate better with implant quality and ultimate outcomes.(17,18) Therefore, data from the dosimetry for this trial were reanalyzed according to the D90 (Table 4). No correlation was found between the D90 and biochemical failure.
Table 4.
D90 in Patients with Biochemical Failure by Biochemical Definition
| D90 | Total | Biochemical Failure Definition |
||
|---|---|---|---|---|
| Per Protocol | ASTRO | Blasko | ||
| n | 91* | 8 | 34 | 35 |
| Mean ± SD | 128.7 ± 35.6 | 139.8 ± 28.8 | 133.0 ± 31.5 | 130.3 ± 37.0 |
| Median | 123.0 | 133.5 | 129.0 | 128.0 |
| Range | 64.0 – 225.0 | 106 – 186 | 64 – 184 | 64 – 186 |
Abbreviations: ASTRO=American Society for Therapeutic Radiology and Oncology; D90=dose delivered to 90% of the prostate
Three patients did not have D90 information.
Discussion
TRUS guided transperineal implantation of radioactive I-125 seeds into the prostate is an accepted standard treatment for low risk prostate cancer patients. The ability of single institutions to perform this procedure with excellent results and low toxicity has been established.(12,13) However, a coordinated multi-institutional experience had not existed previously, so this study was developed to create a structure for performing prostate brachytherapy in this setting. With the successful conduct of this trial, the RTOG was able to launch other studies of low dose-rate prostate brachytherapy to expand the research mission of the Group. Further, this study sought to use a prospective clinical trial mechanism to determine whether the results of prior reports could be reproduced.
Early results of this trial looked promising regarding PSA control and toxicity.(1) This follow up report showing an 8-year PSA control of 92% and an overall survival of 88% is consistent with the single institution results and consistent with the long term results of EBRT or surgery for a similar population of organ confined prostate cancer patients.(12–16)
These excellent control rates are achieved with relatively low acute and late toxicities. Only 3% (n=3 patients) of the 94 evaluable patients had Grade 3 late toxicities, all GU in nature, and there were no Grade 4 or 5 toxicities either acute or late. These toxicity rates also compare well with single institution results and other treatments of surgery or EBRT.(12–16)
It is important to note that in order to accrue patients to this trial, all 27 institutions had to submit a practice or “dry run” implant that was centrally reviewed.(1) This may explain why the overall quality of the implants was acceptable. Likewise it is important to note that given that no institution accrued more than 8 patients, (therefore, a truly multi-institutional experience) the results are likely reflective of the implants done via the preplan technique across the United States and Canada.
Conclusion
Results of this RTOG trial 98-05 (a multi-institutional trial of brachytherapy for localized adenocarcinoma of the prostate) has revealed that this type of trial was successfully completed by the RTOG. Excellent biochemical control rates and overall survival in addition to low toxicity with a median follow-up of 8 years shows that these results are very comparable to other single institution brachytherapy series, to external beam irradiation series, and surgery results. If the RTOG experience is representative of the larger radiation oncology community then these results should provide reassurance that prostate brachytherapy can be performed well in most institutions performing this procedure across North America.
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lawton CA, DeSilvio M, Lee WR, et al. Results of a phase II trial of transrectal ultrasound-guided permanent radioactive implantation of the prostate for definitive management of localized adenocarcinoma of the prostate (RTOG 98-05) Int J Rad Onc Biol & Phys. 2007;67(1):39–47. doi: 10.1016/j.ijrobp.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Feigenberg SJ, Lee WR, DeSilvio ML, Winter K, Pisansky TM, Bruner DW, Lawton C, Morton G, Baikadi M, Sandler H. Health-Related Quality of Life In Men Receiving Prostate Brachytherapy on RTOG 98–05. Int J Radiat Oncol Biol Phys. 2005;62 (4):956–964. doi: 10.1016/j.ijrobp.2004.12.061. [DOI] [PubMed] [Google Scholar]
- 3.Dose and Volume Specification for Reporting Interstitial Therapy. International Commission on Radiation Units and Measurements; Bethesda, MD: 1997. ICRU Report 58. [Google Scholar]
- 4.AAPM Report #51. Dosimetry of Interstitial Brachytherapy Sources, Reprinted from Medical Physics. 1995 February;22(2):209–234. doi: 10.1118/1.597458. [DOI] [PubMed] [Google Scholar]
- 5.Cancer Therapy Evaluation Program (CTEP) 2010 Feb 26; Web. < http://ctep.info.nih.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_20>.
- 6.Cox JD, Stetz J, Pajack TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment for Cancer (EORTC) Int J Rad Onc Biol & Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 7.Dhom G, Degro S. Therapy of prostatic cancer and histopathologic follow-up. Prostate. 1982;3:531–542. doi: 10.1002/pros.2990030602. [DOI] [PubMed] [Google Scholar]
- 8.Roach M, Hanks G, Thames H, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Rad Onc Biol & Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 9.American Society for Therapeutic Radiology and Oncology Consensus Panel. Consensus statement: Guidelines for PSA following radiotherapy. Int J Rad Onc Biol & Phys. 1997;37:1035–1041. [PubMed] [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assoc. 1958;53:457–481. [Google Scholar]
- 11.Kalbfleish JD, Prentice RL. The statistical analysis of failure time data. New York: John Wiley & Sons; 1980. pp. 167–169. [Google Scholar]
- 12.Potters L, Morgenstein C, Calugaru E. 12 year outcomes following permanent prostate brachytherapy in patients with clinically localized prostate cancer. Journal of Urology. 2005;173:1562–1566. doi: 10.1097/01.ju.0000154633.73092.8e. [DOI] [PubMed] [Google Scholar]
- 13.Zelefsky M, Kuban D, Levy L, et al. Multi-institutional analysis of long-term outcome for states T1-T2 prostate cancer treated with permanent seed implantation Int. Journ of Rad Onc Biol & Phys. 2007;67(2):327–333. doi: 10.1016/j.ijrobp.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 14.Bill-Axelson A, Holmberg L, Filen F, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian Prostate Cancer Group-4 randomized trial. JNCI. 2008;100(16):1144–1154. doi: 10.1093/jnci/djn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephenson A, Kattan M, Easthan J, et al. Prostate cancer – specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. JCO. 2009;27(26):4300–4305. doi: 10.1200/JCO.2008.18.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kupelian PA, Potters L, Khuntia D, et al. Radical prostatectomy, external beam radiotherapy < 72 Gy, external beam radiotherapy ≥ 72 Gy, permanent seed implantation, or combined seeds/external beam radiotherapy for stage T1–2 prostate cancer. Int J Rad Onc Bio Phys. 2004;58(1):25–33. doi: 10.1016/s0360-3016(03)00784-3. [DOI] [PubMed] [Google Scholar]
- 17.Stock RG, Stone NN, Lo YC, et al. Post implant dosimetry for 125 iodine prostate implants. Definitions and factors affecting outcome. Intern J Rad Onc Biol & Phys. 2000;48(3):899–906. doi: 10.1016/s0360-3016(00)00707-0. [DOI] [PubMed] [Google Scholar]
- 18.Stock RG, Stone NN, Tabert A, et al. A dose-response study for I-125 prostate implants. Intern J Rad Onc Biol & Phys. 1998;41(1):101–108. doi: 10.1016/s0360-3016(98)00006-6. [DOI] [PubMed] [Google Scholar]