Abstract
Objective
Brain-computer interface (BCI) technology might be useful for rehabilitation of motor function. This speculation is based on the premise that modifying the EEG will modify behavior, a proposition for which there is limited empirical data. The present study examined the possibility that voluntary modulation of sensorimotor rhythm (SMR) can affect motor behavior in normal human subjects.
Methods
Six individuals performed a cued-reaction task with variable warning periods. A typical variable foreperiod effect was associated with SMR desynchronization. SMR features that correlated with reaction times were then used to control a two-target cursor movement BCI task. Following successful BCI training, the reaction time task was embedded within the cursor movement task.
Results
Voluntarily increasing SMR beta rhythms was associated with longer reaction times and decreasing SMR beta rhythms with shorter reaction times.
Conclusions
Voluntary modulation of EEG SMR can affect motor behavior.
Significance
These results encourage studies that integrate BCI training into rehabilitation protocols and examine its capacity to augment restoration of useful motor function.
Keywords: reaction time, EEG, brain-computer interface
Introduction
Brain activity produces electrical signals that are detectable on the scalp, on the cortical surface or within the brain. Brain–computer interfaces (BCIs) translate these signals into outputs that allow the user to communicate without the participation of peripheral nerves and muscles (Wolpaw et al., 2002). BCI research has used various brain signals to provide a variety of communication and control options (Donchin et al., 2000; Pfurtscheller et al., 1993; Wolpaw and McFarland, 2004). A common feature of these studies is that the user alternates between two or more brain states within a short period of time in order to modulate a signal that is used for communication or control.
Sensorimotor rhythms (SMRs) have been successfully used as signals for BCI devices by a number of investigators (e.g., Kostov and Polak, 2000; Pfurtscheller et al., 1993; Wolpaw et al., 1991; Wolpaw and McFarland, 2004). The SMR represents alpha and beta range frequencies recorded over central scalp locations that are reactive to movement and motor imagery. The behavioral correlate of alpha-band rhythms varies with the specific recording site, being associated with vision over posterior scalp (Berger, 1930), motor behavior over central areas (Chatrian, 1976) and auditory function when recorded with MEG over temporal areas (Niedermeyer, 1997). In addition, movement of specific body parts such as the hands or feet is associated with distinct foci of alpha and beta band desynchronization (Pfurtscheller and Lopes da Silva, 1999). Thus these rhythms appear to reflect the functions of the specific cortical areas from which they originate.
Several authors have suggested the possibility that SMR-based BCI technology could also be used to facilitate rehabilitation (Daly and Wolpaw, 2008; Dobkin, 2007). In particular, BCI technology based on sensorimotor rhythms (SMR) might be useful for the rehabilitation of motor disorders given the association of these signals with normal movement and movement imagery (McFarland et al., 2000; Pfurtscheller and Neuper, 1997). However, it is unclear if there is any change in neuromuscular behavior associated with either long-term training-related changes in SMR control or short-term volitional changes in SMR amplitude.
A number of studies have explored the use of neurofeedback training to induce long-term changes in sensorimotor rhythms in users without disabilities (Egner and Gruzlier, 2001 and 2004; Rasey et al., 1996; Vernon et al., 2003). The intent of these studies was to show that altering SMR would also alter behavior. The neurofeedback approach provides users with feedback for altering SMR in a single direction for an extended period of time. As noted by Vernon (2005), an implicit assumption underlying neurofeedback is that the training procedure will lead to long-term changes in the EEG outside of the training context, which will be associated with changes in behavior. Vernon (2005) concludes that evidence for these assumptions is generally lacking. For example, Egner et al. (2004) found that healthy participants who learned to enhance low beta (11.7-14.6 Hz) at Cz did not show the expected increased beta band activity when tested after training.
These studies did not explore the effects of short-term and bidirectional alterations in SMR amplitude on neuromuscular behavior. We hypothesized that short-term changes in SMR amplitude were associated with changes in behavior. We first identified SMR features which discriminated between the Go and NoGo conditions of a reaction time task. Next, individuals were trained to modulate these SMR features bidirectionally in a cursor movement task. Then, a simple reaction time task was embedded in the cursor movement task. In this way we examined how modulation of SMR features affects reaction time.
Methods
Users
The BCI users were six healthy adults, 3 women and 3 men, aged 26-64, who had no previous BCI experience. In addition, one individual was dropped from the study for failure to learn the task and another was dropped for producing excessive EMG activity. All gave informed consent for the study, which was reviewed and approved by the New York State Department of Health Institutional Review Board.
The BCI user sat in a reclining chair facing a video screen and was instructed to remain motionless. BCI operation and data collection were supported by the general-purpose BCI software platform BCI2000 (Schalk et al., 2004) in conjunction with a 64-channel SA Instrumentation amplifier and a Data Translation DT-3003 64 channel A/D board. EEG was recorded from 64 scalp locations (Sharbrough et al., 1991) by 9-mm tin electrodes embedded in a cap (Electro-cap International) and referenced to an electrode on the right ear, and was digitized at 160 Hz and stored for later analysis. Each user completed 2-3 sessions/wk.
Reaction Time Task
The purpose of the reaction time task was to identify EEG features that changed with behavioral performance. During each trial of the initial reaction task the user watched a yellow ball move horizontally across the video screen from left to right over a 4-sec period. Between 1125 and 1625 msec after the ball started to move, a 25 msec warning stimulus was sounded. An imperative stimulus followed either 500, 1000 or 2000 msec after the warning stimulus. The participant was instructed to press a button on a game pad with their right hand immediately after the imperative stimulus if both the warning and imperative stimuli were low tones (i.e. Go cue, MIDI note 35) or to not respond if the stimuli were high tones (i.e. NoGo cue, MIDI note 70). The delay between the imperative stimulus and the button press was recorded as the reaction time. Each trial was preceded by a 1000 msec pretrial pause with the target present but without cursor movement and concluded with a 1000 msec feedback period followed by a 1500 msec inter-trial interval.
Each session of the reaction time task consisted of 8 3-min runs separated by a 1-min rest. Each participant did four sessions of the reaction task on separate days. This provided approximately 140 trials of each of the 6 Go/NoGo by delay trial types for analysis.
Cursor Movement Task
The spectra of Laplacian derivations (McFarland et al., 1997) for channels over central scalp locations were computed from an autoregressive model of order 16 (McFarland and Wolpaw, 2008). Spectral bins within the mu and beta range (i.e., 9-24 Hz) at specific channels that best differentiated Go from NoGo trials in the reaction time task were selected as control features for cursor movement. Then, every 50 ms, the frequency spectrum of the previous 400-ms segment from each electrode was computed and the logarithms of the amplitudes in specific 3-Hz-wide frequency bands were the EEG features. One or more of these features comprised the control signal (i.e., the independent variable) in a linear equation that specified vertical cursor movement (McFarland et al., 2006). That is, if ΔV was vertical cursor movement, Sv was the control signal for vertical movement, bv was the gain, and av was the mean value of Sv for the user’s previous performance,
| (1) |
The screen appearance during a trial was identical to that during the reaction task with the exception that now, in addition to the constant horizontal velocity, the ball moved vertically under control of the participant’s SMR features and a red target was present on the right edge of the screen. This target appeared on either the top half or the bottom half of the right edge 1 sec prior to the appearance of the ball and remained on the screen until the ball reached that edge (i.e., for 4 sec while the ball was present). The participant was instructed to guide the ball to contact the target. For targets on the top edge this required that the participant increase the amplitude of the SMR feature. For targets on the bottom edge this required that the participant decrease the SMR feature. During the 1000 msec feedback period at the end of the trial, the target turned yellow if it was hit by the ball, and disappeared if it was missed. The next trial began after an 1500 msec inter-trial interval. This paradigm is essentially identical to our previously described methods (e.g., McFarland et al., 2005).
Each session of the cursor movement task consisted of 8 3-min runs separated by a 1-min rest. Participants were trained until it was judged that they had mastered the task.
Combined Cursor Movement and Reaction Time Task
In the final phase the cursor movement task was combined with the reaction task. The cursor moved horizontally across the screen at a constant velocity for 4 sec while its vertical velocity was controlled by the SMR features. A Go or NoGo imperative stimulus sounded between 1625 and 3625 msec after the ball started to move. During this phase there was no warning stimulus. There were 5 testing sessions. During the first two runs of the first session the participants only performed the reaction time task as a warm-up. These data were not included in later analyses. Thereafter participants pressed the response button or withheld responding as appropriate while simultaneously controlling the ball position so as to contact the target.
Results
Reaction Time Task
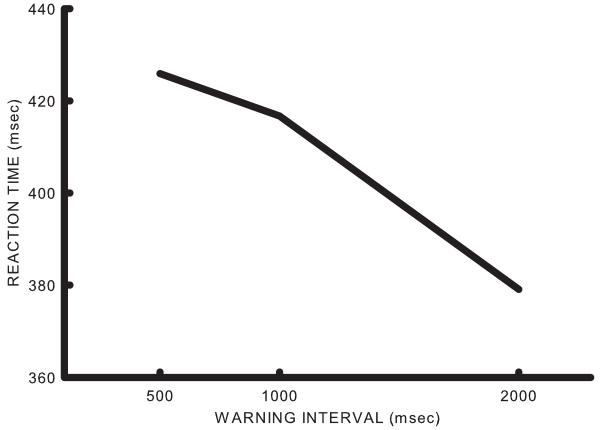
The average reaction time on Go trials as a function of the interval between the warning stimulus and the imperative stimulus (warning interval) is shown in Figure 1. Analysis of variance indicated that warning interval was significant (df= 2/10, F= 265.87, p<0.0001). Inspection of Figure 1 shows that reaction times decreased as warning periods increased (replicating previous findings). There were very few responses on NoGo trials so these data were not analyzed further.
Figure 1.
Average reaction time in six participants as a function of the warning stimulus-imperative stimulus interval. Note that reaction time shortens as the warning-imperative interval lengthens.
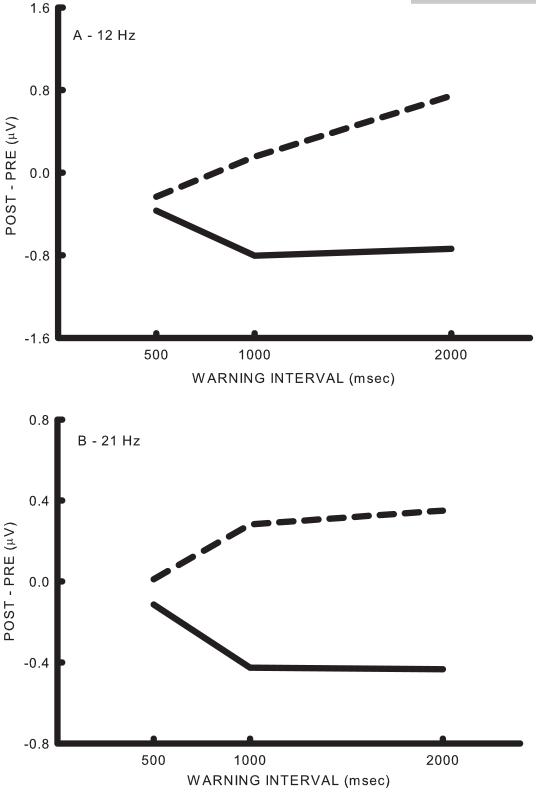
Figure 2 shows average change in SMR amplitudes from the last 500 msec of the pre-warning interval to the last 500 msec of the post-warning interval at C3 on Go and NoGo trials as a function of warning interval. In Figure 2a, the bin centered at 12 Hz (3 Hz wide) showed similar activity on Go and NoGo trials but diverged at 1000 and 2000 msec with increased SMR synchronization occurring on NoGo trials and desynchronization occurring on Go trials. Similar results were obtained with the bin centered at 21 Hz as shown in Figure 2b. Analysis of variance on these data with trial type (Go vs NoGo), warning interval (500, 1000 and 2000 msec) and bin (12 vs 21 Hz) as factors resulted in significant effects for trial type (df= 1/5, 11.86, p<0.0184), delay (df= 2/10, F= 4.46, p<0.0413) and the type x delay interaction (df= 2/10, F= 8.32, p<0.0074). This is consistent with the diverging trends over warning interval shown in Figure 2.
Figure 2.
Effects of warning stimulus-imperative stimulus interval on SMR. A. The difference between the last 500 msec of the post-warning stimulus interval and the last 500 msec of the pre-warning stimulus interval for the 12 Hz (11-13) bin. The solid line represents the average of the Go trials and the dashed line represents the average of the NoGo trials. Note that the two trial types are similar for the 500 msec interval and diverge thereafter. B. The difference between the last 500 msec of the post-warning stimulus interval and the last 500 msec of the pre-warning stimulus interval for the 21 Hz (20-22) bin.
Time-frequency plots for the change in EEG at C3 from baseline during the reaction time task for two of the participants are shown in Figure 3. Stimulus-locked potentials comprised high-frequency oscillations and do not appear in these plots. Subtraction of averaged evoked potentials did not produce qualitative differences and therefore was not performed in this analysis. Initial SMR desynchronization occurs approximately 750 msec after the Go warning stimulus, independent of the warning interval. Desynchronization is maintained until after the response to the imperative stimulus. EEG features that best differentiated Go from NoGO trials and that showed a variable foreperiod effect were selected based on visual inspection of similar plots for each subject. These features were used for cursor movement.
Figure 3.
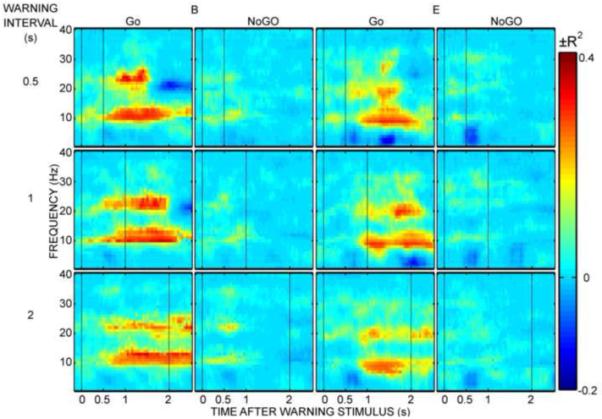
Time-frequency plots for two participants (B and E). The warning and imperative stimulus times are indicated with vertical dashed lines. The 500 msec, 1000 msec, and 2000 msec warning intervals are plotted in the top, middle and bottom rows, respectively. Go and NoGo trial averages are plotted in the first and second column, respectively, for each subject. The color at each time-frequency feature represents the variance in EEG spectral amplitude accounted for by trial condition vs. baseline.
Cursor Movement Task
The features used for training and the number of training sessions are shown in Table 1. A summary of performance in individual subjects is shown in Figure 4. The r2 topographies at the feature used for training indicates that control of EEG activity was spatially focused over central areas in each user. Amplitude and r2 spectra indicate that control was focused in narrow spectral bands in each user. Together they provide evidence that each user learned to modulate SMR activity.
Table 1.
Features used for training, the number of training sessions, and the average percent correct during the final three sessions of training and percent correct during the five sessions of testing
| Subject | Features | Training Sessions |
Percent Correct training |
Percent Correct testing |
|---|---|---|---|---|
| A | C3 - 20 Hz | 14 | 67.3 | 62.9 |
| B | C3 - 12 and 22 Hz | 4 | 91.5 | 76.3 |
| C | CP3- 22 Hz | 13 | 67.5 | 62.2 |
| D | CP3- 10 and 22 Hz | 10 | 79.6 | 78 |
| E | C3- 10 and 23 Hz | 6 | 85.7 | 70.8 |
| F | C1- 23 Hz | 9 | 80.9 | 84.2 |
Figure 4.
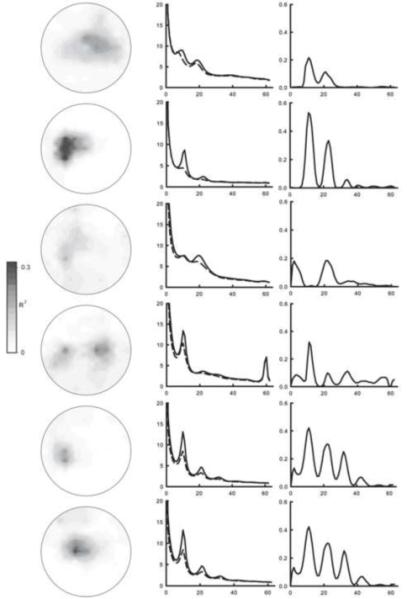
Summary of individual performance over the last three days of training. The left column shows r2 topographies at the frequency used for training (when 2 frequencies were used the higher frequency is shown). The middle column shows voltage spectra for the channel used for training. The solid line represents the average for targets on the top of the right edge of the screen and the dashed line represents targets on the bottom of the right edge. The right column shows spectra of the correlation between target position and amplitudes for the channel used for training.
The average percent correct for each user during the final three training sessions as well as the average for all test sessions are shown in Table 1. The average percent correct over all participants during the last three sessions of training was 78.8.
Combined Cursor Movement and Reaction Time Task
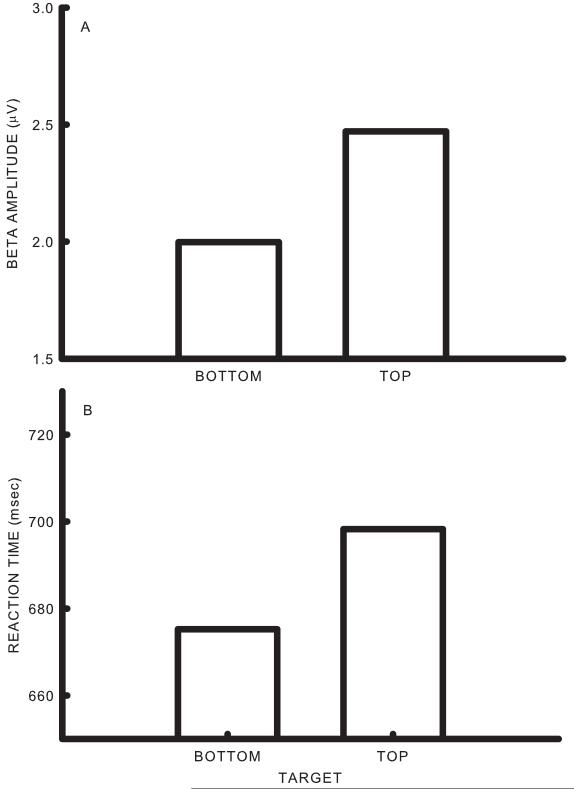
The average percent correct over all participants during the five test sessions was 72.4. While performance was somewhat lower during testing than training, this effect did not reach significance (df= 1/5, F= 4.47, p<0.0882). The difference between the average amplitude of the EEG bin used as the control feature for bottom and top targets during the test phase is shown in Figure 5a. An analysis of variance indicated that the effects of target position were significant (df= 1/5, F= 12.32, p<0.0171).The difference between the average reaction time on Go trials for bottom and top targets during the test phase is shown in Figure 5b. Analysis of variance indicated that the effect of target position was significant (df= 1/5, F= 51.31, p<0.0008). Thus the target that was associated with larger SMR values was also associated with longer reaction times.
Figure 5.
Summary of testing effects. A. Average amplitude of the control feature during bottom and top targets. B. Average reaction times for Go trials during bottom and top targets.
Table 2 summarizes results for individuals during testing. All participants had greater amplitudes for the training features for targets on the top edge of the screen compared to targets on the bottom edge, an effect that was highly significant in each users’ data. All participants had longer reaction times during trials with targets on the top edge compared to those with targets on the bottom edge, but this effect was only significant in 3 of 6 participants. Finally, only 2 of 6 participants had significant positive correlations between SMR amplitudes and reaction times as computed within individuals from scores from individual trials.
Table 2.
Test Effects
| Subject | SMR top |
SMR btm |
p< | Reaction Time top |
Reaction Time btm |
p< | Beta-RT corr |
|---|---|---|---|---|---|---|---|
| A | 4.17 | 3.55 | 0.0005 | 790.5 | 760.8 | 0.0207 | 0.01 |
| B | 3.21 | 2.4 | 0.0001 | 580.4 | 563.4 | 0.1613 | 0.01 |
| C | 4.06 | 2.98 | 0.0001 | 754.6 | 739 | 0.1723 | 0.02 |
| D | 7.6 | 5.49 | 0.0001 | 519.9 | 491.8 | 0.0154 | 0.10* |
| E | 1.75 | 1.37 | 0.0001 | 789.2 | 770.4 | 0.14 | 0.11* |
| F | 1.62 | 1.31 | 0.0001 | 750.8 | 718.2 | 0.0246 | 0.02 |
Discussion
When warning intervals vary randomly within a block of trials, longer intervals typically result in shorter reaction times (Langer et al., 2010; Niemi and Naatanen, 1981; Stuss et al., 2005; Vallesi et al., 2008; Woodworth, 1938). This is known as the variable foreperiod effect. The design of the reaction time task in the present study is somewhat atypical in that we varied the warning interval in a Go/NoGo paradigm whereas the typical design uses a simple reaction time paradigm. Nonetheless we obtained the typical variable foreperiod effect.
The first task of the present study can be described as a variable foreperiod Go/NoGo delayed response task. In a Go/NoGo delayed response task, the subject is informed as to whether or not a response will be required, but must wait for an imperative stimulus to respond. This provides a period of time during which the effects of response preparation on the EEG can be evaluated. Several studies have examined the effects of response preparation on EEG spectral features with this paradigm. Using a constant foreperiod of 1.5 sec, Alegre et al. (2004) report desynchronization in alpha and beta bands at C3 during the Go foreperiod with no effect during the NoGo foreperiod. In a Go/NoGo paradigm with a variable foreperiod, Alegre et al (2006) report alpha and beta desynchronization only at longer intervals following the Go warning stimulus and no change following the NoGo warning stimulus. These results are consistent with our findings for go stimuli, but differ with respect to our findings with NoGo stimuli.
Recently there has been considerable speculation as to the possible functional role of oscillations in nervous system functioning (e.g., Uhlhaas et al., 2009). Egner and Gruzelier (2004) assign a unitary cognitive process to SMRs. Many groups associate SMRs with the activation state of specific neural networks (e.g., Pfurtscheller and Lopes da Silva, 1999; Engel and Fries, 2010; Jones et al., 2010). They view this process to be highly specific, with patterns of sensorimotor activation and deactivation occurring simultaneously in different parts of motor cortex. Another possibility is that EEG rhythms represent a mere epiphenomenon of behaviorally-irrelevant synchronization of cortical activity (Kilgard et al., 2007). We believe that the results of the combined task of the present study are consistent with the former suggestion that EEG rhythms have a functional role.
Previous studies have reported that operant conditioning of SMRs in a single direction can lead to reduced seizure occurrence in individuals with epilepsy (Sterman and Friar, 1972; Kuhlman, 1978; Tan et al., 2009) and to improved behavior in individuals with attentional disorders (Arns et al., 2009). In this study, bidirectional modulation of SMR features resulted in decreased reaction time when subjects were attempting to decrease SMR amplitude compared to when subjects were attempting to increase SMR amplitude. To our knowledge, this is the first report that bidirectional modulation of SMRs can alter behavior. That is, although there have been prior studies of EEG training effects on behavior, these have not involved phasic, task-specific training. Our results suggest that the SMR is more than a mere epiphenomenon but it remains unclear whether SMR modulation affects behavior through an ephaptic effect (Anastassiou et al., 2010) or through changes in the underlying neural activity. While the present results show that modulation of SMR is sufficient to affect reaction time there is always the possibility that some third factor accounts for these effects.
Training individuals to voluntarily modulate SMRs may have potential as a therapeutic modality. Our results show that the rapid bi-directional modulation of SMRs can produce trial-to-trial variations in reaction time. Given the rapid rate at which these effects occur, it is likely that any successful strategy will need to conceptualize the training process as one that teaches individuals phasic, task-appropriate activation and inhibition of specific brain regions. In contrast, there is little evidence to support the notion that training unidirectional changes in the SMR will produce lasting effects (Vernon, 2005). In addition, it is likely that the behavioral associations of a given brain rhythm is site-specific. For example, SMRs at different central recording sites are associated with different movements and movement imageries (McFarland et al., 2000; Pfurtscheller and Neuper, 1997). Thus the development of effective training strategies should rely on both task-specific and site-specific associations.
Parkinson’s disease represents a good example of a movement disorder that might benefit from this methodology as it is associated with increased beta band amplitude (Kuhn et al., 2009) and weaker modulation of beta band activity (Dushanova et al., 2010). Additionally, the degree of suppression of beta band activity in the subthalamic nucleus correlates inversely with reaction time (Williams et al., 2005) and motor impairment (Doyle et al., 2005). It is conceivable that training individuals with Parkinson’s disease to modulate beta band SMR in a task-appropriate manner may improve motor function, particular voluntary motor function which seems to have a stronger association with beta than reaction time (Pogosyan et al., 2009).
It is important to note that the effects observed in the present study were not large. While the change in SMR amplitude between top and bottom targets was statistically significant in all subjects, the change in reaction time was only significant in 3 of 6 subjects. The weaker reaction time effect could be explained by weak association of the trained features with the cognitive requirements of the reaction time task. Indeed, only 2 of 6 subjects showed a correlation between the trained feature and reaction time. It is also possible that this cursor control task does not demand sufficient modulation of SMR amplitude to evoke consistent behavioral effects. While training voluntary control of SMRs may have potential clinical applications, realization of this will require further improvements in this technology.
Summary
This study evaluated the impact of phasic changes in SMR amplitude on Go/NoGo reaction time. Features for SMR control were selected from data collected during an initial period using a warning stimulus during which a typical variable foreperiod effect was obtained. These BCI users were subsequently trained to move a cursor by either increasing or decreasing these SMR features depending upon the vertical position of targets on the right edge of a video screen. Following training, the cursor movement and reaction time tasks were combined. Reaction times were longer when BCI users voluntarily increased SMRs compared to when they decreased them. These results support a causal role of SMR-related cortical activity in the regulation of behavior.
Research Highlights.
Human subjects learned to modulate voluntarily EEG features associated with a variable foreperiod effect in a warned Go/NoGo reaction time task.
In the absence of any warning, voluntary increase in EEG feature amplitude resulted in slower reaction when compared to voluntary decrease in EEG feature amplitude.
Voluntary biphasic modulation of EEG activity has short-term and location-specific effects on behavior.
Acknowledgments
This work was supported by grants from NIH (HD30146 (NCMRR, NICHD) and EB00856 (NIBIB & NINDS)) and the James S. McDonnell Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alegre M, Gurtubay IG, Labarga A, Iriarte J, Valencia M, Artieda J. Frontal and central oscillatory changes related to different aspects of the motor process: a study in go/no-go paradigms. Exp Brain Res. 2004;159:14–22. doi: 10.1007/s00221-004-1928-8. [DOI] [PubMed] [Google Scholar]
- Alegre M, Imirizaldu L, Valencia M, Iriarte J, Arcocha J, Artieda J. Alpha and beta changes in cortical oscillatory activity in a go/nogo randomly-delayed-response choice reaction paradigm. Clin Neurophysiol. 2006;117:16–25. doi: 10.1016/j.clinph.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Anastassiou CA, Montgomery SM, Barahona M, Buzsaki G, Koch C. The effect of spatially inhomogenous extracellular electric fields on neurons. J Neurosci. 2010;30(5):1925–1936. doi: 10.1523/JNEUROSCI.3635-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arns M, de Ridder S, Strehl U, Breteler M, Coenen A. Efficacy of neurofeedback treatment in ADHD: the effects on inattention, impulsivity and hyperactivity: a meta-analysis. Clin EEG Neurosci. 2009;40:180–189. doi: 10.1177/155005940904000311. [DOI] [PubMed] [Google Scholar]
- Berger H. Uber das Elektrenkephalogramm des Menschen II. Journal of Psychology and Neurology. 1930;40:160–179. [Google Scholar]
- Chatrian GE. The mu rhythm. In: Reymond A, editor. Handbook of Electroencephalogr Clin Neurophysiol: the EEG in the waking adult. Elsevier; Amsterdam: 1976. pp. 46–69. [Google Scholar]
- Daly JJ, Wolpaw JR. Brain-computer interfaces in neurological rehabilitation. Lancet Neurol. 2008;7:1032–1043. doi: 10.1016/S1474-4422(08)70223-0. [DOI] [PubMed] [Google Scholar]
- Dobkin BH. Brain-computer interface technology as a tool to augment plasticity and outcomes for neurological rehabilitation. J Physiol (Lond) 2007;579:637–642. doi: 10.1113/jphysiol.2006.123067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin E, Spencer KM, Wijesinghe R. The mental prosthesis: assessing the speed of a P300-based brain-computer interface. IEEE Trans Rehabil Eng. 2000;8:174–179. doi: 10.1109/86.847808. [DOI] [PubMed] [Google Scholar]
- Doyle L, Kuhn A, Hariz M, Kupsch A, Schneider G-H, Brown P. Levodopa-induced modulation of subthalamic beta oscillations during self-paced movements in patients with Parkinson’s disease. Eur J Neurosci. 2005;21:1403–1412. doi: 10.1111/j.1460-9568.2005.03969.x. [DOI] [PubMed] [Google Scholar]
- Dushanova J, Philipova D, Nikolova G. Beta and gamma frequency-range abnormalities in parkinsonian patients under cognitive sensorimotor task. J Neurol Sci. 2010;293:51–58. doi: 10.1016/j.jns.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Egner T, Gruzelier JH. Learned self-regulation of EEF frequency components affects attention and event-related potentials in humans. Neuroreport. 2001;12:4155–4159. doi: 10.1097/00001756-200112210-00058. [DOI] [PubMed] [Google Scholar]
- Egner T, Gruzelier JH. EEG biofeedback of low beta band components: frequency-specific effects on variables of attention and event-related potentials. Clin Neurophysiol. 2004;115:131–139. doi: 10.1016/s1388-2457(03)00353-5. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations- signaling the status quo? Current Directions in Neurobiology. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Vazquez JL, Engineer ND, Pandya PK. Experience dependent plasticity alters cortical synchronization. Hear Res. 2007;229:171–179. doi: 10.1016/j.heares.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostov A, Polak M. Parallel man-machine training in development of EEG-based cursor control. IEEE Trans Rehabil Eng. 2000;8:203–205. doi: 10.1109/86.847816. [DOI] [PubMed] [Google Scholar]
- Kuhn A, Tsui A, Aziz T, Ray N, Brucke C, Kupsch A, Schneider G-H, Brown P. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp Neurol. 2009;215:380–387. doi: 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Kuhlman WN. EEG feedback training of epileptic patients: clinical and electroencephalographic analysis. Electroencephalogr Clin Neurophysiol. 1978;45:699–710. doi: 10.1016/0013-4694(78)90138-4. [DOI] [PubMed] [Google Scholar]
- Langer R, Steinborn MB, Chatterjee A, Sturm W, Willmes K. Mental fatigue and temporal preparation in simple reaction-time performance. Acta Psychol (Amst) 2010;133:64–72. doi: 10.1016/j.actpsy.2009.10.001. [DOI] [PubMed] [Google Scholar]
- McFarland DJ, McCane LM, David SV, Wolpaw JR. Spatial filter selection for EEG-based communication. Electroencephalogr Clin Neurophysiol. 1997;103:386–394. doi: 10.1016/s0013-4694(97)00022-2. [DOI] [PubMed] [Google Scholar]
- McFarland DJ, Miner LA, Vaughan TM, Wolpaw JR. Mu and beta rhythm topographies during motor imagery and actual movement. Brain Topogr. 2000;12:177–186. doi: 10.1023/a:1023437823106. [DOI] [PubMed] [Google Scholar]
- McFarland DJ, Sarnacki WA, Vaughan TM, Wolpaw JR. Brain-computer interface (BCI) operation: signal and noise during early training sessions. Clin Neurophysiol. 2005;116:56–62. doi: 10.1016/j.clinph.2004.07.004. [DOI] [PubMed] [Google Scholar]
- McFarland DJ, Wolpaw JR. Sensorimotor rhythm-based brain-computer interface (BCI): model order selection for autoregressive spectral analysis. J Neural Eng. 2008;5:155–162. doi: 10.1088/1741-2560/5/2/006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedermeyer E. Alpha rhythms as physiological and abnormal phenomena. Int J Psychophysiol. 1997;26:31–49. doi: 10.1016/s0167-8760(97)00754-x. [DOI] [PubMed] [Google Scholar]
- Niemi P, Naatanen R. Foreperiod and simple reaction time. Psychol Bull. 1981;89:133–162. [Google Scholar]
- Pfurtscheller G, Flotzinger D, Kalcher J. Brain-computer interface- a new communication device for handicapped persons. Journal of Microcomputer Applications. 1993;16:293–299. [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C. Motor imagery activates primary sensorimotor area in humans. Neurosci Lett. 1997;239:65–68. doi: 10.1016/s0304-3940(97)00889-6. [DOI] [PubMed] [Google Scholar]
- Pogosyan A, Gaynor LD, Eusebio A, Brown P. Boosting cortical activity at beta-band frequencies slows movement in humans. Curr Biol. 2009;19:1637–1641. doi: 10.1016/j.cub.2009.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasey HW, Lubar JF, McIntyre A, Zoffuto AC, Abbot PL. EEG Biofeedback for the enhancement of attentional processing in normal college students. J Neurother. 1996;1:15–21. [Google Scholar]
- Schalk G, McFarland DJ, Hinterberger T, Birbaumer N, Wolpaw JR. BCI2000: a general-purpose brain-computer interface (BCI) system. IEEE Trans Biomed Eng. 2004;51:1034–1043. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- Sharbrough F, Chatrian CE, Lesser RP, Luders H, Nuwer M, Picton TW. American Electroencephalographic Society guidelines for standard electrode position nomenclature. Journal of Clin Neurophysiol. 1991;8:200–202. [PubMed] [Google Scholar]
- Sterman MB, Friar L. Suppression of seizures in an epileptic following sensorimotor EEG feedback training. Electroencephalogr Clin Neurophysiol. 1972;33:89–95. doi: 10.1016/0013-4694(72)90028-4. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Shallice T, Picton TW, Binns MA, MacDonald R, Borowiec A, Katz DI. Multiple frontal systems controlling response speed. Neuropsychologia. 2005;43:396–417. doi: 10.1016/j.neuropsychologia.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Tan G, Thornby J, Hammond DC, Strehl U, Canady B, Arnemann K, Kaiser DA. Meta-analysis of EEG biofeedback in treating epilepsy. Clin EEG Neurosci. 2009;40:173–179. doi: 10.1177/155005940904000310. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Rodriguez E, Rotarska-Jagiela A, Singer W. Neural synchrony and the development of cortical networks. Trends Cogn Sci. 2008;14:72–80. doi: 10.1016/j.tics.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Vallesi A, McIntosh AR, Shallice T, Stuss DT. When time shapes behavior: fMRI evidence of brain correlates of temporal monitoring. J Cogn Neurosci. 2008;26:1116–1126. doi: 10.1162/jocn.2009.21098. [DOI] [PubMed] [Google Scholar]
- Vernon DJ. Can neurofeedback training enhance performance? An evaluation of the evidence with implications for future research. Appl Psychophysiol Biofeedback. 2005;30:347–364. doi: 10.1007/s10484-005-8421-4. [DOI] [PubMed] [Google Scholar]
- Vernon D, Egner T, Cooper N, Compton T, Neilands C, Sheri A, Gruzelier J. The effect of training distinct neurofeedback protocols on aspects of cognitive performance. Int J Psychophysiol. 2003;47:75–85. doi: 10.1016/s0167-8760(02)00091-0. [DOI] [PubMed] [Google Scholar]
- Williams D, Kuhn A, Kupsch A, Tijssen M, van Bruggen G, Speelman H, Hotton G, Loukas C, Brown P. The relationship between oscillatory activity and motor reaction time in the parkinsonian subthalamic nucleus. Eur J Neurosci. 2005;21:249–258. doi: 10.1111/j.1460-9568.2004.03817.x. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, Vaughan TM. Brain-computer interfaces for communication and control. Clin Neurophysiol. 2002;113:767–791. doi: 10.1016/s1388-2457(02)00057-3. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, McFarland DJ. Control of a two-dimensional movement signal by a non-invasive brain-computer interface. Proc Natl Acad Sci U S A. 2004;51:17849–17854. doi: 10.1073/pnas.0403504101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR, McFarland DJ, Neat GW, Forneris CA. An EEG-based brain-computer interface for cursor control. Electroencephalogr Clin Neurophysiol. 1991;78:252–259. doi: 10.1016/0013-4694(91)90040-b. [DOI] [PubMed] [Google Scholar]
- Woodworth RS. Experimental Psychology. Holt & Co; New York: 1938. [Google Scholar]