Abstract
Retinoid-related orphan receptor (ROR)α4 is the major RORα isoform expressed in adipose tissues and liver. In this study we demonstrate that RORα-deficient staggerer mice (RORαsg/sg) fed with a high-fat diet (HFD) exhibited reduced adiposity and hepatic triglyceride levels compared with wild-type (WT) littermates and were resistant to the development of hepatic steatosis, adipose-associated inflammation, and insulin resistance. Gene expression profiling showed that many genes involved in triglyceride synthesis and storage, including Cidec, Cidea, and Mogat1, were expressed at much lower levels in liver of RORαsg/sg mice. In contrast, overexpression of RORα in mouse hepatoma Hepa1–6 cells significantly increased the expression of genes that were repressed in RORαsg/sg liver, including Sult1b1, Adfp, Cidea, and ApoA4. ChIP and promoter analysis suggested that several of these genes were regulated directly by RORα. In addition to reduced lipid accumulation, inflammation was greatly diminished in white adipose tissue (WAT) of RORαsg/sg mice fed with an HFD. The infiltration of macrophages and the expression of many immune response and proinflammatory genes, including those encoding various chemo/cytokines, Toll-like receptors, and TNF signaling proteins, were significantly reduced in RORαsg/sg WAT. Moreover, RORαsg/sg mice fed with an HFD were protected from the development of insulin resistance. RORαsg/sg mice consumed more oxygen and produced more carbon dioxide, suggesting increased energy expenditure in this genotype. Our study indicates that RORα plays a critical role in the regulation of several aspects of metabolic syndrome. Therefore, RORα may provide a novel therapeutic target in the management of obesity and associated metabolic diseases.
Keywords: staggerer mice, obesity, metabolic syndrome, macrophage, insulin-resistance, diabetes, retinoid-related orphan receptors
obesity is a major global health concern. In the United States alone, 30% of the general population is obese and an estimated 66% of all adults are overweight (40). Obesity is associated with an elevated risk of several pathologies, including Type 2 diabetes, cardiovascular disease, nonalcoholic fatty liver disease (NAFLD), cancer, and airway disease (18, 36). The molecular links between obesity and these diseases are beginning to be understood. It is now well recognized that obesity is associated with chronic low-grade inflammation and that inflammatory processes play an important role in obesity and related pathologies (18, 44, 53). Infiltration of macrophages and various T-lymphocytes in hypertrophic adipose tissues have been considered as important early events in the development of obesity-associated complications (14, 38, 57) and the release of proinflammatory cytokines by adipose tissue plays a key role in the development of insulin resistance, NAFDL, and cardiovascular disease.
Retinoid-related orphan receptors RORα, β, and γ (NR1F1–3) constitute a subfamily of nuclear receptors that regulate gene transcription by binding as a monomer to ROR-response elements (RORE) in the regulatory region of target genes (15, 20, 35). Recent studies have demonstrated that RORs function as ligand-dependent transcription factors (20, 23, 30, 48, 55). RORs have been implicated in the regulation of a wide variety of physiological processes, including cerebellar development, metabolism, circadian rhythm, cellular immunity, bone formation, hypoxia signaling, and inflammatory responses (4, 9, 11, 12, 16, 19, 20, 24, 29, 31–33, 42, 43, 47, 49, 54, 58, 60).
In the present study, we used the staggerer mice (RORαsg/sg), a natural mutant strain containing a deletion within the RORα gene, to obtain further insights into the physiological roles of RORα in energy homeostasis. We show that RORαsg/sg mice, but not RORγ−/− mice, were less susceptible to age- and high-fat diet (HFD)-induced obesity and hepatic steatosis. Moreover, RORαsg/sg mice were protected against insulin-resistance and adipose-associated inflammation. Gene expression profiling by microarray analysis revealed differential gene regulation in liver and white adipose tissue (WAT) between wild-type (WT) and RORαsg/sg mice maintained on an HFD. These include genes involved in hepatic triglyceride accumulation and inflammatory genes. Our study further supports evidence that RORα plays a critical role in the regulation of lipid/energy homeostasis and metabolic syndrome. As ligand-dependent transcription factor, RORα may provide a novel therapeutic target for the management of obesity and associated diseases, such as diabetes and NAFLD.
MATERIALS AND METHODS
Experimental animals.
Heterozygous C57BL/6 staggerer (RORα+/sg) mice were purchased from Jackson Laboratories (Bar Harbor, ME). RORγ−/− and double knockout RORαsg/sg/RORγ−/− (DKO) were described previously (24). Littermate WT mice were used as controls. Mice were supplied ad libitum with mash NIH-A31 formula (1:1 ratio with water). To study diet-induced obesity, 8 to 12 wk old male mice were fed a mash HFD (D12492; Research Diets, New Brunswick, NJ) for 6 wk, unless indicated otherwise. Adiposity was determined with an Piximus densitometer (Lunar, Madison, WI). All animal protocols followed the guidelines outlined by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the National Institute of Environmental Health Sciences (NIEHS).
Cell culture and stable cell lines.
Murine hepatoma Hepa1–6 cells were cultured in DMEM with 10% FBS. Hepa1–6(E) and Hepa1–6(RORα) cells, stably expressing Flag-RORα, were established by infecting parental cells with pLXIN(Empty) (Clontech, Palo Alto, CA) and pLXIN-3xFlag-RORα retrovirus, followed by G418 selection. The expression and nuclear localization of RORα protein were confirmed by Western blot analysis and immunofluorescent staining, respectively. Samples of 5 separate clones were examined by quantitative real-time PCR (QRT-PCR) and chromatin immunoprecipitation (ChIP) analysis.
RNA isolation.
RNA from mouse tissues was isolated with RNeasy mini or midi kit (Qiagen, Valencia, CA) as described previously (24). The quality and integrity of the RNA was assessed by a Bioanalyzer (Agilent, Santa Clara, CA) and agarose gel electrophoresis.
Histology and immunostaining.
Adipose and liver specimens were fixed in 4% paraformaldehyde and paraffin-embedded, and tissue sections (5 μm) were stained with hematoxylin-eosin. To detect macrophages, sections of WAT were stained with antiactive caspase 3 (Promega, Madison, WI) or F4/80 antibody (Santa Cruz Biotechology, Santa Cruz, CA) and an avidin-biotin-peroxidase detection system. F4/80-positive cells in at least six randomly selected fields in sections from five different mice were counted.
Microarray analysis.
Microarray analysis was performed with WAT and liver as described detail in supplementary information.1 The data discussed in this publication have been deposited in the National Center for Biotechnology Information's Gene Expression Omnibus at http://www.ncbi.nlm.nih.gov/geo #GSE23736.
QRT-PCR.
The RNA was reversed-transcribed using a high capacity cDNA archive kit according to the manufacturer's instructions (Applied Biosystems, Foster City, CA). QRT-PCR reactions were performed as described previously (24) with SYBRG and TaqMan systems. Primers are listed in Supplemental Table S1A. All results were normalized relatively to the 18S or GAPDH transcripts.
ChIP assay.
ChIP analysis was performed using a ChIP assay kit (Millipore, Billerica, MA) according to the manufacturer's protocol with minor modifications. Liver tissues from WT and RORαsg/sg mice were homogenized using a polytron PT 3000 (Brinkmann Instruments). After cross-linking with 4% formaldehyde at room temperature for 20 min, cross-linked chromatin was sheered by sonication and then incubated with anti-RORα antibody (sc-6062; Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight. Antibody-chromatin complexes were collected with protein G agarose beads for 2 h. After sequential washes, complexes were eluted and reversed cross-linked by incubation with NaCl at 65°C overnight. After RNase A and proteinase K digestions, the ChIPed-DNA was purified. The fold amount of the ChIPed-DNA relative to each input DNA was determined by quantitative PCR (QPCR). All the QPCR reactions were carried out in triplicate. For ChIP assay using Hepa1–6 cells, cells were cross-linked with 4% formaldehyde for 10 min and cross-linked chromatin sheered and immunoprecipitated as described above. Immunoprecipitation was performed by anti-Flag M2 affinity gel (Sigma-Aldrich). Primer sequences for ChIP-QPCR are listed in Supplemental Table S1B.
Reporter assays.
To analyze the activity of the mouse Adfp promoter, the region between −2043 to +64, which contains two putative ROREs, 5′-TTTGTAGGTGA (RORE1) and 5′-GAAAGAGGTCA (RORE2), was amplified and cloned into the luciferase reporter plasmid pGL4.10 (Promega) and referred to as pGL4.10-Adfp(2). To determine the role of these ROREs, the “GG” in each RORE was mutated to “AA” using QuickChange site-directed mutagenesis kit (Stratagene). The expression vectors 3xFlag-CMV10-RORα4 and 3xFlag-CMV10-Rev-Erbα were described previously (52). Reporter assays were performed in hepatoma Huh-7 cells as reported previously (25, 52).
Analysis of blood and liver lipids.
Blood levels of glucose, cholesterol, triglycerides, and high-density lipoprotein (HDL) were determined using the Cobas Mira Classic Chemistry System (Roche Diagnostics Systems, Montclair, NJ). The chemical reagents for all assays were purchased from Equal Diagnostics (Exton, PA). Serum insulin levels were analyzed with an insulin radioimmunoassay kit from Millipore (St. Charles, MO). To measure liver lipid content, tissues were homogenized and lipids extracted as previously described (61). Lipids were then dissolved in a mixture of 60 μl of tert-butanol and 40 μl of Triton X-100/methanol (2:1) mix. Triglyceride and cholesterol levels were measured with Stanbio assay kits (Stanbio Laboratory, Boerne, TX) (54). Serum leptin levels were analyzed by Elisa using a kit from RayBiotech (Norcross, GA). Steatocrit was analyzed using the perchloric acid method as described (51).
LabMaster metabolic analysis.
Metabolic parameters including, oxygen consumption (VO2), CO2 production (VCO2), and respiratory exchange ratio were analyzed with a LabMaster system (TSE Systems, Chesterfield, MO) using WT and RORαsg/sg mice fed either with a normal diet or HFD for 6 wk. The average values during light period and dark period were calculated. Student's t-test was used to calculate the P value.
Glucose tolerance test and insulin tolerance test.
After an overnight fast, WT and RORαsg/sg mice were injected intraperitoneally with glucose (2 g/kg) or insulin (0.75 U/kg) (Eli Lilly, Indianapolis, IN) to examine glucose tolerance test (GTT) and insulin tolerance test (ITT), respectively. Levels of glucose were analyzed every 20 min for up to 2 h with glucose test strips (Nova Biomedical, Waltham, MA).
Isolation of the stromal-vascular fraction and flow cytometry analysis.
To isolate the stromal-vascular fraction (SVF) cells, epididymal white adipose tissue (eWAT) was isolated from WT and RORαsg/sg mice fed with an HFD for 18 wk. After chopping and collagenase treatment for 2 h at 37°C, cell pellets were centrifuged and resuspended in DMEM containing 10% FBS. The cell suspension was filtrated through 70-μm mesh. After centrifugation, red blood cells were removed by treatment with 1× RBC lysis buffer (eBioscience, San Diego, CA). Remaining cells were centrifuged and washed two times in DMEM containing 10% FBS. Fluorescence-conjugated monoclonal antibodies for F4/80 (Invitrogen, Camarillo, CA) and CD11b (BD Biosciences) were used to detect macrophage population. CD3, CD4, and CD8 (BD Biosciences) were used for the analysis of T lymphocytes. Isotype control antibodies were used for negative control, and dead cells were excluded by 7-amino-actinomycin D or propidium iodine staining. Stained cells were analyzed with the BD LSR II Flow cytometer (Becton Dickinson) using FACSDiVa software.
RESULTS
RORαsg/sg mice were resistant to age-induced obesity.
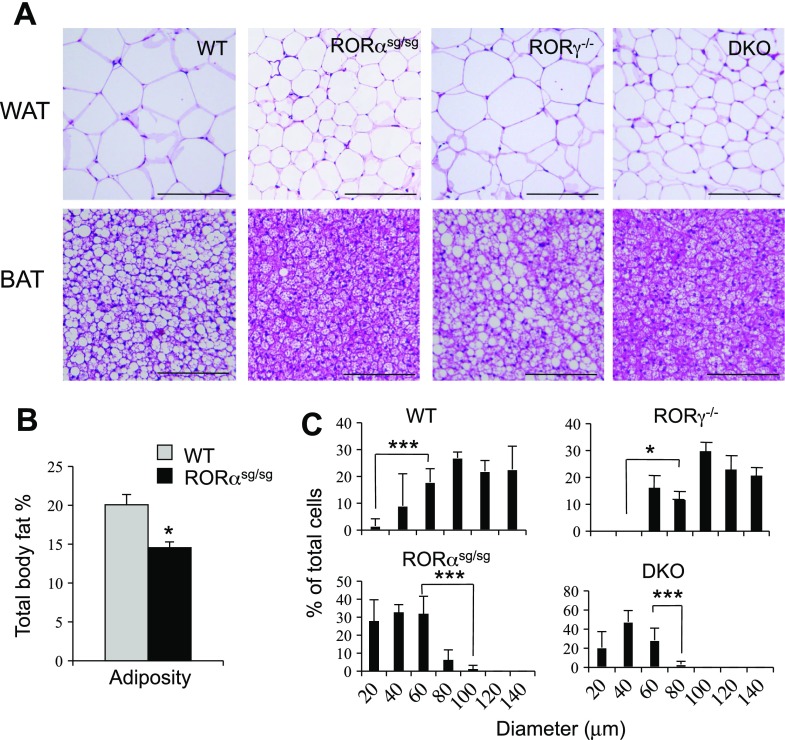
Previous studies indicated a role for RORs in the regulation of several metabolic pathways and energy homeostasis (1, 20, 24, 31). To study the metabolic functions of RORα and RORγ further, we examined whether loss of ROR function had any effect on age-induced obesity. Histological analysis showed that WAT and brown adipose tissues (BAT) of 1 yr old male WT mice became hypertrophic in agreement with a recent report (Fig. 1A) (13). In sharp contrast, 1 yr old male RORαsg/sg mice were greatly resistant to the development of age-induced WAT and BAT hypertrophy (Fig. 1A). Moreover, analysis of the adiposity, measured by Piximus densitometry (dual energy X-ray absorptiometry), showed that RORαsg/sg mice exhibited a significantly lower total body fat index compared with WT littermates (Fig. 1B). Aged RORγ−/− mice developed adipose hypertrophy to a similar extent as WT littermates, while DKO appeared as resistant to age-induced obesity as RORαsg/sg mice (Fig. 1A). The average diameter of adipocytes in WT and RORγ−/− mice was more than twice that in RORαsg/sg and DKO mice (105 μm in WT vs. 40 μm in RORαsg/sg) (Fig. 1C), suggesting that the reduced adiposity observed in RORαsg/sg mice was largely due to reduced triglyceride accumulation. Together, these observations suggested that loss of RORα, but not RORγ, had a major effect on lipid accumulation in adipose tissues.
Fig. 1.
RORα-deficient staggerer mice (RORαsg/sg) mice, but not RORγ−/− mice, display a reduced adiposity. A: representative hematoxylin-eosin (H&E)-stained sections of white adipose tissue (WAT) and brown adipose tissue (BAT) from aged wild-type (WT), RORαsg/sg, RORγ−/−, and RORαsg/sg/RORγ−/− (DKO) male mice. Scale bar indicates 200 μm. B: the total body fat percentage of 1 yr old male WT (n = 9) and RORαsg/sg (n = 5) mice was determined by a Piximus densitometer. C: comparison of the cell size of WAT adipocytes from 1 yr old WT, RORαsg/sg, and RORγ−/− male mice. Cell diameters in 6 randomly selected sections of WAT from 3 mice in each group were measured, and the percentages of the different cell sizes calculated and plotted. P values: WT vs. RORαsg/sg < 0.001, WT vs. DKO < 0.001, WT vs. RORγ−/− > 0.05; *P < 0.02, ***P < 0.001.
RORαsg/sg mice were resistant to HFD-induced obesity.
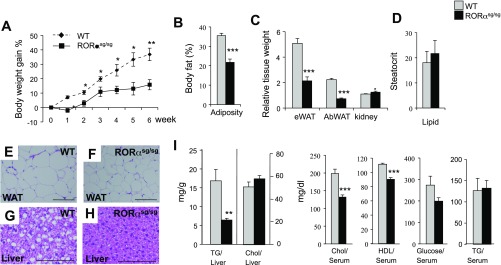
To determine whether RORαsg/sg mice were also protected against diet-induced obesity, 8 to 10 wk old RORαsg/sg and littermate WT control mice were fed with an HFD for 6 wk, and their total body weight was monitored (Supplemental Fig. S1). RORαsg/sg mice gained relatively less weight than their WT littermates (Fig. 2A). By the end of the feeding period, the average body weight of WT mice increased by 35%, while RORαsg/sg mice gained only 16% of their body weight. Analysis of the adiposity by Piximus densitometry showed that RORαsg/sg(HFD) mice exhibited a significantly lower total body fat index than WT(HFD) littermates (Fig. 2B). Moreover, the relative weight of epididymal and abdominal WAT in RORαsg/sg(HFD) mice was 57 and 65% of that of WT(HFD) mice, respectively (Fig. 2C), whereas the kidney weight index was slightly increased in RORαsg/sg(HFD) mice. These observations indicate that HFD-fed RORαsg/sg mice [RORαsg/sg(HFD)] exhibited a reduced fat mass compared with their WT counterparts.
Fig. 2.
RORαsg/sg mice are resistant to diet-induced obesity and hepatic steatosis. A: 10 wk old male mice (WT, n = 6; RORαsg/sg, n = 7) were fed a high-fat diet (HFD) for 6 wk, and weight gain was monitored. The percent body weight gain was calculated based on the body weight at the start of the HFD. B: total body fat mass was analyzed by Piximus densitometry. C: comparison of the relative weights of kidneys, epididymal white adipose tissue (eWAT), and abdominal WAT (AbWAT) after 6 wk on an HFD. D: comparison of the steatocrit in feces of WT(HFD) and RORαsg/sg(HFD) male mice (WT, n = 5; RORαsg/sg, n = 6). E–H: representative H&E-stained sections of liver and WAT from WT(HFD) and RORαsg/sg(HFD) male mice. Scale bar indicates 200 μm. I: comparison of hepatic triglyceride (TG) and cholesterol (Chol) levels, and serum Chol, HDL, glucose, and TG levels in WT(HFD) and RORαsg/sg(HFD). Data present means ± SE; *P < 0.05, **P < 0.01, ***P < 0.001.
Histological analysis of adipose tissue and liver sections revealed that RORαsg/sg(HFD) mice contained considerably smaller adipocytes and accumulated significantly fewer hepatic lipid droplets than their WT(HFD) littermates (Fig. 2, E–H). The latter was supported by biochemical analysis showing significantly lower hepatic triglyceride accumulation in RORαsg/sg(HFD) mice than in WT(HFD) mice (Fig. 2I). The serum concentrations of triglycerides were not significantly changed, but total cholesterol and HDL and low-density lipoprotein levels were significantly reduced in RORαsg/sg mice compared with WT mice (Fig. 2I). Together, these observations indicate that RORαsg/sg(HFD) mice were significantly protected against HFD-induced obesity and hepatic steatosis. These results are consistent with a role for RORα in the regulation of lipid metabolism in adipose tissues and liver (20, 24, 31). Because no difference was observed in the acid steatocrit between WT(HFD) and RORαsg/sg(HFD) mice the protection to metabolic syndrome in RORαsg/sg(HFD) mice appeared not to be related to increased lipid excretion (Fig. 2D).
QRT-PCR analysis showed that the RORα4 is the predominant RORα isoform expressed in WAT, BAT, and liver (Supplemental Fig. S2A), indicating that both liver and adipose tissues are targets for gene regulation by RORα. RORα mRNA expression in WT mice did not significantly change in adipose tissues or liver after fasting but increased by 70% in liver of HFD-fed mice (Supplemental Fig. S2B).
Comparison of gene expression profiles.
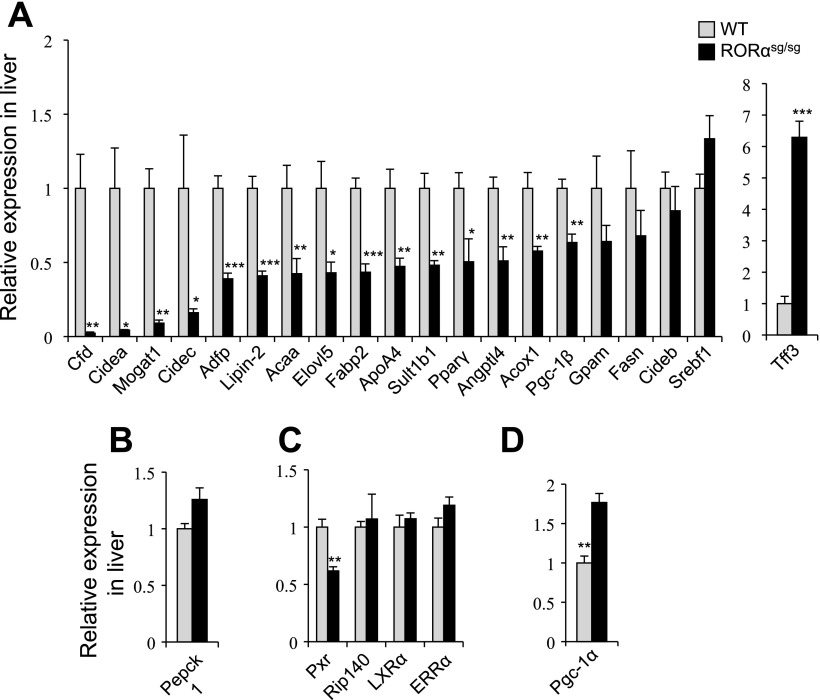
To obtain insights into the mechanism by which loss of RORα prevented HFD-induced obesity and hepatic steatosis, we analyzed the gene expression profiles in liver from WT and RORαsg/sg mice by microarray analysis. This analysis revealed a large number of differences in hepatic gene expression (http://www.ncbi.nlm.nih.gov/geo; accession number GSE23736) between RORαsg/sg and WT mice (Supplemental Table S2). In liver, loss of RORα affected the expression of many genes that regulate lipid homeostasis. The cell death-inducing DFFA-like effector c (Cidec), also termed fat-specific protein (FSP27), and cell death-inducing DFFA-like effector a (Cidea), two proteins that play a critical role in triglyceride accumulation (26, 39, 62), and monoacylglycerol O-acyltransferase 1 (Mogat1), which is part of an alternative pathway of triglyceride synthesis, were among the genes most strongly suppressed in RORαsg/sg liver. The expression of several other genes implicated in fatty acid homeostasis was also repressed in RORαsg/sg liver. These included acyl-coenzyme A (CoA) thioesterases, Acot3 and Acot4, which hydrolyze acyl-CoAs to free fatty acids and CoASH, fatty acid binding protein 5 (Fabp5), adipose differentiation-related protein (Adfp, also termed perilipin 2), lipin 2 (Lpin2), angiopoietin-like 4 (Angptl4), and fibroblast growth factor 21 (Fgf21).
Among other changes, the expression of a several sulfotransferases, cytochrome P450 enzymes, and ATP-binding cassette proteins was significantly reduced in liver of RORαsg/sg(HFD) mice compared with WT counterparts. Sult1e1, which was previously found to be greatly upregulated in RORαsg/sg mice fed with a normal diet (24), was the most dramatically induced gene in the liver of RORαsg/sg(HFD) mice. These data are consistent with previous studies showing that RORα is involved in the regulation of phase I and II metabolic genes (24, 31). Several genes involved in circadian rhythm, including the nuclear receptor Rev-Erbα, and the clock genes Per3 and Cry2, were downregulated in RORαsg/sg(HFD) liver, consistent with the reported regulatory function of RORα in circadian rhythm. The expression of the early growth response genes, Egr1 and Egr2, was decreased more than fourfold in RORαsg/sg(HFD) mice, while PPARα and PXR expression was decreased by 50% (Supplemental Table S2). The suppression of hepatic expression of several differentially expressed genes, including Cidea, Cidec, Mogat1, Adfp, Lpin2, ApoA4, and complement factor D (Cfd; adipsin), and the induction of trefoil factor 3 (Tff3) in RORαsg/sg were confirmed by QRT-PCR (Fig. 3). The expression of Srebf, ERRα, LXRα, and Rip140, known regulators of energy and lipid homeostasis, was not significantly altered in RORαsg/sg liver.
Fig. 3.
Reduced expression of several lipogenic genes in liver of RORαsg/sg mice fed an HFD. The expression of TG synthesis-related genes (A), gluconeogenesis-related genes (B), nuclear receptors (C), and fatty acids oxidation-related gene (D) was analyzed in liver by QRT-PCR from WT and RORαsg/sg mice fed an HFD (WT, n = 5; RORαsg/sg, n = 5). Data present means ± SE, *P < 0.05, **P < 0.01, ***P < 0.001.
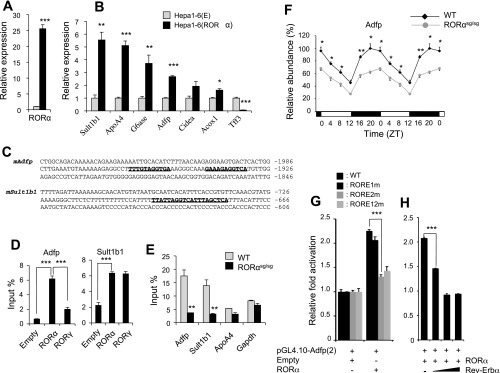
To further evaluate the expression of RORα-responsive genes in a gain-of-function model, we compared the expression of several genes in mouse hepatoma cells Hepa1–6(E) and Hepa1–6(RORα), stably expressing empty vector and RORα, respectively (Fig. 4A). The expression of Sult1b1, ApoA4, Adfp, and Cidea mRNA was significantly induced, while that of Tff3 was repressed in Hepa1–6(RORα) cells (Fig. 4B) consistent with the hepatic gene expression pattern in RORαsg/sg mice (Fig. 3). To determine whether any of the genes were direct transcriptional targets of RORα, the sequence of the upstream regulatory region of several RORα-responsive genes was analyzed for the presence of ROREs. Putative ROREs were identified within the 3 kb promoter region of several genes, including Adfp and Sult1b1 (Fig. 4C). ChIP analysis with Hepa1–6(E) and Hepa1–6(RORα) cells indicated that RORα was recruited to the ROREs in the Adfp and Sult1b1 gene promoters, suggesting that these two genes are direct targets of RORα (Fig. 4D). The latter was supported by ChIP analysis using liver from WT and RORαsg/sg mice (Fig. 4E). The recruitment of RORα to the Adfp and Sult1b1 gene promoters in the liver of WT mice was largely abolished in liver of RORαsg/sg mice (Fig. 4E). The direct regulation of Adfp by RORα was supported by reporter gene analysis. Figure 4G shows that RORα was able to enhance the activation of the Luc reporter driven by the 2 kb proximal Adfp promoter, which contains two putative ROREs (Fig. 4, C and G). Mutation of RORE2, but not RORE1, greatly abolished the increased activation by RORα (Fig. 4G), suggesting that the activation by RORα was largely mediated through RORE2. Although ApoA4 was induced in Hepa1–6(RORα) cells, RORα was not associated with the proximal promoter region suggesting that this gene is regulated by either an RORE further up-/downstream or by an indirect mechanism.
Fig. 4.
Several RORα-responsive genes are regulated directly by RORα. A: comparison of RORα mRNA expression in Hepa1–6(E) and Hepa1–6(RORα) cells stably expressing empty vector or RORα, respectively. B: increased expression of several RORα-responsive genes in Hepa1–6(RORα) compared with Hepa1–6(E) cells. Expression was analyzed by QRT-PCR. C: identification of 2 putative ROREs (boldfaced and underlined) in the promoter of Adfp and Sul1b1 genes. RORα is associated with RORE-containing regulatory regions of the Adfp and Sult1b1 genes. ChIP analysis was performed with Hepa1–6(E), Hepa1–6(RORα), or hepa1–6(RORγ) using anti-Flag M2 antibody (D) or with liver from WT or RORαsg/sg mice using an RORα antibody as described in materials and methods (E). F: the expression of Adfp was examined in liver of WT and RORαsg/sg mice during the circadian time. At each time point 4 mice for each group were analyzed. G: RORα increased Adfp proximal promoter activity through RORE2. Hepatoma Huh-7 cells were cotransfected with pCMVβ-Gal, 3xFlag-CMV10-RORα4 or empty vector, and pGL4.10-Adfp(2) or the reporter plasmid in which RORE1, RORE2, or both (RORE1/2m) were mutated as indicated. The relative luciferase reporter activity was determined 48 h later. H: Rev-Erbα inhibited the enhanced activation of the Adfp promoter by RORα. Cells were cotransfected with 3xFlag-CMV10-RORα4 and pGL4.10-Adfp(2) and 3xFlag-CMV10-Rev-Erbα as indicated and processed as in G. Data present means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001.
Several studies have shown a relationship between the regulation of clock genes, circadian rhythm, and metabolism (2). However, no change in Npas2, Bmal1, or Clock mRNA expression was found in the liver and WAT between WT and RORαsg/sg mice (52, not shown). We therefore examined the circadian expression of the RORα target gene Adfp. Figure 4F shows that Adfp exhibited an oscillatory pattern of expression during circadian time. Loss of RORα reduced the overall expression of Adfp but did not affect the phase of the oscillation, while loss of RORγ had no effect (Fig. 4F and Supplemental Fig. S3). Interestingly, the lowest expression of Adfp occurred between Zeitgeber time (ZT) 8 and 12, a time at which Rev-ERbα is optimally expressed. Rev-Erbα functions as repressor and can compete with RORs for binding to ROREs. Figure 4H shows that Rev-Erbα inhibited the activation of the Adfp promoter by RORα consistent with the hypothesis that it negatively regulates Adfp by competing for the same RORE. Moreover, it suggests that the downregulation of Adfp at ZT10 may be at least in part mediated by Rev-Erbα.
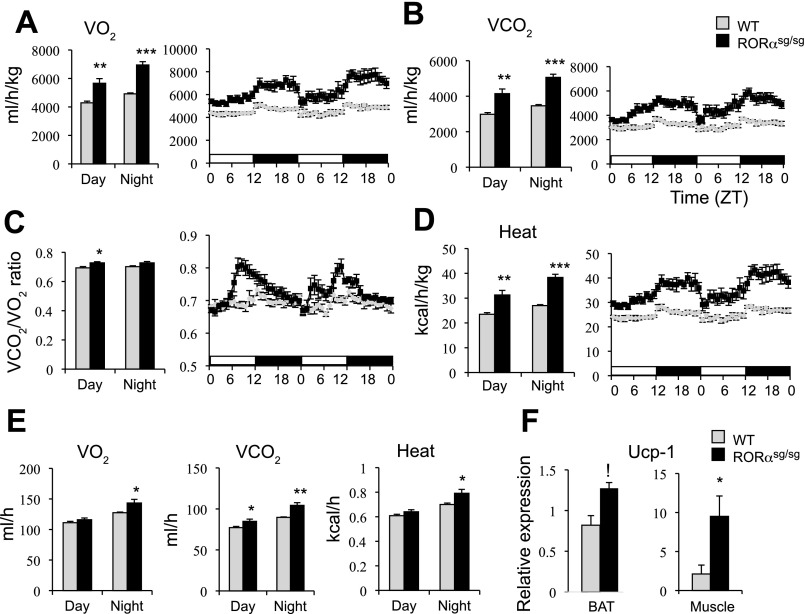
Energy expenditure was increased in RORαsg/sg mice.
Although the relative food consumption was higher in RORαsg/sg mice than WT mice, particularly during the light phase, RORαsg/sg mice were leaner than WT mice (Supplemental Fig. S4A). Consistent with previous observations (31), the increased food intake may relate to the reduced leptin expression in WAT and the decreased level of circulating leptin in RORαsg/sg mice (Supplemental Fig. S4, B and C), which may enhance the appetite in these mice. To determine whether RORαsg/sg mice exhibited increased energy expenditure, the VO2, VCO2, VCO2/VO2, and heat emission were compared between WT(HFD) and RORαsg/sg(HFD) mice over a 2-day period. A debate has been ongoing on what is the best way to normalize for energy expenditure (5, 22, 34). Therefore, in addition to the unadjusted data (per mouse, Fig. 5E), we presented our data relative to free fat mass (Fig. 5, A, B, and D). As shown in Fig. 5, A and B, RORαsg/sg(HFD) mice exhibited significantly elevated VO2 and VCO2 calculated either as ml·h−1·kg−1 fat free mass or per mouse; however, the difference was most pronounced during the dark phase. VCO2/VO2 was also increased in RORαsg/sg mice vs. control mice particularly during the second part of the light phase (Fig. 5C). Together, these results suggested that the RORαsg/sg(HFD) mice had a higher rate of energy expenditure than their WT littermates. The higher rate of energy expenditure was partly due to an increase in heat generation (Fig. 5D). A hypothesis that was supported by the elevated expression of Ucp-1 in BAT, but particularly in the skeletal muscle of RORαsg/sg mice (Fig. 5F). Ucp-1 diverts energy derived from mitochondrial electron transport chain and generation of ATP into heat production. RORαsg/sg mice (8–10 wk old) maintained on a normal diet also showed increased VO2, VCO2, and energy expenditure compared with their WT counterparts (Supplemental Fig. S5). However, at this age the percentage total body fat and lean mass were not significantly different between WT and RORαsg/sg mice (Supplemental Fig. S5E).
Fig. 5.
RORαsg/sg(HFD) mice have increased energy expenditure. A–D: oxygen consumption (VO2) and carbon dioxide generation (VCO2) by WT(HFD) and RORαsg/sg(HFD) were analyzed by indirect calorimetry over a period of two 12 h light/12 h dark cycles. VO2, VCO2, VCO2/VO2, and heat generation were computed per kg free fat mass (WT, n = 5; RORαsg/sg, n = 6). E: comparison of the absolute values (per mouse) of VO2, VCO2, and heat generation between WT and RORαsg/sg mice. F: comparison of Ucp1 expression in BAT and muscle of WT and RORαsg/sg mice. Data present means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001.
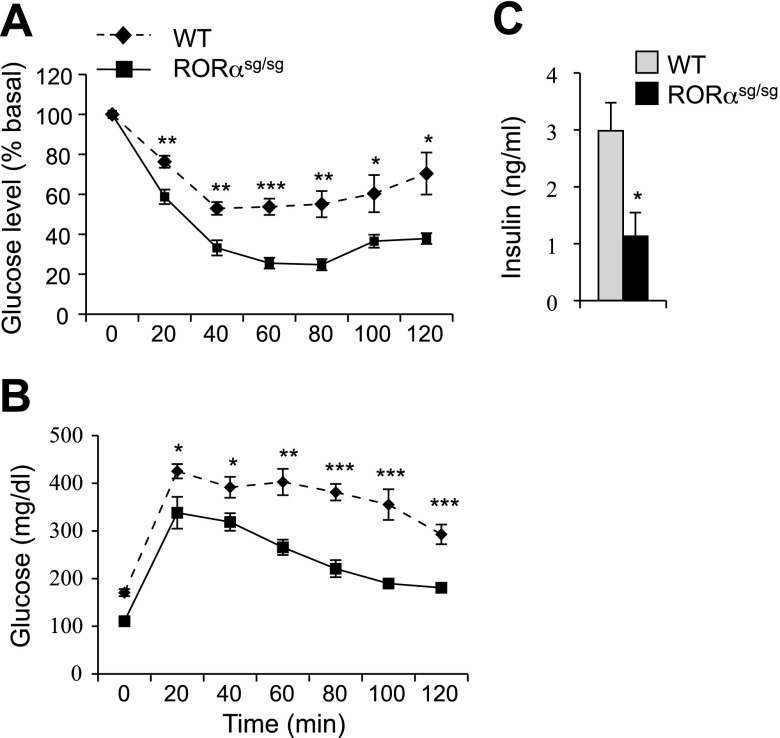
RORαsg/sg mice were protected against HFD-induced insulin-resistance.
Obesity greatly increases the risk of developing insulin resistance and glucose intolerance, key indicators of the development of Type 2 diabetes (18, 44). As indicated by ITT and GTT analyses, WT mice maintained on an HFD for 10 wk developed insulin resistance and glucose intolerance (Fig. 6, A and B). In sharp contrast, RORαsg/sg mice remained insulin sensitive and glucose tolerant. Moreover, blood insulin levels were significantly lower in RORαsg/sg(HFD) mice compared with WT(HFD) mice (Fig. 6C). The data suggest that RORαsg/sg(HFD) mice were protected against developing diabetes. No significant difference in ITT and GTT was observed between 8–10 wk old WT and RORαsg/sg mice fed a normal diet (Supplemental Fig. S6).
Fig. 6.
RORαsg/sg mice are protected against HFD-induced insulin resistance and glucose intolerance. A, B: glucose tolerance test (WT, n = 6; RORαsg/sg, n = 6) and insulin tolerance test (WT, n = 8; RORαsg/sg, n = 6) were performed in WT(HFD) and RORαsg/sg(HFD) mice. Glucose levels were analyzed every 20 min for up to 2–2.5 h. C: blood insulin levels were analyzed in WT(HFD) and RORαsg/sg(HFD) female mice (WT, n = 5; RORαsg/sg, n = 8). Data represent means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001.
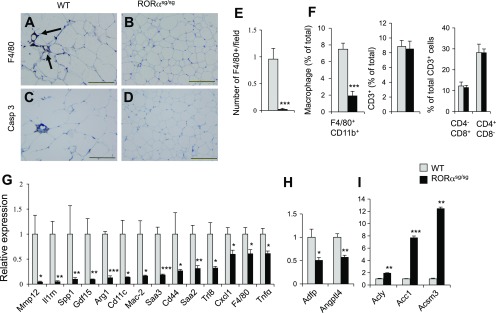
Loss of RORα function reduced WAT-associated inflammation.
Inflammation plays a key role in the development of obesity-associated pathologies, including NAFLD and insulin resistance. A strong link has been established between adipose tissue-associated inflammation and metabolic syndrome (18, 38, 44, 53). Consistent with these concepts, immunohistochemical staining showed that crown-like structures (CLS), representing F4/80-positive macrophages, were frequently found in WAT from WT(HFD) mice. In contrast, F4/80-positive cells were rarely seen in WAT from RORαsg/sg (HFD) mice (Fig. 7, A, B, E). Moreover, active caspase-3+ apoptotic cells were also regularly observed in WAT of WT(HFD) mice, but not in RORαsg/sg (HFD) mice (Fig. 7, C and D). The decrease in F4/80+ macrophages was supported by flow cytometric analysis of inflammatory cell populations of SVF isolated from eWAT. This analysis showed that the percentage of SVF-associated macrophages (F4/80+/Cd11b+) was greatly reduced in RORαsg/sg(HFD) mice (Fig. 7F). No significant difference was observed in the percentages of CD3+, CD4+CD8−, and CD4−CD8+ T lymphocytes between RORαsg/sg and WT mice (Fig. 7F). Together, these observations suggested that loss of RORα greatly diminished the development of obesity-associated inflammation in WAT. The inhibition of WAT-associated inflammation in RORαsg/sg(HFD) mice was supported by the reduced expression of numerous immune and inflammatory response genes, including several chemo/cytokines and their receptors, and Toll-like receptors (Supplemental Table S3). The expression of TNFα, Ccl2, serum amyloid 3 (Saa3), interleukin 1 receptor antagonist (Il1rn), matrix metallopeptidase 12 (Mmp12), secreted phosphoprotein 1 (Ssp1; osteopontin), Cd44, and the macrophage markers, Mac-2, Mrc2, Mpeg1, and F4/80, was significantly reduced in WAT of RORαsg/sg(HFD) mice compared with their WT counterparts (Fig. 7G). Increased expression of many of these genes, including Il1rn, Ssp1, and Cd44, has been linked to obesity-associated inflammation and insulin resistance (3, 28, 45, 46).
Fig. 7.
WAT-associated inflammatory response is significantly reduced in WAT of RORαsg/sg(HFD) mice. A, B: macrophage infiltration into eWAT was greatly reduced in RORαsg/sg(HFD) mice. F4/80+ macrophages were identified by immunohistochemical staining as “crown-like structures” (arrows). Scale bar indicates 200 μm. C, D: reduced apoptosis in eWAT of RORαsg/sg(HFD) mice compared with WT(HDF). Apoptotic cells were stained with an antibody for active-caspase 3. E: the number of F4/80-positive cells were decreased in WAT of RORαsg/sg(HFD) than WT(HFD) mice (WT, n = 5; RORαsg/sg, n = 5). F: stromal-vascular fraction cells from eWAT of WT(HFD) and RORαsg/sg(HFD) were examined by flow cytometry analysis as described in materials and methods. The percentage of the macrophage population (F4/80+CD11b+ cells) was significantly reduced, but T cell population (CD3+ cells) and CD4+ and CD8+ subpopulations were unaffected in RORαsg/sg(HFD) mice (WT, n = 9; RORαsg/sg, n = 5). G, H: induction of inflammatory genes and metabolic in WAT was greatly decreased in RORαsg/sg(HFD) for 6 wk. I: induction of lipogenic genes in WAT of RORαsg/sg(HFD) mice. Gene expression was analyzed by QRT-PCR (5 male mice in each group). Data present means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001.
In addition to inflammatory genes, the expression of a number of metabolic genes was differentially regulated between WAT of WT(HFD) and RORαsg/sg(HFD). Expression of Adfp and Angptl4, which are involved in the regulation of lipid metabolism (6, 27), was repressed in both WAT and liver of RORαsg/sg(HFD) mice (Supplemental Tables S2 and S3, Fig. 7H). Despite the reduced adiposity, the expression of several genes involved in lipogenesis, including acetyl-Coenzyme A carboxylase a and b (Acaca and Acacb), acyl-CoA synthetase medium-chain family member 3 (Acsm3), and Srebf was enhanced in WAT of RORαsg/sg(HFD) mice (Fig. 7I and Supplemental Table S3). Interestingly, Srebf has been reported to induce Acaca expression, and, therefore, the increase in Srebf might be causally related to the upregulation of Acaca in RORαsg/sg(HFD) WAT. Among other changes, the expression of a several cytochrome P450 genes and steroid metabolic genes were altered in RORαsg/sg(HFD) WAT (Supplemental Table S3) consistent with a role of RORα in the regulation of several metabolic pathways (24, 31).
DISCUSSION
In this study, we show that loss of RORα protects mice against age- and diet-induced metabolic syndrome, whereas RORγ null mice behaved as WT mice. Specifically, RORαsg/sg mice remained lean when maintained on an HFD and showed a markedly reduced susceptibility to the development of hepatic steatosis, adipose tissue-associated inflammation, and insulin resistance. Our study extends previous observations (24, 31) and supports the hypothesis that the nuclear receptor RORα plays a critical role in the control of energy balance and lipid homeostasis.
The liver of aged RORαsg/sg mice and mice fed an HFD contained smaller lipid droplets and significantly lower levels of triglycerides compared with WT mice. The accumulation of hepatic triglycerides is regulated at multiple levels, including uptake, transport, synthesis, storage of lipids, and lipolysis. Our gene-profiling analysis revealed that the expression of a number of lipogenic genes was significantly reduced in the liver of RORαsg/sg mice. These included Cidec and Cidea, which regulate triglyceride storage, the size of lipid droplets, and lipolysis and which have been reported to be highly induced in hepatic steatosis (17, 39, 41). In addition, the expression of several genes implicated in the main pathway of triglyceride synthesis (37), including glycerol-3-phosphate acyltransferase (Gpam or Gpat1) and acyl-glycerol-3-phosphate acyltransferase 9 (Agpat9), and Mogat1, which is part of an alternative, less-studied pathway of triglyceride synthesis, was significantly reduced in liver of RORαsg/sg mice. The combined effects on the regulation of multiple genes may have accounted for the reduced lipid accumulation in the liver of RORαsg/sg mice.
The reduced expression of several other genes, including Adfp, Angptl4, Fgf21, and Gdf15, may also have contributed to the decreased accumulation of hepatic triglycerides in RORαsg/sg mice. The expression of several of these genes was reported to be induced in obesity and all four proteins have been implicated in the control of lipid and/or glucose homeostasis (7, 8, 10, 59). Adfp (or perilipin 2) is a major lipid droplet protein present in cells that accumulate triglycerides, and loss of Adfp has been reported to reduce triglycerides levels and to relieve hepatosteatosis in leptin-deficient ob/ob mice (7). Thus, the reduced levels of Adfp expression observed in RORαsg/sg mice might contribute to the protection against hepatic steatosis in these mice.
It is well recognized that obesity is associated with low-grade systemic inflammation and that inflammation plays a key role in obesity-linked pathologies, such as hepatic steatosis and insulin resistance (18, 44, 53). The accumulation of various immune cells, including macrophages and T lymphocytes, in adipose tissues and the production of a variety of proinflammatory cyto- and chemokines are critical events in the development of obesity-associated inflammation (14, 38, 57). Our study supports the growing recognition of a relationship between obesity and adipose tissue-associated inflammation. Our gene expression profiling showed that many proinflammatory and immune-regulatory genes, including those encoding various interleukins, chemokines, chemo/cytokine receptors, TNF-α, Toll-like receptors, and metalloproteinases, were expressed at significantly lower levels in the WAT of HFD-fed RORαsg/sg mice compared with their WT counterparts, suggesting that RORαsg/sg mice develop considerably less inflammation when challenged with an HFD. This was supported by the greatly reduced infiltration of macrophages in WAT of RORαsg/sg mice as indicated by the rare presence of CLS and the lower percentage of SVF-associated F4/80+/Cd11b+ macrophages in WAT of HFD-fed RORαsg/sg mice. Moreover, the expression of several macrophage markers, including F4/80, Mac-2, Mpeg1, and Msr1, was significantly decreased in RORαsg/sg mice. ATF3, a basic leucine zipper-type transcription factor, is one of the transcription factors whose expression was significantly reduced in WAT of RORαsg/sg mice (Supplemental Table S3). Although ATF3 expression is greatly increased in obese adipose tissue and correlates with macrophage accumulation, it functions as a negative feedback mechanism to attenuate obese-macrophage activation (50). Recent studies have shown that WAT contains different macrophage populations. Obesity induces a shift from mannose receptor (Mrc1 or Cd206) positive, Cd11c+ macrophages to Mrc1-negative macrophages that express Ccr2, 3, and 5. The expression of both Mrc1 and these chemokine receptors was downregulated in RORαsg/sg WAT, consistent with a reduction in all three macrophage populations.
Although T-lymphocytes have been reported to play a role in recruiting macrophages to WAT (38), no significant difference was observed in the percentage of CD4+ or CD8+ T lymphocytes in WAT of WT(HFD) and RORαsg/sg(HFD) mice. RORα has been implicated in anti- as well as proinflammatory responses, including the inhibition of NF-κB signaling and induction of Th17 differentiation, respectively (9, 60). The diminished inflammatory response in WAT of RORαsg/sg(HFD) mice is consistent with reports showing reduced inflammatory responses in allergen-induced airway inflammation and inhibition of experimental encephalomyelitis in RORαsg/sg mice (19, 60).
RORαsg/sg mice maintained on an HFD remained insulin sensitive and glucose tolerant, suggesting that these mice were less susceptible to obesity-induced Type 2 diabetes. The expression of a number of genes implicated in the regulation of insulin resistance was found to be downregulated in RORαsg/sg mice. Loss of Adfp has been reported to improve insulin resistance in leptin-deficient ob/ob mice (7). Thus, the reduced expression of Adfp observed in RORαsg/sg mice may have contributed to the protection against insulin resistance. The interleukin-1 receptor antagonist (Il1rn) and Ssp1 were among the genes most dramatically repressed in WAT of RORαsg/sg mice. IL1rn has been reported to be highly upregulated in WAT of obese humans and to regulate insulin sensitivity (21, 45), while Il1rn-deficient mice exhibited decreased adiposity and increased energy expenditure (46). Ssp1 expression was found to be elevated in obesity, while neutralization of Ssp1 was shown to inhibit obesity-induced inflammation and insulin resistance (3, 28). Thus, the downregulation of Adfp, Il1rn, Ssp1, and likely other genes may have collaboratively promoted insulin sensitivity in RORαsg/sg mice through their interrelated effects on inflammation, adipogenesis, and energy expenditure.
Exogenous expression of RORα in mouse hepatoma Hepa1–6 significantly increased the expression of several genes that were found to be repressed in RORαsg/sg liver, including Sult1b1, Adfp, Cidea, and ApoA4. It is likely that certain RORα-responsive genes are regulated directly by RORα through its interaction with ROREs in their respective regulatory region, while others are controlled by indirect mechanisms (20). A recent study identified Fgf21 as a direct target of RORα transcriptional regulation in HepG2 cells (56). The regulation of Fgf21 by RORα is supported by our microarray analysis showing that expression of Fgf21 is reduced in RORαsg/sg liver. Examination of the sequence of the upstream regulatory region of a number of RORα-responsive genes identified putative ROREs in several genes. ChIP analysis indicated that RORα protein was recruited to the RORE-containing promoter region of Adfp and Sult1b1 in the liver of WT mice, but not in that of RORαsg/sg mice. Moreover, RORα was recruited to the promoter regions of Adfp and Sult1b1 in Hepa1–6(RORα) cells. These observations suggested that Adfp and Sult1b1 are direct target genes of RORα. Interestingly, both RORα and RORγ were recruited to the Sult1b1 promoter, whereas the Adfp promoter was RORα-selective. These data are consistent with our previous observations showing redundancy between RORα and RORγ in the regulation of certain genes, while other genes are regulated in an ROR isotype-selective manner (24). The direct regulation of Adfp by RORα was supported by reporter gene analysis. RORα was able to enhance the Adfp promoter activity, while mutation of RORE2 abolished this activation.
Several studies have demonstrated an interrelationship between clock proteins and various metabolic functions (2). The effect of RORα on clock gene regulation could be a component of the mechanism by which RORα controls metabolism. However, Bmal1, Npas2, and Clock expression were not changed in the liver and WAT of RORαsg/sg mice (52, and not shown). Interestingly, in contrast to RORα, Adfp exhibits a strong oscillatory pattern of expression during circadian time with the lowest level of expression at ZT8–12, a time at which Rev-Erbα is most highly expressed. We provide evidence that both Rev-Erbα and RORα play a role in the circadian regulation of Adfp likely by competing for the same RORE.
Obesity is a consequence of an imbalance between energy intake and expenditure. The decreased adiposity in RORαsg/sg mice is not due to reduced food intake nor due to an increase in lipid excretion. Indirect calorimetric analysis of 2 mo old WT and RORαsg/sg mice fed either a normal or HFD showed that VO2, VCO2, and heat generation were significantly enhanced in RORαsg/sg mice on the basis of unadjusted data or those adjusted for free fat mass. The hypothesis of increased energy expenditure was also supported by the upregulation of Ucp-1 in BAT and particularly in the skeletal muscle of RORαsg/sg mice. Thus, the elevated energy expenditure observed in RORαsg/sg mice may at least in part be responsible for the reduced weight gain and resistance to hepatic steatosis and insulin insensitivity.
In summary, our study demonstrates that RORαsg/sg mice are protected from age- and diet-induced hepatic steatosis, obesity-associated inflammation, and insulin resistance. Loss of RORα inhibited the hepatic expression of several genes implicated in lipid accumulation and storage as well as the expression of several inflammatory genes in WAT. We provide evidence indicating that some of these genes are regulated directly by RORα. Recent studies have suggested that the activity of RORα can be regulated by natural and synthetic compounds (30, 55), which raises hopes that RORα may provide a novel therapeutic target in the management and prevention of obesity and related pathologies, such as diabetes.
GRANTS
This research was supported by the Intramural Research Program of the NIEHS (Z01-ES-101586).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Drs. Kristin Lichti-Kaiser and X. Li (NIEHS) for valuable comments on the manuscript.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Austin S, Medvedev A, Yan ZH, Adachi H, Hirose T, Jetten AM. Induction of the nuclear orphan receptor RORgamma during adipocyte differentiation of D1 and 3T3–L1 cells. Cell Growth Differ 9: 267–276, 1998. [PubMed] [Google Scholar]
- 2. Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science 330: 1349–1354, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bertola A, Deveaux V, Bonnafous S, Rousseau D, Anty R, Wakkach A, Dahman M, Tordjman J, Clement K, McQuaid SE, Frayn KN, Huet PM, Gugenheim J, Lotersztajn S, Le Marchand-Brustel Y, Tran A, Gual P. Elevated expression of osteopontin may be related to adipose tissue macrophage accumulation and liver steatosis in morbid obesity. Diabetes 58: 125–133, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boukhtouche F, Doulazmi M, Frederic F, Dusart I, Brugg B, Mariani J. RORalpha, a pivotal nuclear receptor for Purkinje neuron survival and differentiation: from development to ageing. Cerebellum 5: 97–104, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Butler AA, Kozak LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes 59: 323–329, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang BH, Chan L. Regulation of triglyceride metabolism. III. Emerging role of lipid droplet protein ADFP in health and disease. Am J Physiol Gastrointest Liver Physiol 292: G1465–G1468, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Chang BH, Li L, Saha P, Chan L. Absence of adipose differentiation related protein upregulates hepatic VLDL secretion, relieves hepatosteatosis and improves whole body insulin resistance in leptin-deficient mice. J Lipid Res 51: 2132–2142, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cuevas-Ramos D, Almeda-Valdes P, Aguilar-Salinas CA, Cuevas-Ramos G, Cuevas-Sosa AA, Gomez-Perez FJ. The role of fibroblast growth factor 21 (FGF21) on energy balance, glucose and lipid metabolism. Curr Diabetes Rev 5: 216–220, 2009. [DOI] [PubMed] [Google Scholar]
- 9. Delerive P, Monte D, Dubois G, Trottein F, Fruchart-Najib J, Mariani J, Fruchart JC, Staels B. The orphan nuclear receptor ROR alpha is a negative regulator of the inflammatory response. EMBO Rep 2: 42–48, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dostalova I, Roubicek T, Bartlova M, Mraz M, Lacinova Z, Haluzikova D, Kavalkova P, Matoulek M, Kasalicky M, Haluzik M. Increased serum concentrations of macrophage inhibitory cytokine-1 in patients with obesity and type 2 diabetes mellitus: the influence of very low calorie diet. Eur J Endocrinol 161: 397–404, 2009. [DOI] [PubMed] [Google Scholar]
- 11. Duez H, Duhem C, Laitinen S, Patole PS, Abdelkarim M, Bois-Joyeux B, Danan JL, Staels B. Inhibition of adipocyte differentiation by RORalpha. FEBS Lett 583: 2031–2036, 2009. [DOI] [PubMed] [Google Scholar]
- 12. Duez H, Staels B. The nuclear receptors Rev-erbs and RORs integrate circadian rhythms and metabolism. Diab Vasc Dis Res 5: 82–88, 2008. [DOI] [PubMed] [Google Scholar]
- 13. Erickson SK. Nonalcoholic fatty liver disease. J Lipid Res 50, Suppl: S412–S416, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 15: 930–939, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giguere V, Tini M, Flock G, Ong E, Evans RM, Otulakowski G. Isoform-specific amino-terminal domains dictate DNA-binding properties of ROR alpha, a novel family of orphan hormone nuclear receptors. Genes Dev 8: 538–553, 1994. [DOI] [PubMed] [Google Scholar]
- 16. Gold DA, Gent PM, Hamilton BA. RORalpha in genetic control of cerebellum development: 50 staggering years. Brain Res 1140: 19–25, 2006. [DOI] [PubMed] [Google Scholar]
- 17. Gong J, Sun Z, Li P. CIDE proteins and metabolic disorders. Curr Opin Lipidol 20: 121–126, 2009. [DOI] [PubMed] [Google Scholar]
- 18. Hotamisligil GS. Inflammation and metabolic disorders. Nature 444: 860–867, 2006. [DOI] [PubMed] [Google Scholar]
- 19. Jaradat M, Stapleton C, Tilley SL, Dixon D, Erikson CJ, McCaskill JG, Kang HS, Angers M, Liao G, Collins J, Grissom S, Jetten AM. Modulatory role for retinoid-related orphan receptor alpha (RORalpha) in allergen-induced lung inflammation. Am J Respir Crit Care Med 174: 1299–1309, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal 7: e003, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Juge-Aubry CE, Somm E, Giusti V, Pernin A, Chicheportiche R, Verdumo C, Rohner-Jeanrenaud F, Burger D, Dayer JM, Meier CA. Adipose tissue is a major source of interleukin-1 receptor antagonist: upregulation in obesity and inflammation. Diabetes 52: 1104–1110, 2003. [DOI] [PubMed] [Google Scholar]
- 22. Kaiyala KJ, Morton GJ, Leroux BG, Ogimoto K, Wisse B, Schwartz MW. Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes 59: 1657–1666, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kallen JA, Schlaeppi J, Bitsch F, Geisse S, Geiser M, Delhon I, Fournier B. X-ray structure of the RORa LBD at 1.63A: structural and functional data that cholesterol or a cholesterol derivative is the natural ligand of RORa. Structure 10: 1697–1707, 2002. [DOI] [PubMed] [Google Scholar]
- 24. Kang HS, Angers M, Beak JY, Wu X, Gimble JM, Wada T, Xie W, Collins JB, Grissom SF, Jetten AM. Gene expression profiling reveals a regulatory role for ROR alpha and ROR gamma in phase I and phase II metabolism. Physiol Genomics 31: 281–294, 2007. [DOI] [PubMed] [Google Scholar]
- 25. Kang HS, Kim YS, ZeRuth G, Beak JY, Gerrish K, Kilic G, Sosa-Pineda B, Jensen J, Pierreux CE, Lemaigre FP, Foley J, Jetten AM. Transcription factor Glis3, a novel critical player in the regulation of pancreatic beta-cell development and insulin gene expression. Mol Cell Biol 29: 6366–6379, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keller P, Petrie JT, De Rose P, Gerin I, Wright WS, Chiang SH, Nielsen AR, Fischer CP, Pedersen BK, MacDougald OA. Fat-specific protein 27 regulates storage of triacylglycerol. J Biol Chem 283: 14355–14365, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kersten S. Regulation of lipid metabolism via angiopoietin-like proteins. Biochem Soc Trans 33: 1059–1062, 2005. [DOI] [PubMed] [Google Scholar]
- 28. Kiefer FW, Zeyda M, Gollinger K, Pfau B, Neuhofer A, Weichhart T, Saemann MD, Geyeregger R, Schlederer M, Kenner L, Stulnig TM. Neutralization of osteopontin inhibits obesity-induced inflammation and insulin resistance. Diabetes 59: 935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim EJ, Yoo YG, Yang WK, Lim YS, Na TY, Lee IK, Lee MO. Transcriptional activation of HIF-1 by RORalpha and its role in hypoxia signaling. Arterioscler Thromb Vasc Biol 28: 1796–1802, 2008. [DOI] [PubMed] [Google Scholar]
- 30. Kumar N, Solt LA, Conkright JJ, Wang Y, Istrate MA, Busby SA, Garcia-Ordonez RD, Burris TP, Griffin PR. The benzenesulfoamide T0901317 [N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]-benzenesulfonamide] is a novel retinoic acid receptor-related orphan receptor-alpha/gamma inverse agonist. Mol Pharmacol 77: 228–236, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lau P, Fitzsimmons RL, Raichur S, Wang SC, Lechtken A, Muscat GE. The orphan nuclear receptor, RORalpha, regulates gene expression that controls lipid metabolism: staggerer (SG/SG) mice are resistant to diet-induced obesity. J Biol Chem 283: 18411–18421, 2008. [DOI] [PubMed] [Google Scholar]
- 32. Lau P, Nixon SJ, Parton RG, Muscat GE. RORalpha regulates the expression of genes involved in lipid homeostasis in skeletal muscle cells: caveolin-3 and CPT-1 are direct targets of ROR. J Biol Chem 279: 36828–36840, 2004. [DOI] [PubMed] [Google Scholar]
- 33. Lind U, Nilsson T, McPheat J, Stromstedt PE, Bamberg K, Balendran C, Kang D. Identification of the human ApoAV gene as a novel RORalpha target gene. Biochem Biophys Res Commun 330: 233–241, 2005. [DOI] [PubMed] [Google Scholar]
- 34. MacLean PS. Comment on: Kaiyala et al. (2010) Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes 60: e3, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Medvedev A, Yan ZH, Hirose T, Giguere V, Jetten AM. Cloning of a cDNA encoding the murine orphan receptor RZR/ROR gamma and characterization of its response element. Gene 181: 199–206, 1996. [DOI] [PubMed] [Google Scholar]
- 36. Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289: 76–79, 2003. [DOI] [PubMed] [Google Scholar]
- 37. Nagle CA, Klett EL, Coleman RA. Hepatic triacylglycerol accumulation and insulin resistance. J Lipid Res 50, Suppl: S74–S79, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15: 914–920, 2009. [DOI] [PubMed] [Google Scholar]
- 39. Nishino N, Tamori Y, Tateya S, Kawaguchi T, Shibakusa T, Mizunoya W, Inoue K, Kitazawa R, Kitazawa S, Matsuki Y, Hiramatsu R, Masubuchi S, Omachi A, Kimura K, Saito M, Amo T, Ohta S, Yamaguchi T, Osumi T, Cheng J, Fujimoto T, Nakao H, Nakao K, Aiba A, Okamura H, Fushiki T, Kasuga M. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J Clin Invest 118: 2808–2821, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA 288: 1728–1732, 2002. [DOI] [PubMed] [Google Scholar]
- 41. Puri V, Ranjit S, Konda S, Nicoloro SM, Straubhaar J, Chawla A, Chouinard M, Lin C, Burkart A, Corvera S, Perugini RA, Czech MP. Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc Natl Acad Sci USA 105: 7833–7838, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Raichur S, Fitzsimmons RL, Myers SA, Pearen MA, Lau P, Eriksson N, Wang SM, Muscat GE. Identification and validation of the pathways and functions regulated by the orphan nuclear receptor, ROR alpha1, in skeletal muscle. Nucleic Acids Res 38: 4296–4312, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Raspe E, Duez H, Gervois P, Fievet C, Fruchart JC, Besnard S, Mariani J, Tedgui A, Staels B. Transcriptional regulation of apolipoprotein C-III gene expression by the orphan nuclear receptor RORalpha. J Biol Chem 276: 2865–2871, 2001. [DOI] [PubMed] [Google Scholar]
- 44. Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 118: 2992–3002, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Somm E, Cettour-Rose P, Asensio C, Charollais A, Klein M, Theander-Carrillo C, Juge-Aubry CE, Dayer JM, Nicklin MJ, Meda P, Rohner-Jeanrenaud F, Meier CA. Interleukin-1 receptor antagonist is upregulated during diet-induced obesity and regulates insulin sensitivity in rodents. Diabetologia 49: 387–393, 2006. [DOI] [PubMed] [Google Scholar]
- 46. Somm E, Henrichot E, Pernin A, Juge-Aubry CE, Muzzin P, Dayer JM, Nicklin MJ, Meier CA. Decreased fat mass in interleukin-1 receptor antagonist-deficient mice: impact on adipogenesis, food intake, and energy expenditure. Diabetes 54: 3503–3509, 2005. [DOI] [PubMed] [Google Scholar]
- 47. Stapleton CM, Jaradat M, Dixon D, Kang HS, Kim SC, Liao G, Carey MA, Cristiano J, Moorman MP, Jetten AM. Enhanced susceptibility of staggerer (RORalphasg/sg) mice to lipopolysaccharide-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol 289: L144–L152, 2005. [DOI] [PubMed] [Google Scholar]
- 48. Stehlin-Gaon C, Willmann D, Zeyer D, Sanglier S, Van Dorsselaer A, Renaud JP, Moras D, Schule R. All-trans retinoic acid is a ligand for the orphan nuclear receptor RORbeta. Nat Struct Biol 10: 820–825, 2003. [DOI] [PubMed] [Google Scholar]
- 49. Steinmayr M, Andre E, Conquet F, Rondi-Reig L, Delhaye-Bouchaud N, Auclair N, Daniel H, Crepel F, Mariani J, Sotelo C, Becker-Andre M. Staggerer phenotype in retinoid-related orphan receptor alpha-deficient mice. Proc Natl Acad Sci USA 95: 3960–3965, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Suganami T, Yuan X, Shimoda Y, Uchio-Yamada K, Nakagawa N, Shirakawa I, Usami T, Tsukahara T, Nakayama K, Miyamoto Y, Yasuda K, Matsuda J, Kamei Y, Kitajima S, Ogawa Y. Activating transcription factor 3 constitutes a negative feedback mechanism that attenuates saturated Fatty acid/toll-like receptor 4 signaling and macrophage activation in obese adipose tissue. Circ Res 105: 25–32, 2009. [DOI] [PubMed] [Google Scholar]
- 51. Takahashi N, Li F, Hua K, Deng J, Wang CH, Bowers RR, Bartness TJ, Kim HS, Harp JB. Increased energy expenditure, dietary fat wasting, and resistance to diet-induced obesity in mice lacking renin. Cell Metab 6: 506–512, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Takeda Y, Kang HS, Angers M, Jetten AM. Retinoic acid-related orphan receptor gamma directly regulates neuronal PAS domain protein 2 transcription in vivo. Nucleic Acids Res (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6: 772–783, 2006. [DOI] [PubMed] [Google Scholar]
- 54. Wada T, Kang HS, Angers M, Gong H, Bhatia S, Khadem S, Ren S, Ellis E, Strom SC, Jetten AM, Xie W. Identification of oxysterol 7alpha-hydroxylase (Cyp7b1) as a novel retinoid-related orphan receptor alpha (RORalpha) (NR1F1) target gene and a functional cross-talk between RORalpha and liver X receptor (NR1H3). Mol Pharmacol 73: 891–899, 2008. [DOI] [PubMed] [Google Scholar]
- 55. Wang Y, Kumar N, Solt LA, Richardson TI, Helvering LM, Crumbley C, Garcia-Ordonez RD, Stayrook KR, Zhang X, Novick S, Chalmers MJ, Griffin PR, Burris TP. Modulation of retinoic acid receptor-related orphan receptor alpha and gamma activity by 7-oxygenated sterol ligands. J Biol Chem 285: 5013–5025, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang Y, Solt LA, Burris TP. Regulation of FGF21 expression and secretion by retinoic acid receptor-related orphan receptor alpha. J Biol Chem. 285: 15668–15673 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Woods A, James CG, Wang G, Dupuis H, Beier F. Control of chondrocyte gene expression by actin dynamics: a novel role of cholesterol/Ror-alpha signalling in endochondral bone growth. J Cell Mol Med 13: 3497–3516, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xu A, Lam MC, Chan KW, Wang Y, Zhang J, Hoo RL, Xu JY, Chen B, Chow WS, Tso AW, Lam KS. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proc Natl Acad Sci USA 102: 6086–6091, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORalpha and RORgamma. Immunity 28: 29–39, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhou J, Zhai Y, Mu Y, Gong H, Uppal H, Toma D, Ren S, Evans RM, Xie W. A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J Biol Chem 281: 15013–15020, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhou Z, YonToh S, Chen Z, Guo K, Ng CP, Ponniah S, Lin SC, Hong W, Li P. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat Genet 35: 49–56, 2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.