Abstract
Hibernation as manifested in ground squirrels is arguably the most plastic and extreme of physiological phenotypes in mammals. Homeostasis is challenged by prolonged fasting accompanied by heterothermy, yet must be facilitated for survival. We performed LC and GC-MS metabolomic profiling of plasma samples taken reproducibly during seven natural stages of the hibernator's year, three in summer and four in winter (each n ≥ 5), employing a nontargeted approach to define the metabolite shifts associated with the phenotype. We quantified 231 named metabolites; 106 of these altered significantly, demarcating a cycle within a cycle where torpor-arousal cycles recur during the winter portion of the seasonal cycle. A number of robust hibernation biomarkers that alter with season and winter stage are identified, including specific free fatty acids, antioxidants, and previously unpublished modified amino acids that are likely to be associated with the fasting state. The major pattern in metabolite levels is one of either depletion or accrual during torpor, followed by reversal to an apparent homeostatic level by interbout arousal. This finding provides new data that strongly support the predictions of a long-standing hypothesis that periodic arousals are necessary to restore metabolic homeostasis.
Keywords: Ictidomys tridecemlineatus, Spermophilus, γ-glutamyl, biliverdin, N-acetyl
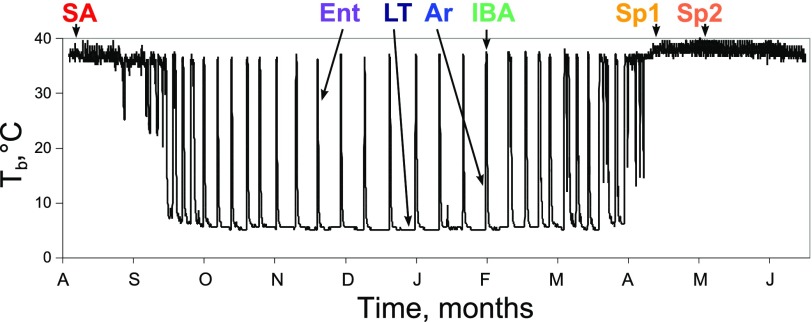
a year in the life of a hibernator begins with reproduction in spring, followed by growth in summer, and then massive storage of fat as fall approaches. Hibernation, characterized by months of fasting and dramatic oscillations between states of cold and warm body temperature (Tb), ensues only after adequate fat stores are deposited (7). Most of the fall and winter months are spent in a state of deep torpor in which metabolic, heart, and respiratory rates are reduced to <5% of their summer equivalents, and body temperature declines to as low as 0°C, or even below (5). However, all hibernating mammals spontaneously reverse torpor at regular intervals by elevating metabolic rate and employing strictly endogenous mechanisms of heat production including shivering and nonshivering thermogenesis. In the thirteen-lined ground squirrel, Ictidomys tridecemlineatus, elevated metabolic rate and Tb (∼37°C) are maintained in each interbout arousal for ∼10–12 h before dropping again to initiate the next ∼2 wk bout of torpor. Thus, the circannual hibernation rhythm can be viewed as a cycle between summer homeothermy and winter heterothermy, the latter of which is itself a cycle between torpor and arousal (see Fig. 1). These dramatic physiological shifts of hibernation have been postulated to result from intrinsic metabolic cycles (41) that may be revealed by metabolic profiling (42).
Fig. 1.
Body temperature (Tb) of a 13-lined ground squirrel over 10 mo: plasma sampling strategy. x-Axis, time in months; y-axis, intra-abdominal Tb in °C. Stages are described in experimental procedures. Sp1, spring group 1, n = 5; Sp2, spring group 2, n = 7; SA, summer active, n = 9; Ent, entrance, n = 7; LT, late torpor, n = 6; Ar, arousing, n = 6; IBA, interbout aroused, n = 6.
Previous work to measure metabolite changes in hibernators documents a number of alterations in liver (2, 32, 36), brain (21, 40), brown adipose tissue (13), bile (4), and blood (1, 9, 24, 31) that begin to assess the metabolites cycling in association with hibernation. These data suggest a two-switch model for the circannual rhythm of hibernation whereby the torpor-arousal cycle is temporally segregated from the summer-winter cycle such that torpor-arousal occurs only in winter (36). However, because of limitations of previous studies in either the number of metabolites or the number of physiological stages examined, insufficient data are available to address whether intrinsic metabolic cycles underlie the two oscillations of circannual hibernation.
Here we report results from an unbiased gas (GC) and liquid chromatography (LC)-mass spectroscopy (MS)-based metabolomic screen (12, 26, 38) comparing multiple individuals representing seven stages of the hibernator's circannual cycle. From a database containing 1,205 named compounds, 231 were identified in ground squirrel plasma. Quantitative differences among the samples were determined by statistical analyses and a machine learning classification tool. The shifts in metabolite levels were consonant with the physiologic shifts undertaken by the animal during the circannual hibernation rhythm, robustly delineating both a seasonal cycle and an embedded intrawinter cycle of torpor and arousal. A common pattern of metabolites that accumulate or deplete during torpor but are then restored during each interbout arousal provides the first strong supporting evidence for the hypothesis that hibernators rewarm to rebalance levels of metabolites that deplete or accumulate at the low Tb of torpor (28). In addition, these new findings define a biomarker signature for metabolite oscillations throughout the hibernator's year, thereby substantially widening our understanding of how animals orchestrate and survive the extreme physiology of hibernation.
EXPERIMENTAL PROCEDURES
Animals
Procurement.
Animals were purchased from the University of Wisconsin thirteen-lined ground squirrel captive breeding program at Oshkosh. Squirrels were transported in July of 2007 or 2008 to Colorado. Animals were initially kept in 14 h light-10 h dark conditions, which were altered over time to mimic natural light cycles. They were given cat chow ad libitum until late September or early October when they were transferred to an environmental chamber that functions as a hibernaculum. The chamber was maintained at 4°C in constant darkness; food and water were removed after the animals entered torpor but provided in the spring when torpor bouts naturally reduced in length and the time spent euthermic increased.
Monitoring.
Ground squirrels received surgical abdominal implants of radiotelemeters (VM-FH disks, Minimitter) and data loggers (iButton, Embedded Data Systems) in early September. Real-time Tb was recorded every 10 min to enable remote monitoring and collection of tissues with precision at all winter stages of hibernation (Fig. 1). Animals euthanized between early spring and late summer comprised three groups: spring active 1 (Sp1): after spontaneous spring arousal and maintenance of euthermic Tb for >10 days in the dark and cold (4°C) hibernaculum; spring active 2 (Sp2): in late spring/early summer months after displaying absence of torpor for 6–39 days, hibernaculum was warmed during this stage to 18°C; summer active (SA): in summer months (July-August). The last four groups were euthanized based upon Tb assessed by remote telemetry during the winter hibernation season where they were continuously housed in a 4°C hibernaculum in constant darkness: entrance (Ent), entering into torpor with Tb 27–23°C; late torpor (LT), typically after 7–10 days of torpor calculated as 80–95% of previous torpor bout length with Tb 4°C; arousing (Ar), during spontaneous natural arousal from torpor with Tb 7–12°C; interbout aroused (IBA), ∼3 h after reaching euthermic Tb of 35–37°C following a torpor bout and spontaneous natural arousal.
Tissue collection.
Animals were deeply anesthetized with isoflurane; when unresponsive to toe pinch, squirrels were euthanized by exsanguination via cardiac puncture. A 10 ml syringe with 23-gauge needle was internally coated with ACD for this purpose (75 mM Na citrate, 38 mM citric acid, and 124 mM glucose, filter sterilized). After blood was collected, the syringe was inverted, the needle removed, and blood was gently expelled into 2 ml microcentrifuge tubes on ice containing 10 μl/ml ACD. These tubes were inverted, and cells were pelleted for 10 min at 4°C and 8,154 g. The supernatants were transferred to a new tube, divided into aliquots, snap-frozen in liquid nitrogen, and stored at −80°C. Samples were shipped on dry ice to Metabolon (Durham, NC) for metabolomic analysis.
Metabolite Analysis
Plasma samples were prepared for LC and GC separation and MS analysis at Metabolon (Durham, NC). The LC/MS portion of the platform was based on a Waters ACQUITY UPLC and a Thermo-Finnigan LTQ mass spectrometer, which consisted of an electrospray ionization source and linear ion-trap mass analyzer as previously described (12). In brief, the sample extract was split into two aliquots, dried, then reconstituted in acidic or basic LC-compatible solvents, each of which contained 11 or more injection standards at fixed concentrations. One aliquot was analyzed using acidic positive ion optimized conditions, and the other using basic negative ion optimized conditions in two independent injections using separate dedicated columns. Extracts reconstituted in acidic conditions were gradient-eluted using water and methanol both containing 0.1% formic acid, while the basic extracts, which also used water-methanol, contained 6.5 mM ammonium bicarbonate. The MS analysis alternated between MS and data-dependent MS2 scans using dynamic exclusion. The GC/MS analysis was performed as previously described (33). The samples were redried under vacuum desiccation for a minimum of 24 h prior to being derivatized under dried nitrogen using bistrimethyl-silyl-triflouroacetamide. This procedure results in a sample containing free plasma metabolites and any compounds bound noncovalently to carrier proteins. The GC column was 5% phenyl with a temperature ramp from 40 to 300°C in 16 min. Samples were analyzed on a Thermo-Finnigan Trace DSQ fast-scanning single-quadrupole mass spectrometer using electron impact ionization. The instrument was tuned and calibrated daily for mass resolution and mass accuracy. Signatures for each metabolite were identified by matching to a database of 1,205 authentic compound standards (12). Quantitative comparisons of each compound in each sample were based on integrated peak ion counts of the quantification ion peak (typically the largest peak in the spectrum) and were adjusted for minor day-to-day instrument gain drift as described (26). Null values were imputed with the minimum value detected for that compound among all samples based on the assumption that the values were below the level of detection. Representative data for glycerol are provided in Supplemental Fig. S1.1 For simplicity of comparison in the figures, all data for each compound were scaled to the median; i.e., 1.0 on the y-axis represents the median value among all samples in which that compound was detected.
Data Analysis
Unsupervised classification with Random Forests (RF) analysis was done in R (39) using 50,000 trees. The data are being assessed for intrinsic variability, thus the output is unitless apart from the “dimensions” shown in Fig. 2 axes. Hibernation stage-dependent alterations in plasma metabolite concentration were assessed by ANOVA (also in R) followed by Hochberg multiple test correction (22) and then Tukey tests to identify pair-wise changes (plotted in Excel, Microsoft). Hierarchical clustering of all 106 compounds found to have q < 0.05 by ANOVA was scaled and visualized by heat map. Box plots were generated in R; triangle is the mean scaled quantity; bold horizontal line is the median quantity; colored region indicates center 50th percentile; outside horizontal lines indicate 100th percentile; circles represent outliers; x-axis, hibernation stages as delineated in Fig. 1; y-axis, scaled quantity. Metabolites shown in line plots in Figs. 4 and 5 were selected based on Tukey statistical values, e.g., Ent=LT=Ar is defined as Tukey P ≥ 0.05 for Ent compared with LT, LT compared with Ar and Ent compared with Ar. Sp1≠Ent is defined as Tukey P < 0.05 for Ent compared with Sp1. The inclusion rules were as follows: for Fig. 4, B and C: Sp1=Sp2=SA and Ent=LT=Ar=IBA, but Sp1≠Ent, LT, Ar, or IBA; Sp2≠Ent, LT, Ar, or IBA; and SA≠Ent, LT, Ar, or IBA. For Fig. 4, E and F: Sp1=Sp2=SA=Ent=IBA, but LT≠Sp1, Sp2, SA, Ent, or IBA, and Ar≠Sp1, Sp2, SA, Ent, or IBA. Due to variability among the summer groups, they were omitted in the figure, but the statistical parameters were as follows for Fig. 4, H and I: LT=Ar, Ent≠LT or Ar≠IBA and Ent≠Sp1, Sp2, or SA. For Fig. 5B: LT≠ Sp1, Sp2, SA, Ent, Ar, and IBA. For Fig. 5D: Ar≠ Sp1, Sp2, SA, Ent, LT, and IBA. It is noteworthy that although 53 of the 106 significantly altering metabolites did not meet these rigorous patterning criteria, they nevertheless exhibited significant shifts between animals in at least two physiological states. Data for these metabolites are reported in Supplemental Table S1 and Supplemental Fig. S2.
Fig. 2.
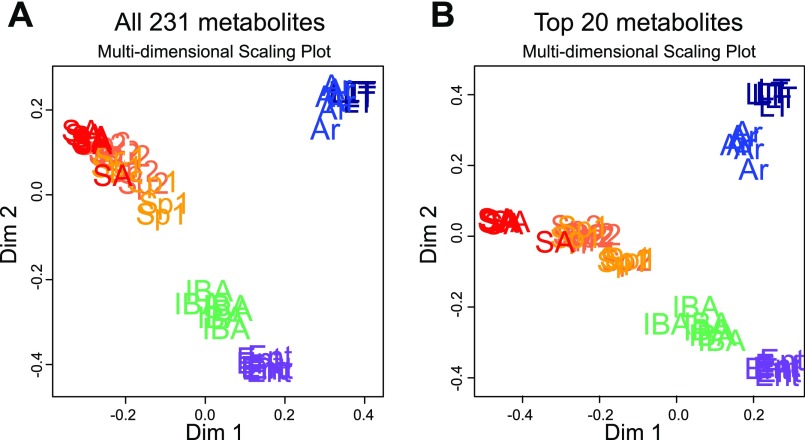
Unsupervised classification of plasma metabolites groups individual animals by physiological status and identifies the metabolites that most strongly contribute to their separation. A: all 231 metabolite compounds were assessed using Random Forests (RF) analysis. B: RF plot using only the top 20 metabolites from A. The top 20 RF metabolic compounds in order of impact from most to least are: urea, allantoin, trans-4-hydroxyproline, N-acetylornithine, methionine, alanine, γ-glutamyltryptophan, 3-hydroxydecanoate, 2-hydroxyisobutyrate, pyridoxate, biliverdin, trigonelline (N-methylnicotinate), kynurenine, cholesterol, propionylcarnitine, glycine, erythronate, creatinine, glycerol, and myristoleate (14:1n5).
Fig. 4.
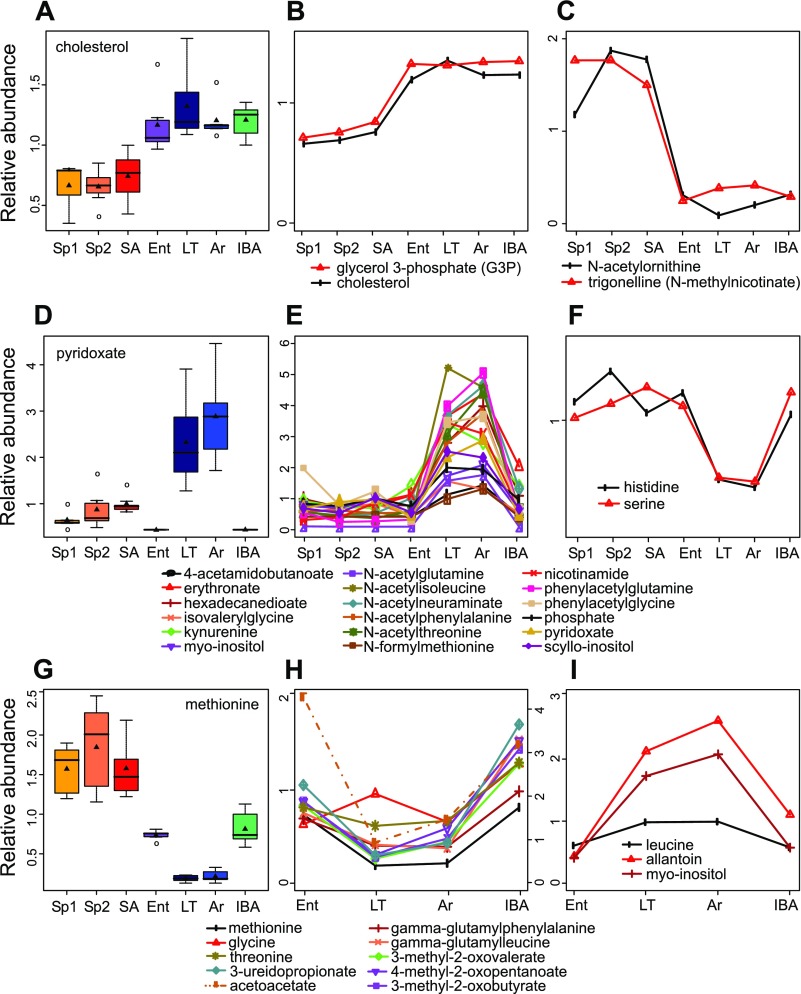
Common patterns in metabolite abundance display seasonal shifts, intrawinter shifts, and a combination of the 2. x-Axes, physiological stage at time of euthanasia; y-axes, relative metabolite abundance. A: the abundance pattern for cholesterol represents a seasonal shift. Four metabolites that reflect the fasting state and transition to lipid metabolism in winter were significantly different from all summer stages to all winter stages, 2 higher in winter (B), and 2 lower (C). See experimental procedures for statistical parameters and box plot features. D: the pattern for pyridoxate represents a common pattern with intrawinter shifts in which winter cold states, LT and Ar, were higher than all other stages (E); these compounds included vitamin B pathway metabolites and modified and unmodified amino acids (AAs); 2 unmodified AAs showed a decrease in LT and Ar relative to all other groups (F). G: methionine demonstrated flux within winter in addition to a seasonal shift. A similar pattern combining seasonal and intrawinter flux was found for additional AAs, degradation products of branched-chain AAs, modified AAs, and a ketone body (acetoacetate, indicated with a broken line and its own scale on the right side y-axis) (H, I). Only metabolites with ANOVA q < 0.05 are shown.
Fig. 5.
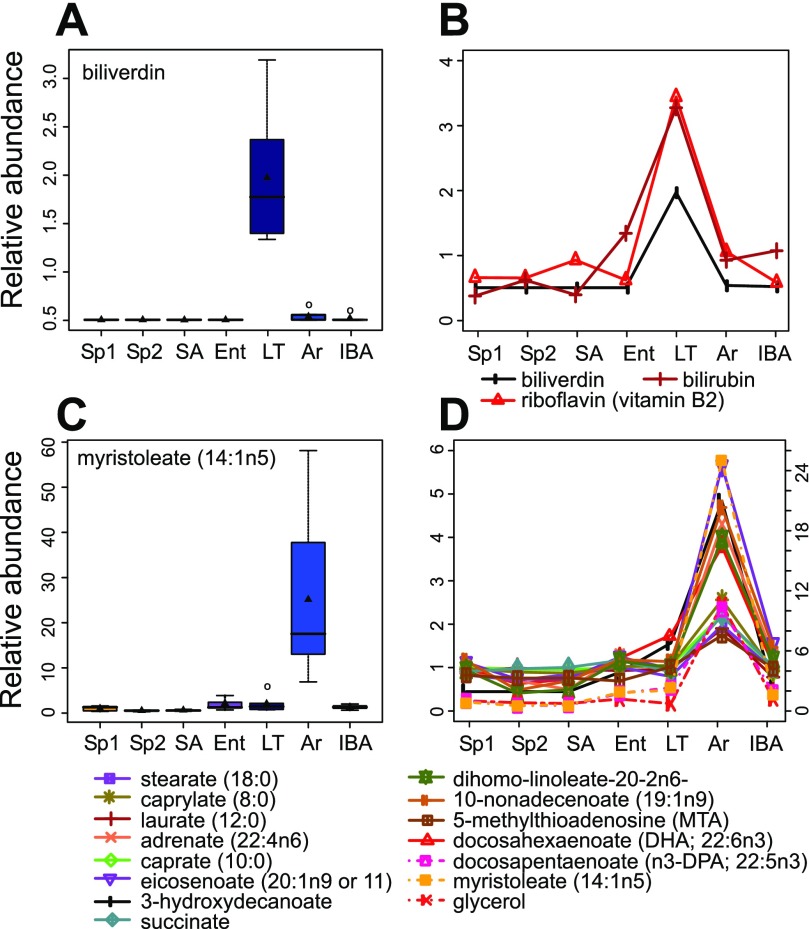
Remaining metabolites accumulate in and deplete from the plasma during torpor and arousing stages. Axes are as in Fig. 4. A: biliverdin accumulates during torpor and rapidly depletes from the plasma upon arousal. In total, 3 compounds (B) involved in heme catalysis and redox balance were significantly higher in LT and shared this pattern. C: the long chain free fatty acid (FFA) myristoleate exemplified the final common metabolite pattern in this dataset; upon initiation of arousal (Tb < 13°C), derivatives of triacylglycerols including glycerol and medium, long, and very long chain FFAs accumulated in the plasma and then depleted by 3 h into IBA (D). Myristoleate, glycerol, and docosapentaenoate are indicated with broken lines and their scale is on the right side y-axis. ANOVA q < 0.05.
RESULTS
A comprehensive understanding of the biochemical underpinnings of hibernation depends on systematic and reproducible sampling of the multiple phenotypic stages that fully represent the natural oscillations of the circannual rhythm. Plasma samples were collected and processed identically to identify metabolites for at least five animals in each sample group encompassing spring emergence (housed conventionally or in the hibernaculum), midsummer, and then four stages from the torpor-arousal cycle of midwinter hibernation (Fig. 1). The samples contained 231 named compounds that were then evaluated for quantitative differences.
Unsupervised Classification of Metabolites
Unsupervised classification of the identified metabolites with RF unambiguously partitioned the animals into three major distinct clusters containing all of the animals representing: 1) the three homeothermic spring and summer groups; 2) both winter groups with low Tb, torpid (LT) and early arousing (Ar); and 3) both winter groups with elevated Tb, IBA and Ent (Fig. 2A). Unsupervised learning is a type of machine learning in which, unlike ANOVA, the sample treatment groups are unlabeled so that clustering and classification in no way depends upon predetermined or biased groupings. Because this analysis was done without selecting compounds or defining biological groups, i.e., the algorithm considered only the abundance of each of the 231 metabolites from each individual, these three groups minimally reflect three dominant metabolite profiles in plasma that underlie circannual hibernation. Repeating the RF analysis using only the top 20 classifiers identified by the initial analysis results in the same general pattern (Fig. 2B). Thus, these compounds provide biomarkers that define the dominant metabolic transitions of circannual hibernation. In order of their influence, the top 20 metabolites are: urea, allantoin, trans-4-hydroxyproline, N-acetylornithine, methionine, alanine, γ-glutamyltryptophan, 3-hydroxydecanoate, 2-hydroxyisobutyrate, pyridoxate, biliverdin, trigonelline (N-methylnicotinate), kynurenine, cholesterol, propionylcarnitine, glycine, erythronate, creatinine, glycerol, and myristoleate (14:1n5). These metabolites are involved in a range of pathways including nitrogen turnover, redox control, amino acid metabolism, and lipid metabolism.
Statistical Analysis
A statistical approach (ANOVA) was used independently to examine the plasma metabolite data after grouping samples by physiological status. Of the 231 compounds assessed, 106 were found to alter significantly among at least two of the seven stages of the hibernator's year, again indicating a dynamic metabolic signature linked to the known physiological states. As seen with the RF analysis, these significantly altering compounds revealed two major distinct shifts when visualized using heat map. One major shift was seasonal, distinguishing the nonhibernating (Sp1, Sp2, and SA) from the hibernating (Ent, LT, Ar, and IBA) stages, and the other major shift was among the winter stages, separating the LT and Ar groups at lower Tbs from the IBA and Ent groups at higher Tbs (Fig. 3). A further evaluation of the number of significant pair-wise differences that separate the groups (Table 1, Tukey P < 0.05) revealed that the two spring groups were indistinguishable, SA was most similar to the two spring groups, IBA was most similar to Ent, and LT was most similar to Ar; these findings mirror, and thus substantiate, the RF groupings. Furthermore, a greater number of metabolite differences were found in comparing LT and Ar to the summer groups than in comparing LT and Ar to the other two winter groups, Ent and IBA. This finding supports the conclusion that the winter warm groups, IBA and Ent, have a distinct winter hibernation metabolic profile. For this to be true, a transition in metabolic signature must occur from euthermic summer animals to euthermic winter animals, a seasonal transition that is not founded on Tb alone.
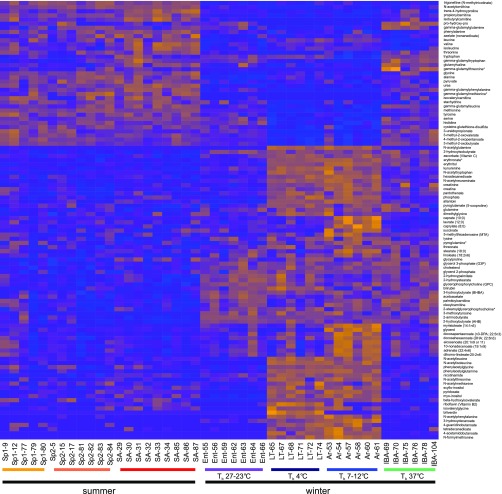
Fig. 3.
Statistical analysis reveals that 13-lined ground squirrel blood plasma metabolites alter significantly with seasonal and intrawinter hibernation cycles. Relative abundances of metabolites as determined by tandem mass spectroscopy (MS/MS) peak integration were analyzed by ANOVA followed by Hochberg multiple test correction. We sorted 106 metabolites (q < 0.05) using hierarchical clustering and visualized them with heat map. Orange represents higher relative metabolite abundance, and blue lower. Labels at bottom provide physiological stage (Fig. 1) and animal number.
Table 1.
A count of pairwise comparisons demonstrates that most differences are seasonal or between the winter cold and warm stages
| Sp2 | SA | Ent | LT | Ar | IBA | |
|---|---|---|---|---|---|---|
| Sp1 | 0 | 10 | 19 | 56 | 65 | 19 |
| Sp2 | 10 | 23 | 66 | 77 | 24 | |
| SA | 38 | 79 | 86 | 36 | ||
| Ent | 42 | 52 | 12 | |||
| LT | 26 | 46 | ||||
| Ar | 60 |
Tukey values (P < 0.05) comparing sample groups were counted for each pair of groups. Sp1, spring active 1; Sp2, spring active 2; SA, summer active; Ent, entrance (into torpor); LT, late torpor; Ar, arousing; IBA, interbout aroused.
Patterns of Plasma Metabolites
Several recurring metabolite patterns were found in these data. Those in which the changes were seasonal (Fig. 4, A–C), intrawinter (Fig. 4, D–F), or some combination of both (Fig. 4, G–I) were prevalent patterns that were apparent in the unsupervised classification and in the statistical analysis. The pattern that reflects a seasonal shift in metabolites is consistent with an altered set point for the homeostatic level of these metabolites between the summer homeothermic and winter heterothermic phases of the circannual rhythm of hibernation. In the simplest form of this pattern, all three summer groups differed from all four winter groups, but there were no differences among individuals within either the three summer or four winter groups (using ANOVA q < 0.05, Tukey P < 0.05; Fig. 4, A–C). Only four metabolites were identified that meet these strict criteria (Fig. 4, B and C), but trends toward this pattern were not uncommon in the dataset (Fig. 3, Supplemental Fig. S2). Cholesterol and glycerol 3-phosphate were higher in the plasma during all of the winter states compared with the summer states, whereas N-acetylornithine and trigonelline were lower during hibernation. Another pattern and the one most frequently observed in the dataset was that in which the plasma metabolite was invariant in all of the groups except LT and Ar (Fig. 4, D–F). Within this pattern, by far the most metabolites accumulated in LT and Ar (Fig. 4E), but two metabolites depleted (Fig. 4F) relative to all other states. This pattern likely reflects an imbalance caused by the low Tb of torpor that is followed by restoration (i.e., a return to homeostasis) during the brief period of euthermic interbout arousal. A cluster of previously unreported modified amino acids dominated this pattern, accumulating in the blood during torpor and then depleting early in IBA. Interestingly, of the 14 proteinogenic free and unmodified amino acids identified here, all but two of them, lysine and glutamine, were lower in winter relative to summer with some variability among the winter stages. A third common pattern observed in the data was a hybrid between the previous two; i.e., all of the winter samples differed from all of the summer samples, but, within winter, LT and Ar also differed from Ent and IBA. This pattern suggests the altered level between summer and winter could not be maintained during the low Tb of torpor but was rapidly restored upon rewarming in IBA (Fig. 4, G–I). As with the second pattern listed above, the metabolites exhibiting this pattern included a number of modified and unmodified amino acids in addition to amino acid precursors, allantoin and a ketone, acetoacetate. Considered together, these patterns reveal what appears to be a seasonal alteration in homeostatic set point for some plasma metabolites, and a prevalence of amino acids and amino acid derivatives that alter significantly with the cycles of torpor and arousal.
Another pattern was clearly demonstrated by biliverdin (Fig. 5A) in which LT was significantly different, and higher, than all other groups. All three compounds, biliverdin, bilirubin, and riboflavin (Fig. 5B), which fit into this pattern, were greatly depleted from the plasma very early in the rewarming stage, and all three are involved in redox regulation (20, 35). The changing levels of these molecules, particularly their rapid depletion at rewarming, are consistent with their playing a central role in ameliorating the potential damage inflicted by oxidative reperfusion upon arousal.
A final pattern in the data was found in a group of 15 metabolites that accumulated in plasma during the initial process of Ar (i.e., before Tb reached 13°C) and then rapidly depleted such that Ar was the only group differing significantly from all of the others (Fig. 5, C and D). Most of these demonstrated an increase between two- and fivefold, but one exceeded 25-fold (myristoleate, Fig. 5C). Significantly, this group comprised medium (6–12 carbons) and long chain (>12 carbons) free fatty acids (FFA), glycerol, and succinate. This pattern is consistent with a rapid and coordinated mobilization of the triacylglycerols stored in white adipocytes to provide energy to fuel arousal.
DISCUSSION
The changes in plasma metabolites that were measured in this study robustly characterize distinct physiological states of the circannual hibernation rhythm; specifically, plasma metabolic profiles define a seasonal shift that distinguishes between summer and winter euthermia and also differentiate among the stages of winter heterothermy. The plasma metabolite profile accurately reflects the physiological state of the animal at the time of sample collection as evidenced by the results of RF analysis. The top 20 metabolites identified by this method are representative of all >200 identified metabolites because they differentiate the states of hibernation in a manner similar to the full dataset and provide biomarkers to distinguish animals based on their physiological state. Here we use the term biomarker to refer to the plasma metabolites that shift quantitatively in abundance during hibernation cycles. Relative measurements from one animal to another of these molecules are capable of distinguishing among six of these seven stages of hibernation. Interestingly, two of the seven sampled groups were not distinguished by plasma metabolite levels; the two homeothermic spring groups, those housed in the cold and dark, Sp1, did not differ from those housed conventionally, Sp2, reflecting the circannual rhythm of hibernation rather than the differing environmental conditions.
These data provide strong support for a proposed two transition (“two switch”) model in which the torpor-arousal cycles that comprise heterothermy are enabled only in the winter phase of a summer-winter cycle (36). The observed segregation of the spring and summer samples from all of the winter samples was predicted by this model, as were clear separations between torpid and aroused hibernators. Because of the increased number of physiological states (seven) and metabolites (231) analyzed in this study, however, many additional insights into the metabolic oscillations underlying the multiple phenotypic transitions of hibernation were revealed, the key findings from this study are summarized in Fig. 6.
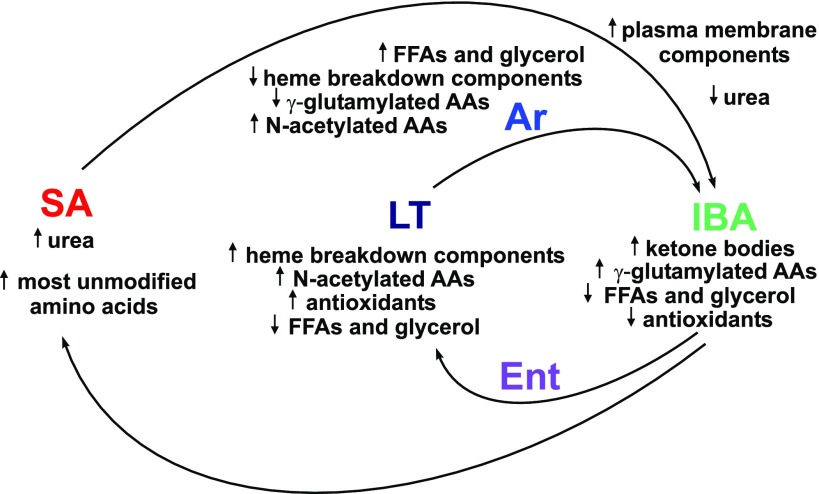
Fig. 6.
Clusters of plasma metabolites shift in abundance with the nested metabolic cycles of hibernation. Sampling points are in color; arrows indicate relative abundance at a particular sampling point.
Approximately half of the measured metabolites altered significantly in at least one of the seven sampled stages during the hibernator's year yet appeared to return to a specific set point during one of the two cycles, or both. For example, plasma methionine (Fig. 4G) was elevated in SA and then decreased in Ent; after further decrease in LT it remains low during arousal then recovers to the Ent level early in IBA. Upon spring emergence from hibernation, once the animals have cycled back into the summer phase of the summer-winter switch, the higher level was restored in the Sp1 and Sp2 animals. This pattern indicates that plasma methionine is maintained at a relatively constant level which is seasonally adjusted to be lower in winter than in summer. Torpor challenges the animal's ability to maintain the winter level (likely because the rates of reactions that produce plasma methionine compared with those that remove it become imbalanced at low Tb), which is then restored only upon a return to euthermia. Related patterns were found for many plasma metabolites, reflecting changing homeostatic set points with either seasonal or torpor-arousal cycles, or both.
A small collection of metabolic compounds changed exclusively with season. For example, a novel winter homeostatic set point in the blood was established for cholesterol and glycerol 3-phosphate; each of these molecules is key in the regulation of lipid metabolism and plasma membrane composition. Other compounds shifted among the winter stages but repeatedly returned to what appeared to be a unique winter set point; some amino acids were in this category such as leucine and methionine. The most abundant pattern was one in which both winter warm groups were similar to summer, but metabolites either depleted or, more often, accrued during a torpor bout and then quickly returned to original levels in IBA. This pattern of accumulation and depletion coordinate with the torpor/arousal cycle harkens back to a long-standing but relatively untested hypothesis that the energetically costly periodic arousals of hibernation are needed to restore metabolic and biochemical homeostasis via mechanisms that only function at euthermic temperatures (28). This hypothesis predicts that molecules that accumulate or deplete during torpor are restored upon arousal. The present data provide robust support for the re-establishment of euthermic homeostatic levels for numerous metabolites during each interbout arousal.
Among the metabolites that accumulated during torpor and were reduced again by the next entrance stage was a cluster of N-acetylated amino acids. A recent study revealed that 84% of human proteins undergo NH2-terminal acetylation (3). Most proteins lose the NH2-terminal methionine by cleavage and are subsequently acetylated (29). When proteins undergo proteolysis, the NH2-terminal residue along with its acetyl adduct is released and circulates freely. Occurrence of particular amino acid residues at the NH2 termini of proteins thus determines the prevalence of the acetylated form by this mechanism; 95% of all N-acetylated termini comprise only a handful of amino acids: N-acetyl serine, alanine, glycine, and methionine. The next most common are valine and aspartate (34). In the present dataset of hibernator blood plasma, despite being listed in the metabolite database, there were no measurable amounts of N-acetylated serine, alanine, valine or aspartate. The accumulation of these N-acetylated amino acids has been associated with inherited pathology that results in neurological damage manifested as seizures, mild mental retardation or autism. Their accumulation in urine results from a deficiency of aminoacylase 1, a kidney enzyme that catabolizes free N-acetylated amino acids into acetic acid and the free amino acid (37). In contrast, the N-acetylated amino acids that accumulate in hibernators differ markedly; only methionine occurs in both lists. Instead, along with methionine, N-acetylated glutamine, phenylalanine, tryptophan, isoleucine, leucine, and threonine significantly altered during torpor. Hence we conclude that the accumulation of these particular acetylated amino acids in torpor reflects a mechanism distinct from their accrual due simply to cleavage of the modified NH2-terminal amino acids of proteins. Instead, there appears to be a selection for the modified form of a subset of amino acids that are not normally enriched in the NH2 termini of proteins. Six of these seven amino acids are essential amino acids, all except glutamine; the accumulation of their acetylated forms raises the intriguing possibility of a salvage mechanism (27) that spares and recycles essential amino acids for use in new protein synthesis during the long winter fast. This is the first report of modified essential amino acids that alter in concordance with fasting or starvation physiology, although previous efforts have been made to discover the fate of the diet-derived essential amino acids in human long-term fasting (14). In contrast, other than the fact that most of them are lower in one of the winter stages than in summer (Fig. 3 and Supplemental Fig. S2), the unmodified forms demonstrate highly variable levels and lack a consistent pattern, results that have been reported previously based on a targeted approach (24). Discovery of the coordinated changes in clusters of modified amino acids may have been particularly enabled in this study because the unique combination of the broad screening strategy with the multiple sampling points allowed measurement and identification of unanticipated modified forms that accumulated at the low Tb of torpor.
A second unanticipated cluster of modified amino acids that altered with the torpor and arousal cycles were the gamma-glutamylated (γ-glu) amino acids (Figs. 3 and 4H and Supplemental Fig. S2). With the exception of glutamine, all of the amino acids that carried this modification were also essential amino acids. These were at low levels during torpor and arousing stages and reached their highest levels in plasma by IBA. These modified amino acids are derived by transfer of the γ-glutamyl moiety from glutathione and glutathione conjugates onto extracellular free amino acids by the enzyme γ-glutamyl transpeptidase as part of the γ-glutamyl cycle. Several tissues are known to express the enzyme, including endothelial cells at the blood brain barrier. The γ-glutamyl modification confers uptake of these amino acids into the endothelial cells in a selective manner that is distinct from uptake of free amino acids. Once inside the cells, the γ-glutamyl modification is removed by γ-glutamyl cyclotransferase to produce pyroglutamate (5-oxoproline) and the associated free amino acid (18). Pyroglutamate stimulates uptake of amino acids by way of Na+-dependent transporters, thereby controlling amino acid content and availability. Because pyroglutamate is converted to glutamate, the latter is also delivered by this mechanism. These metabolites and pathways may be particularly significant in the brains of hibernators for several reasons. First, glutamate can prevent toxic nitrogen accumulation (36); alternatively, it can be recycled to glutathione to protect against oxidative damage (8). The final and most intriguing possibility is that higher levels of glutamate maintain torpor by activating N-methyl-d-aspartate-type glutamatergic receptors that actively inhibit arousal (17). Thus, the depletion of γ-glu amino acids as observed in our late torpor animals may result in reduced glutamate receptor activation and stimulate arousal from torpor.
Also included among the metabolites that accumulate and deplete with torpor and arousal is allantoin, a derivative of urate that is a potent antioxidant. Although this metabolite was consistently high on the list of metabolites that are capable of separating the ground squirrels by physiological stage in this experiment, we were unable to find previous mention of it in association with the phenotype of hibernation, although its precursor urate was assessed during induced arousal in arctic ground squirrels and plasma levels demonstrated a similar pattern to our results here with allantoin (40). The enzyme urate oxidase or uricase acts to convert urate to allantoin in most mammals other than the great apes, resulting in an accumulation of allantoin that is excreted in the urine. The role and fate of allantoin in hibernators is not known, but kidney function hyperactivates during IBA from a dormant state during torpor (Ref. 23 and direct observation), consistent with depletion of this factor from the plasma in IBA.
In addition to the strong patterns of seasonal resetting and torpor-arousal recovery of homeostasis, this dataset revealed two clusters of metabolites with clear functional implications for hibernation. One of these was a group of three compounds that accumulated during torpor but rapidly diminished early in the arousal process, while the animals were still at low Tb. Biliverdin and bilirubin are heme catabolites, and all three compounds have been implicated in redox regulation (35), riboflavin primarily in the context of fatty acid oxidation (20). It appears likely that they are taken up rapidly by other tissues, including liver which demonstrated a perfectly reciprocal pattern of both flavin and biliverdin levels in the same species (32). Similar to our results, bilirubin was found elevated during torpor in plasma in jerboas (30) and in bile of torpid golden-mantled ground squirrel (4). Another potent antioxidant, ascorbate, also accumulated in 13-lined ground squirrel plasma during torpor and depleted upon arousal (Supplemental Fig. S2) as has been noted previously in this species and in the arctic ground squirrel (8, 40). The antioxidant properties of these molecules may be used in the liver in this protective role where massive oxidation of fatty acids results in production of oxygen radicals as the animals rewarm. The same may be true for other organs, including the brain, that exploit fatty acid moieties as their primary hibernation fuel.
The second cluster with a more obvious functional role was that of FFAs and glycerol that are released into the plasma in what appears to be a wave of triacylglycerol catabolism, most likely in white adipose tissue upon initiation of rewarming; these triacylglycerol constituents were found at very low levels in the plasma in LT, high in Ar, and low again by IBA. This early rise of glycerol and FFAs during spontaneous natural arousal in 13-lined ground squirrels matches results obtained in an artificially induced arousal of arctic ground squirrels (16) and is consistent with systemic use of medium and long chain FFAs as fuel for hibernation. The energy stored in FFAs may be recovered by β-oxidation to generate ATP or to be converted to heat via nonshivering thermogenesis in brown adipose tissue; the latter process is important for rewarming from torpor and is also now recognized as having therapeutic implications for human obesity (6). In addition, two-carbon acetyl group derivatives of these FFAs are the building blocks for hepatic ketogenesis (10, 11), which provides ketone bodies for use in target tissues including heart and brain (1, 25). We also found a 10-fold increase in the plasma reservoir of glycerol at arousing. During hibernation, the building blocks for gluconeogenesis are scarce because amino acids are spared (11), thus this rush of glycerol provides a valuable source of three-carbon units for the gluconeogenesis that occurs during each interbout euthermic period (16, 36).
Our findings complement, validate, and expand those of a recent study (31) that examined plasma metabolites from the same species but in fewer stages: spring, LT, and IBA. As with our study, about half of the measured metabolites altered among the hibernation stages, and of the 25 metabolites identified in their study, those that were also found in ours yielded the same abundance trends. Specifically, tyrosine, methionine, carnitine, propionylcarnitine, butyrylcarnitine, and pantothenic acid correlated with our data for spring, LT, and IBA measurements. In addition, we recovered numerous modified versions of most of these molecules including γ-glutamyl, N-acetyl, methoxy, isovaleryl, N-formyl, oleoyl, and palmitoylated forms (see Fig. 3 and Supplemental Fig. S2) that were not identified previously. While their precise roles remain to be elucidated, it is clear from the present data that modified amino acids, namely N-acetylated and γ-glu amino acids, are specifically linked to the torpor-arousal cycles of hibernation. The additional sampling times in the present study serve to complete the cyclical pattern within winter as well as to demarcate the summer-winter cycle.
Hibernators differ profoundly from other mammals in that they are able to establish dramatically differing homeostatic set points, most conspicuously illustrated by their defense of a dynamic Tb during the torpor-arousal cycle (15, 19). The groups of compounds mobilizing in a concerted fashion as revealed by this study speak to tightly controlled mechanisms that act together to achieve broad homeostatic control during the physiological extremes of hibernation. We identified a number of robust hibernation biomarkers that alter with season and winter stage. Our results also clearly demonstrate clusters of molecules whose levels are re-established during the brief euthermic periods that punctuate torpor bouts, a finding that strongly supports the predicted but undemonstrated necessity of recurring winter rewarming to mobilize metabolites and re-establish systemic homeostasis. Numerous challenges to homeostasis (e.g., obesity, insulin resistance, hypothermia, ischemia-reperfusion) that are considered pathological in humans occur naturally and reversibly in a hibernator. Recapitulating various aspects of hibernation will be useful in human medicine but will only be possible with a more complete understanding of the molecular components that define and drive its cycles.
GRANTS
This work was supported by National Institutes of Health Grants 5T15LM-009451 to A. Karimpour-Fard, R01LM-008111 and R01LM-009254 to L. E. Hunter, and R01HL-089049 to S. L. Martin.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Martin laboratory for assistance with the animals and helpful comments on the manuscript, also D. Alexander at Metabolon, Inc. for helpful communication and preparation of Supplemental Fig. S1.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Andrews MT, Russeth KP, Drewes LR, Henry PG. Adaptive mechanisms regulate preferred utilization of ketones in the heart and brain of a hibernating mammal during arousal from torpor. Am J Physiol Regul Integr Comp Physiol 296:R383–R393, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Armstrong C, Staples JF. The role of succinate dehydrogenase and oxaloacetate in metabolic suppression during hibernation and arousal. J Comp Physiol [B] 180:775–783, 2010. [DOI] [PubMed] [Google Scholar]
- 3. Arnesen T, Van Damme P, Polevoda B, Helsens K, Evjenth R, Colaert N, Varhaug JE, Vandekerckhove J, Lillehaug JR, Sherman F, Gevaert K. Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans. Proc Natl Acad Sci USA 106:8157–8162, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baker JA, van Breukelen F. Bile constituents in hibernating golden-mantled ground squirrels (Spermophilus lateralis). Comp Hepatol 8:2, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barnes BM. Freeze avoidance in a mammal: body temperatures below 0°C in an arctic hibernator. Science 244: 1593–1595, 1989. [DOI] [PubMed] [Google Scholar]
- 6. Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmuller A, Gordts PL, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J. Brown adipose tissue activity controls triglyceride clearance. Nat Med 17:200–205, 2011. [DOI] [PubMed] [Google Scholar]
- 7. Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev 83:1153–1181, 2003. [DOI] [PubMed] [Google Scholar]
- 8. Drew KL, Osborne PG, Frerichs KU, Hu Y, Koren RE, Hallenbeck JM, Rice ME. Ascorbate and glutathione regulation in hibernating ground squirrels. Brain Res 851:1–8, 1999. [DOI] [PubMed] [Google Scholar]
- 9. Drew KL, Tøien Ø, Rivera PM, Smith MA, Perry G, Rice ME. Role of the antioxidant ascorbate in hibernation and warming from hibernation. Comp Biochem Physiol C Toxicol Pharmacol 133:483–492, 2002. [DOI] [PubMed] [Google Scholar]
- 10. Epperson LE, Dahl TA, Martin SL. Quantitative analysis of liver protein expression during hibernation in the golden-mantled ground squirrel. Mol Cell Proteomics 3:920–933, 2004. [DOI] [PubMed] [Google Scholar]
- 11. Epperson LE, Rose JC, Carey HV, Martin SL. Seasonal proteomic changes reveal molecular adaptations to preserve and replenish liver proteins during ground squirrel hibernation. Am J Physiol Regul Integr Comp Physiol 298:R329–R340, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 81:6656–6667, 2009. [DOI] [PubMed] [Google Scholar]
- 13. Fedotcheva NI, Litvinova EG, Kamzolova SV, Morguno IG, Amerkhanov ZG. Mitochondrial metabolites in tissues as indicators of metabolic alterations during hibernation. Cryo Lett 31:392–400, 2010. [PubMed] [Google Scholar]
- 14. Felig P, Owen OE, Wahren J, Cahill GF., Jr Amino acid metabolism during prolonged starvation. J Clin Invest 48:584–594, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Florant GL, Heller HC. CNS regulation of body temperature in euthermic and hibernating marmots (Marmota flaviventris). Am J Physiol Regul Integr Comp Physiol 232:R203–R208, 1977. [DOI] [PubMed] [Google Scholar]
- 16. Galster WA, Morrison PR. Gluconeogenesis in arctic ground squirrels between periods of hibernation. Am J Physiol 228:325–330, 1975. [DOI] [PubMed] [Google Scholar]
- 17. Harris MB, Milsom MK. Is hibernation facilitated by an inhibition of arousal? In: Life in the Cold, Eleventh International Hibernation Symposium edited by Heldmaier G, Klingenspor M. Berlin: Springer, 2000, p. 241–250. [Google Scholar]
- 18. Hawkins RA, O'Kane RL, Simpson IA, Vina JR. Structure of the blood-brain barrier and its role in the transport of amino acids. J Nutr 136:218S–226S, 2006. [DOI] [PubMed] [Google Scholar]
- 19. Heller HC, Colliver GW, Bread J. Thermoregulation during entrance into hibernation. Pflügers Arch 369:55–59, 1977. [DOI] [PubMed] [Google Scholar]
- 20. Henriques BJ, Olsen RK, Bross P, Gomes CM. Emerging roles for riboflavin in functional rescue of mitochondrial beta-oxidation flavoenzymes. Curr Med Chem 17:3842–3854, 2010. [DOI] [PubMed] [Google Scholar]
- 21. Henry PG, Russeth KP, Tkac I, Drewes LR, Andrews MT, Gruetter R. Brain energy metabolism and neurotransmission at near-freezing temperatures: in vivo (1)H MRS study of a hibernating mammal. J Neurochem 101:1505–1515, 2007. [DOI] [PubMed] [Google Scholar]
- 22. Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75:4, 1988. [Google Scholar]
- 23. Hong SK. Renal function during hypothermia and hibernation. Am J Physiol 188: 137–150, 1957. [DOI] [PubMed] [Google Scholar]
- 24. Klain GJ, Whitten BK. Plasma free amino acids in hibernation and arousal. Comp Biochem Physiol 27:617–619, 1968. [DOI] [PubMed] [Google Scholar]
- 25. Krilowicz BL. Ketone body metabolism in a ground squirrel during hibernation and fasting. Am J Physiol Regul Integr Comp Physiol 249:R462–R470, 1985. [DOI] [PubMed] [Google Scholar]
- 26. Lawton KA, Berger A, Mitchell M, Milgram KE, Evans AM, Guo L, Hanson RW, Kalhan SC, Ryals JA, Milburn MV. Analysis of the adult human plasma metabolome. Pharmacogenomics 9:383–397, 2008. [DOI] [PubMed] [Google Scholar]
- 27. Lindner HA, Tafler-Naumann M, Rohm KH. N-acetylamino acid utilization by kidney aminoacylase-1. Biochimie 90:773–780, 2008. [DOI] [PubMed] [Google Scholar]
- 28. Lyman CP, Willis JS, Malan A, Wang LCH. Hibernation and Torpor in Mammals and Birds. New York: Academic Press, 1982, p. 317. [Google Scholar]
- 29. Martinez A, Traverso JA, Valot B, Ferro M, Espagne C, Ephritikhine G, Zivy M, Giglione C, Meinnel T. Extent of N-terminal modifications in cytosolic proteins from eukaryotes. Proteomics 8:2809–2831, 2008. [DOI] [PubMed] [Google Scholar]
- 30. Mountassif D, Kabine M, Latruffe N, El Kebbaj MS. Prehibernation and hibernation effects on the d-3-hydroxybutyrate dehydrogenase of the heavy and light mitochondria from liver jerboa (Jaculus orientalis) and related metabolism. Biochimie 89:1019–1028, 2007. [DOI] [PubMed] [Google Scholar]
- 31. Nelson CJ, Otis JP, Carey HV. Global analysis of circulating metabolites in hibernating ground squirrels. Comp Biochem Physiol D 5:265–273, 2010. [DOI] [PubMed] [Google Scholar]
- 32. Nelson CJ, Otis JP, Martin SL, Carey HV. Analysis of the hibernation cycle using LC-MS-based metabolomics in ground squirrel liver. Physiol Genomics 37:43–51, 2009. [DOI] [PubMed] [Google Scholar]
- 33. Ohta T, Masutomi N, Tsutsui N, Sakairi T, Mitchell M, Milburn MV, Ryals JA, Beebe KD, Guo L. Untargeted metabolomic profiling as an evaluative tool of fenofibrate-induced toxicology in Fischer 344 male rats. Toxicol Pathol 37:521–535, 2009. [DOI] [PubMed] [Google Scholar]
- 34. Polevoda B, Sherman F. Nalpha-terminal acetylation of eukaryotic proteins. J Biol Chem 275:36479–36482, 2000. [DOI] [PubMed] [Google Scholar]
- 35. Schipper HM, Song W, Zukor H, Hascalovici JR, Zeligman D. Heme oxygenase-1 and neurodegeneration: expanding frontiers of engagement. J Neurochem 110:469–485, 2009. [DOI] [PubMed] [Google Scholar]
- 36. Serkova NJ, Rose JC, Epperson LE, Carey HV, Martin SL. Quantitative analysis of liver metabolites in three stages of the circannual hibernation cycle in 13-lined ground squirrels by NMR. Physiol Genomics 31:15–24, 2007. [DOI] [PubMed] [Google Scholar]
- 37. Sommer A, Christensen E, Schwenger S, Seul R, Haas D, Olbrich H, Omran H, Sass JO. The molecular basis of aminoacylase 1 deficiency. Biochim Biophys Acta 1812:685–690, 2011. [DOI] [PubMed] [Google Scholar]
- 38. Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 457:910–914, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 39. Team RDC. R: A Language and Environment for Statistical Computing. http://www.R-Project.org.
- 40. Tøien Ø, Drew KL, Chao ML, Rice ME. Ascorbate dynamics and oxygen consumption during arousal from hibernation in Arctic ground squirrels. Am J Physiol Regul Integr Comp Physiol 281:R572–R583, 2001. [DOI] [PubMed] [Google Scholar]
- 41. Tu BP, McKnight SL. Metabolic cycles as an underlying basis of biological oscillations. Nat Rev Mol Cell Biol 7:696–701, 2006. [DOI] [PubMed] [Google Scholar]
- 42. Tu BP, Mohler RE, Liu JC, Dombek KM, Young ET, Synovec RE, McKnight SL. Cyclic changes in metabolic state during the life of a yeast cell. Proc Natl Acad Sci USA 104:16886–16891, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.