Abstract
Skin and soft tissue infections (SSTIs) are the second most common infection encountered in hospitals. Management decisions have become increasingly complex due to the prevalence of resistant pathogens, the wide array of licensed antimicrobials and the availability of potent oral agents and of out-patient parenteral antibiotic therapy. Daptomycin is one of the newer therapeutic agents licensed for complex SSTI management. Rapid cidality, good soft tissue penetration, once daily IV bolus administration and activity against resistant Gram-positive infections make daptomycin an attractive option both in hospitalized and community treated patients. A comprehensive review of the evidence for and experience with daptomycin and its use in SSTIs is presented.
Keywords: SSTI, MRSA, OPAT, outpatient parenteral antibiotic therapy, lipopeptide
Introduction
Daptomycin is the first cyclic lipopeptide and many of its qualities favor its use in complicated skin and soft tissue infections (cSSTIs). It was approved at a dose of 4 mg/kg for this indication by the Food and Drug Administration (FDA) in the United States in 2003 and subsequently in Europe in 2006.1 Daptomycin also has approval for use in Staphylococcus aureus bacteremia and right-sided endocarditis at a dose of 6 mg/kg. Beiras-Fernandez et al have provided a fuller review of daptomycin’s other clinical applications previously in this journal.2 Herein we review published data and experience with daptomycin in cSSTI.
Skin and soft tissue infections: clinical features and microbiology
Skin and soft tissue infections (SSTIs) can be defined as a suppurative microbial invasion of the epidermis and subcutaneous tissues that induce either a local or systemic host response. SSTIs are characterized by induration, erythema, warmth and pain or tenderness3 and range from mild self-limiting furunculosis to life-threatening necrotizing fasciitis.
Complicated SSTIs (cSSTIs) are those either involving deep soft tissue, or requiring significant surgical intervention (such as infected ulcers, burns, and major abscesses), or those in which a significant underlying disease state complicates the response to treatment (for example diabetes mellitus, obesity, immune deficiency, or underlying venous or arterial insufficiency).4 Various severity stratifications have been developed including one by Eron et al,3 forming the basis of the UK and CREST guidelines, and one by Ki and Rotstein in Canada.5 Such classifications are designed to alert the clinician to the level of care required and the need for ancillary therapies, as well as guiding the choice and route of administration of antibiotic therapy, but they remain to be validated. A recent retrospectively validated severity classification, using an adaptation of the Eron classification, stratifies patients with SSTI based on the presence of the systemic inflammatory response and the physiological standardized early warning score (SEWS) and suggests that the presence of co-morbidities is less significant in predicting outcome.6
The main etiological agents implicated in SSTIs are the Gram-positive organisms, Staphylococcus aureus (S. aureus), and the beta-hemolytic streptococci (Groups A, B, C and G). Methicillin-resistant S. aureus (MRSA) infections have risen in prominence over the last 20 years, comprising 59% of S. aureus isolates in a recent study in the USA7 and >10% of isolates in 19 out of the 28 countries in the 2009 European Antimicrobial Resistance Survey.8 The 2 distinct epidemiological forms of MRSA, community acquired (CaMRSA) and healthcare associated (HaMRSA) have quite different clinical features, largely as a consequence of the presence of the Panton Valentine leucocidin toxin in CaMRSA (Table 1). Increasingly however, considerable clinical overlap has been observed as HaMRSA presents in the community and CaMRSA emerges and spreads in healthcare facilities. Local prevalence of MRSA is not only important from the infection control perspective but also the empirical choice of antibiotic for SSTI. UK MRSA guidelines state that if the local prevalence of MRSA exceeds 10% of S. aureus isolates, then empirical treatment of a suspected S. aureus infection (including SSTI) should include anti-MRSA activity.9
Table 1.
Clinical and epidemiological differences between healthcare associated and community acquired MRSA infection
| Healthcare associated MRSA infection | Community acquired MRSA infection | |
|---|---|---|
| Prior hospitalizations/healthcare contact | Yes | No |
| History of prior antibiotic use | Yes | No |
| Chronic medical conditions, eg, diabetes mellitus, chronic kidney disease, vascular disease, decubitus ulcers | Yes | No |
| Contact sport, or living/working in crowded and/or unsanitary conditions | No | Yes |
| Men who have sex with men | No | Yes |
| Surgical site infections | Yes | No |
| Furuncles, boils and abscesses (recurrent) | Not typically | Typical |
| Severe necrotizing pneumonia | No | Yes |
| Panton Valentine leucocidin toxin producing | No | Yes |
| Antimicrobial resistance | Multidrug resistance | Beta-lactam resistance alone |
Antibiotic therapy for SSTI
Beta lactam antibiotics, in particular the penicillinase stable penicillins (flucloxacillin and cloxacillin) remain the mainstay of treatment for suspected streptococcal and methicillin-sensitive S. aureus infections. Combination therapy with flucloxacillin and benzyl penicillin, although still widely practiced, is outdated.10 In proven penicillin-sensitive infection, rationalization to benzyl penicillin remains appropriate. Addition of intravenous clindamycin in rapidly progressive infections is advised as beta lactam antibiotics may be less effective in the static growth phase as characterized by severe streptococcal infections. Additional Gram-negative cover may also be considered in severe hospital-associated cSSTIs.
Vancomycin, discovered more than 50 years ago, has been the mainstay of therapy in MRSA infections and for patients intolerant or allergic to the beta lactams. Emerging data however suggest slower bacterial clearance and poorer clinical response in vancomycin-treated patients with methicillin-sensitive S. aureus (MSSA infection) compared to those treated with beta lactam agents.11 There are also increasing reports of intermediate vancomycin resistance by a variety of mechanisms.12 These factors, as well as concerns over potential vancomycin toxicity, have led to the development of new anti-MRSA agents such as the oxalodindiones (linezolid), new beta-lactam agents (eg, ceftobiprole and ceftaroline), new glycopeptides (dalbavancin and telavancin), the glycylcycline tigecycline and daptomycin. In addition there is renewed interest in older agents with anti-MRSA activity such as trimethoprim-sulfamethoxozole, clindamycin, sodium fucidate and tetracyclines, particularly in the context of CaMRSA. Of all these agents, linezolid and daptomycin have the largest evidence base supporting their efficacy and safety in cSSTIs.13–16 Linezolid is highly effective in SSTI therapy and has an advantage over other agents used in severe SSTI in that it has an oral formulation which facilitates early IV to oral switch and the potential for earlier hospital discharge. Myelo- and mitochondrial toxicity, along with various drug interactions can reduce its utility, particularly when longer term therapy is required.17 Evidence for daptomycin use in SSTI, is discussed below.
Pharmacology of daptomycin
Pharmacodynamics
Daptomycin demonstrates rapid, concentration-dependent, bactericidal activity in vitro against susceptible and resistant Gram-positive cocci, including MRSA, glycopeptide- and linezolid-resistant S. aureus and vancomycin-resistant enterococcal species.18,19 It has a novel mode of action, inducing cell death by calcium-dependent efflux of potassium following insertion of its lipophilic tail into the cell membrane.20 It shows a strong post-antibiotic effect against S. aureus, and in contrast to the beta lactam antibiotics maintains its activity against bacteria in stationary-phase growth.21
Daptomycin has been shown, in experimentally induced blister fluid in healthy volunteers, to have 68% dermal penetration as measured by the ratio under the concentration-time curve over 24 hours.22 A case report describing daptomycin concentrations in synovial fluid shows similar levels of tissue penetration, with synovial daptomycin levels found to be 70% of those in the serum.23 Furthermore, a recent pharmacokinetic study of daptomycin 6 mg/kg in 10 patients with diabetic foot infection found free plasma concentrations equilibrating completely with soft tissue and metatarsal bone within 3 hours of the start of a 30 minute infusion, and this was not affected by inflammation.24
Daptomycin has no Gram-negative activity, and although there are some data showing in vitro activity against anaerobic Gram-positive organisms, this is difficult to assess due to lack of clinical data to support breakpoints.21 This highlights the need to consider additional antimicrobials when used for cSSTIs where suspicion of mixed infection is high, eg, in necrotic diabetic foot infections.
Daptomycin is highly protein bound (91%) and renally excreted. Normally given as a once daily bolus according to patient weight, it is dosed 48 hourly in patients with a creatinine clearance (CrCl) of <30 mL/minute25 or following dialysis.
Elevation in creatinine phosphokinase (CPK) and associated myopathy was frequently seen in early clinical studies when the daptomycin was administered via multiple daily injections. CPK elevation is rare in short term treated patients receiving 4 mg/kg.15,26 In the Fowler et al study of daptomycin use in bacteremia and endocarditis where it was used at a dose of 6 mg/kg/day, CPK elevations were seen in 6.7% of the daptomycin treated group, but led to discontinuation of therapy in only 3 of the 120 patients (2.5%).27 CPK levels should be monitored weekly, or more often in those with myalgia, or concomitant renal failure, or when drugs associated with elevated CPK levels and myopathy are co-administered.21 The FDA has also recently published a drug safety communication highlighting the potential for developing eosinophilic pneumonia during treatment with daptomycin.28 The pharmacokinetics, safety and efficacy in children have not been established and are under investigation.29 It is pregnancy category B.
Daptomycin’s potential for true pharmacokinetic interactions is low as it does not undergo significant metabolism in vivo, and does not induce or inhibit the cytochrome P450 pathway.1 Although not a drug–drug interaction or side-effect per se, daptomycin may cause a spurious rise in the measured prothrombin time due to an interaction with some test reagents. This can lead to difficulties in therapeutic monitoring for warfarin. The effect can be minimized by drawing the international normalized ratio (INR) blood sample just before the daptomycin is given30,31 or by using alternative reagents.
Review of comparative clinical studies assessing daptomycin use for cSSTIs (Tables 2 and 3)
Table 2.
Characteristics of Daptomycin comparative SSTI studies
| Study | Design | Patient characteristics | Daptomycin (dose, treatment duration) | Comparator (type, dose, treatment duration) |
|---|---|---|---|---|
| Arbeit15 | 2 multicenter evaluator-blinded RCTs | N = 1092, adults with Gram-positive cSSTIs requiring hospitalization and IV antibiotics for ≥4 days | 4 mg/kg IV once daily for 7–14 days | Vancomycin IV 1 g bd for 7–14 days or penicillinase-resistant penicillin IV 4–12 g IV q.d in equally divided doses |
| Davis36 | Prospective open label | N = 56, hospitalized adults, cSSTIs at risk of MRSA, prospectively enroled; 212 historical controls treated with vancomycin | 4 mg/kg IV once daily for 3–14 days | Vancomycin IV dosed according to trough concentrations ≥3 days switched to semi-synthetic penicillin in absence of MRSA infection |
| Katz35 | Multicenter, semi-single blinded RCT | N = 100, adults with cSSTIs at risk of MRSA, requiring IV antibiotics | 10 mg/kg IV once daily for 4 days | Vancomycin IV 1 g bd or semi-synthetic penicillin IV 2 g q4h for ≤14 days |
| Pertel34 | Multicenter, evaluator-blinded RCT | N = 103, adults with SSTI requiring hospitalization/iv antibiotics | 4 mg/kg IV once daily for ≤14 days | Vancomycin IV standard doses for 7–14 days |
| Gollnick33 | Phase IIIb multicenter RCT | N = 189, adults with cSSTI requiring hospitalization | 4 mg/kg once daily for 4–14 days | IV Vancomycin or Teicoplanin for 4–14 days |
Abbreviations: CE, clinically evaluable; ITT, intent-to-treat (all patients who received one or more doses of study medication); RCT, randomized controlled trial; IV, intravenous; cSSTI, complicated skin and soft tissue infections.
Table 3.
Summary of outcomes in published comparative studies of daptomycin in patients with cSSTIs
| Study | Median duration of therapy with study drug (days) | Clinical success for CE population, n/N (%) | Clinical success in patients with MRSA infections | Patients with treatment-related AEs, n/N (%) | ||||
|---|---|---|---|---|---|---|---|---|
| D | C | D | C | D | C | D | C | |
| Arbeit15 | Not known | Not known | 372/446 (83) | 384/456 (84) | 21/28 (75) | 25/36 (69) | 94/534 (18) | 119/558 (21) |
| Phase III multicenter RCTs | ||||||||
| Davis36 | 4 | 7 | 41/53 (77) | 89/212 (42) | 15/15 (100) | 30/30 (100) | 0/56 (0) | 0/212 (0) |
| Prospective open-label, vs historical controls | ||||||||
| Katz35 | 4a | 8a | 32/39 (82) | 37/39 (95) | 24/31 (77) | 27/28 (96) | 20/48 (42) | 11/48 (23) |
| Multicenter RCT | ||||||||
| Pertel34 | 6.1* | 6.2* | 47/47 (100) | 46/47 (98) | NR | NR | 3/50 (6) | 1/51 (2) |
| Multicenter RCT | ||||||||
| Gollnick33 | 8∼ | 7∼ | 53/58 (91.4) | 41/47 (87.2) | NR | NR | 55/97 (56.7) | 51/92 (55.4) |
| Phase IIIb Multicenter RCT | ||||||||
Notes:
Study designed to assess outcomes in a group of patients receiving high-dose daptomycin for 4 days versus comparator for ≤14 days;
Mean length of IV therapy
IV therapy was for at least 4 days.
Abbreviations: CE, clinically evaluable; MRSA, Methicillin-resistant Staphylococcus aureus; AE, adverse events; NR, not recorded; NA, not applicable.
The initial data supporting the use of Daptomycin 4 mg/kg/day in cSSTIs came from two Phase III randomized, investigator blinded, controlled clinical trials, comparing it with vancomycin or a semi-synthetic penicillin. The study population was adults (mean age 51) with cSSTIs who were judged to require hospitalization and parenteral antibiotics for ≥96 hours.15 In total, 1092 patients were included across 139 sites in the USA, Europe, South Africa, Australia and Israel between 1999 and 2001. Exclusion criteria included minor infections, third-degree burns, known bacteremia at enrolment, concomitant infection at another site (osteomyelitis, septic arthritis, or endocarditis) or a requirement for curative surgery (eg, amputation). The primary efficacy end point was the non-inferiority of daptomycin to the comparator in clinical success (resolution of signs and symptoms such that no further antibiotic therapy was required) in the clinically evaluable and intent-to-treat (ITT) populations at a test of cure (TOC) visit (6–20 days after the last dose of therapy).
Baseline characteristics were similar with wound infection comprising 44% of underlying diagnoses, 24% with major abscesses, 12% infected diabetic ulcers and 37% with a systemic inflammatory response (SIRS). Over 80% of patients had the infecting organism identified, 70% of which were S. aureus (10% were MRSA).
The two trials, both individually and collectively, met the predefined statistical criteria for non-inferiority of daptomycin against comparator therapy (<10% difference in the upper limit of the 95% confidence interval between the groups’ success rates). Success in the clinically evaluable and microbiologically evaluable populations was comparable, and no organism-specific difference was observed in response to treatment. In particular daptomycin was as effective as both vancomycin against MRSA (clinical success in 75% of those in the daptomycin arm, 69% in the comparator arm), and penicillinase-resistant penicillin against methicillin-sensitive S. aureus (MSSA) (clinical success in 86% of those in the daptomycin arm, 87% in the comparator arm).15
This study was not designed to assess differences in duration of parenteral therapy between groups, however a post hoc analysis of the study population that received only intravenous therapy (89.8% of the total), found that 63% of the daptomycin-treated group required only 4–7 days of therapy compared with 33% of the comparator arm (P < 0.0001). A further analysis of a subset of the South African enrolled patients with ≤ one co-morbidity showed that the median duration of therapy was shorter in the daptomycin group (7 versus 8 days for the comparator group, P < 0.0001).32
A subsequent Phase IIIb multi-center randomized assessor-blinded study33 compared the efficacy and safety of daptomycin versus vancomycin or teicoplanin for the treatment of cSSTIs. The primary objective was to compare daptomycin to its comparator at day 7–14 for clinical success (complete resolution of clinical signs and symptoms or improvement requiring no additional therapy). Patients with cSSTIs were included if they were expected to receive at least 4 days of IV therapy before step down to oral therapy, if needed, and were randomized (1:1) and stratified by age (≥65 years) and systemic inflammatory response syndrome (SIRS) to receive either daptomycin (4 mg/kg IV once daily [OD]) or a glycopeptide (vancomycin 1 g IV BD or teicoplanin 400 mg IV OD). 189 patients (97 in the daptomycin arm, 92 in the comparator) from 29 centers across Europe received treatment. Baseline demographics were similar, with 1/3 of patients ≥65 years old and SIRS present in 58.8% in the daptomycin arm and 56.5% in the pooled comparator. In this study the median time to switch to oral therapy or end therapy was the same in both groups (8 days), and success rates in the clinically evaluable population were similarly high in both groups (91.4% for daptomycin and 87.2% for the pooled comparator) although in elderly patients there was a trend to a higher clinical success rate with daptomycin than with the pooled comparator (88.9% versus 76.5%, respectively, (Confidence interval [CI] −22.4, 45.1).33
A summary of the outcomes from these 3 studies along with the other main comparative studies discussed in this review are presented in Table 3.
Meta-analysis of daptomycin therapy for cSSTIs
Three randomized controlled trials (RCTs) and 1 prospective comparative study (included in Tables 3 and 4) were included in a recent meta-analysis comparing the effectiveness of daptomycin with other agents in the treatment of SSTIs.16 Three studies only included patients with cSSTIs whilst one included non-complicated SSTIs.34 Vancomycin and semi-synthetic penicillins were the comparator agents used. Short-term high-dose daptomycin therapy (10 mg/kg/day for 4 days) was evaluated in one study.35 In total 1557 patients were evaluated (688 in the daptomycin group). No statistically significant difference in clinical success or toxicity in the clinically evaluable or ITT populations was noted. No firm conclusions on the comparative efficacy of daptomycin verses vancomycin in MRSA infection could be drawn due to significant differences between the studies in the proportion of patients infected with MRSA.
Table 4.
Characteristics of SSTI patient population included in CORE 2004, 2005 and EU-CORE 2006–‘08
| Characteristic | CORE 2004 SSTI subgroup40 (n = 522)% | CORE 2005 SSTI subgroup41,42 (n = 486)% | EU-CORE 2006–200839,44 (n = 484)% |
|---|---|---|---|
| Age > 66∼ | 24 | 21 | 53 |
| CrCl < 30 mL/minute | 26 | 12 | 9 |
| Diabetes | 27 | 30 | 41 |
| cSSTIs | 64 | 70 | 100 |
| Bacteremia | 3 | 5 | NR |
| Prior antibiotic therapy | 67 | 74 | 62 |
| Prior vancomycin | Not reported | 49 | 22* |
| MRSA# | 63 | 52 | 33 |
| Enterococcal sp | 19 | Not reported | 10 |
| VRE** | 8 | Not reported | 3 |
Notes:
Age ≥ 65 in EU-CORE group; NR, not recorded;
Percent of all EU-CORE data from this time period ie, includes the non-SSTI patients;
Percent of all positive isolates that were MRSA positive;
Including both vancomycin resistant Enterococcus faecium and faecalis.
Abbreviations: VRE, vancomycin resistant enterococcus; MRSA, methicillin resistant Staphylococcus aureus; SSTI, skin and soft tissue infections; cSSTI, complicated skin and soft tissue infections; CrCl, creatinine clearance; CORE, Cubicin Outcomes Registry and Experience program; EU-CORE, European Cubicin Outcomes Registry and Experience program.
Two studies have specifically examined duration of IV therapy as an outcome but results have been inconclusive: In a nonrandomized study utilising retrospective vancomycin treated controls,36 median duration of time of IV therapy, as well as time to clinical cure and length of stay was less (4 days [range 2–13] versus 7–8 days [range 3–19]) in the daptomycin arm. Notably a higher proportion of patients with MRSA received daptomycin (Table 4). In a setting of high MRSA prevalence, an unusual study was employed whereby 96 patients were randomized to 4 days of high dose daptomycin (10 mg/kg/day) or 8 days of intravenous vancomycin with the option to switch to a semi-synthetic penicillin in sensitive isolates.35 Both arms could switch to oral therapy following clinical improvement. The study was not statistically powered to detect differences between treatment groups, but a trend towards better outcome in the comparator group with longer therapy was observed. Differences were more pronounced in the MRSA subgroup, which itself has previously been identified as an independent predictor of longer hospital stays and poorer clinical outcome irrespective of therapy.37
A further meta-analysis has looked at the comparative effectiveness of antibiotics for the treatment of MRSA complicated skin and soft tissue infections using a Bayesian statistical approach which enables combining of evidence to handle indirect comparisons.38 It compared treatment of cSSTI by vancomycin, linezolid, daptomycin, tigecycline and the novel glycopeptides dalbavancin and telavancin. Following a literature search which initially identified 1632 papers, it included 13 studies within which they could specifically look at the subset of patients with confirmed MRSA-related cSSTIs. Of these however it only included one paper on daptomycin,15 which only included 28 patients with MRSA infection. No difference in effect between daptomycin (success rate of 78.1%; 95% Bayesian confidence interval [CrI95%]: 54.6%–93.2%) and vancomycin (pooled success rate of 74.7%; CrI95%: 64.1%–83.5%) was demonstrated.
Review of non-comparative studies of daptomycin use in cSSTIs
There is now increasing experience with daptomycin worldwide, with over an estimated 1,000,000 treated patients by mid-2010.39 Clinical experience with daptomycin has been captured within the Cubist-sponsored Cubicin Outcomes Registry and Experience (CORE) programme: a multi-center retrospective observational programme based in the United States, and now by the Novartis Pharma AG-sponsored European registry (EU-CORE). Data have been collected on patients who have received daptomycin outside the trial setting, providing ‘real-life’ experience. The data produced should be interpreted with care due to their retrospective, observational nature and potential inclusion bias.
Patient characteristics and outcomes for those with cSSTIs from CORE 200440 and 2005,41–43 as well as from EU-CORE analysis for January 2006 to August 200839,44 are presented here (Tables 4 and 5).
Table 5.
Clinical outcomes and treatment characteristics of patients treated with daptomycin for cSSTIs in CORE 2004, 2005, 2007 and EU-CORE 2006–‘08
| Study | Patient group | Success rate in CE patients (%) | Success rate in MRSA infections (%) | Mean dose mg/kg (range) | Median duration of therapy (days) |
|---|---|---|---|---|---|
| CORE 200440 | cSSTIs (n = 334) | 96 | 136/144 (97) | 4.5 (2.3–12) | 14 |
| CORE 200541,42 | cSSTIs (n = 333) | 93 | Not known | 4.7 (2–10) | 13 |
| EU-CORE 2006–200839,44 | cSSTIs (484) | 84 | 152/187 (81.5)a | Not knownb | 10 |
Note:
Total MRSA population including non-cSSTIs;
Mean/median dose not recorded, but 44% of patients received 4 mg/kg, and 43% received ≥6 mg/kg.
Abbreviations: cSSTI, complicated skin and soft tissue infection; CE, clinically evaluable; MRSA, methicillin resistant Staphylococcus aureus; CORE, Cubicin Outcomes Registry and Experience program; EU-CORE, European Cubicin Outcomes Registry and Experience program.
MRSA infections make up the majority of positive isolates in the earlier registry groups (63% in 2004, 52% in 2005), but a smaller proportion of the European group perhaps reflecting recent decline in MRSA in some participating European nations. Nonetheless, MRSA still accounted for over 50% of all S. aureus isolates. The high rate of prior antibiotic use has been notable across all registry groups, with glycopeptides and in particular vancomycin being the most common preceding antibiotic. Switch to daptomycin was most commonly observed following treatment failure. The EU-CORE data show similarly low failure rates whether daptomycin was prescribed as first- or second-line therapy (6% versus 8% respectively)39 suggesting perhaps that prior vancomycin therapy does not increase the risk of daptomycin failure.
For patients treated for SSTI, a median dose of 4 mg/kg was observed most frequently with a higher mean dose used in cSSTIs compared with uSSTIs (4.5 mg/kg versus 4.2 mg/kg in 2004 (P < 0.001) and 4.7 mg/kg versus 4.4 mg/kg in 2005). In the most recent data from EU-CORE a significant proportion of those with cSSTIs (43%) received ≥6 mg/kg, possibly indicating the complexity of infections treated, and perhaps increasing confidence in daptomycin’s safety and tolerability at these doses.
Overall clinical success was judged by local investigators as ‘cured’ where no further antibiotic therapy was required, and ‘improved’ where there was clinical improvement but further therapy was required following daptomycin.45 Overall success was >90% in both the CORE 2004 and 2005 studies, and >80% in the EU-CORE registry (Table 5). High success rates were maintained in patients with confirmed MRSA infections. The median time to clinical response, as evidenced by signs and symptoms was 4 days (range 1–32) for patients with cSSTIs (2004 registry).
A multivariate analysis of the CORE 2005 cohort determined that sepsis, ICU stay and creatinine clearance <30 mL/minute were significantly associated with clinical failure.43 However, a subsequent stepwise multivariate regression analysis of a larger data set of patients with S. aureus infections from CORE 2005 to 2007 found that the only independent predictors of clinical failure with daptomycin therapy were the presence of endocarditis, bacteremia, severe renal dysfunction (CrCl < 30 mL/minute) and diabetes mellitus.46 Each of these factors have been found to be associated with increased infection-related complications and mortality in previous studies.27,47 This analysis also suggested that prior treatment with other antibiotics, including vancomycin, did not independently influence treatment outcomes with daptomycin, even if the reason for using daptomycin was prior treatment failure.46
Daptomycin in specific cSSTIs
As previously noted cSSTIs comprise a diverse group of infections with similar etiology but varying environmental and host factors. Differing infection types may present diverse therapeutic challenges. Some of these have been investigated in sub groups of prospective clinical trials whilst others have been retrospectively evaluated in post-marketing studies.
Surgical site infections (SSI)
In the CORE 2007 population,48 118 of the 962 patients had SSI. Of these 104 (11%) met the criteria for an efficacy analysis. Positive microbiology was found in 73% and the majority were S. aureus (59%) with MRSA in 25 (24%). The majority of patients also received concomitant antibiotics however (54%), reflecting the potential polymicrobial nature of these infections.
Overall success (cured or improved) was observed in 91.3% with SSI and no statistical difference was observed between pathogens except for the small number of infections caused by vancomycin-resistant Enterococcus species, where success was observed in 5/8 (63%). On logistic regression vancomycin-resistant enterococcal infection was found to be an independent risk factor for failure (odds ratio 14.2, 95% confidence interval 1.3–154). This observation in a small number of patients has not been reflected in large international surveillance programmes.49–54
Diabetic foot infections
Pharmacokinetic data have shown that daptomycin effectively penetrates the soft tissue and bone in patients with diabetic foot infections.24 The clinical evidence supporting daptomycin use in diabetic foot ulcers (DFUs) largely comes from a subset analysis of the two international Phase III RCTs previously discussed.55 In these studies, 133 patients (12% of the total) had DFU infection and 103 were clinically evaluable. 47 received daptomycin and 56 received either vancomycin or semi-synthetic penicillin. Most infections were monomicrobial and S. aureus was the predominant pathogen, with MRSA isolated in 18.2%. There were no statistically significant differences between the daptomycin and the comparator groups for either the overall clinical outcomes (66% versus 70% respectively) or when analysed by infecting organism.55 Of the 39 patients treated for DFUs in CORE 2004, 35 (90%) were successful (cured or improved).15
Daptomycin in outpatient management of cSSTI
Outpatient parenteral antibiotic therapy (OPAT) is increasingly recognized as a cost-effective management option for patients with SSTIs where the appropriate guidance and expertise are available.30 OPAT enables shorter length of hospital stay or even avoidance of admission in appropriate patients, conferring patient convenience, a significant reduction in hospital costs,56 and also reduced risk of health care associated infection. However, a recent study in Glasgow, where OPAT is well established, shows that the service remains poorly accessed in patients with MRSA-associated SSTIs with only 10 (5.8%) of 173 patients receiving OPAT over a 16 month period and potentially one third of survivors having had the potential to be discharged earlier with either oral therapy or OPAT.57
In the UK for OPAT-managed SSTIs when MRSA is not suspected and there is no history of beta lactam allergy, Ceftriaxone 1–2 g IV daily is the standard of care.58 In suspected MRSA or allergy, teicoplanin is the usual alternative pending IV to oral switch. Current recommendations are for teicoplanin to be administered via a loading regimen of three 400 mg doses 12 hourly followed by daily dosing. As the loading regimen is most easily performed in hospital the opportunity for an avoided admission may be lost. Also higher doses (600–800 mg/day) of teicoplanin are generally preferred by most UK infection specialists. In some centers higher doses of teicoplanin (10–15 mg/kg) are used with a daily loading regimen for 3 days followed by thrice weekly dosing until oral switch is feasible.59 In a recently reported cohort of nearly 1000 OPAT-managed SSTIs (approximately 150 of whom received teicoplanin as per the above regimen), first line OPAT therapy with teicoplanin was identified as an independent risk factor for OPAT failure (as defined by either intolerance/allergy, progression of infection or readmission). Increased rates of failure were largely due to teicoplanin intolerance or allergy. Increased duration of therapy compared to ceftriaxone treated patients may have reflected the intermittent dosing regimen.60
Daptomycin is well suited to OPAT use. It has proven clinical efficacy in cSSTIs and a low and predictable toxicity profile. A half-life of 8–9 hours and a prolonged post-antibiotic effect (>6 hours), allows once-daily IV bolus administration. There is growing clinical experience supporting daptomycin’s use in the OPAT setting.
In CORE 2005, 539 (56.8%) of the clinically evaluable patients received OPAT, either de novo or following inpatient initiation.56 One hundred and seventy seven (32.8%) OPAT treated patients had cSSTI. Proportionally more uncomplicated SSTIs were managed via OPAT (18.4% versus 8.8% managed solely via inpatient antibiotic therapy [IPAT], P < 0.001). cSSTI rates were similar in OPAT and IPAT groups. Cure or improvement was observed in 94.6% OPAT treated patients versus 86.3% for those treated with IPAT alone (chi-squared test <0.001). OPAT was also associated with fewer adverse events, reflecting patient selection for OPAT versus IPAT.
Within EU-CORE between 2006 and 2008,61 153 (13.6%) received daptomycin via OPAT. Thirty four patients (22.1%) were treated for cSSTIs. Overall in OPAT 58 (37.9%) of infections were due to S. aureus, the majority of which were MRSA. Daptomycin was typically administered at 4 mg/kg (n = 55, 35.9%) and 6 mg/kg (n = 79, 51.6%) and clinical success at 30 days post-treatment was observed in 136 (88.9%). Possible adverse events were reported in 16 patients, but all were mild to moderate in severity.
Future developments and areas of controversy
Daptomycin resistance
Daptomycin resistance is rare, with more than 99% susceptibility of Gram-positive isolates in recent large European, North and South American, as well as Australian and New Zealand surveillance studies, including amongst MRSA and vancomycin resistant enterococci (VRE).49–54 To our knowledge resistance has not been reported in patients with SSTIs, but there are several case reports of daptomycin non-susceptibility and/or resistance emerging during treatment in patients with deep-seated infections, highlighting the key role that surgical debridement has in these circumstances.27,62,63
Although some in vitro data have led to concerns regarding increased risk of daptomycin resistance associated with intermediate susceptibility to vancomycin (VISA),64,65 recent clinical data have shown that in the vast majority of cases of VISA, daptomycin remained effective.66 Animal data have shown that increasing the dose of daptomycin can improve its efficacy for S. aureus strains with reduced daptomycin susceptibility,67 therefore using a higher dose may be advisable in these patients until evidence is available to guide management.
Dosing of daptomycin in SSTIs
The current licensed dosing for SSTIs is 4 mg/kg in SSTIs15 and 6 mg/kg in bacteremia.27 There are recent data supporting the safe use of daptomycin at doses up to 12 mg/kg, including in patients with a CrCl of < 30 mL /minute and on hemodialysis, when dosing interval is appropriately adjusted.67,68 Furthermore, the presence of bacteremia, endocarditis, severe renal dysfunction (defined as an initial CrCl < 30 mL/minute) and diabetes mellitus were the variables independently associated with clinical failure of daptomycin therapy.46 Therefore, although the 4 mg/kg dose has been shown to be effective and is appropriate for the majority of patients with SSTIs, it has been suggested that higher dosing at 6 mg/kg should be considered in certain patient groups (Table 6).30 In particular this includes patients at risk of, or proven to have bacteremia, which should include those presenting with a sepsis syndrome or requiring high dependency or intensive care due to cSSTI. In diabetic patients with a SSTI complicating a foot ulcer, the possibility of an osteoarticular infection can be difficult to exclude initially, and therefore higher doses would be advisable in these patients.69 Patient groups at risk of SSTIs who are known to have altered drug pharmacokinetics are also likely to require higher doses. Intravenous drug users (IVDU) have increased drug clearance and are therefore likely to require the 6 mg/kg dose, although clinical data are not yet available to confirm this.27 Another group is patients with burn injuries. In 2008 a pharmacokinetic study looked at nine patients with ≥18% body surface area burns between 7 and 27 days after the burn injury. They demonstrated a decreased area under the curve (AUC), increased volume of distribution and more rapid clearance of daptomycin (as is seen with other antibiotics evaluated in burns patients) compared to normal controls. This is felt to reflect clearance of daptomycin through the burn wound itself. The AUC was reduced by 47% for burns patients with a 6 mg/kg dose, and as its pharmacokinetics are linear, the authors suggest that a dose of 10–12 mg/kg would be required in this group of patients to achieve similar drug exposures to those achieved in healthy volunteers.70
Table 6.
Patients with SSTI in whom initial daptomycin dose should be ≥6 mg/kg
| Associated sepsis syndrome |
| Otherwise suspected or confirmed bacteremia or endocarditis |
| Diabetic foot infection/infected ulcer |
| Suspected underlying osteomyelitis or septic arthritis |
| Intravenous drug user |
| Burns injurya |
| Glycopeptide intermediate-resistant MRSA |
| Consider when creatinine clearance <30 mL/minuteb |
Note:
Consider doses of 10–12 mg/kg;
Dosing interval should be increased to 48 hourly.
Abbreviation: MRSA, methicillin resistant Staphylococcus aureus.
Economics
Drug acquisition costs have been one of the major hurdles in daptomycin becoming a first line treatment option in countries where health care is state funded. However, in a recent Canadian review of cost drivers associated with MRSA infection, antimicrobial therapy only made up 4% of the total cost, while hospitalization was estimated to be by far the largest driver (at 81% of the cost per patient).71 The authors estimated that direct health care cost attributable to MRSA in Canada averaged $82 million in 2004 and could reach $129 million in 2010.
Daptomycin is highly suitable as an empirical parenteral antibiotic option in patients with cSSTI to enable OPAT, particularly when there is a suspicion of MRSA infection or serious beta lactam allergy. More rapid hospital discharge or admission avoidance for patients with SSTI in whom IV therapy is indicated, gives a distinct economic advantage. A study in Ohio reviewed outcomes of the first 50 consecutive patients treated with daptomycin in a community hospital, 31 of whom had cSSTI.72 Out of the 50 patients, 31 (62%) had confirmed MRSA infection. Fourteen patients (28%) transitioned to outpatient daptomycin therapy saving an estimated $102,340 in hospital charges. In 48 (96%), infection resolved with daptomycin therapy. Although this study was limited by its observational retrospective nature, it emphasises the significant cost savings that accompany shorter or no hospitalization in these patients.
Shorter duration of therapy could confer economic advantage, however to date the in vitro rapid cidality of daptomycin has yet to be translated into a demonstrably more rapid clinical response or shorter therapy duration.
More clinical and economic data are required to fully evaluate daptomycin’s cost efficiency in comparison to the glycopeptides and to linezolid. Particularly important questions are whether daptomycin use can achieve quicker clinical improvement and hence shorter course IV therapy in both hospital and OPAT settings compared to the glycopeptides or linezolid and whether this, combined with OPAT use, can translate to shorter hospitalization. To date these questions have not been addressed in clinical trials.
Current international recommendations
The current international guidelines for the management of SSTIs in patients known to have, or at risk of MRSA are summarized in Table 7. Daptomycin is considered a first line option for cSSTIs in the UK14,73 and the USA26 and second line in severe infections in Spain.74 Daptomycin is recommended as one of the options for enabling OPAT in the UK.73
Table 7.
Current international MRSA SSTI guidelines
| Guideline | 1st line | Alternative | OPAT | IVOST | Duration |
|---|---|---|---|---|---|
| Spain74 | |||||
| SSTI (mild) | Clindamycin Doxycycline | Co-trimoxazole | NA | NA | No comment |
| SSTI (more severe) | Linezolid, Vancomycin ± Clindamycin | Tigecycline (polymicrobial) Daptomycin (MIC ≥ 1.5) |
No comment | Linezolid | No comment |
| UK14,73 | |||||
| SSTI (non-hospital) | Doxycycline Clindamycin Rifampicin + (Fusidate or Doxycycline or Trimethoprim) | Linezolid Co-trimoxazole |
Glycopeptide Daptomycin |
Yes | No comment |
| SSTI (hospital) | Glycopeptide Linezolid Daptomycin |
Tigecycline (polymicrobial) Clindamycin |
Glycopeptide Daptomycin |
Clindamycin Linezolid |
No comment |
| USA26 | |||||
| uSSTI | Clindamycin TMP-SMX Tetracycline Linezolid |
No comment | Not applicable | Not applicable | 5–10 days |
| cSSTI | Vancomycin Linezolid Daptomycin Telavancin Clindamycin |
No comment | No comment | Linezolid Clindamycin |
7–14 days |
Abbreviations: NA, Not applicable; OPAT, Outpatient parenteral antibiotic therapy; IVOST, Intravenous to Oral antibiotic Switch Therapy; SSTI, skin and soft tissue infection; uSSTI, uncomplicated SSTI; cSSTI, complicated SSTI.
Summary and recommendations
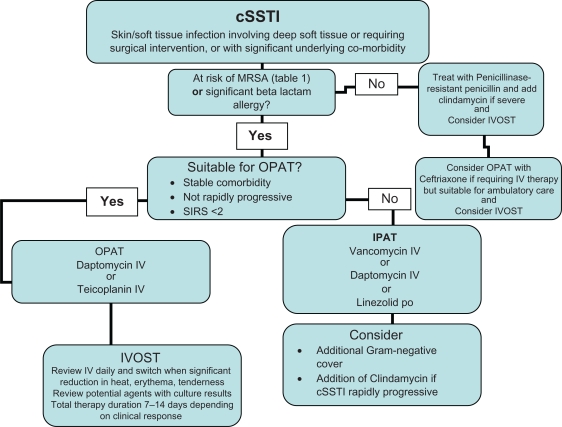
SSTIs form a substantial part of acute hospital care in all countries. Infections with MRSA, the presence of beta lactam allergy and declining efficacy of the glycopeptides requires that alternative antibiotics are available. For inpatients at risk of MRSA or with beta lactam allergy, glycopeptides are likely to remain the first line option in the majority of patients at present. The data presented to date have not demonstrated superiority of daptomycin over glycopeptides in cSSTI, however daptomycin is likely to remain an important second line agent in those failing or intolerant of the glycopeptides. If in vitro rapid cidality data translates to a more rapid clinical improvement in future studies, the significant benefits of shorter course therapy and shorter hospitalization could encourage greater empirical use of daptomycin in cSSTI in hospital. As clinicians become more experienced with the use of OPAT to manage SSTI, and aware of the substantial economic and psychosocial benefits derived from non-inpatient management, daptomycin may become increasingly used, particularly in patients at risk of infection with resistant Gram-positive pathogens. A proposed algorithm for management of cSSTI is shown in Figure 1.
Figure 1.
Proposed antibiotic management algorithm for MRSA cSSTIs.
Abbreviations: OPAT, Outpatient parenteral antibiotic therapy; IPAT, Inpatient parenteral antibiotic therapy; IVOST, Intravenous to oral antibiotic switch therapy.
Footnotes
Disclosure
B White: none to declare. RA Seaton: Principal investigator in Cubist and Novartis sponsored clinical studies of Daptomycin. Investigator initiated studies sponsored by Novartis and Pfizer. Advisory boards for Novartis and Pfizer. Honoraria for speaking at symposia sponsored by Novartis and Pfizer.
No funding was received for the preparation of this manuscript, nor was there any editorial assistance or input from Novartis.
References
- 1.Cubicin summary of product characteristics 2010. Available at: www.emea.europa.eu/humandocs/Humans/EPAR/cubicin/cubicin.htm. Accessed 30 January 2011.
- 2.Beiras-Fernandez A, Vogt F, Sodian R, et al. Daptomycin: a novel lipopeptide antibiotic against Gram-positive pathogens. Infect Drug Resist. 2010;3:95–101. doi: 10.2147/IDR.S6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eron LJ, Lipsky BA, Low DE, et al. Managing skin and soft tissue infections: expert panel recommendations on key decision points. J Antimicrob Chemother. 2003;52(Suppl 1):i3–i17. doi: 10.1093/jac/dkg466. [DOI] [PubMed] [Google Scholar]
- 4.FDA Guidance for Industry: uncomplicated and complicated skin and skin structure infections – developing antimicrobial drugs for treatment. Available from: http://www.fda.org/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071185.pdf. Accessed 30 January 2011.
- 5.Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: a review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol. 2008;19(2):173–184. doi: 10.1155/2008/846453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marwick C, Broomhall J, McCowan C, et al. Severity assessment of skin and soft tissue infections: cohort study of management and outcomes for hospitalized patients. J Antimicrob Chemother. 2011;66(2):387–397. doi: 10.1093/jac/dkq362. [DOI] [PubMed] [Google Scholar]
- 7.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections amongst patients in the emergency department. N Engl J Med. 2006;355(7):666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 8.European Center for Disease Prevention and Control Antimicrobial resistance surveillance in Europe 2009. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm: ECDC;2010. Available from: http://www.ecdc.europa.eu/en/publications/Publications/1011_SUR_annual_EARS_Net_2009.pdf. Accessed 30 January 2011.
- 9.Gemmel CG, Edwards DI, Fraise AP, et al. Guidelines for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the UK. J Antimicrob Chemother. 2006;57(4):589–608. doi: 10.1093/jac/dkl017. [DOI] [PubMed] [Google Scholar]
- 10.Leman P, Mukherjee D. Flucloxacillin alone or combined with benzyl-penicillin to treat lower limb cellulitis: a randomized controlled trial. Emerg Med J. 2005;22(5):342–346. doi: 10.1136/emj.2004.019869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boucher H, Miller LG, Razonable RR. Serious infections caused by Methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2010;51(Suppl 2):S183–S197. doi: 10.1086/653519. [DOI] [PubMed] [Google Scholar]
- 12.Kinney KK. Treatment of infections caused by antimicrobial-resistant Gram-positive bacteria. Am J Med Sci. 2010;340(3):209–217. doi: 10.1097/MAJ.0b013e3181e99aa4. [DOI] [PubMed] [Google Scholar]
- 13.Itani KM, Dryden MS, Bhattacharyya, et al. Efficacy and safety of linezolid versus vancomycin for the treatment of complicated skin and soft-tissue infections proven to be caused by methicillin-resistant Staphylococcus aureus. Am J Surg. 2010;199(6):804–816. doi: 10.1016/j.amjsurg.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 14.Gould FK, Brindle R, Chadwick PR, et al. Guidelines (2008) for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the United Kingdom. J Antimicrob Chemother. 2009;63(5):849–861. doi: 10.1093/jac/dkp065. [DOI] [PubMed] [Google Scholar]
- 15.Arbeit RD, Maki D, Tally FP, Campanaro E, Eisenstein BI. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin Infect Dis. 2004;38(12):1673–1681. doi: 10.1086/420818. [DOI] [PubMed] [Google Scholar]
- 16.Bliziotis IA, Plessa E, Peppas G, Falagas ME. Daptomycin versus other antimicrobial agents for the treatment of skin and soft tissue infections: A meta-analysis. Ann Pharmacother. 2010;44(1):97–106. doi: 10.1345/aph.1M264. [DOI] [PubMed] [Google Scholar]
- 17.Vinh DC, Rubinstein E. Linezolid: a review of safety and tolerability. Journal of Infection. 2009;59(Suppl 1):S59–S74. doi: 10.1016/S0163-4453(09)60009-8. [DOI] [PubMed] [Google Scholar]
- 18.Streit JM, Jones RN, Sader HS. Daptomycin activity and spectrum: a worldwide sample of 6737 clinical Gram-positive organisms. J Antimicrob Chemother. 2004;53(4):669–674. doi: 10.1093/jac/dkh143. [DOI] [PubMed] [Google Scholar]
- 19.Anastasiou DM, Thorne GM, Luperchio S, Alder JD. In vitro activity of daptomycin against clinical isolates with reduced susceptibilities to linezolid and quinuprisitn/dalfopristin. Int J Antimicrob Agents. 2006;28(5):385–388. doi: 10.1016/j.ijantimicag.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Alborn WE, Jr, Allen NE, Preston DA. Daptomycin disrupts membrane potential in growing Staphylococcus aureus. Antimicrob Agents Chemother. 1991;35(11):2282–2287. doi: 10.1128/aac.35.11.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hair PI, Keam SJ. Daptomycin: A review of its use in the management of complicated skin and soft tissue infections and Staphylococcus aureus bacteraemia. Drugs. 2007;67(10):1483–1512. doi: 10.2165/00003495-200767100-00008. [DOI] [PubMed] [Google Scholar]
- 22.Wise R, Gee T, Andrews JM, Dvorchik B, Marshall G. Pharmacokinetics and inflammatory fluid penetration of intravenous daptomycin in volunteers. Antimicrob Agents Chemother. 2002;46(1):31–33. doi: 10.1128/AAC.46.1.31-33.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritchie ND, Lovering AM, Seaton RA. Daptomycin in synovial fluid during treatment of methicillin-resistant Staphylococcus aureus septic arthritis. J Antimicrob Chemother. 2010;65(6):1314–1315. doi: 10.1093/jac/dkq122. [DOI] [PubMed] [Google Scholar]
- 24.Traunmuller F, Schintler MV, Metzler J, et al. Soft tissue and bone penetration abilities of daptomycin in diabetic patients with bacterial foot infections. J Antimicrob Chemother. 2010;65(6):1252–1257. doi: 10.1093/jac/dkq109. [DOI] [PubMed] [Google Scholar]
- 25.Schrievner CA, Fernandez C, Rodvold K, Danziger LH. Daptomycin: a novel cyclic lipopeptide antimicrobial. Am J Health Syst Pharm. 2005;62(11):1145–1158. doi: 10.1093/ajhp/62.11.1145. [DOI] [PubMed] [Google Scholar]
- 26.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of Methicillin-Resistant Staphylococcus aureus infections in adults and children. Clin Inf Dis. 2011;52(3):e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 27.Fowler VG, Jr, Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteraemia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653–665. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- 28.US Food and Drug Administration FDA drug safety communication: eosinophilic pneumonia associated with the use of Cubicin (daptomycin) Available from: www.fda.gov/drugs/drugsafety. Updated 29 July 2010. Accessed 30 January 2011.
- 29.Abdel-Rahman SM, Benziger DP, Jacobs RF, et al. Single-dose pharmacokinetics of daptomycin in children with suspected or proved Gram-positive infections. Paediatr Infect Dis J. 2008;27(4):330–334. doi: 10.1097/INF.0b013e318160edfc. [DOI] [PubMed] [Google Scholar]
- 30.Seaton RA. Daptomycin: rationale and role in the management of skin and soft tissue infections. J Antimicrob Chemother. 2008;62(Suppl 3):iii15–iii23. doi: 10.1093/jac/dkn368. [DOI] [PubMed] [Google Scholar]
- 31.Johns Hopkins POC-IT Center ABX Guide Home page on the Internet. Available from: http://hopkins-abxguide.org Accessed January 2011.
- 32.Krige JE, Lindfield K, Friedrich L, et al. Effectiveness and duration of daptomycin therapy in resolving clinical symptoms in the treatment of complicated skin and skin structure infections. Curr Med Res Opin. 2007;23(9):2147–2156. doi: 10.1185/030079907X219652. [DOI] [PubMed] [Google Scholar]
- 33.Gollnick H, Quist SR, Fierlbeck G, et al. Efficacy and safety of daptomycin versus vancomycin or teicoplanin for the treatment of cSSTIs: a multicenter, randomised, assessor-blind trial. Abstract of the 20th European Congress of Clinical Microbiology and Infectious Diseases; 2010 April 10–13; Vienna, Austria. Poster 1551. [Google Scholar]
- 34.Pertel PE, Eisenstein BI, Link AS, et al. The efficacy and safety of daptomycin versus vancomycin for the treatment of cellulitis and erysipelas. Int J Clin Pract. 2009;633(3):368–375. doi: 10.1111/j.1742-1241.2008.01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katz DE, Lindfield KC, Steenbergen JN, et al. A pilot study of high dose short duration daptomycin for the treatment of patients with complicated skin and skin-structure infections caused by Gram-positive bacteria. Int J Clinc Pract. 2008;62(9):1455–1464. doi: 10.1111/j.1742-1241.2008.01854.x. [DOI] [PubMed] [Google Scholar]
- 36.Davis SL, McKinnon PS, Hall LM, et al. Daptomycin versus vancomycin for complicated skin and skin structure infections: clinical and economic outcomes. Pharmacotherapy. 2007;27(12):1611–1618. doi: 10.1592/phco.27.12.1611. [DOI] [PubMed] [Google Scholar]
- 37.Figtree M, Konecny P, Jennings Z, Goh C, Krikis SA, Miyakis S. Risk stratification and outcome of cellulitis admitted to hospital. J Infect. 2010;60(6):431–439. doi: 10.1016/j.jinf.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 38.Logman JFS, Stephens J, Haider S, et al. Comparative effectiveness of antibiotics for the treatment of MRSA complicated skin and soft tissue infections. Curr Med Res Opin. 2010;26(7):1565–1578. doi: 10.1185/03007995.2010.481251. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Ruiz A, Beiras-Fernandez A, Lehmkuhl H. Clinical experience with daptomycin in Europe: the first 2.5 years. J Antimicrob Chemother. 2011;66(4):912–919. doi: 10.1093/jac/dkq528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owens RC, Jr, Lamp K, Friedrich LV, et al. Postmarketing clinical experience in patients with skin and skin-structure infections treated with daptomycin. Am J Med. 2007;120(10 Suppl 1):S6–S12. doi: 10.1016/j.amjmed.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Brown J, Lamp KC, Friedrich L. Efficacy and safety of daptomycin for the treatment of skin and skin structure infections (SSSI). Abstracts of the Fourth International Symposium on Resistant Gram-positive Infections; Ontario, Canada. 2006. Abstract CPLA27. [Google Scholar]
- 42.Katz DE, Martone WJ. cSSTIs with culture confirmed Staphylococcus aureus treated with daptomycin: Cubicin Outcome Registry and Experience (CORE) 2005 Interim Analysis. Abstracts of the 2006 Annual Conference on Antimicrobial Resistance; 2006 June 26–28; Bethesda, MD. Poster 22:25. National Foundation for Infectious Diseases, Bethesda, MD. [Google Scholar]
- 43.Lamp KC, Friedrich L, Lindfield K. Clinical factors associated with daptomycin outcomes in skin and soft-tissue infections. Abstracts of the Seventeenth European Congress of Clinical Microbiology and Infectious Diseases; 2007 March 31–April 3; Munich, Germany. Poster 837: S213 European Society of Clinical Microbiology and Infectious Diseases, Basel, Switzerland. [Google Scholar]
- 44.Cogo A, Dialiana Z, Gargalianos-Kakolyris P, et al. Complicated skin and soft tissue infections (cSSTIs) treated with daptomycin in the European Cubicin Outcomes Registry and Experience (EU-CORE). Abstracts from the 20th ECCMID (European Congress of Clinical Microbiology and Infectious Diseases); 2010 April 10–13; Vienna, Austria. Poster 1227. [Google Scholar]
- 45.Rolston KV, Segreti J, Lamp KC, Friedrich LV. Cubicin Outcomes Registry and Experience (CORE) methodology. Am J Med. 2007;120(10 Suppl 1):S4–S5. doi: 10.1016/j.amjmed.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Sakoulas G, Brown J, Lamp K, Friedrich LV, Lindfield KC. Clinical outcomes of patients receiving daptomycin for the treatment of Staphylococcus aureus infections and assessment of clinical factors for daptomycin failure: a retrospective cohort study utilizing the Cubicin Outcomes Registry and Experience. Clin Ther. 2009;31(9):1936–1945. doi: 10.1016/j.clinthera.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Bertoni AG, Saydah S, Brancati FL. Diabetes and the risk of infection-related mortality in the US. Diabetes Care. 2001;24(6):1044–1049. doi: 10.2337/diacare.24.6.1044. [DOI] [PubMed] [Google Scholar]
- 48.Chamberlain RS, Culshaw DL, Donovan BJ, Lamp KC. Daptomycin for the treatment of surgical site infections. Surg. 2009;146(2):316–324. doi: 10.1016/j.surg.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 49.Pfaller MA, Sader HS, Jones RN. Evaluation of the in vitro activity of daptomycin against 19615 clinical isolates of Gram-positive cocci collected in North American hospitals (2002–2005) Diagn Microbiol Infect Dis. 2007;57(4):459–465. doi: 10.1016/j.diagmicrobio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Castanheira M, Jones RN, Sader HS. Update of the in vitro activity of daptomycin tested against 6710 Gram-positive cocci isolated in North America (2006) Diagn Microbiol Infect Dis. 2008;61(2):235–239. doi: 10.1016/j.diagmicrobio.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 51.Sader HS, Farrell DJ, Jones RN. Antimicrobial susceptibility of Gram-positive cocci isolated from skin and skin-structure infections in European medical centers. Int J Antimicrob Agents. 2010;36(1):28–32. doi: 10.1016/j.ijantimicag.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 52.Sader HS, Moet G, Jones RN. Update on the in vitro activity of daptomycin tested against 17,193 Gram-positive bacteria isolated from European medical centers (2005–2007) J Chemother. 2009;21(5):500–506. doi: 10.1179/joc.2009.21.5.500. [DOI] [PubMed] [Google Scholar]
- 53.Bell JM, Turnidge JD, Sader HS, Jones RN. Antimicrobial activity and spectrum of daptomcyin: results from the surveillance program in Australia and New Zealand (2008) Pathology. 2010;42(5):470–473. doi: 10.3109/00313025.2010.493869. [DOI] [PubMed] [Google Scholar]
- 54.Gales AC, Sader HS, Ribeiro J, Zoccoli C, Barth A, Pignatari AC. Antimicrobial susceptibility of Gram-positive bacteria isolated in Brazilian hospitals participating in the SENTRY Program (2005–2008) Braz J Infect Dis. 2009;13(2):90–98. doi: 10.1590/s1413-86702009000200004. [DOI] [PubMed] [Google Scholar]
- 55.Lipsky BA, Stoutenburgh U. Daptomycin for treating infected diabetic foot ulcers: evidence from a randomized, controlled trial comparing daptomycin with vancomycin or semi-synthetic penicillins for complicated skin and skin-structure infections. J Antimicrob Chemother. 2005;55(2):240–245. doi: 10.1093/jac/dkh531. [DOI] [PubMed] [Google Scholar]
- 56.Martone WJ, Lindfield KC, Katz DE. Outpatient parenteral antibiotic therapy with daptomycin: insights from a patient registry. Int J Clin Pract. 2008;62(8):1183–1187. doi: 10.1111/j.1742-1241.2008.01824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seaton RA, Coia J, Masterton R, et al. MRSA complicated skin and skin structure infections in Glasgow: evaluation of hospital management and potential for earlier discharge. Abstracts from the 20th European Congress of Clinical Microbiology and Infectious Diseases; 2010 April 10–13; Vienna, Austria. Poster 1873. [Google Scholar]
- 58.Seaton RA, Bell E, Gourlay Y, Semple L. Nurse-led management of uncomplicated cellulitis in the community; evaluation of a protocol incorporating intravenous ceftriaxone. J Antimicrob Chemother. 2005;55(5):764–767. doi: 10.1093/jac/dki092. [DOI] [PubMed] [Google Scholar]
- 59.Lamont E, Seaton RA, Macpherson M, Semple L, Bell E, Thomson AH. Development of teicoplanin dosage guidelines for patients treated within an outpatient parenteral antibiotic therapy (OPAT) programme. J Antimicrob Chemother. 2009;64(1):181–187. doi: 10.1093/jac/dkp147. [DOI] [PubMed] [Google Scholar]
- 60.Seaton RA, Bell E, Bezlyak V, et al. Factors associated with outcome and length of parenteral therapy in outpatient parenteral antibiotic therapy (OPAT) treated patients with skin and soft tissue infections (SSTIs). Abstracts from the 19th European Congress Clinical Microbiology and Infectious Diseases; 2010 April 10–13; Vienna, Austria. Poster 1333. [Google Scholar]
- 61.Seaton RA, Gonzalez-Ramallo VJ, Prisco V, et al. Daptomycin for out-patient parenteral antibiotic therapy (OPAT), a European registry experience. Abstracts from the 7th International Conference of the Hospital Infection Society; 2010 October 10–13; Liverpool, UK. Poster 01.06. [Google Scholar]
- 62.Huang Y, Hsiao C, Liao C, Lee CW, Hsueh PR. Bacteraemia and infective endocarditis cuased by a non-daptomycin-susceptible, vancomycin-intermediate, and methicillin-resistant Staphylococcus aureus strain in Taiwan. J Clin Microbiol. 2008;46(3):1132–1136. doi: 10.1128/JCM.01844-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hayden MK, Rezai K, Hayes RA, Loland K, Quinn JP, Weinstein RA. Development of daptomycin resistance in vivo in methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2005;43(10):5285–5287. doi: 10.1128/JCM.43.10.5285-5287.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cui L, Tominaga E, Neoh HM, et al. Correlation between reduced daptomycin susceptibility and vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother. 2006;50(3):1079–1082. doi: 10.1128/AAC.50.3.1079-1082.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sakoulas G, Alder J, Thauvin-Eliopoulos C, Moellering RC, Jr, Eliopoulos GM. Induction of daptomycin heterogeneous susceptibility in Staphylococcus aureus by exposure to vancomycin. Antimicrob Agents Chemother. 2006;50(4):1581–1585. doi: 10.1128/AAC.50.4.1581-1585.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sader HS, Becker HK, Moet GJ, Jones RN. Antimicrobial activity of daptomycin tested against Staphylococcus aureus with vancomycin MIC of 2 microg/mL isolated in the United States and European hospitals (2006–2008) Diagn Microbiol Infect Dis. 2010;66(3):329–331. doi: 10.1016/j.diagmicrobio.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 67.Moise PA, Hershberger E, Amodio-Groton MI, Lamp KC. Safety and clinical outcomes when utilising high-dose (≥8 mg/kg) daptomycin therapy. Ann Pharmacother. 2009;43(7):1211–1219. doi: 10.1345/aph.1M085. [DOI] [PubMed] [Google Scholar]
- 68.Cunha BA, Eisenstein LE, Hamid NS. Pacemaker-induced Staphylococcus aureus mitral valve acute bacterial endocarditis complicated by persistent bacteraemia from a coronary stent: cure with prolonged/high-dose daptomycin without toxicity. Heart Lung. 2006;35(3):207–211. doi: 10.1016/j.hrtlng.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 69.Lamp KC, Friedrich LV, Medez-Vigo L, Russo R. Clinical experience with daptomycin for the treatment of patients with osteomyelitis. Am J Med. 2007;120(10 Suppl 1):S13–S20. doi: 10.1016/j.amjmed.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 70.Mohr III JF, Ostrosky-Zeichner L, Wainright DJ, Parks DH, Hollenbeck TC, Ericsson CD. Pharmacokinetic evaluation of single-dose intravenous daptomycin in patients with thermal burn injury. Antimicrob Agents Chemother. 2008;52(5):1891–1893. doi: 10.1128/AAC.01321-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goetghebeur M, Landry PA, Han D, Vicente C. Methicillin-resistant Staphylococcus aureus: a public health issue with economic consequences. Can J Infect Dis Med Microbiol. 2007;18(1):27–34. doi: 10.1155/2007/253947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fossaceca C. Outcomes analysis of daptomycin use in a community hospital. Adv Ther. 2007;24(3):517–528. doi: 10.1007/BF02848774. [DOI] [PubMed] [Google Scholar]
- 73.Nathwani D, Morgan M, Masterton RG. Guidelines for UK practice for the diagnosis and management of methicillin-resistant Staphylococcus aureus (MRSA) infections presenting in the community. J Antimicrob Chemother. 2008;61(5):976–994. doi: 10.1093/jac/dkn096. [DOI] [PubMed] [Google Scholar]
- 74.Mensa J, Barberan J, Llinares P, et al. Guidelines for the treatment of infections caused by methicillin-resistant Staphylococcus aureus. Rev Esp Quimioter. 2008;21(4):234–258. [PubMed] [Google Scholar]