Abstract
Objective
Preeclampsia is diagnosed using clinical criteria and in atypical cases the diagnosis may be inaccurate as there are no specific tests to confirm or exclude preeclampsia. This study sought to evaluate the utility of angiogenic biomarkers, sFlt1, sEng and PlGF to distinguish patients with gestational thrombocytopenia and immune thrombocytopenic purpura (ITP) from patients with thrombocytopenia resulting from the HELLP syndrome, a complication of severe preeclampsia.
Methods
Serum was collected and the angiogenic biomarkers of patients with ITP and gestational thrombocytopenia (N=9) were compared to patients with HELLP (N=11) and preeclampsia (N=11). Circulating levels of these angiogenic biomarkers were also compared by gestational age to 1564 randomly selected normotensive women from the Calcium for Preeclampsia Prevention study.
Results
Patients with non-HELLP thrombocytopenia had lower sFlt1 (7.3 +/- 3.8 ng/mL vs 15.5 +/- 5 ng/mL, p<0.001), lower sEng (8.7 +/- 3.6 vs 34 +/- 17, p <0.001) and higher PlGF (484 +/- 412 vs 66.3 +/- 44, p= 0.003) than patients with HELLP syndrome. Angiogenic factor abnormalities in patients with preeclampsia were similar to patients with HELLP syndrome, suggesting a common pathogenesis. Patients with non-HELLP thrombocytopenia had angiogenic profiles similar to normotensive controls, whereas patients with HELLP syndrome had levels higher than the 90th percentile for sFlt1 and sEng and lower than the 10th percentile for PLGF.
Conclusion
Angiogenic biomarkers may be useful in excluding conditions, which mimic preeclampsia.
Keywords: preeclampsia, angiogenic factors, thrombocytopenia
Introduction
Preeclampsia (PE) occurs in 2-7% of pregnancies [1, 2] and is a leading cause of maternal and neonatal morbidity and mortality. Preeclampsia contributes to adverse maternal outcomes such as iatrogenic delivery, increased rate of cesarean section, risk of liver and kidney damage, seizures, abruption, disseminated intravascular coagulation, blood transfusion, prolonged hospitalization and death. It also contributes to adverse fetal and neonatal outcomes including prematurity, intrauterine growth restriction and rarely fetal or neonatal demise [1, 2]. Besides the acute effects on maternal health, there is emerging evidence that preeclampsia, particularly severe early onset preeclampsia, has long term health impacts such as increased risk of future cardiovascular disease, stroke and renal dysfunction[3].
The classic diagnosis of preeclampsia is based on the development of proteinuria and hypertension after 20 weeks gestational age. However, preeclampsia is a heterogeneous disease and can be difficult to diagnose due to the variable onset of hypertension, proteinuria, symptomatology and laboratory abnormalities. The diagnosis can be challenging and time consuming to confirm or exclude, requiring hospitalization, serial blood pressure monitoring, serial blood laboratory studies, and a 24 hour urine collection[4]. Up to 25% of patients may develop atypical preeclampsia or HELLP syndrome (hemolysis, elevated liver enzymes and low platelets), which is difficult to differentiate from other diseases presenting with transaminitis, hemolysis or thrombocytopenia [5, 6].
Many women present for a preeclampsia evaluation with a low platelet count secondary to either gestational thrombocytopenia or immune thrombocytopenic purpura (ITP) or manifest some features associated with preeclampsia, but nevertheless do not have preeclampsia. Currently, there is no serum test either to exclude or confirm preeclampsia. Research from our group and others [7-17] has demonstrated that an imbalance of angiogenic proteins is important in pathogenesis of preeclampsia. Multiple studies have found an increase in antiangiogenic proteins such as soluble fms-like tyrosine kinase 1 (sFlt1) and soluble endoglin (sEng), and a decrease in the proangiogenic protein placental growth factor (PlGF), in the sera of pregnant women at the time of clinical disease [8-14, 16, 17]. Additionally, multiple studies have demonstrated high sensitivity and specificity of sFlt1 [8, 14] and sEng [14] in diagnosing preeclampsia. However, none of these studies have analyzed the utility of these markers to distinguish conditions which mimic preeclampsia, such as chronic renal disease or certain types of thrombocytopenia, from true preeclampsia.
This pilot study sought to evaluate the utility of these biomarkers in distinguishing pregnant women with non-HELLP thrombocytopenia from those with severe preeclampsia and HELLP. We defined non-HELLP thrombocytopenia as pregnant women who had low platelet counts secondary to either gestational thrombocytopenia or ITP and did not have clinical preeclampsia. We hypothesized that sFlt1, sEng and PlGF would distinguish women with PE and HELLP from non-HELLP thrombocytopenia; specifically, patients with PE/HELLP would have increased levels of antiangiogenic proteins and decreased levels of proangiogenic proteins whereas thrombocytopenic patients would not.
Materials and Methods
Women with singleton pregnancies > 20 weeks of gestation presenting to Labor and Delivery or the antepartum service at Beth Israel Deaconess Medical Center (Boston, MA) for a preeclampsia evaluation between 2004-2008 were included in this study. All women gave informed written consent according to the committee on clinical investigations at Beth Israel Deaconess Medical Center who approved the study. The pregnancy outcomes were analyzed by reviewing each case and documenting maternal and fetal outcomes prior to serum analysis by two physicians (BY and SR). Women with new onset hypertension (>140/90) recorded on 2 occasions at least 6 hours apart and proteinuria, defined as 300 mg protein or higher in a 24-hour urine specimen or 0.3 or greater on a protein to creatinine ratio, were considered to have preeclampsia. Women with laboratory abnormalities including hemolysis, elevated liver enzymes, and low platelet counts were defined as HELLP syndrome in accordance with ACOG definition. [18]. Women fit the criteria for gestational thrombocytopenia if they had asymptomatic thrombocytopenia (above 70,000) occurring during the pregnancy with normal platelet counts documented prior to pregnancy[19]. Women were defined as having ITP (immune thrombocytopenic purpura) if the thrombocytopenia had occurred prior to pregnancy (chronic) or had resulted from a viral infection (acute) with persistent platelet counts below 100,000[19]. Three out of the 11 patients with HELLP had been studied previously for angiogenic factor abnormalities [16].
Serum samples were obtained from each patient prior to delivery, stored at -70C, and thawed once for analysis. Samples were analyzed by a single person who was unaware of the clinical outcome. Enzyme-linked immunosorbent assay (ELISA) for sFlt1, sEng and PlGF was performed with commercially available kits, as previously described (R&D systems Inc, Minneapolis, MN). All assays were performed in duplicate and values averaged. If there was >20% difference between duplicate values, the sample was run again. The correlation coefficient between duplicate results for all three biomarkers was 0.99. The intraassay and interassay coefficients of variation were 3.2% and 5.5%, respectively, for sFlt1, 3.0% and 6.3%, respectively, for endoglin and 5.6% and 10.9%, respectively, for PLGF. Significance testing was performed with t tests for comparision of continuous variables and Chi-square test for categorical variables. All probability values were 2-tailed, and a probability value of <0.05 was considered statistically significant.
The normative values for controls were obtained from a random sample of 2200 women from the trial of Calcium for Preeclampsia prevention (CPEP), a randomized, double-blind trial conducted from 1992 to 1995 in healthy nulliparas with singleton pregnancies to evaluate the effects of daily supplementation with calcium or placebo on the incidence and severity of preeclampsia [21]. Serum specimens were requested before 20 weeks of gestation, at 26-29 weeks, and at 36 weeks. Among the randomly selected women there were a total of 1564 who had remained normotensive throughout pregnancy. All 3956 serum specimens obtained from these women at 10-41 weeks of gestation were analyzed for sFlt1, sEng, and PlGF by R&D Systems Analytical Testing Services (Minneapolis, MN). Values were arranged by week of gestation as box (25th -75th percentiles) and whisker (10th and 90th percentiles) plots with a line connecting the median values.
Results
There were 9 women with non-HELLP thrombocytopenia (six women were diagnosed with gestational thrombocytopenia, and three women had ITP). Eleven patients ruled in for preeclampsia and eleven patients had HELLP based on the ACOG definition for these conditions. Characteristics of these patients, who were all non-smokers, are displayed in Table I. One patient in the thrombocytopenia group and 3 patients in the HELLP group received steroids 1-2 days prior to collection of the samples and all patients with HELLP were receiving magnesium for seizure prophylaxis.
Table I.
Demographics and Clinical Characteristics of all patients.
| Thrombocytopenia N=9 |
HELLP N=11 |
Preeclampsia N=11 |
p-values* | |
|---|---|---|---|---|
| Age (years) | 29.7 +/- 7.8 | 32 +/- 6.7 | 30.5 +/- 5.4 | NS |
| Parity | .9 +/- 1.1 | 0.4 +/- 0.5 | 1.2 +/- 1.5 | NS |
| GA (weeks) | 38.2 +/-3.6 | 30.3 +/- 7.1 | 32.0 +/- 2.8 | 0.007, <0.001 |
| BMI | 29.5 +/- 3.2 | 30.8 +/- 8.4 § | 29.5 +/- 4.3 | NS |
| SBP (mm Hg) | 116 +/- 10 | 166 +/- 22 | 160 +/- 19 | <0.001, <0.001 |
| DBP (mm Hg) | 71 +/- 9 | 103 +/- 13 | 95 +/- 8 | <0.001, <0.001 |
| Hematocrit (%) | 33.8 +/- 2.6 | 35.1 +/- 1.9 | 33.5 +/- 3.1 | NS |
| Uric Acid in mg/dl) | 5.2 +/- 1.2 | 6.7 +/- 0.7 | 6.6 +/- 1.0 | NS, 0.04 |
| Creatinine (mg/dl) | 0.5 +/- 0.1 | 0.7 +/- 0.2 | 0.7 +/- 0.3 | NS |
| PLT | 102 +/- 40 | 105 +/- 35 | 251 +/- 71 | NS, <0.001 |
| ALT | 13 +/- 4.3 | 450 +/- 1118 (urine P:C ratio) |
23 +/- 13 | NS |
| Urine analysis | ¥ see note | 5.0 +/- 8.8 | 3.3 +/- 4.3 | |
| Birth Weight (gms) | 3527 +/- 532 | 1762 +/- 1070 | 1869 +/- 828 | 0.0004, 0.0001 |
Mean ± standard deviation. GA = gestational age, BMI = body-mass index (kg/m2), SBP = systolic blood pressure DBP = diastolic blood pressure PLT = platelets × 103 / mm2− AST = aspartate transaminase
p-value of Thrombocytopenia vs. HELLP and Thrombocytopenia vs. Preeclampsia
BMI known for only 5 patients in HELLP group.
2 patients in thrombocytopenia group had 24-hour urine proteins performed: 280 and 299 mg. The remainder of the patients had negative dipsticks
Table II shows the mean values of sFlt1, sEng and PlGF for women in each group. The patients with non-HELLP thrombocytopenia had significantly lower levels of sFlt1 and sEng and higher levels of PlGF compared to women with PE or HELLP. Since sFlt1 acts mainly by antagonizing PlGF and sFlt1 and sEng have been postulated to have a synergistic effect, we also examined the ratio of sFlt1/PlGF and a composite ratio of (sFlt1+sEng)/PlGF. We found that ratio of sFlt1/PlGF as well as composite ratio was significantly lower in patients with non-HELLP thrombocytopenia compared to women with PE and HELLP (Table II).
Table II.
Distribution of the mean values of serum sFlt1, sEng, PlGF, sFlt/PlGF ratio and composite ratio (sFlt1+sEng/PlGF) among patients with non-HELLP thrombocytopenia, HELLP and preeclampsia.
| Thrombocytopenia N=9 |
HELLP N=11 |
Preeclampsia N=11 |
p- values* | |
|---|---|---|---|---|
| sFlt1(ng/mL) | 7.3 +/- 3.8 | 15.5 +/- 5 | 23.5 +/- 4.3 | 0.001, <0.001 |
| sEng(ng/mL) | 8.7 +/- 3.6 | 34 +/- 17 | 43.6 +/- 27.7 | <0.001, 0.001 |
| PlGF(pg/mL) | 484 +/- 412 | 66.3 +/- 44 | 117.4 +/- 63.9 | 0.003, 0.009 |
| sFlt1 / PlGF | 28 +/- 32 | 336 +/- 208 | 286 +/- 200 | <0.001, 0.001 |
| (sFlt1 + sEng) /PLGF | 61 +/- 66 | 1303 +/- 1269 | 870 +/- 752 | 0.009, 0.005 |
Mean ± standard deviation
p-value of Thrombocytopenia vs. HELLP and Thrombocytopenia vs. Preeclampsia
When these values are plotted by gestational age against the normogram obtained from the CPEP trial, the values for all patients with non-HELLP thrombocytopenia were within the normal range while all patients with HELLP syndrome had levels higher than the 90th percentile (sFlt1 and sEng) and lower than the 10th percentile (PlGF). Patients with HELLP syndrome all had statistically significant higher levels of sFlt1: PlGF ratios compared to the normotensive and non-HELLP thrombocytopenia patients except for one patient at 40 weeks of gestation. Similarly, HELLP patients had higher levels of (sFlt1 +sEng) / PlGF except for one patient at 37 weeks and one at 40 weeks (FIGURE 1- A, B, C).
Figure 1.
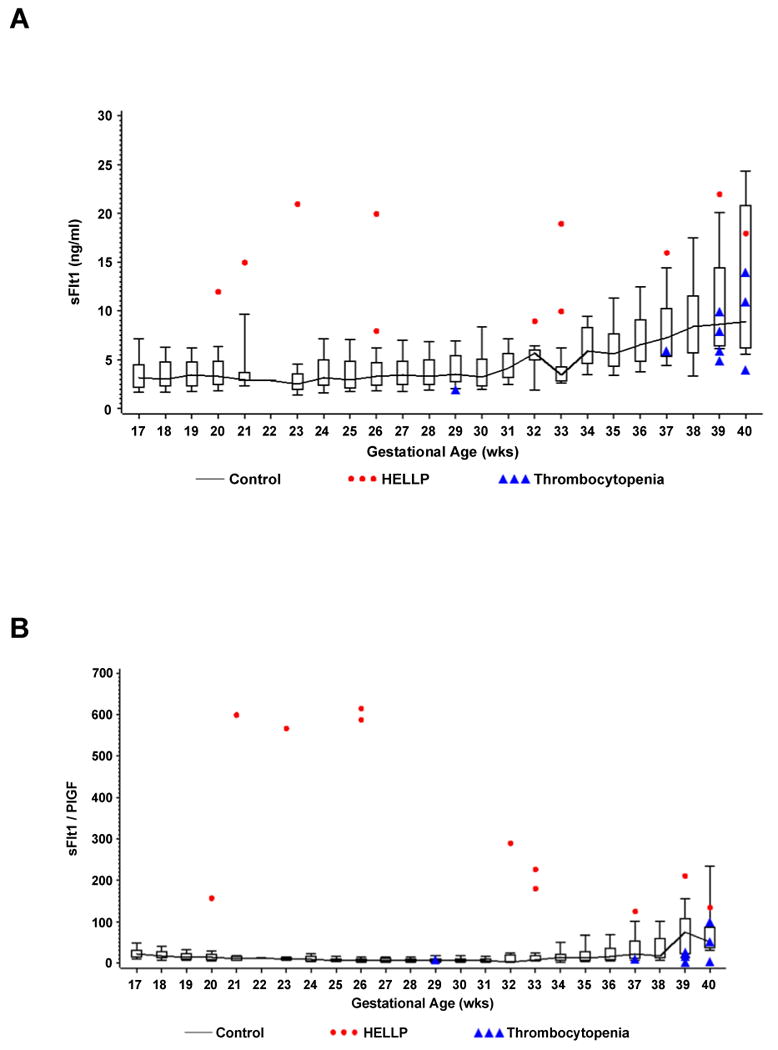
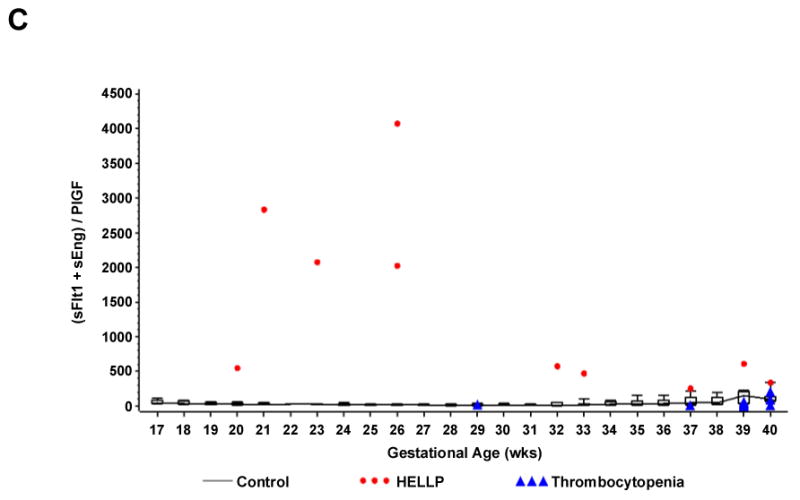
Shows individual values of serum sFlt1 (Panel A), the sFlt1 / PlGF ratio (Panel B), and the composite ratio (sFlt1 +sEng) / PlGF (Panel C) in women with non- HELLP thrombocytopenia (gestational thrombocytopenia or immune thrombocytopenic purpura) or the HELLP syndrome by gestational age. Normal values for each week of gestation were derived from 3956 serum samples of 1564 randomly selected women from the CPEP trial who had remained normotensive throughout pregnancy and displayed as box and whisker plots. Each box represents the 25th through 75th percentiles; the whiskers extend to the 10th and 90th percentiles. The boxes are joined by a line connecting the median values.
Discussion
In this pilot study, we demonstrate that measurement of angiogenic markers may be useful to differentiate non-HELLP thrombocytopenia from thrombocytopenia associated with the HELLP syndrome. Furthermore, we demonstrate that the angiogenic factor abnormalities noted in the HELLP syndrome were similar to patients with preeclampsia, suggesting that the pathogenesis of these two syndromes may be similar.
Thrombocytopenia is one of the laboratory abnormalities used to diagnose HELLP syndrome; therefore, patients with previously undiagnosed thrombocytopenia may present as a challenge to clinicians especially when other features of preeclampsia are present [6]. Gestational thrombocytopenia and severe preeclampsia both have a similar incidence[20] but the clinical significance of these conditions is vastly different. It is important to be able to distinguish patients with ITP and gestational thrombocytopenia from patients with preeclampsia and HELLP as patients with thrombocytopenia (either gestational or ITP) are managed expectantly or treated with oral corticosteroids while patients with HELLP syndrome or severe PE are expeditiously delivered. To make the diagnosis of preeclampsia, many patients are hospitalized for blood pressure monitoring, serial blood draws and 24-hour urine collections to provide diagnostic information for the clinician. The diagnosis of HELLP syndrome can be challenging as 15% of patients with HELLP may present without hypertension or without proteinuria [5]. In non-classic presentations of HELLP and preeclampsia, an accurate means of diagnosis is necessary to ensure proper obstetrical management, particularly at early gestational ages.
Our finding of elevated sFlt1 and sEng levels in preeclampsia and HELLP patients is similar to previous reports [9, 10, 14, 17], which demonstrated elevated concentrations of circulating sFlt1 and sEng and lower concentrations of PlGF in patients with preeclampsia than in control patients, supporting the theory that an imbalance of proangiogenic and antiangiogenic factors may result in preeclampsia. Levine et al showed statistically significant elevated levels of sFlt1 beginning approximately 5 weeks before the onset of preeclampsia compared to control patients within the CPEP trial. Similarly, the PlGF levels were significantly lower in women who had preeclampsia beginning weeks before the onset of clinically apparent disease. [10] A follow-up case-control study from the same group found rising circulating levels of soluble endoglin were accompanied by an increased ratio of sFlt1: PlGF beginning 9-11 weeks before the onset of preterm preeclampsia and 12-14 weeks before the onset of term preeclampsia. [9]
A recent study analyzed the sensitivity and specificity of utilizing sFlt1 and sEng in distinguishing preeclampsia from normotensive patients. This pilot study found 90% sensitivity and 90% specificity for sFlt1 and 90% sensitivity and 95% specificity for sEng [14]. However, this study did not include patients with thrombocytopenia or underlying medical conditions. Prior literature supports the use of the sFlt1 / PlGF ratio and of the composite ratio [(sFlt1+sEng) / PlGF] as a measure incorporating either two or three of the biomarker molecules. [9, 11] Use of these two ratios demonstrated significantly different values between the women with thrombocytopenia and those with preeclampsia and HELLP.
We utilized the normative values for sFlt1, sEng and PlGF based on 1564 normotensive subjects from CPEP This trial showed no difference in obstetrical outcome with or without 2 grams of daily calcium supplementation. [21] The range of values among normotensive women is displayed in box and whisker plots by week of gestational age in Figure 1 (A, B & C). Our patients with non-HELLP thrombocytopenia had similar values as gestational age-matched normal values from the CPEP study while patients with HELLP syndrome had concentrations of antiangiogenic factors that are significantly higher and concentrations of PlGF that are significantly lower than those of the large cohort of normotensive controls from the CPEP study.
Our pilot study supports the use of angiogenic biomarkers by showing statistically significant differences in levels of sFlt1, sEng, PlGF, and angiogenic ratios for patients with non-HELLP thrombocytopenia compared to HELLP. The angiogenic profile of these patients with thrombocytopenia compared to preeclampsia and HELLP is significantly different. This study is the first published, that we are aware of, to use angiogenic biomarkers to help distinguish HELLP from patients who have some features of preeclampsia such as thrombocytopenia but do not actually have the condition. These angiogenic biomarkers may be particularly useful to help diagnose or exclude non-classical presentations of preeclampsia or conditions with similar laboratory abnormalities to those found in HELLP syndrome.
Our study is limited by small sample sizes of the thrombocytopenia, HELLP, and preeclampsia subjects. Additionally, there may have been bias secondary to the selection of patients based on their diagnosis. However, all patients regardless of underlying co-morbidities or conditions were diagnosed based on the ACOG definition of gestational thrombocytopenia, ITP or hypertensive diseases of pregnancy. However, it is reassuring to note that even with small numbers of patients we were able to show statistically significant differences between the groups. We are planning a prospective trial to evaluate the role of these biomarkers as an aid in the diagnosis of preeclampsia and/or HELLP syndrome.
Acknowledgments
SR is supported by NIH grant WRHR 5k12HDOO1255. S.A.K is an investigator of the Howard Hughes Medical Institute. RJL receives salary support from the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Disclosures: S.A.K. reports having served as a consultant to Abbott, Beckman Coulter, Roche, and Johnson & Johnson and having been named coinventor on multiple provisional patents filed by Beth Israel Deaconess Medical Center for the use of angiogenesis-related proteins for the diagnosis and treatment of preeclampsia.
References
- 1.Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102:181–192. doi: 10.1016/s0029-7844(03)00475-7. [DOI] [PubMed] [Google Scholar]
- 2.Hauth JC, Ewell MG, Levine RJ, et al. Pregnancy outcomes in healthy nulliparas who developed hypertension. Calcium for Preeclampsia Prevention Study Group. Obstet Gynecol. 2000;95:24–28. doi: 10.1016/s0029-7844(99)00462-7. [DOI] [PubMed] [Google Scholar]
- 3.Baumwell S, Karumanchi SA. Pre-eclampsia: clinical manifestations and molecular mechanisms. Nephron Clin Pract. 2007;106:c72–81. doi: 10.1159/000101801. [DOI] [PubMed] [Google Scholar]
- 4.Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia-eclampsia. Am J Obstet Gynecol. 2008 doi: 10.1016/j.ajog.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 5.Stella CL, Sibai BM. Preeclampsia: Diagnosis and management of the atypical presentation. J Matern Fetal Neonatal Med. 2006;19:381–386. doi: 10.1080/14767050600678337. [DOI] [PubMed] [Google Scholar]
- 6.Kamen B, Karwal MA, Yankowitz J. Hemolysis and elevated transaminases imitating severe preeclampsia. Obstet Gynecol. 2009;113:545–547. doi: 10.1097/AOG.0b013e31819388d5. [DOI] [PubMed] [Google Scholar]
- 7.Bdolah Y, Lam C, Rajakumar A, et al. Twin pregnancy and the risk of preeclampsia: bigger placenta or relative ischemia? Am J Obstet Gynecol. 2008;198:428 e421–426. doi: 10.1016/j.ajog.2007.10.783. [DOI] [PubMed] [Google Scholar]
- 8.Chaiworapongsa T, Romero R, Kim YM, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 9.Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 10.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 11.Levine RJ, Thadhani R, Qian C, et al. Urinary placental growth factor and risk of preeclampsia. JAMA. 2005;293:77–85. doi: 10.1001/jama.293.1.77. [DOI] [PubMed] [Google Scholar]
- 12.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polliotti BM, Fry AG, Saller DN, Mooney RA, Cox C, Miller RK. Second-trimester maternal serum placental growth factor and vascular endothelial growth factor for predicting severe, early-onset preeclampsia. Obstet Gynecol. 2003;101:1266–1274. doi: 10.1016/s0029-7844(03)00338-7. [DOI] [PubMed] [Google Scholar]
- 14.Salahuddin S, Lee Y, Vadnais M, Sachs BP, Karumanchi SA, Lim KH. Diagnostic utility of soluble fms-like tyrosine kinase 1 and soluble endoglin in hypertensive diseases of pregnancy. Am J Obstet Gynecol. 2007;197:28 e21–26. doi: 10.1016/j.ajog.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Shibata E, Rajakumar A, Powers RW, et al. Soluble fms-like tyrosine kinase 1 is increased in preeclampsia but not in normotensive pregnancies with small-for-gestational-age neonates: relationship to circulating placental growth factor. J Clin Endocrinol Metab. 2005;90:4895–4903. doi: 10.1210/jc.2004-1955. [DOI] [PubMed] [Google Scholar]
- 16.Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 17.Wikstrom AK, Larsson A, Eriksson UJ, Nash P, Norden-Lindeberg S, Olovsson M. Placental growth factor and soluble FMS-like tyrosine kinase-1 in early-onset and late-onset preeclampsia. Obstet Gynecol. 2007;109:1368–1374. doi: 10.1097/01.AOG.0000264552.85436.a1. [DOI] [PubMed] [Google Scholar]
- 18.Diagnosis and management of preeclampsia and eclampsia: ACOG practice bulletin 33. Jan, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Thrombocytopenia in pregnancy: ACOG practice bulletin 6. Sep, 1999. [Google Scholar]
- 20.Burrows RF, Kelton JG. Thrombocytopenia at delivery: a prospective survey of 6715 deliveries. Am J Obstet Gynecol. 1990;162:731–734. doi: 10.1016/0002-9378(90)90996-k. [DOI] [PubMed] [Google Scholar]
- 21.Levine RJ, Hauth JC, Curet LB, et al. Trial of calcium to prevent preeclampsia. N Engl J Med. 1997;337:69–76. doi: 10.1056/NEJM199707103370201. [DOI] [PubMed] [Google Scholar]


