Abstract
Endogenous Cushing syndrome (CS) is caused by excess adrenal glucocorticoid secretion that is adrenocorticotropin (ACTH)-dependent or independent; ACTH-independent adrenocortical causes of CS account for up to 20% of CS in adults, and 15% in children over age 7 years. In younger children, ACTH-independent CS may account for as many as half of CS cases. In both adults and children, adrenocortical lesions causing CS include the common, isolated and sporadic, solitary cortisol-producing adenoma, the rare adrenocortical cancer, and a spectrum of recently recognized, bilateral hyperplasias (bilateral adrenocortical hyperplasias - BAHs): micronodular adrenal disease (MAD) and its pigmented variant, primary pigmented nodular adrenocortical disease (PPNAD) are mostly genetic processes. Macronodular BAHs, ACTH-independent macronodular hyperplasia (AIMAH) or massive macronodular adrenocortical disease (MMAD) are less frequently genetic and almost never present in children (except in McCune-Albright syndrome); they present often with atypical CS in middle-aged or elderly adults. The majority of benign adrenocortical tumors associated with CS are associated with defects of the cyclic AMP signaling pathway, whereas adrenal cancer is linked to aberrant expression of growth factors and germline or somatic mutations of tumor suppressor genes such as TP53. Adrenalectomy is the preferred mode of treatment for all adrenocortical causes of CS.
Keywords: adrenal tumors, Cushing syndrome, cyclic AMP signaling, macronodular hyperplasia, primary pigmented nodular adrenocortical disease
Introduction
Endogenous Cushing syndrome (CS) is a rare disorder [1]. There are significant differences in the pathophysiology and epidemiology of hypercortisolemia among age groups [2]; different criteria are being used for the confirmation and differential diagnosis of this disorder in children [3, 4]. CS may be caused by corticotrophin (ACTH)-producing pituitary tumors, a disorder also known as ‘Cushing disease’ (CD), or by corticotrophin (ACTH)-independent, cortisol-producing adrenocortical tumors (ADTs). In children, ectopic production of ACTH is extraordinarily rare [2, 5]: it has been reported only in a handful of cases confined at the extremes of pediatric age, infants with neuroblastomas or other neuroendocrine tumors [5] and adolescents with carcinoids, sporadic or in the context of multiple endocrine neoplasia type-1. Bilateral adrenocortical hyperplasias (BAHs) are far more common as causes of CS in children than in older patients [1, 2].
Epidemiology
Adrenocortical neoplasms account for less than 0.5% of all clinically significant tumors; however, autopsy studies indicate that as many as 10% of adults over the age of 40 years may have an ADT, usually a simple nodule that is not larger than 1 cm; up to 36% may have micronodular hyperplasia [6]. CS is a manifestation of approximately one third of all ADTs. In children, a significant number of ADTs presenting with CS are malignant, but the opposite is true in adults. There is a female-to-male predominance for ADTs in all ages (although this is probably not true for infants and toddlers).
Clinical presentation
In most patients the onset of CS is rather insidious [1–4]. The most common presenting symptom of the syndrome is weight gain, although it is not universally present. Pathognomonic for CS in childhood is weight gain associated with growth retardation [7]. Other common problems reported include facial plethora, headaches, hypertension, hirsutism, amenorrhea, and hypogonadism (or delayed sexual maturation in children) [8]. Virilization is rare in ADTs unless the tumor produces adrenal androgens in addition to glucocorticoids; skin manifestations, including acne, violaceous striae and bruising and acanthosis nigricans are also common. Sleep disruption, muscular weakness and mental changes are also frequent.
Diagnostic evaluation of ACTH-independent CS
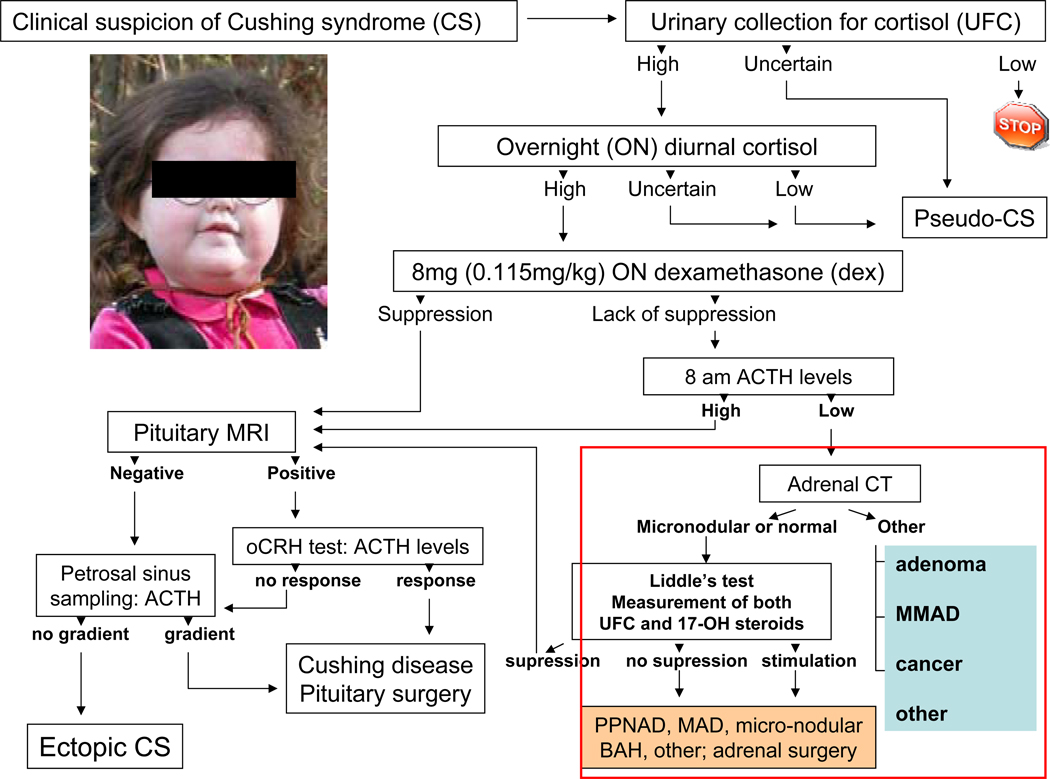
Diagnostic testing for CS is complicated by developmental differences in the regulation of hypothalamic-pituitary-adrenal (HPA) axis between young and older individuals, as well as other factors, such as exposure to steroid hormones, medications and other exogenous substances, stress, and chronic illness [3, 8]; these factors may influence normal values for several of the tests employed in the work-up for CS [2–4, 8]. In children, the diagnosis of CS is facilitated by the inhibitory effects of hypercortisolemia on height gain [7]. Indeed, the deceleration of growth velocity with a concurrent and unabated weight gain are the hallmark of CS in childhood [7–9]. In contrast, in lieu of these apparent signs, extensive biochemical investigation is needed for the confirmation of the diagnosis of CS in adult patients [1–4], especially if their situation is complicated by moderate weight gain and other conditions, which collectively have been called “pseudo-Cushing” states [10]. Adult patients may also have ectopic sources of ACTH as causes of CS in up to 10–15% of the total number of cases [11]. In general, the diagnostic evaluation proposed in Figure 1 is what we recommend for both adults and children with CS [1–4, 8, 10–12] (Figure 1).
Figure 1.
Diagnostic work-up of CS – in the box (right lower panel) adrenocortical causes of CS
The appropriate therapeutic interventions in CS depend on accurate diagnosis and classification of the disease. The history and clinical evaluation (including growth charts in children) are important to make the initial diagnosis. Upon suspicion of the syndrome, laboratory and imaging confirmations are necessary. An algorithm of the diagnostic process is presented in Figure 1. The first step in the diagnosis of CS is to document hypercortisolism. This step is usually done in the outpatient setting. Because of the circadian nature of cortisol and ACTH, isolated cortisol and ACTH measurements are not of great value in diagnosis. One excellent screening test for hypercortisolism is a 24hour urinary free cortisol (UFC) excretion corrected for body surface area. A normal 24-hour UFC value is < 70 ug/m2/day (with the radioimmunoassay values). Falsely high UFC may be obtained because of physical and emotional stress, chronic and severe obesity, pregnancy, chronic exercise, depression, alcoholism, anorexia, narcotic withdrawal, anxiety, malnutrition and excessive water intake (more than 5L/day). These conditions may lead to sufficiently high UFCs to cause what is known as pseudo-CS. On the other hand, falsely low UFC may be obtained mostly with inadequate collection. Another baseline test for the establishment of the diagnosis of CS is a low dose dexamethasone suppression test; the cortisol cut-off level should be <1.8 ug/dl (50nmol/L); if it is greater than 1.8 ug/dl, further evaluation is necessary. If the response to both the 1mg dexamethasone overnight suppression test and the 24hour UFC are both normal, a diagnosis of CS may be excluded with the following caveat: 5–10% of patients may have intermittent or periodic cortisol hypersecretion and may not manifest abnormal results to either test. If periodic or intermittent CS is suspected, continuous follow up of the patients is recommended. Diurnal plasma cortisol variation, including midnight cortisol values, is a very good test for the establishment of the diagnosis of CS. In our institution, it has become the test of choice for the confirmation of endogenous hypercortisolemia and is routinely done in patients with confirmed elevated urinary cortisol levels on the outside. There are several caveats for the interpretation of the test of which the most important ones are: 1) The venous catheter has to be placed at least two hours before the test; and 2) if the patient comes from another time zone, a 1-hour-per-day adjustment should be taken into account prior to obtaining the test. In general, serum cortisol levels are drawn at 11:30PM and 12:00MN and at 7:30AM and 8:00AM, while the patient is lying in bed and asleep; mid-night cortisol levels above 5 ug/dl are abnormal and confirm the diagnosis of Cushing syndrome, whereas an inverted diurnal rhythm is seen in BAHs and some other adrenal tumors. If one of the tests is suggesting CS or, if there is any question about the diagnosis, tests that distinguish between pseudo-Cushing states and CS may be obtained. One such test is the combined dexamethasone-CRH test. Once the diagnosis of CS is confirmed there are several tests to distinguish ACTH-dependent disease from the ACTH-independent syndrome. A spot plasma ACTH may be measured; if this measurement is <5pmol/l it is indicative of ACTH-independent Cushing syndrome, although the sensitivity and specificity of a single ACTH measurement are not high because of the great variability in plasma ACTH levels and the instability of the molecule after the sample’s collection. Even if one assumes that the sample was collected and processed properly (collected on ice and spun down immediately in a refrigerated centrifuge for plasma separation; the sample should then be immediately processed or frozen at −20°C), ACTH levels that are between 5 and 20 pmol/l are not informative in this era of high sensitivity assays; levels above 20 pmol/l are more suggestive of an ACTH-dependent condition, but again that is not a certainty until single ACTH levels are repeatedly over 70pmol/l.
The standard six-day low- and high dose dexamethasone suppression test (Liddle’s test) is used to differentiate Cushing disease from ectopic ACTH secretion and adrenal causes of CS. In the classic form of this test, after 2 days of baseline urine collection, 0.5 mg of dexamethasone (adjusted per weight for children <70kg by dividing the dose by 70 and multiplying by the weight of the child) every 6-hours are given per os starting at 6.00 am on day #3 (“low dose” phase of the test) for a total of 8 doses (2 days); this is continued with a 2 mg dose of dexamethasone per os (adjusted per weight for children <70kg by dividing the dose by 70 and multiplying by the weight of the child) on day #5 (“high dose” phase of the test) given every 6 hours for another 8 doses (final 2 days). UFCs and 17-hydroxysteroid (17OHS) excretion are measured at baseline, during, and 1 day after the end of the dexamethasone administration. Approximately 90% of patients with Cushing disease will have suppression of cortisol and 17-hydroxysteroid values, whereas less than 10% of patients with ectopic ACTH secretion will have suppression. UFC values should suppress to 90% of baseline value and 17-hydroxysteroid excretion should suppress to less than 50% of baseline value. The criteria are similar if one uses serum cortisol values obtained at 8 am of the morning after the last dose of dexamethasone, e.g. serum cortisol on day #7 should be 90% of baseline serum cortisol values (obtained at 8 am the day before dexamethasone administration).
The Liddle test has been modified to 1) giving 2 mg every 6-hours (without the preceding low-dose phase); 2) administering dexamethasone intravenously over 5 hours at a rate of 1mg/hour; or 3) giving a single high dose of dexamethasone (8mg, in children adjusted for weight<70kg) at 11pm and measuring the plasma cortisol level the following morning. This overnight, high dose dexamethasone test has sensitivity and specificity values similar to those of the classic Liddle’s test: a 50% suppression of serum cortisol levels from baseline is what differentiates Cushing disease (more than 50% suppression) from other causes of CS (adrenal or ectopic ACTH production) (less than 50% suppression) [12]. An oCRH stimulation test may also be obtained for the differentiation of Cushing disease from ectopic ACTH secretion [11], but it is less useful in the diagnosis of ADTs [8].
In addition to the biochemical testing, the most useful tests in the diagnosis of cortisol-producing ADTs are imaging (computed tomography-CT or magnetic resonance imaging-MRI) and Liddle’s test (especially in the diagnosis of BAHs). CT is preferred over MRI for cortical (versus medullary) tumors because it allows for better delineation of the adrenal contour (Figure 2). With the use of contrast material, adrenal CT is an excellent diagnostic tool in the investigation of ADTs although more testing is required for the delineation of a cortisol producing benign ADT. Most adrenocortical carcinomas are unilateral and quite large by the time they are detected. Ultrasound may not be used to image the adrenal glands for the diagnostic work up of CS, because its sensitivity and accuracy is much less than CT or MRI. Catheterization studies may not be used to confirm the source of cortisol secretion in ADTs.
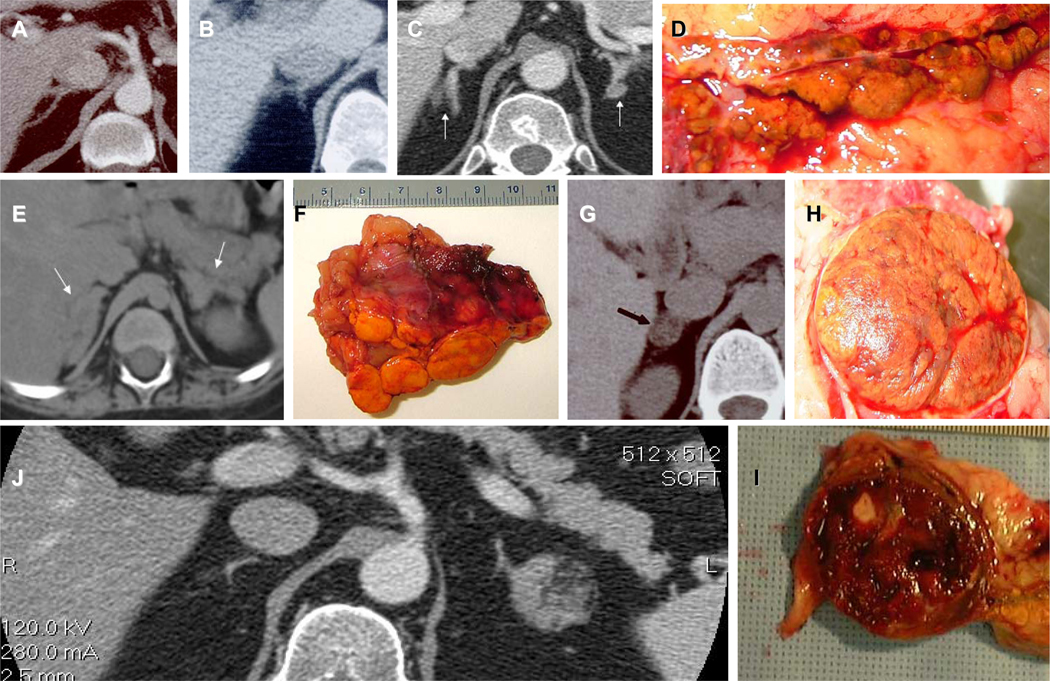
Figure 2.
A, B, C, Computed tomography (CT) of the adrenal glands from 3 patients with micronodular BAHs; D. the right adrenal gland with PPNAD from the patient whose CT is shown in C: multiple small, pigmented nodules are seen that are characteristic of PPNAD; E. CT from a patient with MMAD/AIMAH and CS with massive enlargement of the adrenal glands on both sides (arrows) and F. her left adrenal with visible multiple macronodules; G. CT from an adolescent with a common, solitary, cortisol-producing adrenal adenoma (arrow); H. The tumor from the patient in G: this is a classic cortisol-producing adenoma with yellow fat and brown discoloration the result of pigment (lipofuscin and rarely neuromelanin). J. CT from a patient with a left-sided cortisol-producing tumor that was inhomogeneous with hemorrhage and calcifications; I. The tumor from patient J: a calcification and blood are visible; the tumor was premalignant.
Histological types of benign ADTs causing CS
Benign ADTs causing CS include the common adrenocortical adenoma (ADA) and BAHs [13] such as primary pigmented nodular adrenocortical disease (PPNAD) and corticotropin (ACTH)-independent macronodular adrenocortical hyperplasia (AIMAH) - also known as massive macronodular adrenocortical disease (MMAD). The various types of adrenocortical lesions, their histology and other information are given in Table 1. The common cortisol-producing ADA of the zona fasciculata needs little introduction, although histological variants of this common lesion do exist.
Table 1.
Adrenocortical causes of cushing syndrome
| Adrenocortical lesions | Age group | Histopathology | Genetics | Gene/locus |
|---|---|---|---|---|
| I. BENIGN | ||||
| 1. Common adenoma | all ages | adenoma of the zona fasciculata | MEN 1, FAP, MAS, HLRCS, CNC, Carney triad, other; | menin, APC, GNAS, FH, PRKAR1A; 2p16, 9q34, other; |
| 2. Macronodular hyperplasias (multiple nodules more than 1 cm each) | ||||
| Bilateral macro-adenomatous hyperplasia (BMAH | middle age | distinct adenomas (usually 2 or 3) with internodular atrophy | MEN 1, FAP, MAS, HLRCS, other; isolated (AD); other | menin, APC GNAS, FH, ectopic GPCRs |
| BMAH of childhood (c-BMAH) | infants, very young children | as above; occasional microadenomas | McCune-Albright syndrome (MAS) | GNAS |
| ACTH-independent macronodular adrenocortical hyperplasia (AIMAH), also known as massive macronodular adrenocortical disease (MMAD) (AIMAH/MMAD) | middle age | Adenomatous hyperplasia (multiple) with internodular hyperplasia of the zona fasciculata | Isolated, AD | ectopic GPCRs; WISP-2 & Wnt-signaling; 17q22-24, other |
| 3. Micronodular hyperplasias (multiple nodules less than 1 cm each) | ||||
| Isolated primary pigmented nodular adrenocortical disease (i-PPNAD) | children; young adults | micro-adenomatous hyperplasia with (mostly) internodular atrophy and nodular pigment (lipofuscin) | Isolated; AD | PRKAR1A, PDE11A; 2p16; other |
| Carney complex (CNC) -associated primary pigmented nodular adrenocortical disease (c-PPNAD) | children; young and middle ages | micro-adenomatous hyperplasia with (mostly) internodular atrophy and (mainly nodular) pigment (lipofuscin) | CNC (AD) | PRKAR1A, 2p16; other |
| Isolated micro-nodular adrenocortical disease (i-MAD) | mostly children; young adults | micro-adenomatous with hyperplasia of the surrounding zona fasciculata and limited or absent pigment | isolated, AD; other; | PDE11A, other; 2p12-p16, 5q, other |
| II. MALIGNANT | ||||
| 1. Cancer (sporadic) | all ages | mitotic figures, atypia of cortical cells; capsular invasion; metastases | isolated | TP53, β-catenin, INHA; 2p, 2q, 9q, 11q, other |
| 2. Cancer (syndromic) | children; young adults | as above | LFS (AD); BWS, RTS, other | TP53, CHK22, IGF2, other |
| 3. Brazil variant | children; young adults | as above; milder clinical course | AD; other | TP53, INHA, SF1; 9q34 amplification; other |
Abbreviations: MEN 1=multiple endocrine neoplasia type 1; FAP=familial adenomatous polyposis (polyposis coli); MAS=McCune-Albright syndrome; HLRCS=hereditary leiomyomatosis and renal cancer syndrome; FH=fumarate hydratase; AD=autosomal dominant; CNC=Carney complex; GPCR=G-protein coupled receptors; LFS=Li-Fraumeni syndrome; BWS=Beckwith-Widemann syndrome; RTS=Rubinstein-Taybi syndrome
In all ages, the most common ADT causing CS is a unilateral adenoma (Figure 2); however, up to 10% of patients may have bilateral tumors [13]. Table 1 lists no less than 6 types of BAHs. They are divided into two groups of disorders, macro- and micro-nodular hyperplasias on the basis of the size of the associated nodules (Figure 2). In macronodular disorders, the greatest diameter of each nodule exceeds 1 cm; in the micronodular group nodules are less than 1 cm. Although nodules less than 1cm can occur in macronodular disease (especially the form associated with McCune-Albright syndrome), and single large tumors may be encountered in PPNAD (especially in older patients), the size criterion has biologic relevance, as we rarely see a continuum in the same subject: most patients are either macro- or micro-nodular. There are two additional basic characteristics that we use in this classification of BAHs [13]: that of the presence of pigment and that of status (hyperplasia or atrophy) of the surrounding cortex. Pigment in adrenocortical lesions is rarely melanin; most of the pigmentation in both ADAs and BAH that produce cortisol is lipofuscin (Figure 2). The latter appears macroscopically as light brown to, some times, dark brown or even black discoloration of the tumorous or hyperplastic tissue; microscopically, lipofuscin can be seen but it is better detected by electron microscopy.
PPNAD is a genetic disorder with the majority of cases associated with Carney complex, a syndrome of multiple endocrine gland abnormalities in addition to lentigines and myxomas; the adrenal glands in PPNAD are most commonly normal or even small in size with multiple pigmented nodules surrounded by an atrophic cortex (Figure 3). The nodules are autonomously functioning resulting in the surrounding atrophy of the cortex. Children and adolescents with PPNAD frequently have periodic or atypical CS [14, 15].
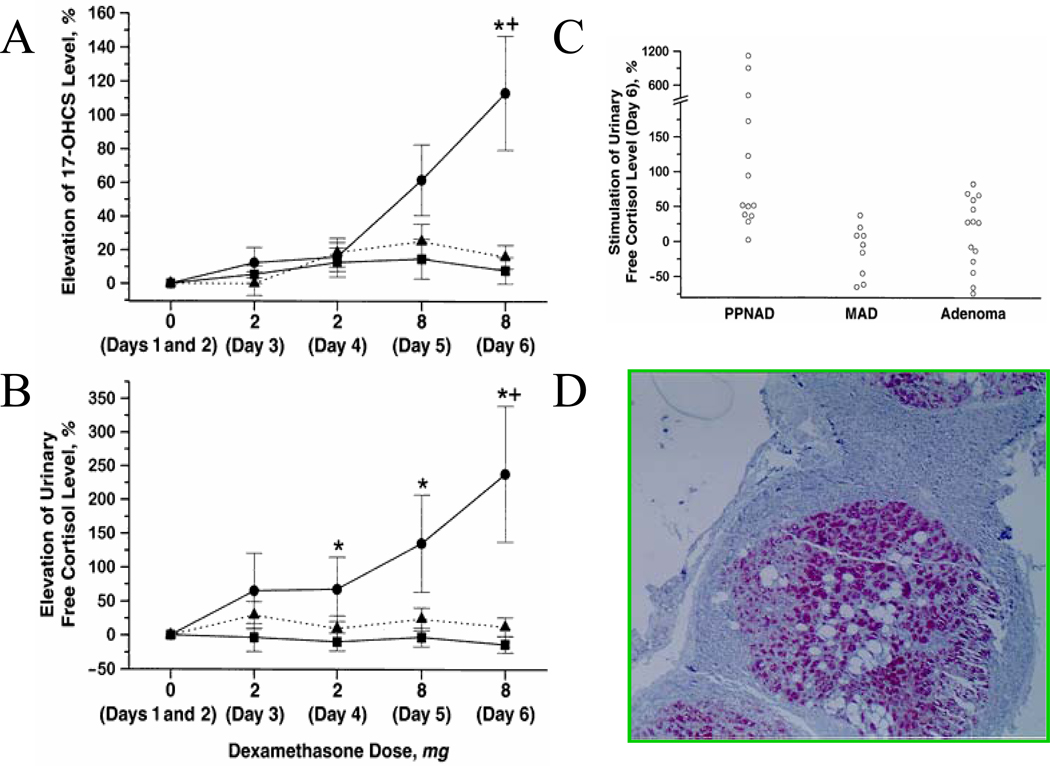
Figure 3.
Paradoxical stimulation of UFC (A) and 17OHS (B) in patients with PPNAD (closed circles) versus those with MMAD (triangles) and solitary cortisol-producing adenomas (squares) during the course of Liddle’s test. C. 50% increase of UFCs on day#6 of the test detects more than 70% of the patients with PPNAD versus other cortisol producing tumors of the adrenal cortex. D. PPNADs express synaptohysin within the cortical nodules, an unexpected feature for a cortical lesion.
AIMAH/MMAD is another rare disease, which leads to CS [16, 17]. The adrenal glands are massively enlarged with multiple, huge nodules that are typical, yellow-to-brown cortisol-producing adenomas (Figure 2). Most cases of MMAD are sporadic, although few familial cases have been described; in those, the disease appears in children. In some patients with MMAD, cortisol levels appear to increase with food ingestion (food-dependent CS) and in response to posture and other activities [16]. In these patients, aberrant expression of the neuroendocrine G-protein-coupled receptors has been demonstrated in their adrenocortical tissue. Food-dependent CS has not been described in younger patients or in children, although bilateral macronodular adrenal hyperplasia can also be seen in McCune Albright syndrome [17]. In this syndrome there is a somatic mutation of the GNAS gene leading to constitutive activation of the Gsα protein and continuous, non-ACTH-dependent stimulation of the adrenal cortex. CS in MAS is rare and usually presents in the infantile period (before 6 months of age); interestingly, a few children have had spontaneous resolution of their CS [13, 14].
Because both PPNAD and MMAD and other BAHs can present with bilateral adrenal masses (Figure 2), a useful biochemical test is the 6-day-long Liddle’s test, modified to identify stimulation of UFC secretion, rather than suppression [18]. In this test, patients with micronodular forms of BAH respond with a gradual increase of UFC and 17-OHS secretion in response to the administration of dexamethasone (by 2 days of 0.5 mg dexamethasone q6hours and 2 days of 2 mg dexamethasone q6 hours) (Figure 4). Although the cause of this “paradoxical” rise in glucocorticoid synthesis by the adrenal cortex in response to dexamethasone is not known, it is a glucocorticoid receptor-mediated phenomenon [19] that may be due to the abnormal expression of various substances by the PPNAD cortex (such as synaptophysin and others, Figure 4) [20].
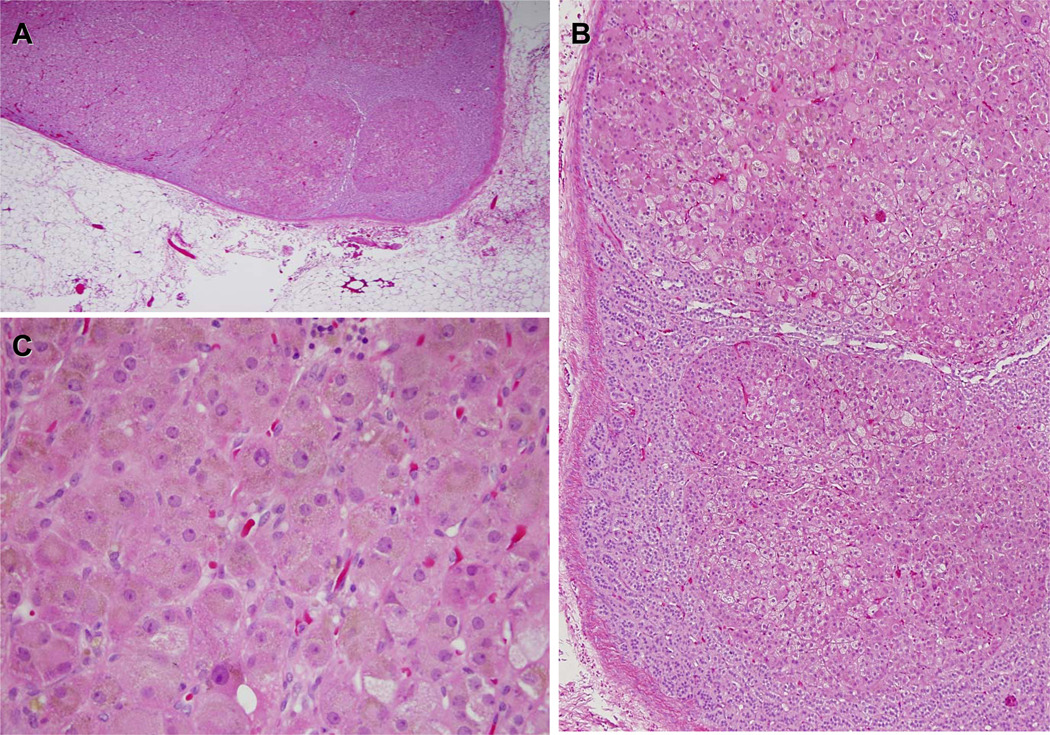
Figure 4.
A, B, and C, lower (×5) and higher (×10 and ×40) magnification, respectively, of hematoxylin and eosin stainings of the patient whose adrenal is shown in Figure 2D. The tissue demonstrates characteristic features of PPNAD such as multiple small nodules (A) surrounded by mostly atrophic or normal cortex (B) and cells that contain pigment (C) that is in most cases lipofuscin. Most PPNADs are due to PRKAR1A mutations but not all; isolated PPNAD (not associated with CNC) is frequently not associated with PRKAR1A mutations and this patient’s PRKAR1A coding sequence was normal.
Adrenal cancer and CS
Malignant neoplasias of the adrenal cortex account for 0.05–0.2% of all cancers, with an approximate prevalence of two new cases per million of population per year; adrenal cancer occurs at all ages, from early infancy to the eighth decade of life [21, 22]. A bimodal age distribution has been reported, with the first peak occurring before age of 5 years, and the second in the fourth to fifth decade. In all published series, females predominate, accounting for 65–90% of the reported cases. Several studies have shown a left-sided prevalence in adrenal cancer; however, others have reported a right-sided preponderance. In approximately 2–10% of the patients, adrenal cancer is found bilaterally. Overall, there appears to be a higher prevalence of adrenocortical carcinoma among patients with incidentally discovered adrenal masses than in the general population, although numerical estimates vary widely in the literature. Among the radiologically detectable masses, independent of size, one in 1,500 lesions may be an adrenal carcinoma; using the 5 cm cut-off as the most commonly accepted criterion for clinical investigation of an ADT, carcinoma may be found in as many as 7% of the patients with adrenal lesions [23].
In some areas of the world, higher incidence of adrenal cancer, especially in children, has been documented. This is particularly true for Southern Brazil, where enviromental mutagens and a frequent TP53 mutation have been postulated as the relevant pathogenic event [24]. In these areas, evaluation of incidentally discovered adrenal masses may be necessary for lesions smaller than 5 cm. Although the incidence of adrenal indidentalomas appears to be higher in some familial neoplasia syndromes like multiple endocrine neoplasia type-1 (MEN 1) and familial adenomatous polyposis (FAP), it is unclear whether this finding is accompanied by a higher predisposition to adrenal cancer.
CS is most common among pediatric patients with adrenal carcinoma present with a hormonal syndrome which makes their detection easier and leads to their early surgical resection and medical treatment. CS is less frequent among adults with the disease.
Clinical and molecular genetics of ADTs associated with CS
As we already mentioned, aberrant cAMP signaling has been linked to genetic forms of cortisol excess that lead to CS [25], mostly BAHs. Macro-nodular adrenocortical hyperplasia may be due to GNAS mutations associated with, either McCune-Albright syndrome or sporadic ADTs. Micro-nodular BAH, and its better-known variant, PPNAD, may be caused by germline inactivating mutations of the PRKAR1A gene [26]. Most patients with PPNAD also have CNC, as mentioned above [27].
Over the last several years, it has become apparent that there are several forms of micro-nodular BAH that are not caused by germline inactivating mutations of the PRKAR1A gene (Table 1). We described one such case associated with an atypical, episodic, form of CS in a young child [14]. Her adrenal histology showed moderate diffuse cortical hyperplasia, multiple capsular deficits, and massive circumscribed and infiltrating extra-adrenal cortical excrescences that in many cases formed micronodules that were non-pigmented. Synaptophysin, a marker for PPNAD, also stained the nodules, in addition to the surrounding cortex [14, 20].
Recently, we reported that inactivating mutations of the PDE11A and PDE8B genes could be found in a subgroup of patients with PPNAD and other forms of BAH [28–30]. PDE11A is a dual-specificity phosphodiesterase catalyzing the hydrolysis of both cAMP and cGMP; it is expressed in several endocrine tissues, including the adrenal cortex [28, 29]. The PDE11A gene was mapped to the 2q31-35 chromosomal region and tumors from patients with PDE11A-inactivating mutations demonstrated 2q allelic losses (51). The PDE11A locus, like that of other PDEs, has a complex genomic organization; of the four possible splice variants, only A4 appears to be expressed in the adrenal cortex, whereas A1 is ubiquitous, and A2 and A3 have a more limited expression pattern. More recent data show that PDE11A is widely expressed in adrenocortical tissue and its expression appears to be modified in a variety of tumors beyond PPNAD and other forms of BAH.
PDE11A mutations and polymorphisms were found as low penetrance predisposing factors to ADTs [29] The PDE11A data support the notion that this gene is not necessarily causative of BAH but that is associated with a low penetrance predisposition to the development of BAH and possibly other ADTs leading to CS and, perhaps, other conditions.
More recently, a single PDE8B mutation was identified in a young child with BAH and CS [30]; PDE8B is another cAMP-specific PDE with wide expression in endocrine tissues, including the adrenal cortex. Its involvement in ADT formation beyond this rare case of iMAD remains to be seen.
BAHs and cAMP signaling
The cause of all forms of BAH studied to date appears to be linked to increased cAMP signaling. However, the histopathological changes in the adrenal glands of patients with the various mutations or functional abnormalities of this pathway differ significantly (Figure 2). PRKAR1A mutations are associated with the pigmented micronodular variant of BAH that is known as PPNAD, whereas PDE11A (and possibly PDE8B) mutations appear to be predisposing to a variety of lesions from isolated PPNAD to non-pigmented micronodular hyperplasia; GNAS mutations are associated with the macronodular and clearly non-pigmented forms of BAH. It is also interesting that sporadic ADTs (without any family history) can be associated with somatic mutations in all three of these genes.
It is noteworthy that in all forms of BAH associated with increased cAMP signaling there are patients with mutations in one of the causative genes that do not present with overt CS. The frequency with which carriers of mutations in one of these genes present with “classic” Cushing syndrome appears to be higher in PRKAR1A mutation carriers than in PDE11A, PDE8B, or GNAS-associated disease with significant, however, inter-individual variability and without a clear genotype-phenotype correlation. Interestingly, the age at which CS presents in these disorders is exactly the reverse, with Mc-Cune Albright syndrome patients (GNAS mutation carriers) presenting almost always in infancy, whereas at least some of the patients with PDE11A, PDE8B mutations appear to present mostly in early childhood years and PRKAR1A-mutation carriers in late adolescence and young adulthood. Thus, a number of factors are likely to affect the expression of these mutations, developmental, hormonal, and perhaps, gender-related. The presence of allelic losses of the corresponding normal allele in adrenal tissues seems to also be a determining factor in the development of disease associated with PRKAR1A and PDE11A, PDE8B mutations, since all these genes were identified using LOH studies [26, 28, 30].
PDE11A and PDE8B are the first PDEs to be linked to an inherited condition associated with tumor formation but may not be the only enzyme of this large family of proteins that predisposes to tumors. Our genome-wide dataset [28] suggests that other PDEs are likely to be involved in adrenal tumorigenesis in a similar manner: not by causing tumors per se, but by being a predisposing factor. Very little is known about PDE11A, PDE8B or other PDEs in adrenocortical tissue which, however, appears to exhibit significant PDE activity in vitro. Our preliminary data suggest that several PDEs are expressed in the cortex; PDE11A is expressed at levels that are higher than those of most other such enzymes with the exception of PDE8B [28, 30].
The high frequency of PDE11A-inactivating mutations in the population [29], the possibility that other members of this large family of proteins are involved in ADT formation, and the identification of clinically-silent carriers [28–30] raise an interesting question: is it possible that PDE11A and PDE8B mutations (or mutations in a similar gene) underlie the high frequency of “incidentalomas” [6] in the general population? At the moment, this question can not be answered; larger and prospective studies need to be performed.
Surgical treatment of ADTs causing CS
Patients with benign ADTs are operated today mostly via a laparoscopic procedure (LP) that is preferred for both bilateral and unilateral lesions. LP has minimized morbidity and improvement is immediate after resection in these patients who are hypertensive pre-operatively or have other complications of CS [31]. Replacement with glucorticoids is necessary for up to a year after surgery for patients with unilateral adrenalectomies, whereas for patients after a bilateral procedure replacement with both gluco- and mineralocorticoids is necessary for life. For patients with cancer, the treatment of all primary tumors is also surgical, although open laparotomy for staging is preferable over LP. If complete resection of an adrenocortical carcinoma cannot be achieved, as much as possible of the tumor should be removed. Solitary recurrences or metastases should also be removed surgically, if possible. Long-term disease-free status has been produced by complete resection of adrenocortical carcinoma, whereas long-term remissions have followed surgical resection of hepatic, pulmonary, or cerebral metastases. Therapy with o,p'-DDD (mitotane) is initiated either as an adjuvant to surgical treatment or for patients with inoperable cancer [32, 33]. o,p'-DDD is an adrenocytolytic agent which is given at maximally tolerated oral doses (up 10g/m2/day). It ameliorates the endocrine syndrome in approximately two-thirds, whereas tumor regression or arrest of growth has been observed in as many as one-third of the patients. Occasionally, for the correction of hypercortisolism, steroid synthesis inhibitors (aminoglutethimide, metyrapone, trilostane, ketoconazole) or glucocorticoid antagonists (RU 486) are required. Patients taking mitotane (o,p'-DDD) may develop hypoaldosteronism or hypocortisolism, and fludrocortisone or hydrocortisone should be added as needed. Radiation therapy is occasionally helpful for palliation of metastases.
Concluding remarks
CS caused by ADTs is most commonly caused by a solitary adenoma; BAHs are a more frequent cause of CS than previously thought. Defects of the cAMP signaling pathway are frequent in ADTs associated with CS. Cancer associated with CS is extremely rare. Surgical advances have made ADTs causing CS a disease that is cured in most cases with the exception of cancer.
Acknowledgements
This research was supported (in part) by the Intramural Research Program of the NIH/NICHD
REFERENCES
- 1.Orth DN. Cushing's syndrome. N Engl J Med. 1995;332:791–803. doi: 10.1056/NEJM199503233321207. [DOI] [PubMed] [Google Scholar]
- 2.Newell-Price JF, Trainer PF, Besser MF, Grossman AB. The diagnosis and differential diagnosis of Cushing's syndrome and pseudo-Cushing's states. Endocr Rev. 1998;19:647–672. doi: 10.1210/edrv.19.5.0346. [DOI] [PubMed] [Google Scholar]
- 3.Nieman LK. Diagnostic tests for Cushing's syndrome. Ann N Y Acad Sci. 2002;970:112–118. doi: 10.1111/j.1749-6632.2002.tb04417.x. [DOI] [PubMed] [Google Scholar]
- 4.Boscaro MF, Barzon LF, Fallo FF, Sonino N. Cushing's syndrome. Lancet. 2001;357:783–791. doi: 10.1016/S0140-6736(00)04172-6. [DOI] [PubMed] [Google Scholar]
- 5.Normann T, Hanven J, Mjolnerod O. Cushing syndrome in an infant associated with neuroblastoma in two ectopic adrenal glands. J Pediatr Surg. 1971;6:169–175. doi: 10.1016/0022-3468(71)90313-7. [DOI] [PubMed] [Google Scholar]
- 6.Saeger W, Reinhard K, Reinhard C. Hyperplastic and Tumorous Lesions of the Adrenals in an Unselected Autopsy Series. Endocr Pathol. 1998;9:235–239. doi: 10.1007/BF02739963. [DOI] [PubMed] [Google Scholar]
- 7.Magiakou MA, Mastorakos G, Chrousos GP. Final stature in patients with endogenous Cushing's syndrome. J Clin Endocrinol Metab. 1994;79:1082–1085. doi: 10.1210/jcem.79.4.7962277. [DOI] [PubMed] [Google Scholar]
- 8.Batista DL, Riar J, Keil M, Stratakis CA. Diagnostic tests for children who are referred for the investigation of Cushing syndrome. Pediatrics. 2007;120:e575–e586. doi: 10.1542/peds.2006-2402. [DOI] [PubMed] [Google Scholar]
- 9.Lebrethon MC, Grossman AB, Afshar FF, Plowman PN, Besser GMFAU, Savage MO. Linear growth and final height after treatment for Cushing's disease in childhood. J Clin Endocrinol Metab. 2000;85:3262–3265. doi: 10.1210/jcem.85.9.6817. [DOI] [PubMed] [Google Scholar]
- 10.Papanicolaou DA, Yanovski JA, Cutler GB, Jr, Chrousos GP, Nieman LK. A single midnight serum cortisol measurement distinguishes Cushing's syndrome from pseudo-Cushing states. J Clin Endocrinol Metab. 1998;83:1163–1167. doi: 10.1210/jcem.83.4.4733. [DOI] [PubMed] [Google Scholar]
- 11.Nieman LK, Oldfield EH, Wesley R, Chrousos GP, Loriaux DL, Cutler GB., Jr A simplified morning ovine corticotropin-releasing hormone stimulation test for the differential diagnosis of adrenocorticotropin-dependent Cushing's syndrome. J Clin Endocrinol Metab. 1993;77:1308–1312. doi: 10.1210/jcem.77.5.8077325. [DOI] [PubMed] [Google Scholar]
- 12.Dichek HL, Nieman LK, Oldfield EH, Pass HI, Malley JD, Cutler GB., Jr A comparison of the standard high dose dexamethasone suppression test and the overnight 8-mg dexamethasone suppression test for the differential diagnosis of adrenocorticotropin-dependent Cushing's syndrome. J Clin Endocrinol Metab. 1994;78:418–422. doi: 10.1210/jcem.78.2.8106630. [DOI] [PubMed] [Google Scholar]
- 13.Stratakis CA, Boikos SA. Genetics of adrenal tumors associated with Cushing's syndrome: a new classification for bilateral adrenocortical hyperplasias. Nat Clin Pract Endocrinol Metab. 2007;3:748–757. doi: 10.1038/ncpendmet0648. [DOI] [PubMed] [Google Scholar]
- 14.Gunther DF, Bourdeau I, Matyakhina L, Cassarino D, Kleiner DE, Griffin K, Courkoutsakis N, Abu-Asab M, Tsokos M, Keil M, Carney JA, Stratakis CA. Cyclical Cushing syndrome presenting in infancy: an early form of primary pigmented nodular adrenocortical disease, or a new entity? J Clin Endocrinol Metab. 2004 Jul;89:3173–3182. doi: 10.1210/jc.2003-032247. [DOI] [PubMed] [Google Scholar]
- 15.Sarlis NJ, Chrousos GP, Doppman JL, Carney JA, Stratakis CA. Primary pigmented nodular adrenocortical disease: reevaluation of a patient with carney complex 27 years after unilateral adrenalectomy. J Clin Endocrinol Metab. 1997;82:1274–1278. doi: 10.1210/jcem.82.4.3857. [DOI] [PubMed] [Google Scholar]
- 16.Bourdeau I, Lampron A, Costa MH, Tadjine M, Lacroix A. Adrenocorticotropic hormone-independent Cushing's syndrome. Curr Opin Endocrinol Diabetes Obes. 2007;14:219–225. doi: 10.1097/MED.0b013e32814db842. [DOI] [PubMed] [Google Scholar]
- 17.Stratakis CA, Kirschner LS. Clinical and genetic analysis of primary bilateral adrenal diseases (micro- and macronodular disease) leading to Cushing syndrome. Horm Metab Res. 1998;30:456–463. doi: 10.1055/s-2007-978914. [DOI] [PubMed] [Google Scholar]
- 18.Stratakis CA, Sarlis N, Kirschner LS, Carney JA, Doppman JL, Nieman LK, Chrousos GP, Papanicolaou DA. Paradoxical response to dexamethasone in the diagnosis of primary pigmented nodular adrenocortical disease. Ann Intern Med. 1999;131:585–591. doi: 10.7326/0003-4819-131-8-199910190-00006. [DOI] [PubMed] [Google Scholar]
- 19.Bourdeau I, Lacroix A, Schürch W, Caron P, Antakly T, Stratakis CA. Primary pigmented nodular adrenocortical disease: paradoxical responses of cortisol secretion to dexamethasone occur in vitro and are associated with increased expression of the glucocorticoid receptor. J Clin Endocrinol Metab. 2003;88:3931–3937. doi: 10.1210/jc.2002-022001. [DOI] [PubMed] [Google Scholar]
- 20.Stratakis CA, Carney JA, Kirschner LS, Willenberg HS, Brauer S, Ehrhart-Bornstein M, Bornstein SR. Synaptophysin immunoreactivity in primary pigmented nodular adrenocortical disease: neuroendocrine properties of tumors associated with Carney complex. J Clin Endocrinol Metab. 1999;84:1122–1128. doi: 10.1210/jcem.84.3.5549. [DOI] [PubMed] [Google Scholar]
- 21.Allolio B, Hahner S, Weismann D, Fassnacht M. Management of adrenocortical carcinoma. Clin Endocrinol (Oxf) 2004;60:273–287. doi: 10.1046/j.1365-2265.2003.01881.x. [DOI] [PubMed] [Google Scholar]
- 22.Libè R, Fratticci A, Bertherat J. Adrenocortical cancer: pathophysiology and clinical management. Endocr Relat Cancer. 2007;14:13–28. doi: 10.1677/erc.1.01130. [DOI] [PubMed] [Google Scholar]
- 23.Grumbach MM, Biller BM, Braunstein GD, Campbell KK, Carney JA, Godley PA, Harris EL, Lee JK, Oertel YC, Posner MC, Schlechte JA, Wieand HS. Management of the clinically inapparent adrenal mass ("incidentaloma") Ann Intern Med. 2003;138:424–429. doi: 10.7326/0003-4819-138-5-200303040-00013. [DOI] [PubMed] [Google Scholar]
- 24.Ribeiro RC, Sandrini F, Figueiredo B, Zambetti GP, Michalkiewicz E, Lafferty AR, DeLacerda L, Rabin M, Cadwell C, Sampaio G, Cat I, Stratakis CA, Sandrini R. An inherited p53 mutation that contributes in a tissue-specific manner to pediatric adrenal cortical carcinoma. Proc Natl Acad Sci U S A. 2001;98:9330–9335. doi: 10.1073/pnas.161479898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stratakis CA. Genetics of adrenocortical tumors: gatekeepers, landscapers and conductors in symphony. Trends Endocrinol Metab. 2003;14(9):404–410. doi: 10.1016/j.tem.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26(1):89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 27.Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab. 2001;86(9):4041–4046. doi: 10.1210/jcem.86.9.7903. [DOI] [PubMed] [Google Scholar]
- 28.Horvath A, Boikos S, Giatzakis C, Robinson-White A, Groussin L, Griffin KJ, Stein E, Levine E, Delimpasi G, Hsiao HP, Keil M, Heyerdahl S, Matyakhina L, Libè R, Fratticci A, Kirschner LS, Cramer K, Gaillard RC, Bertagna X, Carney JA, Bertherat J, Bossis I, Stratakis CA. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet. 2006;38:794–800. doi: 10.1038/ng1809. [DOI] [PubMed] [Google Scholar]
- 29.Horvath A, Giatzakis C, Robinson-White A, Boikos S, Levine E, Griffin K, Stein E, Kamvissi V, Soni P, Bossis I, de Herder W, Carney JA, Bertherat J, Gregersen PK, Remmers EF, Stratakis CA. Adrenal hyperplasia and adenomas are associated with inhibition of phosphodiesterase 11A in carriers of PDE11A sequence variants that are frequent in the population. Cancer Res. 2006;66:11571–11575. doi: 10.1158/0008-5472.CAN-06-2914. [DOI] [PubMed] [Google Scholar]
- 30.Horvath A, Mericq V, Stratakis CA. Mutation in PDE8B, a cAMP-specific Phosphodiesterase in Adrenal Hyperplasia. N. Engl. J. Med. 2008;358:750–752. doi: 10.1056/NEJMc0706182. [DOI] [PubMed] [Google Scholar]
- 31.Gallagher SF, Wahi M, Haines KL, Baksh K, Enriquez J, Lee TM, Murr MM, Fabri PJ. Trends in adrenalectomy rates, indications, and physician volume: A statewide analysis of 1816 adrenalectomies. Surgery. 2007;142:1011–1021. doi: 10.1016/j.surg.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 32.Schteingart DE. Adjuvant mitotane therapy of adrenal cancer - use and controversy. N Engl J Med. 2007;356:2415–2418. doi: 10.1056/NEJMe078087. [DOI] [PubMed] [Google Scholar]
- 33.Terzolo M, Angeli A, Fassnacht M, Daffara F, Tauchmanova L, Conton PA, Rossetto R, Buci L, Sperone P, Grossrubatscher E, Reimondo G, Bollito E, Papotti M, Saeger W, Hahner S, Koschker AC, Arvat E, Ambrosi B, Loli P, Lombardi G, Mannelli M, Bruzzi P, Mantero F, Allolio B, Dogliotti L, Berruti A. N Engl J Med. 2007;356:2372–2380. doi: 10.1056/NEJMoa063360. [DOI] [PubMed] [Google Scholar]