Abstract
Sonic hedgehog (Shh) is a morphogen‐regulating crucial epithelial‐mesenchymal interactions during embryonic development, but its signalling pathway is considered generally silent in post‐natal life. In this study, we demonstrate that Shh is de novo expressed after injury and during regeneration of the adult skeletal muscle. Shh expression is followed by significant up‐regulation of its receptor and target gene Ptc1 in injured and regenerating muscles. The reactivation of the Shh signalling pathway has an important regulatory role on injury‐induced angiogenesis, as inhibition of Shh function results in impaired up‐regulation of prototypical angiogenic agents, such as vascular endothelial growth factor (VEGF) and stromal‐derived factor (SDF)‐1alpha, decreased muscle blood flow and reduced capillary density after injury. In addition, Shh reactivation plays a regulatory role on myogenesis, as its inhibition impairs the activation of the myogenic regulatory factors Myf‐5 and MyoD, decreases the up‐regulation of insulin‐like growth factor (IGF)‐1 and reduces the number of myogenic satellite cells at injured site. Finally, Shh inhibition results in muscle fibrosis, increased inflammatory reaction and compromised motor functional recovery after injury. These data demonstrate that the Shh pathway is functionally important for adult skeletal muscle regeneration and displays pleiotropic angiogenic and myogenic potentials in post‐natal life. These findings might constitute the foundation for new therapeutic approaches for muscular diseases in humans.
Keywords: sonic hedgehog, skeletal muscle regeneration, angiogenesis, myogenesis
Introduction
The Hedgehog (Hh) signalling pathway is an evolutionarily conserved system that is critical for tissue morphogenesis during development. There are three known Hh family members in mammals, named Sonic (Shh), Desert (Dhh) and Indian hedgehog (Ihh). Signalling occurs through the interaction of Hh proteins with the transmembrane receptor Patched1 (Ptc1), activation of the transmembrane molecule Smoothened (Smo) and nuclear translocation of transcriptional factors belonging to the Gli family, which are eventually responsible for the up‐regulation of Hh‐target genes [1]. Importantly, Ptc1 is among the Hh‐target genes itself, thus being both a component, as well as a transcriptional target of the Hh signalling pathway [1].
In the embryo, Shh, together with Wnts, regulates multiple aspects of normal myogenesis, including the expression of myogenic regulatory factors (MRFs), the development, survival and proliferation of the epaxial and hypaxial muscle lineages, and the selection of muscle fibre types [2, 3]. The Shh pathway is considered generally silent in post‐natal life. However, we have previously demonstrated that ischaemia is able to reactivate the Shh pathway in the adult skeletal muscle and myocardium [4, 5]. We have also shown that exogenous administration of Shh might have beneficial effects in experimental models of hindlimb ischaemia, myocardial infarction and diabetic peripheral neuropathy [5, 6, 7]. In the present study, we evaluated whether the Hh pathway is important for the regeneration of injured skeletal muscles in the adult. We investigated the possibility that the Hh pathway is post‐natally reactivated upon non‐ischaemic muscular injuries and studied its functional role on angiogenesis and myogenesis during muscle regeneration.
Materials and methods
Animals and models of muscle injury
C57BL/6J mice were from Jackson Labs. NLS‐Ptc1‐LacZ mice were kindly provided by Dr. Matthew Scott, Stanford University, Stanford, CA. NLS‐Ptc1‐LlacZ mice carry a non‐disruptive insertion of the LacZ reporter gene containing a nuclear localization signal (NLS) upstream of the Ptc1 coding region. Ptc1 expression corresponds to LacZ expression in post‐natal tissues and is not altered by LacZ insertion [4, 5, 6, 7]. Two injury models were used: (i) mechanical crush and (ii) cardiotoxin (CTX) injection of the tibialis anterior (TA) muscle. Injuries were carried out as previously described [8, 9]. In both models, contralateral muscles were used as internal controls. Mice were 8–12 weeks old at the time of experiments. Muscle injuries were performed under anaesthesia. Mice were sacrificed with an overdose of ketamine. Experiments were approved by our local Ethics Committee.
Real‐time (RT) PCR
Muscles were harvested at different time‐points after injury. RT‐PCR was performed as described previously [4]. Results are presented as average fold‐induction of gene expression in injured muscles, compared to contralateral tissues.
In situ hybridization
Skeletal muscles were harvested 2 days after injury and immediately immersion fixed overnight in 4% paraformaldehyde, paraffin‐embedded and sectioned longitudinally at 7–8 μm. Shh in situ hybridization was performed with digoxigenin‐labelled sense and antisense cRNA probes, as previously described [4].
LacZ immunofluorescence and histochemistry in nls‐Ptc1‐lacZ Mice
These analyses were performed 4 days after CTX injury. Staining was done as described previously [4, 5, 6, 7]. Briefly, for X‐gal histochemistry, tissues were fixed in 0.2% gluteraldehyde, washed, stained overnight at 37°C in 1 mg/mL X‐gal, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM MgCl2, 0.01% sodium deoxycholate, 0.02% Nonidet P‐40, 50 mM Na2HPO4 pH8 and visualized as paraffin sections. For β‐gal immunofluorescent staining, tissues were harvested and immediately frozen in OCT. Cryostat sections were then stained with anti‐β‐gal monoclonal antibody (Promega, Promega Corp, San Leandro, CA, USA).
Double immunofluorescent staining
Sections of β‐gal‐stained muscles were also used for Myf5, MyoD, vimentin, F4/80, CD31 and alpha‐SM‐actin immunostaining. For Myf5 and MyoD, primary antisera were rabbit polyclonal anti‐Myf‐5 and anti‐MyoD antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), with a FITC‐conjugated goat anti‐rabbit IgG as secondary antibody (ICL, Inc., Newberg, OR, USA). Vimentin, CD31 and alpha‐SM‐actin staining were performed as previously described [4, 6]. For F4/80, we used a rat anti‐mouse antibody (Serotec, Raleigh, NC, USA), with FITC‐conjugated goat anti‐rat IgG (Invitrogen, Invitrogen Corp, Carlsbad, CA, USA) secondary antibody.
Culture experiments
C2C12 cells were cultivated in differentiation medium and treated for 72 hrs with 4 μg/ml Shh or vehicle. 5‐Bromo‐2′‐deoxyuridine (BrdU) (10 μM) was added to the culture medium for the last 24 hrs. Cells were fixed and stained with a peroxidase‐coupled anti‐BrdU antibody (Roche Diagnostics, Indianapolis, IN, USA) and luminescence was measured. All the experiments were performed in absence or presence of 1 μM of the Shh inhibitor cyclopamine. Cyclopamine inhibits Hh signalling through interaction with Smo and is commonly used to block Hh activity [10, 11, 12, 13]. Cyclopamine was kindly provided by Drs. Lynn James and Dale Gardner of the USDA ARS, Poisonous Plant Research Labs, Logan, UT. Experimental conditions were based on previously published results [14]. All experiments were performed in triplicate.
In vivo inhibition of the Shh pathway
Unilateral injection of CTX was carried out in 36 C57BL/6J mice. Eighteen mice received intraperitoneal injections of cyclopamine, at a concentration of 1 mg/ml, at the dose of 10 mg/kg/day, starting 1 day before injury, until sacrifice. The remaining mice (n= 18) received saline and served as control. The Hh‐inhibitory efficiency of cyclopamine treatment was proved in preliminary experiments by the abolishment of β‐gal activity in CTX‐injured muscles of NLS‐Ptc1‐LacZ mice (data not shown). In an additional set of experiments, cyclopamine was administered to uninjured mice, in order to exclude any potential toxic and/or non‐specific effect of the compound. Uninjured mice treated with cyclopamine did not show any behavioural, anatomical, morphological, or functional alteration. Muscle blood flow and motor function were not altered by cyclopamine treatment (data not shown).
In vivo expression of VEGF, SDF‐1alpha, IGF‐1, Myf‐5 and MyoD
Six cyclopamine‐treated mice and six controls were sacrificed 4 days after CTX injury. Muscles were harvested and the local expression levels of VEGF165, SDF‐1alpha and IGF‐1 proteins were quantified by ELISA (R&D Systems, Minneapolis, MN, USA), as previously described [4, 5, 6]. Results are presented as ratio between injured muscle and contralateral side. Protein extracts were also used for Myf5 and MyoD Western blotting, performed as previously reported [15]. Protein expression was compared by densitometric analysis.
Quantification of activated satellite cells, capillary density, fibrosis and epimysial reaction
Six cyclopamine‐treated and six control mice were sacrificed 4 days after CTX injury. Activated satellite cells were identified by positive immunostaining for Myf5 or MyoD. Other six cyclopamine‐treated and six control mice were sacrificed 10 days after CTX injection for quantification of capillary density, fibrosis and epimysial thickness (ET). Capillary density was evaluated by fluorescein‐labelled Griffonia Simplicifolia BS‐1 lectin staining (Vector Labs, California, CA, USA), as previously described [7, 16]. Fibrosis was evaluated by Van Gieson staining, as previously reported [5]. ET was determined by Gomori’s Trichrome staining, as previously described [17]. Results are presented as ratio between injured and contralateral muscles. Calculations were done on five sections per muscle. Analyses were performed in a blinded fashion by two independent investigators.
Regional blood flow
Mice treated with cyclopamine or saline underwent evaluation of regional blood flow of the TA muscle by laser Doppler flowmetry (PeriFlux System 5002, Perimed, Richmond, VA, USA), performed in triplicate in three different areas of the muscle, before CTX injection and at different time‐points after injury, as previously described [4, 6]. Results are presented as ratio between injured and contralateral muscles. Analyses were performed in a blinded fashion by two independent investigators.
Motor function
The gastrocnemius muscle was unilaterally injured by CTX injection in twelve C57BL/6J mice. Six mice were treated with cyclopamine and six with saline. Motor function was measured using a grip strength meter (Columbus Instruments, Columbus, OH, USA), before injury and at different time‐points after injury. The force measurement was recorded in four separate trials. The analyses were performed in a blinded fashion by two independent investigators.
Statistical analyses
Results are expressed as mean ± S.D. Group differences were analysed by Student’s t‐test. Differences were considered statistically significant at a value of P < 0.05.
Results
Post‐natal recapitulation of the Shh pathway in injured and regenerating skeletal muscle
Two days after mechanical crush, there was a significant increase in Shh mRNA level in injured TA compared to contralateral muscle (Fig. 1A). At this time‐point, also muscles injured with CTX exhibited a significant increase of Shh mRNA (Fig. 1B). In both models, Shh mRNA remained significantly higher in injured muscles at day 4 and 7 after injury (Fig. 1A and B). At day 10, Shh mRNA expression was not different in injured and control tissues (Fig. 1A and B). No significant modification of Ihh and Dhh expression was documented in crushed and CTX‐injected TA at any of the investigated time‐points (Fig. 1C–F). Since Ptc1 is a Shh‐target gene and its expression constitutes evidence of Shh activity [4, 5, 6, 7], the mRNA levels of this gene were also analysed. We found that both mechanical and toxic injuries of the skeletal muscle were followed by significant up‐regulation of Ptc1 mRNA. This effect was detectable at day 2, 4 and 7 after injury, with the highest expression level being observed at day 4, when Ptc1 mRNA was increased about 5 fold in injured muscles, compared to controls (Fig. 1G and H).
Figure 1.
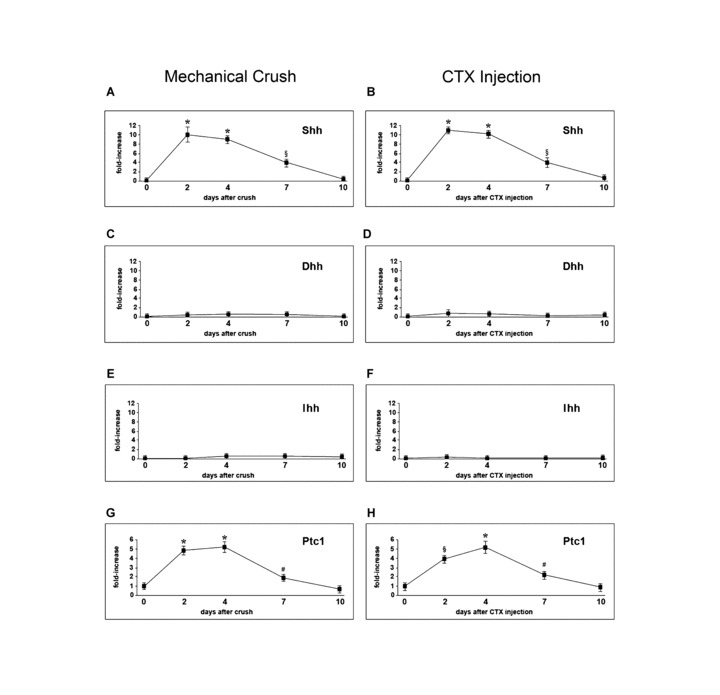
Shh pathway activation in injured skeletal muscle. (A, B) Shh mRNA is increased in TA muscle after mechanical crush and CTX‐injection, with maximum up‐regulation 2 days after injury (*P < 0.001). Expression is significantly increased also at day 4 (*P < 0.001) and 7 (§P < 0.01) and returns to normal at day 10. (C–F) Dhh and Ihh mRNA expression levels are unchanged after mechanical crush and CTX injection. (G, H) Ptc1 mRNA is significantly increased 2, 4 and 7 days after mechanical crush and CTX‐injury (*P < 0.001, §P < 0.01, #P < 0.05).
In order to identify the source of Shh production in injured skeletal muscles, we performed in situ hybridization analyses. Two days after both mechanical crush and CTX injection, a strong Shh‐positive signal was detected in skeletal muscle fibres surrounding the injured area (Fig. 2A and B). These findings indicate that surviving skeletal muscle fibres are responsible for Shh production following injury.
Figure 2.
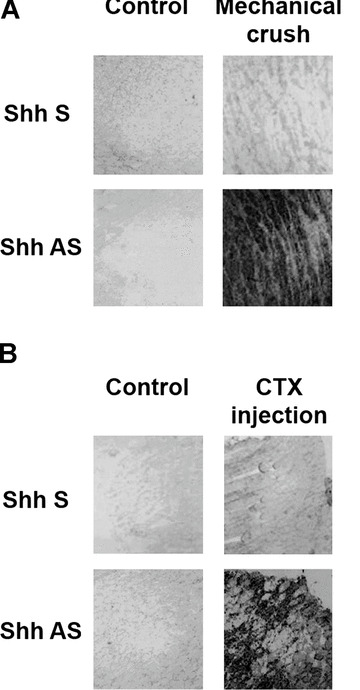
In situ hybridization for Shh after skeletal muscle injury. (A) Two days after mechanical crush, Shh expression is detectable in skeletal muscle fibres surrounding the injured area. (B) Also after CTX injection, there is strong Shh‐positive signal in muscle fibres within the injured tissue.
Inhibition of Shh impairs angiogenesis in vivo
To test the functional importance of the Shh signalling during muscle regeneration, CTX injury was performed in mice treated with daily systemic injections of the Shh inhibitor cyclopamine. Mice treated with saline solution were used as controls. First, we evaluated whether Shh has a regulatory role on angiogenesis, a process that is crucial for tissue repair. It is known that non‐ischaemic injuries of the adult skeletal muscle induce up‐regulation of the angiogenic molecules Vascular Endothelial Growth Factor (VEGF) and Stromal‐derived Factor (SDF)‐1alpha [18, 19, 20]. These cytokines are overexpressed during muscle regeneration and regulate several cellular and molecular mechanisms of angiogenesis, including migration and proliferation of endothelial cells and trafficking and homing of bone marrow‐derived stem cells. We found that, 4 days after injury, VEGF up‐regulation was significantly reduced in injured muscles of mice treated with the Shh inhibitor cyclopamine (Fig. 3A). Similarly, the up‐regulation of SDF‐1alpha was significantly reduced in injured muscles of mice treated with cyclopamine (Fig. 3B). Following these observations, we hypothesized that the negative effects of Shh inhibition on the expression levels of VEGF and SDF‐1alpha could be mirrored by a decrement of the angiogenic response that physiologically follows muscle injury. As hypothesized, capillary density was reduced upon inhibition of the Shh pathway (Fig. 3C and D). Injured muscles of cyclopamine‐treated mice displayed 49 ± 10 vessels/section, compared to 88 ± 7 vessels/section of muscles of saline‐treated animals (P < 0.01) (Fig. 3E). Likewise, laser Doppler flowmetry showed profound alterations of muscle blood flow in animals treated with cyclopamine (Fig. 3F).
Figure 3.
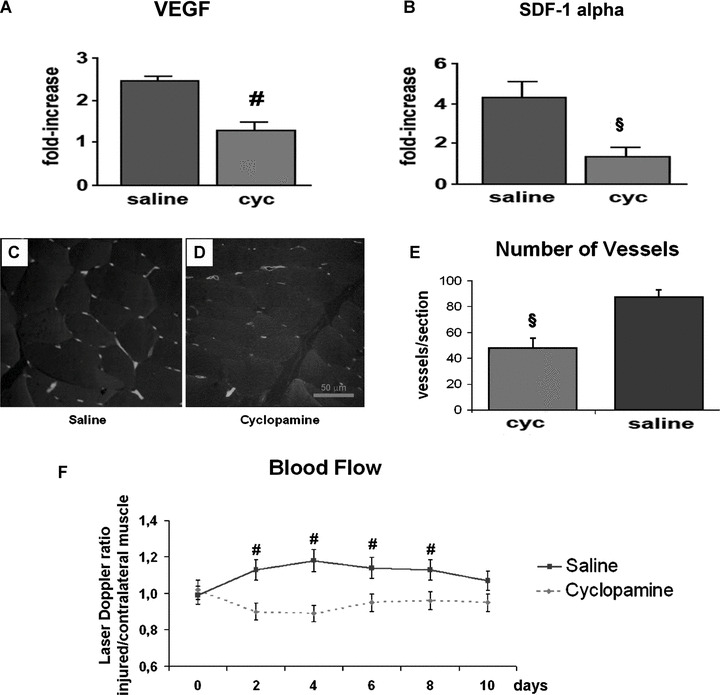
Inhibition of Shh reduces the angiogenic response to injury. (A) In CTX‐injured muscles, local production of VEGF is significantly reduced by treatment with the Shh inhibitor cyclopamine (cyc) (#P < 0.05). (B) Cyclopamine treatment reduces the up‐regulation of SDF‐1alpha in CTX‐injured muscles (§P < 0.01, #P < 0.05).
Inhibition of Shh impairs the up‐regulation of myogenic factors and reduces the number of activated satellite cells in vivo
Insulin‐like growth factor (IGF)‐1 is known to be important in the regulation of myogenesis. This growth factor enhances the proliferation and differentiation of muscle satellite cells (MSCs) and also stimulates muscle hypertrophy during muscle regeneration, concomitant with satellite cell activation [21, 22, 23, 24]. We found that, in muscles of mice injured with CTX and treated with cyclopamine, the up‐regulation of IGF‐1 was significantly reduced (Fig. 4A). Muscle injury is also characterized by the activation of myogenic regulatory factors (MRFs), such as Myf5 and MyoD. These molecules are expressed by activated MSCs and represent a biological marker of regeneration. Inhibition of Shh signalling significantly impaired the up‐regulation of Myf5 and MyoD after injury (Fig. 4B and C). Also the number of activated satellite cells in the site of injury, identified as cells expressing either Myf5 or MyoD, was significantly reduced in the muscles of mice treated with cyclopamine (Fig. 4D and E).
Figure 4.
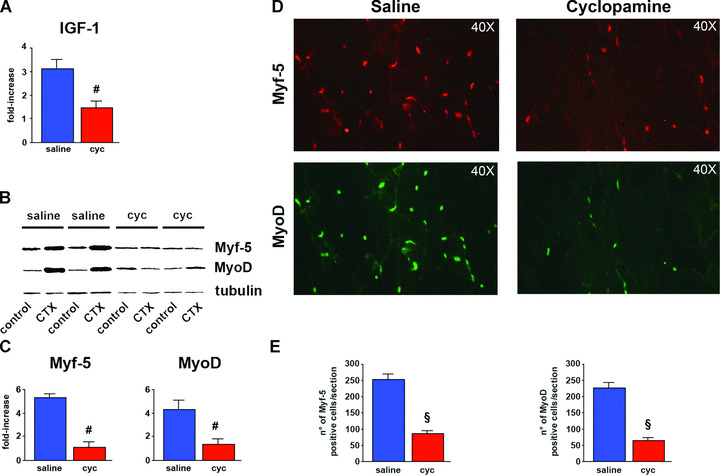
Inhibition of Shh decreases local production of myogenic factors and reduces the number of activated MSCs in vivo. (A) In CTX‐injured muscles, local production of IGF‐1 is significantly reduced by cyc (#P < 0.05). (B) Western blotting analysis showing impaired up‐regulation of both Myf5 and MyoD in CTX‐injured muscles of mice treated with cyc. (C) Quantification of Western blotting data by densitometric analysis showing significant difference of Myf5 and MyoD protein expression between saline‐ and cyc‐treated animals (#P < 0.05). (D) Immunofluorescent staining for Myf5 and MyoD 4 days after CTX‐injury, showing substantial reduction of both Myf5‐ and MyoD‐positive cells in injured muscles of mice treated with cyc. (E) Quantification of Myf5 and MyoD histologic analyses, demonstrating that the number of Myf5‐positive cells is significantly reduced in muscles of mice treated with cyc (|P < 0.01). Also the number of MyoD‐positive cells is significantly reduced upon inhibition of Shh activity (§P < 0.01).
Shh direct effects on satellite cells in vitro and in vivo
To evaluate whether Shh has direct effects on satellite cells in injured and regenerating skeletal muscle, we used NLS‐Ptc1‐LacZ mice. The muscles of these animals were injured with CTX and analysed by β‐gal‐immunostaining. Based on the data obtained by RT‐PCR, histological analyses were performed 4 days after injury, when the maximum up‐regulation of Ptc1 mRNA was observed. We detected several X‐gal‐positive cells in foci of muscle regeneration (Fig. 5A and B). Since Ptc1 is a downstream transcriptional target of Shh and its expression corresponds to LacZ expression in post‐natal tissues of NLS‐Ptc1‐LacZ mice [4, 5, 6, 7], X‐gal‐positive cells represent Shh‐responding elements. By performing double immunofluorescent analyses for Myf5 and MyoD, we found that several β‐gal‐positive cells were also immunopositive for these MRFs (Fig. 5C and D), indicating that activated MSCs directly respond to Shh stimulation in injured and regenerating muscles. We also performed immunostaining for CD31 (an endothelial cell‐specific marker) and alpha‐SM‐actin (a smooth muscle cell‐specific marker) and found no co‐localization with β‐gal (data not shown). We also performed immunostaining for F4/80 (a macrophage‐specific marker) and found that some macrophages were occasionally positive for β‐gal (data not shown). However, this finding was not confirmed in other experiments and has not been included in this study. Finally, we found that some β‐gal‐positive cells were positive for vimentin, a molecule expressed by interstitial mesenchymal cells, such as undifferentiated muscle precursor cells and fibroblasts (data not shown).
Figure 5.
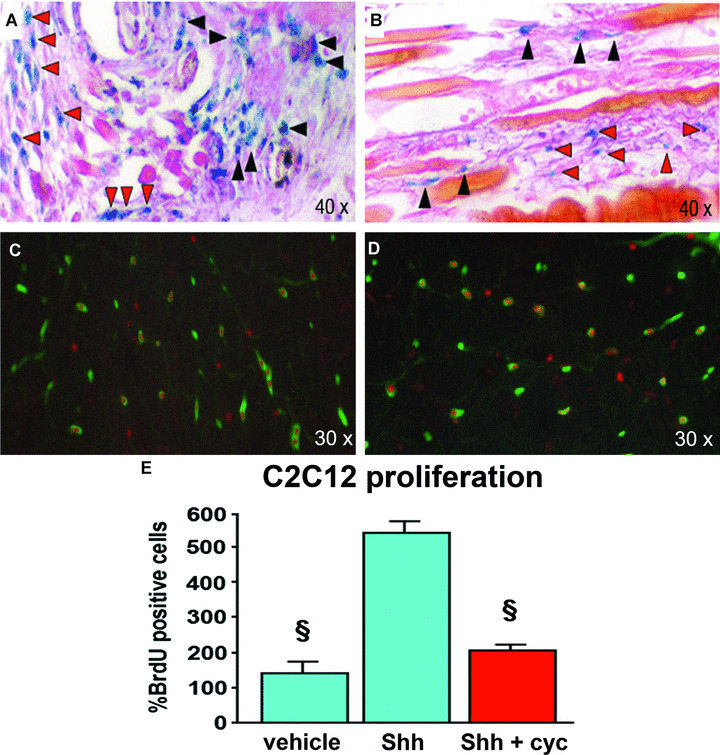
Direct effect of Shh on MSCs in vivo and in vitro. (A, B) In NLS‐Ptc1‐LacZ mice, β‐gal‐ositive cells can be detected around and between injured fibres, 4 days after CTX injection. Many β‐gal‐positive cells are characterized by small size and are located on the surface of injured myofibres (black arrowheads) or between them (red arrowheads). (C) Double immunofluorescent staining for β‐gal (nuclear red staining) and Myf5 (green staining) double positive cells in injured skeletal muscle. (D) Double immunofluorescent staining showing β‐gal (nuclear red staining) and MyoD (green staining) double positive cells in injured skeletal muscle. (E) The proliferation of myoblastic C2C12 cells is significantly increased by Shh and reduced by cyc (§P < 0.01).
Following the demonstration that Shh directly induces Ptc1 up‐regulation in MSCs in vivo, we performed in vitro experiments to evaluate the effects of Shh treatment and inhibition on the proliferation of myoblast cells. Exposure of mouse myoblast C2C12 cells to Shh in culture resulted in significant increase in BrdU incorporation in comparison to untreated cells (Fig. 5E). This effect was significantly reduced by cyclopamine (Fig. 5E).
These data corroborate earlier studies that have documented the ability of Shh to regulate activity and functions of MSCs in vitro[14, 25]. They also provide the first demonstration that Shh directly acts on adult MSCs in vivo.
Inhibition of Shh impairs skeletal muscle repair and functional recovery
Muscles of mice treated with cyclopamine showed a greater extent of fibrosis than controls (Fig. 6A–C). Similarly, ET, a marker of skeletal muscle inflammatory reaction, was greater in mice treated with cyclopamine than controls (Fig. 6D and E). The ratio between ET in injured and contralateral muscles, measured 10 days after CTX injection, was significantly higher in mice treated with cyclopamine than in controls (Fig. 6F). Finally, we evaluated whether Shh function is required for efficient functional recovery after injury. CTX injection was performed unilaterally in the gastrocnemius muscle of mice treated with cyclopamine or saline. Inhibition of Shh caused significant reduction of muscle strength at day 6, 8 and 10 after injury (Fig. 6G).
Figure 6.
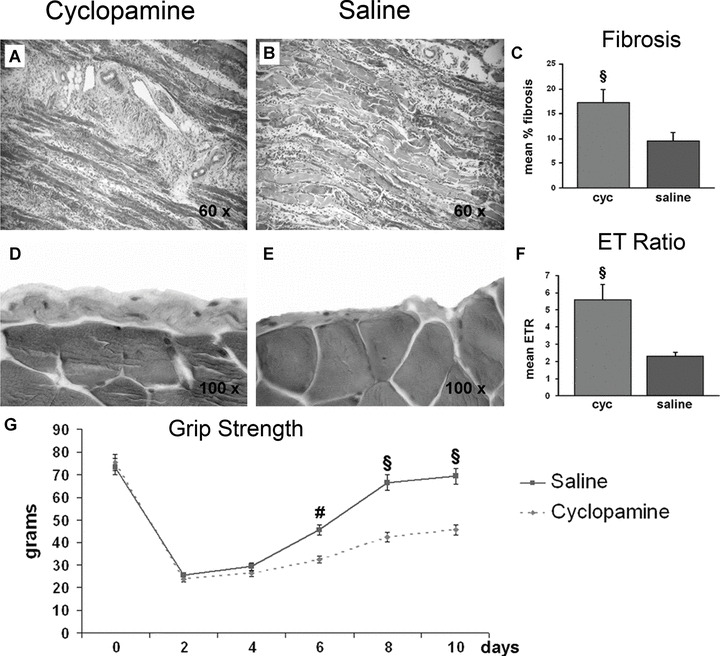
Inhibition of Shh increases fibrosis and epimysial thickness and reduces motor functional recovery. (A, B) Representative images of fibrosis (blue staining) in muscles of mice treated with cyc and saline. (C) The mean percentage of fibrosis is significantly higher in the cyc group than controls (§P < 0.01). (D, E) Representative images of ET (blue staining) in muscles of mice treated with cyc and saline. (F) The ratio between ET at the injured and contralateral side is significantly higher in mice treated with cyc than controls (§P < 0.01). (G) Cyc treatment reduces motor functional recovery after CTX injury. In saline‐treated animals, grip strength returns to normal levels by day 10 after injury. In comparison, grip strength is significantly reduced in mice treated with cyc at day 6, 8 and 10 after injury (#P < 0.05, §P < 0.01).
Discussion
In the present study, we show that the adult skeletal muscle resurrects the embryonic Shh pathway to guide regeneration after injury. The reactivation of this developmental pathway is functionally important, since its inhibition results in impaired production of angiogenic and myogenic secreted factors, decreased up‐regulation of the MRFs Myf5 and MyoD, impairment of the angiogenic response to injury, reduction of the number of activated satellite cells at the damage site, increased fibrosis and inflammatory reaction and compromised muscle functional recovery.
These results were obtained by inhibiting the Shh pathway by using cyclopamine, a plant alkaloid that is able to block Shh activity by interacting with Smo [10, 11, 12, 13]. Cyclopamine does not selectively block the Shh pathway, but also inhibits the Dhh and Ihh signalling pathways [10, 11, 12, 13]. However, we have found Shh up‐regulation, but no Dhh or Ihh up‐regulation, in the muscle injury models used in this study. Therefore, the modifications observed in cyclopamine‐treated mice have to be attributed to the inhibition of the Shh activity. In addition, we also administered cyclopamine to healthy uninjured mice, in order to exclude any potential toxic and/or non‐specific effect of the compound. In these animals, treatment with cyclopamine did not show any behavioural, anatomical, morphological or functional alteration. In particular, muscle blood flow and motor function were not altered.
The discovery that Shh is de novo expressed in injured adult skeletal muscle and its activity is functionally important for regeneration is consistent with the concept that, in addition to its classical role during embryogenesis, Shh participates in a number of physiologic and pathologic conditions in post‐natal life. In this respect, the contribution of Shh to tissue regeneration has already been reported for heart, skin, bone, corneal epithelium, peripheral nerve and spinal cord [5, 26, 27, 28, 29, 30, 31, 32]. Our findings shed new light on fundamentally important cellular and molecular mechanisms involved in muscle repair in the adult, ranging from angiogenesis to myogenesis.
The fact that Shh plays a regulatory role on angiogenesis during muscle regeneration is important, because several recent key observations have demonstrated that myogenesis and angiogenesis are interdependent and contribute to skeletal muscle regeneration in an integrated fashion. Indeed, angiogenesis‐related factors are produced by skeletal muscle in response to non‐ischaemic injuries and mouse models of impaired skeletal muscle regeneration are characterized by delayed angiogenesis and reduced production of the prototypical angiogenic agent VEGF [18, 19, 33]. In addition, VEGF promotes regeneration in transplanted skeletal muscles, plays a role in myoblast migration and survival and, following glycerol‐ or cardiotoxin‐induced damage, markedly improves muscle fibre reconstitution [34, 35]. Very recently, it has also been shown that VEGF gene transfer promotes skeletal muscle regeneration and function in a mouse model of muscular dystrophy [36]. Other recent studies have identified SDF‐1alpha as an important chemokine for muscle biology. In the embryo, the inactivation of the SDF‐1alpha receptor CXCR4 results in impaired limb myogenesis [37]. SDF‐1alpha is increased in certain inflammatory myopathies in the adult [38]. In post‐natal life, the SDF‐1alpha receptor CXCR4 is expressed by both endothelial progenitor cells and MSCs [39]. Importantly, MSCs respond to muscle‐derived SDF‐1alpha by activating multiple intracellular mechanisms related to chemotaxis and phosphorylation of myogenic transcription factors [39]. Taken together, these findings demonstrate that VEGF and SDF‐1alpha contribute to muscle regeneration not only by increasing blood flow and inducing growth of new vascular structures, but also by directly stimulating myogenesis. The close inter‐relationship existing between the vascular system and myogenesis has been further strengthen by the recent discovery that cells derived from blood vessels of human skeletal muscle can regenerate skeletal muscle, similarly to embryonic mesoangioblasts [40].
The main result of our study is that satellite cells directly respond to Shh in the setting of muscle injury in vivo. MSCs are a population of myogenic precursors that respond to stimuli such as injury and exercise by proliferating and committing to a myoblast cell fate and are primarily responsible for muscle regeneration. Upon activation, satellite cells differentiate and fuse to form new myofibres or repair damaged ones [41]. The fact that Ptc1 is up‐regulated in activated satellite cells in vivo indicates that Shh might have direct effects on these cells in the adult. This is also supported by the evidence that Shh inhibition reduces the number of activated satellite cells at the damage site, impairs the up‐regulation of Myf5 and MyoD that physiologically occurs in regenerating muscles, and increases muscle fibrosis. Our findings corroborate recently published data that have demonstrated the ability of Shh to regulate proliferation and differentiation of adult MSCs in vitro[14, 42]. We have not investigated the molecular mechanisms through which Shh regulates Myf5 and MyoD expression in adult muscles. However, based on the role played by Shh during embryonic myogenesis, it is possible to speculate that the transcription factor Gli is crucial for these processes. Indeed, it is known that a Gli‐binding site in the Myf5 epaxial somite enhancer is necessary for the specification of epaxial muscle progenitor cells and that, in the embryo, Gli interacts with several important myogenic pathways, such as those regulated by Wnt, Frizzled, Numb and beta‐catenin [43, 44, 45, 46, 47]. In addition, it has been recently demonstrated that Shh induces MAPK/ERK and phosphoinositide 3‐kinase (PI3K)‐dependent Akt phosphorylation in adult myoblasts in vitro and that Shh‐induced Akt phosphorylation is required for its promotive effects on muscle cell proliferation and differentiation [42].
Our study shows that Shh inhibition results in decreased muscle regeneration after injury. This was demonstrated at the functional level, by showing impaired motor functional recovery after injury, and at the morphological level, by providing evidence of increased fibrosis in injured muscles. The fact that cyclopamine treatment increases fibrosis in injured muscles is consistent with previous data by our group that have shown that Shh gene therapy reduces fibrosis in animal models of myocardial ischaemia [5]. Increased fibrosis in cyclopamine‐treated mice might be explained in several ways. First, it could be an indirect consequence of impaired angiogenesis, which might reduce myofibre viability, favour tissue necrosis and promote apoptosis. Alternatively, it might be due to the fact that, as discussed previously, angiogenic cytokines have direct effects on muscle cells and myogenesis [18, 19, 33, 34, 35, 36]. In this respect, it is intriguing to note that some VEGF‐ and SDF‐1alpha‐induced intracellular mechanisms, such as the PI3K/Akt and MAP kinase signalling pathways, in addition to be important for endothelial cell survival, migration and proliferation, are also involved in muscle survival, differentiation and regeneration [48, 49, 50, 51, 52, 53]. An additional explanation for the development of fibrosis in cyclopamine‐treated animals might be the decreased production of IGF‐1. Indeed, IGF‐1 is a protein with potent anti‐apoptotic functions in skeletal muscle cells [51, 54] and its reduced expression might enhance cell death. Finally, as discussed above and consistent with previous published data [42], increased fibrosis in cyclopamine‐treated mice might be due, at least in part, to inhibition of the direct effects displayed by Shh on MSC number and activity.
In conclusion, our study unveils multiple, novel and unexpected functional roles for the Shh pathway in the adult skeletal muscle. The effects of this morphogen range from induction of angiogenesis to regulation of myogenesis. Further examination of these processes, in an integrated fashion, might provide important new information on the pathobiology of muscle regeneration, potentially constituting the foundation for new therapeutic approaches for muscular diseases in humans.
Acknowledgements
We are grateful to Drs. Lynn James and Dale Gardner of the USDA ARS, Poisonous Plant Research Labs, Logan, UT, for providing the Hh inhibitor cyclopamine. This study was supported in part by NIH grant (no. HL089684) and by Muir (Italian Ministry of University and Research) grant (no. fIRB‐RBID08MAFS) both awarded to Dr. Roberto Pola.
References
- 1. Kogerman P, Grimm T, Kogerman L, et al. Mammalian suppressor‐of‐fused modulates nuclear‐cytoplasmic shuttling of Gli‐1. Nat Cell Biol. 1999; 1: 312–9. [DOI] [PubMed] [Google Scholar]
- 2. Borycki AG, Brunk B, Tajbakhsh S, et al. Sonic hedgehog controls epaxial muscle determination through Myf5 activation. Development. 1999; 126: 4053–63. [DOI] [PubMed] [Google Scholar]
- 3. Kruger M, Mennerich D, Fees S, et al. Sonic hedgehog is a survival factor for hypaxial muscles during mouse development. Development. 2001; 128: 743–52. [DOI] [PubMed] [Google Scholar]
- 4. Pola R, Ling LE, Aprahamian TR, et al. Postnatal recapitulation of embryonic hedgehog pathway in response to skeletal muscle ischemia. Circulation. 2003; 108: 479–85. [DOI] [PubMed] [Google Scholar]
- 5. Kusano KF, Pola R, Murayama T, et al. Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signaling. Nat Med. 2005; 11: 1197–204. [DOI] [PubMed] [Google Scholar]
- 6. Pola R, Ling LE, Silver M, et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001; 7: 706–11. [DOI] [PubMed] [Google Scholar]
- 7. Kusano KF, Allendoerfer KL, Munger W, et al. Sonic hedgehog induces arteriogenesis in diabetic vasa nervorum and restores function in diabetic neuropathy. Arterioscler Thromb Vasc Biol. 2004; 24: 2102–7. [DOI] [PubMed] [Google Scholar]
- 8. Mitchell CA, McGeachie JK, Grounds MD. Cellular differences in the regeneration of murine skeletal muscle: a quantitative histological study in SJL/J and BALB/c mice. Cell Tissue Res. 1992; 269: 159–66. [DOI] [PubMed] [Google Scholar]
- 9. Hirata A, Masuda S, Tamura T, et al. Expression profiling of cytokines and related genes in regenerating skeletal muscle after cardiotoxin injection: a role for osteopontin. Am J Pathol. 2003; 163: 203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cooper MK, Porter JA, Young KE, et al . Teratogen‐mediated inhibition of target tissue response to Shh signaling. Science. 1998; 280: 1603–7. [DOI] [PubMed] [Google Scholar]
- 11. Taipale J, Chen JK, Cooper MK, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000; 406: 1005–9. [DOI] [PubMed] [Google Scholar]
- 12. Berman DM, Karhadkar SS, Hallahan AR, et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002; 297: 1559–61. [DOI] [PubMed] [Google Scholar]
- 13. Incardona JP, Gaffield W, Kapur RP, et al . The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development. 1998; 125: 3553–62. [DOI] [PubMed] [Google Scholar]
- 14. Koleva M, Kappler R, Vogler M, et al. Pleiotropic effects of sonic hedgehog on muscle satellite cells. Cell Mol Life Sci. 2005; 62: 1863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McMahon CD, Popovic L, Oldham JM, et al. Myostatin‐deficient mice lose more skeletal muscle mass than wild‐type controls during hindlimb suspension. Am J Physiol Endocrinol Metab. 2003; 285: E82–7. [DOI] [PubMed] [Google Scholar]
- 16. Pola R, Aprahamian TR, Bosch‐Marce M, et al. Age‐dependent VEGF expression and intraneural neovascularization during regeneration of peripheral nerves. Neurobiol Aging. 2004; 25: 1361–8. [DOI] [PubMed] [Google Scholar]
- 17. Desgranges P, Barbaud C, Caruelle JP, et al. A substituted dextran enhances muscle fiber survival and regeneration in ischemic and denervated rat EDL muscle. FASEB J . 1999; 13: 761–6 [DOI] [PubMed] [Google Scholar]
- 18. Wagatsuma A, Tamaki H, Ogita F. Sequential expression of vascular endothelial growth factor, Flt‐1, and KDR/Flk‐1 in regenerating mouse skeletal muscle. Physiol Res. 2006; 55: 633–40. [DOI] [PubMed] [Google Scholar]
- 19. Wagatsuma A. Endogenous expression of angiogenesis‐related factors in response to muscle injury. Mol Cell Biochem. 2007; 298: 151–9. [DOI] [PubMed] [Google Scholar]
- 20. Brzóska E, Grabowska I, Hoser G, et al. Participation of stem cells from human cord blood in skeletal muscle regeneration of SCID mice. Exp Hematol. 2006; 34: 1262–70. [DOI] [PubMed] [Google Scholar]
- 21. Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin‐like growth factor system in myogenesis. Endocr Rev. 1996; 17: 481–517. [DOI] [PubMed] [Google Scholar]
- 22. Seale P, Rudnicki MA. A new look at the origin, function, and “stem‐cell” status of muscle satellite cells. Dev Biol. 2000. 15; 218: 115–24. [DOI] [PubMed] [Google Scholar]
- 23. Adams GR, Haddad F. The relationships among IGF‐1, DNA content, and protein accumulation during skeletal muscle hypertrophy. J Appl Physiol. 1996; 81: 2509–16. [DOI] [PubMed] [Google Scholar]
- 24. Adams GR, McCue SA. Localized infusion of IGF‐I results in skeletal muscle hypertrophy in rats. J Appl Physiol. 1998; 84: 1716–22. [DOI] [PubMed] [Google Scholar]
- 25. Elia D, Madhala D, Ardon E, et al. Sonic hedgehog promotes proliferation and differentiation of adult muscle cells: Involvement of MAPK/ERK and PI3K/Akt pathways. Biochim Biophys Acta. 2007; 1773: 1438–46. [DOI] [PubMed] [Google Scholar]
- 26. Asai J, Takenaka H, Kusano KF, et al. Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell‐mediated microvascular remodeling. Circulation. 2006; 113: 2413–24. [DOI] [PubMed] [Google Scholar]
- 27. Levy V, Lindon C, Harfe BD, et al . Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell. 2005; 9: 855–61. [DOI] [PubMed] [Google Scholar]
- 28. Miyaji T, Nakase T, Iwasaki M, et al. Expression and distribution of transcripts for sonic hedgehog in the early phase of fracture repair. Histochem Cell Biol. 2003; 119: 233–7. [DOI] [PubMed] [Google Scholar]
- 29. Saika S, Muragaki Y, Okada Y, et al. Sonic hedgehog expression and role in healing corneal epithelium. Invest Ophthalmol Vis Sci. 2004; 45: 2577–85. [DOI] [PubMed] [Google Scholar]
- 30. Akazawa C, Tsuzuki H, Nakamura Y, et al. The upregulated expression of sonic hedgehog in motor neurons after rat facial nerve axotomy. J Neurosci. 2004; 24: 7923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bambakidis NC, Wang RZ, Franic L, et al . Sonic hedgehog‐induced neural precursor proliferation after adult rodent spinal cord injury. J Neurosurg. 2003; 99: 70–5. [DOI] [PubMed] [Google Scholar]
- 32. Bunn JR, Canning J, Burke G, et al. Production of consistent crush lesions in murine quadriceps muscle–a biomechanical, histomorphological and immunohistochemical study. J Orthop Res. 2004; 22: 1336–44. [DOI] [PubMed] [Google Scholar]
- 33. Ochoa O, Sun D, Reyes‐Reyna SM, et al. Delayed angiogenesis and VEGF production in CCR2 ‐/‐ mice during impaired skeletal muscle regeneration. Am J Physiol Regul Integr Comp Physiol. 2007; 293: R651–61. [DOI] [PubMed] [Google Scholar]
- 34. Smythe GM, Lai MC, Grounds MD, et al . Adeno‐associated virus‐mediated vascular endothelial growth factor gene therapy in skeletal muscle before transplantation promotes revascularization of regenerating muscle. Tissue Eng. 2002; 8: 879–91. [DOI] [PubMed] [Google Scholar]
- 35. Germani A, Di Carlo A, Mangoni A, et al. Vascular endothelial growth factor modulates skeletal myoblast function. Am. J. Pathol. 2003; 163: 1417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Messina S, Mazzeo A, Bitto A, et al. VEGF overexpression via adeno‐associated virus gene transfer promotes skeletal muscle regeneration and enhances muscle function in mdx mice. FASEB J. 2007; 21: 3737–46. [DOI] [PubMed] [Google Scholar]
- 37. Odemis V, Lamp E, Pezeshki G, et al. Mice deficient in the chemokine receptor CXCR4 exhibit impaired limb innervation and myogenesis. Mol Cell Neurosci . 2005; 30: 494–05. [DOI] [PubMed] [Google Scholar]
- 38. De Paepe B, Creus KK, De Bleecker JL. Chemokines in idiopathic inflammatory myopathies. Front Biosci. 2008; 13: 2548–77. [DOI] [PubMed] [Google Scholar]
- 39. Ratajczak MZ, Majka M, Kucia M, et al. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF‐1 by muscle‐derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem Cells 2003; 21: 363–71. [DOI] [PubMed] [Google Scholar]
- 40. Dellavalle A, Sampaolesi M, Tonlorenzi R, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007; 9: 255–67. [DOI] [PubMed] [Google Scholar]
- 41. Cossu G, Mavilio F. Myogenic stem cells for the therapy of primary myopathies: wishful thinking or therapeutic perspective J Clin Invest. 2000; 105: 1669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Elia D, Madhala D, Ardon E, et al. Sonic hedgehog promotes proliferation and differentiation of adult muscle cells: involvement of MAPK/ERK and PI3K/Akt pathways. Biochim Biophys Acta. 2007; 1773: 1438–46. [DOI] [PubMed] [Google Scholar]
- 43. Borycki A, Brown AM, Emerson CP Jr. Shh and Wnt signaling pathways converge to control Gli gene activation in avian somites. Development. 2000; 127: 2075–87. [DOI] [PubMed] [Google Scholar]
- 44. Gustafsson MK, Pan H, Pinney DF, et al. Myf5 is a direct target of long‐range Shh signaling and Gli regulation for muscle specification. Genes Dev. 2002; 16: 114–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McDermott A, Gustafsson M, Elsam T, et al. Gli2 and Gli3 have redundant and context‐dependent function in skeletal muscle formation. Development. 2005; 132: 345–57. [DOI] [PubMed] [Google Scholar]
- 46. Holowacz T, Zeng L, Lassar AB. Asymmetric localization of numb in the chick somite and the influence of myogenic signals. Dev Dyn. 2006; 235: 633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Borello U, Berarducci B, Murphy P, et al. The Wnt/beta‐catenin pathway regulates Gli‐mediated Myf5 expression during somitogenesis. Development. 2006; 133: 3723–32. [DOI] [PubMed] [Google Scholar]
- 48. Takahashi A, Kureishi Y, Yang J, et al. Myogenic Akt signaling regulates blood vessel recruitment during myofiber growth. Mol Cell Biol. 2002; 22: 4803–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′‐kinase/Akt signal transduction pathway. Requirement for Flk‐1/KDR activation. J Biol Chem. 1998; 273: 30336–43. [DOI] [PubMed] [Google Scholar]
- 50. Fujio Y, Guo K, Mano T, et al. Cell cycle withdrawal promotes myogenic induction of Akt, a positive modulator of myocyte survival. Mol Cell Biol. 1999; 19: 5073–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Alessi DR, Andjelkovic M, Caudwell B, et al. Mechanism of activation of protein kinase B by insulin and IGF‐1. EMBO J. 1996; 15: 6541–51. [PMC free article] [PubMed] [Google Scholar]
- 52. Bodine SC, Stitt TN, Gonzalez M, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo . Nat Cell Biol. 2001; 3: 1014–9. [DOI] [PubMed] [Google Scholar]
- 53. Rommel C, Bodine SC, Clarke BA, et al. Mediation of IGF‐1‐induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001; 3: 1009–13. [DOI] [PubMed] [Google Scholar]
- 54. Mourkioti F, Rosenthal N. IGF‐1, inflammation and stem cells: interactions during muscle regeneration. Trends Immunol. 2005; 26: 535–42. [DOI] [PubMed] [Google Scholar]


