Abstract
Nanoparticle-based molecular imaging has emerged as an interdisciplinary field which involves physics, chemistry, engineering, biology, and medicine. Single-walled carbon nanotubes (SWCNTs) have unique properties which make them suitable for applications in a variety of imaging modalities, such as magnetic resonance, near-infrared fluorescence, Raman spectroscopy, photoacoustic tomography, and radionuclide-based imaging. In this review, we will summarize the current state-of-the-art of SWCNTs in molecular imaging applications. Multifunctionality is the key advantage of nanoparticles over traditional approaches. Targeting ligands, imaging labels, therapeutic drugs, and many other agents can all be integrated into the nanoparticle to allow for targeted molecular imaging and molecular therapy by encompassing many biological and biophysical barriers. A multifunctional, SWCNT-based nanoplatform holds great potential for clinical applications in the future.
Keywords: Single-walled carbon nanotubes (SWCNTs), molecular imaging, positron emission tomography (PET), Raman spectroscopy, near-infrared fluorescence (NIRF), photoacoustic tomography (PAT)
Introduction
The discovery of multi-walled carbon nanotubes (MWCNTs) and the synthesis of single-walled carbon nanotubes (SWCNTs) in the early 1990s opened up a new arena for research on the nanoscale1-3. A recent search in PubMed for “carbon nanotube” returned more than 5000 publications. Carbon nanotubes (CNTs) are generally produced by three major techniques: electric arc discharge, laser ablation, and thermal or plasma-enhanced chemical vapor deposition (CVD)4. A number of purification techniques have been developed for CNTs such as physical separation and gas/liquid phase oxidation in combination with chemical treatment5.
Characterization of CNTs generally includes scanning electron microscopy (SEM), transmission electron microscopy (TEM), atomic force microscopy (AFM), scanning tunneling microscopy (STM), and many others6. Electron microscopy techniques, including TEM and SEM, are among the most popular choices to image CNTs7-9. Intriguingly, the confined space inside SWCNTs has been utilized for high resolution (HR) TEM imaging of the dynamic behavior of individual small molecules10,11. These findings opened up the possibility of investigating the biological activities of these molecules on an individual basis, which may potentially be applied to a wide range of systems.
Nanotechnology, an interdisciplinary research field involving physics, chemistry, engineering, biology, and medicine, has great potential for early detection, accurate diagnosis, and personalized treatment of diseases. One of the most important foundations for nanotechnology is the new physical properties arising from the nanoscale phenomena. With the size of many orders of magnitude smaller than human cells, nanoparticles (such as SWCNTs) can offer unprecedented interactions with biomolecules both on the surface of and inside the cells, which may revolutionize disease diagnosis and treatment. Over the last decade, there have been numerous nanotechnology centers established world wide and it is expected that nanotechnology will mature into a clinically useful field in the near future12,13. To date, the most well-studied nanoparticles include quantum dots (QDs)14, CNTs15, nanoshells16, paramagnetic nanoparticles17, and many others18,19.
The unique chemical and physical properties of CNTs, including but not limited to electrical conductance, high mechanical stiffness, light weight, transistor behavior, piezo-resistance, thermal conductivity, luminescence, electrochemical bond expansion, and their versatile chemistry, make them superb materials for a broad spectrum of applications ranging from energy storage (e.g. H2, Li) to nano-sensors to drug/gene delivery vehicles. Several excellent review articles have been published on these topics6,20-22. Over the last several years, imaging studies with SWCNTs have flourished. In this review, we will summarize the current state-of-the-art molecular imaging applications with SWCNTs.
Molecular imaging
The field of molecular imaging, “the visualization, characterization and measurement of biological processes at the molecular and cellular levels in humans and other living systems”23, has expanded tremendously over the last decade. In general, molecular imaging modalities include molecular magnetic resonance imaging (MRI), magnetic resonance spectroscopy (MRS), optical bioluminescence, optical fluorescence, targeted ultrasound, single photon emission computed tomography (SPECT), and positron emission tomography (PET)24. Continued development and wider availability of scanners dedicated to small animal imaging studies, which can provide a similar in vivo imaging capability in mice, primates, and humans, can enable smooth transfer of knowledge and molecular measurements between species thereby facilitating clinical translation.
Molecular imaging takes advantage of the traditional diagnostic imaging techniques and introduces molecular imaging agents (probes) to measure the expression of indicative molecular markers at different stages of diseases. It can give whole body readout in an intact system which is much more relevant and reliable than in vitro/ex vivo assays; reduce the workload and speed up the drug development process; provide more statistically accurate results since longitudinal studies can be performed in the same animal which serves as its own control; aid in lesion detection in patients and patient stratification; and help in individualized treatment monitoring and dose optimization25,26. Non-invasive detection of various molecular markers can allow for much earlier diagnosis, earlier treatment, and better prognosis that will eventually lead to personalized medicine.
A number of methods have been explored for the chemical functionalization of CNTs which is a prerequisite for their biomedical applications27. The focus of this review is on SWCNTs only and MWCNTs will not be discussed. Over the last several years, SWCNTs have been explored in almost every single molecular imaging modality, including magnetic resonance (MR), optical, SPECT, and PET imaging. Possessing unique physical properties, SWCNTs have also been investigated with several other imaging techniques such as Raman spectroscopy and photoacoustic tomography (PAT).
MRI with SWCNTs
MRI detects the interaction of protons (or certain other nuclei) with each other and with the surrounding molecules in a tissue of interest28. Different tissues have different relaxation times that can result in endogenous MR contrast. Exogenous contrast agents can further enhance this by selectively shortening either the T1 (longitudinal) or T2 (transverse) relaxation time29,30. The MR image can be weighted to detect the differences in either T1 or T2 by adjusting certain parameters during data acquisition. Traditionally, Gd3+-chelates have been used to enhance the T1 contrast31 and iron oxide nanoparticles have been used to increase the T2 contrast17.
The application of Gd3+-functionalized SWCNTs in MRI has been reported32. The nanoscale loading and confinement of aquated Gd3+ ion clusters within ultra-short (20 - 80 nm) SWCNTs was found to superparamagnetic, with a MRI efficacy of 40 times greater than the Gd3+-based contrast agents in current clinical use32. In a follow-up study, it was reported that these Gd3+-containing SWCNTs are also sensitive to the pH value of the environment33. The r1/pH response between pH 7.0 and 7.4 was 10 times greater than any other MR contrast agent tested. More importantly, these Gd3+-containing SWCNTs were very stable and maintained their integrity when challenged by buffer solution, serum, heat, as well as pH cycling. These findings suggested that such Gd3+-containing SWCNTs might be useful in the clinic for the early detection of cancer, where the extracellular pH of tumors can drop to pH 7.0 or below. However, the effect of morphology and length distribution of the nanotubes on the MRI characteristics of these Gd3+-containing SWCNTs is not clear based on these studies.
The major disadvantage of MRI is its inherent low sensitivity, which can only be partially compensated by working at higher magnetic fields (4.7 - 14 T), acquiring data for much longer time periods, and using exogenous contrast agents. Although proof-of-principle studies have been reported for molecular MRI of several targets34, whether molecular MRI can significantly improve patient management remains to be elucidated in future studies. Currently, it is unclear whether Gd3+-containing SWCNTs can be molecularly targeted and performs well in animal studies, which needs to be demonstrated before any clinical applications can be in place.
Optical imaging with SWCNTs
Due to their strong light quenching properties, direct fluorescent labeling of SWCNTs has not been very successful for optical imaging applications. However, optical properties of SWCNTs can also be manipulated without covalent modification. Wrapping SWCNTs with fluorescently labeled polymer, poly(vinylpyrrolidone) (PVP), was able to make individual SWCNTs observable by a fluorescent microscope35. Subsequently, SWCNTs have been conjugated with QDs and the supramolecular assembly could be stably dispersed under physiological conditions and readily visualized by fluorescence microscopy36.
The major drawback of optical imaging in living subjects is the poor tissue penetration and intense scattering of light37. Near-infrared (NIR, 700 - 900 nm) optical imaging can provide opportunities for rapid and cost-effective pre-clinical evaluation in small animal models, since the absorbance spectra for all biomolecules reach minima in the NIR region which gives a clear spectral window38,39. Taking advantage of their intrinsic NIR fluorescence (NIRF) signal, the uptake of SWCNTs into macrophage-like cells has been studied40. In one report, SWCNTs suspended in air over trenches were imaged using their intrinsic NIRF emission41. Recently, semiconducting SWCNTs were used as NIRF tags for selective probing of cell surface receptors and cellular imaging42. After conjugation to antibodies specific for various cell surface markers, cell binding of the SWCNT-antibody conjugate was readily detected by NIRF microscopy (Fig. 1a).
Fig. 1.
NIRF imaging of SWCNTs. (a) NIRF images of antigen-positive and control cells treated with a SWCNT-antibody conjugate. (b) NIR emission (color-coded for intensity) from SWCNTs in the gut of a living larva, viewed through the larval cuticle. Left: the black branching structures are part of the trachea system that brings air in from openings in the cuticle surface (upper right); Right: Boluses of food containing SWCNTs in a loop of the gut of a living larva. Scale bars in (b) are 50 μm (left) and 100 μm (right), respectively. (Reprinted with permission from 42,45. © 2008 American Chemical Society.)
Although these reports suggested that SWCNTs are useful NIRF tags for sensitive and selective biological detections and imaging in vitro, and potentially in vivo, aqueous surfactant suspensions of SWCNTs were found to be very sensitive to various environmental conditions such as the concentration, pH, and/or salinity. To solve this problem, combination of SWCNTs previously suspended in sodium dodecylbenzene sulfonate (SDBS) with biocompatible PVP, which can be polymerized in situ to entrap the SWCNT-SDBS micelles, was reported to yield much more stable luminescent, individualized SWCNTs across a broad pH range43. Such stable suspensions were demonstrated by imaging individual SWCNTs on the surface of live human embryonic kidney cells. In another report, single particle tracking of endocytosis and exocytosis of SWCNTs in cells was achieved using an inverted microscope with a 785 nm laser excitation and a 2D InGaAs imaging array44. This study gave the first conclusive evidence of SWCNT exocytosis and showed that the rate of exocytosis closely matches the rate of endocytosis with negligible temporal offset.
NIRF imaging of SWCNTs have been reported in small living organisms such as the Drosophila melanogaster (fruit flies)45. The NIRF signal of SWCNTs was imaged in intact living larvae (Fig. 1b), and individual SWCNTs in dissected tissue specimens were then imaged, structurally identified, and counted to estimate a biodistribution. Comparing with fluorescent dyes or QDs, the more popular fluorescent agents, the quantum efficiency of SWCNTs is much lower. Therefore, NIRF imaging of SWCNTs is quite challenging in live animals (e.g. mice and rats). In one study, individualized, chemically pristine SWCNTs were intravenously administered to rabbits and monitored ex vivo through their characteristic NIRF signal in the blood sample and excised tissue46. No adverse effect from low-level SWCNT exposure was detected by either behavior or pathological examination.
Besides the low quantum efficiency, the biggest limiting factor for in vivo imaging applications of SWCNTs, another limitation of NIRF microscopy of SWCNTs is the diffraction limit to resolution, which is highly relevant for cell-based imaging where good resolution is desirable. Recently, a non-perturbing, far-field optical technique was reported which can allow sub-wavelength mapping of single-molecule chemical reaction sites on semiconducting SWCNTs47. This technique can enable precise localization of excitonic luminescence regions along the nanotube axis in unprecedented detail. X-ray fluorescence has also been applied, for the first time, to study macrophages exposed to unpurified and purified SWCNTs48. In this study, investigation of the elemental distributions allowed one to image SWCNT localization within a cell, as well as detect the chemical modification of the cell after SWCNT internalization.
Among all molecular imaging modalities, no single modality is perfect and sufficient to obtain all the necessary information for a particular question24. For example, it is difficult to accurately quantify fluorescence signal in living subjects, particularly in deep tissues; MRI has high resolution yet it suffers from low sensitivity. Combination of multiple imaging modalities can offer synergistic advantages over any single modality alone. In one report, heterostructured complexes formed from magnetic iron oxide nanoparticles and NIRF SWCNT were investigated as multimodal imaging agents49. The complex showed distinct NIRF and visible/NIR absorbance features, corresponding to the various nanotube species. AFM and cryo-TEM images showed DNA-encapsulated complexes composed of an approximately 3 nm iron oxide particle attached to a SWCNT on one end. Macrophage cells that engulf the DNA-wrapped complexes were imaged using both MRI and NIR mapping, indicating that such multifunctional complexes could potentially be useful in multimodal biomedical imaging.
For clinical applications, optical imaging will only be possible in limited sites such as the tissues and lesions close to the surface of the skin, tissues accessible by endoscopy, and intraoperative visualization14. Due to the many limitations of the fluorescence properties of SWCNTs, they are not suitable for fluorescence imaging applications in human studies although they can be very useful in cell- and small animal-based investigations. Several other optical imaging techniques currently under active development, such as Raman spectroscopy and PAT50,51, may have potential clinical relevance/applications in the future.
Raman spectroscopy with SWCNTs
Raman spectroscopy, which can differentiate the spectral fingerprint of many molecules, can have excellent multiplexing capabilities52,53. Narrow spectral features can be readily separated from the broadband autofluorescence in Raman spectroscopy since it is a scattering phenomenon instead of absorption/emission in fluorescence imaging. However, the inherently weak magnitude of the Raman effect limits its sensitivity which severely hampered the biomedical applications of Raman spectroscopy. SWCNT has an intense Raman peak produced by the strong electron-phonon coupling which causes efficient excitation of tangential vibration in the SWCNT upon light exposure54. In 2007, a laser-scanning optical microscope was developed to measure the low-temperature Raman scattering spectra of individual SWCNTs55. The laser-scanning scheme of the microscope can allow for highly repeatable imaging over large sample areas. Meanwhile, anodized aluminum oxide perforated in an organized fashion was used to examine the surface enhanced Raman scattering (SERS) of SWCNTs at micron length and a large signal enhancement was observed56.
Near-field Raman imaging and spectroscopy has been employed to study the localized vibrational modes along SWCNTs synthesized by different methods57. Direct comparison between arc-discharge and CVD grown SWCNTs can allow one to rule out the artifacts induced by the supporting substrate. Simultaneous near-field photoluminescence and Raman imaging of SWCNTs revealed that near-field enhancement is much stronger for photoluminescence than for Raman scattering, likely due to the low quantum yield of SWCNTs58. Near-field Raman spectroscopy studies of single isolated SWCNTs have also been reported, and the near-field origin of the image contrast was confirmed by the measured dependence of the Raman scattering signal on tip-sample distance and the unique polarization properties59. Subsequently, Raman mapping and 3D real optical imaging of SWCNTs with near-field resolution was achieved60.
Raman spectroscopy and confocal Raman imaging of ultralong SWCNTs grown by the “fast-heating” CVD method has been reported61. It was demonstrated that larger structural changes resulting in a full chirality change can occur in these SWCNTs to produce a metal-to-semiconductor intramolecular junction. Raman spectroscopy is also applicable in evaluating the purity of SWCNTs. Unlike other popular analytical techniques such as SEM, TEM, thermogravimetric analysis (TGA), solution phase NIR spectroscopy and Raman spectroscopy can be used to quantitatively compare arbitrary samples of bulk SWCNT materials of different purities62.
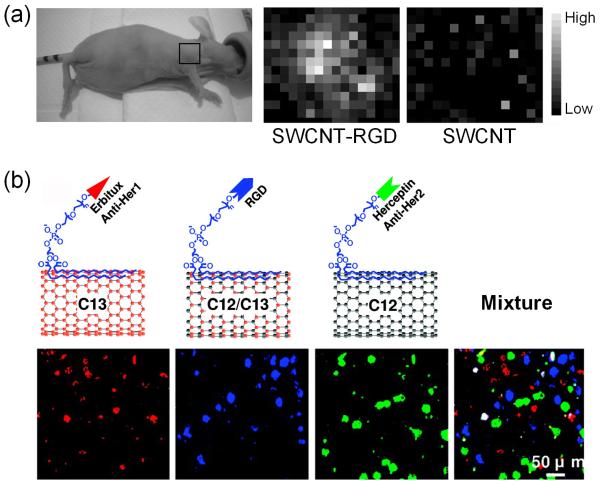
In a pioneering study, SWCNTs and other SERS nanoparticles were used to demonstrate whole-body Raman imaging, nanoparticle pharmacokinetics, multiplexing, as well as in vivo tumor targeting, with an imaging system adapted for small-animal Raman imaging63. As a proof-of-principle, the ability to detect tumor targeting with peptide-conjugated SWCNTs in tumor-bearing mice was confirmed which set the foundation for continued investigation of Raman imaging in living subjects. Subsequently, an optimized Raman microscope was used to further evaluate tumor targeting and localization of SWCNTs in mice64. The molecular target and targeting ligand used in these studies was integrin αvβ3 and an Arginine-Glycine-Aspartic acid (RGD, potent integrin αvβ3 antagonist) containing peptide respectively, one of the most extensively studied and validated receptor-ligand pair over the last decade66,67. Raman imaging commenced over three days revealed increased accumulation of SWCNT-RGD in the U87MG human glioblastoma tumor (integrin αvβ3-positive68,69) than the unconjugated SWCNTs (Fig. 2a). These reports strongly supported further development of non-invasive Raman imaging as a tool to asses the efficacy of new diagnostic strategies and therapies in small animal models, which may eventually lead to improvements in cancer patient care.
Fig. 2.
Raman imaging of SWCNTs. (a) Left: Photograph of an integrin αvβ3-positive tumor-bearing mouse depicting tumor area scanned with Raman spectroscopy (black box). Right: Raman tumor maps of mice receiving SWCNT-RGD conjugate or unconjugated SWCNT at 72 h post-injection. (b) Deconvoluted confocal Raman spectroscopy images of a mixture of three cell lines after incubation with a mixture of three different, molecularly targeted SWCNT conjugates. Each of the SWCNT conjugate has a different Raman signature (due to different carbon isotope composition) and was conjugated with a targeting moiety for a different molecular target. (Reprinted with permission from 64,70. © 2008 American Chemical Society.)
One major advantage of Raman spectroscopy is its superb potential for multiplexed imaging. In one abovementioned study, although multiplexed Raman imaging in vivo was demonstrated using different SERS nanoparticles with different Raman signatures, multiplexing was not achieved with differently functionalized SWCNTs since they all have the same Raman properties63. Recently, multiplexed Raman imaging of live cells with isotopically modified SWCNTs was reported70. SWCNTs with different isotope (12C and/or 13C) compositions, which in turn exhibit distinct Raman G-band peaks, were investigated in this study. Different cancer cells over-expressing specific cell surface receptors, the human epidermal growth factor receptor 1 (Her1)71,72, integrin αvβ3, and human epidermal growth factor receptor 2 (Her2)72, were used for receptor-specific targeting. Three differently “colored” SWCNTs were each conjugated with a different targeting ligand which recognizes one specific receptor, allowing for multicolor Raman imaging of cells in a multiplexed manner (Fig. 2b). The Raman signal of SWCNTs was very stable against photobleaching, thus enabling long-term imaging and tracking. Since the Raman excitation and scattering photons of these SWCNTs are in the NIR region, these SWCNT conjugates may be useful for future in vivo imaging applications.
Antibody-functionalized SWCNTs have recently been used as multicolor Raman labels for highly sensitive, multiplexed protein detection in an arrayed format73. When combined with SERS substrates, the strong Raman intensity of SWCNT tags affords protein detection sensitivity in sandwich assays down to the femtomolar level. By conjugating different antibodies to pure 12C- and 13C-SWCNTs, multiplexed SWCNT Raman-based protein detection was demonstrated. Although this work focused on antibody-antigen interactions, the use of SWCNT Raman tags for specific targeting and detection may also be applicable for probing protein-protein interactions and/or nucleic acid hybridization. In future studies, SWCNTs with controlled diameters (which exhibit distinctive Raman peaks in their radial-breathing modes depending on the diameter74) may also be investigated for multicolor Raman imaging.
The primary limitations of Raman imaging in larger subjects are those also faced by other optical techniques. Even in the NIR region, light penetration beyond a few centimeters of tissue is quite difficult. The use of laser, which is typically used to generate the Raman signal of SWCNTs, may also damage tissues and distort the signals if the intensity of the laser is too strong. The key advantage of Raman imaging over fluorescence imaging is the superb multiplexing capability and lack of confounding background signal from autofluorescence. In addition, the use of SWCNTs as Raman tags is more advantageous than other Raman nanoparticles because of the inherent Raman signature of SWCNTs, which requires no further labeling or encapsulation to produce a Raman signal.
Photoacoustic tomography with SWCNTs
PAT is a cross-sectional imaging technique based on the photoacoustic effect75. In PAT, the tissue is usually irradiated by a short-pulsed laser beam to produce thermal and acoustic impulse responses. Locally absorbed light is converted into heat, which is further converted to a pressure rise via thermoelastic expansion of the tissue. The initial pressure rise propagates in the tissue as an ultrasonic wave, referred to as a photoacoustic wave, which is detected by ultrasonic transducers placed outside the tissue to produce electric signals. The electric signals are then amplified, digitized, and transferred to a computer to form a PAT image.
PAT can offer higher spatial resolution and slightly better tissue penetration than most other optical imaging techniques. A number of contrast agents for photoacoustic imaging have been suggested76-78, yet most was not used for molecular imaging of specific targets. In a recent study, SWCNT conjugated with cyclic RGD peptides was used as a contrast agent for photoacoustic imaging of tumors in living mice79. Intravenous administration of the SWCNT-RGD conjugate to tumor-bearing mice showed eight times greater photoacoustic signal in the tumor than mice injected with non-targeted SWCNTs (Fig. 3). Taking advantage of the intrinsic Raman signal of the SWCNT, the in vivo PAT imaging results were further validated ex vivo with Raman microscopy (Fig. 3). With regard to sensitivity, a concentration of 50 nM of SWCNTs was found to produce a photoacoustic signal equivalent to the mouse tissue (the background signal) in this study79. However, the minimum detectable concentration of SWCNTs is likely less than that since the PAT images were acquired before and after the administration of the contrast agent, which makes it possible to separate the signal of the SWCNT from the background.
Fig. 3.
Validation of the in vivo PAT images by ex vivo Raman microscopy. Photographs of the tumors in mice and the corresponding photoacoustic subtraction images (green) shown as horizontal slices through the tumors. After the photoacoustic scan, the tumors were excised and scanned using a Raman microscope (red). Mice injected with unconjugated SWCNT showed both low photoacoustic and Raman signals compared with mice injected with SWCNT-RGD. (Reprinted with permission from 79. © 2008 Nature Publishing Group.)
One major limitation of this prototype imaging system is that the data acquisition time is quite long. More than twenty minutes were required to produce a single PAT image of a 100 mm3 sized tumor. However, PAT compares very favorably to other imaging modalities with its precise depth information, sub-millimeter resolution, and nanomolar sensitivity. With further improvement in background reduction, hardware and image reconstruction software, and the use of lasers with high repetition rates, it is likely that PAT will find wide uses in the future in both basic research and clinical care. While the photoacoustics research community continues to discover new phenomena and invent new technologies, several companies are actively commercializing PAT instrument. Therefore, PAT will continue to be a highly vibrant research field in the years to come.
Radionuclide-based imaging with SWCNTs
Radionuclide-based imaging techniques (PET and SPECT) have much more clinical relevance, hence are much more widely used in the clinic, than molecular MRI and optical imaging. Not only is there no tissue penetration limit for these techniques, PET and SPECT are also highly sensitive (down to the picomolar level) and quantitative80. SPECT imaging detects gamma rays81. Internal radiation is administered through inhaling, ingesting, or injecting a low mass amount of radiolabeled pharmaceuticals. A collimator is used to only allow the emitted gamma photon to travel along certain directions to reach the detector, which ensures that the position on the detector accurately represents the source of the gamma ray. The gamma camera can be used in planar imaging to obtain 2D images, or in SPECT imaging to obtain 3D images. The most common radioisotopes used for SPECT imaging include 99mTc (t1/2: 6.0 h), 111In (t1/2: 2.8 d), and radioiodine.
In one early study, water-soluble hydroxylated SWCNTs were labeled with 125I (t1/2: 60.2d) to study the distribution in mice82. It was concluded that these SWCNTs moved easily among the compartments and tissues of the body and behaved as small molecules although their apparent molecular weight was tremendously large. In a later report, water-soluble SWCNT was functionalized with the chelating molecule diethylentriaminepentaacetic acid (DTPA) and labeled with 111In for imaging purposes83. It was suggested that the intravenously administered SWCNTs were not retained in any of the reticuloendothelial system (RES) organs (liver or spleen) and were rapidly cleared from the circulation system through the renal excretion route. Urine excretion studies of these SWCNTs followed by electron microscopy analysis of urine samples revealed that a certain fraction of the SWCNTs were excreted intact.
Because of the use of lead collimators to define the angle of incidence, SPECT imaging has a very low detection efficiency (< 10−4 times the emitted number of gamma rays)84. PET, on the other hand, has much higher detection efficiency (up to ~10%). It was first developed in the 1970s85 and dedicated PET scanners for small animal studies were first reported in the late 1990s86.
The biodistribution of 64Cu (t1/2: 12.7 h) labeled SWCNTs in mice has been investigated by PET, ex vivo biodistribution, and Raman spectroscopy87. It was found that these SWCNTs are highly stable in vivo and the surface PEG chain length can significantly affect its blood concentration and biodistribution. Effectively PEGylated SWCNTs exhibited relatively long circulation half-life (about 2 h) and low uptake by the RES. Most importantly, efficient targeting of integrin αvβ3-positive tumors in mice was achieved with SWCNTs coated with PEG chains linked to cyclic RGD peptides87. The intrinsic Raman signature of the SWCNTs was used to directly probe their presence in mice tissues and confirm the radionuclide-based imaging results (Fig. 4). All in vivo and ex vivo experiments confirmed that there was minimal renal uptake of the SWCNT-RGD conjugate, and the majority of the conjugate accumulated in the tumor and the RES. After evaluating the pharmacokinetics, confirming the tumor-targeting efficacy, and demonstrating the lack of toxicity to animals88, the potential use of SWCNT as a nanoplatform for integrated multimodality imaging and molecular therapy is currently being explored89.
Fig. 4.
In vivo targeted PET imaging with SWCNTs. (a) Schematic illustration of the non-covalently functionalized SWCNT. The hydrophobic chains (blue segments) bind strongly to the sidewall of the SWCNT, and the PEG chains render water solubility. RGD peptide can allow for integrin αvβ3 targeting and DOTA molecules on the SWCNT can complex 64Cu for PET imaging. (b) 2D projection of the PET images of U87MG tumor-bearing mice at 8 h post-injection of SWCNT-RGD without and with (denoted as “Block”) co-injection of an excess amount of RGD peptide, which demonstrated receptor specificity in vivo. The arrowheads indicate the tumors. (c) Raman spectra of tissue homogenate provided direct evidence of SWCNT presence in the tumor and the liver. (Reprinted with permission from 87. © 2007 Nature Publishing Group.)
Subsequently, tumor-targeting SWCNT constructs with covalently attached monoclonal antibodies, radiometal chelates, and fluorescent probes were investigated90. These constructs were found to be specific for the cancer cells, both in vitro and in vivo, they were designed to target. In a follow-up study, PET imaging was carried out to determine the tissue biodistribution and pharmacokinetics of 86Y (t1/2: 14.7 h) labled SWCNTs in a mouse model91. It was found that 86Y cleared from the blood within 3 hours and distributed predominantly to the kidneys, liver, spleen and bone. Although the activity that accumulated in the kidney cleared with time, the whole-body clearance was quite slow. Recently, biodistribution of radiolabeled SWCNT-oligonucleotide conjugates was also investigated in mice92.
Radiolabeled nanoparticles represent a new class of probes which has enormous potential for clinical applications. Different from other molecular imaging modalities where typically the nanoparticle itself is detected, radionuclide-based imaging detects the radiolabel rather than the nanoparticle. The nanoparticle distribution is measured indirectly by assessing the localization of the radionuclide, which can provide quantitative measurement of the tumor targeting efficacy and pharmacokinetics only if the radiolabel on the nanoparticle is stable enough under physiological conditions. However, dissociation of the radionuclide (typically metal) from the chelator, and/or the radionuclide-containing polymer coating from the nanoparticle, may occur which can cause significant difference between the nanoparticle distribution and the radionuclide distribution.
In several studies, SWCNTs were reported to undergo either complete or partial renal clearance in mice, with little uptake by the liver or other organs of the RES82,83,90. These findings defy the general trend of high RES uptake for nanoparticles93,94 and deserve further investigation/validation. Direct measurement of the SWCNT in various tissues using its intrinsic Raman signal, as well as rigorous validation of the stability of the radiolabel on the nanoparticle, should always be carried out to obtain more reliable experimental results87. Studies have shown that typically only molecules less than 70 kDa (a few nanometers in diameter) undergo renal clearance95-97. The SWCNTs used in these reports are more than 100 nm in length, even up to a few micrometers82,83,87,90. It is very unlikely that these SWCNTs can be cleared from the kidney (dissociated radiolabel and polymer coating can, however) unless severe kidney damage has occurred.
Conclusion
Nanotechnology has touched upon every single modality of the molecular imaging arena19. The future of nanomedicine lies in multifunctional nanoplatforms which combine both therapeutic components and multimodality imaging. The ultimate goal is that nanoplatform-based agents can allow for efficient, specific in vivo delivery of therapeutic agents (drugs, genes, etc.) without systemic toxicity, and the dose delivered as well as the therapeutic efficacy can be accurately measured non-invasively over time. The unique physical and chemical properties of SWCNTs have made them versatile contrast agents for a wide variety of imaging modalities (Table 1). Not limited to imaging applications only, SWCNTs have also been widely used in many other areas, such as in material science and as drug/gene delivery vehicles6,20-22. Intriguingly, the intrinsic optical absorbance of SWCNTs has been used for photothermal killing of cancer cells upon continuous NIR radiation98. Together, the versatile chemistry of SWCNTs in combination with their intrinsic optical properties can lead to a multifunctional nanoplatform for multimodality molecular imaging and therapy (Fig. 5). Much remains to be done before this can be a clinical reality and many factors need to be optimized, among which are biocompatibility, pharmacokinetics, in vivo targeting efficacy, the ability to escape the RES, cost-effectiveness, and acute/chronic toxicity.
Table 1.
The unique physical and chemical properties of SWCNTs have made them versatile contrast agents for a number of imaging modalities.
Fig. 5.
A multifunctional SWCNT-based platform incorporating multiple receptor targeting, multimodality imaging, and multiple therapeutic entities. Not all functional moieties will be necessary and only suitably selected components are needed for each individual application.
Like most new technologies, there are concerns about the possible side effects caused by the use of SWCNTs. A large number of publications have appeared on this topic and the preliminary results highlighted the difficulties in evaluating the toxicity of SWCNTs. A variety of parameters including the structure, size distribution and surface area, surface chemistry and surface charge, agglomeration state, as well as the purity of the SWCNT sample, have considerable impact on the reactivity of SWCNTs. In rodent studies, pristine SWCNTs were capable of producing inflammation, epithelioid granulomas (microscopic nodules), fibrosis, and biochemical/toxicological changes in the lungs99-101. However, several recent reports have clearly shown that stably functionalized SWCNTs, when intravenously injected, displayed near-complete clearance from the main organs in a few months88,102. Further, no toxic side effect of SWCNTs to mice was observed in necropsy, histology, and blood chemistry measurements. Clearly, robust chemistry of the SWCNT surface modification is the key to success in future biomedical and clinical applications. The future of SWCNTs in nanomedicine looks bright and development of SWCNT-based nanoplatforms for biomedical applications must proceed in tandem with the assessment of any toxicological side effects. Much further studies on well-characterized SWCNTs are necessary to determine their safety profile as well as the environmental impact.
The most promising clinical applications of SWCNT-based agents will be in cardiovascular medicine, where there is much less biological barrier for efficient delivery of nanoparticles, and in oncology, where the leaky tumor vasculature can allow for better tissue penetration than in normal organs/tissues. SWCNT-based nanosensors6,20 and in vivo imaging are both critical for future optimization of patient management. Ex vivo diagnostics in combination with in vivo diagnostics can provide a synergistic approach that neither strategy alone can offer. While imaging can give a whole body perspective of the disease state in patients, detection of blood and urine markers of the disease can provide invaluable and complimentary information of the biological responses to therapeutic intervention. Upon further development and validation, nanoplatform-based approaches (both ex vivo nanosensor and in vivo imaging) will eventually be able to predict which patients will likely respond to a specific molecular therapy and monitor their response to personalized therapy. With unique and versatile chemical/physical properties, SWCNTs have the potential to profoundly impact disease diagnosis and patient management in the future.
Acknowledgements
Funding was provided, in part, by the UW School of Medicine and Public Health’s Medical Education and Research Committee through the Wisconsin Partnership Program. Ting Gao acknowledges the support from Tyco Electronics.
REFERENCES
- [1].Iijima S. Nature. 1991;354:56. [Google Scholar]
- [2].Iijima S, Ichihashi T. Nature. 1993;363:603. [Google Scholar]
- [3].Bethune DS, Kiang CH, de Vries MS, Gorman G, Savoy R, Vazquez J, et al. Nature. 1993;363:605. [Google Scholar]
- [4].Yan Y, Chan-Park MB, Zhang Q. Small. 2007;3:24. doi: 10.1002/smll.200600354. [DOI] [PubMed] [Google Scholar]
- [5].Pillai SK, Ray SS, Moodley M. J. Nanosci. Nanotechnol. 2007;7:3011. doi: 10.1166/jnn.2007.921. [DOI] [PubMed] [Google Scholar]
- [6].Yun YH, Dong Z, Shanov V, Heineman WR, Halsall HB, Bhattacharya A, et al. Nanotoday. 2007;2:30. [Google Scholar]
- [7].Zhu H, Suenaga K, Hashimoto A, Urita K, Hata K, Iijima S. Small. 2005;1:1180. doi: 10.1002/smll.200500200. [DOI] [PubMed] [Google Scholar]
- [8].Porter AE, Gass M, Muller K, Skepper JN, Midgley PA, Welland M. Nat. Nanotechnol. 2007;2:713. doi: 10.1038/nnano.2007.347. [DOI] [PubMed] [Google Scholar]
- [9].Homma Y, Takagi D, Suzuki S, Kanzaki KI, Kobayashi Y. J. Electron. Microsc. 2005;54(Suppl 1):i3. doi: 10.1093/jmicro/54.suppl_1.i3. [DOI] [PubMed] [Google Scholar]
- [10].Morgan DA, Sloan J, Green ML. Chem. Commun. 2002;20:2442. doi: 10.1039/b207594f. [DOI] [PubMed] [Google Scholar]
- [11].Liu Z, Yanagi K, Suenaga K, Kataura H, Iijima S. Nat. Nanotechnol. 2007;2:422. doi: 10.1038/nnano.2007.187. [DOI] [PubMed] [Google Scholar]
- [12].Thayer AM. Chem. Eng. News. 2007;85:15. [Google Scholar]
- [13].Kawasaki ES, Player A. Nanomedicine. 2005;1:101. doi: 10.1016/j.nano.2005.03.002. [DOI] [PubMed] [Google Scholar]
- [14].Cai W, Hsu AR, Li ZB, Chen X. Nanoscale Res. Lett. 2007;2:265. doi: 10.1007/s11671-007-9061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lacerda L, Bianco A, Prato M, Kostarelos K. Adv. Drug. Deliv. Rev. 2006;58:1460. doi: 10.1016/j.addr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- [16].Hirsch LR, Gobin AM, Lowery AR, Tam F, Drezek RA, Halas NJ, et al. Ann. Biomed. Eng. 2006;34:15. doi: 10.1007/s10439-005-9001-8. [DOI] [PubMed] [Google Scholar]
- [17].Thorek DL, Chen AK, Czupryna J, Tsourkas A. Ann. Biomed. Eng. 2006;34:23. doi: 10.1007/s10439-005-9002-7. [DOI] [PubMed] [Google Scholar]
- [18].Ferrari M. Nat. Rev. Cancer. 2005;5:161. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- [19].Cai W, Chen X. Small. 2007;3:1840. doi: 10.1002/smll.200700351. [DOI] [PubMed] [Google Scholar]
- [20].Sinha N, Ma J, Yeow JT. J. Nanosci. Nanotechnol. 2006;6:573. doi: 10.1166/jnn.2006.121. [DOI] [PubMed] [Google Scholar]
- [21].Lacerda L, Raffa S, Prato M, Bianco A, Kostarelos K. Nanotoday. 2007;2:38. [Google Scholar]
- [22].Foldvari M, Bagonluri M. Nanomedicine. 2008;4:183. doi: 10.1016/j.nano.2008.04.003. [DOI] [PubMed] [Google Scholar]
- [23].Mankoff DA. J. Nucl. Med. 2007;48:18N. [PubMed] [Google Scholar]
- [24].Massoud TF, Gambhir SS. Genes Dev. 2003;17:545. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- [25].Rudin M, Weissleder R. Nat. Rev. Drug Discov. 2003;2:123. doi: 10.1038/nrd1007. [DOI] [PubMed] [Google Scholar]
- [26].Cai W, Rao J, Gambhir SS, Chen X. Mol. Cancer Ther. 2006;5:2624. doi: 10.1158/1535-7163.MCT-06-0395. [DOI] [PubMed] [Google Scholar]
- [27].Balasubramanian K, Burghard M. Small. 2005;1:180. doi: 10.1002/smll.200400118. [DOI] [PubMed] [Google Scholar]
- [28].Pathak AP, Gimi B, Glunde K, Ackerstaff E, Artemov D, Bhujwalla ZM. Methods Enzymol. 2004;386:3. doi: 10.1016/S0076-6879(04)86001-4. [DOI] [PubMed] [Google Scholar]
- [29].Zhang Z, Nair SA, McMurry TJ. Curr. Med. Chem. 2005;12:751. doi: 10.2174/0929867053507379. [DOI] [PubMed] [Google Scholar]
- [30].Pautler RG, Fraser SE. Curr. Opin. Immunol. 2003;15:385. doi: 10.1016/s0952-7915(03)00073-6. [DOI] [PubMed] [Google Scholar]
- [31].de Roos A, Doornbos J, Baleriaux D, Bloem HL, Falke TH. Magn. Reson. Annu. 1988:113. [PubMed] [Google Scholar]
- [32].Sitharaman B, Kissell KR, Hartman KB, Tran LA, Baikalov A, Rusakova I, et al. Chem. Commun. 2005;31:3915. doi: 10.1039/b504435a. [DOI] [PubMed] [Google Scholar]
- [33].Hartman KB, Laus S, Bolskar RD, Muthupillai R, Helm L, Toth E, et al. Nano Lett. 2008;8:415. doi: 10.1021/nl0720408. [DOI] [PubMed] [Google Scholar]
- [34].Sosnovik DE, Weissleder R. Curr. Opin. Biotechnol. 2007;18:4. doi: 10.1016/j.copbio.2006.11.001. [DOI] [PubMed] [Google Scholar]
- [35].Didenko VV, Moore VC, Baskin DS, Smalley RE. Nano Lett. 2005;5:1563. doi: 10.1021/nl050840h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bottini M, Cerignoli F, Dawson MI, Magrini A, Rosato N, Mustelin T. Biomacromolecules. 2006;7:2259. doi: 10.1021/bm0602031. [DOI] [PubMed] [Google Scholar]
- [37].Cheong WF, Prahl SA, Welch AJ. IEEE J. 1990;26:2166. [Google Scholar]
- [38].Frangioni JV. Curr. Opin. Chem. Biol. 2003;7:626. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- [39].Cai W, Shin DW, Chen K, Gheysens O, Cao Q, Wang SX, et al. Nano Lett. 2006;6:669. doi: 10.1021/nl052405t. [DOI] [PubMed] [Google Scholar]
- [40].Cherukuri P, Bachilo SM, Litovsky SH, Weisman RB. J. Am. Chem. Soc. 2004;126:15638. doi: 10.1021/ja0466311. [DOI] [PubMed] [Google Scholar]
- [41].Lefebvre J, Austing DG, Bond J, Finnie P. Nano Lett. 2006;6:1603. doi: 10.1021/nl060530e. [DOI] [PubMed] [Google Scholar]
- [42].Welsher K, Liu Z, Daranciang D, Dai H. Nano Lett. 2008;8:586. doi: 10.1021/nl072949q. [DOI] [PubMed] [Google Scholar]
- [43].Duque JG, Cognet L, Parra-Vasquez AN, Nicholas N, Schmidt HK, Pasquali M. J. Am. Chem. Soc. 2008;130:2626. doi: 10.1021/ja0777234. [DOI] [PubMed] [Google Scholar]
- [44].Jin H, Heller DA, Strano MS. Nano Lett. 2008;8:1577. doi: 10.1021/nl072969s. [DOI] [PubMed] [Google Scholar]
- [45].Leeuw TK, Reith RM, Simonette RA, Harden ME, Cherukuri P, Tsyboulski DA, et al. Nano Lett. 2007;7:2650. doi: 10.1021/nl0710452. [DOI] [PubMed] [Google Scholar]
- [46].Cherukuri P, Gannon CJ, Leeuw TK, Schmidt HK, Smalley RE, Curley SA, et al. Proc. Natl. Acad. Sci. U.S.A. 2006;103:18882. doi: 10.1073/pnas.0609265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cognet L, Tsyboulski DA, Weisman RB. Nano Lett. 2008;8:749. doi: 10.1021/nl0725300. [DOI] [PubMed] [Google Scholar]
- [48].Bussy C, Cambedouzou J, Lanone S, Leccia E, Heresanu V, Pinault M, et al. Nano Lett. 2008;8:2659. doi: 10.1021/nl800914m. [DOI] [PubMed] [Google Scholar]
- [49].Choi JH, Nguyen FT, Barone PW, Heller DA, Moll AE, Patel D, et al. Nano Lett. 2007;7:861. doi: 10.1021/nl062306v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yang X, Skrabalak SE, Li ZY, Xia Y, Wang LV. Nano Lett. 2007;7:3798. doi: 10.1021/nl072349r. [DOI] [PubMed] [Google Scholar]
- [51].Qian X, Peng XH, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, et al. Nat. Biotechnol. 2008;26:83. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- [52].Cao YC, Jin R, Mirkin CA. Science. 2002;297:1536. doi: 10.1126/science.297.5586.1536. [DOI] [PubMed] [Google Scholar]
- [53].Sun L, Yu C, Irudayaraj J. Anal. Chem. 2007;79:3981. doi: 10.1021/ac070078z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Jorio A, Saito R, Dresselhaus G, Dresselhaus MS. Philos. Transact. A Math. Phys. Eng. Sci. 2004;362:2311. doi: 10.1098/rsta.2004.1443. [DOI] [PubMed] [Google Scholar]
- [55].Zhang L, Aite S, Yu Z. Rev. Sci. Instrum. 2007;78:083701. doi: 10.1063/1.2768924. [DOI] [PubMed] [Google Scholar]
- [56].Zhang C, Abdijalilov K, Grebel H. J. Chem. Phys. 2007;127:044701. doi: 10.1063/1.2752498. [DOI] [PubMed] [Google Scholar]
- [57].Anderson N, Hartschuh A, Cronin S, Novotny L. J. Am. Chem. Soc. 2005;127:2533. doi: 10.1021/ja045190i. [DOI] [PubMed] [Google Scholar]
- [58].Hartschuh A, Qian H, Meixner AJ, Anderson N, Novotny L. Nano Lett. 2005;5:2310. doi: 10.1021/nl051775e. [DOI] [PubMed] [Google Scholar]
- [59].Hartschuh A, Sanchez EJ, Xie XS, Novotny L. Phys. Rev. Lett. 2003;90:095503. doi: 10.1103/PhysRevLett.90.095503. [DOI] [PubMed] [Google Scholar]
- [60].Atalay H, Lefrant S. J. Nanosci. Nanotechnol. 2004;4:749. doi: 10.1166/jnn.2004.106. [DOI] [PubMed] [Google Scholar]
- [61].Doorn SK, Zheng L, O’Connell J, Zhu M,Y, Huang S, Liu J. J. Phys. Chem. B. 2005;109:3751. doi: 10.1021/jp0463159. [DOI] [PubMed] [Google Scholar]
- [62].Itkis ME, Perea DE, Jung R, Niyogi S, Haddon RC. J. Am. Chem. Soc. 2005;127:3439. doi: 10.1021/ja043061w. [DOI] [PubMed] [Google Scholar]
- [63].Keren S, Zavaleta C, Cheng Z, de la Zerda A, Gheysens O, Gambhir SS. Proc. Natl. Acad. Sci. U.S.A. 2008;105:5844. doi: 10.1073/pnas.0710575105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zavaleta C, de la Zerda A, Liu Z, Keren S, Cheng Z, Schipper M, et al. Nano Lett. 2008;8:2800. doi: 10.1021/nl801362a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Cai W, Chen X. Anti-Cancer Agents Med. Chem. 2006;6:407. doi: 10.2174/187152006778226530. [DOI] [PubMed] [Google Scholar]
- [66].Cai W, Chen X. J. Nucl. Med. Suppl. 2008;2:113S. doi: 10.2967/jnumed.107.045922. [DOI] [PubMed] [Google Scholar]
- [67].Cai W, Niu G, Chen X. Curr. Pharm. Des. 2008;14:2943. doi: 10.2174/138161208786404308. [DOI] [PubMed] [Google Scholar]
- [68].Cai W, Chen K, Li ZB, Gambhir SS, Chen X. J. Nucl. Med. 2007;48:1862. doi: 10.2967/jnumed.107.043216. [DOI] [PubMed] [Google Scholar]
- [69].Cai W, Wu Y, Chen K, Cao Q, Tice DA, Chen X. Cancer Res. 2006;66:9673. doi: 10.1158/0008-5472.CAN-06-1480. [DOI] [PubMed] [Google Scholar]
- [70].Liu Z, Li X, Tabakman SM, Jiang K, Fan S, Dai H. J. Am. Chem. Soc. 2008;130:13540. doi: 10.1021/ja806242t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Cai W, Chen K, He L, Cao Q, Koong A, Chen X. Eur. J. Nucl. Med. Mol. Imaging. 2007;34:850. doi: 10.1007/s00259-006-0361-6. [DOI] [PubMed] [Google Scholar]
- [72].Cai W, Niu G, Chen X. Eur. J. Nucl. Med. Mol. Imaging. 2008;35:186. doi: 10.1007/s00259-007-0560-9. [DOI] [PubMed] [Google Scholar]
- [73].Chen Z, Tabakman SM, Goodwin AP, Kattah MG, Daranciang D, Wang X, et al. Nat. Biotechnol. 2008;26:1285. doi: 10.1038/nbt.1501. [DOI] [PubMed] [Google Scholar]
- [74].Rao AM, Richter E, Bandow S, Chase B, Eklund PC, Williams KA, et al. Science. 1997;275:187. doi: 10.1126/science.275.5297.187. [DOI] [PubMed] [Google Scholar]
- [75].Wang LV. Med. Phys. 2008;35:5758. doi: 10.1118/1.3013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kim G, Huang SW, Day KC, O’Donnell M, Agayan RR, Day MA, et al. J. Biomed. Opt. 2007;12:044020. doi: 10.1117/1.2771530. [DOI] [PubMed] [Google Scholar]
- [77].Ku G, Wang LV. Opt. Lett. 2005;30:507. doi: 10.1364/ol.30.000507. [DOI] [PubMed] [Google Scholar]
- [78].Liao CK, Huang SW, Wei CW, Li PC. J. Biomed. Opt. 2007;12:064006. doi: 10.1117/1.2812704. [DOI] [PubMed] [Google Scholar]
- [79].De la Zerda A, Zavaleta C, Keren S, Vaithilingam S, Bodapati S, Liu Z, et al. Nat. Nanotechnol. 2008;3:557. doi: 10.1038/nnano.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Cai W, Chen X. Front. Biosci. 2007;12:4267. doi: 10.2741/2386. [DOI] [PubMed] [Google Scholar]
- [81].Peremans K, Cornelissen B, Van Den Bossche B, Audenaert K, Van de Wiele C. Vet. Radiol. Ultrasound. 2005;46:162. doi: 10.1111/j.1740-8261.2005.00031.x. [DOI] [PubMed] [Google Scholar]
- [82].Wang H, Wang J, Deng X, Sun H, Shi Z, Gu Z, et al. J. Nanosci. Nanotechnol. 2004;4:1019. doi: 10.1166/jnn.2004.146. [DOI] [PubMed] [Google Scholar]
- [83].Singh R, Pantarotto D, Lacerda L, Pastorin G, Klumpp C, Prato M, et al. Proc. Natl. Acad. Sci. U.S.A. 2006;103:3357. doi: 10.1073/pnas.0509009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Chatziioannou AF. Proc. Am. Thorac. Soc. 2005;2:533. doi: 10.1513/pats.200508-079DS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Phelps ME, Hoffman EJ, Mullani NA, Ter-Pogossian MM. J. Nucl. Med. 1975;16:210. [PubMed] [Google Scholar]
- [86].Cherry SR, Shao Y, Silverman RW, Meadors K, Siegel S, Chatziioannou A, et al. IEEE Trans. Nucl. Sci. 1997;44:1161. [Google Scholar]
- [87].Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, et al. Nat. Nanotechnol. 2007;2:47. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- [88].Liu Z, Davis C, Cai W, He L, Chen X, Dai H. Proc. Natl. Acad. Sci. U.S.A. 2008;105:1410. doi: 10.1073/pnas.0707654105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Liu Z, Chen K, Davis C, Sherlock S, Cao Q, Chen X, et al. Cancer Res. 2008;68:6652. doi: 10.1158/0008-5472.CAN-08-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].McDevitt MR, Chattopadhyay D, Kappel BJ, Jaggi JS, Schiffman SR, Antczak C, et al. J. Nucl. Med. 2007;48:1180. doi: 10.2967/jnumed.106.039131. [DOI] [PubMed] [Google Scholar]
- [91].McDevitt MR, Chattopadhyay D, Jaggi JS, Finn RD, Zanzonico PB, Villa C, et al. PLoS ONE. 2007;2:e907. doi: 10.1371/journal.pone.0000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Villa CH, McDevitt MR, Escorcia FE, Rey DA, Bergkvist M, Batt CA, et al. Nano Lett. 2008 doi: 10.1021/nl801878d. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Brannon-Peppas L, Blanchette JO. Adv. Drug Deliv. Rev. 2004;56:1649. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- [94].Chavanpatil MD, Khdair A, Panyam J. J. Nanosci. Nanotechnol. 2006;6:2651. doi: 10.1166/jnn.2006.443. [DOI] [PubMed] [Google Scholar]
- [95].Caliceti P, Veronese FM. Adv. Drug Deliv. Rev. 2003;55:1261. doi: 10.1016/s0169-409x(03)00108-x. [DOI] [PubMed] [Google Scholar]
- [96].Wu AM, Senter PD. Nat. Biotechnol. 2005;23:1137. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- [97].Soo Choi H, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, et al. Nat. Biotechnol. 2007;25:1165. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Kam NW, O’Connell M, Wisdom JA, Dai H. Proc. Natl. Acad. Sci. U.S.A. 2005;102:11600. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Donaldson K, Aitken R, Tran L, Stone V, Duffin R, Forrest G, et al. Toxicol. Sci. 2006;92:5. doi: 10.1093/toxsci/kfj130. [DOI] [PubMed] [Google Scholar]
- [100].Kolosnjaj J, Szwarc H, Moussa F. Adv. Exp. Med. Biol. 2007;620:181. doi: 10.1007/978-0-387-76713-0_14. [DOI] [PubMed] [Google Scholar]
- [101].Lam CW, James JT, McCluskey R, Arepalli S, Hunter RL. Crit. Rev. Toxicol. 2006;36:189. doi: 10.1080/10408440600570233. [DOI] [PubMed] [Google Scholar]
- [102].Schipper ML, Nakayama-Ratchford N, Davis CR, Kam NW, Chu P, Liu Z, et al. Nat. Nanotechnol. 2008;3:216. doi: 10.1038/nnano.2008.68. [DOI] [PubMed] [Google Scholar]