Abstract
Cardiac hypertrophy is associated with upregulation of vascular endothelial growth factor (VEGF) in the myocardium. Here, we evaluated the effects of a decoy VEGF receptor on heart morphology and function to a murine model of pressure overload hypertrophy. Mice were administered adenoviral vector encoding a decoy VEGF receptor (Ad-Flk), and their hearts were subjected to pressure overload by transverse aortic constriction (TAC). Treatment with Ad-Flk led to a net reduction in capillary density in hearts subjected to TAC. Ad-Flk also led to a reduction in TAC-induced cardiac hypertrophy and promoted left ventricle dilatation and a loss in contractile function. Treatment with Ad-Flk markedly increased myocardial fibrosis and collagen gene upregulation. In contrast, Ad-Flk had no effect on any of these parameters in sham-treated mice. Administration of a VEGF trap reagent diminished pressure overload cardiac hypertrophy and promoted the progression to heart failure but had no effect on sham-treated animals. These findings suggest that VEGF is required to maintain myocardial capillary density and that reductions in the vascular bed are associated with the transition from compensatory hypertrophy to failure.
Keywords: heart failure, remodeling, endothelial growth factors, hypertrophy
Cardiac hypertrophy is initially an adaptive response to preserve cardiac function in response to several forms of stress.1,2 However, after sustained external load, hearts can evolve to a state of decompensated hypertrophy resulting in cardiac dilation and loss of contractile function. Whereas it is known that overload-induced cardiac hypertrophy involves the participation of angiotensin II,3 endothelin-1,4 and fibroblast growth factor-2,5 the molecular mechanisms responsible for the transition from compensated to decompensated hypertrophy are poorly defined.
Myocardial ischemia and diminished myocardial blood flow are predictors of poor prognosis in heart failure.6 Pressure or volume overload–induced cardiac hypertrophy is associated with a reduction in capillary density in a number of animals models.7 In addition, a reduced capillary bed has been described for the left ventricular hypertrophy that occurs in the intact parts of the heart with myocardial infarction.8 Recently, we have shown that a reduction in cardiac capillary density promotes contractile dysfunction in a transgenic mouse model where a constitutively active form of Akt1 is expressed from a cardiac-specific promoter.9 These results suggest that impaired vasculature could contribute to the transition from compensated to decompensated cardiac hypertrophy. However, this hypothesis has not been directly tested in a model of pathological hypertrophy, such as that induced by pressure overload of the heart.
Vascular endothelial growth factor (VEGF) is an endothelial cell mitogen that has an essential role in both vasculogenesis and angiogenesis.10 VEGF regulates multiple angiogenic cellular responses, including survival, migration, and differentiation, through activation of Akt signaling within endothelial cells.11 VEGF is secreted from cardiomyocytes in response to extracellular stimuli.12–15 Mice engineered to express only a single spliced isoform of VEGF-A gene (VEGF120) or mice with cardiac-specific deletion of VEGF-A exhibit reduced capillary density and impaired contractility.16,17 These reports led us to the hypothesize that VEGF may be required to maintain the capillary bed under conditions of cardiac stress.
It has been shown previously that intravenous administration of an adenoviral vector encoding the ligand-binding domain of VEGF receptor 2 (Flk1) fused to murine IgG2a Fc leads to systemic VEGF secretion and inhibition of angiogenesis in both tumor18–20 and ischemic hindlimb21 models of vessel growth. Here, we used the adenoviral vector encoding the ectodomain of Flk1 in a murine model of pressure overload hypertrophy. This treatment resulted in reduced myocardial capillary density, accelerated contractile dysfunction, and pathological cardiac remodeling. These findings indicate that VEGF-dependent capillarization is essential for compensatory cardiac hypertrophy in response to pressure overload.
Methods
Animals
Study protocols were approved by the Institutional Animal Care and Use Committee at Boston University. Ten-week–old male C57BL/6 mice were used in this study. Transverse aortic constriction (TAC) was performed as described previously in detail.22 Sham-treated animals underwent open chest surgery but not transverse aortic constricting. After 2 weeks of surgery, mice were subjected to transthoracic echocardiography and cardiac catheterization to determine heart rate, proximal aortic pressure, and left ventricular end-diastolic (LVED) pressure. Animals were then euthanized, and the hearts were weighed and harvested for additional analysis.
Adenovirus-Mediated Gene Transfer
Adenovirus vectors encoding Flk1-Fc and control Fc fragment were described previously.19 We injected 2×108 plaque-forming units of Ad-Flk1-Fc (Ad-Flk) or Ad-control Fc (ad-cont) into the jugular vein of mice 3 days before TAC.
Echocardiography
Transthoracic echocardiography was performed with an Acuson 256 sector scanner equipped with a 13-MHz broadband transducer. All of the recordings were performed with conscious animals.23
Quantitative Real-Time PCR
Total RNA was prepared by Qiagen using protocols provided by the manufacturer. cDNA was produced using ThermoScript RT-PCR Systems (Invitrogen). Real-time PCR was performed as described previously.24 Transcript levels of atrial natriuretic peptide (ANP), VEGF-A, and collagen III was determined as the relative number of transcripts to those of glyceraldehydes-3-phosphate dehydrogenase and normalized to the mean value of control hearts. Primers for ANP, VEGF-A, collagen III, and glyceraldehydes-3-phosphate dehydrogenase were as described.24–26
Histological Analysis
Heart sections were prepared as described27 and were stained with TRITC conjugated BS-1 lectin to evaluate capillary density, fluorescein isothiocyanate-conjugated wheat germ agglutinin to evaluate myofiber size, and Masson’s trichrome for detection of myocardial interstitial fibrosis. To determine the capillary density, myofiber size, and myocardial interstitial fibrosis, we selected ≥10 fields randomly and calculated as described previously with the image analysis software NIH IMAGE.28
Statistics Analysis
All of the data are presented as mean±SEM. Comparisons among groups were made by 1-way ANOVA, followed by Scheffe F test. A level of P<0.05 was accepted as statistically significant.
Results
VEGF Receptor Decoy Reduces Capillary Density in Hearts Subjected to TAC
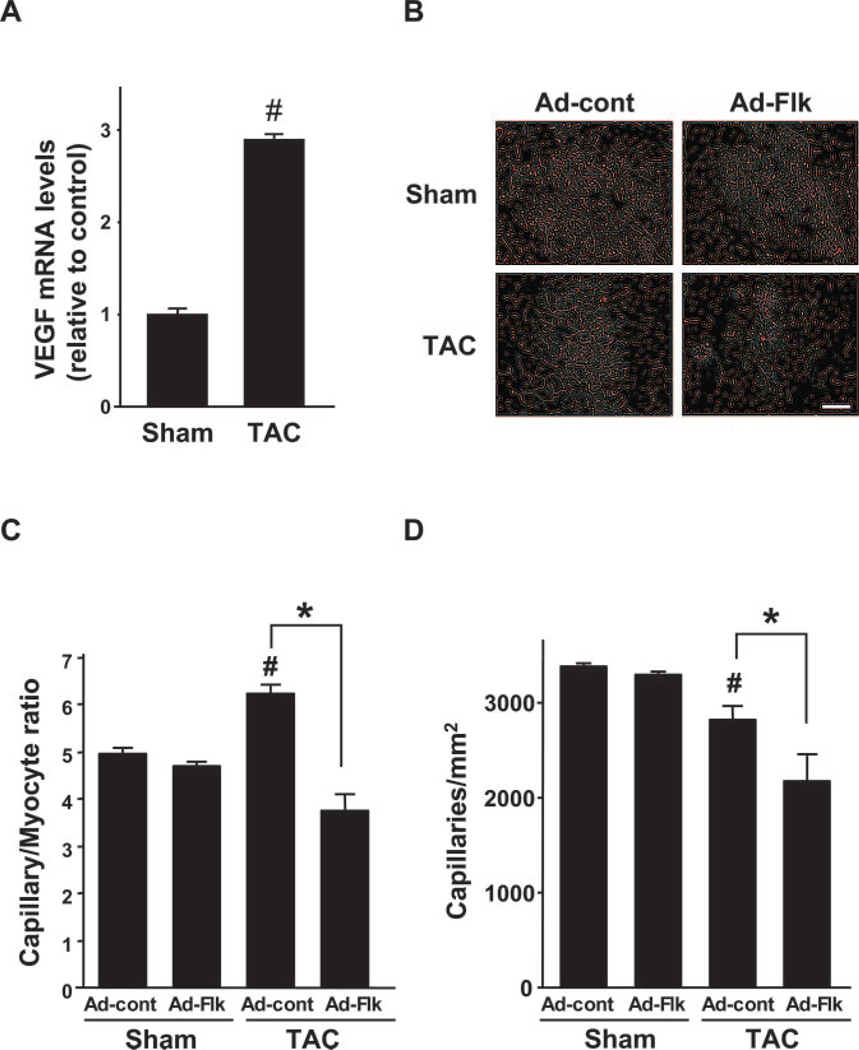
Consistent with previous results,29,30 TAC led to a 2.9-fold (P<0.01) increase in VEGF-A transcript expression (Figure 1A). To investigate the links between coronary vasculature and compensatory cardiac hypertrophy, an adenoviral vector encoding a decoy VEGF receptor fused to the Fc fragment of IgG2a (Ad-Flk) or a control vector expressing only the Fc fragment (Ad-cont) was delivered to mice, and hearts were subjected to sham surgery or pressure overload resulting from TAC. Based on prior experiments,9 2×108 plaque-forming units of Ad-Flk or ad-cont were injected via the jugular vein. As shown in Table, there were no significant differences in body weight in each experimental group at 2 weeks after surgery or sham treatment. Mice exposed to TAC showed a significant increase in proximal aortic systolic blood pressure and heart rate compared with sham in both Ad-Flk and Ad-cont experimental groups.
Figure 1.
Treatment with VEGF receptor decoy decreases the coronary vascular bed in hearts subjected to TAC. (A) TAC leads to an increase in VEGF-A transcript expression. (B) Representative images of TRITC-conjugated BS-1 lectin stained heart sections. Scale bars: 50 0 µm. (C) Quantitative analysis of capillary/myocyte ratio in Ad-cont– or Ad-Flk–treated mice at 2 weeks after sham operation or TAC. (D) Quantitative analysis of capillary density in Ad-cont– or Ad-Flk–treated mice 2 weeks after sham operation or TAC. Results are presented as mean±SEM (n=4 to 6 each). *P<0.05. #P<0.05 vs Ad-cont with sham.
Body Weight, Heart Rate, and Proximal Aortic Systolic Blood Pressure in Ad-cont– or Ad-Flk–Treated Mice at 14 Days After Surgery
| Variable (n=4–6) |
Sham | TAC | ||
|---|---|---|---|---|
| Ad-cont | Ad-Flk | Ad-cont | Ad-Flk | |
| Body weight (g) | 21.8±0.5 | 21.9±0.5 | 21.1±0.7 | 21.2±1.0 |
| Systolic blood pressure (mm Hg) | 99±3 | 101±4 | 149±10* | 142±13* |
| Heart rate (bpm) | 488±32 | 485±26 | 558±30* | 586±30* |
Results are presented as mean±SEM.
P<0.05 vs Ad-cont sham.
Capillary status was evaluated by histology to determine the effect of Ad-Flk on the coronary vasculature (Figure 1B). In Ad-cont mice, TAC led to an increase in capillary/myocyte ratio at 2 weeks (Figure 1C), but the net myocardial capillary density decreased significantly (Figure 1D). Treatment with Ad-Flk blocked the increase in capillary/myocyte ratio and led to a further reduction in the capillary density in the myocardium relative to the Ad-cont group. In contrast, Ad-Flk had no effect on capillary/myocyte ratio or capillary density in sham-operated mice.
VEGF Receptor Decoy Reduces Impaired Cardiac Hypertrophy in Response to TAC
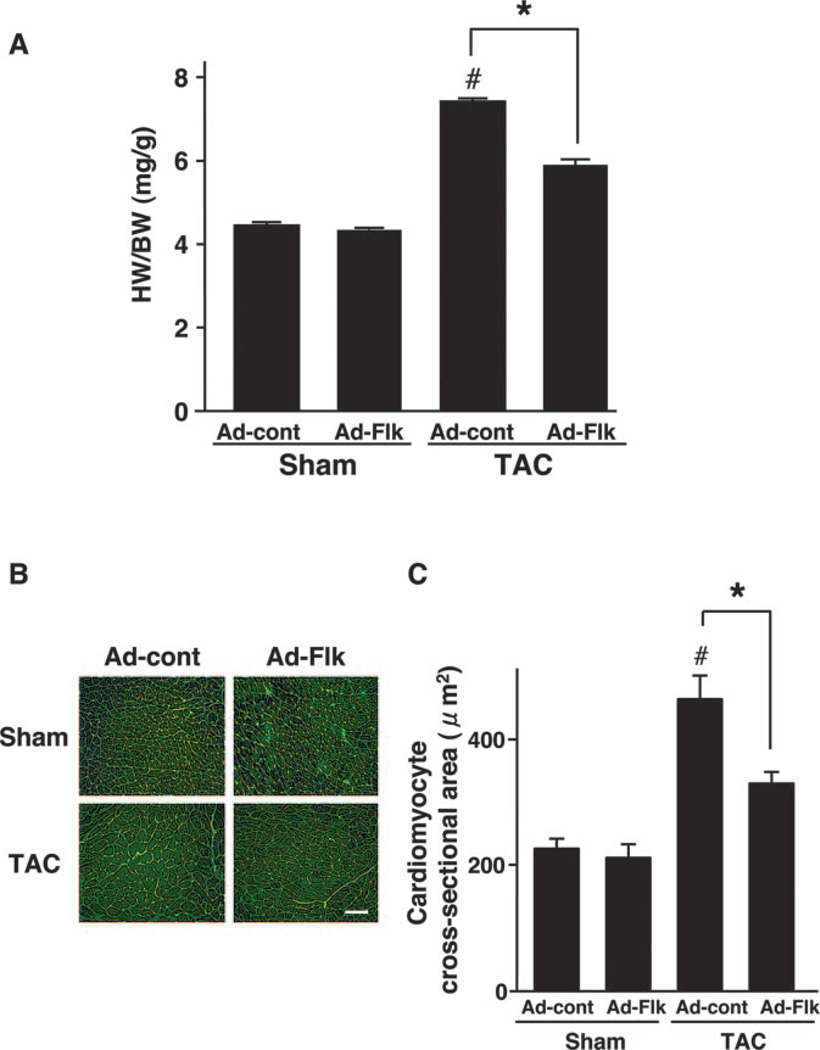
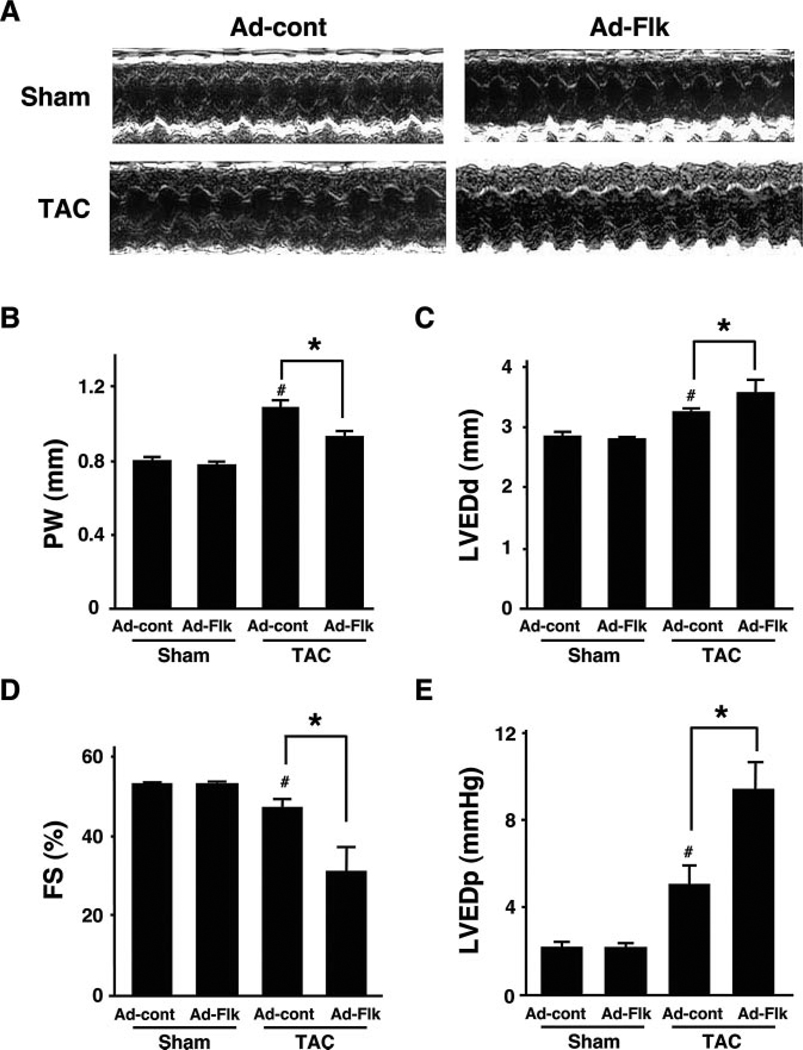
The heart weight/body weight ratio was significantly increased by TAC in control mice (Figure 2A). Treatment with Ad-Flk led to a 54% decrease in TAC-induced cardiac growth. However, treatment with Ad-Flk had no effect on heart size in sham-treated animals. Analysis of myocyte cross-sectional area in histological sections corroborated these findings (Figure 2B). The calculated myocyte size increased in control mice that underwent TAC, but this increase was largely blocked in mice that were administered Ad-Flk before TAC (Figure 2C). Ad-Flk administration to sham mice had no effect on myocyte cross-sectional area. Echocardiographic measurements also showed that interventricular septum (data not shown) and posterior wall thickness (Figure 3A and 3B) were significantly increased by TAC in Ad-cont–treated mice, but these increases were suppressed by the administration of Ad-Flk before TAC.
Figure 2.
Treatment with VEGF receptor decoy attenuates pressure overload induced cardiac hypertrophy in hearts subjected to TAC. (A) Heart weight/body weight ratio in Ad-cont– or Ad-Flk–treated mice 2 weeks after sham operation or TAC. *P<0.05. (B) Representative images of wheat germ agglutinin (WGA)-stained heart sections. Scale bars: 50 µm. (C) Quantitative analysis of cardiac myocyte cross-sectional area in Ad-cont– or Ad-Flk–treated mice 2 weeks after sham operation or TAC. Data were obtained from analysis of WGA-stained heart sections. Results are presented as mean±SEM (n=4 to 6 each). *P<0.05. #P<0.05 vs Ad-cont with sham.
Figure 3.
VEGF receptor decoy promotes LV dilatation and contractile dysfunction in hearts subjected to TAC. (A) Representative M-mode echocardiogram for Ad-cont–or Ad-Flk–treated mice 2 weeks after sham operation or TAC. (B through E) Posterior wall thickness (PW), LVED dimension (LVEDd), %FS, and LVED pressure, respectively, in Ad-cont– or Ad-Flk–treated mice 2 weeks after sham operation or TAC. Results are presented as mean±SEM (n=4 to 6 each). *P<0.05. #P<0.05 vs Ad-cont with sham.
VEGF Receptor Decoy Accelerates Contractile Dysfunction and Pathological Cardiac Remodeling
LVED dimension was significantly increased, and percentage of fractional shortening (%FS) was significantly decreased in Ad-cont–treated mice 2 weeks after TAC (Figure 3C and 3D). Treatment with Ad-Flk promoted the enlargement of LVED dimension and further decreased %FS in mice who underwent TAC (Figure 3E). In contrast, treatment with Ad-Flk had no effect on echocardiographic or hemodynamic parameters in sham-operated mice.
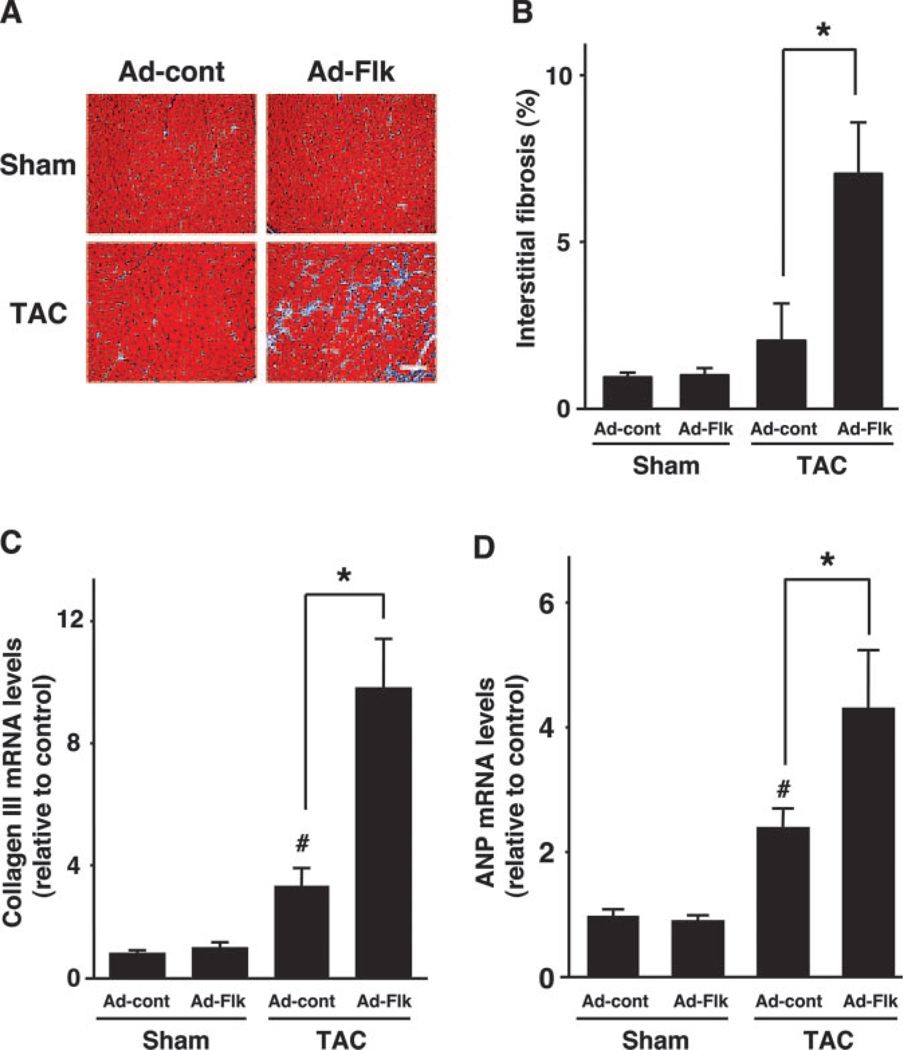
Myocardial interstitial fibrosis was evaluated to further investigate the consequence of diminished capillary density on cardiac remodeling (Figure 4A). There was a trend toward increased fibrosis after TAC in mice treated with Ad-cont, but this was not statistically significant (Figure 4B). However, treatment with Ad-Flk led to a large increase in fibrosis in the hearts subjected to TAC. Consistent with this observation, collagen III gene expression was significantly increased by Ad-Flk treatment in hearts subjected to TAC (Figure 4C). Finally, TAC led to an increase in the expression of the fatal-type cardiac gene ANP, and this upregulation was more pronounced in mice treated with Ad-Flk than Ad-cont (Figure 4D). Ad-Flk did not detectably affect fibrosis or collagen and ANP expression in sham-operated mice.
Figure 4.
VEGF receptor decoy treatment promotes pathological cardiac remodeling in hearts. (A) Representative images of Masson’s trichrome (MT) staining of heart sections. Scale bars: 50 µm. (B) Quantitative analysis of myocardial interstitial fibrosis in Ad-cont– or Ad-Flk–treated mice 2 weeks after sham operation or TAC. (C) Collagen III mRNA expression in Ad-cont– or Ad-Flk–treated mice 2 weeks after sham operation or TAC. (D) Atrial natriuretic peptide (ANP) mRNA expression in Ad-cont– or Ad-Flk–treated mice 2 weeks after sham operation or TAC. Results are presented as mean±SEM (n=4 to 6 each). *P<0.05. #P<0.05 vs Ad-cont with sham.
Discussion
In the present study, we demonstrated that sequestration of endogenous VEGF impairs adaptive cardiac hypertrophy in response to pressure overload. We show that pressure overload leads to an upregulation of VEGF-A expression and an increase in the capillary/myocyte ratio. However, there is a net reduction in capillary density (capillaries/mm2), because the increase in capillarization (≈1.2-fold increase in capillaries/myocyte) does not keep pace with myocyte growth (≈2-fold increase in myocyte cross-sectional area). In animals treated with the ectodomain of Flk1, there was no increase in the capillary/myocyte ratio and, thus, a greater reduction in coronary capillary density after aortic constriction. This corresponded to a rapid transition to heart failure as characterized by decreased %FS, increased LVED dimension and pressure, increased ANP and collagen III gene expression, and increased myocardial interstitial fibrosis. These findings suggest that VEGF is essential for coronary vascular network growth under conditions that promote compensatory cardiac hypertrophy and that a reduction in available VEGF contributes to the rapid progression from compensatory cardiac hypertrophy to failure. Notably, treatment with Flk-Fc had no effect on coronary capillary density or function in normal hearts of sham-treated animals. These data suggest that, in the absence of growth stimuli, normal contractile function can be maintained without the need for VEGF.
Previous studies have shown that VEGF is upregulated in myocardium in pathological conditions such as myocardial infarction,31,32 pressure overload,29,30 and hemodynamic overload.33 In vitro, it has been shown that VEGF is secreted from cardiomyocytes in response to various extracellular stimuli.12–15 Presumably, VEGF functions as a paracrine factor that is required to recruit new vessels as the heart grows, similar to what has been described for skeletal muscle hypertrophy.34 VEGF may also be required to preserve the myocardial capillary bed in pressure-overloaded hearts through its antiapoptotic actions on endothelial cells.35 Despite the upregulation in cardiac expression of VEGF under conditions of cardiac stress,29–33 “pathological” cardiac hypertrophy can be associated with a net loss of capillary density in some animal models.7,8 In contrast, cardiac growth associated with development, nutritional input, or vigorous exercise is associated with maintained or increased capillary density.7,36 Thus, the availability of VEGF may play a role in determining whether the phenotype of the growing heart is “physiological” or “pathological.”9
Another finding of this study is that treatment with VEGF receptor decoy attenuated the extent of hypertrophy induced by pressure overload. Therefore, stress-induced heart growth may depend on the status of the vascular bed in a manner that is similar to tumor19 and fat pad37 growth. It has also been reported that knockout mice lacking cardiac VEGF exhibit a diminished capillary density and a small heart,17 suggesting that the endothelium is a source of growth factors for the heart during development. Similarly, liver or pancreas development requires factors released from newly formed endothelial cells for normal growth.38,39 Thus, the identification of paracrine factors released by coronary endothelial cells could lead to the identification of novel regulators or myocyte hypertrophy and function.40
In conclusion, our work shows that reductions in VEGF-dependent capillary density promote the rapid progression from compensatory cardiac hypertrophy to failure in pressure overloaded hearts. Thus, the status of the coronary vasculature can play a role in controlling heart function under conditions of cardiac stress. In this regard, patients with hypertrophic cardiomyopathy typically exhibit risk factors associated with endothelial cell dysfunction,41–43 and this combination could exacerbate the transition to heart failure. Finally, these data indicate that antiangiogenesis agents that target VEGF for the treatment of cancer could be detrimental for heart function in patients who also experience hypertrophic cardiomyopathy.
Acknowledgments
This work was supported by National Institutes of Health grants HL77774, HL66957, AR40197, and AG15052 to K.W. We thank Ann Bialik for technical assistance.
References
- 1.Gerdes AM, Kellerman SE, Moore JA, Muffly KE, Clark LC, Reaves PY, Malec KB, McKeown PP, Schocken DD. Structural remodeling of cardiac myocytes in patients with ischemic cardiomyopathy. Circulation. 1992;86:426–430. doi: 10.1161/01.cir.86.2.426. [DOI] [PubMed] [Google Scholar]
- 2.Olivetti G, Quaini F, Lagrasta C, Cigola E, Ricci R, Maestri R, Anversa P. Cellular basis of ventricular remodeling after myocardial infarction in rats. Cardioscience. 1995;6:101–106. [PubMed] [Google Scholar]
- 3.Senbonmatsu T, Ichihara S, Price E, Jr, Gaffney FA, Inagami T. Evidence for angiotensin II type 2 receptor-mediated cardiac myocyte enlargement during in vivo pressure overload. J Clin Invest. 2000;106:R1–R5. [PubMed] [Google Scholar]
- 4.Ito H, Hiroe M, Hirata Y, Fujisaki H, Adachi S, Akimoto H, Ohta Y, Marumo F. Endothelin ETA receptor antagonist blocks cardiac hypertrophy provoked by hemodynamic overload. Circulation. 1994;89:2198–2203. doi: 10.1161/01.cir.89.5.2198. [DOI] [PubMed] [Google Scholar]
- 5.Schultz JE, Witt SA, Nieman ML, Reiser PJ, Engle SJ, Zhou M, Pawlowski SA, Lorenz JN, Kimball TR, Doetschman T. Fibroblast growth factor-2 mediates pressure-induced hypertrophic response. J Clin Invest. 1999;104:709–719. doi: 10.1172/JCI7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Heuvel AF, van Veldhuisen DJ, van der Wall EE, Blanksma PK, Siebelink HM, Vaalburg WM, van Gilst WH, Crijns HJ. Regional myocardial blood flow reserve impairment and metabolic changes suggesting myocardial ischemia in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2000;35:19–28. doi: 10.1016/s0735-1097(99)00499-4. [DOI] [PubMed] [Google Scholar]
- 7.Hudlicka O, Brown M, Egginton S. Angiogenesis in skeletal and cardiac muscle. Physiol Rev. 1992;72:369–417. doi: 10.1152/physrev.1992.72.2.369. [DOI] [PubMed] [Google Scholar]
- 8.Anversa P, Beghi C, Kikkawa Y, Olivetti G. Myocardial infarction in rats. Infarct size, myocyte hypertrophy, and capillary growth. Circ Res. 1986;58:26–37. doi: 10.1161/01.res.58.1.26. [DOI] [PubMed] [Google Scholar]
- 9.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci W, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999;77:527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- 11.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 12.Maruyama K, Mori Y, Murasawa S, Masaki H, Takahashi N, Tsutusmi Y, Moriguchi Y, Shibazaki Y, Tanaka Y, Shibuya M, Inada M, Matsubara H, Iwasaka T. Interleukin-1 β upregulates cardiac expression of vascular endothelial growth factor and its receptor KDR/flk-1 via activation of protein tyrosine kinases. J Mol Cell Cardiol. 1999;31:607–617. doi: 10.1006/jmcc.1998.0895. [DOI] [PubMed] [Google Scholar]
- 13.Zheng W, Seftor EA, Meininger CJ, Hendrix MJ, Tomanek RJ. Mechanisms of coronary angiogenesis in response to stretch: role of VEGF and TGF-β. Am J Physiol Heart Circ Physiol. 2001;280:H909–H917. doi: 10.1152/ajpheart.2001.280.2.H909. [DOI] [PubMed] [Google Scholar]
- 14.Funamoto M, Fujio Y, Kunisada K, Negoro S, Tone E, Osugi T, Hirota H, Izumi M, Yoshizaki K, Walsh K, Kishimoto T, Yamauchi-Takihara K. Signal transducer and activator of transcription 3 is required for glycoprotein 130-mediated induction of vascular endothelial growth factor in cardiac myocytes. J Biol Chem. 2000;275:10561–10566. doi: 10.1074/jbc.275.14.10561. [DOI] [PubMed] [Google Scholar]
- 15.Levy AP, Levy NS, Loscalzo J, Calderone A, Takahashi N, Yeo KT, Koren G, Colucci WS, Goldberg MA. Regulation of vascular endothelial growth factor in cardiac myocytes. Circ Res. 1995;76:758–766. doi: 10.1161/01.res.76.5.758. [DOI] [PubMed] [Google Scholar]
- 16.Carmeliet P, Ng YS, Nuyens D, Theilmeier G, Brusselmans K, Cornelissen I, Ehler E, Kakkar VV, Stalmans I, Mattot V, Perriard JC, Dewerchin M, Flameng W, Nagy A, Lupu F, Moons L, Collen D, D’Amore PA, Shima DT. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat Med. 1999;5:495–502. doi: 10.1038/8379. [DOI] [PubMed] [Google Scholar]
- 17.Giordano FJ, Gerber HP, Williams SP, VanBruggen N, Bunting S, Ruiz-Lozano P, Gu Y, Nath AK, Huang Y, Hickey R, Dalton N, Peterson KL, Ross J, Jr, Chien KR, Ferrara N. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proc Natl Acad Sci U S A. 2001;98:5780–5785. doi: 10.1073/pnas.091415198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker CM, Farnebo FA, Iordanescu I, Behonick DJ, Shih MC, Dunning P, Christofferson R, Mulligan RC, Taylor GA, Kuo CJ, Zetter BR. Gene therapy of prostate cancer with the soluble vascular endothelial growth factor receptor Flk1. Cancer Biol Ther. 2002;1:548–553. doi: 10.4161/cbt.1.5.176. [DOI] [PubMed] [Google Scholar]
- 19.Kuo CJ, Farnebo F, Yu EY, Christofferson R, Swearingen RA, Carter R, von Recum HA, Yuan J, Kamihara J, Flynn E, D’Amato R, Folkman J, Mulligan RC. Comparative evaluation of the antitumor activity of anti-angiogenic proteins delivered by gene transfer. Proc Natl Acad Sci U S A. 2001;98:4605–4610. doi: 10.1073/pnas.081615298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tseng JF, Farnebo FA, Kisker O, Becker CM, Kuo CJ, Folkman J, Mulligan RC. Adenovirus-mediated delivery of a soluble form of the VEGF receptor Flk1 delays the growth of murine and human pancreatic adenocarcinoma in mice. Surgery. 2002;132:857–865. doi: 10.1067/msy.2002.127680. [DOI] [PubMed] [Google Scholar]
- 21.Jacobi J, Tam BY, Wu G, Hoffman J, Cooke JP, Kuo CJ. Adenoviral gene transfer with soluble vascular endothelial growth factor receptors impairs angiogenesis and perfusion in a murine model of hindlimb ischemia. Circulation. 2004;110:2424–2429. doi: 10.1161/01.CIR.0000145142.85645.EA. [DOI] [PubMed] [Google Scholar]
- 22.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang XP, Liu YH, Rhaleb NE, Kurihara N, Kim HE, Carretero OA. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol. 1999;277:H1967–H1974. doi: 10.1152/ajpheart.1999.277.5.H1967. [DOI] [PubMed] [Google Scholar]
- 24.Ouchi N, Shibata R, Walsh K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res. 2005;96:838–846. doi: 10.1161/01.RES.0000163633.10240.3b. [DOI] [PubMed] [Google Scholar]
- 25.Murtuza B, Suzuki K, Bou-Gharios G, Beauchamp JR, Smolenski RT, Partridge TA, Yacoub MH. Transplantation of skeletal myoblasts secreting an IL-1 inhibitor modulates adverse remodeling in infarcted murine myocardium. Proc Natl Acad Sci U S A. 2004;101:4216–4221. doi: 10.1073/pnas.0306205101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu P, Zhang D, Swenson L, Chakrabarti G, Abel ED, Litwin SE. Minimally invasive aortic banding in mice: effects of altered cardiomyocyte insulin signaling during pressure overload. Am J Physiol Heart Circ Physiol. 2003;285:H1261–H1269. doi: 10.1152/ajpheart.00108.2003. [DOI] [PubMed] [Google Scholar]
- 27.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izumiya Y, Kim S, Izumi Y, Yoshida K, Yoshiyama M, Matsuzawa A, Ichijo H, Iwao H. Apoptosis signal-regulating kinase 1 plays a pivotal role in angiotensin II-induced cardiac hypertrophy and remodeling. Circ Res. 2003;93:874–883. doi: 10.1161/01.RES.0000100665.67510.F5. [DOI] [PubMed] [Google Scholar]
- 29.Shyu KG, Liou JY, Wang BW, Fang WJ, Chang H. Carvedilol prevents cardiac hypertrophy and overexpression of hypoxia-inducible factor-1 α and vascular endothelial growth factor in pressure-overloaded rat heart. J Biomed Sci. 2005;12:409–420. doi: 10.1007/s11373-005-3008-x. [DOI] [PubMed] [Google Scholar]
- 30.Hilfiker-Kleiner D, Hilfiker A, Kaminski K, Schaefer A, Park JK, Michel K, Quint A, Yaniv M, Weitzman JB, Drexler H. Lack of JunD promotes pressure overload-induced apoptosis, hypertrophic growth, and angiogenesis in the heart. Circulation. 2005;112:1470–1477. doi: 10.1161/CIRCULATIONAHA.104.518472. [DOI] [PubMed] [Google Scholar]
- 31.Heba G, Krzeminski T, Porc M, Grzyb J, Ratajska A, Dembinska-Kiec A. The time course of tumor necrosis factor-α, inducible nitric oxide synthase and vascular endothelial growth factor expression in an experimental model of chronic myocardial infarction in rats. J Vasc Res. 2001;38:288–300. doi: 10.1159/000051057. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Brown LF, Hibberd MG, Grossman JD, Morgan JP, Simons M. VEGF, flk-1, and flt-1 expression in a rat myocardial infarction model of angiogenesis. Am J Physiol. 1996;270:H1803–H1811. doi: 10.1152/ajpheart.1996.270.5.H1803. [DOI] [PubMed] [Google Scholar]
- 33.Kim CH, Cho YS, Chun YS, Park JW, Kim MS. Early expression of myocardial HIF-1α in response to mechanical stresses: regulation by stretch-activated channels and the phosphatidylinositol 3-kinase signaling pathway. Circ Res. 2002;90:E25–E33. doi: 10.1161/hh0202.104923. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi A, Kureishi Y, Yang J, Luo Z, Guo K, Mukhopadhyay D, Ivashchenko Y, Branellec D, Walsh K. Myogenic Akt signaling regulates blood vessel recruitment during myofiber growth. Mol Cell Biol. 2002;22:4803–4814. doi: 10.1128/MCB.22.13.4803-4814.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujio Y, Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem. 1999;274:16349–16354. doi: 10.1074/jbc.274.23.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shiojima I, Yefremashvili M, Luo Z, Kureishi Y, Takahashi A, Tao J, Rosenzweig A, Kahn CR, Abel ED, Walsh K. Akt signaling mediates postnatal heart growth in response to insulin and nutritional status. J Biol Chem. 2002;277:37670–37677. doi: 10.1074/jbc.M204572200. [DOI] [PubMed] [Google Scholar]
- 37.Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 39.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 40.Brutsaert DL. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev. 2003;83:59–115. doi: 10.1152/physrev.00017.2002. [DOI] [PubMed] [Google Scholar]
- 41.Dimitrow PP, Krzanowski M, Nizankowski R, Szczeklik A, Dubiel JS. Comparison of the effect of verapamil and propranolol on response of coronary vasomotion to cold pressor test in symptomatic patients with hypertrophic cardiomyopathy. Cardiovasc Drugs Ther. 2000;14:643–650. doi: 10.1023/a:1007871032421. [DOI] [PubMed] [Google Scholar]
- 42.Dimitrow PP, Krzanowski M, Surdacki A, Nizankowski R, Szczeklik A, Dubiel JS. Impaired response of the forearm resistance but not conductance vessels to reactive hyperemia in hypertrophic cardiomyopathy. Angiology. 1999;50:267–272. doi: 10.1177/000331979905000401. [DOI] [PubMed] [Google Scholar]
- 43.Dimitrow PP, Surdacki A, Dubiel JS. Verapamil normalizes the response of left ventricular early diastolic filling to cold pressor test in asymptomatic and mildly symptomatic patients with hypertrophic cardiomyopathy. Cardiovasc Drugs Ther. 1997;11:741–746. doi: 10.1023/a:1007758006452. [DOI] [PubMed] [Google Scholar]