Abstract
Hyaluronic acid is a hygroscopic macromolecule formed by the polymerisation of glucuronic acid and N-acetylglucosamine disaccharide. It is a primary component of the extracellular matrix in various body tissues. Ihe use of topical Hyaluronic acid in the treatment of oral ulcers has been recently reported. This article reviews the mechanism of action, indications and efficacy of topical Hyaluronic acid gel in the management of oral ulcers.
Keywords: Hyaluronic acid, treatment, oral ulcers
Introduction
Hyaluronic acid (HA) is a linear polymer of glucuronic acid and N-acetylglucosamine disaccharide. The main function of HA includes tissue healing including activation and moderation of the inflammatory responses, promotion of cell proliferation, migration, and angiogenesis.[1] It also promotes re-epithelization via proliferation of basal keratinocytes. HA is a hygroscopic macromolecule and its solutions are highly osmotic. In the oral mucosa, this property enables to control of tissue hydration during periods of inflammatory process or response to tissue injury resulting in ulcer formation.
Molecular Structure and Availability
High-molecular-weight HA [Figure 1] acts as an anti-inflammatory molecule and has been shown to be beneficial in supporting gingival health. It is the most abundant high-molecular-weight glycosaminoglycan (GAG) in the extracellular matrix of soft periodontal tissues.[2] Studies indicate that HA exhibits beneficial anti-inflammatory and antibacterial activity in the treatment of gingivitis and periodontitis.[3,4] Topically, HA has been used as a 0.2% solution for the treatment of recurrent aphthous ulcers in clinical trials.[5,6] HA is commercially available as sodium hyaluronatein combination with polyvinylpyrrolidone (PVP) and glycyrrhetinic acid.[7] The combination was launched as Gelclair® sachets (Sinclair Pharma, UK Ltd) in 2002 and received US Food and Drug Administration (US FDA) approval for management of oral mucositis associated with chemotherapy. PVP, an important constituent of this preparation, has remarkable properties such as good chemical and biological inertness, very low toxicity, high media compatibility, and crosslinkable flexibility, which gives stability to the formulation.
Figure 1.
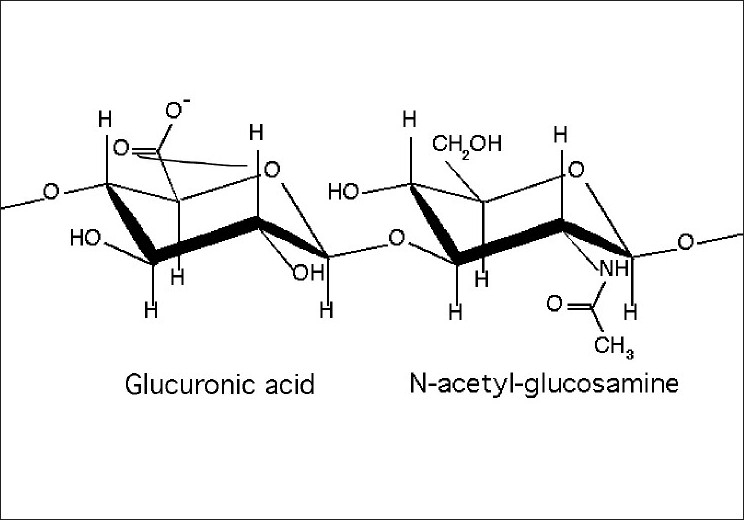
Biochemical structure of hyaluronic acid
Mechanism of Action (Gelclair® or PVP-SH gel)
Topical HA 0.2% or PVP-SH gel forms a protective coating around the oral cavity to shield exposed or sensitized nerve endings from overstimulation [Figure 2]. Each of the ingredients in PVP-SH performs a specific role, as listed here:-
Figure 2.
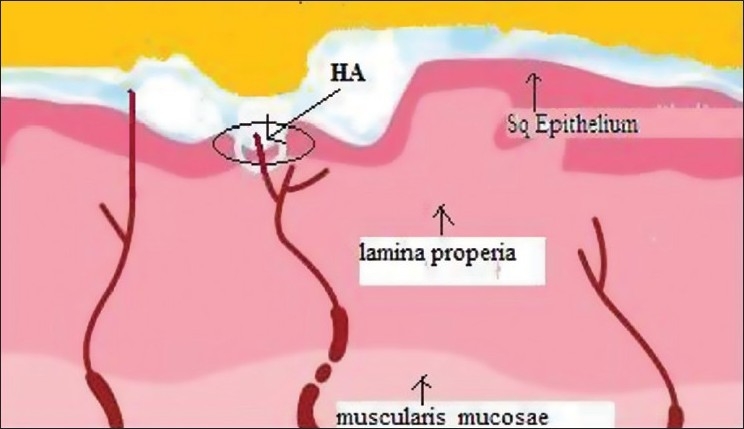
Topical hyaluronic acid (HA) forms a barrier film on exposed nerve endings
Polyvinylpyrrolidone – It is a hydrophilic polymer with muco-adherent and film-forming properties, which enhances tissue hydration.
Hyaluronic acid (as sodium hyaluronate) – It coats the oral mucosa, enhances tissue hydration, and accelerates healing.
Glycyrrhetinic acid – It is a breakdown product of glycyrrhizin, the active component of licorice and has anti-inflammatory properties that aid in ulcer healing. It is also used as a flavoring agent.
Indications
Recurrent and generalized aphthous oral ulcers
Ulcers due to drug reactions
Ulcers following the use of dental amalgam as a restorative material
Ulcers due to orthodontic brackets and wires
Ulcers due to ill-fitting dentures
Ulcers due to diseases as lichen planus,[9] Behcet's disease
Traumatic ulcers
Ulcers due to radiotherapy/chemotherapy
Post-vaporization of oral lesions with CO2 laser[12]
Directions for Use
PVP-SH gel is available in 15-ml single-use sachets.[7] The entire contents of the single-dose PVP-SH sachet are dissolved in 40 ml or two tablespoons of water. The mixture is stirred and used immediately as an oral rinse for at least a minute or as long as possible to coat the tongue, palate, throat, buccal mucosa and all oral tissue thoroughly, and then spit out. Depending on the discomfort caused by oral ulcers, PVP-SH gel can be used at least three times per day or as needed. It is advised not to eat or drink for at least one hour following use. In children under the age of 6 years who may be unable to rinse or gargle, the gel can be applied undiluted with a cotton bud.
Efficacy of Topical HA
Nolan et al,[5] evaluated the efficacy of a topical HA preparation (0.2%) in the management of recurrent aphthous in 120 patients in a randomized, placebo-controlled, double-blind trial. Patients treated with topical HA recorded few ulcers on day 5 of the investigation than those treated with placebo (P < 0.001). Also, the occurrence of new ulcers was lower in the HA-treated group on day 4 when compared with placebo-treated group (P = 0.047).[2]
Lee et al,[6] tested the efficacy of topical 0.2% HA gel on recurrent oral ulcers in 33 patients with recurrent aphthous ulceration or Behcet's disease. The patients were asked to use topical 0.2% HA gel twice daily for 2 weeks. The subjective parameters investigated s included number of ulcers, healing period and visual analogue scale (VAS) for pain. The objective assessment included number of ulcers, maximal area of ulcer, and inflammatory signs, which were inspected by a physician. After 2 weeks, a subjective reduction in the number of ulcers was observed in 72.7% of the patients. A decrease in the ulcer healing period was observed in 72.7% of the patients, and 75.8% experienced improvement in VAS for pain. Objective inspection of the ulcers revealed a reduction in the number of ulcers in 57.6% of the patients, and a decrease in the area of 78.8% of the ulcers was seen. Among the inflammatory signs, swelling and local heat showed significant improvement after treatment.
Advantages of Topical HA in Oral Ulcers
HA 0.2% or PVP-SH gel offers advantages over topical steroids in that it is safe to be used in all patients including infants and pregnant women, in whom there may be reluctance to use steroids. It can be used in all grades of oral ulceration[7,8] [Table 1].
Table 1.
WHO grading of oral mucositis

Contraindications
The only contraindication is known history of allergy or hypersensitivity to HA or any of the ingredients in the PVP-SH gel.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Ialenti A, Di Rosa M. Hyaluronic acid modulates acute and chronic inflammation. Agents Actions. 1994;43:44–7. doi: 10.1007/BF02005763. [DOI] [PubMed] [Google Scholar]
- 2.Sukumar S, Drízhal I. Hyaluronic acid and periodontitis. Acta Medica (Hradec Kralove) 2007;50:225–58. [PubMed] [Google Scholar]
- 3.Pistorius A, Martin M, Willershausen B, Rockmann P. The clinical application of hyaluronic acid in gingivitis therapy. Quintessence Int. 2005;36:531–8. [PubMed] [Google Scholar]
- 4.Mendes RM, Silva GA, Lima MF, Calliari MV, Almeida AP, Alves JB, et al. Sodium hyaluronate accelerates the healing process in tooth sockets of rats. Arch Oral Biol. 2008;53:1155–62. doi: 10.1016/j.archoralbio.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Nolan A, Baillie C, Badminton J, Rudralingham M, Seymour RA. The efficacy of topical hyaluronic acid in the management of recurrent aphthous ulceration. J Oral Pathol Med. 2006;35:461–5. doi: 10.1111/j.1600-0714.2006.00433.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee JH, Jung JY, Bang D. The efficacy of topical 0.2% hyaluronic acid gel on recurrent oral ulcers: comparison between recurrent aphthous ulcers and the oral ulcers of Behçet's disease. J Eur Acad Dermatol Venereol. 2008;22:590–5. doi: 10.1111/j.1468-3083.2007.02564.x. [DOI] [PubMed] [Google Scholar]
- 7.Smith T. Gelclair: Managing the symptoms of oral mucositis. Hosp Med. 2001;62:623–6. doi: 10.12968/hosp.2001.62.10.1666. [DOI] [PubMed] [Google Scholar]
- 8.Buchsel PC. Polyvinylpyrrolidone-sodium hyaluronate gel (Gelclair): A bioadherent oral gel for the treatment of oral mucositis and other painful oral lesions. Expert Opin Drug Metab Toxicol. 2008;4:1449–54. doi: 10.1517/17425255.4.11.1449. [DOI] [PubMed] [Google Scholar]
- 9.Nolan A, Badminton J, Maguire J, Seymour RA. The efficacy of topical hyaluronic acid in the management of oral lichen planus. J Oral Pathol Med. 2009;38:299–303. doi: 10.1111/j.1600-0714.2008.00739.x. [DOI] [PubMed] [Google Scholar]
- 10.Higuchi Y, Ansai T, Awano S, Soh I, Yoshida A, Hamasaki T, et al. Salivary levels of hyaluronic acid in female patients with dry mouth compared with age-matched controls: A pilot study. Biomed Res. 2009;30:63–8. doi: 10.2220/biomedres.30.63. [DOI] [PubMed] [Google Scholar]
- 11.Yuan J, Tohara H, Mikushi S, Hoshino T, Yue B, Uematsu H. The effect of “Oral Wet” for elderly people with xerostomia-the effect of oral rinse containing hialuronan. Kokubyo Gakkai Zasshi. 2005;72:106–10. doi: 10.5357/koubyou.71and72.106. [DOI] [PubMed] [Google Scholar]
- 12.Hita-Iglesias P, Torres-Lagares D, Gutiérrez-Pérez JL. Evaluation of the clinical behaviour of a polyvinylpyrrolidone and sodium hyalonurate gel (Gelclair) in patients subjected to surgical treatment with CO2 laser. Int J Oral Maxillofac Surg. 2006;35:514–7. doi: 10.1016/j.ijom.2005.12.004. [DOI] [PubMed] [Google Scholar]


