Abstract
Imiquimod is a small molecule with adjuvant pro-inflammatory effects that can be topically delivered as a cream for treating external genital and perianal warts. In our report, two Chinese males at the ages of 25 and 22 years were treated with imiquimod 5% cream for recurrent condyloma accuminatum, three times per week for 18 and 12. weeks, respectively. Depigmentation were noted and gradually enlarged in the treated areas after the two patients discontinued imiquimod. Therefore, clinicians should be made aware of the possible pigmentary changes associated with application of this cream.
Keywords: Genital, imiquimod, vitiligo
Introduction
Imiquimod 5% cream is an immune response modifier, currently approved for use in external genital and perianal warts, superficial basal cell carcinoma, and actinic keratoses.[1] The antiviral and antitumoral activity seems to be dependent on focal activation of the immune system with induction, synthesis, and release of cytokines.[2] Local side effects, such as erythema, edema, erosion, and crusting are frequently observed. The induction of vitiligo-like depigmentation has been reported rarely.[3–8] In this paper, we describe two cases of genital vitiligo following use of imiquimod 5% cream.
Case Reports
Case 1
A 25-year-old Chinese male with genital warts for 7 months was diagnosed as condyloma accuminatum and treated initially with external application of 5-fluorouracil injection, trichloroacetic acid solution, and so on. The lesions disappeared completely without obviously pigmentary change. After approximately 2 months, new lesions appeared on his coronoid sulcus of penis and imiquimod 5% cream was prescribed for three times weekly. After 8 weeks of continuous use, the lesions gradually grew down and subsidized, erythema and anabrosis occurred in the areas treated with imiquiod. He was instructed to stop using imiquimod and provided rivanol solution and zinc oil for external using, but the patient persisted in using imiquimod cream up to 18 weeks for being afraid of recurrence of the lesions. One month after the drug withdrawal, the patient observed a depigmentation macula on his penis, which initially looked like a rice grain and gradually enlarged without subjective symptoms. Dermatological inspection discovered an ivory-white patch of nearly 4 cm × 2 cm with irregular edge on the patient's coronoid sulcus and corpus penis [Figure 1], without any vegetations in external genitals and vitiligo-like maculae on other sites.
Figure 1.
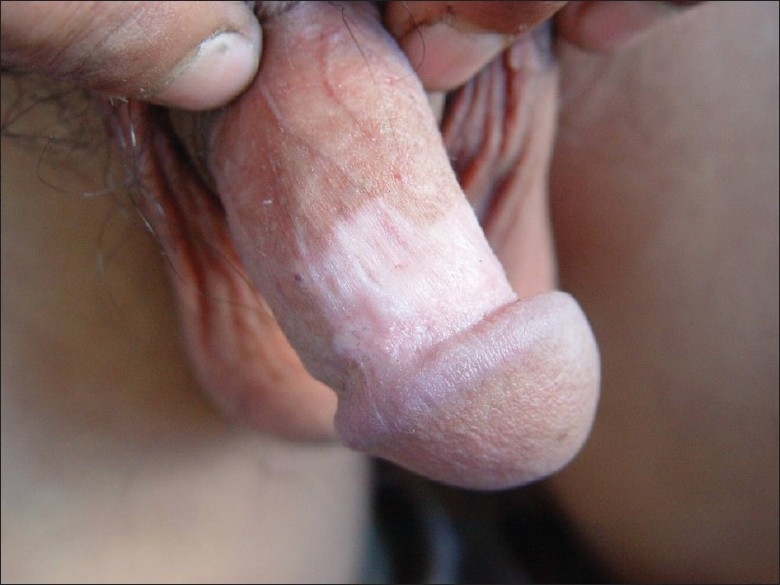
An ivory-white patch of nearly 4 cm × 2 cm with irregular edge on the patient's coronoid sulcus and corpus penis
Case 2
A 22-year-old male, with condyloma accuminata of the penis, treated initially with cryotherapy with only mild improvement. Imiquimod 5% cream was applied to the warts every other night; there was not significant side effect, except for moderate erythema and edema. After 12 weeks of continuous use, there was a complete resolution of lesions, without any pigmentary changes. New lesions appeared and imiquimod was used again. Since moderate to severe local inflammatory reactions, and subsequently vitiligo-like patches in the areas treated with imiquimod, the patient stopped using imiquimod and returned to see us 2 months later. He complained that the vitiligo-like patches had been enlarging gradually, especially during the period of last weeks. On examination, there were depigmented patches with irregular pigmented edges on his penis. He had not previous history of vitiligo, and no other depigmented areas were found elsewhere. The patient refused our request for biopsies of these lesions and refused to have them photographed.
Discussion
Imiquimod is an immune-response modifier for the topical treatment of external genital and perianal warts.[1] It is generally a well-tolerated drug, its most frequent side effects are erythema, flaking, scabbing, edema, and excoriation. However, imiquimod treatment associated pigmentary changes have been reported. The US Food and Drugs Administration lists 68 reports of pigmentary changes related to imiquimod from 1997 to 2003, including 43 reports of depigmentation, 7 of vitiligo, 1 of hypopigmentation, and 17 of hyperpigmentation.[2] Other four cases of vitiligo-like depigmentation following use of imiquimod have been reported.[3–8]
The possible mechanisms of hypopigmentation associated with imiquimod treatment include antibodies found in sera of patients with vitiligo to nonpigment cell antigens, cytoplasmic pigment cell antigens and pigment cell-surface antigens, stimulation by imiquimod of both the innate immune response and cell-mediated adaptive immunity, and increased sensitivity of melanocytes to oxidative stress.[2] Furthermore, imiquimod enhances antigen presentation by stimulating CD8+ T-cell activation and inducing Langerhans cell maturation.[9] Vitiligo-like depigmentation may result from the destruction of melanocytes by CD8+ T-cells directed to melanocyte surface antigens after antigen presentation is enhanced by imiquimod.[10] In addition, the release of NO was promoted indirectly by imiquimod; it induced cascade reaction of free radical and resulted in apoptosis of melanocytes.
Some authors[7,8] speculated that imiquimod might have acted as a triggering factor in a susceptible patient who had a positive family history of vitiligo. They also suggested that clinicians should be cautious when prescribing imiquimod in patients with a personal of family history of vitiligo. However, our patient and other reported case had no personal or family history of vitiligo. In addition, the depigmentation did not extend beyond the treatment area, suggesting a local factor was responsible.
In case 1, during the period of using imiquimod, erythema, dropsy or anabrosis appeared on the treated areas several times, without pigmentary changes were observed. However, 1 month after discontinuing imiquimod, there was a depigmented macula appeared and primarily confined to the site of imiquimod treatment and enlarged gradually. It suggested that depigmentation maculae resulted from local factors, instead of postinflammatory hypopigmentation. We presume that imiquimod as a precipitating factor induced local immunologic abnormality, eventually, vitiligo-like lesions were caused.
In view of the mode of action of imiquimod, vitiligo-like hypopigmentation following a course of topical imiquimod treatment is, therefore, not unexpected. As the use of imiquimod expands, clinicians should be made aware of the possible pigmentary changes associated with its use, particularly when used in visible areas. Furthermore, clinicians should keep in mind that not all individuals treated with imiquimod will result in pigmentary change. Each case should be individually examined, the course of action determined, and the patient monitored while receiving imiquimod therapy.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Majewski S, Marczak M, Mlynarczyk B, Benninghoff B, Jablonska S. Imiquimod is a strong inhibitor of tumor cell-induced angiogenesis. Int J Dermatol. 2005;44:14–9. doi: 10.1111/j.1365-4632.2004.02318.x. [DOI] [PubMed] [Google Scholar]
- 2.Mashiah J, Brenner S. Possible mechanisms in the induction of vitiligo-like hypopigmentation by topical imiquimod. Clin Exp Dermatol. 2008;33:74–6. doi: 10.1111/j.1365-2230.2007.02520.x. [DOI] [PubMed] [Google Scholar]
- 3.Serrão VV, Páris FR, Feio AB. Genital vitiligo-like depigmentation following use of imiquimod 5% cream. Eur J Dermatol. 2008;18:342–3. doi: 10.1684/ejd.2008.0402. [DOI] [PubMed] [Google Scholar]
- 4.Jacob SE, Blyumin M. Vitiligo-like hypopigmentation with poliosis following treatment of superficial basal cell carcinoma with imiquimod. Dermatol Surg. 2008;34:844–5. doi: 10.1111/j.1524-4725.2008.34158.x. [DOI] [PubMed] [Google Scholar]
- 5.Senel E, Seckin D. Imiquimod-induced vitiligo-like depigmentation. Indian J Dermatol Venereol Leprol. 2007;73:423. doi: 10.4103/0378-6323.37065. [DOI] [PubMed] [Google Scholar]
- 6.Al-Dujaili Z, Hsu S. Imiquimod-induced vitiligo. Dermatol Online J. 2007;13:10. [PubMed] [Google Scholar]
- 7.Brown T, Zirvi M, Cotsarelis G, Gelfand JM. Vitiligo-like hypopigmentation associated with imiquimod treatment of genital warts. J Am Acad Dermatol. 2005;52:715–6. doi: 10.1016/j.jaad.2004.10.861. [DOI] [PubMed] [Google Scholar]
- 8.Stefanaki C, Nicolaidou E, Hadjivassiliou M, Antoniou C, Katsambas A. Imiquimod-induced vitiligo in a patient with genital warts. J Eur Acad Dermatol Venereol. 2006;20:755–6. doi: 10.1111/j.1468-3083.2006.01533.x. [DOI] [PubMed] [Google Scholar]
- 9.Eedy DJ. Imiquimod: A potential role in dermatology? Br J Dermatol. 2002;147:1–6. doi: 10.1046/j.1365-2133.2002.04945.x. [DOI] [PubMed] [Google Scholar]
- 10.Stanley MA. Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential. Clin Exp Dermatol. 2002;27:571–7. doi: 10.1046/j.1365-2230.2002.01151.x. [DOI] [PubMed] [Google Scholar]


