Abstract
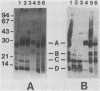
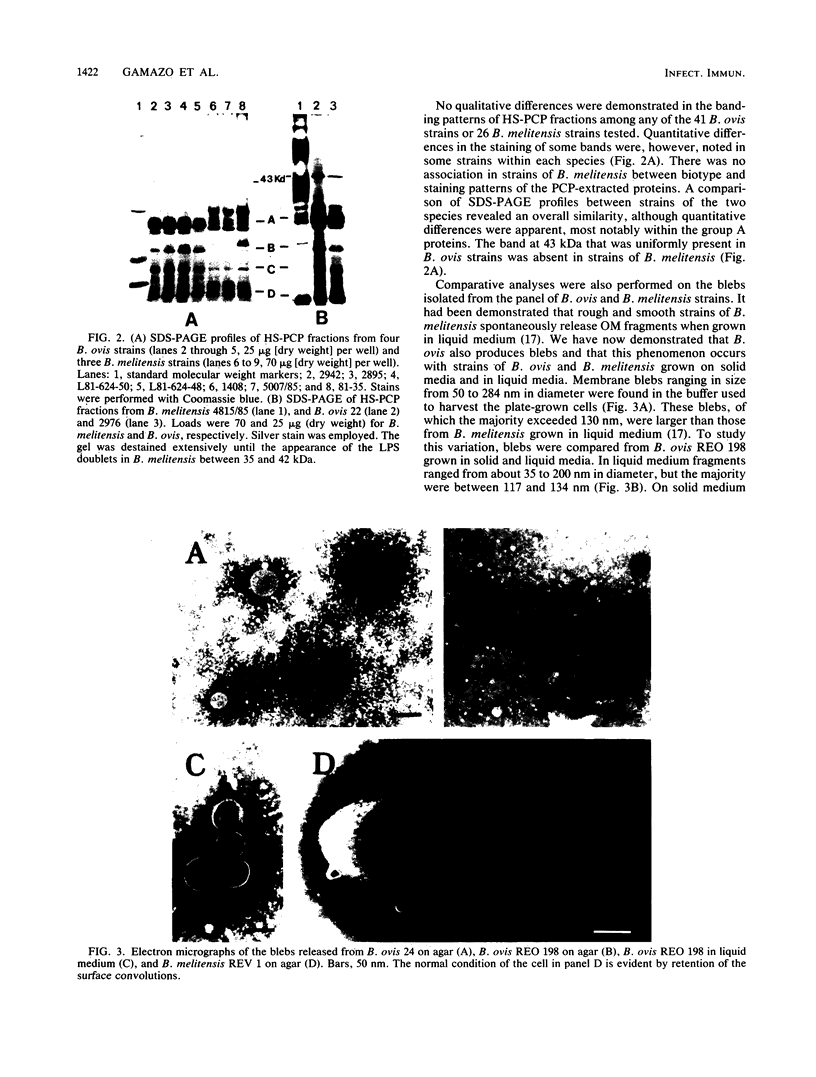
Sheep infected with Brucella ovis produce antibody responses to the rough lipopolysaccharide and to proteins present in hot saline (HS) extracts of B. ovis (J. I. Riezu-Boj, I. Moriyón, J. M. Blasco, C. M. Marín, and R. Díaz, J. Clin. Microbiol. 23:938-942, 1986). The distribution and antigenic relatedness of proteins in HS extracts and in outer membrane blebs were established by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting for 41 strains of B. ovis and 26 strains of Brucella melitensis of diverse geographic origin. Five major groups of proteins were identified in HS extracts of B. ovis that had been freed of rough lipopolysaccharide: proteins of 43 kilodaltons (kDa), group A (25.5 to 32.0 kDa), group B (21.5 to 22.5 kDa); group C (18.0 to 19.5 kDa), and group D (13.0 to 15.5 kDa). Group A, B, C, and D proteins were also present in blebs. The profiles of proteins in HS extracts or blebs from strains of both Brucella species were very similar. Cross-reactions were demonstrated among HS extracts and blebs of all strains tested in immunoblots performed with an antiserum against the HS extract of a reference strain of B. ovis. Evidence was also provided of an antigenic relationship between group 3 proteins of the outer membrane and some of the proteins in groups A, B, and C. The conservation of these antigens and their immunogenicity in infected animals provide promise that they may serve as components of an effective subcellular vaccine for ovine brucellosis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afzal M., Tengerdy R. P., Ellis R. P., Kimberling C. V., Morris C. J. Protection of rams against epididymitis by a Brucella ovis-vitamin E adjuvant vaccine. Vet Immunol Immunopathol. 1984 Oct;7(3-4):293–304. doi: 10.1016/0165-2427(84)90087-4. [DOI] [PubMed] [Google Scholar]
- Baldwin C. L., Verstreate D. R., Winter A. J. Immune response of cattle to Brucella abortus outer membrane proteins measured by lymphocyte blastogenesis. Vet Immunol Immunopathol. 1985 Aug;9(4):383–396. doi: 10.1016/0165-2427(85)90067-4. [DOI] [PubMed] [Google Scholar]
- Bascoul S., Cannat A., Huguet M. F., Serre A. Studies on the immune protection to murine experimental brucellosis conferred by Brucella fractions. I. Positive role of immune serum. Immunology. 1978 Aug;35(2):213–221. [PMC free article] [PubMed] [Google Scholar]
- Blasco J. M., Marín C. M., Barberán M., Moriyón I., Díaz R. Immunization with Brucella melitensis Rev 1 against Brucella ovis infection of rams. Vet Microbiol. 1987 Sep;14(4):381–392. doi: 10.1016/0378-1135(87)90029-0. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Claxton P. D. Brucella ovis vaccination of rams. A comparison of two commercial vaccines and two methods of vaccination. Aust Vet J. 1968 Feb;44(2):48–54. doi: 10.1111/j.1751-0813.1968.tb04953.x. [DOI] [PubMed] [Google Scholar]
- Douglas J. T., Rosenberg E. Y., Nikaido H., Verstreate D. R., Winter A. J. Porins of Brucella species. Infect Immun. 1984 Apr;44(1):16–21. doi: 10.1128/iai.44.1.16-21.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubray G., Limet J. Evidence of heterogeneity of lipopolysaccharides among Brucella biovars in relation to A and M specificities. Ann Inst Pasteur Microbiol. 1987 Jan-Feb;138(1):27–37. doi: 10.1016/0769-2609(87)90051-2. [DOI] [PubMed] [Google Scholar]
- Dubray G., Plommet M. Structure et constituants des Brucella. Caractérisation des fractions et propriétés biologiques. Dev Biol Stand. 1976;31:68–91. [PubMed] [Google Scholar]
- Ekdahl M. O., Money D. F., Martin C. A. Some aspects of epididymitis of rams in New Zealand. N Z Vet J. 1968 May;16(5):81–82. doi: 10.1080/00480169.1968.33750. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Gamazo C., Moriyón I. Release of outer membrane fragments by exponentially growing Brucella melitensis cells. Infect Immun. 1987 Mar;55(3):609–615. doi: 10.1128/iai.55.3.609-615.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoni J. M., Davis F. E., Palade G. E. Protein blotting in uniform or gradient electric fields. Anal Biochem. 1985 Jan;144(1):32–40. doi: 10.1016/0003-2697(85)90080-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Limet J., Plommet A. M., Dubray G., Plommet M. Immunity conferred upon mice by anti-LPS monoclonal antibodies in murine brucellosis. Ann Inst Pasteur Immunol. 1987 May-Jun;138(3):417–424. doi: 10.1016/s0769-2625(87)80052-1. [DOI] [PubMed] [Google Scholar]
- Luchsinger D. W., Anderson R. K. Longitudinal studies of naturally acquired Brucella abortus infection in sheep. Am J Vet Res. 1979 Sep;40(9):1307–1312. [PubMed] [Google Scholar]
- Madraso E. D., Cheers C. Polyadenylic acid-polyuridylic acid (poly A : U) and experimental murine brucellosis. II. Macrophages as target cells of poly A : U in experimental brucellosis. Immunology. 1978 Jul;35(1):77–84. [PMC free article] [PubMed] [Google Scholar]
- McGowan B. Epididymitis in rams: effect of vaccination and culling on the clinical incidence of the disease. Cornell Vet. 1979 Jan;69(1):67–72. [PubMed] [Google Scholar]
- McGowan B., Harrold D. R. Epididymitis in rams: studies on vaccine efficacy. Cornell Vet. 1979 Jan;69(1):73–76. [PubMed] [Google Scholar]
- Montaraz J. A., Winter A. J. Comparison of living and nonliving vaccines for Brucella abortus in BALB/c mice. Infect Immun. 1986 Aug;53(2):245–251. doi: 10.1128/iai.53.2.245-251.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaraz J. A., Winter A. J., Hunter D. M., Sowa B. A., Wu A. M., Adams L. G. Protection against Brucella abortus in mice with O-polysaccharide-specific monoclonal antibodies. Infect Immun. 1986 Mar;51(3):961–963. doi: 10.1128/iai.51.3.961-963.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Pitt M. W., Jones L. M., Schurig G. G., Berman D. T. Purification and characterization of smooth and rough lipopolysaccharides from Brucella abortus. J Bacteriol. 1979 May;138(2):361–369. doi: 10.1128/jb.138.2.361-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyon I., Berman D. T. Effects of nonionic, ionic, and dipolar ionic detergents and EDTA on the Brucella cell envelope. J Bacteriol. 1982 Nov;152(2):822–828. doi: 10.1128/jb.152.2.822-828.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyon I., Berman D. T. Isolation, purification, and partial characterization of Brucella abortus matrix protein. Infect Immun. 1983 Jan;39(1):394–402. doi: 10.1128/iai.39.1.394-402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers D. M., Jones L. M., Varela-Diaz V. M. Studies of antigens for complement fixation and gel diffusion tests in the diagnosis of infections caused by Brucella ovis and other Brucella. Appl Microbiol. 1972 May;23(5):894–902. doi: 10.1128/am.23.5.894-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Wu H. C. Proteins of the outer membrane of gram-negative bacteria. Annu Rev Microbiol. 1980;34:369–422. doi: 10.1146/annurev.mi.34.100180.002101. [DOI] [PubMed] [Google Scholar]
- Pardon P. Resistance against a subcutaneous Brucella challenge of mice immunized with living or dead Brucella or by transfer of immune serum. Ann Immunol (Paris) 1977 Nov-Dec;128(6):1025–1037. [PubMed] [Google Scholar]
- Pavlov H., Hogarth M., McKenzie I. F., Cheers C. In vivo and in vitro effects of monoclonal antibody to Ly antigens on immunity to infection. Cell Immunol. 1982 Jul 15;71(1):127–138. doi: 10.1016/0008-8749(82)90502-0. [DOI] [PubMed] [Google Scholar]
- Plommet M., Plommet A. M. Immune serum-mediated effects on brucellosis evolution in mice. Infect Immun. 1983 Jul;41(1):97–105. doi: 10.1128/iai.41.1.97-105.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezu-Boj J. I., Moriyón I., Blasco J. M., Marín C. M., Diaz R. Comparison of lipopolysaccharide and outer membrane protein-lipopolysaccharide extracts in an enzyme-linked immunosorbent assay for the diagnosis of Brucella ovis infection. J Clin Microbiol. 1986 May;23(5):938–942. doi: 10.1128/jcm.23.5.938-942.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SULITZEANU D. Passive protection experiments with Brucella antisera. J Hyg (Lond) 1955 Jun;53(2):133–142. doi: 10.1017/s0022172400000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos J. M., Verstreate D. R., Perera V. Y., Winter A. J. Outer membrane proteins from rough strains of four Brucella species. Infect Immun. 1984 Oct;46(1):188–194. doi: 10.1128/iai.46.1.188-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift B. L., Craddock F., Hancock H. A., Jensen R., Thomas G. M., Trueblood M. S., Weibel J. Ram epididymitis: A clinical report. Theriogenology. 1982 Mar;17(3):343–347. doi: 10.1016/0093-691x(82)90094-2. [DOI] [PubMed] [Google Scholar]
- Swift B. L., Maki L. R. Immunologic studies on three ram epididymitis bacterins. Cornell Vet. 1968 Oct;48(4):659–665. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Alphen L., Lugtenberg B., Rietschel E. T., Mombers C. Architecture of the outer membrane of Escherichia coli K12. Phase transitions of the bacteriophage K3 receptor complex. Eur J Biochem. 1979 Nov;101(2):571–579. doi: 10.1111/j.1432-1033.1979.tb19752.x. [DOI] [PubMed] [Google Scholar]
- Verstreate D. R., Creasy M. T., Caveney N. T., Baldwin C. L., Blab M. W., Winter A. J. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982 Mar;35(3):979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreate D. R., Winter A. J. Comparison of sodium dodecyl sulfate-polyacrylamide gel electrophoresis profiles and antigenic relatedness among outer membrane proteins of 49 Brucella abortus strains. Infect Immun. 1984 Oct;46(1):182–187. doi: 10.1128/iai.46.1.182-187.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- West D. M., Johnstone A. C., Bruere A. N., Chapman H. M. Epiphysitis in rams following vaccination against Brucella ovis infection. N Z Vet J. 1978 May;26(5):133–134. doi: 10.1080/00480169.1978.34518. [DOI] [PubMed] [Google Scholar]