Abstract
Prior studies have supported a role for mesolimbic dopaminergic mechanisms in the regulation of maternal behavior. Accordingly, the ventral tegmental area (VTA) and its dopaminergic projections to the nucleus accumbens (NAc) and medial prefrontal cortex (mPFC) have been implicated in both the onset and maintenance of normal maternal behavior. To date, studies of direct manipulation of VTA neurochemistry at the onset of maternal behavior have been limited. The current study was undertaken to directly test the hypothesis that enhancement of dopaminergic transmission in the mesolimbic dopamine system can stimulate maternal activity using a pup-induced virgin model. Nulliparous female rats were stereotaxically infused with pertussis toxin (PTX 0, 0.1, or 0.3 μg/hemisphere) into the VTA to chronically stimulate the activity of dopaminergic projection neurons. After 3 days of recovery, maternal responding to donor pups was tested daily, and latency (in days) to full maternal behavior was recorded. Intra-VTA PTX treatment produced a robust dose-dependent decrease in maternal behavior latency, and a long-lasting increase in locomotor activity. These effects were associated with significantly decreased dopamine D1 receptor mRNA expression in the NAc. No effects of PTX treatment on mesolimbic dopamine utilization or mPFC receptor expression were observed. The findings indicate that chronic neural activation in the VTA accelerates the onset of maternal behavior in virgin female rats via modification of the NAc dopamine D1 receptor.
Keywords: maternal behavior, pertussis toxin, ventral tegmental area, nucleus accumbens, dopamine receptors, gene expression
1. INTRODUCTION
The mesolimbic dopamine system has been well-studied for its role in motivated behavior [5, 23, 30]. This system includes the ventral tegmental area (VTA), and its dopaminergic projections to the nucleus accumbens (NAc, mesoaccumbens) and medial prefrontal cortex (mPFC, mesocortical). These substrates are activated by drugs of abuse and natural reinforcers (e.g. food and sex), and mediate appetitive responses such as drug-seeking [29], feeding, and sexual behavior [5, 23, 30]. Activation of the mesolimbic dopamine system also appears to underlie maternal motivation and associated behaviors [28, 34, 39, 58]. The observation that postpartum rats will bar press for access to pups [33, 62] suggests that pups provide a motivational stimulus for maternal responding [48, 60]. Indeed, a number of studies now exist indicating that intact dopamine neurotransmission is necessary for the expression of normal maternal behavior [41, 53].
Studies of mesolimbic involvement in maternal behavior indicate that both mesoaccumbens and mesocortical terminal fields represent critical structures. With regard to the NAc, acute exposure of maternally-experienced and postpartum rats to foster pups increases dopamine release in this region as measured by in vivo microdialysis [1, 2, 18]. Administration of dopamine D1 or D2 receptor antagonists into the NAc disrupts maternal behavior in postpartum females [28, 40, 42], an effect which is also observed following systemic administration [9]. Interestingly, stimulation of NAc D1 (but not D2) receptors via direct agonist infusion stimulates maternal behavior in postpartum rats [52, 54]. Though fewer studies have focused on the mPFC, findings suggest that intact function of this area is also necessary for normal maternal responding. For example, tetrodotoxin- and GABA-induced inactivation [12], as well as excitotoxic lesions of this region [3], abolish pup retrieval in lactating rats. These observations are supported by reports of increased neuronal activity in the mPFC in response to suckling stimulation from pups [13, 14]. Collectively, the data strongly implicate NAc and mPFC neurochemistry in the mediation of maternal behavior.
In addition to mesolimbic terminal fields, accumulating evidence also suggests that VTA dopamine cell firing mediates the rewarding properties of pup stimuli, as well as the display of maternal behavior in postpartum rats. Inactivation of the VTA via direct infusion of bupivacaine disrupts pup preference of parturient rats in a conditioned place preference paradigm [49]. Similarly, blockade of dopaminergic activation in the VTA via GABA-A and GABA-B receptor stimulation disrupts postpartum maternal behavior [41]. A comparable effect is observed following dopamine-specific 6-OHDA lesions of the VTA [19]. These observations are consistent with the finding that maternal behavior in lactating rats is associated with increased electrical activity in the VTA [21].
As indicated above, most efforts to elucidate the role of the mesolimbic dopamine system in maternal behavior have involved spontaneously maternal postpartum rats. However, using the postpartum model, it is difficult to dissociate maternal behaviors driven by hormonal stimulation and/or physiological demands (e.g. lactation) from those mediated specifically by the incentive value of pups. In this regard, the pup-induced virgin female rat model [6, 7, 10, 16, 45, 48] offers practical advantages. In this model, normally-cycling adult virgin females can be induced (sensitized) to behave maternally through continuous contact with foster pups for approximately 4–7 days. Indeed, the pup-induced model may be a useful tool for the delineation of more specific neural mechanisms underlying maternal motivation [46].
While the pup-induced model may allow for a more comprehensive understanding of mesolimbic involvement in maternal behavior, few mechanistic studies have been conducted. Specifically, lesions of the NAc have been shown to disrupt maternal responding in virgin female rats [34]. Conversely, tail-pinch has been reported to facilitate maternal behavior [55], possibly through stress-induced activation of the mesoaccumbens pathway. Infusion of morphine into the VTA also facilitates the onset of maternal behavior in this model [38, 57], an effect apparently mediated indirectly via disinhibition of dopaminergic cells [25]. Though suggestive, these findings provide only indirect evidence for the specific role of mesolimbic dopamine activation in pup-induced maternal responding.
The present study was undertaken to more fully characterize mesolimbic involvement in maternal behavior using the pup-induced virgin model. To accomplish this, we infused the VTA with pertussis toxin (PTX), a protein that catalyzes the ADP-ribosylation Gi/Go protein α subunits [17]. Intra-VTA PTX treatment is believed to chronically disinhibit dopamine projection neurons through general inactivation of Gi/Go protein-coupled receptors, including the inhibitory GABA-B receptor and dopamine D2 auto receptor [8, 24, 26, 32, 51, 59]. These long-lasting biochemical effects have been reported to transiently increase dopamine transmission the NAc [35, 51], and robustly augment several dopamine-mediated behaviors including psychostimulant sensitization [36, 37, 50, 61, 63]. Thus, we hypothesized that intra-VTA PTX treatment would enhance the rate of induction of sensitized maternal responding via stimulation of mesolimbic dopamine function. The findings indicate that VTA stimulation accelerates the onset of maternal behavior via adaptation of dopamine neurotransmission.
2. EXPERIMENTAL METHODS AND PROCEDURE
2.1. Experimental Animals
Experimental animals were virgin, adult female Sprague-Dawley rats (175–200 g, approximately 6 weeks of age) purchased from Charles River Breeding Laboratories (Crl:CD(SD)BR; Kingston, NY). All animals were housed in standard plastic laboratory cages (40 cm × 20 cm × 18 cm) on a 14:10 h light:dark cycle (lights on at 0500 hr) in temperature-controlled (21–24°C) rooms. Food and water were provided ad libitum. Animals were acclimated to the housing conditions for at least 7 days prior to experimentation, and were maintained in accordance with the National Research Council (NRC) Guide for the Care and Use of Laboratory Animals. All procedures were approved by the Institutional Animals Care and Use Committee of Tufts University.
2.2. Experimental Design and Procedures
For this study, 3 experiments were conducted using separate groups of animals according to the following design and procedures:
2.2.1. Experiment I
This experiment was conducted to determine the effects of intra-VTA PTX treatment on maternal responding in adult virgin female rats. Following a 1-week acclimation period, animals were randomly assigned to one of three intra-VTA treatment conditions: PTX low-dose (0.1 μg/hemisphere, n=10), PTX high-dose (0.3μg/hemisphere, n=11) or PTX control (heat-inactivated PTX, 0.3 μg/hemisphere, n=9) via bilateral stereotaxic infusion. Following surgery, animals were individually-housed and were allowed to recover. Beginning on post-treatment day 4, all animals were tested for maternal responding to donor pups within the home cage. Testing continued daily until animals displayed 2 consecutive days of full maternal behavior (FMB). Latency (in days) to FMB was used as the dependent measure. To verify the biological activity of PTX within the VTA [37], all animals were tested for spontaneous home cage locomotor activity on the evening of post-treatment days 18–19.
2.2.2. Experiment II
Experiment I was designed to specifically test the effects of intra-VTA PTX treatment on maternal responding. To determine whether the effect of intra-VTA PTX treatment on maternal behavior is temporally associated with changes in locomotor activity, Experiment II was conducted using a separate set of animals. The treatment procedure was identical to that described for Experiment I, except that two groups were studied: intra-VTA infusion of high-dose PTX (0.3 μg/hemisphere, n=7) or its inactive control (0.3 μg/hemisphere, n=5). Following surgery, animals were individually-housed and allowed to recover undisturbed for 6 days, receiving only standard husbandry care. On post-treatment day 6, animals were transported to the motor activity testing room, and were tested for spontaneous home cage locomotor activity (1900–0500 hr). The chosen time-frame for testing (post-treatment day 6–7) was based on the results of Experiment I, indicating that the effects of intra-VTA PTX on maternal behavior emerge at post-treatment day 7.
2.2.3. Experiment III
Experiment III was conducted using a separate set of animals to determine whether the effect of intra-VTA PTX treatment on maternal responding is associated with changes forebrain dopamine function. Group assignments and treatment procedures for Experiment III were identical to those described for Experiment II: intra-VTA infusion of high-dose PTX (0.3 μg/hemisphere, n=5) or its inactive control (0.3 μg/hemisphere, n=6). Following surgery, animals recovered undisturbed for 6 days, receiving only standard husbandry care. On post-treatment day 7, animals were euthanized and the NAc and mPFC were assayed for dopamine and DOPAC content, as well as dopamine receptor mRNA expression (D1 and D2). The chosen time-frame for this analysis was based on the results of Experiment I, indicating that the effects of intra-VTA PTX on maternal behavior emerge at post-treatment day 7.
2.3. PTX Infusion into the VTA
Prior to infusion of experimental substances, a pilot study of 6 animals was conducted using dye injection to optimize stereotaxic coordinates and angles to be used for the adult female Sprague-Dawley rat (data not shown). Following optimization, Pertussis Toxin (salt-free) was obtained from List Biological Laboratories (Campbell, CA) and prepared in 0.9% NaCl (0.1 and 0.3 μg/0.5 μl). For the control condition, PTX (0.3 μg/0.5 μl of 0.9% NaCl) was heat-inactivated by boiling at 100°C for 5 min [11]. For stereotaxic infusion, animals were first anesthetized using isoflurane (3–5% to effect and adjusted to 1–3% for maintenance). The head was shaved and the skin prepared with alternating isopropyl alcohol (70%)/providone-iodine washes. Animals were then mounted in a stereotaxic frame (Stoelting Co., Wood Dale, IL) with the incisor bar set to 3.3 mm below horizontal zero. Sterile 2% lidocaine (100 μl, s.c.) was administered to the scalp, and a midline incision (1.5 cm) was made. Bilateral burr holes (1 mm) were drilled in the area of the skull overlying the VTA. A Hamilton Syringe (Hamilton Company, Reno, NV) was lowered into the VTA according to the following optimized coordinates [43]: (A–P) −4.5 mm from bregma, (L) +/−2.3 mm from the midline, and (D–V) −8.3 mm from the skull surface at a 10° angle from vertical. PTX (0.1 or 0.3 μg/hemisphere) or its control (heat inactivated PTX; 0.3 μg/hemisphere) was then delivered bilaterally into the VTA via the Hamilton syringe (0.5 μl/hemisphere) over a period of 1 min. A diffusion time of 30 s was allowed prior to syringe withdrawal. Following infusions, the wound was irrigated with sterile saline, and closed with 3–4 nylon sutures (Monosof, 4.0). The incision site was swabbed with Neosporin ointment, and the animal was treated with a single dose of Carprofen (5.0 mg/kg, s.c.) for management of postoperative pain. Animals were then monitored until full mobility was regained, and were individually housed for the remainder of the experiment.
2.4. Maternal Behavior Testing
The virgin sensitization model used in this study has been well characterized (6, 7, 10, 32, 45, 46]. Briefly, on day 4 post-treatment, experimental animals were exposed to 3 freshly-fed donor pups (3–10 days postnatal) in the home cage. Maternal behavior was monitored for 15 minutes following the start of exposure. Behaviors quantified were latency for retrieval of pups, grouping pups, and crouching over pups in the nest. After the initial 15 minute measurement, spot checks of mother-pup position were conducted every 15 min for a total of 1 hour. Pups remained in the cage overnight, were removed on the following morning, and placed with a lactating donor female for a minimum of 24 hr. Experimental animals were then re-exposed to 3 healthy donor pups as described above. The procedure continued until animals displayed 2 consecutive days of FMB, defined as retrieval, grouping, and crouching over pups. Latency (in days) to FMB was defined as the number of days prior to the first display of FMB (i.e. day 1 of FMB minus 1 day). Animals not reaching this criterion within 13 days of testing were assigned a maximum latency of 12 days.
2.5. Home Cage Locomotor Activity
Home cage locomotor activity testing was conducted over a 10-hour period during the dark phase (1900–0500 hr). Individual animals were tested using the SmartFrame Cage Rack System® (Hamilton/Kinder, Poway, CA) in a dedicated testing room. The system consisted of individual PC-interfaced horizontal photobeam frames. Each frame contained 12 photobeams (8Lx4W) which continuously tracked the movement of animals within the home cage. Raw activity data were collected and reduced using MotorMoniter® software (Hamilton/Kinder). All data were analyzed and expressed in the form of photobeam breaks.
2.6. Tissue Harvesting and Brain Dissection
Brains were harvested between 1300–1500 h. Animals were exposed for 30 s to CO2 and decapitated. Brains were rapidly removed, frozen in methylbutane, and stored at −80°C. At the time of dissection, brains were cryostat-sectioned (−20°C), and stereotaxic infusion sites were verified by gross observation. Specifically, brains were sectioned coronally in 25 μm increments until a region of interest was revealed. One millimeter micropunches were obtained using a biopsy punch (diameter = 1 mm; depth = 1 mm). Micropunches were collected bilaterally from the NAc (core and shell combined) and the mPFC (anterior cingulate, infralimbic, prelimbic cortex combined), based on the atlas of Paxinos and Watson [43]. Micropunches were placed into RNAse-free microcentrifuge tubes and stored at −80°C prior to processing for mRNA, dopamine, and DOPAC content.
2.7. Gene Expression Analysis
Total RNA was isolated from micropunches using the RNeasy Lipid Mini Kit® (Qiagen, Valencia, CA) following the manufacturer’s protocol. RNA yields were measured using the NanoDrop® ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE). From each RNA sample, 50 ng of RNA were used to prepare cDNA using the RETROscript® (Ambion, Austin, TX) protocol with heat denaturation. Real time RT-PCR was performed on 2.75 ng of cDNA per well using an ABI Prism 7700 (Applied Biosystems, Foster City, CA). All PCR primers were purchased from Applied Biosystems. Assay ID and accession numbers were as follows: D1 receptor - Rn03062203_s1 and NM-012546.2, D2 receptor - Rn01418275_m1 and NM_012547.1, and beta-actin Rn00667869_m1 and NM_031144.2. Quantification of mRNA expression was accomplished using the Pfaffl method [44], in which expression of the beta-actin gene (housekeeping gene) in each sample is used to normalize cycle threshold (CT) values for genes of interest. Thus, a relative expression value for each gene of interest (with respect to the control group) was calculated for individual brain samples [44].
2.8. HPLC Measurement of Tissue Dopamine and DOPAC Content
Micropunched tissue was homogenized in a HPLC mobile phase (MDTM-70-1332; ESA, Chelmsford, MA) consisting of the following: NaH2PO4 75 mM, EDTA 25 μM, octanesulfonic acid sodium salt 1.7 mM, triethylamine 100 μl/L; 10% acetonitrile, pH 3.0 with phosphoric acid. Homogenates were centrifuged and supernatants were analyzed for dopamine and dihydroxyphenylacetic acid (DOPAC) content using a DECADE Digital Electrochemical Amperometric Detector (Antec Leyden, Netherlands). Mobile phase was delivered to the detector at a rate of 0.2 ml/min using an LC1150 Solvent Delivery System (GBC Scientific Equipment, Victoria, Australia). Catecholamines were separated on a ThermoHypersil-Keystone column (150 × 2.1 mm, pore size 5.0 μm). WinChrom® data management software (GBC Separations, Hubbardston, MA) was used for data analysis. Tissue protein content was determined using the Bradford method (Bio-Rad, Hercules, CA). Dopamine and DOPAC (ng/mg protein) were determined based on comparison to an external standard curve. Turnover of dopamine for each brain region was calculated using the DOPAC/DA ratio.
2.9. Statistical Analysis
All analyzed data were obtained from animals determined to have accurate stereotaxic infusion sites (see Tissue Harvesting and Brain Dissection). Maternal behavior data were analyzed using the Fisher’s Exact Test (number of responders) and Kruskal-Wallis One-Way ANOVA on Ranks (latency). Locomotor activity data were analyzed using Two-Way repeated measures ANOVA and Student’s t-test. Dopamine, DOPAC, and gene expression data were analyzed using Student’s t-test. Post-hoc analyses were conducted using the Dunn’s Test for non-parametric data, and the Student Newman-Keuls (SNK) test for parametric data. All statistical results are presented in detail in the figure captions. Statistical significance was defined as P<0.05.
3. RESULTS
3.1. Experiment I: Effects of Intra-VTA PTX on Maternal Behavior
Experiment I was conducted to determine the effects of intra-VTA PTX treatment on maternal responding in adult virgin female rats. Figure 1 (Panel A) depicts the effects of PTX on the cumulative percentage of animals responding maternally at each of 12 consecutive test days. By day 2 of testing, animals in the high-dose PTX began to respond maternally. By day 4 of testing, a significantly larger number of high-dose PTX animals reached FMB compared to the control group (Fisher’s Exact Tests). In high-dose animals, a trend of increasing responsiveness continued until day 6 of testing, at which time 95 percent of animals reached criteria for FMB, and 100 percent by day 11. Animals in the low-dose PTX group began to respond with FMB by day 4 of testing. This group exhibited a linear increase in the percentage of animals reaching criteria, with 95 percent fully maternal on day 10, and 100 percent by day 12. In contrast, no animals in the control group met the criteria for FMB until day 5 of testing. Moreover, after day 6, the percentage of control group animals responding maternally reached a plateau, with 60 percent of animals achieving FMB by the end of testing (day 12). It is important to note that all animals responding maternally within each initial 15 min observation period continued to maintain contact with pups in the nest. Thus, once initiated, maternal responding persisted, regardless of treatment group. Figure 1 (Panel B) illustrates the median latency to display FMB for all treatment groups. Kruskal-Wallis One-Way ANOVA on Ranks indicated that the median latency for the high-dose group (4 days) was significantly lower than that of the control group (8 days). The decreased latency observed in the low-dose group (5.5 days) did not attain significance.
Figure 1.
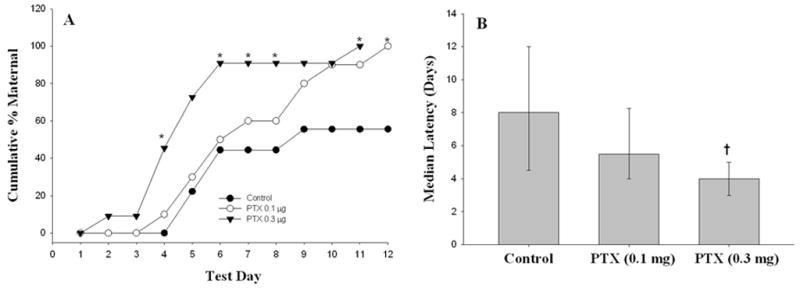
Intra-VTA PTX treatment stimulates maternal behavior. Adult, virgin female rats were infused bilaterally into the VTA with PTX (0.1 or 0.3 μg/hemisphere) or its inactive control. Groups of 9–11 animals were tested for maternal responding to donor pups beginning on post-treatment day 4. Panel A: Data are cumulative percentage displaying FMB. Fisher’s Exact Tests – *P<0.05 vs. Control. Panel B: Data are median latency to FMB. Percentiles (95th and 25th) are represented by the upper and lower horizontal lines, respectively. Kruskal-Wallis One-Way ANOVA on Ranks – H= (2)9.130, P = 0.010. †P<0.05 vs. Control.
One week following maternal behavior testing (day 18–19 post-treatment), animals were tested for spontaneous home cage locomotor activity overnight to verify the biological activity of intra-VTA PTX. Figure 2 illustrates the dose-dependent effects of PTX treatment on locomotor activity. When analyzed over a 10-hour testing period (Panel A), Two-Way repeated measures ANOVA revealed significant effects of Group, Time, and a Group x Time interaction (see figure caption for ANOVA statistics). Post-hoc (SNK) tests indicated that animals treated with high-dose PTX displayed significantly more locomotor activity (photobeam breaks) over the 10-hour testing period when compared to low-dose PTX and control groups. Likewise, when collapsed across time (Panel B), One-Way ANOVA revealed a significant Group effect, with the high-dose PTX group engaging in significantly more locomotor activity compared to low-dose PTX and control groups (SNK). While the activity of animals in the low-dose PTX group was elevated, this effect did not attain significance. Overall, the findings indicate that intra-VTA PTX treatment decreases maternal response latency in virgin female rats, and produces long-lasting increases in overall motor activity in the same animals.
Figure 2.
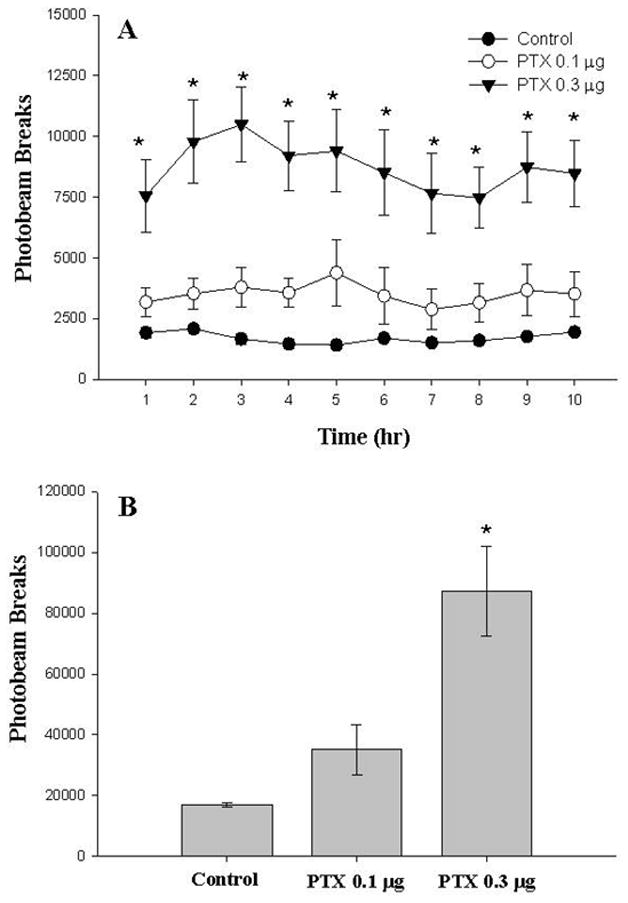
Locomotor effects of intra-VTA PTX on post-treatment day 18–19. Adult, virgin female rats were infused bilaterally into the VTA with PTX (0.1 or 0.3 μg/hemisphere) or its inactive control. Locomotor activity was monitored over 10 hours (dark phase) on post-treatment day 18–19. Data are mean photobeam breaks (± SEM) for groups of 9–11 animals. Panel A (1 hour intervals): Two-Way repeated measures ANOVA Group [F(2, 243)=12.99, P<0.001], Time [F(9, 243)=4.4, P<0.001], Group X Time [F(18, 243)=1.812, P<0.01]. Panel B (collapsed across 10 hours): One-Way ANOVA – Group [F(2, 27)=12.32, P<0.001]. SNK: *P<0.05 vs. all groups.
3.2. Experiment II: Effects of Intra-VTA PTX on Locomotor Activity
Experiment I indicated that intra-VTA PTX treatment significantly reduces maternal behavior latency within 1 week of treatment, and produces increased locomotor activity that can be observed at 18–19 days post-treatment. However, since locomotor activity could not be measured during maternal behavior testing, the contribution of increased (nonspecific) motor activity to reduced latency cannot be ruled out. To specifically address this, a separate set of animals was bilaterally infused with either high-dose (0.3 μg) PTX or heat-inactivated PTX (control), and tested for locomotor activity overnight at post-treatment day 6–7 (corresponding to the median FMB latency in high-dose PTX-treated animals). Animals treated with PTX showed a moderate increase in locomotor activity (photobeam breaks) over the 10-hour test period when compared to controls (Figure 3). However, Two-Way repeated measures ANOVA failed to indicate a significant effect of PTX over time (Panel A). Likewise, when collapsed across time (Panel B), t-test indicated that the difference in locomotor activity controls and PTX-treated animals did not attain significance. This finding suggests that the effect of intra-VTA PTX on maternal responding is not associated with significant differences in motor activation. However, these findings should be interpreted with caution, due to the significant variability observed in the PTX-treated group, which reduced the overall experimental power of these analyses.
Figure 3.
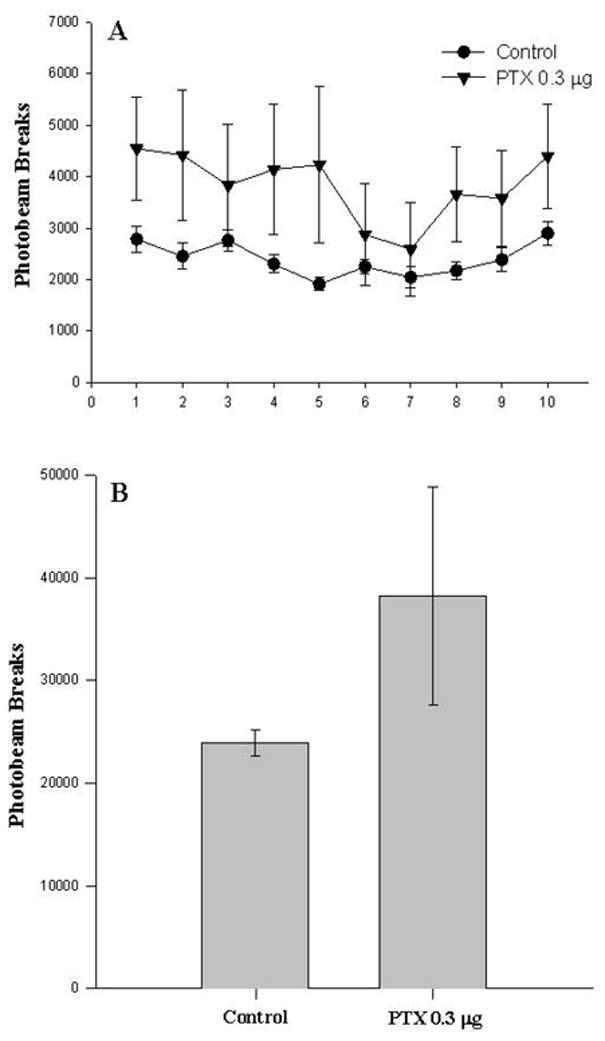
Locomotor effects of intra-VTA PTX on post-treatment day 6–7. Adult, virgin female rats were infused bilaterally into the VTA with PTX (0.3 μg/hemisphere) or its inactive control. Locomotor activity was monitored over 10 hours (dark phase) on post-treatment day 6, a time-frame corresponding with the emergence of stimulated maternal responding. Data are mean photobeam breaks (± SEM) for groups of 5–7 animals. Panel A (1 hour intervals): Two-Way repeated measures ANOVA Group [F(1, 90)=1.257, P<0.288], Time [F(9, 90)=3.56, P<0.001], Group X Time [F(9, 90)=1.53, P<0.150]. Panel B (collapsed across 10 hours): Student's t-test [T(10)=1.12, P<0.288].
3.3. Experiment III: Effects of Intra-VTA PTX on Forebrain Dopamine Function
Experiment III was conducted to determine whether the effect of intra-VTA PTX treatment on maternal responding is associated with changes forebrain dopamine function. For this experiment, separate sets of animals were bilaterally infused with either high-dose (0.3 μg) PTX or heat-inactivated PTX (control) and were euthanized on post-treatment day 7 (corresponding to the median FMB latency in high-dose PTX-treated animals). Table 1 depicts dopamine content and turnover in the NAc and mPFC following intra-VTA PTX treatment. Analysis of tissue DOPAC and dopamine concentration, as well as dopamine utilization (DOPAC/dopamine), indicated no treatment effects in either brain region. However, analysis of dopamine receptor expression in the NAc revealed decreased D1 mRNA in PTX-treated animals relative to controls (Figure 4, left panel). While NAc D2 mRNA expression was also decreased in PTX-treated animals, this effect did not attain significance (P<0.106). In contrast, there was a general trend was toward elevated D1 and D2 mRNA in the mPFC of PTX-treated animals (Figure 4, right panel). Though this did not attain significance, the statistics should be interpreted cautiously given the high degree of variability and low sample size. Overall, the findings indicate that increased maternal responsiveness following intra-VTA PTX treatment is associated with altered dopamine receptor mRNA expression in the NAc.
Table 1.
Mean DOPAC and Dopamine Content (+/−SEM) in the NAc and mPFC following Intra-VTAPTX Treatment
| Brain Region | Intra-VTA Treatment | Sample Size | DOPAC (ng/mg protein) | Dopamine (ng/mg protein) | DOPAC/DA Ratio |
|---|---|---|---|---|---|
| N. Accumbens | Control | 6 | 31.58 (2.60) | 169.30 (14.81) | 0.132 (0.004) |
| PTX (0 3 μg) | 6 | 25.20 (7.24) | 107.45 (34.86) | 0.168 (0.02) | |
| T(10)=0.83, P<0.43 | T(10)=1.63, P<0.134 | T(0)=1.51, P<0.161 | |||
| mPFC | Control | 6 | 58.43 (24.60) | 143.06 (52.32) | 0.152 (0.019) |
| PTX (0 3 μg) | 6 | 85.67 (48.70) | 242.53 (105.85) | 0.139 (0.028) | |
| T(10)=0.50, P<0.628 | T(10)=0.84, P<0.419 | T(10)=0.37, P<0.719 |
Figure 4.
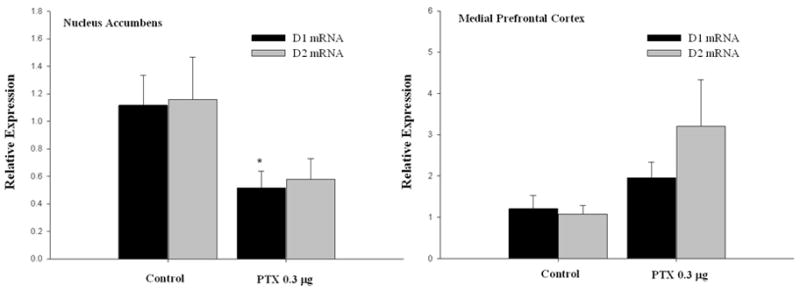
Intra-VTA PTX treatment alters forebrain DA receptor mRNA expression. Adult, virgin female rats were infused bilaterally into the VTA with PTX (0.3 μg/hemisphere) or its inactive control. Animals were euthanized on post-treatment day 7, a timeframe corresponding with the emergence of stimulated maternal responding. Data are mRNA expression relative to control (± SEM) for groups of 5–6 animals. Left panel (nucleus accumbens): Student’s t-tests, D1 mRNA - [T(9)=2.511, *P<0.033 vs. control], D2 mRNA - [T(9)=1.79, P<0.106]. Right panel (medial prefrontal cortex): Student’s t-tests, D1 mRNA - [T(9)=1.463, P<0.178], D2 mRNA - [T(9)=1.69, P<0.124].
4. DISCUSSION
Prior studies indicate that blockade of mesolimbic dopamine transmission disrupts the display of maternal behavior in the postpartum rat [28, 34, 39, 58]. Therefore, we tested the hypothesis that chronic stimulation of this system via intra-VTA PTX treatment would increase maternal responding. Using a pup-induced virgin model, we found that intra-VTA PTX treatment produced a dose-dependent decrease in latency to respond maternally, with the high dose reducing maternal response time by approximately 50%. This effect did not appear to be associated with a significant change in locomotor activity or forebrain dopamine turnover at the time of maternal behavior testing. However, stimulation of maternal responding was associated with a decrease in NAc D1 receptor mRNA expression. The results indicate that the mesolimbic dopamine system can be driven to accelerate the onset of maternal activity, thereby providing support for the role of this system in maternal behavior.
Numerous studies have demonstrated a variety of long-lasting behavioral alterations following a single intracranial PTX infusion in rats [e.g. 11, 22, 47, 63]. PTX is an AB5 exotoxin that is taken up into cells where it catalyzes the long-lasting ADP-ribosylation of Gi/Go protein α subunits. This action freezes G proteins in their GDP-bound (inactive) state, effectively blocking inhibitory G protein-coupled receptor signal transduction. The result of this modification is an unopposed increase in intracellular cyclic AMP accumulation and downstream cellular activity [15, 17]. In the midbrain, the K+ channel-coupled dopamine D2 autoreceptor and GABA-B receptor are among the inhibitory influences on dopamine cell firing [8, 24, 26, 32, 51, 59]. Both receptors are Gi/Go -coupled, and thus represent targets for PTX action. Indeed, PTX infusion into the VTA has previously been reported to increase mesolimbic dopamine transmission [35, 50]. This increased mesolimbic activity purportedly underlies the ability of PTX to sensitize rats to dopamine agonists and stress, and to facilitate the development of psychostimulant sensitization [36, 37, 49, 50, 61, 63]. Thus, the present study design was based on the prediction that intra-VTA PTX treatment would similarly accelerate pup-induced maternal responding.
The development of maternal responding in cycling, virgin female rats following daily pup exposure has been well-documented [6, 7, 10, 16, 48, 45]. In many respects, maternal behavior induced via pup exposure is very similar to that observed in lactating rats [16]. This pup-induction paradigm offers an advantage in that it allows for the examination of neural substrates mediating innate motivation in the absence of the physiological changes associated with pregnancy, parturition, and lactation. It is for this reason that we used the pup-induction model to study the effect of intra-VTA PTX treatment on maternal behavior. We found that daily exposure of the control group to pups resulted in a median FMB latency of 8 days. These findings are consistent with those reported previously [46, 48]. In contrast, median FMB latencies for high- and low-dose PTX groups were 4 and 5.5 days, respectively. Moreover, there were some animals that first displayed maternal behavior as early as day 1 in the high-dose PTX group. These results demonstrate a clear PTX-induced acceleration of maternal activity.
Prior studies have reported that intra-VTA PTX produces robust increases in spontaneous locomotor activity which develop over time, and are both dose-dependent and long-lasting [37]. These locomotor effects can be potentiated by systemic or intra-NAc treatment with amphetamine or apomorphine [36, 37]. A similar effect was observed in the present study, wherein PTX-treated animals previously tested for maternal behavior showed dose-dependent elevations of spontaneous motor activity at 18–19 days post-treatment. By comparison, the magnitude of PTX-induced locomotor behavior was not as large when tested at 6–7 days post-treatment. Indeed, activity levels of high-dose PTX-treated animals tested at this earlier time point were approximately 60 percent smaller those observed on post-treatment days 18–19 (cf. Figure 2). This is consistent with findings indicating that the motor activating effects of intra-VTA PTX develop with a latency of at least 4–6 days, peak at approximately 28 days, and decline to control levels after 60 days [37]. Taken together, it may be concluded that behaviors secondary to increased motor activation (e.g. increased contact with pups [56]) likely did not play a significant role in the effect of intra-VTA PTX on maternal responding. Further, the results indicate that the PTX regimen used herein was biologically active, and produced predictable dose- and time-dependent behavioral effects.
Studies investigating the effects of intra-VTA PTX on dopamine metabolism have yielded variable results, which appear to depend upon the time of assay. For example, Steketee et al. [51] found that infusion of PTX into the A10 region increased dopamine metabolism in the NAc and the mPFC for up to 4 days, while ADP-ribosylation persisted for up to 30 days post-treatment. Studies examining the limbic forebrain (including NAc) at later time points following PTX treatment showed no change in basal extracellular dopamine at day 14 [50] and no change in tissue dopamine content or dopamine turnover at days 15–16 [63]. Similarly, the present study did not find an effect of intra-VTA PTX treatment on DOPAC, dopamine, or dopamine turnover in either the NAc or mPFC when studied at post-treatment day 7. Therefore, the observation of accelerated maternal responding following PTX treatment does not appear to be temporally associated with changes in forebrain dopamine synthesis or metabolism.
In contrast to the aforementioned findings, RT-PCR analysis of gene expression revealed a significant (>50%) decrease in dopamine D1 receptor mRNA in the NAc of animals infused with high-dose PTX into the VTA. This effect was observed at 7 days post-surgery, a time corresponding to the observed enhancement of maternal behavior. A similar decrease in expression was observed for D2 receptor mRNA, but this failed to attain significance. In the mPFC, no effect of PTX on D1 or D2 receptor expression was observed. Therefore, the effect of PTX on pup-induced maternal behavior, though not temporally associated with changes in dopamine utilization per se, appears to involve postsynaptic adaptations in dopamine receptor signaling within the NAc.
In the present study, the mechanism(s) for PTX-induced suppression of NAc D1 receptor gene expression and its role in maternal responding are unclear. To our knowledge, the effect of intra-VTA PTX on forebrain dopamine receptor gene or protein expression has not been reported previously. However, Narayanan et al. [37] reported that the locomotor activating effect of intra-VTA PTX can be blocked by systemic treatment with a D1 (but not D2), receptor antagonist. This group also found that intra-VTA PTX treatment augmented amphetamine-induced c-Fos activation in the NAc, an effect purportedly mediated via supersensitive D1 receptors [35]. These findings are comparable to those demonstrating that locomotor activation in amphetamine- and cocaine-sensitized animals is associated with the development of D1 receptor supersensitivity [20, 27]. Interestingly, D1 receptor activation is also necessary for the expression of postpartum maternal behavior [52, 54]. Thus, it is conceivable that the down-regulation of D1 gene expression observed in the present study represents an adaptive response to PTX-induced supersensitivity, and that this lasting modification mediates enhanced maternal motivation. In support of this, a recent report has associated decreased NAc D1 mRNA with increased motivation to search for food reward [4]. Thus, when interpreted in the context of the studies cited above, the current findings support the hypothesis that NAc D1 receptor adaptations underlie both sensitization to drugs of abuse and maternal responding, and perhaps incentive motivation in general.
In conclusion, the findings reported herein indicate that chronic activation of the mesolimbic dopamine system via intra-VTA PTX treatment accelerates the onset of maternal behavior in a virgin, pup-induced model. This effect is associated with decreased dopamine D1 receptor mRNA expression in the NAc, a finding that is consistent with D1 receptor supersensitivity [35]. Thus, the neural correlate of PTX-induced maternal behavior is similar to that observed in other models of drug [27] and food [4] reward. The results suggest common neural substrates for a variety of reward-related phenomena, including maternal motivation.
Highlights.
We tested the hypothesis that VTA stimulation would enhance maternal activity.
A pup-sensitized virgin female rat model of maternal behavior was used.
VTA stimulation via pertussis toxin resulted in acceleration of maternal behavior.
Behavior was associated with decreased D1 receptor mRNA in the nucleus accumbens.
Mesolimbic dopamine system can be driven to accelerate maternal behavior in the rat.
Acknowledgments
This research was supported by NIH Grants HD19789 (RSB) and GM074869 (EDG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Afonso VM, Grella SL, Chatterjee D, Fleming AS. Previous maternal experience affects accumbal dopaminergic responses to pup-stimuli. Brain Res. 2008;1198:115–23. doi: 10.1016/j.brainres.2007.12.042. [DOI] [PubMed] [Google Scholar]
- 2.Afonso VM, King S, Chatterjee D, Fleming AS. Hormones that increase maternal responsiveness affect accumbal dopaminergic responses to pup- and food-stimuli in the female rat. Hormones and Beh. 2009;56:11–23. doi: 10.1016/j.yhbeh.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Afonso VM, Sison M, Lovic V, Fleming AS. Medial prefrontal cortex lesions in the female rat affect sexual and maternal behavior and their sequential organization. Behav Neurosci. 2007;121:515–26. doi: 10.1037/0735-7044.121.3.515. [DOI] [PubMed] [Google Scholar]
- 4.Alsiö J, Olszewski PK, Norbäck AH, Gunnarsson ZE, Levine AS, Pickering C, Schiöth HB. Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neurosci. 2010;171:779–87. doi: 10.1016/j.neuroscience.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 5.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 6.Bridges RS, Scanlan VF. Maternal memory in adult, nulliparous rats: effects of testing interval on the retention of maternal behavior. Dev Psychobiol. 2005;46:13–18. doi: 10.1002/dev.20038. [DOI] [PubMed] [Google Scholar]
- 7.Bridges RS, Thankey KP, Scanlan VF. Duration of daily test pup exposure in adult, nulliparous rats alters maternal behavior induction rats: implications for animal use numbers. Contemp Top Lab Anim Sci. 2004;43:28–31. [PubMed] [Google Scholar]
- 8.Bunney BS, Walters JR, Roth RH, Aghajanian GK. Dopaminergic neurons: effect of antipsychotic drugs and amphetamine on single cell activity. J Pharmacol Exp Ther. 1973;185:560–71. [PubMed] [Google Scholar]
- 9.Byrnes EM, Rigero BA, Bridges RS. Dopamine antagonists during parturition disrupt maternal care and the retention of maternal behavior in rats. Pharmacol Biochem Behav. 2002;73:869–75. doi: 10.1016/s0091-3057(02)00941-3. [DOI] [PubMed] [Google Scholar]
- 10.Cosnier J, Couturier C. Maternal behavior induced in adult castrated rats. C R Soc Biol. 1966;160:789–91. [PubMed] [Google Scholar]
- 11.Culm KE, Lim AM, Onton JA, Hammer RP., Jr Reduced Gi and Go protein function in the rat nucleus accumbens attenuates sensorimotor gating deficits. Brain Res. 2003;982:12–18. doi: 10.1016/s0006-8993(03)02880-4. [DOI] [PubMed] [Google Scholar]
- 12.Febo M, Felix-Ortiz AC, Johnson TR. Inactivation or inhibition of neuronal activity in the medial prefrontal cortex largely reduces pup retrieval and grouping in maternal rats. Brain Res. 2010;1325:77–88. doi: 10.1016/j.brainres.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Febo M, Ferris CF. Development of cocaine sensitization before pregnancy affects subsequent maternal retrieval of pups and prefrontal cortical activity during nursing. Neuroscience. 2007;148:400–12. doi: 10.1016/j.neuroscience.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferris CF, Kulkarni P, Sullivan JM, Jr, Harder JA, Messenger TL, Febo M. Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci. 2005;25:149–56. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fields TA, Casey PJ. Signalling functions and biochemical properties of pertussis toxin-resistant G-proteins. Biochem J. 1997;321:561–71. doi: 10.1042/bj3210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleming AS, Rosenblatt JS. Maternal behavior in the virgin and rat. J Comp Physiol Psychol. 1974;86:957–72. doi: 10.1037/h0036414. [DOI] [PubMed] [Google Scholar]
- 17.Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–49. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 18.Hansen S, Bergvall AH, Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol Biochem Behav. 1993;45:673–76. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- 19.Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K. Mesotelencephalic dopamine system and reproductive behavior in the female rat: effects of ventral tegmental 6-hydroxydopamine lesions on maternal and sexual responsiveness. Behav Neurosci. 1991;105:588–98. doi: 10.1037//0735-7044.105.4.588. [DOI] [PubMed] [Google Scholar]
- 20.Henry DJ, White FJ. Repeated cocaine administration causes persistent enhancement of D1 dopamine receptor sensitivity within the rat nucleus accumbens. J Pharmacol Exp Ther. 1991;258:882–90. [PubMed] [Google Scholar]
- 21.Hernandez-Gonzalez M, Navarro-Meza M, Prieto-Beracoechea CA, Guevara MA. Electrical activity of prefrontal cortex and ventral tegmental area during rat maternal behavior. Behav Process. 2005;70:132–43. doi: 10.1016/j.beproc.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Hummel M, Unterwald EM. Intra-accumbens pertussis toxin sensitizes rats to the locomotor activating effects of a single cocaine challenge. Brain Res. 2003;965:100–107. doi: 10.1016/s0006-8993(02)04142-2. [DOI] [PubMed] [Google Scholar]
- 23.Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Rev. 1999;31(1):6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 24.Innis RB, Aghajanian GK. Pertussis toxin blocks autoreceptor-mediated inhibition of dopaminergic neurons in the rat substantia nigra. Brain Res. 1987;411:139–43. doi: 10.1016/0006-8993(87)90690-1. [DOI] [PubMed] [Google Scholar]
- 25.Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–8. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Rev. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- 27.Kalivas PW, Stewart J. Dopamine neurotransmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev. 1991;16:223–44. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- 28.Keer SE, Stern JM. Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol Behav. 1999;67:659–69. doi: 10.1016/s0031-9384(99)00116-x. [DOI] [PubMed] [Google Scholar]
- 29.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–11. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koob GF. Hedonic valence, dopamine and motivation. Mol Psychiatry. 1996;1:186–9. [PubMed] [Google Scholar]
- 31.Kristal MB. The biopsychology of maternal behavior in nonhuman mammals. ILAR J. 2009;50:51–63. doi: 10.1093/ilar.50.1.51. [DOI] [PubMed] [Google Scholar]
- 32.Lacey MG, Mercuri NB, North RA. On the potassium conductance increase activated by GABAB and dopamine D2 receptors in rat substantia nigra neurones. J Physiol. 1988;401:437–53. doi: 10.1113/jphysiol.1988.sp017171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 2000;108:215–31. doi: 10.1016/s0166-4328(99)00170-9. [DOI] [PubMed] [Google Scholar]
- 34.Li M, Fleming AS. The nucleus accumbens shell is critical for normal expression of pup-retrieval in postpartum female rats. Behav Brain Res. 2003;145:99–111. doi: 10.1016/s0166-4328(03)00135-9. [DOI] [PubMed] [Google Scholar]
- 35.Narayanan S, Dalia A, Wallace L, Uretsky N. Effect of pertussis toxin injected into the ventral tegmental area on amphetamine-induced Fos protein in the nucleus accumbens. Brain Res Bull. 1997;43:435–9. doi: 10.1016/s0361-9230(97)00030-0. [DOI] [PubMed] [Google Scholar]
- 36.Narayanan S, Dalia A, Wallace LJ, Uretsky NJ. Enhancement of the locomotor response to apomorphine in pertussis toxin-treated animals depends on the site of pertussis toxin injection into the ventral tegmental area. Brain Res. 1997;748:263–6. doi: 10.1016/s0006-8993(96)01379-0. [DOI] [PubMed] [Google Scholar]
- 37.Narayanan S, Wallace L, Uretsky N. Spontaneous and Drug-Stimulated Locomotor Activity after the Administration of Pertussis Toxin into the Ventral Tegmental Area. J Psychiatry Neurosci. 1996;21:172–80. [PMC free article] [PubMed] [Google Scholar]
- 38.Neumann A, Hoey RF, Daigler LB, Thompson AC, Kristal MB. Ingestion of amniotic fluid enhances the facilitative effect of VTA morphine on the onset of maternal behavior in virgin rats. Brain Res. 2009;1261:29–36. doi: 10.1016/j.brainres.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- 40.Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM, Smith CD. The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav Neurosci. 2005;119:1588–604. doi: 10.1037/0735-7044.119.6.1588. [DOI] [PubMed] [Google Scholar]
- 41.Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Frontiers in Neuroendocrinology. 2009;30:46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Parada M, King S, Li M, Fleming AS. The roles of accumbal dopamine D1 and D2 receptors in maternal memory in rats. Behav Neurosci. 2008;122:368–76. doi: 10.1037/0735-7044.122.2.368. [DOI] [PubMed] [Google Scholar]
- 43.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. New York: Academic Press; 1986. [Google Scholar]
- 44.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenblatt JS. Nonhormonal basis of maternal behavior in the rat. Science. 1967;156:1512–4. doi: 10.1126/science.156.3781.1512. [DOI] [PubMed] [Google Scholar]
- 46.Scanlan VF, Byrnes EM, Bridges RS. Reproductive experience and activation of maternal memory. Behav Neurosci. 2006;120:676–86. doi: 10.1037/0735-7044.120.3.676. [DOI] [PubMed] [Google Scholar]
- 47.Schroeder JA, Hummel M, Unterwald EM. Distribution of Gi/o protein signaling in the nucleus accumbens results in a D1 dopamine receptor-mediated hyperactivity. Pharmacol Biochem Beh. 2004;79:93–9. doi: 10.1016/j.pbb.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Seip KM, Morrell JI. Exposure to pups influences the strength of maternal motivation in virgin female rats. Physiol Behav. 2008;95:599–608. doi: 10.1016/j.physbeh.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seip KM, Morrell JI. Transient inactivation of the ventral tegmental area selectively disrupts the expression of conditioned place preference for pup- but not cocaine-paired contexts. Behav Neurosci. 2009;123:1325–38. doi: 10.1037/a0017666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steketee JD, Kalivas PW. Sensitization to psychostimulants and stress after injection of pertussis toxin into the A10 dopamine region. J Pharmacol Exp Ther. 1991;259:919–24. [PubMed] [Google Scholar]
- 51.Steketee JD, Striplin CD, Murray TF, Kalivas PW. Pertussis toxin in the A10 region increases dopamine synthesis and metabolism. J Neurochem. 1992;58:811–6. doi: 10.1111/j.1471-4159.1992.tb09329.x. [DOI] [PubMed] [Google Scholar]
- 52.Stolzenberg DS, McKenna JB, Keough S, Hancock R, Numan MJ, Numan M. Dopamine D1 receptor stimulation of the nucleus accumbens or the medial preoptic area promotes the onset of maternal behavior in pregnancy-terminated rats. Behav Neurosci. 2007;121:907–19. doi: 10.1037/0735-7044.121.5.907. [DOI] [PubMed] [Google Scholar]
- 53.Stolzenberg DS, Numan M. Hypothalamic interaction with the mesolimbic DA system in the control of the maternal and sexual behaviors in rats. Neurosci Biobehav Rev. 2011;35:826–47. doi: 10.1016/j.neubiorev.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 54.Stolzenberg DS, Zhang KY, Luskin K, Ranker L, Bress J, Numan M. Dopamine D(1) receptor activation of adenylyl cyclase, not phospholipase C, in the nucleus accumbens promotes maternal behavior onset in rats. Horm Behav. 2010;57:96–104. doi: 10.1016/j.yhbeh.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 55.Szechtman H, Siegel HI, Rosemblatt JS, Komisaruk BR. Tail-pinch facilitates onset of maternal behavior in rats. Physiol Behav. 1977;19:807–9. doi: 10.1016/0031-9384(77)90319-5. [DOI] [PubMed] [Google Scholar]
- 56.Terkel J, Rosenblatt JS. Aspects of non-hormonal maternal behavior in the rat. Hormones Behav. 1971;2:161–71. [Google Scholar]
- 57.Thompson AC, Kristal MB. Opioid stimulation in the ventral tegmental area facilitates the onset of maternal behavior in rats. Brain Res. 1996;743:184–201. doi: 10.1016/s0006-8993(96)01041-4. [DOI] [PubMed] [Google Scholar]
- 58.Vernotica EM, Rosenblatt JS, Morrell JL. Microinfusion of cocaine into the medial preoptic area or nucleus accumbens transiently impairs maternal behavior in the rat. Behav Neurosci. 1999;113:377–90. doi: 10.1037//0735-7044.113.2.377. [DOI] [PubMed] [Google Scholar]
- 59.Wang RY. Dopaminergic neurons in the rat ventral tegmental area. II. Evidence for autoregulation. Brain Res. 1981;3:141–51. [Google Scholar]
- 60.Wansaw MP, Pereira M, Morrell JI. Characterization of maternal motivation in the lactating rat: Contrasts between early and late postpartum responses. Horm Behav. 2008;54:294–301. doi: 10.1016/j.yhbeh.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weinstein DM, Narayanan S, Byrnes JJ, Uretsky NJ, Wallace LJ. Comparison of sensitization elicited by amphetamine and pertussis toxin: characterization of locomotor behavior and limbic dopamine release. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:885–97. doi: 10.1016/s0278-5846(97)00087-0. [DOI] [PubMed] [Google Scholar]
- 62.Wilsoncroft VE. Babies by bar-press: maternal behavior in the rat. Behav Res Methods Instrum. 1969;1:229–30. [Google Scholar]
- 63.Zhang J, Engel JA, Hjorth S, Svensson L. Changes in the acoustic startle response and prepulse inhibition of acoustic startle in rats after local injection of pertussis toxin into the ventral tegmental area. Psychopharmacol. 1995;119:71–8. doi: 10.1007/BF02246056. [DOI] [PubMed] [Google Scholar]


