Abstract
The objective of this study was to develop a technique for analyzing spatial patterns of cartilage loss in the medial femoral condyle (MF), and to study MF cartilage loss in participants of the Osteoarthritis Initiative. Using a 0.7mm sagittal double echo at steady state (DESS) sequence, 160 osteoarthritic knees from 80 participants with varying degrees of medial joint space narrowing were imaged at baseline and 1-year follow-up. MF cartilage was segmented and cartilage loss determined. Rate of change varied significantly (P = 0.0067) along the anterior-posterior extension of the MF, with the greatest changes (−45 μm, −2.7%) observed 30–60° posterior to the trochlear notch. The rate was greater in the central MF after excluding peripheral aspects of the MF from analysis. Sensitivity to change was greatest at 45–75° (standardized response mean = −0.32) but was minimally affected by medial-lateral trimming. In conclusion, the greatest sensitivity to change was achieved when analyzing the posterior aspect of the central, weight-bearing MF.
Keywords: cartilage, osteoarthritis, image analysis, 3 tesla, subregions
Osteoarthritis (OA) is a disease characterized by structural changes of all diarthrodial tissues, including the articular cartilage (1,2). Cartilage loss is known to occur in a spatially heterogeneous manner and is assumed to correspond to sites of high mechanical stress (3). Radiography attempts to estimate cartilage loss from femorotibial joint space narrowing (JSN) in a defined position of the knee (4,5), but weight-bearing knee radiographs are conventionally acquired in full extension, although biomechanical studies suggest the greatest femorotibial contact stresses occur with the knee in 24–28° flexion (3). Moreover, it has been arthroscopically observed that cartilage loss in the femoral condyle frequently occurs more posteriorly than shown by conventional standing-view radiographs (6), potentially explaining the lack of correlation reported between JSN and arthroscopic joint status (7). Several authors have therefore recommended that radiographs be taken in knee flexion (6,8,9).
Vignon et al. (10) reported that the decrease in the minimum joint space width of OA knees over 2 years was 0.17 ± 0.75 mm (mean ± standard deviation) in standing anterior-posterior radiographs (standardized response mean = SRM = 0.23; change not significant), and 0.24 ± 0.50 mm in a fluoroscopically controlled posterior-anterior Lyon schuss view taken in 20–30° of knee flexion (SRM = 0.48; P = 0.007). These findings suggest that greater cartilage loss occurs in regions of the medial femoral condyle (MF) located somewhat posterior to regions that are in contact with the medial tibia when the knee is fully extended. The small standard deviation also suggests that cartilage loss is more uniform across participants at this location than at that measured using conventional anterior-posterior radiographs. A cross-sectional study confirmed that the Lyon schuss view was superior in detecting JSN compared with the extended anterior-posterior view (11).
However, radiographic measurements are known to be confounded by meniscal subluxation or extrusion (12–14), they depend on the specific flexion angle of the knee, and are two-dimensional. Therefore, and because the cartilage surfaces are not in direct contact in all parts of the joint, radiography cannot reveal the spatial patterns of cartilage loss within the knee. MRI, in contrast, can directly visualize articular cartilage and can be used to analyze articular cartilage dimensions (morphology) quantitatively (1,2,5). A valuable source of high-quality MRI datasets is the Osteoarthritis Initiative (OAI), a program targeted at identifying sensitive biomarkers of symptomatic knee OA, with a particular focus on MRI surrogate markers. The primary objectives of the current study were
to develop an MRI-based technique for determining the cartilage thickness in various regions of interest (ROIs) along the anterior-posterior extension of the MF
to allow exclusion of peripheral areas of the MF from the analysis by cropping anterior-posterior ROIs at the medial and lateral margins (medial-lateral trimming) since peripheral areas are prone to partial volume effects in sagittal MR images and inclusion may desensitize measurements of change
to determine the spatial pattern of cartilage loss along the anterior-posterior dimension of the MF longitudinally over 1 year, with and without medial-lateral trimming, using 3-T MRI data from participants of the OAI
A secondary objective was to explore whether the spatial pattern of cartilage loss varied between participants with different grades of JSN. The general aim was to identify which subregion(s) of the MF display the greatest and most uniform cartilage loss in OA and may therefore be most suitable for investigating effects of structure-modifying therapy in clinical trials.
MATERIALS AND METHODS
Subject Selection
The subsample studied was drawn from the first half (2678 cases) of the OAI cohort (public-use data sets 1.2.1 for the clinical data and 1.B.1 for the imaging data), a multicenter, population-based, longitudinal, cohort study designed to identify biomarkers for the development and/or progression of symptomatic knee OA (http://www.oai.ucsf.edu/datarelease/). Participants in the OAI cohort span a diversity of ethnic backgrounds and are generally aged between 45 and 79 years. Participants with contraindications to 3-T MRI, bilateral end-stage knee OA, an inability to walk without aids, and rheumatoid or other inflammatory arthritis were excluded from the OAI cohort.
The subpopulation studied here (n = 80) was from a study comparing cartilage loss in both knees of OA participants, where one knee displayed JSN (advanced radiographic OA) and the contralateral knee did not (early radiographic OA). The inclusion criteria were (a) Body Mass Index (BMI) >25; (b) chronic frequent pain (most days of a month within the past 12 months), or at least “occasional” pain (pain in past 12 months, but not most days of a month) in the knee with less medial JSN (mJSN; two of the 80 participants had at least “occasional” pain, but both knees displayed definite OARSI grade ≥2 osteophytes; (c) mJSN grade (15) 1 (n = 47), grade 2 (n = 35), or grade 3 (n = 8) in one knee and no or less mJSN in the contralateral knee (73 cases displayed no mJSN in the contralateral knee and seven cases of mJSN grade 2 or 3 displayed grade 1 in the contralateral knee); and (d) no lateral JSN in both knees (n = 77) or a lower grade of lateral JSN than mJSN in the same knee (n = 3).
The 80 participants were selected by first using files of the 160 participants from the OAI progression subcohort (OAI public-use datasets 0.1.1, 0.B.1, and 1.B.1), for which adjudicated readings of fixed flexion radiographs between the four imaging sites and a central reading site were already available (16,17). Based on the adjudicated readings, 19 (of 160) cases fulfilled the selection criteria. Another 61 cases were selected by using the radiographic readings from the four imaging sites of all 2678 cases released from the OAI to date (OAI public-use data sets 1.2.1 and 1.B.1), but excluding the 160 cases mentioned above. The selection criteria for these 61 participants were confirmed by central readings, provided by an experienced musculoskeletal radiologist (A.G.) and adjudicated by a rheumatologist (D.H.) in case of discrepancies between the site and central readings.
MR Image Acquisition and MR Image Segmentation
Baseline and 1-year follow-up MR images of both knees were acquired by the OAI at four imaging sites (University of Maryland School of Medicine [Baltimore], the Ohio State University [Columbus], the University of Pittsburgh, and the Memorial Hospital of Rhode Island [Pawtucket]), using 3-T MR systems (Siemens Magnetom Trio, Erlangen, Germany) and quadrature transmit-receive knee coils (USA Instruments, Aurora, OH), as described previously (18,19). Sagittal double echo at steady state (DESS) images with 0.7mm slice thickness and water excitation were acquired as described previously (18,19) (Fig. 1). The DESS sequence has been shown to produce cartilage thickness data consistent with that of previously validated spoiled gradient recalled sequences and to display a similar test-retest precision (reproducibility) (18,19). Additional details on the OAI MRI protocol were recently published (20). The OAI study protocol, amendments, and informed consent documentation were reviewed and approved by local institutional review boards.
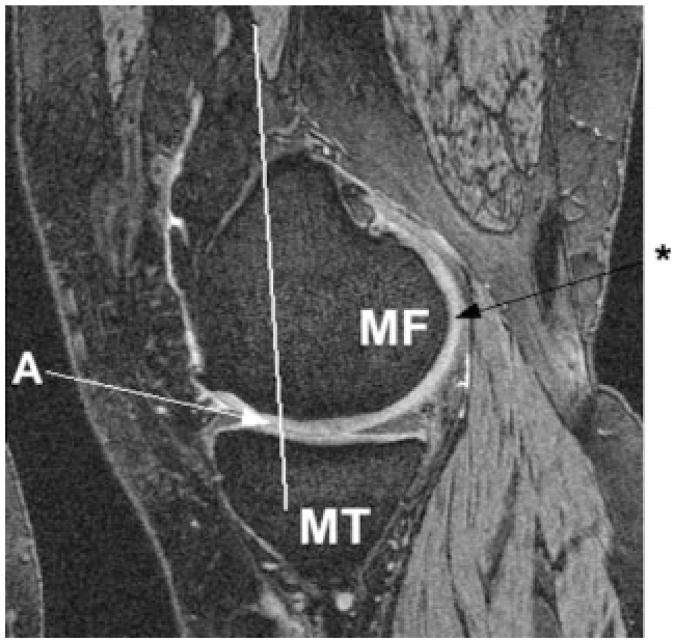
FIG. 1.
Sagittal double echo at steady state image of the medial femorotibial joint, showing the medial tibia (MT), the MF condyle, the anterior clipping plane of MF (trochlear notch) through landmark “A”, and the point marked at the posterior end of the femoral condyle (*).
Images were shipped from the OAI coordinating center to the image analysis center (Chondrometrics GmbH, Ainring, Germany) and analyzed in pairs (baseline and 1-year follow-up). The seven readers, each with more than 3 years’ experience in cartilage segmentation and with previous experience in segmenting sagittal DESS images from the OAI pilot studies (18,19), were blinded to the order of the image acquisition. Manual segmentation of the MF cartilage was performed using custom software (Chondrometrics GmbH). To separate the MF from the femoral trochlea, readers marked (a) a line running parallel to the femoral shaft through the most posterior end of the trochlear notch (Fig. 1, Fig. 2), (b) a point marking the most posterior end of the MF (Fig. 1, Fig. 2), and (c) a point marking the most posterior end of the lateral femoral condyle prior to segmentation. From these anatomic landmarks, a plane separating the MF from the femoral trochlea was constructed using the vector along the femoral shaft and the connection of the most posterior points of both condyles (Fig. 2). The latter was necessary to correct for rotation of the knee vs the sagittal image plane during image acquisition. The readers then segmented the cartilage surface and the bone interface of the MF posterior to the plane dividing it from the femoral trochlea (anterior clipping plane). Denuded areas were included in the segmentation by marking the bone interface only, but not the cartilage surface. Segmentation was performed for the entire MF, including the posterior part where the cartilage surface and bone interface meet near the transition of the distal femoral metaphysic and the femoral shaft. Segmentations running anterior toward the trochlea were cropped automatically at the anterior clipping plane (Fig. 1). An appropriate choice of anatomic landmarks and segmentation of the MF cartilage were quality controlled by a single expert reader who double checked all slices of all datasets.
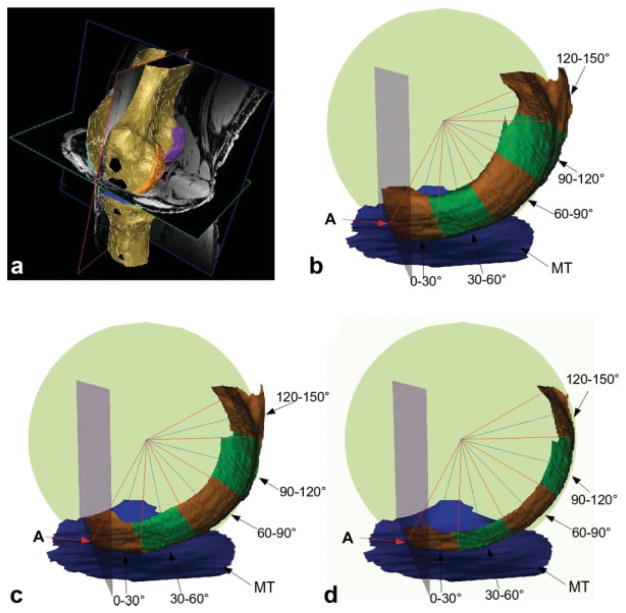
FIG. 2.
a: 3D reconstruction of a knee MRI data set showing the anterior clipping plane (red frame), separating the medial femoral condyle cartilage (orange) from the trochlear cartilage (turquoise). b: 3D reconstruction illustrating the regional subdivision of the MF condyle in anatomic relationship with the medial tibia (MT). The figure also shows the anterior clipping plane (gray) through landmark “A” and the sphere (green) that was fitted to the MF. The borders between the subregions not shown are indicated using blue lines. c: 3D reconstruction of MF with medial-lateral trimming showing the central 66.7% of the MF. d: 3D reconstruction of MF with medial-lateral trimming showing the central 33.3% of the MF.
Regional Algorithm Development and Application
The segmentations of the bone interface and cartilage surface were used to construct triangulated three-dimensional (3D) representations of the entire cartilage surface and the total area of subchondral bone (tAB) of the MF (21). To define a reliable frame of reference for a reproducible decomposition of these surfaces, a fast converging, geometric sphere fitting algorithm (22) was applied to determine a center “C” for the tAB of the MF. The nonlinear least-squares estimates for the sphere parameters (radius “R”, center “C”) were computed by minimizing the orthogonal error distance vectors, which were given for each vertex of the tAB by the vector connecting the vertex and the closest point on the sphere. The Gauss-Newton method (22) was used to iteratively optimize the parameter vector for “R” and “C”.
As a second component of the frame of reference, the point of the tAB where the central slice of the segmented MF intersected the anterior clipping plane, was computed as the anterior landmark “A” (Fig. 1, Fig. 2). A clipping plane through the center of the sphere “C” was constructed from the vector connecting “A” and “C”, and the vector connecting the most posterior points of the MF and lateral femoral condyle.
The determination of the anterior-posterior regions of interest on the 3D surface reconstruction was then performed by rotating the clipping plane around the axis parallel to the connection of the most posterior points of the MF and lateral femoral condyle, and through the center of the sphere “C”. The plane was rotated in 5° steps, starting with 0° at the anterior landmark “A” and ending with 150° (see below). In order to limit noise resulting from a choice of too small subregions, nine overlapping, 30° subregions, each shifted by 15° from the previous region (0–30°, 15–45°, 30–60°, etc.), were constructed by merging the six subsequent primary subregions (5° strips) between 0° and 150° in the anterior-posterior direction (Fig. 2). This was considered a reasonable compromise for determining regions of interest (ROI) ROIs of adequate size while obtaining sufficiently detailed spatial information, given the clinical context.
To study the cartilage morphology also in central (medial-lateral) “trimmed” areas of the MF, the tAB was trimmed at the internal and external border, focusing on a central third (33.3%) or central two thirds (66.7%) of the MF (and excluding the peripheral parts from the analysis), compared to an analysis of the entire medial-lateral extension of the MF (100%). Finally, mean cartilage thickness over the entire tAB (ThCtAB; including denuded areas with 0 mm cartilage thickness, but not osteophytes (23)) was computed for the entire MF and for each of the nine anterior-posterior subregions, with and without the medial-lateral trimming described above.
The methods above were applied to each knee and time point separately, without the use of registration techniques. The mean and standard deviation of the difference in ThCtAB (μm) between baseline and 1-year follow-up image acquisitions was determined in all 160 knees and in subgroups with different mJSN grades (n = 73 with no mJSN [grade 0]; n = 54 mJSN grade 1; n = 33 mJSN grade 2 or 3). Knees with mJSN grades 2 and 3 were pooled because only eight knees displayed mJSN grade 3. The mean change was also expressed in percent, by relating the mean change across participants (or across subgroups with different mJSN grades) to the mean baseline value for the MF or MF subregions. As a measure of the sensitivity to change, the standardized response mean (SRM = mean change/standard deviation of change) was calculated for the MF and its subregions. This study was not designed or powered to determine region(s) with statistically greater change or higher SRM compared to other regions, but to explore the spatial pattern of cartilage loss along the anterior-posterior subregions of the femur with and without medial-lateral trimming. To confirm whether the magnitude of change was different across the subregions, an analysis of variance of repeated measures was applied to the longitudinal changes in ThCtAB across the nine anteroposterior subregions, and to those of the entire MF with and without medial-lateral trimming (33.3, 66.7, and 100%, respectively). No statistical testing was performed between different mJSN grades.
RESULTS
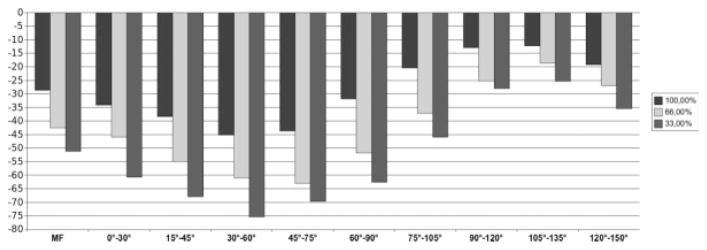
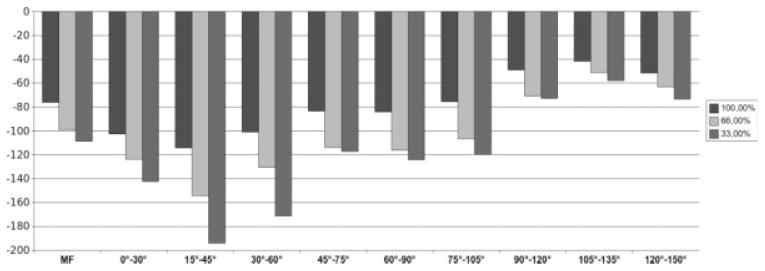
The change in cartilage thickness over 1 year in the entire MF was −29 ± 100 μm across all 160 knees (−1.7%; SRM = −0.29, P < 0.001 for 1-year follow-up vs baseline). Subregions located at 0–90° displayed higher rates of change, whereas subregions located more posterior (>90°) displayed lower rates of change, as compared to the entire MF (Table 1; Fig. 3). The analysis of variance of repeated measures confirmed that the rate of change differed between the anterior-posterior subregions (P = 0.0067). The region located at 30–60° displayed the greatest rate of change (−45 ± 167 μm; −2.7%; Fig. 3 and Table 1); the region located at 45–75°, the greatest SRM (−0.32; Table 1).
Table 1.
Mean change (MC [%]) and SRM of the mean cartilage thickness (ThCtAB) in the MF Condyle (Total MF) Over 1 Year and in Overlapping Subregions of the MF Located 0–150° Posterior to the Trochlear Notch
| 100% ml ext.
|
Central 66.7%
|
Central 33.3%
|
|||||
|---|---|---|---|---|---|---|---|
| MC [%] | SRM | MC [%] | SRM | MC [%] | SRM | ||
| All (n = 160) | Total MF | −1.7 | −0.29 | −2.2 | −0.33 | −2.6 | −0.33 |
| 0–30° | −2.0 | −0.19 | −2.5 | −0.19 | −3.1 | −0.23 | |
| 15–45° | −2.3 | −0.21 | −3.0 | −0.23 | −3.6 | −0.25 | |
| 30–60° | −2.7 | −0.27 | −3.4 | −0.28 | −4.2 | −0.29 | |
| 45–75° | −2.6 | −0.32 | −3.5 | −0.36 | −3.9 | −0.32 | |
| 60–90° | −1.8 | −0.28 | −2.7 | −0.36 | −3.3 | −0.35 | |
| 75–105° | −1.1 | −0.17 | −1.9 | −0.25 | −2.3 | −0.26 | |
| 90–120° | −0.7 | −0.12 | −1.3 | −0.18 | −1.4 | −0.17 | |
| 105–135° | −0.7 | −0.12 | −0.9 | −0.14 | −1.2 | −0.16 | |
| 120–150° | −1.1 | −0.19 | −1.3 | −0.2 | −1.7 | −0.22 | |
| mJSN 0 (n = 73) | Total MF | −0.5 | −0.12 | −0.9 | −0.17 | −1.1 | −0.19 |
| 0–30° | −0.8 | −0.1 | −1.2 | −0.13 | −1.7 | −0.17 | |
| 15–45° | −1.1 | −0.14 | −1.3 | −0.15 | −1.4 | −0.14 | |
| 30–60° | −1.4 | −0.2 | −1.6 | −0.18 | −1.8 | −0.17 | |
| 45–75° | −1.1 | −0.17 | −1.4 | −0.18 | −1.4 | −0.15 | |
| 60–90° | −0.1 | −0.02 | −0.5 | −0.08 | −0.5 | −0.08 | |
| 75–105° | 0.5 | 0.09 | 0.1 | 0.01 | 0.0 | 0.00 | |
| 90–120° | 0.2 | 0.05 | −0.2 | −0.04 | −0.3 | −0.05 | |
| 105–135° | −0.1 | −0.03 | −0.4 | −0.07 | −0.7 | −0.11 | |
| 120–150° | −0.4 | −0.08 | −0.7 | −0.11 | −0.8 | −0.13 | |
| mJSN 1 (n = 54) | Total MF | −1.5 | −0.21 | −2.2 | −0.26 | −2.8 | −0.31 |
| 0–30° | −1.1 | −0.08 | −1.3 | −0.09 | −2.1 | −0.14 | |
| 15–45° | −1.1 | −0.08 | −1.6 | −0.1 | −2.2 | −0.15 | |
| 30–60° | −2.1 | −0.17 | −3.2 | −0.21 | −3.7 | −0.25 | |
| 45–75° | −2.9 | −0.34 | −4.3 | −0.41 | −5.3 | −0.46 | |
| 60–90° | −2.1 | −0.36 | −3.5 | −0.5 | −4.8 | −0.52 | |
| 75–105° | −1.4 | −0.23 | −2.3 | −0.35 | −3.1 | −0.36 | |
| 90–120° | −0.8 | −0.12 | −1.3 | −0.17 | −1.4 | −0.16 | |
| 105–135° | −0.4 | −0.06 | −0.6 | −0.08 | −1.0 | −0.11 | |
| 120–150° | −0.9 | −0.14 | −1.1 | −0.16 | −1.7 | −0.20 | |
| mJSN 2 or 3 (n = 33) | Total MF | −5.1 | −0.85 | −6.4 | −0.86 | −7.1 | −0.64 |
| 0–30° | −8.0 | −0.63 | −9.5 | −0.57 | −10.9 | −0.48 | |
| 15–45° | −9.4 | −0.79 | −12.4 | −0.75 | −15.4 | −0.58 | |
| 30–60° | −8.6 | −0.74 | −11.7 | −0.74 | −15.9 | −0.54 | |
| 45–75° | −7.0 | −0.6 | −10.4 | −0.64 | −11.7 | −0.44 | |
| 60–90° | −6.0 | −0.63 | −8.7 | −0.7 | −9.8 | −0.59 | |
| 75–105° | −4.6 | −0.51 | −6.4 | −0.56 | −7.3 | −0.51 | |
| 90–120° | −2.8 | −0.4 | −3.7 | −0.46 | −3.8 | −0.38 | |
| 105–135° | −2.2 | −0.39 | −2.5 | −0.36 | −2.7 | −0.36 | |
| 120–150° | −2.8 | −0.43 | −3.0 | −0.42 | −3.4 | −0.40 | |
Results are given for the entire medial-lateral extension (100% ml ext.), the central 66.7%, and the central 33.3% of the MF, after trimming the medial and lateral edges for all knees (n = 160), knees without medial joint space narrowing (mJSN grade 0; n = 73), knees with mJSN grade 1 (n = 54), and mJSN grade 2 or 3 knees (n = 33).
FIG. 3.
Analysis of all knees (n = 160). Bar chart showing the average change (μm) in mean cartilage thickness (ThCtAB) over 1 year for the total MF condyle and for subregions of the MF located 0–150° posterior to the trochlear notch. Results are shown for the entire medial-lateral extension (100%) and for the central 66.7% and 33.3% of the MF, respectively.
Trimming the medial-lateral edges of the MF increased (P < 0.001; analysis of variance of repeated measures) the rate of change and SRM for the central 66.7% (−51 ± 154 μm; −2.2%; SRM −0.33) and the central 33.3% of the tAB (−42 ± 129 μm; −2.6%; SRM −0.33) compared to the total MF (see above). This principal finding applied to all nine anteroposterior subregions (Table 1; Fig. 3).
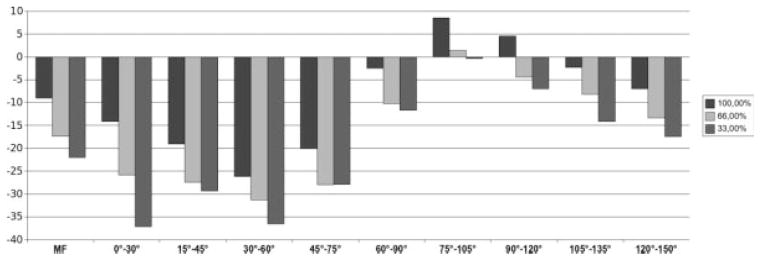
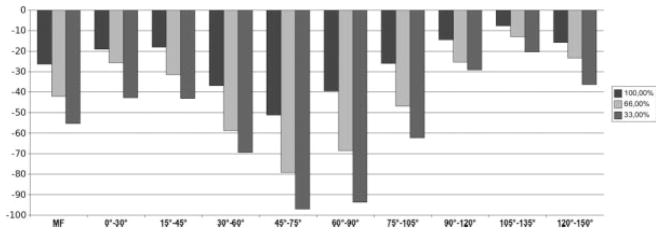
The rate of change and SRM for ThCtAB of the MF over 1 year increased with higher grades of mJSN (Figs. 4 to 6; Table 1). Also, some subtle variations in the spatial pattern of the change were observed between mJSN grades (Table 1): In mJSN grade 0 knees, the greatest rate of change was at 30–60° (Fig. 4); in mJSN grade 1 knees, at 45–75° (Fig. 5); and in mJSN grade 2 or 3 knees, at 15–45° (Fig. 6). The greatest SRM in mJSN grade 0 knees was at 30–60° (−0.20), in mJSN grade 1 knees, at 60–90° (−0.36); and in mJSN grade 2 or 3 knees, at 15–45° (−0.79). In mJSN grade 0 or 1 knees, the SRM in certain subregions exceeded that of the total MF, but this was not the case for higher mJSN grades.
FIG. 4.
Analysis of knees without medial joint space narrowing (mJSN grade 0; n = 73). Bar chart showing the average change (μm) in mean cartilage thickness (ThCtAB) over 1 year for the total MF condyle and for subregions of the MF located 0–150° posterior to the trochlear notch. Results are shown for the entire medial-lateral extension (100%) and for the central 66.7% and 33.3% of the MF, respectively.
FIG. 6.
Analysis of knees with medial joint space narrowing (mJSN grade 2 or 3; n = 33). Bar chart showing the average change (μm) in mean cartilage thickness (ThCtAB) over 1 year for the total MF condyle and for subregions of the MF located 0–150° posterior to the trochlear notch. Results are shown for the entire medial-lateral extension (100%) and for the central 66.7% and 33.3% of the MF, respectively.
FIG. 5.
Analysis of knees with medial joint space narrowing (mJSN grade 1; n = 54). Bar chart showing the average change (μm) in mean cartilage thickness (ThCtAB) over 1 year for the total MF condyle and for subregions of the MF located 0–150° posterior to the trochlear notch. Results are shown for the entire medial-lateral extension (100%) and for the central 66.7% and 33.3% of the MF, respectively.
Medial-lateral trimming resulted in greater rates of change, particularly in knees with mJSN grades 2 or 3 (e.g., −16% in the central 33.3% of the 30–60° subregion; Table 1). The SRM, however, was only consistently greater in trimmed than non-trimmed regions of the MF in mJSN grade 1 knees, but not in mJSN grade 0 or grade 2 or 3 knees. The greatest SRMs observed were −0.85 for the total MF without trimming and −0.86 for the central 66.7% of the MF in mJSN grade 2 or 3 knees.
DISCUSSION
The primary objective of this study was to develop an MRI-based technique for analyzing the spatial pattern of cartilage loss in the MF condyle based on anterior-posterior subregions, with and without medial-lateral trimming, and to determine changes in cartilage thickness over 1 year with 3-T MRI in participants of the OAI. Additionally we explored whether the spatial pattern of cartilage loss depended on radiographic OA status, namely, the grade of mJSN. We found that the rate of change varied significantly between anterior-posterior subregions of the MF, with greatest changes observed 30–60° posterior to the trochlear notch. The rate of change was greater in central aspects than over the entire medial-lateral extension of the MF after trimming the edges medially and laterally. Sensitivity to change was greatest in the 45–75° subregions but was only marginally affected by medial-lateral trimming.
The algorithm was designed to compute cartilage thickness (ThCtAB) in overlapping regions and to achieve sufficient spatial resolution to track regional patterns of cartilage loss in the MF while keeping the size of each subregion large enough to reduce noise in the data. Our results show that the algorithm provided here is capable of capturing significant differences in the rate of change between anterior-posterior subregions in OA knees with frequent knee pain and various degrees of radiographic involvement. Additionally, the algorithm was designed to allow for various degrees of medial-lateral trimming, to exclude peripheral areas of the MF, which are not orientated perpendicular to the image plane and in which therefore the bone interface and cartilage surface are not as clearly delineated due to partial volume effects. The results show that rates of change measured in the central areas of the MF were significantly higher than for the entire medial-lateral extension of the MF, although sensitivity to change (SRM) was minimally affected after excluding peripheral aspects of the MF. This must, however, be put into context of the acquisition protocol, which involved a 0.7mm sagittal DESS sequence with near-isotropic spatial resolution (18,20). Many other longitudinal OA studies relied on 1.5mm sagittal images (1,2) and, excluding the peripheral parts of the MF, may be more effective at lower spatial resolution because partial-volume effects increase with greater slice thickness. In contrast to a previously published algorithm (24,25), which focused on the weight-bearing region of the femur only, the current algorithm covers the entire femoral condyle in anterior-posterior direction. A previous report has separated the femur into the trochlea (femoral trochlea), as well as into anterior, central, and posterior aspects of the femoral condyles, but has not used a finer grid of ROIs or applied medial-lateral trimming (26).
A limitation of the current technique is that, in comparison with a landmark-based registration algorithms (i.e., Saha et al. (27)), the precise spatial comparability of the subregions between subjects is likely less between different subjects than when also taking into account several specific aspects of the individual shape of each femoral condyle. Another limitation of the study is that data used were originally collected to compare the rate and sensitivity of change in participants with mJSN in one knee and no JSN in the contralateral knee (28). Therefore, only the MF, but not the lateral femoral condyle, was examined. Application of the algorithm to the lateral femoral condyle, however, is straightforward, and its application to the MF in the current study allowed us to study the spatial pattern of cartilage loss across knees with well-defined grades of medial radiographic disease. As this study represents work in an exploratory cohort, however, the findings should be replicated in a larger validation cohort.
The results of this study support previous radiographic findings that JSN in OA is more severe when knees are flexed to 20–30° (6,8–11) since the posterior aspects of the weight-bearing region of the femoral condyle (30–75°) displayed greater rates of change than those located somewhat more anterior. One reason the posterior aspect of the MF located at >90° displayed smaller rates of change than the total MF may be that these areas are not involved in load-bearing during frequent activities, such as walking, but only during very deep knee bends, an activity likely avoided in people with knee OA. Another reason why the weight-bearing regions of the femur that are in contact with the tibia in 20–30° flexion display greater rates of change than the more anterior region of the condyles is that greatest femorotibial contact stresses occur with the knee in 24–28° flexion (3).
Previous papers using coronal imaging protocols have relied on a femoral ROI, defined as covering 60% of the slices between the trochlear notch and the most posterior aspect of the femoral condyle (18,19,25,29,30). To relate the current findings to these coronal ROIs, we applied this 60% criterion to all 160 knees investigated and found the posterior end of the 60% ROI to be located at 56.9 ± 3.7°. The coronal 60% ROI thus includes most of the femoral ROI with the greatest rate of change (30–60°) but does not entirely include the ROI with the greatest SRM (45–75°). Coronal images are limited since partial-volume effects are too high posterior (>60%) to support segmentation (18), but when segmenting the weight-bearing part of the MF condyle in sagittal images (18), the posterior cutoff value for the weight-bearing region can be set more posterior. When applying a 75% cutoff, for example, the posterior end of the femoral ROI is located at 73.5 ± 4.8° in the knees investigated here. This femoral ROI would thus include the regions with the highest SRM and the regions with the greatest rates of change across all mJSN grades. Our data indicate that there may be subtle differences in the spatial pattern of femoral cartilage loss between knees with different mJSN grade, potentially due to alterations in femorotibial biomechanics. However, this finding should be confirmed in future work since the current study was not powered to conclusively answer this question.
In conclusion, this study presents an MRI-based technique for analyzing the spatial pattern of cartilage loss in the femoral condyle (MF) based on anterior-posterior subregions, with and without medial-lateral trimming. When applying the algorithm to an exploratory cohort of 160 knees examined longitudinally over 1 year with 3-T MRI, we found the rate of change to vary significantly between anterior-posterior subregions of MF. The greatest changes were observed 30–60° posterior to the trochlear notch, and the greatest sensitivity to change, 45–75° posterior to the notch. When trimming the MF medially and laterally to analyze its central aspect, the rate of change was greater than for the total medial-lateral extension of the MF, but sensitivity to change was minimally affected. These results indicate that the highest sensitivity to change in cartilage thickness of the MF in OA may be achieved when focusing on an ROI covering the posterior aspect of the weight-bearing (central) region of the MF condyle.
Acknowledgments
We thank the following readers for dedicated data segmentation: Gudrun Goldmann, Linda Jakobi, Manuela Kunz, Dr. Susanne Maschek, Sabine Mühlsimer, Annette Thebis, and Dr. Barbara Wehr. We thank Martin Hudelmaier for performing the quality control readings at data entry and Susanne Maschek for the quality control readings of the segmentations. The image analysis of this study was funded by Eli Lilly & Co, IN. The images were acquired by the OAI, a public-private partnership composed of five contracts (N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261, N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners of the OAI include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation.
References
- 1.Eckstein F, Burstein D, Link TM. Quantitative MRI of cartilage and bone: degenerative changes in osteoarthritis. NMR Biomed. 2006;19:822–854. doi: 10.1002/nbm.1063. [DOI] [PubMed] [Google Scholar]
- 2.Eckstein F, Cicuttini F, Raynauld JP, Waterton JC, Peterfy C. Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): morphological assessment. Osteoarthritis Cartilage. 2006;14(suppl 1):46–75. doi: 10.1016/j.joca.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 3.Maquet P, Van De BA, Simonet J. The weight-bearing surfaces of the femoro-tibial joint. Acta Orthop Belg. 1976;42(suppl 1):139–143. [PubMed] [Google Scholar]
- 4.Brandt KD, Mazzuca SA, Conrozier T, Dacre JE, Peterfy CG, Provvedini D, Ravaud P, Taccoen A, Vignon E. Which is the best radiographic protocol for a clinical trial of a structure modifying drug in patients with knee osteoarthritis? J Rheumatol. 2002;29:1308–1320. [PubMed] [Google Scholar]
- 5.Guermazi A, Burstein D, Conaghan P, Eckstein F, Hellio Le Graverand-Gastineau MP, Keen H, Roemer FW. Imaging in osteoarthritis. Rheum Dis Clin North Am. 2008;34:645–687. doi: 10.1016/j.rdc.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Messieh SS, Fowler PJ, Munro T. Anteroposterior radiographs of the osteoarthritic knee. J Bone Joint Surg Br. 1990;72:639–640. doi: 10.1302/0301-620X.72B4.2380220. [DOI] [PubMed] [Google Scholar]
- 7.Brandt KD, Fife RS, Braunstein EM, Katz B. Radiographic grading of the severity of knee osteoarthritis: relation of the Kellgren and Lawrence grade to a grade based on joint space narrowing, and correlation with arthroscopic evidence of articular cartilage degeneration. Arthritis Rheum. 1991;34:1381–1386. doi: 10.1002/art.1780341106. [DOI] [PubMed] [Google Scholar]
- 8.Marklund T, Myrnerts R. Radiographic determination of cartilage height in the knee joint. Acta Orthop Scand. 1974;45:752–755. doi: 10.3109/17453677408989685. [DOI] [PubMed] [Google Scholar]
- 9.Railhac JJ, Fournie A, Gay R, Mansat M, Putois J. A radiologic study of the knee in an antero-posterior incidence with light flexion and standing up position. Its interest in the diagnosis of femoro-tibial osteoarthrosis (author’s transl) J Radiol. 1981;62:157–166. [PubMed] [Google Scholar]
- 10.Vignon E, Piperno M, Le Graverand MP, Mazzuca SA, Brandt KD, Mathieu P, Favret H, Vignon M, Merle-Vincent F, Conrozier T. Measurement of radiographic joint space width in the tibiofemoral compartment of the osteoarthritic knee: comparison of standing anteroposterior and Lyon schuss views. Arthritis Rheum. 2003;48:378–384. doi: 10.1002/art.10773. [DOI] [PubMed] [Google Scholar]
- 11.Merle-Vincent F, Vignon E, Brandt K, Piperno M, Coury-Lucas F, Conrozier T, Mathieu P, Hellio Le Graverand MP. Superiority of the Lyon schuss view over the standing anteroposterior view for detecting joint space narrowing, especially in the lateral tibiofemoral compartment, in early knee osteoarthritis. Ann Rheum Dis. 2007;66:747–753. doi: 10.1136/ard.2006.056481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams JG, McAlindon T, Dimasi M, Carey J, Eustace S. Contribution of meniscal extrusion and cartilage loss to joint space narrowing in osteoarthritis. Clin Radiol. 1999;54:502–506. doi: 10.1016/s0009-9260(99)90846-2. [DOI] [PubMed] [Google Scholar]
- 13.Gale DR, Chaisson CE, Totterman SM, Schwartz RK, Gale ME, Felson D. Meniscal subluxation: association with osteoarthritis and joint space narrowing. Osteoarthritis Cartilage. 1999;7:526–532. doi: 10.1053/joca.1999.0256. [DOI] [PubMed] [Google Scholar]
- 14.Hunter DJ, Zhang YQ, Tu X, LaValley M, Niu JB, Amin S, Guermazi A, Genant H, Gale D, Felson DT. Change in joint space width: hyaline articular cartilage loss or alteration in meniscus? Arthritis Rheum. 2006;54:2488–2495. doi: 10.1002/art.22016. [DOI] [PubMed] [Google Scholar]
- 15.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(suppl A):1–56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Eckstein F, Maschek S, Wirth W, Hudelmaier M, Hitzl W, Wyman B, Nevitt M, Le Graverand MP. One year change of knee cartilage morphology in the first release of participants from the Osteoarthritis Initiative progression subcohort: association with sex, body mass index, symptoms and radiographic osteoarthritis status. Ann Rheum Dis. 2009;68:674–679. doi: 10.1136/ard.2008.089904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter DJ, Niu J, Zhang Y, Totterman S, Tamez J, Dabrowski C, Davies R, Le Graverand MP, Luchi M, Tymofyeyev Y, Beals CR. Change in cartilage morphometry: a sample of the progression cohort of the Osteoarthritis Initiative. Ann Rheum Dis. 2009;68:349–356. doi: 10.1136/ard.2007.082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckstein F, Hudelmaier M, Wirth W, Kiefer B, Jackson R, Yu J, Eaton CB, Schneider E. Double echo steady state magnetic resonance imaging of knee articular cartilage at 3 tesla: a pilot study for the Osteoarthritis Initiative. Ann Rheum Dis. 2006;65:433–441. doi: 10.1136/ard.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckstein F, Kunz M, Hudelmaier M, Jackson R, Yu J, Eaton CB, Schneider E. Impact of coil design on the contrast-to-noise ratio, precision, and consistency of quantitative cartilage morphometry at 3 tesla: a pilot study for the osteoarthritis initiative. Magn Reson Med. 2007;57:448–454. doi: 10.1002/mrm.21146. [DOI] [PubMed] [Google Scholar]
- 20.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hohe J, Ateshian G, Reiser M, Englmeier KH, Eckstein F. Surface size, curvature analysis, and assessment of knee joint incongruity with MRI in vivo. Magn Reson Med. 2002;47:554–561. doi: 10.1002/mrm.10097. [DOI] [PubMed] [Google Scholar]
- 22.Ahn SJ, Rauh W, Warnecke H-J. Least squares orthogonal distances fitting of circle, sphere, ellipse, hyperbola and parabola. Pattern Recognition. 2001;34:2283–2303. [Google Scholar]
- 23.Eckstein F, Ateshian G, Burgkart R, Burstein D, Cicuttini F, Dardzinski B, Gray M, Link TM, Majumdar S, Mosher T, Peterfy C, Totterman S, Waterton J, Winalski CS, Felson D. Proposal for a nomenclature for magnetic resonance imaging based measures of articular cartilage in osteoarthritis. Osteoarthritis Cartilage. 2006;14:974–983. doi: 10.1016/j.joca.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging. 2008;27:737–744. doi: 10.1109/TMI.2007.907323. [DOI] [PubMed] [Google Scholar]
- 25.Wirth W, Hellio Le Graverand MP, Wyman BT, Maschek S, Hudelmaier M, Hitzl W, Nevitt M, Eckstein F. Regional analysis of femorotibial cartilage loss in a subsample from the Osteoarthritis Initiative progression subcohort. Osteoarthritis Cartilage. 2009;17:291–297. doi: 10.1016/j.joca.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelletier JP, Raynauld JP, Berthiaume MJ, Abram F, Choquette D, Haraoui B, Beary JF, Cline GA, Meyer JM, Martel-Pelletier J. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9:R74. doi: 10.1186/ar2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saha PK, Zhang H, Sonka M, Christensen GE, Rajapakse CS. Active index model: a unique approach for regional quantitative morphometry in longitudinal and cross-sectional studies. Proc SPIE. 2007;6512:65121B1–65121B12. [Google Scholar]
- 28.Eckstein F, Benichou O, Wirth W, Nelson DR, Maschek S, Hudelmaier M, Kwoh K, Guermazi A, Hunter D for the OAI Investigators. Magnetic resonance imaging based cartilage loss in painful contra-lateral knees with and without radiographic joint space narrowing: data from the Osteoarthritis Initiative (OAI) Arthritis Care Res. 2009 doi: 10.1002/art.24791. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hellio Le Graverand MP, Buck RJ, Wyman BT, Vignon E, Mazzuca SA, Brandt KD, Piperno M, Charles HC, Hudelmaier M, Hunter DJ, Jackson C, Kraus VB, Link TM, Majumdar S, Prasad PV, Schnitzer TJ, Vaz A, Wirth W, Eckstein F. Change in regional cartilage morphology and joint space width in osteoarthritis participants versus healthy controls: a multicenter study using 3.0 tesla MRI and Lyon schuss radiography. Ann Rheum Dis. 2008 Dec 22; doi: 10.1136/ard.2008.099762. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Eckstein F, Wirth W, Hudelmaier M, Stein V, Lengfelder V, Cahue S, Marshall M, Prasad P, Sharma L. Patterns of femorotibial cartilage loss in knees with neutral, varus, and valgus alignment. Arthritis Rheum. 2008;59:1563–1570. doi: 10.1002/art.24208. [DOI] [PubMed] [Google Scholar]