Abstract
Grape polyphenols confer potential health benefits, including prevention of neurodegenerative diseases. To determine the absorption and tissue distribution of the complex grape polyphenol mixture, 14C-labeled polyphenols were biosynthesized by grape cell suspension cultures, during co-incubation with radioisotopically labeled sucrose, and fractionated into polyphenolic subfractions. The pharmacokinetics and distribution of grape polyphenols into blood, brain, and peripheral interstitial fluid were determined by tracking the 14C label. The blood peak 14C concentration of the fractions ranged from 15 minutes to 4 hours. Absorption and tissue distribution varied greatly between fractions. Concentrations in interstitial fluid were lower than in blood. The amount of residual label in the brain at 24 hours ranged from 0.1% to 1.7% of the dose, depending on the fraction. 14C label found in the brain tissue and brain microdialysate indicated that grape polyphenols or their metabolites are able to cross the blood–brain barrier. Using 14C-labeled plant polyphenols it is possible to track the compounds or their metabolic products into any tissue and determine distribution patterns in spite of low concentrations. A central question regarding the potential role of dietary polyphenolics in neurodegenerative research is whether they are bioavailable in the brain. Our observations indicate that some grape-derived polyphenolics do reach the brain, which suggests their potential value for applications in neurodegenerative disorders.
Key Words: blood–brain barrier, brain kinetics, grape, grape cell culture, interstitial fluid kinetics, neurodegenerative diseases, pharmacokinetics, polyphenols, tissue distribution
Introduction
As health care improves and people live longer, neurodegenerative diseases emerge as a major factor in the degradation of quality of life of an individual and pose an increasing financial burden on society.1 Current pharmacological treatments only slow the progress of these diseases, but do not provide a cure. Therefore prevention of neurodegenerative diseases becomes an important approach to maintaining the health of older populations. Oxidative stress, which can cause protein damage and inflammatory processes, is a contributing factor to neurodegenerative diseases.2 Botanical supplements may afford neuroprotection by reduction in these oxidative stress and inflammatory processes. A number of studies have shown potential benefits of grape polyphenols in prevention or amelioration of Alzheimer's disease.3–5 Understanding the mechanisms of the neuroprotective effect of grape polyphenols and effectively using them to decrease the incidence of neurodegenerative diseases will require an understanding of the pharmacokinetics and tissue distribution of polyphenols in these extremely complex mixtures.
The study reported here used a number of technologies to determine the kinetics and tissue distribution of different grape polyphenolic fractions with different composition containing anthocyanins and/or proanthocyanidins ranging from monomers to hexamers. 14C-labeled grape polyphenols were produced in tissue culture and fractionated into 23 fractions. These were combined into five fractions of similar chemical composition and polarity. Rats were dosed orally with the labeled fractions, and blood concentrations were measured over a 24-hour period. Ultrafiltrate (UF) probes were used to collect subcutaneous interstitial fluid (ISF) samples. Microdialysis (MD) probes were used to determine kinetics of transfer of labeled materials into the brain. Accelerator mass spectrometry (AMS) was used to measure labeled polyphenols in samples when they became too dilute for measurement by conventional liquid scintillation counting techniques. Urine and feces were also collected to determine excretion of labeled compounds from the body. With these techniques we have been able to assess the absorption and track the distribution of various grape polyphenol fractions. Verification that some of these materials crossed the blood–brain barrier strengthens the rationale that these compounds may exert a direct protective effect on the parts of the brain involved in Alzheimer's disease pathology.
Materials and Methods
Preparation of 14C-labeled grape polyphenol fractions
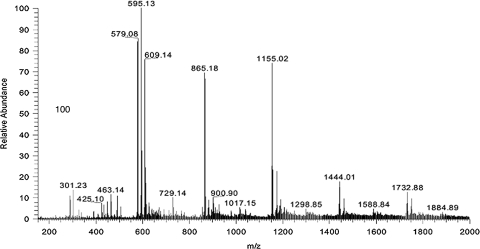
Cell suspension cultures of grape (Vitis hybrid) were produced and maintained as previously described.6–9 Cell cultures were placed in an enclosed polyacrylic labeling chamber, constructed to provide a safe containment space as labeled polyphenolic compounds were biosynthesized by the cells.8 Uniformly labeled [14C]sucrose ([U-14C]sucrose) with specific activity of 10 mCi/mmol (374 MBq/mmol) in a crystalline solid (MP Biomedicals Inc., Irvine, CA, USA) was used as the source of label delivered to the metabolizing cell cultures. An aliquot (5 mL) of 14C stock solution (85 μCi/mL) was added to the grape cell cultures (50 mL; final label concentration, 7 μCi/mL) according to procedures described by Yousef et al.7 Radiolabeled cultures were harvested, filtered, and extracted, and the resulting crude extract was fractionated on Toypearl (HW-40F; Tosoh Bioscience LLC, Montgomeryville, PA, USA) followed by silica gel (type 60, 10–40 μm, CaSO4 binder S-6503; Sigma Chemical Co., St. Louis, MO, USA).7 Twenty-three fractions of increasing polarity were obtained. Fractions with similar chemical composition were combined, and this resulted in five major fractions, labeled I–V (Table 1). Fractions I–V were used for animal testing, and the enrichment level (μCi of 14C per unit volume or mass) was determined. The combination of fractions was based on anthocyanin and/or proanthocyanidin content, to study their absorption and tissue distribution in the in vivo model. The first and second fractions (less polar) contained mainly proanthocyanindins, whereas later fractions were mainly anthocyanins (more polar). A high-performance liquid chromatography (HPLC)-mass spectrometry spectrum of fraction III showing a mixture of proanthocyanidins and anthocyanins is presented in Figure 1. The confirmation of compounds identified in each of the major fractions has been reported previously.7
Table 1.
Chemical Composition and 14C Activity of Labeled Grape Fractions
| Fraction | Composition | Enrichment (μCi/mL) | Percentage of enrichment |
|---|---|---|---|
| I | Monomeric proanthocyanidins, fatty acids, and triglycerides | 12 | 5% |
| II | Proanthocyanidin monomers (catechin/epicatechin), dimers to hexamers, traces of anthocyanins (cyanidin and peonidin glycosides) | 8 | 7% |
| III | Proanthocyanidin dimers and trimers (in smaller concentration compared to fraction II), higher concentrations of anthocyanin glycosides (cyanidin, peonidin, delphinidin) and piceid acid (the glucoside of trans-resveratrol) | 15 | 10% |
| IV | Anthocyanin 3-O-glycosides, particularly peonidins and cyanidin glycosides | 20 | 69% |
| V | Anthocyanin glycosides (mostly peonidins and cyanidins) | 11 | 9% |
Cell suspension cultures of grape (Vitis hybrid) were cultured with [14C]sucrose. Filtered and extracted cultures were fractionated on Toypearl followed by silica gel.
FIG. 1.
Electrospray ionization positive-ion mode mass spectrum of the major radiolabeled grape cell culture fraction III. Proanthocyanidins included monomers (m/z 289), dimers (m/z 579), trimers (m/z 865), tetramers (m/z 1,155), pentamers (m/z 1,444), and hexamers (m/z 1,732) based on catechin and/or epicatechin molecules. Anthocyanins included peonidin (m/z 301), cyanidin 3-O-p-coumarylglucoside (m/z 595), and delphinidin 3-O-cis-p-coumarylglucoside (m/z 609).
Study design
The goals of this study were to determine the absorption and distribution of the labeled fractions in blood, peripheral ISF, and brain. Rats were implanted with jugular catheters for blood sampling. Subcutaneous ISF was sampled with UF probes, and cerebral ISF was sampled with MD probes as described in detail elsewhere.10,11 The target for MD probes was chosen to be the hippocampus because changes in this region occur early in Alzheimer's disease.12,13 Concentrations of label in blood, subcutaneous ISF, and brain MD were profiled over 24 hours. At the end of the pharmacokinetic study the animals were sacrificed, and the brains were harvested for residual 14C content and radiographic film analysis.
Animals
All animal studies were conducted in accordance with federal guidelines and were approved by the Purdue Animal Care and Use Committee (Purdue University, West Lafayette, IN, USA). Thirteen male Sprague-Dawley rats (weighing ∼250 g, 56–61 days old) were obtained from Harlan (Indianapolis, IN, USA). They were maintained on AIN-93M rat chow and acclimated for 3–5 days before surgery. The number of animals per group was determined by the amount of material in the fraction.
Determination of probe recoveries
The brain MD probes and the UF probes are constructed with polyacrylonitrile membranes with a 30,000 dalton molecular size cutoff. In vitro probe recovery studies were done for UF-6-12 probes (catalog number MF-7023; Bioanalytical Systems, Inc., West Lafayette) and MD probes as described elsewhere.14 For MD a 4-mm membrane probe (catalog number MD-2204; Bioanalytical Systems) and a 1 μL/minute flow rate of artificial cerebrospinal fluid were used. It was not possible to perform in vivo MD probe recoveries because the fractions were not completely soluble in artificial cerebrospinal fluid.
Surgical implantation of catheters and probes
Rats were implanted with jugular catheters and subcutaneous UF-3-12 probes (Bioanalytical Systems) under sterile conditions as described previously15 to sample blood and ISF, respectively. Anesthesia was induced with isoflurane (3–5%) in an anesthesia chamber and maintained with a mask (1.5–3% isoflurane). When MD probe guides were implanted, anesthesia was induced with a 10:1 mixture of ketamine (100 mg/mL) and xylazine (100 mg/mL) at a dose of 0.1 mL/100 g. Animals were placed in a stereotaxic frame, and a guide cannula (Bioanalytical Systems) for the MD probe was implanted in the hippocampus. Buprenorphine (0.03 mg/kg) was administered after all surgical procedures to alleviate pain.
After surgery, the rats were placed in a Culex® automated blood sampling and caging system (Bioanalytical Systems). The catheters were automatically flushed with heparinized saline to maintain patency. The UF probes were connected to a small peristaltic pump (model P720; Instech Laboratories, Plymouth Meeting, PA, USA). The pump was run continuously to maintain probe patency. The rats were allowed to recover from surgery for 2 days before the pharmacokinetic and distributions studies were done.
Pharmacokinetic and distribution studies
Rats were fasted for 8 hours. Stylets were removed from guide cannulas and replaced with a 4-mm brain MD probes (Bioanalytical Systems). The probe was perfused with artificial cerebrospinal fluid at the rate of 1 μL/minute. Baseline serum and subcutaneous ISF and brain microdialysate samples were collected. Rats were dosed with 0.5 mL of one of the grape polyphenol fractions—I (n = 2), II (n = 2), III (n = 3), IV (n = 4), or V (n = 2)—by gavage. Because the different fractions had different 14C enrichment levels, the doses varied from 4 to 10 μCi. The Culex automated blood sampling system was programmed to collect blood samples over a 24-hour period. The samples were stored in a refrigerated fraction collector, and the blood was automatically replaced with saline by the Culex. Hourly samples of ISF were collected in a second refrigerated fraction collector. Urine and feces were also collected to determine what percentage of the dose was lost during the first 24 hours. After 24 hours the rats were killed by CO2 overdose. The vascular system was flushed with cold saline to remove blood from organs. The skull was opened, and the brain was removed, placed in a coronal brain matrix (Roboz Surgical, Gaithersburg, MD, USA), and frozen at –80°C. After the brain was frozen it was sliced into 1-mm slices using the brain matrix as a guide. Each slice was cut in half, and one-half was analyzed for 14C label. The other half was reserved for analysis on radiographic film to determine if there were areas of concentration of label.
Sample preparation and analysis
Serum was separated from blood. No preparation was needed for the ISF samples. One hundred microliters of recovery solutions, serum, ISF, and urine was placed in scintillation vials with 15 mL of EcoLite™ scintillation fluid (MP Biomedicals) and counted in a β-scintillation counter. Brain slices were analyzed by two methods depending on the fraction. Brain slices from rats dosed with grape fractions I, II, and III, which had lower uptake into the brain and had 14C levels too low to be analyzed by β-scintillation counter, were sent to the Purdue Rare Isotope Measurement (PRIME) Laboratory for analysis by AMS. Brain slices from rats dosed with fractions IV and V, which had too much radioactivity for AMS analysis, were homogenized in 2 mL of cold phosphate-buffered saline. One milliliter of the homogenate was placed in scintillation vials with 15 mL of scintillation fluid and counted in the β-scintillation counter.
Brain MD 14C levels were too low to be counted in a β-scintillation counter, and these samples were therefore analyzed by AMS in the PRIME Laboratory. Samples to be measured by AMS were dried by vacuum centrifugation. The samples were then combusted to graphite, which was placed in the ion source of the AMS to determine 14C.
The feces were ground and then homogenized with normal saline. One hundred microliters of the homogenate was placed in scintillation vials with 15 mL of scintillation fluid and counted in the β-scintillation counter. Because different fractions had different enrichment levels, all results were expressed as a percentage of the dose.
Results
Grape fractions
The polyphenol content and enrichment of the five grape fractions is given in Table 1. The chemical compositions of these fractions were previously described.7 In brief, the early silica gel fractions (highly nonpolar fractions; II and III) were rich in proanthocyanidins (monomers m/z 289; dimers m/z 578; trimers m/z 867; tetramers m/z 1,155; pentamers m/z 1,444; and hexamers m/z 1,732) based on catechin and/or epicatechin molecules. Later fractions (more polar fractions; IV and V) were composed of a variety of anthocyanins, including cyanidin (m/z 287), peonidin (m/z 301), cyanidin 3-O-glucoside (m/z 449), peonidin 3-O-glucoside (m/z 463), delphinidin 3-O-cis-p-coumarylglucoside (m/z 610), cyanidin 3-O-p-coumarylglucoside (m/z 595, major component), and petunidin 3-O-cis-p-coumarylglucoside (m/z 625), and, in addition, stilbenes such as resveratrol (m/z 391) were also detected in the later fractions. Fractions varied in volume and enrichment levels (Table 1). Fraction IV, rich in anthocyanin 3-O-glycosides, contained 56% of the volume and 69% of the counts.
Plasma kinetics
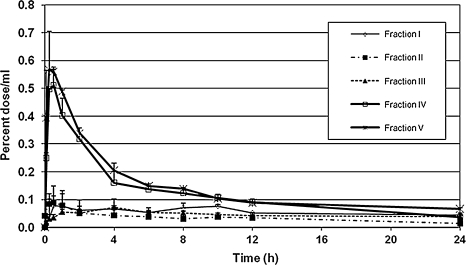
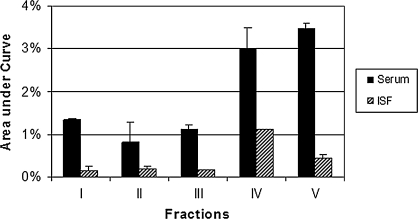
The pharmacokinetics of the different fractions in the serum are illustrated in Figure 2. All concentrations are expressed as a percentage of the dose to account for different enrichment levels of the different fractions. The time to maximum concentration (Tmax) of the different fractions ranged from 15 minutes for fraction V to 4 hours for fraction III. The other fractions reached peak concentrations at approximately 30 minutes. There was a 7.8-fold difference in the maximum concentration (Cmax) between the best and most poorly absorbed fractions. The greatest absorption of label occurred in fraction V, the fraction of highest polarity, which contained a high proportion of anthocyanin glycosides (mostly peonidins and cyanidins). This fraction also had the earliest Tmax. The second highest Cmax occurred in fraction IV, which also contained anthocyanin glycosides. The fractions with the highest Cmax also had the highest elimination rate. The poorly absorbed fractions were eliminated more slowly than the better absorbed fractions. The area under the serum time × concentration curve (AUC) for the fractions (Fig. 3) ranged from 0.08% to 0.35% of dose/mL of serum. The greatest absorption occurred in the anthocyanin glycoside fraction V, and the lowest absorption occurred in the proanthocyanidin monomers to hexamers of fraction II.
FIG. 2.
Serum concentrations (+SEM) of labeled fractions after oral dosing expressed as percentage of dose/mL. There was a 7.8-fold difference in the Cmax between the best and most poorly absorbed fractions. Fractions containing anthocyanin glycosides were better absorbed than proanthocyanidin fractions.
FIG. 3.
AUC (+SEM) of serum and ISF: percentage of dose/mL over a 24-hour period.
Probe recoveries
UF probe recoveries for the fractions were as follows: 98.4% (I), 84.9% (II), 86.1% (III), 92.8% (IV), and 92.5% (V). MD probe recoveries were as follows: 4.5% (I), 7.2% (II), 11.0% (III), 11.2% (IV), and 6.3% (V).
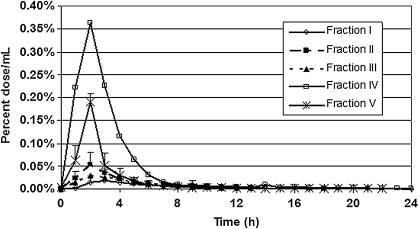
ISF
ISF pharmacokinetics are illustrated in Figure 4. With the exception of fraction I, Tmax occurred later than serum concentrations (2–3 hours). The highest Cmax was achieved by fraction IV with the anthocyanin 3-O-glycosides. The second highest Cmax was achieved by fraction V with the anthocyanin glycosides. Concentrations of the remaining fractions were considerably lower. The relationship between serum and subcutaneous ISF concentrations differed for different fractions. The AUC for the ISF represents the amount of 14C material in the subcutaneous ISF. The AUC for the ISF in all fractions was less than the AUC for the serum (Fig. 3). The highest AUC was achieved by fraction IV with the anthocyanin 3-O-glycosides.
FIG. 4.
Subcutaneous ISF concentrations of labeled fractions for the 24 hours post-dose. The Tmax in ISF occurred later than in serum in four of the five fractions as can be seen by comparison to Figure 1. These peaks occurred between 2 and 3 hours post-dose.
The blood volume of the rat represents 7% of its body weight,16 and the blood plus ISF represent 20% of the body weight.17 Based on these calculations the total percentage dose in the plasma and ISF at Cmax for the five fractions was 2.5 ± 1.4% (I), 2.5 ± 0.10% (II), 1.61 ± 0.12% (III), 15.4 ± 1.58% (IV), and 13.9 ± 1.5% (V).
Brain
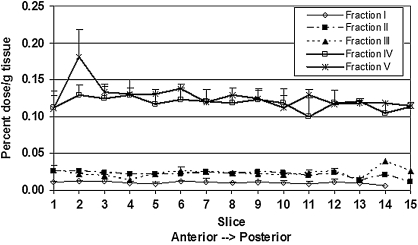
For each fraction the brain slices did not show any regional differences in 14C label accumulation from anterior to posterior slices of the brain (Fig. 5). Radiographic studies of brain slices also showed no areas of increased accumulation. There was a difference in accumulation among the fractions. At 24 hours post-dose, fractions I, II, and III had lower rates of accumulation, which ranged from 0.12% to 0.32% of dose/g of brain (Table 2). Accumulation of label from the fractions of the anthocyanin glycosides, fractions IV and V, was five to 14 times higher than that of fractions I–III. The label remaining in the brain at 24 hours expressed as a fraction of the plasma AUC ranged from 0.127 for monomeric proanthocyanidin fraction (I) to 0.681 for the anthocyanin-3-O-glycoside fraction (V).
FIG. 5.
Average percentage of dose/g (+SEM) in 1-mm brain slices from rats dosed with different fractions of 14C-labeled grape. Fractions I–III with lower plasma and ISF levels also showed lower levels in brain.
Table 2.
Relationship Between Brain and Peripheral 14C for Different Fractions
| Fraction | Brain residual counts (% dose/g)a | Brain residual/serum AUCb | Brain MD AUC/serum AUC |
|---|---|---|---|
| I | 0.123 ± 0.044% | 0.127 ± 0.052 | — |
| II | 0.316 ± 0.047% | 0.447 ± 0.092 | 2.07% |
| III | 0.328 ± 0.056% | 0.307 ± 0.038 | 2.54% |
| IV | 1.741 ± 0.034% | 0.681 ± 0.099 | 2.03% |
| V | 1.712 ± 0.015% | 0.347 ± 0.123 | 2.89% |
Data are mean ± SE values.
Residual 14C in brain 24 hours post-dose of labeled grape fraction.
Ratio of residual 14C in brain 24 hours post-dose to serum AUC.
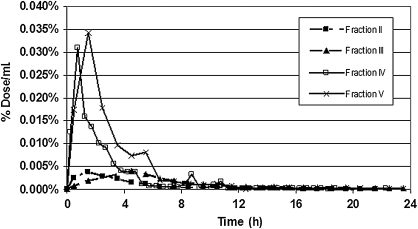
The MD data, illustrated in Figure 6, provide the pharmacokinetic relationships of the different fractions in the brain. Cmax occurred between at 0.75 and 1.5 hours for fractions II, IV, and IV and at 4.5 hours for fraction III. No data were obtained for fraction I because of equipment malfunction. The highest concentrations in brain microdialysate were achieved by fractions IV and V. When AUC values for brain MD were expressed as a percentage of the serum AUC, the results were similar for all fractions (Table 2).
FIG. 6.
Concentration in percentage of dose/mL of 14C label in brain microdialysate obtained from probes placed in the hippocampus.
Urine, feces, and small intestine
The percentage of the dose lost in urine and feces and the percentage dose remaining in the small intestine tissue are shown in Table 3. Urinary losses ranged from 3% to 43%. Fecal losses ranged from 2.7% to 19.4%. From 0.8% to 2.3% of the dose remained in the small intestine tissue.
Table 3.
Percentage of Dose Excreted in Urine and Feces over a 24-Hour Period and Percentage of Dose Remaining in the Small Intestine 24 Hours Post-Dose
| Fraction | Urine | Feces | Small intestine |
|---|---|---|---|
| I | 27 ± 2 | 3.0 ± 2.9 | 2.314 ± 0.002 |
| II | 3 ± 2 | 19.4 ± 6.7 | 0.822 ± 0.067 |
| III | 40 ± 32 | 2.7 ± 1.2 | 1.585 ± 0.016 |
| IV | 16 | 3.4 ± 1.3 | 1.313 ± 0.069 |
| V | 43 ± 12 | 4.8 ± 0.7 | 1.905 ± 0.008 |
Data are mean ± SE values.
Discussion
Polyphenol constituents of grapes and red wine have been purported to have many health benefits, including reduction in the incidence of heart disease and Alzheimer's disease.4,18–20 If these natural products and supplements enriched in them can be demonstrated to be beneficial, they may be a useful intervention to reduce the incidence of these diseases. The advantage of using food-derived supplements is that there may be fewer detrimental side effects. Alternatively, if the active components in the complex mixtures can be identified, it may be possible to isolate and concentrate them to increase the potency and enhance the protective effect. However, unlike drugs, which usually consist of a single active ingredient, botanicals are complex mixtures. The activity may be derived from a single compound, or it may be due to the synergistic action of multiple compounds, which may be required in specific ratios to achieve optimal efficacy. This synergistic effect of multiple components of a botanical was demonstrated by Morré et al.21 in their investigations of the effect of green tea catechins on cancer. Epigallocatechin gallate (10−5 M) is effective in inhibiting the growth of HeLa cells in culture. Epicatechin (10−4 M) alone has no inhibitory power but when added to epigallocatechin gallate increases the latter's inhibitory power 100-fold.
One of the problems in studying complex mixtures found in botanicals is measuring the absorption, distribution, and elimination of the different components. Bioavailability of different polyphenols varies widely.22 In some cases beneficial effects have been demonstrated even though the polyphenol was not found in the target tissue. Feeding resveratrol reduced the amyloid plaque formation associated with neurodegenerative diseases in mouse brains even though no resveratrol or metabolites could be detected in the brain using HPLC detection methods.23
14C-labeled compounds have long been used to investigate bioavailability of drugs24,25 and nutrients. With the introduction of the AMS technology into the tracking of 14C labels it is possible both to use smaller doses and to track compounds with low bioavailabilities, when chemical methods may not be sensitive enough. This gives AMS great potential in the investigation of the bioavailability of phytochemicals.26 AMS has been used to track β-[14C]carotene27 and [14C]lutein28 long-term kinetic studies in human volunteers. The AMS technique can be especially useful for investigating the permeability of the blood–brain barrier where the concentrations can be very low. Diisopropyl fluorophosphate is an organophosphate insecticide. Vogel and co-workers used [14C]diisopropyl fluorophosphate to investigate the effect of repeated low doses on the permeability of the blood–brain barrier.29,30
For the investigation of single compounds the 14C-labeled material can sometimes be synthesized chemically. When investigating the bioavailability of the complex mixtures found in plants chemical synthesis is not possible. However, methods have been developed to grow plant cell culture material with 14C-labeled precursors and fractionate the complex polyphenol-rich mixtures.6–8 By using these labeled fractions it is possible to track the absorption and distribution of the polyphenols and/or their metabolites. In some cases it is possible to track compounds in the brain by traditional methods. Prasain et al.31 found catechin levels of 53.16 ng/g of wet tissue in brain extracts. However, when the concentrations become too low to be measured by HPLC, the distribution into the brain can still be followed with 14C-labeled materials with the extremely sensitive AMS technique.
There is a considerable difference in the degree of absorption of the different grape fractions. Fractions IV and V, consisting mainly of anthocyanin glycosides, mostly peonidins and cyanidins, were better absorbed than the less polar fractions, consisting of proanthocyanidins ranging from monomers to hexamers (Fig. 2). The AUC for these anthocyanin fractions was about twice that of the less polar fractions (Fig. 3). The low absorption of some fractions may be partly explained by the instability of the compounds during the digestive process. Green et al.32 demonstrated using an in vitro model that 80% of catechins are destroyed during the process of digestion. Studies of anthocyanin pharmacokinetics in humans33 from either wine or grape juice showed similar Tmax (0.5–1.5 hours) to fractions IV and V but had a lower serum percentage dose/mL at Cmax. The probable explanation for this is that other components besides anthocyanins in fractions IV and V contributed to the increased amount of label in the serum at Cmax. At 24 hours measurable amounts of all fractions were still found in the serum. Therefore it is possible that with repeated dosing, as would be the case with a dietary supplement, higher serum levels may be achieved.
Because the fractions in this study contained multiple compounds, the pharmacokinetic curves represent the cumulative concentration of all of the compounds in the fraction. There may be a considerable amount of variation in the absorption of the individual components. Some components of the fraction may be much better absorbed than others.
Unless the target tissue for the physiological or pharmacological effect is the blood or vascular system, the compounds must leave the blood and pass into the ISF in order to enter other target tissues. The two anthocyanin glycoside fractions (IV and V) had greater ISF concentrations than the other three fractions. However, the ISF concentrations were not directly correlated with serum concentrations. Fraction IV had a lower serum AUC than fraction V. However, fraction IV had an ISF AUC that was larger than fraction V's by more than a factor of 2. This illustrates that serum concentrations may not always be indicative of the concentrations that reach the target tissue. A similar anomaly between serum and ISF concentrations was seen in the less well-absorbed fractions (I, II, and III). Of these, the fraction with the lowest serum AUC, fraction II, had the highest ISF AUC. Target tissue concentrations may not be predictable from serum concentrations alone.
For compounds to move from the blood to the brain they must cross the blood–brain barrier, which presents a considerable impediment to designing treatments for diseases involving the brain. Measuring the concentration of compounds that do reach the brain also presents challenges because the small amounts that enter the brain may be beyond the limit of detection of standard analytical methods. If MD is used for kinetic studies the low recoveries of MD probes further reduces the concentrations. However, substances present at concentrations of femtomolar or less, which may be beyond the limit of detection by chemical analysis, may still be clinically beneficial. Grape polyphenols are difficult to detect by standard analytical techniques at these concentrations. Use of labeled compounds provides a significant advantage in tracking compounds into the brain because the ultrasensitivity of AMS can be used when concentrations are too low for other methods. All fractions showed some 14C activity in brain tissue. For each fraction the extent of 14C activity from slice to slice and within individual slices was uniform, indicating that there were no areas of specific accumulation. This was supported by the radiographic analysis of brain slices, which also showed no areas of increased accumulation. The total accumulation of label from the different fractions was different (Table 2). However, when the AUC for brain MD was compared to the AUC for serum the percentage of material in the brain was 2–3% of the material in the serum for fractions II–V, indicating that the potential for materials from these fractions for crossing the blood–brain barrier was about equal and that the limiting factor to bioavailability for the brain was absorption.
Urinary and fecal losses were also followed in this study. There was a large variation in the urinary and fecal losses between fractions. The urinary losses ranged from 3% to 43%. There was a great deal of variability in the urinary losses among rats receiving the same fractions. With some fractions, especially fraction II, there was very little urine production, resulting in low urinary losses. Urinary losses represent not only the original compounds but also all metabolites. In human studies of urinary losses of anthocyanins following orally dosed red wine or red grape juice, Frank et al.34 found 0.18–0.23% in the first 7 hours, and Lapidot et al.35 found 1.5–5.1% urinary anthocyanin losses in 12 hours after consumption of red wine. Fecal 14C content for most fractions was low, in the range of 3–5% of the starting material; only fraction II was higher at 19%. It was expected that the fecal losses would be greater in the fractions that were not as well absorbed. There are several possibilities for the low amounts of label found in the fecal material. The microflora in the intestine can utilize the 14C-containing materials as carbon sources and turn the 14C into 14CO2, which can escape as a gas. A small percentage (0.8–2.3%) of the label was absorbed by the intestinal enterocytes and remained in the small intestine (Table 3).
A central question regarding the potential neuroprotective actions of dietary polyphenolics is whether they cross the blood–brain barrier. We found differences in the amounts of compounds or metabolites from different fractions in the brain. In particular, we demonstrated that small-molecular-sized, polar polyphenols, such as anthocyanin 3-O-glycosides and cyanidin glycosides (fraction IV), as well as anthocyanin glucosides such as peonidins and cyanidins (fraction V) or metabolites of these, accumulate in brain tissue. In contrast, dietary proanthocyanidins (fractions I–III) and their metabolites were found in very low levels in brain tissue. Collectively, our evidence suggests that research on developing dietary polyphenols for applications in neurodegenerative disorders should prioritize investigations of smaller polar polyphenols for brain bioavailability and bioactivity. In addition, because there is some overlap in constituent classes between fractions III and IV, but a greater difference in absorption between these fractions, determination of the compounds present in fraction IV but absent in fraction III may indicate which compounds to investigate for brain bioavailability and bioactivity. Future studies will involve separation techniques to determine bioavailability and tissue distribution of the original compounds and metabolites.
Acknowledgments
The authors thank Pamela Lachcik, Jane Einstein, and Tom Kubley for technical assistance. This project was supported by Purdue University-University of Alabama at Birmingham Botanical Center for Age Related Diseases funded by the Office of Dietary Supplements and NCCAM grant P50 AT 00477.
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Alzheimer's Association. Alzheimer's Facts and Figures. 2009. www.alz.org/national/documents/report_alzfactsfigures2009.pdf. [Feb;2010 ]. www.alz.org/national/documents/report_alzfactsfigures2009.pdf
- 2.Thome J. Gsell W. Rösler M, et al. Oxidative-stress associated parameters (lactoferrin, superoxide dismutases) in serum of patients with Alzheimer's disease. Life Sci. 1997;60:13–19. doi: 10.1016/s0024-3205(96)00583-8. [DOI] [PubMed] [Google Scholar]
- 3.Deshane J. Chaves L. Sarikonda KV, et al. Proteomics analysis of rat brain protein modulations by grape seed extract. J Agric Food Chem. 2004;52:7872–7883. doi: 10.1021/jf040407d. [DOI] [PubMed] [Google Scholar]
- 4.Wang J. Ho L. Zhao W, et al. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer's disease. J Neurosci. 2008;28:6388–6392. doi: 10.1523/JNEUROSCI.0364-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho L. Chen LH. Wang J, et al. Heterogeneity in red wine polyphenolic contents differentially influences Alzheimer's disease-type neuropathology and cognitive deterioration. J Alzheimers Dis. 2009;16:59–72. doi: 10.3233/JAD-2009-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lila MA. Yousef GG. Jiang Y. Weaver CM. Sorting out bioactivity in flavonoid mixtures. J Nutr. 2005;135:1231–1235. doi: 10.1093/jn/135.5.1231. [DOI] [PubMed] [Google Scholar]
- 7.Yousef GG. Seigler DS. Grusak MA, et al. Biosynthesis and characterization of 14C-enriched flavonoid fractions from plant cell suspension cultures. J Agric Food Chem. 2004;52:1138–1145. doi: 10.1021/jf035371o. [DOI] [PubMed] [Google Scholar]
- 8.Grusak MA. Rogers RB. Yousef GG. Erdman JWJ. Lila MA. An enclosed-chamber labeling system for the safe 14C-enrichment of phytochemicals in plant cell suspension cultures. In Vitro Cell Dev Biol Plant. 2004;40:80–85. [Google Scholar]
- 9.Hirasuna TJ. Schuler ML. Lackney VK. Spanswick RM. Enhanced-anthocyanin production in grape cell cultures. Plant Sci. 1991;78:120–122. [Google Scholar]
- 10.Janle EM. Kissinger PT. Microdialysis and ultrafiltration. In: Coburn SP, editor; Townsend DW, editor. Advances in Food and Nutrition Research. Vol. 40. Academic Press; San Diego: 1996. pp. 183–196. [DOI] [PubMed] [Google Scholar]
- 11.Leegsma-Vogt G. Janle E. Ash SR. Venema K. Korf J. Utilization of in vivo ultrafiltration in biomedical research and clinical applications. Life Sci. 2003;73:2005–2018. doi: 10.1016/s0024-3205(03)00569-1. [DOI] [PubMed] [Google Scholar]
- 12.Wang L. Swank JS. Glick IE, et al. Changes in hippocampal volume and shape across time distinguish dementia of the Alzheimer type from healthy aging. Neuroimage. 2003;20:667–682. doi: 10.1016/S1053-8119(03)00361-6. [DOI] [PubMed] [Google Scholar]
- 13.Dixon RM. Bradley KM. Budge MM. Styles P. Smith AD. Longitudinal quantitative proton magnetic resonance spectroscopy of the hippocampus in Alzheimer's disease. Brain. 2002;125:2332–2341. doi: 10.1093/brain/awf226. [DOI] [PubMed] [Google Scholar]
- 14.Janle EM. Cregor M. Ultrafiltrate and microdialysis DL probe in vitro recoveries: electrolytes and metabolites. Curr Sep. 1996;15:31–34. [PubMed] [Google Scholar]
- 15.Janle EM. Portocarrero C. Zhu Y. Zhou Q. Effect of long-term oral administration of green tea extract on weight gain and glucose tolerance in Zucker diabetic (ZDF) rats. J Herbal Pharmacother. 2005;5:55–64. [PubMed] [Google Scholar]
- 16.Wang CF. Hegsted DM. Normal blood volume, plasma volume and thiocyanate space in rats and their relation to body weight. Am J Physiol. 1949;156:218–226. doi: 10.1152/ajplegacy.1949.156.2.218. [DOI] [PubMed] [Google Scholar]
- 17.Hanes DA. Weaver CM. Wastney ME. Calcium and oxalic acid kinetics differ in rats. J Nutr. 1999;129:165–169. doi: 10.1093/jn/129.1.165. [DOI] [PubMed] [Google Scholar]
- 18.Orgogozo JM. Dartigues JF. Lafont S, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris) 1997;153:185–192. [PubMed] [Google Scholar]
- 19.Russo A. Palumbo M. Aliano C, et al. Red wine micronutrients as protective agents in Alzheimer-like induced insult. Life Sci. 2003;72:2369–2379. doi: 10.1016/s0024-3205(03)00123-1. [DOI] [PubMed] [Google Scholar]
- 20.Balu M. Sangeetha P. Murali G. Panneerselvam C. Age-related oxidative protein damages in central nervous system of rats: modulatory role of grape seed extract. Int J Dev Neurosci. 2005;23:501–507. doi: 10.1016/j.ijdevneu.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Morré DJ. Morré DM. Sun H, et al. Tea catechin synergies in inhibition of cancer cell proliferation and of a cancer specific cell surface oxidase (ECTO-NOX) Pharmacol Toxicol. 2003;92:234–241. doi: 10.1034/j.1600-0773.2003.920506.x. [DOI] [PubMed] [Google Scholar]
- 22.Singh M. Arseneault M. Sanderson T. Murthy V. Ramassamy C. Challenges for research on polyphenols from foods in Alzheimer's disease: bioavailability, metabolism, and cellular and molecular mechanisms. J Agric Food Chem. 2008;56:4855–4873. doi: 10.1021/jf0735073. [DOI] [PubMed] [Google Scholar]
- 23.Karuppagounder SS. Pinto JT. Xu H, et al. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer's disease. Neurochem Int. 2009;54:111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cotler S. Bugge CJ. Colburn WA. Role of gut contents, intestinal wall, and liver on the first pass metabolism and absolute bioavailability of isotretinoin in the dog. Drug Metab Dispos. 1983;11:458–462. [PubMed] [Google Scholar]
- 25.Cundy KC. Sueoka C. Lynch GR, et al. Pharmacokinetics and bioavailability of the anti-human immunodeficiency virus nucleotide analog 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs. Antimicrob Agents Chemother. 1998;42:687–690. doi: 10.1128/aac.42.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Vuong T. Buchholz BA. Lame MW. Dueker SR. Phytochemical research using accelerator mass spectrometry. Nutr Rev. 2004;62:375–388. doi: 10.1111/j.1753-4887.2004.tb00008.x. [DOI] [PubMed] [Google Scholar]
- 27.Dueker SR. Lin Y. Buchholz BA, et al. Long-term kinetic study of beta-carotene, using accelerator mass spectrometry in an adult volunteer. J Lipid Res. 2000;41:1790–1800. [PubMed] [Google Scholar]
- 28.de Moura FF. Ho CC. Getachew G. Hickenbottom S. Clifford AJ. Kinetics of 14C distribution after tracer dose of 14C-lutein in an adult woman. Lipids. 2005;40:1069–1073. doi: 10.1007/s11745-005-1471-4. [DOI] [PubMed] [Google Scholar]
- 29.Palmblad M. Buchholz BA. Hillegonds DJ. Vogel JS. Neuroscience and accelerator mass spectrometry. J Mass Spectrom. 2005;40:154–159. doi: 10.1002/jms.734. [DOI] [PubMed] [Google Scholar]
- 30.Vogel JS. Keating GA., 2nd Buchholz BA. Protein binding of isofluorophate in vivo after coexposure to multiple chemicals. Environ Health Perspect. 2002;110(Suppl 6):1031–1036. doi: 10.1289/ehp.02110s61031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prasain JK. Peng N. Dai Y, et al. Liquid chromatography tandem mass spectrometry identification of proanthocyanidins in rat plasma after oral administration of grape seed extract. Phytomedicine. 2009;16:233–243. doi: 10.1016/j.phymed.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green RJ. Murphy AS. Schulz B. Watkins BA. Ferruzzi MG. Common tea formulations modulate in vitro digestive recovery of green tea catechins. Mol Nutr Food Res. 2007;51:1152–1162. doi: 10.1002/mnfr.200700086. [DOI] [PubMed] [Google Scholar]
- 33.Bitsch R. Netzel M. Frank T. Strass G. Bitsch I. Bioavailability and biokinetics of anthocyanins from red grape juice and red wine. J Biomed Biotechnol. 2004;2004:293–298. doi: 10.1155/S1110724304403106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frank T. Netzel M. Strass G. Bitsch R. Bitsch I. Bioavailability of anthocyanidin-3-glucosides following consumption of red wine and red grape juice. Can J Physiol Pharmacol. 2003;81:423–435. doi: 10.1139/y03-038. [DOI] [PubMed] [Google Scholar]
- 35.Lapidot T. Harel S. Granit R. Kanner J. Bioavailability of red wine anthocyanins as detected in human urine. J Agric Food Chem. 1998;46:4297–4302. [Google Scholar]