Abstract
Recent studies have shown that deoxygenated human red blood cells (RBCs) converted garlic-derived polysulfides into hydrogen sulfide, which in turn produced vasorelaxation in aortic ring preparations. The vasoactivity was proposed to occur via glucose- and thiol-dependent acellular reactions. In the present study, we investigated the interaction of garlic extracts with human deoxygenated RBCs and its effect on intracellular hemoglobin molecules. The results showed that garlic extract covalently modified intraerythrocytic deoxygenated hemoglobin. The modification identified consisted of an addition of 71 atomic mass units, suggesting allylation of the cysteine residues. Consistently, purified human deoxyhemoglobin reacted with chemically pure diallyl disulfide, showing the same modification as garlic extracts. Tandem mass spectrometry analysis demonstrated that garlic extract and diallyl disulfide modified hemoglobin's beta-chain at cysteine-93 (β-93C) or cysteine-112 (β-112C). These results indicate that garlic-derived organic disulfides as well as pure diallyl disulfide must permeate the RBC membrane and modified deoxyhemoglobin at β-93C or β-112C. Although the physiological role of the reported garlic extract-induced allyl modification on human hemoglobin warrants further study, the results indicate that constituents of natural products, such as those from garlic extract, modify intracellular proteins.
Key Words: diallyl disulfide, garlic extract, hemoglobin, posttranslational modification, red blood cells
Introduction
For centuries, garlic has been used as food and herbal medicine for a variety of human conditions, raging from cardiovascular disease and hyperlipidemia to dementia.1–6 Notable among the benefits attributed to garlic are its blood pressure-lowering and vasodilatation capacities.1,7 Despite several studies that indicated the health benefits of garlic extract for hypertensive patients, the molecular mechanisms underlying the effect of garlic extract on human physiology remain largely unknown. Vasodilation and vasoconstriction are of fundamental importance to cardiovascular function, contributing to matching of blood perfusion with metabolic need of perfused tissues. These processes are mediated by a wide variety of intrinsic and extrinsic molecules: inorganic, organic, amino acids, peptides, and even proteins. Notably, nitric oxide appears to be the endothelium-derived relaxing factor responsible for smooth muscle relaxation. Oxygen itself plays an important role in vasodilation, with hypoxic vasodilatation being one of the major effects of lack of oxygen on vascular tone. More recently, hydrogen sulfide (H2S) has been shown to be an important vasorelaxant, and its quantitative role in control of vascular tone is an active area of current research.8 The identity of the bioactive molecule(s) from garlic extract and the molecular mechanisms underlying its proposed vascular benefit are still poorly understood. Furthermore, it is still unknown whether garlic extracts exert a direct or indirect effect on endothelium or underlying vascular smooth muscle. The molecular mechanisms of garlic's antioxidant activity and lipid-lowering effects are known and may be part of a synergistic effect when coupled to garlic's effect on red blood cell (RBC) metabolism.9,10 A plausible explanation is that garlic extract's constituents may serve as (or be processed to become) signaling molecules, exerting physiological changes through their interactions with metabolites or protein receptors that trigger beneficial cellular responses. On the other hand, membrane-permeable garlic extract constituents may also exert a direct effect on intracellular proteins, thus eliciting a physiological change. Thus, in this report we investigated the effect of garlic extracts on intraerythrocytic deoxygenated hemoglobin.
It has been proposed recently that the vasoactivity of garlic arises from the interaction of organic disulfides found in garlic extracts with deoxygenated RBCs, producing glucose- and thiol-dependent vasoactive H2S.8 The results provide a plausible explanation for the role of garlic extract in vasodilatation, supporting the hypothesis that garlic-derived compounds may be converted to bioactive substances that influence cellular function. The study did not explore the interaction of garlic extract constituents with intraerythrocytic hemoglobin molecules. In order to determine if garlic extract constituents could modify intracellular proteins, garlic extract were incubated with deoxygenated RBCs, as in the previous study.8 After incubation, hemoglobin was purified and subjected to BioMass calculation and deconvolution tool of BioWorks software (Thermo Fisher Scientific, Waltham, MA, USA) and tandem mass spectrometry (MS). Our results showed, for the first time, a garlic-induced allyl modification of intraerythrocytic hemoglobin cysteinyl residues.
Materials and Methods
Preparation and treatment of RBCs and hemoglobin purification
Human RBCs were collected with EDTA as anticoagulant, after obtaining informed consent, according to procedures approved by the University of Puerto Rico-Rio Piedras Campus Institutional Review Board (San Juan, PR). The cells were used within 1 week of collection. Prior to study, the RBCs were washed three times by centrifugation with a minimum of a fivefold excess of phosphate-buffered saline (PBS) containing 500 μM diethylenetriaminepentaacetic acid, to chelate extraneous transition metals. RBCs were resuspended to a final hematocrit of about 10%. Deoxygenated RBCs were prepared by extensive cycling of vacuum treatments and argon flushing, in rubber-stoppered 5-mL tubes. Deoxygenation was ascertained spectrophotometrically (i.e., deoxyhemoglobin peak at 555 nm vs. oxyhemoglobin peaks at 540 and 576 nm). Garlic extracts were prepared as previously described.8 In brief, 2–3 g of garlic was ground together in a ceramic mortar and pestle with 2–3 mL of deionized water containing 500 μM diethylenetriaminepentaacetic acid (1 mL/g of garlic) and squeezed through cheesecloth, and the exudates were diluted with PBS containing diethylenetriaminepentaacetic acid (1:10 vol/vol). The garlic extract was aged at least 15 minutes after preparation and was always used within 2 hours. RBCs were incubated with garlic extract (100 μL/mL of RBCs) for 1 hour at room temperature. Iodoacetamide, which permeates through the RBC membrane, was used. Stock iodoacetamide solutions were prepared freshly in PBS containing DPTA and added to RBCs to a final concentration of 10 mM. RBCs were preincubated with iodoacetamide at room temperature for 30 minutes. Excess iodoacetamide was removed by multiple washings of the RBCs with PBS containing DPTA. RBCs were monitored visually and spectrophotometrically during the incubation with garlic extract at room temperature for 1 hour. At appropriate intervals, aliquots of RBC reaction mixtures were removed from the stoppered vials and centrifuged in 1.5-mL Eppendorf tubes. Lysates from washed RBCs were prepared inside the centrifuge tube, 3–5 volumes of cold deionized water containing diethylenetriaminepentaacetic acid were added, and the mixture was then centrifuged at 20,000 g for 5 minutes. The lysates were then spun down (3,000 g) on Sephadex G-25 spin columns equilibrated with 25 mM ammonium bicarbonate. Hemoglobin was collected in the void volume, its concentration was determined, and the preparation was then used for mass spectrometric analysis. For treatment with diallyl disulfide (DADS) (catalog number 32621, Sigma-Aldrich, St. Louis, MO, USA), RBCs (1 mL) were incubated in the presence of 273 mmol of DADS at room temperature for 18 hours. DADS is partly soluble in aqueous solutions. Therefore, the solution containing RBCs and DADS was gently mixed to promote the diffusion of the insoluble compound. After the 18-hour incubation, RBCs were collected, and hemoglobin was purified as explained above.
Protein MS analysis
BioMass analysis
Purified human hemoglobin was diluted in a solution containing acetonitrile:water (50:50 vol/vol) and 0.2% formic acid. The diluted hemoglobin was directly infused into the electrospray ionization source at 1 pmol/μL. The ionization source was set at a spray voltage of 2.0 kV, capillary temperature of 250°C, and capillary voltage of 165 V. The ionized molecules were analyzed in a LTQ mass spectrometer (Thermo Fisher Scientific). The mass spectra were recorded for 2 minutes. The data acquired were then analyzed using the BioMass calculation and deconvolution tool of BioWorks software. The parameters for the BioMass calculation deconvolution were set as follow: enable averaging, enable smoothing (Gaussian; 3), deconvoluted spectrum (12,000–17,000), adduct ion mass [ + proton (+1.008)], and mass step size 0.25 units. The atomic mass obtained from the convoluted mass spectrum was verified manually using the equation (MW + nH+)/n = [MW + (n + 1)H+]/(n + 1), where MW is the molecular weight.
Tandem MS
Purified human hemoglobin (100 pmol) was subjected to trypsin digestion in digestion buffer (40 mM ammonium bicarbonate, 10% acetonitrile, and trypsin [1 μg]) overnight (18 hours) at 37°C. The reaction mixture was dried on a speed vacuum, and the tryptic peptides were resuspended in acetonitrile:water (50:50 vol/vol) containing 0.2% formic acid. The solution containing the tryptic peptides was infused directly into the electrospray ionization source at 1 pmol/μL (spray voltage, 1.5 kV; capillary temperature, 200°C). The ionized peptides were infused into the LTQ mass spectrometer for tandem MS analysis. The peptides were identified by correlating MS-MS spectra with sequences from the National Center for Biotechnology Information nonredundant protein and EST databases using SEQUEST (ThermoFisher) database search algorithms. Only those peptides identified that passed selection filters imposed to the database search were taken into consideration [Xcorr higher than 1.5 (+1), 2.0 (+2) or 2.5 (+3); Delta Score >0.1; 10 or more b and y ions; MS2 intensity of >5 × 10−4; peptide probability >E × 10−2].
Results
Garlic extract modifies human beta-hemoglobin
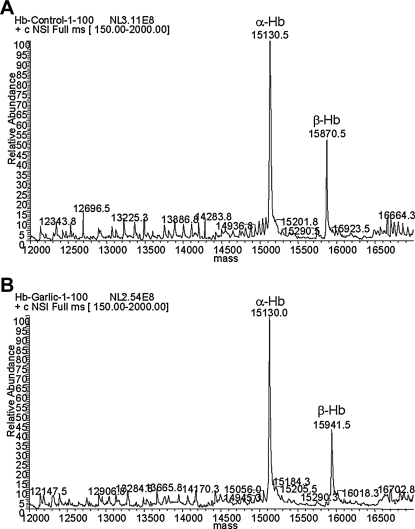
Human RBCs incubated with garlic extract induced the rapid production of H2S. The production of H2S is derived from the decomposition of allicin. Allicin is the main organosulfur compound found in garlic, and it is produced from the amino acid alliin.11 The production of allicin from the decomposition of alliin depends on the enzyme alliinase, which is activated when garlic is crushed. Allicin by-products such as diallyl sulfide, DADS, and diallyl trisulfide are rapidly formed in aqueous solutions. Of these three allicin by-products, DADS is the most prevalent (66%) in comparison to the other sulfides produced.11 These organosulfur compounds could modify the reactive cysteine residues found in the alpha-chain (position 104) and beta-chain (positions 93 and 112) of the hemoglobin molecule. In order to determine if human hemoglobin is modified by garlic extract, freshly collected and prepared human RBCs were incubated with fresh garlic extract. After incubation in an argon atmosphere, RBCs were collected by centrifugation and washed. As negative control, hemoglobin was extracted from untreated RBCs. The purified hemoglobin molecules were subjected to BioMass analyses. The mass spectrum of hemoglobin purified from control-treated RBCs showed the two major subunits of the hemoglobin molecule, the alpha- (15,130.5) and beta- (15,870.5) chains, as expected (Fig. 1A). Interestingly, however, the hemoglobin purified from the garlic-treated RBCs showed a mass shift of the peak representing the beta-chain but not the alpha-chain (Fig. 1B). The detected mass shift was, approximately, 71 atomic mass units (amu). The result indicates that a compound present in garlic extract can modify intraerythrocytic human hemoglobin, specifically the beta-chain.
FIG. 1.
Garlic extract modifies human beta-hemoglobin (Hb). Freshly prepared human red blood cells were treated with either (A) water or (B) garlic extract. Hb was purified from the treated red blood cells and subjected to BioMass analysis. (A) Hb purified from control samples showed two major peaks at 15,130.5 and 15,870.5, corresponding to alpha- and beta-chain, respectively. (B) In contrast, Hb purified from garlic extract-treated samples showed a mass shift of the peak corresponding to beta-chain. The hemoglobin beta-chain of the garlic extract showed a molecular mass of 15,941.5, a difference of 71 atomic mass units. The spectra are representative of at least three independent experiments. NL, normalization level; +c NSI Full ms, positive mode nanospray ionization full mass spectra.
Garlic extract modifies human beta-hemoglobin inside RBCs
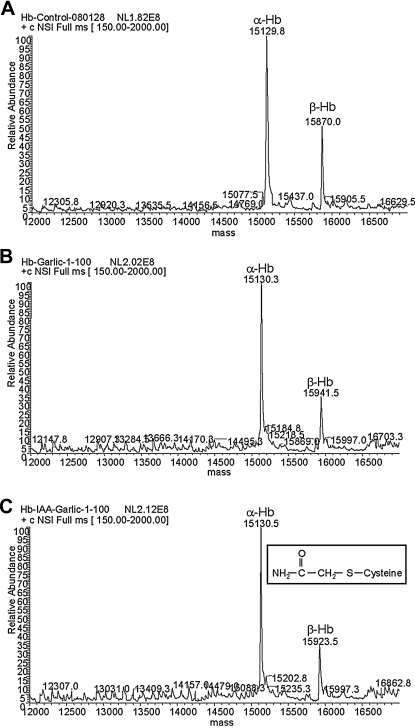
The modification of beta-hemoglobin was obtained after incubation of RBCs with raw garlic extract. The result suggests that a compound present in the garlic extract could diffuse (actively or passively) through the plasma membrane and modify the hemoglobin inside the RBCs. In order to demonstrate that hemoglobin is modified inside RBCs, the cells were preincubated with iodoacetamide. Iodoacetamide is a membrane-permeable compound that is known to modify cysteine residues. After incubation with iodoacetamide, garlic extract was added to the preparation. The RBCs were collected and washed, and the hemoglobin molecules were purified. As the control, RBCs were treated with vehicle solution and garlic extract alone. As expected, hemoglobin purified from control samples did not show a change in the molecular mass of the alpha- or beta-chain (Fig. 2A), and the garlic extract-treated samples produced the previously detected mass shift on the beta-chain (Fig. 2B). Conversely, the hemoglobin purified from the RBCs treated with iodoacetamide and garlic extract showed a mass shift of 53 amu (Fig. 2C). The expected change in mass for iodoacetamide-cysteine is 58 amu, 5 mass units more than the calculated mass. The mass deviation could be due to decomposition during the ionization process and/or accuracy of the deconvoluted mass spectrum because incubation with iodoacetamide alone results in the same molecular shift (data not shown). Nevertheless, the results indicate that the hemoglobin beta-chain is modified by a membrane-permeable compound present in garlic extract, which could be blocked by iodoacetamide.
FIG. 2.
Garlic extract modifies human beta-Hb inside red blood cells. Freshly prepared human red blood cells were treated with (A) vehicle, (B) garlic extract alone, or (C) iodoacetamide (IAA)/garlic extract. The purified Hb proteins from all samples were subjected to BioMass analysis. (A) Control sample showed peaks corresponding to unmodified Hb alpha- and beta-chains. (B) The purified Hb protein from garlic extract-treated samples showed the mass shift in the beta-chain, as previously detected. (C) The purified Hb from the IAA-pretreated samples showed the alpha-chain at a mass similar to the control, but the beta-chain showed a mass shift of 53 atomic mass units compared to control samples, indicating that the membrane-permeable IAA modified human beta-Hb and interfered with garlic extract-induced modification. The mass spectra are representative of at least three independent experiments.
DADS modifies hemoglobin beta-chain
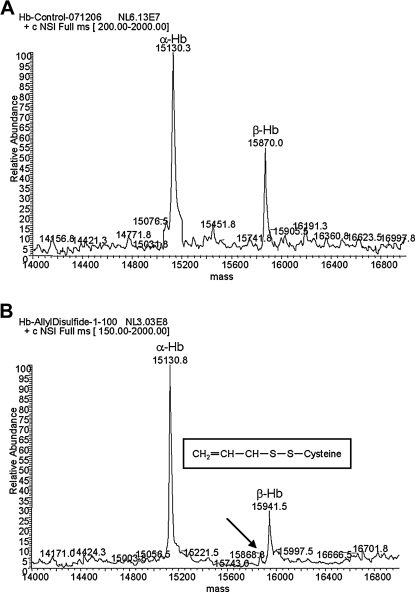
The garlic extract-induced modification of hemoglobin beta-chain was not observed when the garlic extract was heated, indicating that the membrane-permeable compound present in garlic extract has to be volatile (data not shown). Consistently, processed garlic contains a variety of organosulfur volatiles, including DADS.12 The mass shift of approximately 71 amu suggests that the garlic extract membrane-permeable compound could induce a single S-allyl-cysteine modification on the hemoglobin beta-chain. Therefore, the detected modification can be induced by DADS present in the garlic extract. In order to test this hypothesis, freshly prepared human deoxygenated RBCs were incubated with pure DADS (Sigma-Aldrich). The RBCs were incubated at room temperature in the presence of DADS for 18 hours. The hemoglobin purified from untreated RBCs showed the expected molecular mass of alpha- and beta-chains (Fig. 3A). In contrast, hemoglobin purified from DADS-treated RBCs showed a 71 amu mass shift as observed in garlic extract-treated RBCs (compare Figs. 1B and 3B). Importantly, the incubation of RBCs with purified DADS for shorter periods of times resulted in incomplete modification of hemoglobin beta-chain, producing a mixture of unmodified and modified beta-chain (data not shown). Even after 18 hours of incubation, traces of unmodified beta-chain were detected (Fig. 3B, arrow). DADS is an oil-based and volatile organosulfur compound that is poorly soluble in water. Therefore, the rate of the reaction will be determined by the diffusion rate of DADS in water and its absorption by the RBCs. This result indicates that the decomposition of DADS induces an S-allyl modification of cysteine residues on the hemoglobin beta-chain (Fig. 3C, inset). Importantly, based on the molecular weight shift detected, the beta-chain contains only one allyl-sulfide modification, similar to the change observed with garlic extract.
FIG. 3.
Diallyl disulfide modifies human Hb beta-chain. Freshly prepared human red blood cells were treated with either (A) vehicle or (B) diallyl disulfide for 18 hours at room temperature. Hb was purified from the samples and subjected to BioMass analysis. (A) The control sample, consistently, showed the same mass for both alpha- and beta-chains of Hb. (B) The diallyl disulfide-treated sample showed a mass shift for the beta-chain of 71 atomic mass units, similar to the one detected for the samples treated with garlic extract. A trace of unmodified beta-chain was still detected after 18 hours of incubation (arrow). The results suggest that diallyl disulfide induced the formation of a S-allyl-cysteine adduct on the beta-chain molecule (inset).
Hemoglobin beta-chain is modified at specific cysteine residues
The identification of DADS as one source of garlic extract-induced hemoglobin modification suggests that reactive cysteine residues on the beta-chain may be modified. In order to identify the site of modification, hemoglobin purified from control RBCs and RBCs treated with garlic extract (data not shown) and DADS was subjected to tandem MS analysis (Table 1). The purified modified hemoglobin was subjected to trypsin digestion, and the peptides were assessed by mass spectrometry. The recorded MS-MS spectra were compared to the human database. Peptides from both alpha- and beta-chains of hemoglobin were identified. The database was probed, in particular, taking into consideration possible additions of 71 amu to cysteine residues. In the purified hemoglobin from control samples no modified cysteine was seen (Table 1A). However, two peptides containing modified cysteine residues were identified in the DADS-treated sample (Table 1B, bold). These peptides correspond to cysteine at positions 93 and 112 of the beta-chain (β-93C and β-112C, respectively). Consistent with the BioMass analysis, no modification was seen of the cysteine at position 104 of the hemoglobin alpha-chain. The identification of these two modified cysteine residues suggests that the beta-chain can sustain S-allyl modification at either cysteine but not at both cysteines at the same time. The detection of ions is affected by different parameters intrinsic to the mass spectrometer and sample preparation. The main factors that affect detection of a specific ion are abundance, ionization suppression, space charging (i.e., ionic interaction at the ion trap), and scan time. Because of these inherent factors of MS analysis, the lack of detection of an expected ion (i.e., peptide) does not mean that it is not present in the analyzed sample. The lack of detection of the peptide with the unmodified cysteine in the treated sample (Table 1B) could be explained by a reduction in the abundance of these peptides due to a change in the protein conformation that interferes with trypsin digestion, reducing the amount of peptides produced, ionic interactions (i.e., space charging) at the ion trap, or variation in ionization potential from sample to sample. It is difficult to determine which of these factors contributed to the lack of detection. Nevertheless, the results indicated that specific cysteine residues in the beta-chain are modified by DADS.
Table 1.
Hemoglobin Beta-Chain Is Modified at Specific Cysteine Residues
| Peptide | MH+ | z | P (pro) P (pep) | Score XC | Coverage DeltaCn |
|---|---|---|---|---|---|
| (A) Untreated Hb | |||||
| Alpha globin; alpha-2 globin (Homo sapiens) | 7.99E-13 | 70.30 | |||
| K.VADALTNAVAHVDDMPNALSALSDLHAHK.L | 2,996.48944 | 3 | 1.53E-05 | 5.30 | 0.55 |
| K.TYFPHFDLSHGSAQVK.G | 1,833.89186 | 2 | 2.93E-07 | 4.46 | 0.61 |
| K.VGAHAGEYGAEALER.M | 1,529.73430 | 2 | 1.08E-11 | 4.29 | 0.60 |
| K.FLASVSTVLTSK.Y | 1,252.71473 | 2 | 2.06E-04 | 3.60 | 0.53 |
| K.KVADALTNAVAHVDDMPNALSALSDLHAHK.L | 3,124.58441 | 3 | 7.99E-13 | 6.06 | 0.55 |
| K.LRVDPVNFK.L | 1,087.62586 | 2 | 2.43E-04 | 2.46 | 0.25 |
| K.LLSHCLLVTLAAHLPAEFTPAVHASLDK.F | 2,967.61246 | 2 | 7.46E-04 | 4.41 | 0.69 |
| K.TYFPHFDLSHGSAQVK.G | 1,833.89186 | 3 | 1.36E-06 | 2.96 | 0.58 |
| K.LRVDPVNFK.L | 1,087.62586 | 2 | 1.45E-03 | 2.49 | 0.41 |
| Beta globin; Hb beta-chain (H. sapiens) | 2.96E-12 | 100.27 | |||
| K.SAVTALWGK.V | 932.51999 | 1 | 8.78E-03 | 1.66 | 0.18 |
| R.FFESFGDLSTPDAVMGNPK.V | 2,058.94772 | 2 | 6.95E-05 | 4.12 | 0.47 |
| K.VLGAFSDGLAHLDNLK.G | 1,669.89080 | 2 | 2.96E-12 | 5.24 | 0.43 |
| K.VVAGVANALAHK.Y | 1,149.67387 | 2 | 1.07E-07 | 3.82 | 0.46 |
| K.VNVDEVGGEALGR.L | 1,314.66482 | 2 | 9.37E-05 | 4.34 | 0.51 |
| K.EFTPPVQAAYQK.V | 1,378.70014 | 2 | 2.27E-03 | 2.56 | 0.26 |
| R.LLGNVLVCVLAHHFGK.E | 1,719.97269 | 2 | 3.12E-03 | 2.40 | 0.26 |
| K.LHVDPENFR.L | 1,126.56398 | 2 | 1.72E-04 | 2.38 | 0.33 |
| K.VNVDEVGGEALGR.L | 1,314.66482 | 1 | 9.71E-08 | 3.32 | 0.54 |
| K.SAVTALWGK.V | 932.51999 | 2 | 2.91E-05 | 2.21 | 0.50 |
| K.KVLGAFSDGLAHLDNLK.G | 1,797.98576 | 2 | 3.96E-11 | 5.31 | 0.58 |
| K.GTFATLSELHCDK.L | 1,421.67294 | 2 | 2.02E-06 | 2.84 | 0.40 |
| (B) Diallyl disulfide-treated Hb | |||||
| Alpha globin; alpha-2 globin (H. sapiens) | 1.42E-11 | 40.27 | |||
| K.KVADALTNAVAHVDDMPNALSALSDLHAHK.L | 3,124.58441 | 2 | 3.34E-06 | 3.67 | 0.52 |
| K.TYFPHFDLSHGSAQVK.G | 1,833.89186 | 2 | 4.69E-07 | 4.47 | 0.62 |
| K.VADALTNAVAHVDDMPNALSALSDLHAHK.L | 2,996.48944 | 2 | 5.67E-06 | 5.48 | 0.64 |
| K.VGAHAGEYGAEALER.M | 1,529.73430 | 2 | 1.42E-11 | 3.81 | 0.55 |
| Beta globin; Hb beta-chain (H. sapiens) | 1.14E-08 | 90.25 | |||
| K.EFTPPVQAAYQK.V | 1,378.70014 | 2 | 1.14E-08 | 2.61 | 0.44 |
| K.GTFATLSELHC*DK.L | 1,493.67294 | 2 | 1.55E-05 | 3.44 | 0.52 |
| K.KVLGAFSDGLAHLDNLK.G | 1,797.98576 | 2 | 3.19E-06 | 4.60 | 0.49 |
| K.LHVDPENFR.L | 1,126.56398 | 2 | 6.72E-04 | 2.55 | 0.32 |
| R.LLGNVLVC*VLAHHFGK.E | 1,791.97269 | 2 | 9.17E-03 | 2.77 | 0.40 |
| K.SAVTALWGK.V | 932.51999 | 2 | 8.84E-05 | 2.38 | 0.41 |
| K.VLGAFSDGLAHLDNLK.G | 1,669.89080 | 2 | 1.39E-08 | 4.91 | 0.51 |
| K.VVAGVANALAHK.Y | 1,149.67387 | 2 | 2.33E-05 | 3.63 | 0.51 |
The purified HB protein from either (A) control or (B) DADS-treated samples was digested with trypsin and subjected to tandem mass spectrometry analysis. Peptides identified from the alpha- and beta-chains of Hb are shown. The modified cysteine residues on the beta-chain from the DADS-treated samples are indicated in bold in B.
Discussion
Folk knowledge of a role for garlic in wound healing, antisepsis, blood pressure, and general health dates back centuries. Garlic in the Mediterranean diet has been thought to be one of the reasons people of that region are less susceptible to cardiovascular disease. However, the molecular mechanisms that mediate garlic-dependent health benefits are poorly understood. The present results showed that a garlic-derived allyl disulfide group modifies the intraerythrocytic hemoglobin beta-chain in the β-93C or β-112C residue. Only one cysteine per beta-chain is modified at any given time because BioMass analysis indicated a mass shift of 71 amu, corresponding to one allylation per beta-chain, suggesting that modification at one cysteine precludes or blocks the other (in the same beta-chain) from being modified. Adult human hemoglobin molecules are composed of two beta- and two alpha-chains. Because BioMass analysis is done under denaturing conditions, the data do not provide information concerning whether the tetramers are modified symmetrically, i.e., when a tetramer is allylated, it is done so concertedly, with both β-93C or β-112C residues at the same time, or whether there is a statistical distribution that after one cysteine gets modified it prevents allylation of the other in a beta-chain. Thus, a hemoglobin molecule may contain a modified β-93C in one beta-chain and β-112C in the other beta-chain, or it may have either both β-93C or both β-112C residues of the beta-chains. The results presented do not exclude any of these possibilities. Nevertheless, the results showed a complete mass shift of the peak corresponding to the modified beta-chain, suggesting that allylation takes placed in the context of the tetrameric hemoglobin molecule. Importantly, modifications, such as S-nitrosylation, on the identified cysteine residues have been shown to modulate the biological function of the hemoglobin molecule.
This is the first time that a garlic-derived compound has been shown to modify a protein in the intracellular milieu, suggesting a plausible mechanism that Allium-derived and possibly other “natural products” may exert physiological changes. Although the structural and physiological roles of the identified modification on hemoglobin remain to be determined, the results provide evidence to confirm that the cellular proteome interacts with and is affected by foodstuffs consumed and the environment to which it is exposed. Most studies on the effects of environmental factors are focused on genome–environmental agent interactions. However, the “proteome–environment” interaction, driven by diet variations within the population, may exert a more rapid or invasive change in physiology, acclimation, and/or adaptation. The results presented in this work are leading to further studies of covalent and noncovalent modifications that natural products may exert on the proteome. In conclusion, the results presented in this work may lead to a greater appreciation of the richness of the phenomenon of garlic-induced vasodilatation as well as documented evidence for further research into the changes that natural products exert on the proteome.
Acknowledgments
This work was supported, in part, by a National Institutes of Health/Center of Biomedical Research Excellence grant (5P20RR016439) to J.B. and I.E.V. and by the Defense Advanced Projects Agency and The Bonaventis Research Institute, Puerto Rico to J.B. The authors thank Drs. Brad Weiner and Manuel Gomez for their support in the establishment of the Protein Mass Spectrometry Facility at the College of Natural Sciences.
Author Disclosure Statement
The authors do not have any commercial association that may represent a conflict of interest in connection with the results reported.
References
- 1.Anim-Nyame N. Sooranna SR. Johnson MR. Gamble J. Steer PJ. Garlic supplementation increases peripheral blood flow: a role for interleukin-6? J Nutr Biochem. 2004;15:30–36. doi: 10.1016/j.jnutbio.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Borek C. Antioxidant health effects of aged garlic extract. J Nutr. 2001;131(3 Suppl):1010S–1015S. doi: 10.1093/jn/131.3.1010S. [DOI] [PubMed] [Google Scholar]
- 3.Borek C. Garlic reduces dementia and heart-disease risk. J Nutr. 2006;136(3 Suppl):810S–812S. doi: 10.1093/jn/136.3.810S. [DOI] [PubMed] [Google Scholar]
- 4.Ohnishi ST. Ohnishi T. In vitro effects of aged garlic extract and other nutritional supplements on sickle erythrocytes. J Nutr. 2001;131(3 Suppl):1085S–1092S. doi: 10.1093/jn/131.3.1085s. [DOI] [PubMed] [Google Scholar]
- 5.Takasu J. Uykimpang R. Sunga MA. Amagase H. Niihara Y. Aged garlic extract is a potential therapy for sickle-cell anemia. J Nutr. 2006;136(3 Suppl):803S–805S. doi: 10.1093/jn/136.3.803S. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka S. Haruma K. Yoshihara M, et al. Aged garlic extract has potential suppressive effect on colorectal adenomas in humans. J Nutr. 2006;136(3 Suppl):821S–826S. doi: 10.1093/jn/136.3.821S. [DOI] [PubMed] [Google Scholar]
- 7.Ried K. Frank OR. Stocks NP. Fakler P. Sullivan T. Effect of garlic on blood pressure: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2008;8:13. doi: 10.1186/1471-2261-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benavides GA. Squadrito GL. Mills RW, et al. Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci USA. 2007;104:17977–17982. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjee SK. Mukherjee PK. Maulik SK. Garlic as an antioxidant: the good, the bad and the ugly. Phytother Res. 2003;17:97–106. doi: 10.1002/ptr.1281. [DOI] [PubMed] [Google Scholar]
- 10.Koseoglu M. Isleten F. Atay A. Kaplan YC. Effects of acute and subacute garlic supplement administration on serum total antioxidant capacity and lipid parameters in healthy volunteers. Phytother Res. 2010;24:374–378. doi: 10.1002/ptr.2953. [DOI] [PubMed] [Google Scholar]
- 11.Amagase H. Clarifying the real bioactive constituents of garlic. J Nutr. 2006;136(3 Suppl):716S–725S. doi: 10.1093/jn/136.3.716S. [DOI] [PubMed] [Google Scholar]
- 12.Amagase H. Petesch BL. Matsuura H. Kasuga S. Itakura Y. Intake of garlic and its bioactive components. J Nutr. 2001;131(3 Suppl):955S–962S. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]