Abstract
Pluripotent mesenchymal stem cells (MSCs) are bone marrow stromal progenitor cells that can differentiate into osteogenic, chondrogenic, adipogenic, and myogenic lineages. Several signaling pathways have been shown to regulate the lineage commitment and terminal differentiation of MSCs. Here, we conducted a comprehensive analysis of the 14 types of bone morphogenetic protein (BMPs) for their abilities to regulate multilineage specific differentiation of MSCs. We found that most BMPs exhibited distinct abilities to regulate the expression of Runx2, Sox9, MyoD, and PPARγ2. Further analysis indicated that BMP-2, BMP-4, BMP-6, BMP-7, and BMP-9 effectively induced both adipogenic and osteogenic differentiation in vitro and in vivo. BMP-induced commitment to osteogenic or adipogenic lineage was shown to be mutually exclusive. Overexpression of Runx2 enhanced BMP-induced osteogenic differentiation, whereas knockdown of Runx2 expression diminished BMP-induced bone formation with a decrease in adipocyte accumulation in vivo. Interestingly, overexpression of PPARγ2 not only promoted adipogenic differentiation, but also enhanced osteogenic differentiation upon BMP-2, BMP-6, and BMP-9 stimulation. Conversely, MSCs with PPARγ2 knockdown or mouse embryonic fibroblasts derived from PPARγ2−/− mice exhibited a marked decrease in adipogenic differentiation, coupled with reduced osteogenic differentiation and diminished mineralization upon BMP-9 stimulation, suggesting that PPARγ2 may play a role in BMP-induced osteogenic and adipogenic differentiation. Thus, it is important to understand the molecular mechanism behind BMP-regulated lineage divergence during MSC differentiation, as this knowledge could help us to understand the pathogenesis of skeletal diseases and may lead to the development of strategies for regenerative medicine.
Introduction
Mesenchymal stem cells (MSCs) hold great promise for tissue bioengineering and regenerative medicine. MSCs are adherent marrow stromal cells that can self-renew and differentiate into osteogenic, chondrogenic, adipogenic, and myogenic lineages [1–5]. Although primarily residing in within the bone marrow compartment [5–7], MSCs have been isolated from periosteum, trabecular bone, adipose tissue, synovium, skeletal muscle, and deciduous teeth [8]. MSCs represent a very small fraction (e.g., 0.001–0.01%) of the total population of nucleated cells in the marrow [3]. Currently, the biologic properties of MSCs, specifically with respect to their existence in the adult organism and postulation of their biological niche, are not well understood. It is essential to understand the molecular mechanisms that govern the stem cell potential and lineage-specific differentiation of MSCs because MSCs without directed differentiation may become tumorigenic in vivo [9, 10.
Several signaling pathways have been implicated in regulating stem cell self-renewal and lineage commitment [11–16]. Bone morphogenetic proteins (BMPs) play an important role in regulating cell proliferation and differentiation during development [17, 18 and have been shown to play an important role in stem cell biology [19, 20. BMPs belong to the TGF-β superfamily and consist of at least 15 members in humans [17,18,21, 22. Genetic disruptions of BMPs have resulted in various skeletal and extraskeletal abnormalities during development [21, 23. BMPs fulfill their signaling activity by interacting with the heterodimeric complex of two transmembrane serine/threonine kinase receptors, BMPR type I and BMPR type II [17]. The activated receptor kinases phosphorylate the transcription factors Smads 1, 5, or 8, which in turn form a heterodimeric complex with Smad4 in the nucleus and activate the expression of target genes in concert with other coactivators [17].
Although not well understood, BMPs play an important role in regulating osteoblast differentiation and subsequent bone formation [22,24–27]. We have demonstrated that several BMPs are potent inducers of osteogenesis of MSCs [27–32], and that osteogenic BMPs (e.g., BMP-2, BMP-6, and BMP-9) regulate a distinct set of downstream targets that may play a role in regulating BMP-induced osteoblast differentiation [27,30–33]. BMP-2, BMP-4, and BMP-7, in coordination with other signaling molecules or supplementary cofactors, have been shown to promote preadipocyte differentiation [34–46]. However, the biological effects of BMPs on multiple lineage commitment and terminal differentiation of MSCs have not comprehensively analyzed.
In this study, using a well-characterized mesenchymal stem cell line C3H10T1/2 [47] and primary mesenchymal progenitor cells, we conducted a comprehensive analysis of 14 types of BMPs for their ability to induce lineage-specific differentiation of MSCs. BMPs were shown to exhibit distinct abilities to regulate expression of the master regulators of four different lineages. When the osteogenic and adipogenic lineages were further analyzed, BMP-2, −4, −6, −7, and −9 were shown to effectively induce both adipogenic and osteogenic differentiation of MSCs in vitro and in vivo, although the commitment of MSCs to osteoblast or adipogenic lineage was shown to be mutually exclusive. Overexpression of Runx2 synergizes with BMP-induced osteogenic differentiation, while exerting not detectable effects on adipogenic differentiation. However, overexpression of PPARγ2 promotes both osteogenic and adipogenic differentiation in vitro and in vivo. Conversely, knockdown or deletion of PPARγ2 not only inhibited adipogenic differentiation, but also diminished BMP-induced ossification, suggesting that PPARγ2 may also play an important role in osteogenic differentiation. Taken together, findings from our in vitro and in vivo studies suggest that BMPs may play an important role in regulating multilineage commitment and terminal differentiation of MSCs. Understanding the regulatory mechanisms behind BMP-regulated lineage divergence of MSC differentiation may lead to the development of novel therapeutic strategies for human disorders (such as osteoporosis and obesity) and for regenerative medicine.
Materials and Methods
Cell culture and chemicals
HEK293 and C3H10T1/2 cell lines were obtained from the ATCC (Manassas, VA), and were maintained in complete DMEM and Basal Medium Eagle, respectively. Unless indicated otherwise, all chemicals were purchased from Sigma-Aldrich or Fisher Scientific.
Adenoviral vectors expressing BMPs, PPARγ2, and Runx2
Recombinant adenoviruses expressing the 14 types of human BMPs (i.e., AdBMPs) were generated as previously described [28,29, 48. The coding regions for mouse PPARγ2 and Runx2 were amplified by PCR and subcloned into a shuttle vector for recombinant adenovirus generation using the AdEasy system [48, 49. The resultant vectors are designated as AdPPARγ2 and AdRunx2. An analogous adenovirus expressing GFP only (i.e., AdGFP) was used as a control [49–51].
Quantitative real-time PCR (qPCR) analysis
The qPCR was carried out as described [31–33]. Ten micrograms of total RNA were used to generate cDNA templates by reverse transcription with hexamer and Superscript II reverse transcriptase (Invitrogen). The first strand cDNA products were further diluted 5- to 10-fold and used as qPCR templates. The qPCR primers (Supplementary Table 1; Supplementary Table 1 is available online at http://www.liebertpub.com/scd) were 18-mers, designed by using the Primer3 program, http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi, to amplify the 3′-end (approximately 120 bps) of the mouse Runx2, Sox9, MyoD, or PPARγ2 genes. SYBR Green-based qPCR analysis was carried out by using the Opticon DNA Engine (M J Research). The specificity of each qPCR reaction was verified by melting curve analysis and further confirmed by resolving the PCR products on 1.5% agarose gels. Ten-fold serially diluted pUC19 was used as a standard. Triplicate reactions were carried out for each sample. All samples were normalized by the expression level of GAPDH.
Alkaline phosphatase activity
Alkaline phosphatase activity was assessed by the colorimetric assay and/or histochemical staining assay as previously described [28–33].
Construction and verification of Runx2 and PPARγ2 knockdown vectors
We used our recently developed pSOS system to select and validate efficacious short interfering double-stranded RNA (siRNA) target sites of mouse Runx2 and PPARγ2 [52]. Briefly, we designed four pairs of oligonucleotides containing siRNA target sites for mouse Runx2 or PPARγ2 using Dharmacon's siDESIGN program (Supplementary Table 1). The oligo pairs were annealed and subcloned into the Sfi I site of pSOS, resulting in pSOS-simRunx2 or pSOS-simPPARγ2. To assess the knockdown efficiency, we further subcloned the coding regions of mouse Runx2 and PPARγ2 into the pSOS-simRunx2 and pSOS-simPPARγ2, resulting in pSOS-simRunx2-WT and pSOS-simPPARγ2-WT, respectively. The pSOS-simRunx2-WT and pSOS-simPPRAγ2-WT were transfected into HEK-293 cells, and GFP signal levels were used to assess the silencing efficiency of different siRNA target sites [52]. Authenticity of PCR amplified sequences and the oligonucleotide cassettes were verified by DNA sequencing. Cloning and construction details are available upon request.
Establishment of stable C3H10-Runx2-kd and C3H10-PPARγ2-kd lines
As the pSOS is a retrovirus-based vector, we pooled the three pSOS-simRunx2 or pSOS-simPPARγ2 plasmids and packaged retroviral viruses by transfecting HEK-293 cells with the packaging plasmid pAmpho [52]. The produced retroviral supernatants were used to infect subconfluent C3H10T1/2 cells (e.g., usually 3–4 rounds of infection within 24–36 h). The infected cells were selected against blasticidin S (Invitrogen) for 5–7 days. The empty pSOS vector was used to establish a control stable line. The stable lines were designated as C3H10-Runx-kd, C3H10-PPARγ2, and C3H10-Control.
PPARγ2 null mouse embryonic fibroblasts (MEFs)
MEFs derived from animals with the homozygous and heterozygous deletion of PPARγ2 (i.e., MEF-PPARγ2−/− and MEF-PPARγ2+/−) were obtained from Bruce Spiegelman of Dana Farber Cancer Institute.
Immunofluorescence staining
Immunofluorescence staining was carried out as described [31, 32. Briefly, subconfluent C3H10-Runx-kd and C3H10-Control cells were fixed with methanol at −20°C for 15 min and washed with PBS. The fixed cells were permeabilized, blocked with 10% goat serum, and followed by incubation with anti-Runx2 antibody. After washing, the cells were incubated with anti-mouse IgG secondary antibody labeled with Alexa Fluor 594 (Molecular Probes). The presence of Runx2 protein was examined under a fluorescence microscope. Stains without the primary antibody, or with control IgG, were used as negative controls.
Stem cell implantation
The use and care of animals were approved by the Institutional Animal Care and Use Committee. Subconfluent C3H10T1/2, its derivative lines, or MEFs were infected with AdBMPs, AdGFP, and/or AdPPARγ2 or AdRunx2 for 15 h, and collected for subcutaneous injection (5 × 106 cells per injection) into the flanks of athymic nude (nu/nu) mice (five animals per group, 4 to 6–week-old, male, Harlan Sprague Dawley). Five weeks after implantation, animals were sacrificed and the implantation sites were retrieved for microCT analysis and histologic evaluation and other stains.
Hematoxylin and Eosin, Masson's Trichrome, and Alcian Blue staining
Retrieved tissues were decalcified, fixed in 10% formalin overnight, and embedded in paraffin. Serial sections of the embedded specimens were stained with hematoxylin and eosin (H&E). Masson's Trichrome stain was carried out as described [29]. Deparaffinized and rehydrated sections were stained with 1% Alcian Blue pH 2.5.
Oil Red-O staining
Decalcified and paraformaldehyde fixed tissues were subjected to frozen sectioning. The sections were rinsed with PBS, water and ethanol, and then stained with freshly prepared Oil Red-O solution (six parts saturated Oil Red-O dye in isopropanol plus four parts water) at 37°C for 15 min, followed by washing with 70% ethanol and PBS.
Transmission electron microscopy analysis
For transmission electron microscopy, MSCs were infected with AdGFP or AdBMPs for 7 or 14 days, and fixed with 0.5% glutaraldehyde (in 0.1 M sodium phosphate buffer, pH 7.4) at 4°C for 30 min. The fixed cells were collected by using cell scrappers followed by a brief centrifugation. The cell pellets were washed with 0.1 M sodium barbital buffer, pH 9.4, 2–3 times, and incubated in alkaline phosphatase (ALP) substrate solution (0.1 M barbital buffer, pH 9.4, 0.1 M β-glycerophosphate, 0.5 M MgCl2, and 0.2 M CaCl2) at room temperature for 60 min, followed by washing with 0.1 M sodium barbital buffer, pH 9.4 or PBS. The cell pellets were then incubated in precold 0.05 M lead nitrate for 4 min at room temperature and washed extensively with PBS. The cell pellets were fixed for additional 2 h in 2.5% glutaraldehyde, and postfixed for 2 h with 1% osmium tetroxide. The cell pellets were dehydrated in an ascending ethanol series washes and embedded in Epon 812. Serial ultrathin sections were stained with uranyl acetate and lead citrate, and then examined using a Zeiss 900 electron microscope. Magnifications, 7,000× to 12,000×.
MicroCT analysis
The cone-beam micro-CT system used for acquiring mouse data consists of a microfocal X-ray source, an orthogonally-mounted rotary stage with object holder, and a CsI-coupled CMOS detector. The microfocal X-ray source (MX-20, Faxitron, USA) comprises of a tungsten anode with beryllium exit window, and it has a focal spot of 20 μm and was operated at 28 KeV. The 14-bit digital camera (Bioptics, USA) includes a CsI scintillator plate and a 2048 × 1024 array of CMOS thin-film transistors and photodiodes with a pixel size of 50 μm. The new developed back projection-filtration (BPF) algorithm with a unit appodization function was used for reconstructing volumetric mouse images from the acquired cone-beam data. Volume-rendered views were generated with opacity-weighted compositing and gradient-based shading (VolView 2.0: Kitware, Inc) techniques.
Results
BMPs exert distinctive abilities to induce lineage-specific regulators of mesenchymal progenitor cells
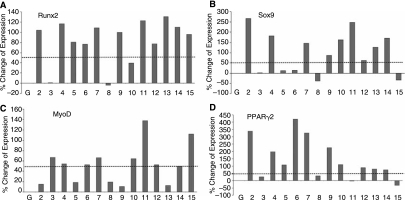
We previously demonstrated that BMP-2, −6, and −9 are the most potent inducers of osteoblast differentiation [22,27–32]. Here, we sought to further determine how the 14 BMPs regulate multilineage commitment and differentiation of MSCs. We focused on four commonly known lineages derived from MSCs, and analyzed the expression of the known respective lineage-specific regulators: Runx2 (osteogenic), Sox9 (chondrogenic), PPARγ2 (adipogenic), and MyoD (myogenic). Most BMPs, except BMP-3 and BMP-8, exhibit a modest ability to up-regulate Runx2 expression (Fig. 1A). These results are intriguing because, although they are consistent with the recent observation that BMP-3 has been shown to inhibit osteogenesis [53], we and others have demonstrated that only BMP-2, −4, −6, −7, and 9 can induce de novo bone formation [29, 54. One possibility is that Runx2 is not only associated with osteogenesis. When the expression of chondrogenic regulator Sox9 was examined, we found that osteogenic BMP-2, −4, and −7 significantly induced Sox9 expression, whereas BMP-11, −10, −13, and −14 also up-regulated Sox9 expression (Fig. 1B). However, most BMPs, except BMP-11 and 15, exhibited a weak ability to induce the expression of the myogenic regulator MyoD under the same conditions (Fig. 1C). Lastly, we analyzed the expression of the adipogenic regulator PPARγ2 and found that BMP-2, −4, −5, −6, −7, −9, and −10 effectively induced PPARγ2 expression in MSCs (Fig. 1D).
FIG. 1.
Quantitative analysis of the four lineage-specific regulators affected by the 14 types of BMPs in C3H10T1/2 MSCs. BMP-regulated expression of the lineage-specific regulators in MSCs was assessed with quantitative real-time PCR. The MSCs were infected with AdBMPs or AdGFP for 5 days prior to RNA isolation for real time-PCR analysis. (A) Expression of osteoblastic lineage regulator Runx2. (B) Expression of chondrogenic lineage regulator Sox9. (C) Expression of myogenic lineage regulator MyoD. (D) Expression of adipogenic lineage regulator PPARγ2. The dotted lines indicate 50% upregulation of gene expression.
BMPs regulate both osteogenic and adipogenic differentiation
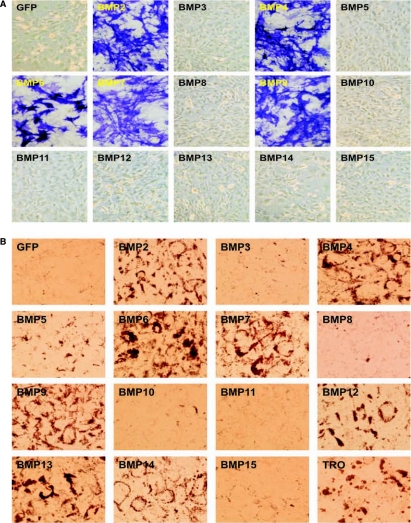
The overlapping osteogenic and chondrogenic activities of several BMPs may not be surprising, as chondrogenesis and osteogenesis are closely related and well-coordinated processes during skeletal development. In fact, endochondrondral ossification is the major bone formation process for long bone growth [22]. However, we were intrigued by the strong adipogenic activity of several osteogenic BMPs. Although not well defined, it has long been speculated that the disrupted balance between osteogenesis and adipogenesis may be responsible for certain diseases, such as age-related osteoporosis [55–61]. We sought to further dissect the osteogenic versus adipogenic activity of the BMPs in MSCs. Consistent with our earlier findings, only BMP-2, −4, −6, −7, and −9 of the 14 BMPs were shown to induce the early osteogenic marker alkaline phosphatase in MSCs (Fig. 2A). Under the same conditions, the above five osteogenic BMPs were shown to effectively induce adipogenic differentiation, as assessed with Oil Red-O staining (Fig. 2B). We also analyzed the expression of the late marker of adipogenic differentiation lipoprotein lipase (LPL) upon BMP stimulation, and found that the five osteogenic BMPs exhibited the greatest ability to induce LPL expression in C3H10T1/2 cells (data not shown). Interestingly, BMP-12, −13, and −14 also exhibited a modest ability to induce terminal adipogenic differentiation, while BMP-5 and BMP-10, although able to induce PPARγ2 expression (Fig. 1D), did not induce significant Oil Red-O staining under the same assay conditions (Fig. 2B). Similar results were also obtained using primary MEFs and primary marrow stromal cells, although these progenitor cells exhibited a detectable level of spontaneous differentiation (data not shown).
FIG. 2.
BMP-induced osteogenic and adipogenic differentiation in MSCs. (A) Osteogenic differentiation. C3H10T1/2 cells were infected with AdBMPs or AdGFP for 7 days, followed by histochemical staining of early osteogenic marker ALP activity. (B) Adipogenic differentiation. C3H10T1/2 cells were infected with AdBMPs or AdGFP for 20 days, followed by Oil Red-O staining. PPARγ agonist Troglitazone (TRO, 10 μM) was used as a positive control.
BMP-regulated osteogenic and adipogenic lineage commitment of MSCs is mutually exclusive
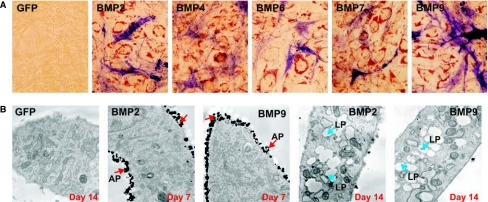
We next examined whether MSCs committed to adipogenesis or osteogenesis were derived from distinct cell populations. Briefly, C3H10T1/2 cells were infected with adenovirus vectors expressing BMP-2, −4, −6, −7, or −9, or GFP control [22,28,29,31,32,48,50, 51. At day 14, cells were first stained for alkaline phosphatase activity, followed by Oil Red-O staining. As shown in Figure 3A, as an early osteogenic marker, alkaline phosphatase staining was still readily detectable in the cells stimulated with all five osteogenic BMPs at day 14. The osteogenic BMP-stimulated C3H10T1/2 cells also exhibited positive Oil Red-O staining. These double staining experiments suggest that the BMP-induced osteogenic and adipogenic lineage commitments of mesenchymal progenitor cells may be mutually exclusive, although the progenitor cells undergoing osteogenic or adipogenic differentiation are closely located and are usually intertwined. These findings were also confirmed by electron microscope analysis. As shown in Fig. 3B, BMP-2 and BMP-9 effectively induced the cytoplasm membrane-bound ALP activity in certain cells, and lipid accumulation in other cells. It was also noted that the ALP positive cells were metabolically active and highly rich in organelles, such as mitochondria and ER apparatuses. Under this assay condition, we did not observe any cells that exhibited both high ALP activity and lipid accumulation, suggesting that BMP-induced osteogenic and adipogenic lineage commitment is mutually exclusive.
FIG. 3.
BMP-induced osteogenic and adipogenic differentiation in MSCs is mutually exclusive. (A) ALP and Oil Red-O costaining. C3H10T1/2 cells were infected with AdBMP-2, −4, −6, −7, −9, or AdGFP. At Day 14, cells were fixed and first stained for ALP activity, followed by Oil Red-O staining. (B) Transmission electron microscope analysis of BMP-induced MSC osteogenic and adipogenic differentiation. C3H10T1/2 cells were infected with the indicated AdBMP-2, AdBMP-9, or AdGFP. At Day 7 and Day 14, cells were processed for transmission electron microscopy (TEM) analysis. Magnification, 12,000×. Abbreviations: AP, membrane-bound ALP activity; LP, accumulated lipid droplets.
BMPs regulate both osteogenic and adipogenic differentiation of mesenchymal progenitor cells in vivo
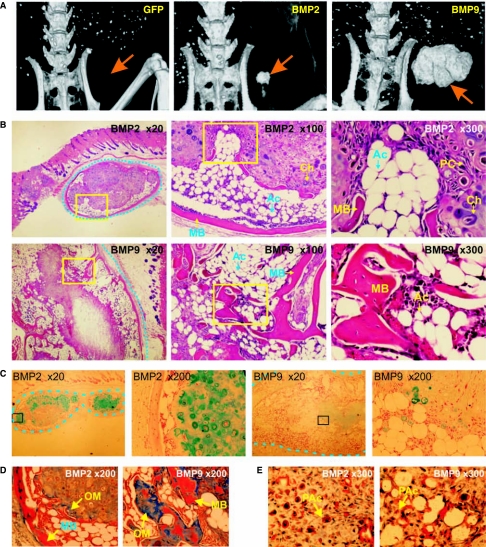
We then determined whether BMPs could induce both osteogenic and adipogenic differentiation in vivo. We conducted a series of stem cell transplantation experiments, in which MSCs transduced with AdBMP-2, AdBMP-9, or AdGFP, were injected subcutaneously into athymic nude mice. After 5 weeks, the animals were sacrificed for microCT imaging and the implanted cell masses were retrieved for histologic evaluation. While no palpable mass was formed in the animals injected with AdGFP-infected cells, the AdBMP-2 or AdBMP-9–infected cells formed solid cell/tissue masses, and larger masses were found in the animals injected with BMP-9–expressing MSCs (Fig. 4A). These results suggest that these BMPs may not only promote lineage specific differentiation but also may be important for the early proliferation and repopulation of the MSCs. Consistent with the in vitro findings, BMP-2 and −9 induced both osteogenesis and adiogenesis, while chondrogenesis was readily detectable in some of these samples, most notably BMP-2–transduced MSC masses. Representative histologic findings from AdBMP-2 and AdBMP-9 infected cell implants are shown in Fig. 4B. Consistent with our earlier findings [29], these findings indicate that BMP-2 induces predominantly endochondral ossification, while BMP-9 primarily induces intramembraneous ossification. The results were confirmed when the retrieved MSCs masses were stained with Alcian Blue. A significant number of Alcian Blue-stained positive cells were found in BMP-2–treated MSC implants, whereas a scattered few positive cells were detected in BMP-9–transduced MSC implants (Fig. 4C). Nevertheless, mature mineralized bone-like matrix was readily found in both BMP-2 and BMP-9–tranduced MSC implants, as demonstrated by Masson's Trichrome staining (Fig. 4D). Oil Red-O staining revealed that both mature adipocytes and preadipocytes were readily found in BMP-2 or BMP-9–transduced MSC implants (Fig. 4E). Overall, our results confirm the in vitro findings, and suggest that some of these BMPs may regulate both osteogenic and adipogenic lineage commitment and terminal differentiation of MSCs.
FIG. 4.
BMP-2 and BMP-9 induce both osteogenic and adipogenic differentiation in vivo. (A) MicroCT analysis of the tissue masses formed by GFP, BMP-2, or BMP-9–transduced MSCs. Transduced cells were implanted subcutaneously (5 × 106 cells per injection) into the flanks of athymic nude mice. After 5 weeks, animals were sacrificed and subjected to microCT analysis and histologic evaluation. Arrows indicate injection sites. (B) Histology of the tissue masses formed by BMP2- or BMP-9–transduced MSCs. Serial sections of decalcified tissues were stained with H&E. Dotted lines indicate the margin of masses. Boxed regions indicate the subsequently magnified areas. (C) Cartilage formation assessed with Alcian Blue staining. Serial sections were stained with Alcian Blue. Cartilage matrix was stained blue. (D) Mineralized bone matrix assessed with Masson's Trichrome staining. Serial sections of the decalcified tissues were stained with Masson's Trichrome. Fully mineralized bone matrix was stained red, while unmineralized osteoid matrix was stained light or dark blue. (E) Adipocyte formation assessed with Oil Red-O staining. Decalcified tissues were frozen sectioned and stained with Oil Red-O. Lipid-containing/producing cells, including preadipocytes, were stained red. Abbreviations: Ac, adipocyte; Ch, chondrocyte; MB, mineralized bone matrix; OM, osteoid matrix; PAc, preadipocyte; PC, progenitor cells.
Overexpression of Runx2 synergizes BMP-induced ALP activity without affecting adipogenic differentiation
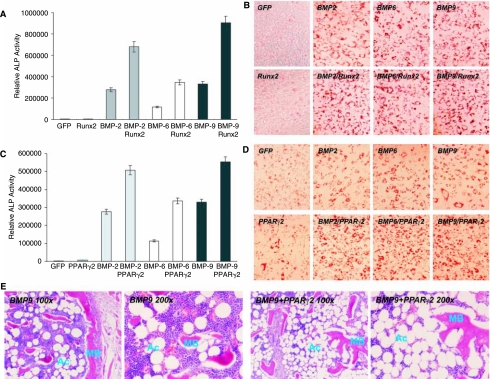
We next examined the role of Runx2, a well-established regulator of osteoblast differentiation, in BMP-9-induced MSC differentiation. Using an adenoviral vector expressing Runx2, we found that overexpression of Runx2 in C3H10T1/2 cells resulted in approximately 140%, 190%, and 170% increase in ALP activity upon BMP-2, BMP-6, and BMP-9 stimulation, respectively (Fig. 5A). Similar results were obtained for ALP histochemical staining assays (Supplementary Fig. 3A) (Supplementary Fig. 3 is available online at http://www. liebertpub.com/scd). However, overexpression of Runx2 did not inhibit, if not increase the adipogenic differentiation of MSCs stimulated by BMP-2, −6, and −9 (Fig. 5B). These findings suggest that Runx2 may primarily regulate osteogenic differentiation of MSCs without affecting adipogenic differentiation.
FIG. 5.
Effect of overexpression of Runx2 or PPARγ2 on BMP-induced osteogenic and adipogenic differentiation. (A) Synergistic effect of Runx2 on BMP-induced ALP activity. C3H10T1/2 cells were coinfected with BMP-2, −6, −9, GFP, and/or Runx2 adenoviral vectors. ALP activity was assayed at Day 7. (B) Runx2 exhibits no significant effect on BMP-induced adipogenic differentiation. C3H10T1/2 cells were coinfected with BMP-2, −6, −9, GFP, and/or Runx2 adenoviral vectors. Oil Red-O stain was carried out at Day 14. (C) PPARγ2 enhances BMP-induced ALP activity. C3H10T1/2 cells were coinfected with BMP-2, −6, −9, GFP, and/or PPARγ2 adenoviral vectors. ALP activity was analyzed at Day 7. (D) PPARγ2 augments BMP-induced adipogenic differentiation. C3H10T1/2 cells were coinfected with BMP-2, −6, −9, GFP, and/or PPARγ2 adenoviral vectors. Oil Red-O stain was conducted at Day 14. (E) PPARγ2 overexpression enhances both adipogenic and osteogenic differentiation in vivo. C3H10T1/2 cells were transduced with BMP-9, GFP, and/or PPARγ2 adenoviral vectors and implanted subcutaneously (5 × 106 cells per injection) into the flanks of athymic nude mice. After 5 weeks, animals were sacrificed and subjected to microCT analysis (see Fig. 7B) and histologic evaluation. Abbreviations: Ac, adipocyte; MB, mineralized bone matrix.
Overexpression of PPARγ2 promotes both osteogenic and adipogenic differentiation
The role of PPARγ2 in BMP-induced differentiation of MSCs is rather intriguing. PPARγ2 has been shown to be significantly up-regulated by several BMPs, especially osteogenic BMPs (Fig. 1D) [30]. To examine the role of PPARγ2 in BMP-induced differentiation of MSCs, we used an adenoviral vector to express mouse PPARγ2. As shown in Fig. 5C, overexpression of PPARγ2 enhanced ALP activity by 79%, 63%, and 64% for BMP-2, BMP-6, and BMP-9 stimulated MSCs, respectively. These results were also confirmed by histochemical staining (Supplementary Fig. 3B). As expected, overexpression of PPARγ2 promoted BMP-induced adipogenic differentiation (Fig. 5D). The in vivo stem cell implantation experiments confirmed that overexpression of PPARγ2 promoted both osteogenic and adipogenic differentiation (Fig. 5E and Fig. 7A panels a and b). These findings strongly suggest that PPARγ2 may play an important role in regulating BMP-mediated osteogenic and adipogenic lineage commitment of MSCs.
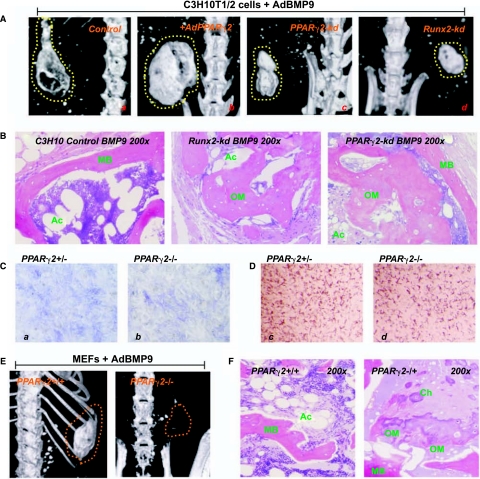
FIG. 7.
The in vivo effect of Runx2 and PPARγ2 depletion on BMP-9–induced osteogenic and adipogenic differentiation. (A) MicroCT analysis of BMP-9–induced bone formation affected by PPARγ2 overexpression, PPARγ2 knock-down, and Runx2 knockdown. Cell lines were transduced with AdBMP-9, AdGFP, and AdBMP-9+AdPPARγ2 for 15 h and subjected to subcanteous implantation (5 × 106 cells per injection) into the flanks of athymic nude mice. After 5 weeks, animals were sacrificed and subjected to microCT analysis and histologic evaluation. Dotted lines indicate the margin of the ossified masses. (B) Histologic evaluation of the ossified masses retrieved from (A). (C) qPCR analysis of PPARγ2 expression in MEFs derived from PPARγ2−/− and PPARγ2−/− animals. (D) BMP-9–induced ALP activity and Oil Red-O stain in PPARγ2+/− and PPARγ2−/− MEFs. The MEFs were infected with AdBMP-9 and stained for ALP activity and Red Oil-O at Day 7 and Day 14, respectively. (E) Genetic deletion of PPARγ2 significantly inhibits BMP-9–induced ossification. BMP-9–transduced MEFs were subcutaneously implanted in athymic nude mice. MicroCT analysis was carried out 5 weeks after implantation. Dotted lines indicate the margin of the palpable masses. (F) Histologic evaluation of the samples retrieved from (E). Abbreviations: Ac, adipocyte; Ch, chondrocyte; MB, mineralized bone matrix; OM, osteoid matrix.
Knockdown of Runx2 or PPARγ2 expression leads to a decrease in BMP-mediated osteogenic differentiation of MSCs
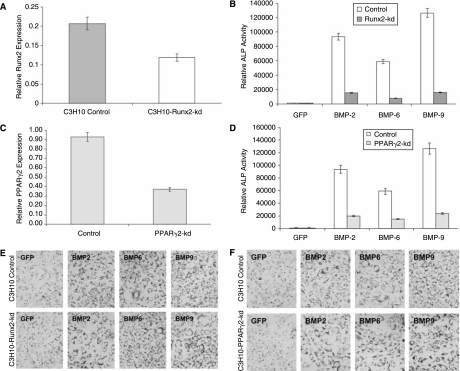
To complement the above overexpression experiments, we sought to further investigate the effect of silencing Runx2 and PPARγ2 on BMP-induced osteogenic and adipogenic differentiation of MSCs. We used our recently developed pSOS system to select and validate the siRNA target sites of mouse Runx2 and PPARγ2 [52]. We chose four candidate siRNA sites that targeted the coding region of mouse Runx2 or PPARγ2 (Supplementary Table 1).We demonstrated that three of the four tested siRNA sites effectively silenced GFP-Runx2 chimeric transcript in transient transfection assays (Supplementary Fig. 1A and B) (Supplementary Fig. 1 is available online at http://www.liebertpub.com/scd). We then pooled the pSOS vectors expressing the three effective siRNA sites and established a stable pool of C3H10T1/2 cells, designated as C3H10-Runx2-kd. The effective silencing of Runx2 expression in this stable line was confirmed by immunofluorescence staining (Supplementary Fig. 1C). The siRNA sites targeting the coding region of mouse PPARγ2 were selected and validated in a similar fashion (Supplementary Fig. 2A and B) (Supplementary Fig. 2 is available online at http://www.liebertpub.com/scd). The pooled three PPARγ2 siRNA sites were used to establish a stable pool of C3H10T1/2 cells, designated as C3H10-PPARγ2-kd. The knockdown efficiency (>50%) of C3H10-Runx2-kd and C3H10-PPARγ2-kd was further confirmed by quantitative real-time PCR assays (Fig. 6A and C). Our results demonstrated that silencing either Runx2 or PPARγ2 expression resulted in a decrease in BMP-induced ALP activity. Specifically, the ALP activity decreased to approximately 16%, 7%, and 13% for BMP-2, BMP-6, and BMP-9-stimulated C3H10-Runx2-kd cells, respectively, compared to that of the empty vector control line C3H10-Control (Fig. 6B). Similarly, compared to that of the empty vector control line C3H10-Control, the ALP activity decreased to approximately 21%, 25%, and 19% for BMP-2, BMP-6, and BMP-9-stimulated C3H10-PPARγ2-kd cells, respectively (Fig. 6D). Similar results were obtained using the histochemical staining assays of ALP activity (Supplementary Fig. 3C and D). On the contrary, the knockdown of either Runx2 or PPARγ2 expression in MSC C3H10T1/2 cells did not significantly affect adipogenic differentiation in vitro, as judged by Oil Red-O staining (Fig. 6E and F).
FIG. 6.
Effect of RNAi-mediated silencing of Runx2 and PPARγ2 on BMP-induced osteogenic and adipogenic differentiation. (A) Verification of silencing Runx2 expression in C3H10-Runx2-kd and empty vector stable lines by qPCR. (B) Runx2 knock-down inhibits BMP-induced ALP activity. C3H10-Runx2-kd and empty vector stable lines were infected with AdBMP-2, AdBMP-6, AdBMP-9, or AdGFP. ALP assays were carried out 7 days after infection. (C) Verification of silencing PPARγ2 expression in C3H10-PPARγ2-kd and empty vector stable lines by qPCR. (D) PPARγ2 knock-down inhibits BMP-induced ALP activity. C3H10- PPARγ2-kd and empty vector stable lines were infected with AdBMP-2, AdBMP-6, AdBMP-9, or AdGFP. ALP assays were carried out 7 days after infection. (E) Runx2 knock-down exerts no significant effect on BMP-induced adipogenic differentiation. C3H10-Runx2-kd and empty vector stable lines were infected with AdBMP-2, AdBMP-6, AdBMP-9, or AdGFP. Oil Red-O stain was carried out 14 days after infection. (F) PPARγ2 knock-down exerts no significant effect on BMP-induced adipogenic differentiation. C3H10-PPARγ2-kd and empty vector stable lines were infected with AdBMP-2, AdBMP-6, AdBMP-9, or AdGFP. Oil Red-O stain was carried out 14 days after infection.
We next performed stem cell implantation experiments to investigate the in vivo effect of silencing Runx2 or PPARγ2 expression on BMP-9–induced bone and fat formation in MSCs. The micoCT imaging demonstrated that knockdown of either Runx2 or PPARγ2 expression in MSCs resulted in the formation of smaller heterotopically ossified masses (Fig. 7A, panel a, vs. panels c and d). Histologic evaluation revealed that osteoid matrix and not fully mineralized bone matrix were readily detected in the samples retrieved from either Runx2 or PPARγ2 knockdown MSC implantation (Fig. 7B). These results were reproducible in three batches of independent experiments. In the Runx2 knockdown samples, there was also a decrease in adipocyte accumulation. Noticeable decrease in adipocyte accumulation was obtained in PPARγ2 knockdown samples. These in vivo results were in general consistent with that of the in vitro findings shown in Figs. 5 and 6. Our findings strongly suggest that PPARγ2, in addition to its critical role in adipogenic differentiation, may play a role in regulating BMP-9-induced terminal differentiation of osteogenic progenitor cells.
Deletion of PPARγ2 results in decreased ossification of BMP-9-stimulated PPARγ2−/− MEFs
Considering a possible partial phenotype of RNAi knockdown of PPARγ2 in C3H10T1/2 cells, we used the MEFs derived from animals in which the PPARγ2 was genetically deleted and investigated that the loss of PPARγ2 would affect BMP-9–induced bone formation. The relative PPARγ2 expression was analyzed in PPARγ2 heterozygous and homozygous MEFs, and confirmed that PPARγ2 level was low in PPARγ2 homozygous MEFs (Fig. 7C). The ALP activity was slightly decreased in PPARγ2 null MEFs upon BMP-9 stimulation, while as expected the adipogenic differentiation was notably decreased in PPARγ2 null MEFs upon BMP-9 stimulation (Fig. 7D). Stem cell implantation experiments revealed that PPARγ2 null MEFs formed smaller palpable masses than that of the wildtype or heterozygous MEFs (data not shown), whereas much low or no detectable ossified masses were observed with microCT imaging analysis in PPARγ2 MEFs injected the animals (Fig. 7E). Histologically, the masses retrieved from PPARγ2 null MEF implantation displayed a feature of more immature or non-mineralized osteoid matrix containing chondrocytes (Fig. 7F). As expected, no significant adipogenic differentiation was observed in PPARγ2 null MEFs derived masses (Fig. 7F). Thus, the results obtained from PPARγ2−/− MEFs are consistent with that from the C3H10-PPARγ2-kd cell. Taken together, these findings suggest that PPARγ2 may play an important role in regulating both osteogenic and adipogenic differentiation of MSCs, although the exact mechanism through which PPARγ2 regulates osteocyte and adipocyte lineage commitment and terminal differentiation remains to be elucidated.
Discussion
Using a well-characterized mesenchymal progenitor cell line C3H10T1/2 [47], we conducted a comprehensive analysis of the 14 types of BMPs for their abilities to regulate multilineage specific differentiation of MSCs. Most BMPs exhibited distinct abilities to regulate expression of the master regulators of four common lineages derived from MSCs. When the osteogenic and adipogenic lineages were analyzed, BMP-2, −4, −6, −7, and −9 were shown to effectively induce both adipogenic and osteogenic differentiation of MSCs in vitro and in vivo. However, the BMP-induced commitment to osteogenic or adipogenic lineage was mutually exclusive. Overexpression of Runx2 enhanced BMP-induced osteogenic differentiation, whereas knockdown of Runx2 expression diminished BMP-induced bone formation. Changes in the Runx2 expression status did not significantly affect BMP-induced adipogenic differentiation in vitro, although there was a slight decrease in adipocyte accumulation in Runx2 knockdown MSC-formed ectopic bone masses. However, overexpression of PPARγ2 promoted both osteogenic and adipogenic differentiation upon BMP-2, −6 and −9 stimulation. Conversely, both knockdown and deletion of PPARγ2 not only reduced adipogenic differentiation but also significantly inhibited osteogenic differentiation, especially the full mineralization of osteoid matrix.
The ability of PPARγ2 to enhance BMP-induced osteogenic differentiation is rather intriguing. It has long been hypothesized that osteoblast and marrow adipocyte lineages may have a close but reciprocal relationship [61]. Changes in the balance between osteogenesis and adipogenesis have been postulated as contributing factors to physiologic and pathologic conditions, such as ageing and osteoporosis [55–62]. A recent study indicated that aging activates adipogenic and suppresses osteogenic programs in marrow stem cells [62]. A decrease in bone volume in age-related osteoporosis is usually accompanied by an increase in marrow adipose tissue [55–59, 60. PPARγ insufficiency was shown to enhance osteogenesis [63], whereas the PPARγ agonist Rosiglitazone causes bone loss in mice [64, 65. Conversely, patients with progressive osseous hyperplasia have heterotopic bone formation within adipose tissue [66]. However, Runx2 null mice exhibited impaired bone formation and reduced adipogenesis [67], suggesting that osteogenic and adipogenic differentiation is closely related. Thus, the possible role of PPARγ2 in BMP-induced osteogenic and adipogenic differentiation of MSCs remains to be defined. Our earlier studies indicate that PPARγ2 is readily up-regulated by osteogenic BMPs in preosteoblast progenitor cells [30], as well as in this study (Fig. 1D). We also demonstrated that PPARγ2 agonist Troglitazone inhibited osteogenic BMP-induced ALP activity in MSCs (Supplementary Fig. 3E and F). While we do not have any satisfactory explanations for the different outcomes of our PPARγ2 overexpression/knockdown/deletion versus Troglitazone treatment and other in vivo observations, it is conceivable that: (1) Troglitazone as a small molecule agonist may exert off-target effect; (2) the action of PPARγ2 may be context-dependent, while we used a rather homogenous population of MSCs; (3) PPARγ2 may interplay with other factors during skeletal development, which is difficult to replicate in our stem cell implantation experiments; and (4) there may be a mechanistic difference between BMP-induced bone formation and developmental osteogenesis. Nonetheless, our findings clearly demonstrated that PPARγ2 may play an important role in regulating BMP-induced osteogenic and adipogenic differentiation, although it remains to be understood what exact role PPARγ2 may play in BMP-induced osteogenesis and adipogenesis of MSCs.
While the roles of BMPs in bone and skeletal development are well recognized [16,22,23, 27, little is known about the role of BMP signaling in adipose development. A recent study reported that mice lacking the BMP-2 regulated zinc finger protein Schnurri-2 exhibited a significant reduction of in white fat mass [68]. Although several transcription factors have been identified as the “master regulators” of muscle, adipose, bone and cartilage differentiation [4, 5, the molecular mechanisms which control the early stages of MSC lineage commitment and possible lineage plasticity remain to be understood.
MSC precursors have been shown to be highly heterogeneous in terms of their multilineage differentiation potential or “plasticity” [3, 69. In fact, only about 30% of the initial adherent bone marrow-derived MSC clones are pluripotent (mostly tri-lineage, osteo/chondo/adipogenic) [3, 69, and the reminder displays a bi-lineage (osteo/chondrogenic) or uni-lineage (osteogenic) potential [69, 70. Nevertheless, MSCs can be rather easily isolated from bone marrow and expanded in vitro, and they have generated a great deal of interest and become a prime target for researchers of tissue regeneration and replacement of damaged tissues, such as bone, cartilage, tendon, and ligament [3,7, 8.
Future investigations should be directed toward understanding how lineage commitment and divergence of MSCs is regulated [15,27, 71. Due to their low frequency and the lack of knowledge on cell surface markers and their location of origin, most information concerning MSCs is derived from in vitro studies. The cell populations could also represent different points of a hierarchy or a continuum of MSC differentiation. These issues reinforce the urgent need for a more comprehensive analysis of the MSC identity and characteristics, which should help to understand the regulatory mechanisms behind lineage determination and terminal differentiation of MSCs. Ultimately, understanding the regulatory mechanisms behind BMP-regulated lineage divergence during MSC differentiation should aid us in understanding the causes of skeletal diseases (such as osteoporosis and obesity) and may lead to the development of novel therapeutic strategies for regenerative medicine.
Supplementary Material
Acknowledgments
We thank Bruce M. Spiegelman of Dana-Farber Cancer Institute, Boston, MA, for providing the PPARγ2 null mouse embryo fibroblasts (MEFs). We also thank Parke-Davis/Pfizer for generously providing Troglitazone. The reported work was supported, in part, by research grants from the American Cancer Society (H.H.L. and T.C.H.), The Brinson Foundation (T.C.H.), the Natural Science Foundation of China and the Chinese Ministry of Science and Technology (QL, #30500602; QK, #30600625; Z.L.D. and T.C.H.), the 863 Project Grant from the Chinese Ministry of Science and Technology (Z.L.D., T.C.H., Q.K., and Q.L.), the Orthopaedic Research and Education Foundation (R.C.H. and H.H.L.), and the National Institutes of Health (Q.K., R.C.H., T.C.H., and H.H.L.).
Author Disclosure Statement
The authors declare no competing financial interests.
References
- 1.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 2.Aubin JE. Bone stem cells. J Cell Biochem Suppl. 1998;31:73–82. [PubMed] [Google Scholar]
- 3.Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD. Moorman MA. Simonetti DW. Craig S. Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Ducy P. Schinke T. Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science. 2000;289:1501–1504. doi: 10.1126/science.289.5484.1501. [DOI] [PubMed] [Google Scholar]
- 5.Caplan AI. Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends in Mol Med. 2001;7:259–264. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- 6.Friedenstein AJ. Piatetzky S., II Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–390. [PubMed] [Google Scholar]
- 7.Baksh D. Song L. Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barry FP. Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Rubio D. Garcia-Castro J. Martin MC. de la Fuente R. Cigudosa JC. Lloyd AC. Bernad A. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 10.Burns JS. Abdallah BM. Guldberg P. Rygaard J. Schroder HD. Kassem M. Tumorigenic heterogeneity in cancer stem cells evolved from long-term cultures of telomerase-immortalized human mesenchymal stem cells. Cancer Res. 2005;65:3126–3135. doi: 10.1158/0008-5472.CAN-04-2218. [DOI] [PubMed] [Google Scholar]
- 11.Molofsky AV. Pardal R. Morrison SJ. Diverse mechanisms regulate stem cell self-renewal. Curr Opin Cell Biol. 2004;16:700–707. doi: 10.1016/j.ceb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Wang J. Wynshaw-Boris A. The canonical Wnt pathway in early mammalian embryogenesis and stem cell maintenance/differentiation. Curr Opin Genet Dev. 2004;14:533–539. doi: 10.1016/j.gde.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Kleber M. Sommer L. Wnt signaling and the regulation of stem cell function. Curr Opin Cell Biol. 2004;16:681–687. doi: 10.1016/j.ceb.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Reya T. Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 15.Luo J. Chen J. Deng ZL. Luo X. Song WX. Sharff KA. Tang N. Haydon RC. Luu HH. He TC. Wnt signaling and human diseases: what are the therapeutic implications? Lab Invest. 2007;87:97–103. doi: 10.1038/labinvest.3700509. [DOI] [PubMed] [Google Scholar]
- 16.Deng ZL. Sharff KA. Tang N. Song WX. Luo J. X L. Chen J. Bennett E. Reid R. Manning D. Xue A. Montag AG. Luu HH. Haydon RC. He T-C. Regulation of osteogenic differentiation during skeletal development. Frontiers in Biosci. 2008;13:2001–2021. doi: 10.2741/2819. [DOI] [PubMed] [Google Scholar]
- 17.Shi Y. Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 18.Attisano L. Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 19.Varga AC. Wrana JL. The disparate role of BMP in stem cell biology. Oncogene. 2005;24:5713–5721. doi: 10.1038/sj.onc.1208919. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J. Li L. BMP signaling and stem cell regulation. Dev Biol. 2005;284:1–11. doi: 10.1016/j.ydbio.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- 22.Luo J. Sun MH. Kang Q. Peng Y. Jiang W. Luu HH. Luo Q. Park JY. Li Y. Haydon RC. He TC. Gene therapy for bone regeneration. Curr Gene Ther. 2005;5:167–179. doi: 10.2174/1566523053544218. [DOI] [PubMed] [Google Scholar]
- 23.Zhao GQ. Consequences of knocking out BMP signaling in the mouse. Genesis. 2003;35:43–56. doi: 10.1002/gene.10167. [DOI] [PubMed] [Google Scholar]
- 24.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 25.Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16:247–252. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- 26.Ducy P. Karsenty G. The family of bone morphogenetic proteins. Kidney Int. 2000;57:2207–2214. doi: 10.1046/j.1523-1755.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- 27.Luu HH. Song WX. Luo X. Manning D. Luo J. Deng ZL. Sharff KA. Montag AG. Haydon RC. He TC. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25:665–677. doi: 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- 28.Cheng H. Jiang W. Phillips FM. Haydon RC. Peng Y. Zhou L. Luu HH. An N. Breyer B. Vanichakarn P. Szatkowski JP. Park JY. He TC. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85-A:1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Kang Q. Sun MH. Cheng H. Peng Y. Montag AG. Deyrup AT. Jiang W. Luu HH. Luo J. Szatkowski JP. Vanichakarn P. Park JY. Li Y. Haydon RC. He TC. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;11:1312–1320. doi: 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- 30.Peng Y. Kang Q. Cheng H. Li X. Sun MH. Jiang W. Luu HH. Park JY. Haydon RC. He TC. Transcriptional characterization of bone morphogenetic proteins (BMPs)-mediated osteogenic signaling. J Cell Biochem. 2003;90:1149–1165. doi: 10.1002/jcb.10744. [DOI] [PubMed] [Google Scholar]
- 31.Peng Y. Kang Q. Luo Q. Jiang W. Si W. Liu BA. Luu HH. Park JK. Li X. Luo J. Montag AG. Haydon RC. He TC. Inhibitor of DNA binding/differentiation helix-loop-helix proteins mediate bone morphogenetic protein-induced osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279:32941–32949. doi: 10.1074/jbc.M403344200. [DOI] [PubMed] [Google Scholar]
- 32.Luo Q. Kang Q. Si W. Jiang W. Park JK. Peng Y. Li X. Luu HH. Luo J. Montag AG. Haydon RC. He TC. Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279:55958–55968. doi: 10.1074/jbc.M407810200. [DOI] [PubMed] [Google Scholar]
- 33.Si W. Kang Q. Luu HH. Park JK. Luo Q. Song WX. Jiang W. Luo X. Li X. Yin H. Montag AG. Haydon RC. He TC. CCN1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3A-induced osteoblast differentiation of mesenchymal stem cells. Mol Cell Biol. 2006;26:2955–2964. doi: 10.1128/MCB.26.8.2955-2964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen TL. Shen WJ. Kraemer FB. Human BMP-7/OP-1 induces the growth and differentiation of adipocytes and osteoblasts in bone marrow stromal cell cultures. J Cell Biochem. 2001;82:187–199. doi: 10.1002/jcb.1145. [DOI] [PubMed] [Google Scholar]
- 35.Gimble JM. Morgan C. Kelly K. Wu X. Dandapani V. Wang CS. Rosen V. Bone morphogenetic proteins inhibit adipocyte differentiation by bone marrow stromal cells. J Cell Biochem. 1995;58:393–402. doi: 10.1002/jcb.240580312. [DOI] [PubMed] [Google Scholar]
- 36.Ji X. Chen D. Xu C. Harris SE. Mundy GR. Yoneda T. Patterns of gene expression associated with BMP-2-induced osteoblast and adipocyte differentiation of mesenchymal progenitor cell 3T3-F442A [In Process Citation] J Bone Miner Metab. 2000;18:132–139. doi: 10.1007/s007740050103. [DOI] [PubMed] [Google Scholar]
- 37.Sottile V. Seuwen K. Bone morphogenetic protein-2 stimulates adipogenic differentiation of mesenchymal precursor cells in synergy with BRL 49653 (rosiglitazone) FEBS Lett. 2000;475:201–204. doi: 10.1016/s0014-5793(00)01655-0. [DOI] [PubMed] [Google Scholar]
- 38.Tang MK. Leung AK. Kwong WH. Chow PH. Chan JY. Ngo-Muller V. Li M. Lee KK. Bmp-4 requires the presence of the digits to initiate programmed cell death in limb interdigital tissues. Dev Biol. 2000;218:89–98. doi: 10.1006/dbio.1999.9578. [DOI] [PubMed] [Google Scholar]
- 39.Bowers RR. Kim JW. Otto TC. Lane MD. Stable stem cell commitment to the adipocyte lineage by inhibition of DNA methylation: Role of the BMP-4 gene. Proc Natl Acad Sci USA. 2006;103:13022–13027. doi: 10.1073/pnas.0605789103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang EA. Israel DI. Kelly S. Luxenberg DP. Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors. 1993;9:57–71. doi: 10.3109/08977199308991582. [DOI] [PubMed] [Google Scholar]
- 41.Taha MF. Valojerdi MR. Mowla SJ. Effect of bone morphogenetic protein-4 (BMP-4) on adipocyte differentiation from mouse embryonic stem cells. Anat Histol Embryol. 2006;35:271–278. doi: 10.1111/j.1439-0264.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- 42.zur Nieden NI. Kempka G. Rancourt DE. Ahr HJ. Induction of chondro-, osteo- and adipogenesis in embryonic stem cells by bone morphogenetic protein-2: effect of cofactors on differentiating lineages. BMC Dev Biol. 2005;5:1. doi: 10.1186/1471-213X-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fux C. Mitta B. Kramer BP. Fussenegger M. Dual-regulated expression of C/EBP-alpha and BMP-2 enables differential differentiation of C2C12 cells into adipocytes and osteoblasts. Nucleic Acids Res. 2004;32:e1. doi: 10.1093/nar/gnh001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mie M. Ohgushi H. Yanagida Y. Haruyama T. Kobatake E. Aizawa M. Osteogenesis coordinated in C3H10T1/2 cells by adipogenesis-dependent BMP-2 expression system. Tissue Eng. 2000;6:9–18. doi: 10.1089/107632700320847. [DOI] [PubMed] [Google Scholar]
- 45.Asahina I. Sampath TK. Hauschka PV. Human osteogenic protein-1 induces chondroblastic, osteoblastic, and/or adipocytic differentiation of clonal murine target cells. Exp Cell Res. 1996;222:38–47. doi: 10.1006/excr.1996.0005. [DOI] [PubMed] [Google Scholar]
- 46.Ahrens M. Ankenbauer T. Schroder D. Hollnagel A. Mayer H. Gross G. Expression of human bone morphogenetic proteins-2 or −4 in murine mesenchymal progenitor C3H10T1/2 cells induces differentiation into distinct mesenchymal cell lineages. DNA Cell Biol. 1993;12:871–880. doi: 10.1089/dna.1993.12.871. [DOI] [PubMed] [Google Scholar]
- 47.Reznikoff CA. Brankow DW. Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973;33:3231–3238. [PubMed] [Google Scholar]
- 48.He TC. Zhou S. da Costa LT. Yu J. Kinzler KW. Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo J. Deng ZL. Luo X. Tang N. Song WX. Chen J. Sharff KA. Luu HH. Haydon RC. Kinzler KW. Vogelstein B. He TC. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat Protoc. 2007;2:1236–1247. doi: 10.1038/nprot.2007.135. [DOI] [PubMed] [Google Scholar]
- 50.He TC. Sparks AB. Rago C. Hermeking H. Zawel L. da Costa LT. Morin PJ. Vogelstein B. Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 51.He TC. Chan TA. Vogelstein B. Kinzler KW. PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo Q. Kang Q. Song WX. Luu HH. Luo X. An N. Luo J. Deng ZL. Jiang W. Yin H. Chen J. Sharff KA. Tang N. Bennett E. Haydon RC. He TC. Selection and validation of optimal siRNA target sites for RNAi-mediated gene silencing. Gene. 2007;395:160–169. doi: 10.1016/j.gene.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 53.Daluiski A. Engstrand T. Bahamonde ME. Gamer LW. Agius E. Stevenson SL. Cox K. Rosen V. Lyons KM. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat Genet. 2001;27:84–88. doi: 10.1038/83810. [DOI] [PubMed] [Google Scholar]
- 54.Li JZ. Li H. Sasaki T. Holman D. Beres B. Dumont RJ. Pittman DD. Hankins GR. Helm GA. Osteogenic potential of five different recombinant human bone morphogenetic protein adenoviral vectors in the rat. Gene Ther. 2003;10:1735–1743. doi: 10.1038/sj.gt.3302075. [DOI] [PubMed] [Google Scholar]
- 55.Meunier P. Aaron J. Edouard C. Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–154. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 56.Burkhardt R. Kettner G. Bohm W. Schmidmeier M. Schlag R. Frisch B. Mallmann B. Eisenmenger W. Gilg T. Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: a comparative histomorphometric study. Bone. 1987;8:157–164. doi: 10.1016/8756-3282(87)90015-9. [DOI] [PubMed] [Google Scholar]
- 57.Rozman C. Reverter JC. Feliu E. Berga L. Rozman M. Climent C. Variations of fat tissue fraction in abnormal human bone marrow depend both on size and number of adipocytes: a stereologic study. Blood. 1990;76:892–895. [PubMed] [Google Scholar]
- 58.Kajkenova O. Lecka-Czernik B. Gubrij I. Hauser SP. Takahashi K. Parfitt AM. Jilka RL. Manolagas SC. Lipschitz DA. Increased adipogenesis and myelopoiesis in the bone marrow of SAMP6, a murine model of defective osteoblastogenesis and low turnover osteopenia. J Bone Miner Res. 1997;12:1772–1779. doi: 10.1359/jbmr.1997.12.11.1772. [DOI] [PubMed] [Google Scholar]
- 59.Verma S. Rajaratnam JH. Denton J. Hoyland JA. Byers RJ. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol. 2002;55:693–698. doi: 10.1136/jcp.55.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Justesen J. Stenderup K. Ebbesen EN. Mosekilde L. Steiniche T. Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 61.Nuttall ME. Gimble JM. Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Curr Opin Pharmacol. 2004;4:290–294. doi: 10.1016/j.coph.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 62.Moerman EJ. Teng K. Lipschitz DA. Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379–389. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akune T. Ohba S. Kamekura S. Yamaguchi M. Chung UI. Kubota N. Terauchi Y. Harada Y. Azuma Y. Nakamura K. Kadowaki T. Kawaguchi H. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ali AA. Weinstein RS. Stewart SA. Parfitt AM. Manolagas SC. Jilka RL. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology. 2005;146:1226–1235. doi: 10.1210/en.2004-0735. [DOI] [PubMed] [Google Scholar]
- 65.Soroceanu MA. Miao D. Bai XY. Su H. Goltzman D. Karaplis AC. Rosiglitazone impacts negatively on bone by promoting osteoblast/osteocyte apoptosis. J Endocrinol. 2004;183:203–216. doi: 10.1677/joe.1.05723. [DOI] [PubMed] [Google Scholar]
- 66.Kaplan FS. Shore EM. Progressive osseous heteroplasia. J Bone Miner Res. 2000;15:2084–2094. doi: 10.1359/jbmr.2000.15.11.2084. [DOI] [PubMed] [Google Scholar]
- 67.Kobayashi H. Gao Y. Ueta C. Yamaguchi A. Komori T. Multilineage differentiation of Cbfa1-deficient calvarial cells in vitro. Biochem Biophys Res Commun. 2000;273:630–636. doi: 10.1006/bbrc.2000.2981. [DOI] [PubMed] [Google Scholar]
- 68.Jin W. Takagi T. Kanesashi SN. Kurahashi T. Nomura T. Harada J. Ishii S. Schnurri-2 controls BMP-dependent adipogenesis via interaction with Smad proteins. Dev Cell. 2006;10:461–471. doi: 10.1016/j.devcel.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 69.Muraglia A. Cancedda R. Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113(Pt 7):1161–1166. doi: 10.1242/jcs.113.7.1161. [DOI] [PubMed] [Google Scholar]
- 70.Osyczka AM. Noth U. O'Connor J. Caterson EJ. Yoon K. Danielson KG. Tuan RS. Multilineage differentiation of adult human bone marrow progenitor cells transduced with human papilloma virus type 16 E6/E7 genes. Calcif Tissue Int. 2002;71:447–458. doi: 10.1007/s00223-001-1090-2. [DOI] [PubMed] [Google Scholar]
- 71.Hong JH. Hwang ES. McManus MT. Amsterdam A. Tian Y. Kalmukova R. Mueller E. Benjamin T. Spiegelman BM. Sharp PA. Hopkins N. Yaffe MB. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.