Summary
Background
The transmission of pathogens via blood transfusion is still a major threat. Expert conferences established the need for a pro-active approach and concluded that the introduction of a pathogen inactivation/reduction technology requires a thorough safety profile, a comprehensive pre-clinical and clinical development and an ongoing hemovigilance program.
Material and Methods
The INTERCEPT Blood System utilizes amotosalen and UVA light and enables for the treatment of platelets and plasma in the same device. Preclinical studies of pathogen inactivation and toxicology and a thorough program of clinical studies have been conducted and an active he-movigilance-program established.
Results
INTERCEPT shows robust efficacy of inactivation for viruses, bacteria (including spirochetes), protozoa and leukocytes as well as large safety margins. Furthermore, it integrates well into routine blood center operations. The clinical study program demonstrates the successful use for very diverse patient groups. The hemovigilance program shows safety and tolerability in routine use. Approximately 700,000 INTERCEPT-treated products have been transfused worldwide. The system is in clinical use since class III CE-mark registration in 2002. The safety and efficacy has been shown in routine use and during an epidemic.
Conclusion
The INTERCEPT Blood System for platelets and plasma offers enhanced safety for the patient and protection against transfusion-transmitted infections.
Keywords: Pathogen inactivation, Platelets, Plasma, Amotosalen
Zusammenfassung
Hintergrund
Die Übertragung von Pathogenen durch Blutkomponenten stellt eine erhebliche Gefahr dar. Fach-konferenzen haben eine proaktive Vorgehensweise zum Schutz gegen transfusionsbedingte Erkrankungen gefordert. Für die Einfuhrung einer Pathogeninaktivierungs/ Reduktionstechnologie bedarf es eines ausreichenden Sicherheitsprofils sowie einer umfangreichen präklinischen und klinischen Entwicklung und eines fortwährenden Hämovigilanzprogramms.
Material und Methoden
Das INTERCEPT Blood System basiert auf dem Einsatz von Amotosalen und UVA-Licht und ermöglicht in demselben Gerät die Behandlung von Plasma und Thrombozy-tenkonzentraten. Präklinische Studien zur Pathogeninaktivierung und Toxikologie und ein umfangreiches klinisches Studienprogramm wurden durchgeführt und ein aktives Hamovigilanzprogramm etabliert.
Ergebnisse
INTERCEPT zeigt ein Höchstmass an Inaktivierung für Viren, Bakterien (inklusive Spirochäten), Protozoen und Leukozyten und ein großes Sicherheitsprofil und ist leicht zu implementieren. Das klinische Studienprogramm demonstriert den erfolgreichen Einsatz bei verschiedensten Patientengruppen. Das Hamovigilanzprogramm zeigt die Sicherheit und Verträglichkeit im Routineeinsatz. Weltweit wurden bis heute zirka 700 000 INTERCEPT-behandelte Produkte transfundiera Das System wurde von den wichtigsten europäischen Behörden zugelassen und ist seit 2002 im klinischen Einsatz. Die Sicherheit und Wirksamkeit wurde in Zeiten der Epidemie sowie im Routineeinsatz gezeigt.
Schlussfolgerung
Das INTERCEPT Blood System für Thrombozyten und Plasma bietet erhöhte Sicherheit für den Patienten und im Besonderen Schutz vor transfusi-onsbedingter Übertragung von Krankheiten durch verschiedenste Pathogene.
Introduction
The transmission of pathogens via blood transfusion is still a major threat. Expert conferences established the need for a pro-active approach and concluded that the introduction of a pathogen inactivation/reduction technology requires a thorough safety profile, a comprehensive pre-clinical and clinical development and an ongoing hemovigilance program [1,2].
Chemical Structure and Characteristics of Amotosalen
The INTERCEPT Blood System for pathogen inactivation utilizes amotosalen, a synthetic psoralen (formerly S-59-HC1) as active compound and UVA light.
Psoralens like 8-methoxypsoralen (8-MOP) or trimethyl-psoralen (TMP) are naturally occurring photoactive substances found in a number of plants such as limes, celery or parsnips [3]. These plants have been recognized for hundreds of years for their beneficial effect on certain skin lesions including vitiligo [4]. To date 8-MOP is approved for the ex-vivo treatment of cutaneous T-cell lymphoma [5], the psoralen UVA (PUVA) therapy for the treatment of psoriasis [6] or in other extracorporeal photopheresis treatments to treat graft rejection syndromes [7]. The average dietary uptake of the naturally occurring psoralens is in the range of 1–2 mg/day.
Amotosalen was developed as the result of extensive studies in which many different psoralens were analyzed for the potential to specifically target nucleic acids and for compatibility with the biological functions of platelet and plasma components. These criteria were met by amotosalen which demonstrated excellent activity against a broad range of pathogens (viruses, bacteria including spirochetes and protozoa) as well as leukocytes due to very high specificity for nucleic acids. Because it has minimal interaction with proteins or other cellular components, the functional properties of the blood components are preserved [8].
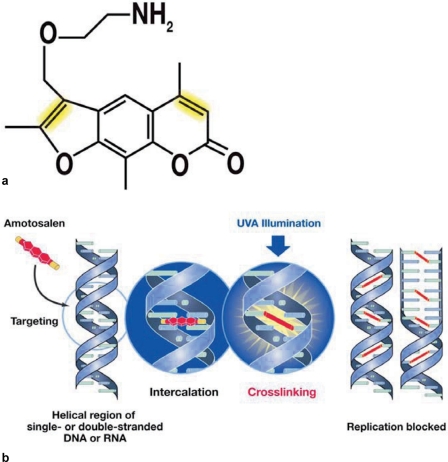
Amotosalen, like all psoralens, is a tricyclic molecule consisting of a furan and a pyrone moiety. The introduction of an amine side chain renders amotosalen highly water soluble and less lipophilic than 8-MOP [8]. It can thus quickly pass cellular membranes, bacterial walls or viral envelopes and readily interact with nucleic acids without interacting with proteins or cellular lipids. The chemical structure of amotosalen is not changed upon cell entry and it is therefore also exceptionally well suited for the inactivation of intracellular pathogens, different from other photoactive compounds currently in use. It readily intercalates into double-helical structures of DNA or RNA where covalent bonds between its reactive groups and pyrimidine bases are formed upon UVA illumination. Even intrastrand reactions are possible. Thus single-stranded nucleic acids also serve as targets. The chemical interaction is very well described, highly specific between amotosalen and the pyrimidine bases and occurs with very high frequency (fig. 1). Therefore, low concentrations of nucleic acids (e.g. from pathogens at very low titers) will specifically react even in the presence of large amounts of other biological materials.
Fig. 1.
a The chemical structure of amotosalen. b The mechanism of action. The INTERCEPT Blood System for platelets and plasma uses a combination of amotosalen (structure as shown) and long wavelength ultraviolet A (UVA) light. The amotosalen compound penetrates cellular and nuclear membranes and intercalates into the helical regions of DNA and RNA. Covalent crosslinks to the nucleic acid base pairs form upon exposure to UVA light (reactive groups highlighted in yellow), blocking DNA and RNA replication.
Theoretically, the formation of one cross-link between the strands of nucleic acids is sufficient to prevent replication of the genome. The frequency with which the interaction between amotosalen molecules and the pyrimdines occurs, i.e. covalent bonds are formed, has been determined for different nucleic acids and organisms. On average cross-links are formed every 83 base pairs. The reaction with nucleic acids does not depend on generation of active oxygen species, which are known to cause damage to the cell, and is effective even in the absence of oxygen. This high frequency of interaction makes replication but also transcription of genes impossible and inhibits DNA repair mechanisms.
Amotosalen has no sequence specificity and thus is efficient for the inactivation of a broad spectrum of pathogens. In addition the amotosalen-nucleic acid cross-linking occurs in the presence of UVA light, but not in its absence. This feature represents another unique safety measure as the reaction can be tightly controlled ex vivo simply by turning the light source on or off.
Process for the INTERCEPT Blood System
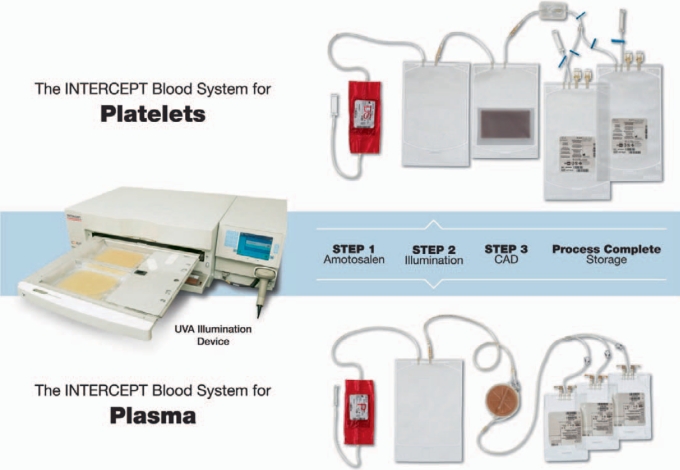
The INTERCEPT technology for pathogen inactivation for platelets or plasma components is based on two major steps: after the mixing of the blood component with the amotosalen (150 μmol/l, final concentration) the suspension is illuminated with UVA light (3 J/cm2); thereafter, residual amotosalen and free photoproducts are adsorbed in a compound adsorption device (CAD) [9, 10, 11]. The illumination device has been specifically designed to allow only UVA light to illuminate the blood components as it is reported that light of shorter wavelength like UVB or UVC has a detrimental effect on the platelets and proteins through the generation of active oxygen species [12, 13]. Therefore, exposure to UVB and UVC light is completely prevented in the INTERCEPT process. The entire procedure is performed in a closed integrated disposable set. Figure 2 shows the INTERCEPT illumination device INT100 and integrated disposable sets for platelets or plasma.
Fig. 2.
The INTERCEPT Blood Systems. The INTERCEPT Blood System for platelets consists of a UVA illuminator and an integrated disposable set, which comes in three formats (SV set, LV set, and DS set) as described in Table 1. The upper panel shows the DS set. The INTERCEPT Blood System for plasma consists of the same UVA illuminator and an integrated disposable set, each comes with 3 storage containers (lower panel).
The platelet or plasma product is attached via one sterile connection to the disposable set and passed through the pouch containing the amotosalen into the illumination container. The set is placed into the Illuminator where the UVA light illumination is performed for 4–6 min. Two products can be illuminated in parallel. After illumination the suspension is transferred into the container with the CAD and, depending on product and the workflow in the blood bank, the adsorption is performed for 10–20 min (plasma) by flow-through or 4–16 h (pending work flow) on an orbital shaker (platelets). Subsequently the platelets or plasma are transferred into the storage bags for either storage at room temperature for up to 7 days (platelets) or freezing (plasma). Hands-on time for the preparation of pathogen-inactivated products with the INTERCEPT Blood System is less than 10 min. Especially when using the dual-storage set for platelets, throughput of one illumination device is at about 40 units/h. This makes the technology very well suited also for large transfusion centers.
The INTERCEPT Blood System offers the highest degree of synergy as a single platform is used for pathogen inactivation of both platelets and plasma. The same illumination device, the same active compound (amotosalen) and very similar production steps are applied. This brings many advantages: installation and maintenance of the device, the training of personnel and the preparation of SOPs are all simplified. In addition having a single device of course saves laboratory space and is cost-saving.
The INTERCEPT Blood System is designed to be broadly compatible with different platelet and plasma collection platforms. The INTERCEPT platelet process can be performed with pooled whole blood-derived platelets from 4–7 buffy coats. The 7 buffy coat approach is of special interest in terms of economics because double-dose products are produced from a single treatment. Apheresis platelets collected with any of the commonly used platforms are well suited for the process as well. Double-dose apheresis collections or pooled buffy coats can be treated with a single process and split into two platelet units for transfusion.
The technology allows for inactivation of platelets in 100% plasma or in different platelet-additive solutions (PAS) such as InterSol™ or SSP+. Both PAS are CE mark-registered for use with the INTERCEPT System. For the approach with additive solutions, 53–68% of the plasma is removed and replaced by the additive solution. Centers changing their production from 100% plasma to additive solution can benefit from the gain of concurrent plasma for fractionation or other purposes.
The functional integrity of the INTERCEPT-treated platelets has been proven in a series of in vitro assays measuring parameters, e.g. glucose consumption, lactate generation, extent of shape change, hypotonic shock response, expression of activation markers like CD 62 or pH values. The hemostatic function of INTERCEPT-treated platelets (adhesion and aggregation capacity) was evaluated under flow conditions in an ex vivo perfusion model over the course of storage for up to 7 days. Lozano et al. [14] found the hemostatic function to be similar to conventional platelets for up to 7 days of storage. A total of 11 clinical trials enrolling more than 1,000 patients have been conducted and demonstrated efficacy and safety for clinical use [15, 16, 17, 18]. INTERCEPT-treated platelets are being used in routine for the support of thrombocytopenic patients whether as prophylaxis or in the course of acute bleeding. A number of EU centers have been producing INTERCEPT platelets in routine production for several years and provide them for the treatment of many different patient groups. A retrospective analysis of 13,000 platelet transfusions in one center providing exclusively INTERCEPT platelets showed no increase in platelet utilization or red blood cell concentrate utilization consistent with retention of the clinical efficiency compared to conventional platelet components [19].
The INTERCEPT plasma process is compatible with either apheresis or whole blood-derived plasma. For whole blood plasma, 2 or 3 units are mixed before processing and used in a single treatment resulting in up to three therapeutic units of plasma each containing 200 ml. Several studies analyzed the retention of the coagulation factors and inhibitors after INTERCEPT treatment. The values determined that factor VIII and fibrinogen showed the largest decline, but for those two as well as for all other factors the values were well in the range for therapeutic plasma units [20, 21, 22].
INTERCEPT-treated plasma has been tested in several clinical trials involving different patient groups. It has been demonstrated to be highly efficient in patients with congenital [23] or acquired coagulopathies [24] as well as for the treatment of patients requiring therapeutic plasma exchange [25]. Moreover, for INTERCEPT plasma products an active hemovigilance program has been established, showing high levels of safety and tolerability and a very low frequency of acute transfusion reactions compared to conventional plasma [26].
The technology is compatible with pathogen inactivation of fresh frozen plasma or previously frozen plasma [27].
Altogether there are 4 different disposable sets available for the treatment of platelets and plasma components enabling for pathogen inactivation of a broad range of component volumes (tables 1, 2).
Table 1.
INTERCEPT Blood System processing sets for platelets
| Parameters | Small volume (SV) set | Large volume (LV) set |
Dual storage (DS) set | |
|---|---|---|---|---|
| PAS | no PAS | |||
| Volume, ml | 255–325 | 300–420 | 255–390 | 300–420 |
| Platelet yields (× 1011) | 2.5–6.0 | 2.5–7.0 | 2.5–7.0 | 2.5–7.0 |
| Ratio plasma | 32–47% | 32–47% | 100% | 32–47% |
| RBC contamination/ml | <4 × 106 | <4 × 106 | <4 × 106 | <4 × 106 |
| CAD, h | 4–16 | 6–16 | 16–24 | 6–16 |
| Storage containers | 1 | 1 | 1 | 2 |
| Storage, days | 7 | 7 | 5 | 7 |
Table 2.
INTERCEPT Blood System processing sets for plasma
| Parameters | Plasma set |
|---|---|
| Volume, ml | 385–650 |
| RBC contamination/ml | <4 × 106 |
| CAD (flow through) | ∼10 min |
| Storage containers | 3 |
| Storage (frozen) | 2 years |
Results from Extensive Toxicology Program Demonstrate High Safety Margins
For any technology with a mechanism of action involving direct interaction with DNA or RNA, whether involving photoactive compounds or high-energy light, it is critical to establish the safety profile.
Amotosalen has been developed according to the standards of the International Conference of Harmonization for drug development [28]. In addition to the recommended studies, further studies were conducted which were pertinent to testing the safety of amotosalen dosed intravenously. Studies were designed according to the assessment of the circumstances of clinical use and from the patients' perspective. Therefore, a broad range of pharmacokinetic and toxicological analyses was performed. A large series of studies has been performed for amotosalen alone as well as for platelet or plasma components treated with the INTERCEPT Blood System with and without the use of the CAD. These included animal studies on acute and chronic toxicology but also studies to assess long-term toxicology like genotoxicity, reproductive toxicology, neonatal and juvenile animal development and carcinogenicity. Importantly, the studies on reproductive toxicity have great impact on the assessment of long-term safety of the product. They were of highest importance for the decision to use INTERCEPT-treated components for the support of pregnant women and women with child-bearing potential. Interestingly, the studies on genotoxicity revealed that the safety profile of amotosalen is much higher than that of the earlier mentioned 8-MOP, which is routinely used for PUVA therapy [29]. Carcinogenicity was analyzed applying the p53 heterogeneous knock-out model. The p53 gene encodes a tumor suppressor, and mice with the respective mutation are prone to the development of cancer at an early age. Therefore, exposure to potential carcinogens provides a sensitive model to assess carcinogenicity. In the studies performed the mice were challenged for 39 weeks, 3 times a week, with amounts of amotosalen 1,000-fold the human clinical exposure. No cancer was observed in any of the animals [29]. Also the pharmacological safety (CNS, cardiovascular and renal system) and furthermore phototoxicity and vein irritation were assessed at large multiples of the anticipated clinical exposures. The studies were done using different animals including rodents, dogs and primates. In addition ADME (absorption, distribution, metabolism and excretion) studies analyzing the metabolism of amotosalen as well as occupational safety studies were conducted to evaluate the safety of personnel working on the production of the blood components (table 3).
Table 3.
Extensive toxicology studies to evaluate the safety of INTERCEPT Blood Systems for platelets and plasma
| Studies | Platelets | Plasma |
|---|---|---|
| Acute toxicology | X | X |
| Repeated dose – 1 month | X | X |
| Repeat dose – 3 months | X | X |
| General pharmacology | X | X |
| Reproductive toxicology | X | X |
| Genotoxicity | X | X |
| Carcinogenicity | X | X |
| Phototoxicity | X | X |
| Neonatal toxicity | X | X |
| ADME | X | X |
| Occupational safety | X | X |
In these studies amotosalen has been used in concentrations representing very high multiples of the actual clinical exposure. No specific target organ toxicity, reproductive toxicity or carcinogenicity was observed [30]. Effects on the CNS or electrocardiogram were only detectable at 30,000-fold the expected clinical exposure. Taken together even these extensive analyses revealed no toxicological relevant effects.
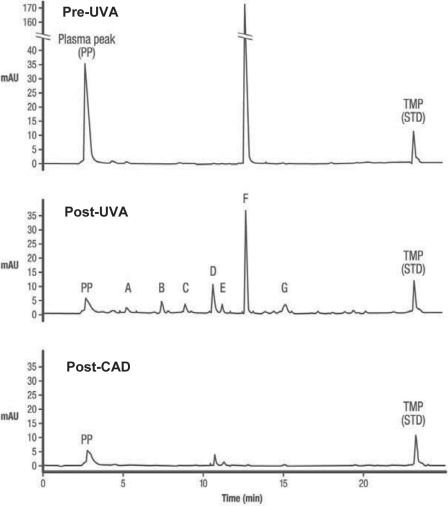
Any method applying a photoreactive compound, independent of the compound being ‘natural’ or synthetic, and UV light will lead to the generation of photoproducts. In this regard especially the high-energy, short-wavelength UVB and UVC light have disadvantages as for example due to the generation of active oxygen species. As mentioned earlier the INTERCEPT technology per se offers a series of safety measures. The most important being the highly specific and controlled mechanism of action during the ex vivo treatment process, i.e. the nature of the active compound and the use of long-wavelength UVA light (320–400 nm). Furthermore, the chemistry of the amotosalen-nucleic acid interaction is very well understood and only occurs upon illumination with UVA light. As soon as the light source is turned off the reaction is stopped. As additional safety measure, the use of the CAD has been shown to reduce the amount of residual amotosalen from 150 μmol/l to approximately 0.5 μmol/l (fig. 3). This is a very selective process as other molecules like proteins do not adsorb to the CAD matrix. This removal of amotosalen and its free photoproducts adds additional control to the process. Animal studies and also phase I and phase II clinical trials performed with healthy volunteers have shown that even without the CAD no toxicity was observed.
Fig. 3.
Amotosalen photodegradation after UVA illumination. HPLC traces of a platelet component containing 150 μmol/l amotosalen before illumination with UVA (Pre-UVA), following exposure to a 3 J/cm2 UVA treatment (Post-UVA), and following minimal incubation with the CAD (Post-CAD). The ordinate is optical density at 300 nm (as measured by the HPLC detector) and the abscissa is time in minutes. Equal volumes (20 μl) were injected onto the HPLC column for each of the traces. The amotosalen photoproducts are labeled A-G with residual amotosalen indicated by peak F. The peaks in labeled ‘PP’ are plasma peaks that are present on HPLC chromatograms of controls without amotosalen. TMP (4.5′,8-trimeth-ylpsoralen) serves as an internal standard for the HPLC assay.
A very thorough analysis has been performed to analyze potential safety risks associated with the INTERCEPT technology. In summary neither the toxicological and pharmacokinetic studies performed for amotosalen nor platelet or plasma components treated with the INTERCEPT process showed any evidence for toxicologically relevant effects even at levels well above the exposure a patient would receive in clinical applications (approximately 1μg/kg). The INTERCEPT Blood System provides a strongly increased safety against transfusion-transmitted diseases without any evidence for a safety risk for the patient.
The toxicological studies for INTERCEPT have been reviewed by North American (Food and Drug Administration) as well as European authorities (TÜV, Paul Ehrlich Institute (Germany); Swiss Medics (Switzerland); Afssaps (France)), and no deficiencies in the toxicology program were identified.
Studies Show Lack of Neo-Antigen Formation due to INTERCEPT Treatment
For the patients' safety and for the survival of the transfused blood components in the recipient, it is important to analyze the possibility of neo-antigen formation. Immune responses could be generated as a result of interactions between the photoreactive compound or its photoproducts and lipids or proteins in the platelet or plasma preparation or the recipient. The major cause of drug-induced immune thrombocytopenia is the result of antigen formation from the drug or its metabolites with platelet surface glycoproteins [31]. Very recently plasma proteomic analyses have demonstrated a lack of interaction of amotosalen with the major platelet surface glycoproteins [32].
One of the hallmarks of the INTERCEPT technology is the high degree of specificity of amotosalen for DNA and RNA and its selective binding to the pyrimidine bases. The vast majority of literature on psoralens describes the selectivity of this class of molecules [33, 34].
In order to evaluate a potential neo-antigen formation as result of the INTERCEPT process, studies were performed analyzing the amount of amotosalen and its photoproducts at each step of the procedure. In these analyses the importance of the compound adsorption device for the removal of residual amotosalen and photoproducts was shown. In the analyses no neo-antigens were detected after the photochemical treatment of platelets or plasma [35].
A sensitive immune assay to detect antibodies to treated platelets and plasma was developed for surveillance in clinical studies. Patient serum samples from INTERCEPT phase III clinical trials (4 trials with INTERCEPT platelets and 3 trials with INTERCEPT plasma) were analyzed for the presence of antibodies to potential amotosalen neo-antigens. These large double-blinded, randomized and controlled trials enrolled hematooncological patients with the need for multiple platelet transfusions. The design was such that patients with continuing or recurrent thrombocytopenia (only a subgroup of the enrolled patients) were eligible for a secondary period with platelet transfusions. This allowed evaluation of immune responses to multiple transfusions of INTERCEPT platelets over a period of time. The time between the two treatment cycles would be optimal for antibody formation to occur [15,16].
Furthermore, in the SPRINT trial the incidence of lym-phocytotoxic antibodies was low and statistically less for the INTERCEPT group as compared to the conventional group. Platelet-specific antibodies were found in 11% of the refractory patients receiving INTERCEPT platelets and in 14% of the patients receiving conventional platelets [15,16].
In summary, the patient samples taken from the clinical trials showed that none of the patients exhibited clinical or laboratory manifestations of neo-antigenicity. Beyond this, no other alteration of platelet membrane proteins was identified based on testing for lymphocytotoxic antibodies and platelet-specific alloantibodies. In addition, an independent expert analysis of the SPRINT trial patient data, revealed no evidence for increased incidence of lung-injury (ALI) after transfusion of INTERCEPT treated platelets when compared to conventional platelets [36]. More than 1,000 patients received INTERCEPT-treated products in the course of clinical trials they were enrolled in. Additionally, the active INTERCEPT hemovigilance program [37, 38, 39] currently performed accumulated data on the safety of about 50,000 transfusions, and in total about 700,000 transfusions of INTERCEPT-treated products have been performed. Altogether, from all the clinical experience there is no evidence for immune response to potential neo-antigen formation in patients repeatedly exposed to INTERCEPT platelets and plasma. Based on these results, one can postulate that exposure to INTERCEPT blood components has a low probability of immune responses to potential neo-antigens.
Since, as discussed earlier, any technology applying photo-reactive substances and light as energy source leads to photo-product formation, the best safeguard against any unwanted side reaction is the integration of an adsorption device or filter to remove the reaction products prior to transfusion to the patient.
An impressive panel of clinical studies enrolling more than 1,000 patients has been performed with INTERCEPT-treated platelet and plasma components including very diverse patient groups and analyzing different endpoints. In the clinical trials with INTERCEPT-treated platelets count increment (CI) and corrected count increment (CI) values, the incidence of acute transfusion reactions and proportion of patients with grade 2 bleeding were assessed as primary end-points. The assessment of the very complex endpoint bleeding is very demanding and should be performed according to WHO criteria; it requires a study of appropriate size to guarantee statistical power. The SPRINT study which analyzed grade 2 bleeding as primary and grade 3 and 4 bleeding as one of the secondary endpoints was a randomized, controlled, double-blinded noninferiority study designed with sufficient size (n = 645) and power to evaluate clinical hemostasis in response to transfusion of INTERCEPT and conventional platelets. The SPRINT trial demonstrated noninferiority of INTERCEPT-treated platelets for WHO grade 2 and grade 3 and 4 bleeding.
In a study with 10 patients diagnosed with acute myelo-genous leukemia (n = 8) and acute lymphoblastic leukemia (n = 2) and 40 study transfusions (28 conventional gamma-irradiated and 12 pathogen-inactivated using the INTERCEPT Blood System), Apelseth et al. [40] reported that platelet dose and quality of platelets are important for optimal immediate transfusion response, whereas duration of transfusion effect is influenced mainly by patient variables. Although they reported lower 1-hour and 24-hour CI and CCI as a result of INTERCEPT treatment, no correlation was observed between platelet CI and change in clinical bleeding status after transfusion.
A recent publication by Kerckhoffs et al [41] reported the outcome of a multicenter, unblinded, randomized, noninferiority trial (HOVON 82) comparing the clinical effectiveness of buffy coat-derived leukoreduced platelet components stored for up to 7 days in plasma with platelets stored in Inter-Sol with and without INTERCEPT treatment, in 99, 85 or 94 patients. The study is powered for platelet CI and CCI, and the results were somewhat comparable with previously reported findings in that transfusing INTERCEPT-treated platelets resulted in lower CI and CCI response in patients [16]. It is important to mention though that in the HO VON 82 study the INTERCEPT-treated platelets were in addition gamma-irradiated to a high percentage. Such treatment is not required for INTERCEPT platelets but causes unneeded damage to the platelets [42] and thus can be causative to the lower CI and CCI values. Interesting are also the results from the TESSI study in which the efficacy of INTERCEPT-treated platelets stored for 6 or 7 days was analyzed. This multicenter randomized, controlled, double-blinded study met its primary endpoint which was the 1-hour CCI value, thus demonstrating noninferiority of INTERCEPT platelets when compared to conventional platelets [18].
Despite the HOVON 82 study being underpowered for bleeding assessment, the authors also reported the observation of more bleeding events in the INTERCEPT arm and therefore concluded that INTERCEPT-treated platelets were inferior to untreated platelets stored in plasma. This conclusion illustrates the challenges in the conduct of platelet transfusion clinical trials. For example, the imbalance in patient randomization and the high rate of off-protocol transfusions in HOVON 82 makes a definitive conclusion questionable. In addition, the recent platelet dose study (PLADO) in 1,200 patients, powered for bleeding assessment with 400 patients in each study arm, clearly showed that low doses of platelet transfusions are not associated with more bleeding [43]. Taken together, powered assessment of hemostasis requires studies significantly larger in size than the HOVON 82 study, with defined per protocol methods and conducted by observers blinded to treatment to insure adequate classification of bleeding. Differences in the methods to assess bleeding, lack of blinding, high degree of off-protocol transfusions and inadequate study size of the HOVON 82 study likely contributed to the discrepant reporting of bleeding as compared to the PLADO and SPRINT studies or the experience from the hemovigilance program and the routine use of INTERCEPT platelets.
Broad Spectrum of Pathogen Inactivation by INTERCEPT
A pathogen inactivation or pathogen reduction technology should be versatile and should be tested against as broad a panel of pathogens as possible. To date the major threat for transfusion-transmitted diseases are bacteria which can grow in platelet products due to storage at room temperature. In addition emerging or re-emerging pathogens are of great concern for transfusion of both platelet and plasma components. This is mostly a result of worldwide travels, immigration of donors and climate changes allowing the vector of a given pathogen to survive in countries in which the conditions have changed favorably.
As described above, the mechanism of action of the INTERCEPT technology, especially the fact that the interaction between amotosalen and DNA or RNA molecules is not sequence-specific, theoretically allows for the inactivation of nearly any even yet unknown pathogen.
The spectrum includes enveloped viruses like HIV-1 and HIV-2 where inactivation capacity has been demonstrated for intracellular as well as cell-free viruses and in addition HIV virus isolates from patients suffering with HIV infection. Other cell-associated viruses like HTLV and CMV can be inactivated at very high degree. The inactivation results of the latter are sufficient to support that centers performing INTERCEPT treatment for platelets could replace CMV serology according to their respective national regulatory authorities. This of course has a major impact on inventory management and cost savings due to decreased expiry rates of platelet components for centers that manage dual inventories and rely on CMV serology to maintain CMV-negative donors [39, 40]. This saving may not be as significant in centers that issue leukoreduced blood components for CMV-negative indications.
The inactivation of intracellular viruses is becoming a more and more important feature of the technology. XMRV/MLV was recently detected in tissues from patients suffering with prostate carcinoma and in blood of patients with chronic fatigue syndrome. Apparently, XMRV/MLV, among other cell types, seems to use also lymphocytes for its replication. Very recently the inactivation of XMRV/MLV with the INTERCEPT Blood System was demonstrated for both platelet and erythrocyte components [44].
Also hepatitis viruses including HBV and HCV can be inactivated very effectively as can HTLV-1 and HTLV-2. The broad spectrum of enveloped viruses for which very efficient inactivation has been demonstrated also includes duck hepatitis B virus, bovine viral diarrhea virus, pseudorabiesvirus, vaccinia virus and the influenza strains H1N1 and H5N1. Most of these studies were performed as infectivity studies in cell cultures and animal models [45].
The efficiency with which non-enveloped viruses can be inactivated varies strongly between different viruses. While ad-enovirus 5 and bluetongue virus are very susceptible to the treatment and for parvovirus B19 an intermediate level of inactivation could be demonstrated (table 4), HAV is not and calicivirus is only to a low degree susceptible to inactivation.
Table 4.
The extent of inactivation (log10 reduction) of viruses in platelet components by the INTERCEPT Blood System (official label claims)
| Viruses | Extent of inactivation* (log10 reduction) |
|---|---|
| Enveloped viruses | |
| HIV-1 (cell-associated)*** | >6.1 |
| HIV-1 (cell-free) | >6.2 |
| Clinical isolate of HIV-1 | >3.4 |
| Clinical isolate of HIV-2 | >2.5 |
| Latent proviral HIV-1 | inactivated to the limit of detection |
| HBV (strain MS-2) | >5.5 |
| HCV (strain Hutchison) | >4.5 |
| HTLV-I (human T-cell lymphotropic virus)*** | 4.7** |
| HTLV-II (human T-cell lymphotropic virus)*** | 5.1** |
| Cell-associated cytomegalovirus (CMV)*** | >5.9 |
| Bovine viral diarrhea virus (BVDV, model virus for human HCV) | >6.0 |
| Duck hepatitis B virus (DHBV, model virus for human HBV) | >6.2 |
| West Nile virus | >6.0 |
| SARS-CoV (human corona virus) | >5.8 |
| Chikungunya virus | >6.4 |
| Influenza A H5N1 virus (avian influenza) | >5.9 |
| Non-enveloped viruses | |
| Bluetongue virus, type 11 | >5.0 |
| Calicivirus | 1.7–2.4 |
| Human adenovirus-5 | >5.9 |
| Parvo (parvovirus B19) | 3.5 to >5.0 |
‘>’ refers to inactivation below the limit of detection of the assay. In some cases assays have a very small dynamic range due to limits on attainable virus titers.
Inherent low-level background in non-infected indicator cells precludes ‘>’ of HTLV.
Intracellular.
Of great interest is the capacity of the INTERCEPT technology to inactivate emerging and re-emerging pathogens. Highly effective inactivation has been demonstrated for example for a large panel of (re)-emerging viruses. These include the coronavirus causing SARS, West Nile virus, the earlier mentioned influenza strains H1N1 and H5N1 as well as chickungunya virus and dengue virus. The versatility and efficiency of the INTERCEPT Blood System was demonstrated when in 2006 a chickungunya epidemic occurred in the French overseas department of He de La Reunion. When about one third of the population was infected by the virus, local blood was suspended. Due to the fact that the INTERCEPT technology was approved by the French regulatory body Afssaps, the INTERCEPT technology for platelets was rapidly implemented. After implementation of the INTERCEPT Blood System no cases of chickungunya transmission via blood components have been reported [46]. Similar experience occurred in Guadeloupe and La Martinique where a more recent dengue outbreak caused severe problems. Also here the INTERCEPT technology helped to provide safe platelet components.
Besides (re)-emerging pathogens, bacteria are a major threat to blood safety especially for platelet components. Therefore, any technology for pathogen inactivation has to achieve the highest level of inactivation. To test the capacity of the INTERCEPT technology to inactivate bacteria, studies with a broad spectrum of aerobic and anaerobic Gram-positive and Gram-negative bacteria as well as spirochetes has been performed [9]. The assays have shown that both slow-growing and fast-growing bacteria as well as samples containing low titers or high titers of bacteria can be effectively inactivated (table 5). The pathogen-inactivated platelet concentrates were retained for up to 5 days, and growth of bacteria upon cultivation was analyzed. Even after this holding period no bacterial growth could be detected in the INTERCEPT-treated products [11]. Bacterial spores, like those from Bacillus cereus, are quite resistant to pathogen inactivation. The vegetative form of B. cereus instead was susceptible to INTERCEPT treatment. It is important to mention that bacteria like B. cereus normally exist in their vegetative form while in blood. They only form spores as a mechanism to survive when the conditions are not favorable. In this regard spores might not be a real important threat for transfusion of blood components. A recent study compared the capacity of bacterial detection methods with the INTERCEPT technology for reducing the risk associated with transfusion of platelet components contaminated with low levels of bacteria. The authors concluded that the bacterial detection method could lead to contaminated products being released for transfusion based on test-negative results while inactivation could reduce the risk [46].
Table 5.
The extent of inactivation (log10 reduction) of bacteria in platelet components by the INTERCEPT Blood System (official label claims)
| Bacterial species | Extent of inactivation* (log10 reduction) |
|---|---|
| Gram-negative bacteria | |
| Escherichia coli | >6.4 |
| Serratia marcescens | >6.7 |
| Klebsiella pneumoniae | >5.6 |
| Pseudomonas aeruginosa | 4.5 |
| Salmonella choleraesuis | >6.2 |
| Yersinia enterocolitica | >5.9 |
| Enterobacter cloacae | 5.9 |
| Gram-positive bacteria | |
| Staphylococcus epidermidis | >6.6 |
| Staphylococcus aureus | 6.6 |
| Streptococcus pyogenes | >6.8 |
| Listeria monocytogenes | >6.3 |
| Corynebacterium minutissimum | >6.3 |
| Bacillus cereus (includes spores) | 3.6 |
| Bacillus cereus (vegetative) | >6.0 |
| Bifidobacterium adolescentis | >6.5 |
| Propionibacterium acnes | >6.7 |
| Lactobacillus species | >6.9 |
| Clostridium perfringens (vegetative form) | >7.0 |
| Spirochete bacteria | |
| Treponema pallidum (syphilis) | ≥6.8 to ≤7.0 |
| Borrelia burgdorferi (Lyme disease) | >6.8 |
‘>’ refers to inactivation below the limit of detection of the assay.
In recent years the number of reports concerning infections with emerging protozoa is increasing. Of biggest concern are Plasmodium falciparum (malaria), Trypanosoma cruzi (chagas disease), Leishmania and Babesia microti. The ability to inactivate also these protozoa with the INTERCEPT technology has been demonstrated recently (table 6) [48, 49].
Table 6.
The extent of inactivation (log10 reduction) of parasites in platelet components by the INTERCEPT Blood System (official label claims)
| Parasites | Extent of inactivation* (log10 reduction) |
|---|---|
| Plasmodium falciparum**(malaria) | ≥6.0 |
| Trypanosoma cruzi (Chagas' disease) | >5.3 |
| Leishmania mexicana (metacyclic promastigote stage) | >5.0 |
| Leishmania major Jish (amastigote stage) | >4.3 |
| Babesia microti (babesiosis) | >5.3 |
‘>’ refers to inactivation below the limit of detection of the assay.
Intracellular.
Besides the infectious pathogens, residual leukocytes can cause severe harm to recipients of blood components. Leukocytes can cause transfusion-associated graft-versus-host disease (TA-GVHD), especially in immunosuppressed patients. Currently, blood components for patients at risk for TA-GVHD are gamma-irradiated to prevent this highly morbid complication.
Furthermore, leukocytes can cause febrile nonhemolytic transfusion reactions as a result of the cytokine/chemokine production [50, 51], and they represent the reservoir for intra-cellular pathogens like for example HIV, EBV, CMV or the newly described XMRV/MLV but also for Leishmania. Although widely implemented, leukofiltration does not remove all of the residual leukocytes in a blood component. A series of experiments has been performed to analyze the inactivation of leukocytes with the INTERCEPT technology (table 7) [52, 53, 54]. In vitro and in vivo studies in the mouse as well as with human T cells showed an inactivation capacity exceeding that of gamma-irradiation [55]. The robustness of the leukocyte inactivation with INTERCEPT has been demonstrated in limiting dilution assays of clonal T-cell proliferation in comparison with gamma-irradiation at 2,500 cGy. While in terms of the degree of inactivation both technologies were comparable, small deviations in the dose of gamma-irradiation could cause the inactivation to fail, whereas INTERCEPT treatment was more robust in this regard and thus exhibits the higher safety margin [56].
Table 7.
High levels of leukocytes are inactivated in platelet components by the INTERCEPT Blood System
| Assay system | Evidence of inactivation |
|---|---|
| In vitro | |
| Limiting dilution assay | >5.4 log10 reduction of viable T cells |
| DNA modification | Approximately one amotosalen adduct per 83 base pairs |
| Polymerase chain reaction | Amplification inhibited by amotosalen – DNA adducts |
| Cytokine synthesis | Elimination of IL-8, IL-1β synthesis during storage |
| In vivo | |
| Murine transfusion model | Prevention of TA-GVHD in a murine parent to F1 transfusion model |
Hei et al. [57] demonstrated the effect INTERCEPT treatment on the cytokine/chemokine production of residual leukocytes during platelet storage. They analyzed the presence of supernatant IL-8 and IL-β by ELISA over a period of 5 and 7 days respectively. While gamma-irradiation causes infrequent strand breaks in the leukocyte DNA and expression of IL-8 and IL-β could be detected, these substances could not be detected after INTERCEPT treatment [57]. Similar findings were described by Cognasse et al. [58, 59]. They demonstrated that INTERCEPT treatment of platelets did not increase the release of platelet derived growth factor-AB, tumor necrosis factor and, most interestingly, soluble CD40 ligand which has been described as implicated in transfusion-associated lung injury [58, 59].
The results regarding the inactivation of leukocytes led to the official label claim that INTERCEPT treatment of platelets may replace gamma-irradiation as a measure to prevent TA-GVHD. Multiple centers in different European countries stopped gamma-irradiation altogether after implementation of the INTERCEPT Blood System and are treating patients at risk for TA-GVHD with non-gamma-irradiated, INTER-CEPT-treated platelet concentrates.
Summary of Log-Reduction Values
The greater the level of inactivation of pathogens like bacteria, viruses, protozoa and spirochetes, the greater is the contribution of such a technology to the safety of the blood components and to the lowering of the risk for the patient. In addition, only a technology with proven high degree of inactivation/reduction of pathogens will eventually allow for changing the paradigm in blood safety from the development and implementation of novel screening methods and donor deferrals to a pro-active approach, namely pathogen inactivation [60]. The INTERCEPT technology provides a high level of pathogen inactivation for a very broad spectrum of pathogens. The values given in tables 4, 5, 6 were the basis for the official label claims granted for the INTERCEPT Blood System from the regulatory body.
Current State of Accreditation in Europe
The INTERCEPT Blood system has been registered with a class III CE mark as a drug device combination. In contrast to a class IIb CE mark this requires a very thorough preclinical and clinical development program. Also any statement regarding the performance of the product has to be approved as official label claim. Therefore, as another difference to class IIb products, no self-declaration can be made but all claims are tested and based on data submitted before the respective claims can be granted. The INTERCEPT Blood System for platelets has gained the CE mark in 2002 and for plasma in 2006, allowing market distribution in Europe for both components.
In addition the INTERCEPT Blood System for platelets and plasma has been approved by the French regulatory authority Afssaps. In Germany the Paul Ehrlich Institute granted the marketing authorization to some centers for platelets, and the respective files for INTERCEPT plasma are in the final stage of review. In Switzerland the SwissMedics approved INTERCEPT for platelets and for plasma.
The official label claims state that ‘INTERCEPT treated platelets or plasma components are not clinically different from conventional components and are infused according to standard clinical practice guidelines’.
There is no patient exclusion, and thus the products can and are being used for all patients including neonates, children, pregnant women, patients undergoing stem cell transplantations and patients under very strong chemotherapeutical treatment.
The CE mark for INTERCEPT-treated platelets officially allows for the storage for up to 7 days (here regional regulations apply). Platelets stored for 7 days are routinely used in several countries with the exception of France and Germany.
The approval by the Paul Ehrlich Institute allows the use of INTERCEPT as alternative to gamma-irradiation and to omit CMV serology.
In addition, in regions where leukodepletion is not mandatory, INTERCEPT can also officially be performed without prior leukodepletion.
All claims regarding the efficacy of the INTERCEPT technology and regarding the inactivation of a given pathogen were officially granted by the regulatory authority and were not the result of self-declaration.
Disclosure Statement
Both authors are employees of Cerus Corporation
References
- 1.Alter HJ. Pathogen reduction: a precautionary principle paradigm. Transfus Med Rev. 2008;22:97–102. doi: 10.1016/j.tmrv.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein HG, Anderson D, Bernardi MJ, Cable R, Carey W, Hoch JS, Robitaille N, Sivilotti ML, Smaill F. Pathogen inactivation: making decisions about new technologies. Report of a consensus conference. Transfusion. 2007;47:2338–2347. doi: 10.1111/j.1537-2995.2007.01512.x. [DOI] [PubMed] [Google Scholar]
- 3.Wagstaff D. Dietary exposure to furocoumarins. Regul Toxicol Pharmacol. 1991;14:261–272. doi: 10.1016/0273-2300(91)90029-u. [DOI] [PubMed] [Google Scholar]
- 4.Shi Y, Lipson SE, Chi DY, et al. Applications of psoralens as probes of nucleic acid structure and function. In: Morrison H, editor. Bioorganic Photochemistry and Nucleic Acids. New York: Wiley; 1990. pp. 341–378. [Google Scholar]
- 5.Gottlieb SL, Wolfe JT, Fox FE, DeNardo BJ, Macey WH, Bromley PG, Lessin SR, Rook AH. Treatment of cutaneous T-cell lymphoma with extracorporeal photopheresis monotherapy and in combination with recombinant interferon alfa: a 10-year experience at a single institution. J Am Acad Dermatol. 1996;35:946–957. doi: 10.1016/s0190-9622(96)90119-x. [DOI] [PubMed] [Google Scholar]
- 6.Honigsmann H. Phototherapy for psoriasis. din Exp Dermatol. 2001;26:343–350. doi: 10.1046/j.1365-2230.2001.00828.x. [DOI] [PubMed] [Google Scholar]
- 7.Taylor A, Gasparro FP. Extracorporeal photoche-motherapy for cutaneous T-cell lymphoma and other diseases. Semin Hematol. 1992;29:132–141. [PubMed] [Google Scholar]
- 8.Wollowitz S. Fundamentals of the psoralen-based Helinx technology for inactivation of infectious pathogens and leukocytes in platelets and plasma. Semin Hematol. 2001;38(suppl 11):4-11. doi: 10.1016/s0037-1963(01)90118-0. [DOI] [PubMed] [Google Scholar]
- 9.Lin L, Dikeman R, Molini B, Lukehart SA, Lane R, Dupuis K, Metzel P, Corash L. Photochemical treatment of platelet concentrates with amotosalen and UVA inactivates a broad spectrum of pathogenic bacteria. Transfusion. 2004;44:1496–1504. doi: 10.1111/j.1537-2995.2004.04125.x. [DOI] [PubMed] [Google Scholar]
- 10.van Rhenen DJ, Vermeij J, Mayaudon V, Hind C, Lin L, Corash L. Functional characteristics of S-59 photochemically treated platelet concentrates derived from buffy coats. Vox Sang. 2000;79:206–214. doi: 10.1159/000056732. [DOI] [PubMed] [Google Scholar]
- 11.Knutson F, Alfonso R, Dupuis K, Mayaudon V, Lin L, Corash L, Hogman CF. Photochemical inactivation of bacteria and HIV in buffy-coat-derived platelet concentrates under conditions that preserve in vitro platelet function. Vox Sang. 2000;78:209–216. doi: 10.1159/000031183. [DOI] [PubMed] [Google Scholar]
- 12.Terpstra FG, van't Wout AB, Schuitemaker H, van Engelenburg FA, Dekkers DW, Verhaar R, de Korte D, Verhoeven AJ. Potential and limitation of UVC irradiation for the inactivation of pathogens in platelet concentrates. Transfusion. 2008;48:304–313. doi: 10.1111/j.1537-2995.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- 13.Verhaar R, Dekkers DW, De Cuyper IM, Ginsberg MH, de Korte D, Verhoeven AJ. UV-C irradiation disrupts platelet surface disulfide bonds and activates the platelet integrin alphallbbeta3. Blood. 2008;112:4935–4939. doi: 10.1182/blood-2008-04-151043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lozano M, Galan A, Mazzara R, Corash L, Escolar G. Leucocyte-reduced buffy coat derived platelet concentrates photochemically treated with amo-tosalen-HQ and ultraviolet A light stored up to 7 days: assessment of hemostatic function under flow conditions. Transfusion. 2006;47:666–671. doi: 10.1111/j.1537-2995.2007.01169.x. [DOI] [PubMed] [Google Scholar]
- 15.van Rhenen D, Gulliksson H, Cazenave JP, Pam-philon D, Ljungman P, Kluter H, Vermeij H, Ka-ppers-Klunne M, de Greef G, Laforet M, Lioure B, Davis K, Marblie S, Mayaudon V, Flament J, Conlan M, Lin L, Metzel P, Buchholz D, Corash L. Transfusion of pooled buffy coat platelet components prepared with photochemical pathogen inactivation treatment: the euroSPRITE trial. Blood. 2003;101:2426–2433. doi: 10.1182/blood-2002-03-0932. [DOI] [PubMed] [Google Scholar]
- 16.McCuUough J, Vesole DH, Benjamin RJ, Slichter SJ, Pineda A, Snyder E, Stadtmauer EA, Lopez-Plaza I, Coutre S, Strauss RG, Goodnough LT, Fridey JL, Raife T, Cable R, Murphy S, Howard F, Davis K, Lin JS, Metzel P, Lin L, Koutsoukos A, Corash L, Buchholz DH, Conlan MG. Therapeutic efficacy and safety of platelets treated with a photochemical process for pathogen inactivation: the SPRINT trial. Blood. 2004;104:1534–1541. doi: 10.1182/blood-2003-12-4443. [DOI] [PubMed] [Google Scholar]
- 17.Janetzko K, Cazenave JP, Kluter H, Kientz D, Michel M, Beris P, Lioure B, Hastka J, Marblie S, Mayaudon V, Lin L, Lin JS, Conlan MG, Flament J. Therapeutic efficacy and safety of photochemically treated apheresis platelets processed with an optimized integrated set. Transfusion. 2005;45:1443–1452. doi: 10.1111/j.1537-2995.2005.00550.x. [DOI] [PubMed] [Google Scholar]
- 18.Lozano M, Knutson F, Tardivel R, Cid J, Maymo R, Lof HM, Roddie HP, Pelly JP, Docherty AR, Sherman CD, Lin L, Propst M, Corash L, Prowse CV. A multi-center study of therapeutic efficacy and safety of platelet components prepared with pathogen inactivation (INTERCEPT) stored for 6 or 7 days prior to transfusion. Vox Sang. 2010;99:13. doi: 10.1111/j.1365-2141.2011.08635.x. [DOI] [PubMed] [Google Scholar]
- 19.Cazenave JP, Isola H, Waller C, Mendel I, Kientz D, Laforet M, Raidot JP, Kandel G, Wiesel ML, Corash L. Use of additive solutions and pathogen inactivation treatment of platelet components in a regional blood center: impact on patient outcomes and component utilization during a 3-year period. Transfusion. 2010 doi: 10.1111/j.1537-2995.2010.02873.x. doi: 10.1111/j.1537-2995. 2010.02873.x. [DOI] [PubMed] [Google Scholar]
- 20.Singh Y, Sawyer L, Pinkoski L, Dupuis K, Hsu J, Lin L, Corash L. Photochemical treatment of plasma with amotosalen and UVA light inactivates pathogens while retaining coagulation function. Transfusion. 2006;46:1168–1177. doi: 10.1111/j.1537-2995.2006.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irsch J, Pinkoski L, Corash L, Lin L. INTERCEPT plasma: comparability with conventional fresh-frozen plasma based on coagulation function – an in vitro analysis. Vox Sang. 2010;98:47–55. doi: 10.1111/j.1423-0410.2009.01224.x. [DOI] [PubMed] [Google Scholar]
- 22.Schlenke P, Hervig T, Isola H, Wiesel ML, Kientz D, Pinkoski L, Singh Y, Lin L, Corash L, Cazenave JP. Photochemical treatment of plasma with amotosalen and UVA light: process validation in three European blood centers. Transfusion. 2008;48:697–705. doi: 10.1111/j.1537-2995.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- 23.de Alarcon P, Benjamin R, Dugdale M, Kessler C, Shopnick R, Smith P, Abshire T, Hamble-ton J, Matthew P, Ortiz I, Cohen A, Konkle BA, Streiff M, Lee M, Corash L, Wages D. Fresh frozen plasma prepared with amotosalen HC1 (S-59) photochemical pathogen inactivation (PCT-FFP): transfusion of patients with congenital factor deficiencies. Transfusion. 2005;45:1362–1372. doi: 10.1111/j.1537-2995.2005.00216.x. [DOI] [PubMed] [Google Scholar]
- 24.Mintz PD, Bass NM, Petz LD, Steadman R, Streiff M, McCuUough J, Burks S, Wages D, VanDoren S, Corash L. Photochemically treated fresh frozen plasma for transfusion of patients with acquired coagulopathy of liver disease. Blood. 2006;107:3753–3760. doi: 10.1182/blood-2004-03-0930. [DOI] [PubMed] [Google Scholar]
- 25.Mintz PD, Neff A, MacKenzie M, Goodnough LT, Hillyer C, Kessler C, McCrae K, Menitove JE, Skikne BS, Damon L, Lopez-Plaza I, Roualt C, Crookston KP, Benjamin RJ, George J, Lin JS, Corash L, Conlan MG. A randomized, controlled phase III trial of therapeutic plasma exchange with fresh frozen plasma prepared with amotosalen and UVA light compared to untreated fresh frozen plasma in thrombotic thrombocytopenic purpura. Transfusion. 2006;46:1693–1704. doi: 10.1111/j.1537-2995.2006.00959.x. [DOI] [PubMed] [Google Scholar]
- 26.Cazenave JP, Waller C, Kientz D, Mendel I, Lin L, Jacquet M, Propst M, Liu W, Corash L, Sundin D, Defoin L, Masse N, Osselaer JC. An active hemovigilance program characterizing the safety profile of 7,483 transfusions with plasma components prepared with amotosalen and UVA photochemical treatment. Transfusion. 2010;50:1210–1209. doi: 10.1111/j.1537-2995.2009.02579.x. [DOI] [PubMed] [Google Scholar]
- 27.de Valensart N, Rapaille A, Goossenaerts E, Sond-ag-Thull D, Deneys V. Study of coagulation function in thawed apheresis plasma for photochemical treatment by amotosalen and UVA. Vox Sang. 2009;96:213–218. doi: 10.1111/j.1423-0410.2008.001147.x. [DOI] [PubMed] [Google Scholar]
- 28.Ciaravino V, McCuUough T, Dayan AD. Pharma-cokinetic and toxicology assessment of INTERCEPT (S-59 AND UVA treated) platelets. Hum Exp Toxicol. 2001;20:533–550. doi: 10.1191/096032701718120319. [DOI] [PubMed] [Google Scholar]
- 29.Tice RR, Gatehouse D, Kirkland D, Speit G. The pathogen reduction treatment of platelets with S-59 HC1 (amotosalen) plus ultraviolet A light: genotoxicity profile and hazard assessment. Mutat Res. 2007;630:50–68. doi: 10.1016/j.mrgentox.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Ciaravino V. Preclinical safety of a nucleic acid-targeted Helinx compound: a clinical perspective. Semin Hematol. 2001;38(suppl 11):12-19. doi: 10.1016/s0037-1963(01)90119-2. [DOI] [PubMed] [Google Scholar]
- 31.Aster RH. Drug-induced immune thrombocytope-nia: an overview of pathogenesis. Semin Hematol. 1999;36:2–6. [PubMed] [Google Scholar]
- 32.Cazenave JP, Ohlmann P, Isola H, Gachet C. Photochemical pathogen inactivation treatment of human plasma (amotosalen+ UVA) has no major impact on the protein pattern assessed by a 2-DIGE proteomic assay. Blood. 2008;112:696. [Google Scholar]
- 33.Gasparro F, Chan G, Edelson RL. Phototherapy and photopharmacology. Yale J Biol Med. 1985;58:519–534. [PMC free article] [PubMed] [Google Scholar]
- 34.Cimino GD, Gamper HB, Isaacs ST, Hearst JE. Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry and biochemistry. Ann Rev Biochem. 1985;54:1154–1193. doi: 10.1146/annurev.bi.54.070185.005443. [DOI] [PubMed] [Google Scholar]
- 35.Lin L, Conlan MG, Tessman J, Cimino G, Porter S. Amotosalen interactions with platelet and plasma components: absence of neoantigen formation after photochemical treatment. Transfusion. 2005;45:1610–1620. doi: 10.1111/j.1537-2995.2005.00554.x. [DOI] [PubMed] [Google Scholar]
- 36.Corash L, Lin JS, Sherman CD, Eiden J. Determination of acute lung injury following repeated platelet transfusions. Blood. 2010 doi: 10.1182/blood-2010-06-293399. doi:10.1182/blood-2010-06-293399. [DOI] [PubMed] [Google Scholar]
- 37.Osselaer JC, Messe N, Hervig T, Bueno J, Castro E, Espinosa A, Accorsi P, Junge K, Jacquet M, Flament J, Corash L. A prospective observational cohort safety study of 5106 platelet transfusions with components prepared with photochemical pathogen inactivation treatment. Transfusion. 2008;48:1061–1071. doi: 10.1111/j.1537-2995.2008.01643.x. [DOI] [PubMed] [Google Scholar]
- 38.Osselaer JC, Cazenave JP, Lambermont M, Gar-raud O, Hidajat M, Barbolla L, Tardivel R, Defoin L, Waller C, Mendel I, Raidot JP, Kandel G, De Meuter R, Fabrigli P, Dehenau D, Arroyo JL, Padron F, Gouezec H, Corral M, Jacquet M, Sundin D, Lin L, Corash L. An active haemov-igilance programme characterizing the safety profile of 7437 platelet transfusions prepared with amotosalen photochemical treatment. Vox Sang. 2008;94:315–323. doi: 10.1111/j.1423-0410.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- 39.Osselaer JC, Doyen C, Defoin L, Debry C, Gof-faux M, Messe N, Hooydonk MV, Bosly A, Lin JS, Lin L, Corash L. Universal adoption of pathogen inactivation of platelet components: impact on platelet and red blood cell component use. Transfusion. 2009;49:1412–1422. doi: 10.1111/j.1537-2995.2009.02151.x. [DOI] [PubMed] [Google Scholar]
- 40.Apelseth TO, Bruserud O, Wentzel-Larsen T, Hervig T. Therapeutic efficacy of platelet transfusion in patients with acute leukemia: an evaluation of methods. Transfusion. 2010;50:766–775. doi: 10.1111/j.1537-2995.2009.02540.x. [DOI] [PubMed] [Google Scholar]
- 41.Kerkhoffs JL, van Putten WL, Novotny VM, Te Boekhorst PA, Schipperus MR, Zwaginga JJ, van Pampus LC, de Greef GE, Luten M, Huijgens PC, Brand A, van Rhenen DJ. Clinical effectiveness of leucoreduced, pooled donor platelet concentrates, stored in plasma or additive solution with and without pathogen reduction. Br J Haematol. 2010;150:209–217. doi: 10.1111/j.1365-2141.2010.08227.x. [DOI] [PubMed] [Google Scholar]
- 42.Slichter SJ, Davis K, Enright H, Braine H, Gern-sheimer T, Kao K, Kickler T, Lee E, McFarland J, McCullough J, Rodey G, Schiffer CA, Woodson R. Factors affecting posttransfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood. 2005;105:4106–4114. doi: 10.1182/blood-2003-08-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slichter SJ, Kaufman RM, Assmann SF, McCul-loough J, Triulzi DJ, Straus RG, Gernsheimer TB, Ness PM, Brecher ME, Josephson CD, Konkle BA, Woodson RD, Ortel TL, Hillyer CD, Sker-ett DL, McCrae KR, Sloan SR, Uhl L, George JN, Aquino VM, Manno CS, McFarland JG, Hess JR, Leissinger C, Granger S. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med. 2010;362:600–613. doi: 10.1056/NEJMoa0904084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mikovits J, Hagen K, Sadowski C, Ruscetti F, Liu W, Lin L. Inactivation of XMRV by the Intercept Blood System in platelet concentrates. Transfusion. 2010;50:211A. [Google Scholar]
- 45.Lin L, Hanson CV, Alter HJ, Jauvin V, Bernard KA, Murthy KK, Metzel P, Corash L. Inactivation of viruses in platelet concentrates by photochemical treatment with amotosalen and long-wavelength ultraviolet light. Transfusion. 2005;45:580–590. doi: 10.1111/j.0041-1132.2005.04316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rasongles P, Angelini-Tibert MF, Simon P, Currie C, Isola H, Kientz D, Slaedts M, Jacquet M, Sundin D, Lin L, Corash L, Cazenave JP. Transfusion of platelet components prepared with photochemical pathogen inactivation treatment during a chikun-gunya virus epidemic in He de La Reunion. Trans fusion. 2009;49:1083–1091. doi: 10.1111/j.1537-2995.2009.02111.x. [DOI] [PubMed] [Google Scholar]
- 47.Nussbaumer W, Allersdorfer D, Grabmer C, Rheinschmidt M, Lin L, Schonitzer D, Lass-Florl C. Prevention of transfusion of platelet components contaminated with low levels of bacteria: a comparison of bacteria culture and pathogen inactivation methods. Transfusion. 2007;47:1125–1133. doi: 10.1111/j.1537-2995.2007.01247.x. [DOI] [PubMed] [Google Scholar]
- 48.VanVoorhis WC, Barrett LK, Eastman RT, Alfonso R, Dupuis K. Trypanosoma cruzi inactivation in human platelet concentrates and plasma by psor-alen (amotosalen HCl) and long-wavelength UV. Antimicrob Agents Chemother. 2003;47:475–479. doi: 10.1128/AAC.47.2.475-479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grellier P, Benach J, Labaied M, Charneau S, Gil H, Monsalve G, Alfonso R, Sawyer L, Lin L, Stei-ert M, Kupuis K. Photochemical inactivation with amotosalen and long-wavelength ultraviolet light of Plasmodium and Babesia in platelet and plasma components. Transfusion. 2008;48:1676–1684. doi: 10.1111/j.1537-2995.2007.01762.x. [DOI] [PubMed] [Google Scholar]
- 50.Heddle NM, Kalma L, Singer J, Richards C, Fedak P, Walker I, Kelton JG. The role of plasma from platelet concentrates in transfusion reactions. N Engl J Med. 1994;331:625–628. doi: 10.1056/NEJM199409083311001. [DOI] [PubMed] [Google Scholar]
- 51.Muylle L, Joos M, Wouters E, Bock RD, Peeter-mens ME. Increased tumor necrosis factor alpha (TNF-alpha), interleukin 1, and interleukin 6 (IL-6) levels in the plasma of stored plateet concentrates: relationship between TNF-alpha and IL-6 levels and febrile transfusion reactions. Transfusion. 1993;33:195–199. doi: 10.1046/j.1537-2995.1993.33393174443.x. [DOI] [PubMed] [Google Scholar]
- 52.Grass JA, Hei DJ, Metchette K, Cimino GD, Wi-esehahn GP, Corash L, Lin L. Inactivation of leukocytes in platelet concentrates by psoralen plus UVA. Blood. 1998;91:2180–2188. [PubMed] [Google Scholar]
- 53.Fiebig E, Hirschkom DF, Maino VC, Grass JA, Lin L, Busch MP. Assessment of donor T-cell function in cellular blood components by the CD69 induction assay: effects of storage, gamma irradiation, and photochemical treatment. Transfusion. 2000;40:761–770. doi: 10.1046/j.1537-2995.2000.40070761.x. [DOI] [PubMed] [Google Scholar]
- 54.Truitt R, Johnson BD, Hanke C, Talib S, Hearst JE. Photochemical treatment with S-59 psoralen and ultraviolet A light to control the fate of naive or primed T lymphocytes in vivo after allogeneic bone marrow transplantation. J Immunol. 1999;163:5145–5156. [PubMed] [Google Scholar]
- 55.Corash L, Lin L. Novel processes for inactivation of leukocytes to prevent transfusion-associated graft-versus-host disease. Bone Marrow Transplant. 2004;33:1–7. doi: 10.1038/sj.bmt.1704284. [DOI] [PubMed] [Google Scholar]
- 56.Schlenke P, Kirchner H, Corash L. Concerning Caspari et al: Pathogen inactivation of cellular blood products – more security for the patient or less? Transfus Med Hemother. 2005;32:45–48. [Google Scholar]
- 57.Hei DJ, Grass J, Lin L, Corash L, Cimino G. Elimination of cytokine production in stored platelet concentrate aliquots by photochemical treatment with psoralen plus ultraviolet A light. Transfusion. 1999;39:239–248. doi: 10.1046/j.1537-2995.1999.39399219279.x. [DOI] [PubMed] [Google Scholar]
- 58.Khan SY, Kelher MR, Heal JM, Blumberg N, Boshkov LK, Phipps R, Gettings KF, McLaughlin NJ, Silhman CC. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–2462. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cognasse F, Osselaer JC, Payrat JM, Chavarin P, Corash L, Garraud O. Release of immune modulation factors from platelet concentrates during storage after photochemical pathogen inactivation treatment. Transfusion. 2008;48:809–813. doi: 10.1111/j.1537-2995.2008.01655.x. [DOI] [PubMed] [Google Scholar]
- 60.Allain JP, Bianco C, Blajchman MA, Brecher ME, Busch M, Leiby D, Lin L, Stramer S. Protecting the blood supplly from emerging pathogens: the role of pathogen inactivation. Transfus Med Rev. 2005;19:110–126. doi: 10.1016/j.tmrv.2004.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]