Summary
Pathogen inactivation systems are in use in many European countries as routine procedures. However, a pathogen inactivation system for erythrocytes is currently not available. Although significant improvements have been made to decrease the incidence of transfusion-transmitted infections, risks remain for infectious disease agents specific to red blood cell concentrates, such as parasitic infections resulting in babesiosis and malaria. The pathogen inactivation system for erythrocytes utilizes S-303 and glutathione for the treatment of red blood cell concentrates. Preclinical studies to assess the pathogen inactivation efficacy and toxicology as well as preliminary clinical studies have been completed. Preclinical studies have shown log reduction for leukocytes, several viruses and bacteria in excess of 4 to 6 logs. Preclinical toxicology studies were conducted to enable the initiation of two phase III clinical studies in the USA for support of acute and chronic anemia. A second-generation system was developed after observation of an unexpected immune response in two chronic anemia patients. Preclinical pathogen inactivation studies, serological evaluations and a clinical study to evaluate survival of S-303-treated erythrocytes have been completed to support advanced development of the S-303 pathogen inactivation system. A functional system for the inactivation of red blood cell concentrates has been completed and is reaching clinical application.
Keywords: Pathogen inactivation, S-303, Erythrocyte, Red cell concentrates
Zusammenfassung
Technologien zur Pathogeninaktivierung sind in zahlreichen europäischen Ländern als Routineverfahren etabliert, jedoch fehlt ein solches für Erythrozyten. Auch vor dem Hintergrund signifikanter Verbesserungen bei der Testung von Blutkomponenten verbleiben Restrisiken, einschließlich von Pathogenen, wie etwa im Bereich der auch durch Erythrozyten übertragenen Parasiten oder von Bakterien. Das Pathogeninaktivierungssystem für Erythrozyten nutzt S-303 und Glutathion im funktionell geschlossenen System. Präklinische Studien haben eine Reduktion von Leukozyten, mehreren Modellviren und von Bakterien um 4–6 Log-Stufen belegt. Präklinische toxikologische Studien wurden durchgeführt und haben zur Initiierung von Phase-III-Studien bei akuter und chronischer Anämie in den USA geführt. Nach Immunantworten in 2 Patienten gegen pathogeninaktivierte Erythrozyten wurde eine neue Generation des Inaktivie-rungssystems entwickelt. Präklinische Inaktivierungs-studien, serologische Evaluationen und eine klinische Studie zur Ermittlung der Überlebensrate von S-303-behandelten Erythrozyten liegen vor; die Daten unter-stützen die Weiterentwicklung des Pathogeninaktivie-rungssystems. Ein funktionelles System zur Inaktivie-rung von Erythrozytenkonzentraten hat die präklinische Entwicklung komplettiert und die erste klinische Anwendung erreicht.
Rationale for Pathogen Inactivation for Red Blood Cells
Pathogen inactivation technologies have been developed and have been introduced into clinical practice to enable inactivation of pathogens and leukocytes in both platelet concentrates and therapeutic plasma. The best investigated technology with demonstration of therapeutic efficacy as well as safety is based on photochemical treatment using UVA and psoralens [1, 2, 3, 4, 5, 6, 7]. More recently, other systems using either riboflavin and broadband UV, methylene blue and white light, or UVC have been further developed and, at least in part, have been shown to provide safe and functional components [8, 9]. For the first time in the history of blood transfusion, physicians have been provided with a proactive means towards sterilization of labile blood components. This holds true in particular if procedures can demonstrate 4–6 log inactivation efficacy for many tested microorganisms [10, 11]. Reactive strategies to blood safety that include current tests and extending the possibilities of additional testing have limitations based on detection sensitivity, specificity and logistics for implementation. Development and implementation of pathogen inactivation technologies for all components would represent a significant milestone to provide the safest blood transfusion therapy [12,13].
However, pathogen inactivation systems have so far not provided a clinically implementable procedure for pathogen inactivation of red blood cells (RBCs), the most commonly used blood component for support of severe anemias and as critical supportive therapy for acute bleeding. While RBC transfusions provide therapeutic benefits and improve patient outcomes by increasing hemoglobin levels and enhancing oxygen delivery to tissues, there are both infectious and noninfectious risks associated with transfusion that may result in unintended adverse outcomes. Risks for transfusion-transmitted infections caused by viruses such as HIV for which intensive testing is in place has been significantly reduced [14]. However, residual risk of transfusion-transmitted infections remains due to emerging viruses [15], bacteria [16], protozoa [17] and residual contaminating leukocytes [18]. Without a pathogen inactivation technology for RBCs, the risk for transfusion-transmitted infections would remain for the majority of patients undergoing blood transfusions.
In addition to infectious risks associated with blood transfusion, there are noninfectious risks that include transfusion-associated graft-versus-host disease (TA-GVHD) [19], transfusion-related acute lung injury (TRALI) [20], acute transfusion reactions [21], allo-immunization to leukocyte antigens [22], microchimerism and immune modulation [23, 24]. Reduction in the incidence of noninfectious risks associated with RBC transfusions has largely relied on leukocyte reduction and gamma irradiation to reduce some of the adverse immune responses. Although not universally implemented, leukocyte depletion has shown the potential to reduce neutrophil priming activity [25], which is believed to be involved in the two-hit hypothesis of TRALI, and recently an association of reduced cardiopulmonary complications with leukocyte depletion has been proposed [26]. Prevention of TA-GVHD by gamma irradiation relies on identification of at-risk patients and is not universally employed because of increased potassium leakage and potential limited storage duration after gamma irradiation [27]. Leukoreduction prior to gamma irradiation may provide an improvement to the RBC quality and extend allowable storage time after gamma irradiation; however, higher levels of extracellular potassium and lactate dehydrogenase compared to non-gamma-irradiated RBCs are still observed [28].
Pathogen and leukocyte inactivation of RBCs is therefore of potential relevance in order to transfuse patients entirely with pathogen-inactivated blood components and thus provide an unprecedented level of blood safety. To achieve this goal, a comprehensive and cost-effective approach to reduce the risks of both infectious and noninfectious complications of RBC transfusion is needed. This approach would ideally inactivate potentially contaminating infectious pathogens, inhibit leukocyte proliferation, inhibit leukocyte cytokine synthesis and antigen presentation, and minimize exposure to allogeneic plasma proteins. The S-303 pathogen and leukocyte inactivation system is being developed to achieve these objectives. The major characteristics and some goals which have already been achieved through this technology shall be discussed in the current review.
Chemical Structure and Characteristics of S-303
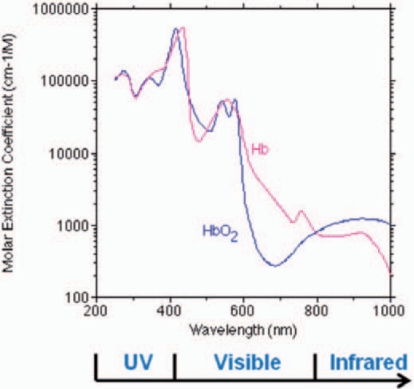
The principle of the psoralen-based pathogen inactivation technology currently in use to inactivate pathogens in platelet concentrates and plasma relies on photo-activation of the psoralen compound, amotosalen, by UVA light (320–400 nm). Naturally, the hemoglobin present in erythrocytes prohibits the use of this technology for pathogen inactivation in RBC concentrates or whole blood due to the efficient absorption of UVA light by hemoglobin (fig. 1), since high energy or very narrow path lengths would be required to obtain effective pathogen inactivation. New techniques are therefore warranted which are independent of UV light activation to achieve DNA/RNA cross-linking. Therefore, a novel technology has been developed to inactivate infectious pathogens and leukocytes in RBC components. The pathogen inactivation system for RBCs uses the chemical S-303 and a quencher glutathione (GSH). S-303 is a modular compound that enables nucleic acid targeting and cross-linking, thereby preventing nucleic acid replication. The structure of the S-303 molecule is shown in figure 2. The molecule is designed to target nucleic acids, cross-link them via a bis-alkylating group and release a negatively charged, nonreactive byproduct, S-300. When S-303 is added to RBCs, the compound rapidly (within seconds to minutes) passes through membranes, including those of cells and viral envelopes, due to its amphipathic character and intercalates into helical regions of nucleic acids. The nonreactive byproduct formed after reaction of the S-303 compound with nucleic acids or by decomposition is S-300. The decomposition kinetics (fig. 3) are rapid (half-life 20 min) at concentrations above 10 nmol/1 slowing considerably at lower S-303 concentrations (half-life greater than 6 h).
Fig. 1.
Absorption spectra of oxygenated and de-oxygenated hemoglobin. Absorption of UV light by hemoglobin requires high energy, long exposures or thin path lengths for sufficient compound activation (psoralens, methylene blue or riboflavin) for effective pathogen inactivation. S-303 is active at neutral pH and does not require activation via an external energy source.
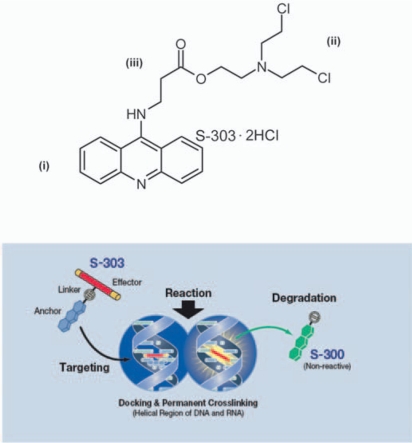
Fig. 2.
The chemical structure of the S-303 molecule and mechanism of action.
S-303 is a nucleic acid-targeted alkylator with three components: i) an acridine anchor that intercalates non-covalently into nucleic acids, ii) a bis-alkylator effector group that reacts with nucleophiles such as DNA and RNA bases, and iii) a small flexible carbon linker containing a labile ester bond that hydrolyzes at neutral pH to yield the non-reactive, negatively charged breakdown product, S-300.
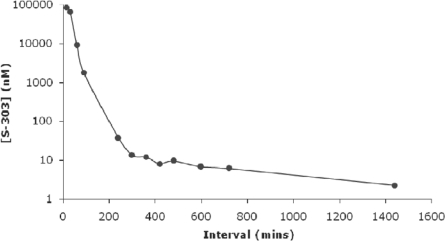
Fig. 3.
Degradation of S-303 molecule in RBCs.
The degradation kinetics of S-303 in RBCs (SAG-M RBC + treatment solution) were measured by an HPLC method for concentrations greater than 1 μmol/l and an LC/MS/MS method for lower concentrations. The limit of quantitation for the LC/MS/MS assay is 0.75 nmol/l. The residual levels of S-303 are shown without the volume exchange step. The level of S-303 after the pathogen inactivation process is below the limit of quantitation.
S-303 has the potential to react with other nucleophiles in a unit of RBCs, including small molecules such as phosphate and water and macromolecules such as proteins. To minimize these nonspecific reactions with proteins, glutathione (GSH), a naturally occurring antioxidant present in most cells at intracellular levels of approximately 5 mmol/1, is included in the process. GSH distributes only in the extracellular plasma space, while S-303 diffuses across membranes and equilibrates inside and outside of cells. This allows GSH to quench extracellular reactions of S-303 without a significant impact on pathogen inactivation.
Mechanisms and Principles of the S-303 Pathogen Inactivation System
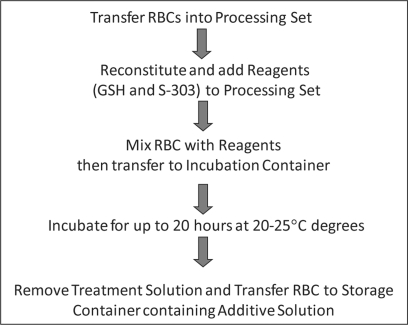
The S-303 pathogen inactivation system for RBCs will be implemented using two disposable systems: one for the reconstitution and delivery of the S-303 and GSH to the RBC unit, and a second for the processing of the RBCs. The system has been designed to enable processing of RBC units in additive solution over a wide range of RBC volumes based on quality control data obtained from blood centers in Germany and other countries in the European Union. Figure 4 shows the process steps involved in the S-303 pathogen inactivation system. A RBC concentrate separated from whole blood or collected via apheresis and leukoreduced is transferred into the integrated processing set. The processing set consists of three containers: one for mixing of the pathogen inactivation reagents (RBCs, GSH and S-303) together with a diluent solution contained in the processing set, a second container for incubating the RBCs and reagents and a third container for storage of the pathogen-inactivated RBCs. For each unit of RBCs, approximately 30 ml of GSH and S-303 in saline are mixed with the RBCs and 140 ml of a diluent solution in the first container to a final concentration of 20 mmol/1 GSH and 0.2 mmol/1 S-303. The RBCs and reagents in the first container are transferred to a second container to allow the pathogen inactivation process to occur (that is nearly complete after 30 min) and for S-303 to decompose to the nonreactive byproduct S-300 (between 6 and 18 h). Following the pathogen inactivation and S-303 decomposition, the RBC unit with reagents and byproducts is centrifuged, the supernatant is removed, and the pathogen-inactivated RBC unit is suspended in additive solution and transferred to a storage container for refrigerated storage up to 35 days.
Fig. 4.
Description of the S-303 treatment process for RBCs.
The RBC unit is transferred to the mixing container of the processing set which contains an aqueous treatment solution. The GSH and S-303 are reconstituted and then sterilely transferred to the mixing container of the processing set resulting in a final concentration of 0.2 mmol/1 S-303 and 20 mmol/1 GSH. Following addition and mixing with GSH and S-303, the treated RBC unit is transferred to the Incubation container. The RBC unit is incubated at room temperature (20–25 °C) for up to 18 h. At the end of the incubation period the RBC unit is centrifuged, the supernatant expressed and the RBC unit transferred to a storage container with SAG-M.
The S-303 pathogen inactivation process combines pathogen inactivation, leukocyte inactivation and reduction in the amount of residual plasma. Thus, this process has the potential to reduce the risks associated with both infectious and noninfectious hazards of RBC transfusion. The volume exchange step described above to remove the byproducts of the S-303 treatment process and GSH after the pathogen inactivation step offers a chance to improve RBC quality. Pathogen-inactivated RBCs have shown higher glucose levels and lower lactate levels derived from metabolism of the RBCs and have demonstrated to lower the extracellular potassium concentration [29]. It is also possible that this treatment may impact some of the adverse outcomes postulated to be associated with storage of RBCs [30].
In vitro data have been collected to demonstrate that there is no detectable volume loss or difference in total hemoglobin compared to conventional RBC units [31]. Furthermore, the pathogen inactivation process has been applied to whole-blood derived RBCs with and without buffy coat depletion (after overnight hold of whole blood). Mean corpuscular hemoglobin concentration, total hemoglobin, hemolysis and ATP levels were not statistically different between S-303-treated and conventional RBCs. Mean cell volume, hematocrit and pH were significantly higher in conventional RBC, but well within reference ranges, whereas extracellular potassium and lactate were significantly lower and glucose higher in S-303-treated RBCs [32].
Seven clinical studies have been conducted with pathogen-inactivated RBCs using S-303 and GSH. Five clinical studies were conducted using RBCs prepared with the first generation of the S-303 pathogen inactivation process, one clinical study was conducted using RBCs prepared with an interim modified process, and one study has been completed with RBC prepared with the second-generation process described in the present article (table 1). Five of these studies were radiolabel recovery studies in healthy subjects and two were phase III studies in patients requiring therapeutic RBC transfusion support.
First-Generation Pathogen Inactivation Process
Three phase I clinical trials were performed with the first-generation S-303 pathogen inactivation process in healthy subjects to evaluate the viability of S-303 RBCs [33]. In the phase IC study, the 24-hour recovery and lifespan of 35-day-old, 51Cr-labeled RBCs were evaluated in 29 subjects in a controlled, randomized cross-over study. 24-hour recovery was 84.5% for control (untreated) RBCs and 81.7% for test (S-303-treated) RBCs. Although these 24-hour recovery values were statistically significantly different (p = 0.048), results for both test and control RBCs exceeded the FDA standard of 75% mean 24-hour recovery with a corresponding standard deviation <9%. S-303 RBCs were not immunogenic in any of the phase I studies, as determined using a standard gel card technique, despite repeated exposures to S-303 RBCs in many study subjects over a 3-year period.
Two phase III clinical studies were initiated to evaluate the major indications for RBC transfusion support: acute blood loss replacement in cardiac surgery patients to ensure adequate tissue oxygenation and chronic transfusion support to correct the anemia and suppress endogenous erythropoiesis in patients with hemoglobinopathies. The phase III clinical studies were suspended when 2 patients in the chronic transfusion trial developed positive cross-match reactions to S-303 RBCs after several transfusions. Data from the trials were analyzed at the time of suspension, and the efficacy results are summarized below.
The phase III trial to evaluate support of acute anemia using S-303 RBCs prepared with the first-generation process was designed as a randomized, controlled, double-blind, noninferiority study [34]. The clinical trial population included patients undergoing cardiovascular surgery expected to require RBC transfusion. Study transfusions (S-303 RBCs or control RBCs) were given as clinically indicated during and up to 7 days after surgery. The primary endpoint was a composite of myocardial infarction, renal failure and death during or within 7 days of the first study transfusion. The primary end-point was selected to evaluate therapeutic efficacy of S-303 RBCs when transfused to prevent morbidity and mortality reflective of inadequate tissue oxygenation due to acute anemia caused by surgical blood loss. Additional secondary endpoints included hemoglobin increment post transfusion, number of RBC units transfused and other blood component usage.
The study was designed to enroll 200 patients to demonstrate noninferiority. At the time of study suspension, 148 patients (74 test and 74 control) had been randomized and treated with study RBCs. Efficacy and safety data were analyzed according to the prespecified statistical analysis plan. S-303 RBCs were found to be as effective as control RBCs based upon the primary endpoint and all secondary endpoints. The composite endpoint for the S-303 RBC group was not inferior to the control RBC group: 22% of test patients and 21% of control patients experienced the composite endpoint (p = 0.02 by noninferiority). In addition, the incidence for each of the components of the primary endpoint was not different between treatment groups [34]. The mean number of RBC units transfused and other blood components transfused as well as the mean hemoglobin increment post transfusion were not different between treatment groups. Although the trial did not achieve its targeted enrollment of 200 patients, the data indicated that S-303 RBCs were not inferior to control RBCs.
The transfusion trial for support of chronic anemia was designed as a randomized, controlled, double-blind, two-treatment period, cross-over, noninferiority trial. The primary end-point was hemoglobin utilization per day of support adjusted for body weight. Secondary endpoints included number of RBC units transfused, hemoglobin increments, reticulocyte count, acute transfusion reactions and global safety. The patient population included patients with hemoglobinopathies (thalassemia and sickle cell anemia) participating in chronic RBC transfusion programs as part of the management of their disease processes. The study was designed to enroll 50 patients with completion of both treatment periods required to demonstrate noninferiority. At the time of suspension of the study, 26 patients had been randomized and started the first treatment period, and 8 patients had initiated the second treatment period. No patient had completed both treatment periods, and therefore efficacy and safety data could not be analyzed as planned. Instead, a post-hoc hypothesis-generating analysis of efficacy was performed for the first treatment period only. 19 patients were considered evaluable for efficacy using the modified analysis plan, based on receipt of 2 or more transfusions (exclusive of wash-in transfusions). As the study was terminated prematurely, the evaluable group was highly heterogeneous, with some patients receiving as few as 2 study RBC transfusions, and others as many as 12 during the first treatment period. Summary of the limited and variable efficacy data for these 19 patients found no statistically significant differences between treatment groups for the primary endpoint or any secondary endpoint [35].
Second-Generation S-303 Pathogen Inactivation System
A recent phase I clinical study was conducted in two centers in the USA to characterize the posttransfusion recovery and lifespan of autologous RBCs prepared with the second-generation S-303 pathogen inactivation process and stored for 35 days prior to transfusion [37]. It was composed as a randomized, single-blind, controlled, two-period cross-over study in healthy adult subjects. 28 subjects were enrolled at 2 study sites, and 26 subjects completed the study. The study was divided into 3 periods: screening and enrollment, treatment period 1 and treatment period 2. Each treatment period consisted of autologous blood donation, treatment of donated components (test or control), infusion of radiolabeled autologous RBCs and collection of blood samples for assessment of RBC recovery and lifespan. RBCs were radiolabeled with 51Cr for assessment of RBC recovery and lifespan and with 99mTc for measurement of blood volume according to ICSH Guidelines. The in vivo 24-hour recovery for both S-303-treated and control RBCs met the FDA criteria of mean recovery greater than 75%, standard deviation of the mean less than 9%, and that minimally 70% of the RBC products administered will have a recovery of at least 75%. The in vitro quality attributes based on the EU and AABB guidelines were met throughout the storage period, and there was an acceptable maintenance of ATP concentration and a more favorable extracellular potassium concentration in the S-303-treated RBC units compared to conventional RBCs [37]. Clinical trials in patients with acute and chronic anemia are being planned based on the results obtained in the phase I clinical trial using the second-generation S-303 pathogen inactivation process for RBCs.
Safety of S-303-Treated Red Blood Cells
Safety assessments for the potential toxicity, carcinogenicity and reproductive and genetic toxicology of S-303- and GSH-treated RBCs and S-300, the major decomposition product of S-303, have been conducted and completed. These studies were designed and performed according to the ICH guidelines with an approach that is consistent with the development of a new pharmaceutical drug. Table 2 listed the studies conducted in rat, dog, mouse and rabbit. The objectives of these studies have been:
-
–
to transfuse S-303-treated red cells to test animals in multiple doses, such as expected clinical exposure of RBCs to treat either acute or chronic anemia,
-
–
to administer S-303, or mainly its degradation product S-300 (which will be the major transfused moiety) at higher doses in animals than would be expected in a clinical setting,
-
–
to evaluate immune response after routine exposure to S-303-treated RBC for neoantigenicity.
Table 2.
Safety studies conducted with the S-303 pathogen inactivation technologya
cA chronic transfusion model in the rabbit was developed to evaluate immune response to S-303-treated RBCs.
Preclinical studies were conducted in dog, rat, mouse and rabbit depending on the study objectives.
Studies to evaluate organ toxicity were conducted with both S-303-treated RBCs and S-300 in both rat and dog.
Based on data from the completed experiments, safety margins have been established based on expected clinical exposure during RBC transfusions and results from the systemic toxicology and carcinogenicity studies that support further clinical development for the S-303 pathogen inactivation system for RBCs. No observations of systemic toxicity or immune response were observed in the toxicology studies that included 6-month chronic exposure in rats and 9-month chronic exposure in dogs.
Immune Response to S-303-Treated Red Blood Cells
During the phase III trial supporting patients with hemoglobinopathies, positive cross-match reactions to pathogen-inactivated RBCs with sera from two patients were detected following exposure to first-generation process S-303 RBCs [38]. In vitro studies demonstrated that the immunoreactivity to S-303 RBCs was a result of low-titer antibodies formed against the acridine moiety of S-303 on the RBC surface [39,40]. Immune reactivity was inhibited by S-303-related acridine compounds, but not GSH. Physiologic activity of the anti-acridine antibody in the patient sera was evaluated using a monocyte monolayer assay [41] in which S-303 RBCs are exposed to the reactive sera and then incubated with monocytes. The patient sera did not induce phagocytosis of S-303 RBCs by monocytes suggesting that the immune response was not clinically relevant and that transfusion of pathogen-inactivated RBCs to these patients would be unlikely to elicit a hemolytic transfusion event.
Observation of the immune response to S-303 RBCs using the first-generation pathogen inactivation process led to the initiation of a follow-up retrospective cross-match analysis of patient sera which revealed that 2 control patients from the acute anemia trial had positive cross-match tests at the end of the study, even though they had never been exposed to S-303 RBCs [42]. This suggested the possibility of reactivity of S-303-treated RBCs with a naturally occurring antibody. Importantly, however, no clinical sequelae were associated with the formation of this antibody, either in the acute or the chronic anemia patients.
In an effort to understand the incidence of this immune reactivity in subjects never exposed to S-303-treated RBCs, two additional studies were conducted. Sera were collected from healthy blood donors and from multiply transfused patients not previously exposed to S-303 RBCs, but who were at risk for development of allo-antibodies due to their transfusion-dependent disease (sickle cell anemia, thalassemia, myelodys-plasia and inflammatory disease) necessitating repeat blood transfusions. In each of these studies, serum samples were tested against RBC aliquots prepared from the same unit: control (untreated) RBCs, and first-generation process S-303 RBCs. A minimum of three RBC units was evaluated with each serum sample. The criterion for a positive response was reactivity of sera with all three S-303 RBC units.
In the healthy volunteer blood donor population, 2 out of 200 sera (1.0%) consistently reacted with all three units of S-303 RBCs prepared with the first-generation process. In the multiply transfused patients, selected because of their increased risk for development of allo-antibodies, 3 out of 186 (1.6%) sera were consistently positive against RBCs prepared with the first-generation process [42].
Development of the second-generation pathogen inactivation process was focused on minimizing the amount of RBC-bound acridine and therefore on reducing the potential for either an immune response after exposure to S-303-treated RBCs or immune reactivity due to a naturally occurring antibody to S-303-treated RBCs.
To evaluate the potential for an immune response after exposure to S-303-treated RBCs, a rabbit chronic transfusion model and flow cytometry assay were developed to replicate the observed immunogenicity [43] and to provide a model system to evaluate improvements to the S-303 pathogen inactivation system. This model is being used to assess the potential for immune response using the second-generation S-303 pathogen inactivation process.
To establish the incidence of immune reactivity with the second-generation S-303 treatment process, an in vitro serology study was initiated to determine what proportion of the population, if any, have reactivity with S-303 RBCs. For this study, approximately 800 serum samples from patients with congenital hemoglobinopathies and no known prior exposure to S-303 and 1,500 samples from healthy donors have been collected and screened for reactivity by gel card cross-match against 3 different units of S-303 RBCs [44]. Preliminary results indicate that there were no positive cross-matches in any of the healthy donor samples screened (200 of the 1,500 samples) and that 2 out of 800 (0.25%) multiply transfused patients, selected because of their increased risk for development of allo-antibodies, demonstrated low-titer, pre-existing reactivity to S-303-treated RBCs [44]. This level is not unexpected as positive cross-matches are a regular occurrence in blood transfusion centers – typical levels of cross-match reactivity range from 1 to 4% – and all blood banks have standard operating procedures in place to ensure safe transfusion of these patients. Furthermore, similar levels of positive reactions to drugs and chemicals have been observed to be due to naturally occurring antibodies [45, 46] that may be a result of environmental exposure to structurally similar chemicals. Additional serology studies are planned to further refine the incidence of naturally occurring antibodies to S-303-treated RBCs.
Pathogen Inactivation Efficacy
Results from feasibility studies evaluating inactivation of bacteria and viruses with the second-generation S-303 pathogen inactivation process have been conducted (table 3 [adapted from 29]). In each of the studies, RBC units were contaminated with the selected pathogen, treated with GSH and S-303, and incubated for 3 h at room temperature (∼20 °C). Inactivation (log10 reduction) was determined independently for each replicate by comparing the organism titer before and after S-303 treatment.
Table 3.
Pathogen inactivation efficacy results based on pilot studies
| Organism | Mean log reductiona |
|---|---|
| Staphylococcus aureus | 5.1 ± 0.3 |
| Yersinia enterocolitica | ≥ 6.8 ± 0.2 |
| Serratia marcescens | 5.1 ± 0.1 |
| Escherichia coli | ≥ 6.7 ± 0.1 |
| HIV | > 5.9 ± 0.1 |
| Bovine viral diarrhea virus | > 4.8 ± 0.1 |
| Bluetongue | ≥ 5 ± 0.04 |
| Human adenovirus type 5 | >7.4 ± 0.2 |
Log reduction was calculated as log (pre-S-303 titer/post-S-303 titer), where titer is expressed as 10× per ml for the mean of four replicates. Bacterial titers were expressed as colony forming units (cfu) per ml and viral titers were expressed as plaque forming units (pfu) per ml. Greater than symbols indicate no detectable residual pathogen based on the highest titer inocula available for evaluation. Inactivation incubations were 3 h at room temperature. Values represent the means ± SD for 4 replicates per experiment.
The bacterial species Staphylococcus aureus, Yersinia enterocolitica, Serratia marcescens and Escherichia coli were chosen for these preliminary studies because they have been associated with RBC transfusion-transmitted sepsis. These pathogens were selected based on the following criteria: S. aureus because it has been shown to contaminate RBC units during venipuncture and is a representative Gram-positive bacteria; and Y. enterocolitica and S. marcescens due to their ability to grow at the low temperatures at which RBCs are stored; in addition, Y. enterocolica, S. marcescens and E. coli also serve as representative Gram-negative bacteria.
HIV was used because of its prior history for transfusion-transmitted infection as well as being a representative enveloped RNA virus. Recent studies have demonstrated greater than 4 log inactivation of XMRV, another retrovirus potentially associated with chronic fatigue syndrome and prostate cancer [47]. Bluetongue virus and human adenovirus type 5 were selected as model nonenveloped RNA and DNA viruses, respectively. Bovine viral diarrhea virus was included because it is an established model for human hepatitis C virus and other flaviviruses such as West Nile and Dengue virus.
Additional studies will be conducted with the second-generation process to include those organisms for which blood is currently tested, or models for them, as well as organisms of significant concern in RBC transfusions (e.g. Plasmodium and Babesia). Proof-of-principle of the S-303 system for inactivation of pathogens of emerging importance in RBCs has been demonstrated using the first-generation S-303 pathogen inactivation system. Inactivation of greater than 6.8 log for Plasmodium falciparum, greater than 4.9 log for Babesia microti, greater than 5.3 log for Trypanosoma cruzi and greater than 6.0 log for West Nile virus have been reported [48]. The scope and sequence of new studies may be modified to reflect newly emerging organisms or organism of particular importance to specific regions.
Development and Implementation of S-303 Treatment System – the Path Forward
The S-303 pathogen inactivation system for RBCs is in clinical development, and phase III clinical studies are planned in the European Union. Proof-of-concept data has been gathered in patients with acute anemia using the first-generation process. A second-generation process has been developed to minimize the potential for an immune response. The in vitro and in vivo studies with S-303 pathogen-inactivated RBCs using the second generation process has shown that the treated RBC retain sufficient viability and biochemical properties to support therapeutic transfusion. Preclinical studies have been completed to support the safety of S-303-treated RBCs for clinical evaluation, and preliminary studies have demonstrated sufficient pathogen inactivation efficacy to address infectious risks of blood transfusion. The technology has also shown promise for application to whole blood [29].
Implementation of the S-303 pathogen inactivation system in different blood centers and even different countries will likely have to fulfil the same quality criteria currently in place for conventional RBC components. Studies have shown that the S-303 pathogen inactivation system can meet these quality criteria [32, 37]. Currently the European Council Guide for the Preparation of Blood Components provides relatively broad requirements for the quality of RBC components, and thus the S-303 pathogen inactivation system will need to be compatible with these broad requirements. The challenge lies in providing guard bands to allow a safe process under any circumstances. For example
-
–
Different preprocessing times have to be taken into account and validated, including overnight storage of whole blood or immediate processing post collection. The currently applied period of generally 3–6 h for ‘self-sterilization’ may not be necessary any longer if pathogen inactivation is to follow, and the time that is gained may be used for additional processing steps required for pathogen inactivation of the RBCs. A period for ‘self-sterilization’ of 3–6 h is generally considered to be adequate to allow the active phagocytosis of any residual bacteria which may be contained in the collected blood unit and which are usually derived from the skin of the donor.
-
–
Different blood donation volumes have to be taken into account (450 or 500 ml) as well as different RBC volumes and hemoglobin contents, depending on whether inline leukocyte depletion is performed directly from the whole blood or from the RBC concentrates.
-
–
The method to obtain RBCs from whole blood donation differs between Europe and the USA where buffy coat depletion is used to separate platelets from the plasma and RBCs. Different centrifugation profiles based on speed, applied g force, time and acceleration/deceleration dynamics will affect the RBC specifications and will vary between countries and blood centers.
-
–
The need for irradiation may be diminished, allowing immediate availability of suitable blood units, the possibility to use the entire inventory, and the use of units at the normal expiration time which is currently shortened in the case of irradiated RBCs [18].
Development of RBC pathogen inactivation systems will have to consider these variables to ensure consistent RBC products. However the S-303 pathogen inactivation technology has the potential to provide a more uniform and higher quality RBC concentrate that will address both the infectious and noninfectious risks of RBC transfusions.
Disclosure Statement
N. Mufti is an employee of Cerus. R. Henschler and E. Seifried are developing the S-303 technology with Cerus under a Joint Collaboration Agreement.
Table 1.
Clinical studies using RBC prepared with the S-303 treatment process
| S-303 treatment processa | Phase | Number of subjects enrolled | Number of subjects receiving S-303 RBCs | Primary endpoint | Reference |
|---|---|---|---|---|---|
| First generation | 1A | 43 | 21 | 24 h recovery: 78.9% (test) and 83.9% (control) | 33 |
| First generation | 1B | 28 | 28 | 24 h recovery: 81.1% (test) | 33 |
| First generation | 1C | part A 29 part B 11 | 40 | part A: 24 h recovery: 81.7% (test) and 84.5% (control) part B: tolerability of full-unit transfusion | 33 |
| First generation | 3 | 148/200c | 74 | incidence of myocardial infarction, renal failure, death: 21.6% (test) and 20.5% (control) | 34 |
| First generation | 3 | 26/50c | 17 | mean Hb transfused (g Hb/kg/day): 1.3 (test) and 0.9 (control) | 35 |
| Interim modified | 1 | 28 | 28 | 24 h recovery: 79.8% (test) and 84.5% (control) | 36 |
| Second generation | 1 | 27 | 26 | 24 h recovery: 88.0% (test) and 90.1% (control) | 37 |
The first-generation process used 0.2 mmol/l S-303 and 2 mmol/l GSH, a compound adsorption device (CAD) and Esol as the additive solution. An interim modified process was a precursor to the second generation process during optimization to reduce the potential for immune response. The modified process used 0.2 mmol/l S-303 and 20 mmol/l GSH (monosodium salt plus one equivalent of NaOH to pH9) and AS-3 (Nutricel) as the additive solution. The second-generation process used 0.2 mmol/l S-303 and 20 mmol/l GSH (monosodium salt, pH 7), a diluent solution and AS-5 (Optisol) as the additive solution.
The phase III clinical trials were halted prematurely. The studies had planned to enroll 200 cardiovascular (CV) surgery patients and 50 chronically transfused patients however the CV surgery patient study had sufficient power to demonstrate noninferiority.
References
- 1.van Rhenen D, Gulliksson H, Cazenave JP, Pamphilon D, Ljungman P, Kluter H, Venneij H, Kappers-Klunne M, de Greef G, Laforet M, Lioure B, Davis K, Marblie S, Mayaudon V, Rament J, Conlan M, Lin L, Metzel P, Buchholz D, Corash L. Transfusion of pooled buffy coat platelet components prepared with photochemical pathogen in-activation treatment: the euroSPRITE trial. Blood. 2003;101:2426–2433. doi: 10.1182/blood-2002-03-0932. [DOI] [PubMed] [Google Scholar]
- 2.McCullough J, Vesole DH, Benjamin RJ, Slichter SJ, Pineda A, Snyder E, Stadtmauer EA, Lopez-Plaza I, Coutre S, Strauss RG, Goodnough LT, Fridey JL, Raife T, Cable R, Murphy S, Howard F, Davis K, Lin JS, Metzel P, Lin L, Koutsoukos A, Corash L, Buchholz DH, Conlan MG. Therapeutic efficacy and safety of platelets treated with a photochemical process for pathogen inactivation: the SPRINT trial. Blood. 2004;104:1534–1541. doi: 10.1182/blood-2003-12-4443. [DOI] [PubMed] [Google Scholar]
- 3.Janetzko K, Cazenave JP, Klüter H, Kientz D, Michel M, Beris P, Lioure B, Hastka J, Marblie S, Mayaudon V, Lin L, Lin JS, Conlan MG, Rament J. Therapeutic efficacy and safety of photochemically treated apheresis platelets processed with an optimized integrated set. Transfusion. 2005;45:1443–1452. doi: 10.1111/j.1537-2995.2005.00550.x. [DOI] [PubMed] [Google Scholar]
- 4.Klein HG, Anderson D, Bernardi MJ, Cable R, Carey W, Hoch JS, Robitaille N, Sivilotti ML, Smaill F. Pathogen inactivation: making decisions about new technologies. Report of a consensus conference. Transfusion. 2007;47:2338–2347. doi: 10.1111/j.1537-2995.2007.01512.x. [DOI] [PubMed] [Google Scholar]
- 5.Mintz PD, Bass NM, Petz LD, Steadman R, Streiff M, McCullough J, Burks S, Wages D, VanDoren S, Corash L. Photochemically treated fresh frozen plasma for transfusion of patients with acquired co-agulopathy of liver disease. Blood. 2006;107:3753–3760. doi: 10.1182/blood-2004-03-0930. [DOI] [PubMed] [Google Scholar]
- 6.Mintz PD, Neff A, MacKenzie M, Goodnough LT, Hillyer C, Kessler C, McCrae K, Menitove JE, Skikne BS, Damon L, Lopez-Plaza I, Roualt C, Crookston KP, Benjamin RJ, George J, Lin JS, Corash L, Conlan MG. A randomized, controlled phase III trial of therapeutic plasma exchange with fresh frozen plasma prepared with amotosalen and UVA light compared to untreated fresh frozen plasma in thrombotic thrombocytopenic purpura. Transfusion. 2006;46:1693–1704. doi: 10.1111/j.1537-2995.2006.00959.x. [DOI] [PubMed] [Google Scholar]
- 7.Cazenave JP, Isola H, Waller C, Mendel I, Kientz D, Laforet M, Raidot JP, Kandel G, Wiesel ML, Corash L. Use of additive solutions and pathogen inactivation treatment of platelet components in a regional blood center: impact on patient out comes and component utilization during a 3-year period. Transfusion. 2010 doi: 10.1111/j.1537-2995.2010.02873.x. doi:10.1111/j.l537-2995.2010.02873.x. [DOI] [PubMed] [Google Scholar]
- 8.The Mirasol Clinical Evaluation Study Group A randomized controlled clinical trial evaluating the performance and safety of platelets treated with MIRASOL pathogen reduction technology. Transfusion. 2010 doi: 10.1111/j.1537-2995.2010.02694.x. doi: 10.1111/j.l537-2995.2010.02694.x. [DOI] [PubMed] [Google Scholar]
- 9.Sandier SG. The status of pathogen-reduced plasma. Transfus Apheresis Sci. 2010 doi: 10.1016/j.transci.2010.09.006. doi:10.1016/j. transci.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Lin L, Dikeman R, Molini B, Lukehart SA, Lane R, Dupuis K, Metzel P, Corash L. Photochemical treatment of platelet concetrates with amotosalen and UVA inactivates a broad spectrum of pathogenic bacteria. Transfusion. 2004;44:1496–1504. doi: 10.1111/j.1537-2995.2004.04125.x. [DOI] [PubMed] [Google Scholar]
- 11.Singh Y, Sawyer L, Pinkoski L, Dupuis K, Hsu J, Lin L, Corash L. Photochemical treatment of plasma with amotosalen and UVA light inactivates pathogens while retaining coagulation function. Transfusion. 2006;46:1168–1177. doi: 10.1111/j.1537-2995.2006.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein HG, Anderson D, Bernardi MJ, Cable R, Carey W, Hoch JS, Robitaille N, Sivilotti ML, Smaill F. Pathogen inactivation: making decisions about new technologies. Report of a consensus conference. Transfusion. 2007;47:2338–2347. doi: 10.1111/j.1537-2995.2007.01512.x. [DOI] [PubMed] [Google Scholar]
- 13.Alter HJ. Pathogen reduction: a precautionary principle paradigm. Transfus Med Rev. 2008;22:97–102. doi: 10.1016/j.tmrv.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perkins HA, Busch MP. Transfusion-associated infections: 50 years of relentless challenges and remarkable progress. Transfusion. 2010;50:2080–2099. doi: 10.1111/j.1537-2995.2010.02851.x. [DOI] [PubMed] [Google Scholar]
- 15.Mohammed H, Linnen JM, Munoz-Jordan JL, Tomashek K, Goster G, Broulik AS, Petersen L, Stramer SL. Dengue virus in blood donations, Puerto Rico, 2005. Transfusion. 2008;48:1348–1354. doi: 10.1111/j.1537-2995.2008.01771.x. [DOI] [PubMed] [Google Scholar]
- 16.Benjamin RJ, Wagner SJ. The residual risk of sepsis: modeling the effect of concentration on bacterial detection in two-bottle culture systems and an estimation of false-negative culture rates. Transfusion. 2007;47:1381–1389. doi: 10.1111/j.1537-2995.2007.01326.x. [DOI] [PubMed] [Google Scholar]
- 17.Leiby DA. Babesiosis and blood transfusion: flying under the radar. Vox Sang. 2006;90:157–165. doi: 10.1111/j.1423-0410.2006.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichols WG, Price TH, Gooley T, Corey L, Boeckh M. Transfusion-transmitted cytomegalo-virus infection after receipt of leukoreduced blood products. Blood. 2003;101:4195–4200. doi: 10.1182/blood-2002-10-3143. [DOI] [PubMed] [Google Scholar]
- 19.Triulzi D, Duquesnoy R. Fatal transfusion-associated graft-versus-host disease in an immunocom-petent recipient of a volunteer unit of red cells. Transfusion. 2006;46:885–888. doi: 10.1111/j.1537-2995.2006.00819.x. [DOI] [PubMed] [Google Scholar]
- 20.Kelher MR, Masuno T, Moore EE, Damle S, Meng X, Song Y, Liang X, Niedzinski J, Geier SS, Khan SY, Gamboni-Robertson F, Silliman CC. Plasma from stored packed red blood cells and MHC class I antibodies causes acute lung injury in a 2-event in vivo rat model. Blood. 2009;113:2079–2087. doi: 10.1182/blood-2008-09-177857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heddle NM, Klama LN, Griffith L, Roberts R, Shukla G, Kelton JG. A prospective study to identify risk factors associated with acute reactions to platelet and red cell transfusions. Transfusion. 1993;33:794–797. doi: 10.1046/j.1537-2995.1993.331094054613.x. [DOI] [PubMed] [Google Scholar]
- 22.Van de Watering L, Hermans J, Wityliet M, Bers-teegh M, Brand A. HLA and RBC immunization after filtered and buffy coat-depleted blood transfusion in cardiac surgery: a randomized controlled trial. Transfusion. 2003;43:765–771. doi: 10.1046/j.1537-2995.2003.00390.x. [DOI] [PubMed] [Google Scholar]
- 23.Lapierre V, Auperin A, Robinet E, Ferrand C, Oubouzar N, Tramalloni D, Saas P, Debaene B, Lasser P, Tiberghien P. Immune modulation and microchimerism after unmodified versus leukoreduced allogeneic red blood cell transfusion in cancer patients: results of a randomized study. Transfusion. 2007;47:1691–1699. doi: 10.1111/j.1537-2995.2007.01344.x. [DOI] [PubMed] [Google Scholar]
- 24.Vamvakas EC, Blajchman MA. Deleterious clinical effects of transfusion-associated immunomodu-lation: fact or fiction. Blood. 2001;97:1180–1195. doi: 10.1182/blood.v97.5.1180. [DOI] [PubMed] [Google Scholar]
- 25.Cardo LJ, Wilder D, Salata J. Neutrophil priming, caused by cell membranes and microvesicles in packed red blood cell units, is abrogated by leukocyte depletion at collection. Transfus Apheresis Sci. 2008;38:117–125. doi: 10.1016/j.transci.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Blumberg N, Heal JM, Gettings KF, Phipps RP, Masel D, Refaai MA, Kirkley SA, Fialkow LB. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukodepletion of blood transfusions. Transfusion. 2010 doi: 10.1111/j.1537-2995.2010.02748.x. doi: 10.1111/j.1537-2995.2010.02748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moroff G, Leitman SF, Luban NL. Principles of blood irradiation, dose validation and quality control. Transfusion. 1997;37:1084–1092. doi: 10.1046/j.1537-2995.1997.371098016450.x. [DOI] [PubMed] [Google Scholar]
- 28.Zimmermann R, Wintzheimer S, Weisbach V, Strobel J, Zingsem J, Eckstein R. Influence of prestorage leukoreduction and subsequent irradiation on in vitro red blood cell (RBC) storage variables of RBCs in additive solution saline-adenine-glucose-mannitol. Transfusion. 2009;49:75–80. doi: 10.1111/j.1537-2995.2008.01920.x. [DOI] [PubMed] [Google Scholar]
- 29.Mufti NA, Erickson AC, North AK, Hanson D, Sawyer L, Corash LM, Lin L. Treatment of whole blood (WB) and red blood cells (RBC) with S-303 inactivates pathogens and retains in vitro quality of stored RBC. Biologicals. 2010;38:14–19. doi: 10.1016/j.biologicals.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 30.Steiner ME, Stowell C. Does red blood cell storage affect clinical outcome? When in doubt, do the experiment. Transfusion. 2009;49:1286–1290. doi: 10.1111/j.1537-2995.2009.02265.x. [DOI] [PubMed] [Google Scholar]
- 31.Henschler R, Janetzko K, Erterek B, Giesen M, Pfeiffer HU, Mufti N, Kraemer A, Erickson A. Characterization of red cell concentrates treated with the S-303 pathogen inactivation system and stored in saline adenine glucose-mannitol (SAGM) Vox Sang. 2010;99(suppl 1):38. [Google Scholar]
- 32.Henschler R, Janetzko K, Erterek B, Giesen M, Terencio J, Luna J, Fernandez X, Rausch P, Erickson A, Mufti N. Evaluation of the S-303 pathogen inactivation (PI) system with RBC components using US and EU processing practices. Transfusion. 2010;50(suppl):75A. [Google Scholar]
- 33.Rios JA, Hambleton J, Viele M, Rugg N, Sinder-mann G, Greenwalt T, Wages D, Cook D, Corash L. Viability of red cells prepared with S-303 pathogen inactivation treatment. Transfusion. 2006;46:1778–1786. doi: 10.1111/j.1537-2995.2006.00973.x. [DOI] [PubMed] [Google Scholar]
- 34.Benjamin RJ, McCullough J, Mintz PD, Snyder E, Spotnitz WD, Rizzo RJ, Wages D, Lin J-S, Wood L, Corash L, Conlan MG. Therapeutic efficacy and safety of red blood cells treated with a chemical process (S-303) for pathogen inactivation: a phase III clinical trial in cardiac surgery patients. Transfusion. 2005;45:1739–1749. doi: 10.1111/j.1537-2995.2005.00583.x. [DOI] [PubMed] [Google Scholar]
- 35.Conlan MG, Vichinsky E, Snyder E, Goodnough L, Labotka R, Eder A, Sloan S, Minniti C, Lin J-S. S-303 pathogen inactivated red blood cells in patients with hemoglobinopathies participating in chronic transfusion programs: preliminary safety and efficacy results. Vox Sang. 2005;89(suppl 1):121. [Google Scholar]
- 36.Cancelas JA, Dumont L, Herschel L, Roger J, Rugg N, Garratty G, Arndt P, Propst M, Corash L, Sundin D, AuBuchon J. A randomized, controlled, 2-period crossover study of recovery and lifespan of radiolabeled autologous 35-day old red blood cells with a modified S 303 treatment for pathogen inactivation. Vox Sang. 2008;95(suppl 1):8. [Google Scholar]
- 37.Cancelas JA, Rugg N, Dumont LJ, Szczeriorkowski ZM, Siegel A, Erickson A, Propst M, North A, Mufti N, Corash L. Comprehensive evaluation of a new process for S-303 pathogen-inactivation of red blood cells. Transfusion. 2010;50(suppl):9A. [Google Scholar]
- 38.Conlan MG, Stassinopoulos A, Garratty G, Wages D, Corash L, Wood L, Sloan SR, Labotka RJ. Antibody formation to S-303-treated RBCs in the setting of chronic RBC transfusion. Blood. 2004;104(suppl):112A. [Google Scholar]
- 39.Conlan MG, Lin L-S, Stassinopoulos A. Investigation of immunoreactivity observed after transfusion of S-303 RBCs in 2 phase III clinical trials in support of acute or chronic anemia. Transfusion. 2005;45(3suppl):29A. [Google Scholar]
- 40.North AK, Castro G, Erickson A, Cook D, Corash L. Characterization of antibodies to red cells prepared with S-303 pathogen inactivation treatment. Vox Sang. 2007;93(suppl 1):167-168. [Google Scholar]
- 41.Arndt PA, Garratty G. A retrospective analysis of the value of the monocyte monolayer assay results for predicting the clinical significance of blood group alloantibodies. Transfusion. 2004;44:1273–1281. doi: 10.1111/j.1537-2995.2004.03427.x. [DOI] [PubMed] [Google Scholar]
- 42.North AK, Garratty G, Schott M, Arndt PA, Castro GM, Erickson AC, Mintz PD, Corash L. A modified process for preparation of S-303 RBC for pathogen inactivation substantially reduces potential for reactivity. Transfusion. 2006;46(suppl):116A. [Google Scholar]
- 43.North AK, Lee V, Erickson A, Moore N. Demonstration of an S-303-induced immune response in a naive rabbit model of chronic transfusion using a sensitive flow cytometry assay. Vox Sang. 2007;93(suppl 1):168. [Google Scholar]
- 44.North A, Propst M, Henschler R, Geisen C, Garratty G, Arndt P, Kattamis A, Cohen A, Pia A, Arslan O, Galanello R. Evaluation of naturally occurring antibodies to pathogen inactivated red blood cells. Transfusion. 2010;50(suppl):38A. [Google Scholar]
- 45.Leger RM, Arndt PA, Garratty G. Serological studies of piperacillin antibodies. Transfusion. 2008;48:2429–2434. doi: 10.1111/j.1537-2995.2008.01852.x. [DOI] [PubMed] [Google Scholar]
- 46.Arndt P, Garratty G, Isaak E, Bolger M, Lu Q. Positive direct and indirect antiglobulin tests associated with oxaliplatin can be due to drug antibody and/or drug-induced nonimmunolgic protein adsorption. Transfusion. 2009;49:711–718. doi: 10.1111/j.1537-2995.2008.02028.x. [DOI] [PubMed] [Google Scholar]
- 47.Mikovits JA, Hagen K, Liu W, Hanson D, Sad-owski C, Ramani VK, Lee KG, Ruscetti FW, Lin L. Inactivation of XMRV in platelet and RBC components prepared with the INTERCEPT Blood System. Rev Antiviral Ther Infectious Dis. 2010;8:36. [Google Scholar]
- 48.Dupuis K, Bernard K, Jones S, Grellier P, Labaid M, Van Voorhis W, Barrett L, Benach J, Monsalve G, Gil-Gil H, Sawyer L. Helinx technology inactivates pathogens of emerging importance in red blood cell concentrates. Blood. 2003;102(suppl 1):816a. [Google Scholar]