Summary
Methylene blue (MB) treated plasma has been in clinical use for 18 years. The current THERAFLEX MB-Plasma has a number of improved features compared with the original Springe methodology. This overview embodies: the biochemical characteristics of MB, the mechanism of the technology, toxicology, pathogen reduction capacity, current position in clinical setting and status within Europe. The THERAFLEX MB (TMB) procedure is a robust, well standardised system lending itself to transfusion setting and meets the current guidelines. The pathogen kill power of the TMB system, like the other available technologies, is not limitless, probably in order of 6 log for most enveloped viruses and considerably less for non-enveloped ones. It does not induce either new antigen or grossly reducing the function and life span of active principle in fresh frozen plasma (FFP). The removal of the residual MB at the end of the process has the beneficial effect of reducing potential toxic impacts. Clinical haemovigilance data, so far, indicate that cell-free MB plasma is effective in all therapeutic setting requiring FFP, besides inconsistent thrombotic thrombocytopenia purpura data, without serious side-effects or toxicity. The current system is in continuous improvement e.g. regarding virus reduction range, illumination device, software used, and process integration in the blood bank setting.
Keywords: Fresh frozen plasma, Methylene blue-inactivated, Pathogen inactivation, Virus inactivation, THERAFLEX MB-Plasma
Zusammenfassung
Mit Methylenblau (MB) behandeltes Plasma wird seit 18 Jahren klinisch eingesetzt. Das aktuell verwendete THERAFLEX-MB-Plasma-Verfahren ist im Vergleich zu der ursprünglichen Methode des DRK-Blutspendedienstes Springe verbessert worden. In diesem Artikel werden die chemischen Eigenschaften von MB, der Mechanismus des Verfahrens, die Toxikologie, die Pathogenreduktionskapazität, der Einfluss auf Plasmaproteine sowie die derzeitige klinische Anwendung und Verwendung in Europa zusam-mengefasst. Das THERAFLEX-MB-Plasma-System ist ein robustes, standardisiertes Verfahren, das sich für Blut-bankeinrichtungen anbietet und den aktuellen Richtlinien entspricht. Die Pathogenreduktionskapazität des Verfahrens ist, wie die aller anderen verfügbaren Methoden, nicht unbegrenzt. Sie liegt im Bereich von zirka 6 log für umhüllte und ist geringer für nichtumhüllte Viren. Der Prozess induziert weder neue Antigene noch reduziert er we-sentlich die Funktion oder Lebensdauer der Plasmaproteine. Das Entfernen des MB am Ende des Prozesses hat den Vorteil, dass mögliche toxikologische Auswirkungen ver-ringert werden. Klinische Erfahrungen und Hämovigilanz-berichte zeigen, dass das zellfreie MB-Plasma in therapeutischen Bereichen, in denen frischgefrorenenes Plasma benötigt wird, abgesehen von widersprüchlichen Daten bei thrombotisch-thrombozytopenischer Purpura, effektiv und ohne schwere Nebenreaktionen odertoxische Effekte ist. Das System wird kontinuierlich weiter entwickelt, z.B. in Bezug auf die Palette der Viren, die Belichtungs-maschine, die verwendete Software und die Integration in die Blutbank.
Introduction
Pathogen safety of fresh frozen plasma (FFP) in most European countries has improved significantly over the past two decades owing to stringent donor selection, strict exclusion criteria and complementary improved screening tests, and the desire for introduction of safer plasma. In certain countries, the safety is further enhanced by universal leucodepletion and pathogen reduction. Universal leucodepletion not only decreases transfusion reactions and HLA alloimmunisation but also provides the benefit of removing cell-associated pathogens such as cytomegalo-virus (CMV) and human T-cell lymphotropic viruses (HTLV) I and II. Pathogen reduction raises the safety margin by inactivating pathogens that have gone undetected during screening due to window periods or test errors. In addition, pathogen reduction provides a proactive safeguard, inactivating emerging pathogens before they enter the blood supply chain and before screening tests have been developed and implemented [1]. It should be noted that the current pathogen reduction procedures are not foolproof. Breakthrough for high viral loads may occur, especially in ramp-up early phase of the infection, and we need also to worry about parvoviruses and hepatitis A as well as vCJD and any evolving viruses that may emerge. Nevertheless, the implementation of virus reduction technologies is a way forward to increase the safety margin.
The adopted technology for pathogen reduction, to achieve rapid acceptance, must be robust, affordable, and clinically effective without grossly reducing the function and the life span of active principles in FFP or being toxic to recipient. Hence this requires continual quality improvement in terms of safety, efficacy, availability and supply management.
A promising development, fit for purpose, is the use of me thy lene blue (MB) for viral reduction of single unit FFP. This technology was originally developed by the Blood Centre of the German Red Cross, Springe, Germany; and MB FFP was first produced routinely for clinical use in 1992 [2]. The emerging THERAFFEX MB-Plasma system (MacoPharma, Tourcoing, France) has a number of improved features and has been used routinely by blood centres in several countries with success. More than 4.4 million MB-treated plasmas including over 1.9 million THERAFLEX MB (TMB) plasma units have been generated to date [3]. This article gives an overview of the chemical characteristics of MB, the mechanism of the technology, toxicology, and pathogen reduction capacity as well as the current position and haemovigilance status within Europe. The manuscript is based on our previous review [4] and updated by some of the recent data and reports on viral reduction topic and European haemovigilance [5, 6, 7, 8, 9, 10, 11, 12].
Biochemical Characteristics of Methylene Blue
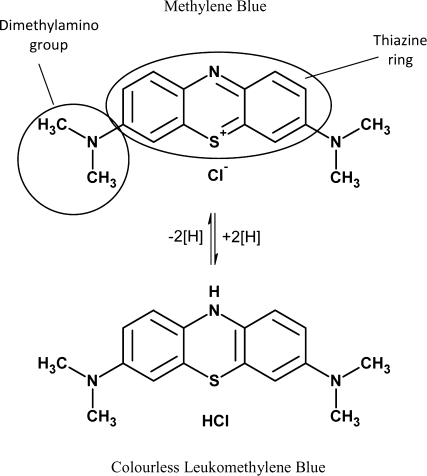
MB is a positively charged phenothiazine derivative. It consists of a thiazine ring and two dimethylamino groups (fig. 1). The chemical name is: 3,7-bis(dimethylamino)-phenothiazine-5-ylium chloride. At room temperature MB is an odourless dark green powder, which is soluble in cold water. The alternative colourless leuco MB is readily oxidized into MB in the presence of oxygen. Since MB is used as active ingredient in several drugs there are monographs in the USP and in the Pharm. Eur.
Fig. 1.
Structure of MB and leukomethylene blue.
MB was first synthesized by Heinrich Caro in 1876. It was the first tar-derived colour in the world for which the synthesis process was patented in 1877 [13]. The substance was used as starting point for the creation of several drugs e.g. for chlorpromazine which was used as antipsychotic [14].
As first medical application MB was used as early as 1891 by Guttmann and Ehrlich to treat malaria [15]. Although it was later replaced by other drugs, there is renewed interest for the treatment of malaria with MB [16]. Some of the other useful applications of MB include: treatment for methaemoglobinaemia recommended by the WHO and the European Commission as antidote [17]; attenuating the pathogenic effects of sepsis; sentinel lymph node biopsy (SFNB) to differentiate the different tissues [18]; chromoperturbation and chromoendoscopy to localise Barrett's metaplasia [19, 20]; inhibition of the actions of nitric oxide that lead to increased blood pressure and myocardial function [21, 22]; treatment of anaphylaxis [23, 24, 25] by giving intravenously 1.5-2 mg/kg body weight [26].
In all the above clinical applications the final amount of MB in the patient is considerably higher than when using MB plasma. Nevertheless, the potential side-effect of treated plasma remains to be fully elucidated by active haemovigilance at national and European levels by main users.
From a historical pathogen reduction standpoint, reduction of viruses by addition of MB to various aqueous solutions and subsequent illumination with visible light has been described as early as 1933 by Perdrau and Todd [27]. Subsequently different authors have described reduction of several pathogens to different extent. This includes Herpes simplex [28], Es-cherichia coli [29], and vaccinia virus [30]. Today it is well established that combined MB light system has a broad spectrum potential for reducing the impact of pathogens transmit-table through transfusion.
Processing Methodology
Two different methods for MB treatment of human plasma for virus reduction are currently in use. Table 1 summarises the key features of the original and the current MB/light treatment systems. The original ‘Springe’ method, developed by the Blood Centre of the German Red Cross Chapters of Lower Saxony, Saxony-Anhalt, Thuringia, Oldenburg and Bremen (NSTOB), institute Springe and commercialised by Grifols and the TMB plasma method originated by MacoPharma.
Table 1.
Comparison of the processing methods used in the original and current methylene blue treatment systems
| Processing steps | Springe | THERAFLEX (Macotronic V) | THERAFLEX (Macotronic B) |
|---|---|---|---|
| 1. Freezing/thawing | yes | no | no |
| 2. Leucodepletion | no | yes* | yes* |
| 3. Addition of MB | 50 μmol/l MB solution volume adjusted to 1 μmol/l | 85 μg of MB dry pill | 85 μg of MB dry pill |
| 4. Illumination | one side | two side | two side |
| fluorescent lamps | sodium lamps | Light emitting diodes | |
| no temperature control | temperature monitoring | temperature monitoring | |
| 5. Removal of MB | no | yes | yes |
0.65 μm membrane filter is integrated in the system.
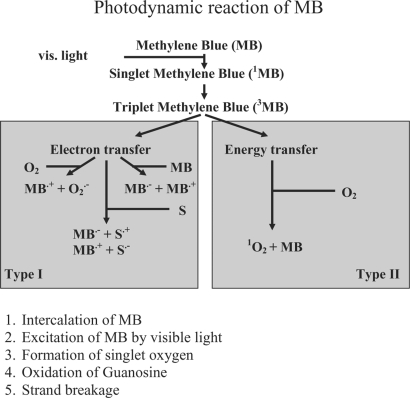
The inactivating process can be divided into five steps as shown in figure 2.
Fig. 2.
The photodynamic mechanism of MB. Type II is the main mechanism seen in plasma [46].
The Springe process required freezing and thawing of plasma units to disrupt leucocytes, releasing intracellular viruses, because MB only partially penetrates cell membrane. An optimised amount of MB solution was then added to the plasma unit to achieve a final concentration of 1 μmol/1, and subsequently the plasma bag was illuminated with fluorescent tubes on one side for 1 h. This process was factory-based. The current improved TMB plasma system lends itself to blood bank-based processing and has obtained a CE class III marking. It employs a 0.65 μm membrane filter (Plasmaflex PLAS4; MacoPharma) which removes residual leucocytes, red cells, platelets and aggregates, and minimises the amount of microvesicles and microparticles [31]. Filtration efficiency is ensured by integrity testing during filter manufacturing and by routine monitoring of residual cell content and filtration time in the blood bank. Moreover, the Blueflex filter is acting as backup system for the PLAS4 filter comprising an identical membrane. The filtered plasma then flows pass a dry pill of 85 μg anhydrous MB chloride which is integrated into the bag system providing an approximate final concentration of 1 μmol/1 for a volume of plasma between 235 and 315 ml, hence aphaeresis plasma needs to be split into the volume within this range.
The illumination is achieved by a microprocessor-controlled device (Macotronic; MacoPharma), ensuring treatment under a Good Manufacturer Practice (GMP) controlled condition where not only both illumination dose and intensity are constantly monitored but also temperature is maintained at ≤22 °C. The use of low-pressure sodium lamps with a peak wavelength of 590 nm instead of fluorescent lamps in the original version reduces the illumination time from 1 h to about 20 min to achieve the required light dose of 180 J/cm2. Before treatment the donation number is captured by barcode reader or optionally by radio frequency identification (RFID). When the same donation number is scanned for a second time, the software will reject illumination. Plasma units that have been treated are indicated by a barcode label comprising all essential information on the illumination process. After treatment, over 90% of the residual MB combined with its photo-activated products is removed by a specially designed filter (Blue-flex; MacoPharma) [32, 33]. Thus, plasma is filtered twice, resulting in virtually cell-free plasma. In the UK, as plasma units are imported in a frozen state, a thawing step is required before MB treatment.
From the operational standpoint further improvement in aphaeresis plasma processing by TMB plasma system has been made optimising the process flow with the use of the LEAN management system principle which enhance the safety and comfort of both the process and the staff [34].
Recently a new Macotronic illumination device has been introduced, Macotronic B. This device is equipped with light-emitting diodes instead of sodium low-pressure lamps. The size of the device was substantially reduced, enhancing operational flexibility. Cross-validation between the Macotronic V and B was done measuring factor V, factor VIII, factor XI, protein S, alpha2-antiplasmin, prothrombin time, activated partial thromboplastin time, thrombin-antithrombin, and total protein content. Results showed equivalence of active components, in particular factor V, factor XI and protein S [35], and factor retention was similar to previously published data [4]. In a study using plasma spiked with pseudorabies virus, bovine diarrhoeal virus or HIV, MB treatment which employed the Macotronic B showed at least an equivalent viral reduction capacity as compared to that of the Macotronic V device [36].
It is pertinent to question whether factor VIII level is a relevant quality indicator of FFP and cryoprecipitate, in particular in developed countries where these products are not indicated for factor VIII replacement [37]. Attempts should be made to introduce more clinically relevant indicators including other labile factors, affected by photoreduction system, such as factor V and XI where FFP is currently in clinical use for [38]. In countries that lack financial resources to obtain pathogen-inactivated factor concentrates, cryoprecipitate derived from MB FFP could be useful in treating haemophilia A, von Willebrand's disease as well as fibrinogen and factor XIII deficiencies. The processing steps for cryoprecipitate should therefore be customised to optimise the recovery of the coagulation components required for clinical use e.g. factor VIII, fibrinogen and factor XIII.
Commercially available fibrin glue products are manufactured from pooled plasma. Cryoprecipitate prepared from MB FFP could be an alternative source which provides the benefit of reduced number of donor exposure. Preparation of fibrin glue from TMB single-donor plasma with a proprietary device revealed comparable performance compared to standard FFP [39].
Pharmacokinetics and Toxicology
There is considerable clinical experience to support that MB has limited toxicity.
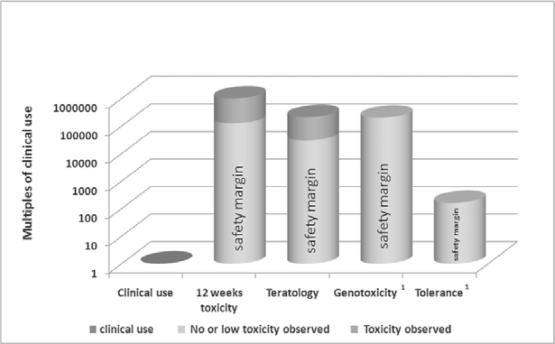
In a recent study using rat models, after 20 mg/kg intravenous infusion, MB shows a biphasic elimination half-life with an initial half-life of only 3 min and a terminal half-life of 12 h. The excretion of MB in urine and faeces was almost complete 96 h after infusion with less than 1 % of the infused MB found in various organs. Azure B, a photoactivated product of MB, revealed a similar pharmacokinetic profile [40]. Furthermore, MB given orally has been found to be well absorbed providing a systemic bioavailability of approximately 50%, hence results from earlier toxicology studies using an oral route [41, 42] can also be applied to intravenous route. Chronic exposure in rats and mice at doses up to 200 mg/kg/day via oral route for 13 weeks did not show signs of toxicity [41]. Studies using animal models and intravenous injections show that MB has a very high safety margin, in the magnitude of 160 to 200,000 times of the clinical dose used in the MB FFP (fig. 3; unpublished data, MacoPharma). Also the photoproducts were tested for toxicological effects. Azure B, reveals similar toxicological profile as MB in vitro, for Azure A and C the results are inconclusive [40, 43].
Fig. 3.
Safety margins of MB-treated plasma using animal models. Concentration in the clinical use column is based on an MB dose of 0.1 μg/kg.
In human, intravenous MB at doses of 1–5 mg/kg has been used mainly in methaemoglobinaemia and cyanide poisoning without the report of serious adverse reactions. The amount of MB used in the current TMB system is 85 μg per plasma unit. Assuming an average volume of a plasma unit of 250 ml, a 70-kg adult transfused with 1,000 ml of TMB FFP will receive approximately 0.005 mg/kg of MB. With the use of MB removal filter which reduces MB and its photoproducts to an average level of 2 μg/1 [32], the transfused residual MB will be 0.00003 mg/kg or 0.5 μg per plasma unit which is well below the requirement of the guidelines for blood transfusion services in the UK and France (below 30 μg/unit) [44].
MB-treated plasma underwent extensive toxicity studies for licensing purpose. In practice the primary goal of toxicity testing is to estimate safety margins to better inform clinical researchers, and preclinical toxicity testing is limited in defining the ultimate safety of the product. Small members of animals in cross-species testing are used to predict response in human. Based on available data, there is considerable evidence to support that TMB plasma has limited toxicity, and so far no neoantigenicity or genotoxicity have been observed. There was no significant passive cutaneous anaphylaxis even at tenfold higher MB concentration in mouse model. Moreover, no antibody formation was detected in rabbits which for prolonged times where repeatedly immunised with autologous photoinactivated plasma [45]. It is expected that more data would become available after extensive post marketing surveillance and haemovigilance.
Pathogen Reduction Capacity
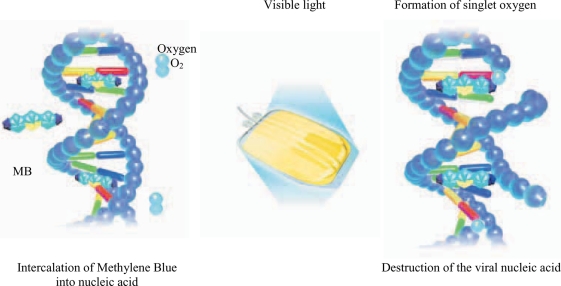
MB is a phenothiazine compound which intercalates into viral nucleic acid, and subsequent illumination generates singlet oxygen leading to guanosine oxidation and destruction of the viral nucleic acid preventing viral replication [46, 47] (fig. 4). In addition, an experiment on HIV 1 has shown that MB treatment also acts on other target sites such as the envelope, core proteins, and reverse transcriptase enzyme [48]. Table 2 shows the viral reduction spectrum and viral reduction capacity of the THERAFLEX system based on infectivity assay in cell cultures [4, 49, 50]. The reduction of enveloped viruses tested is at least 5 logs and its reduction spectrum includes HIV as well as model viruses for HBV and HCV. In addition, it is also effective against West Nile virus (WNV) [49, 51]. For non-enveloped viruses the efficacy is diverse, e.g. approximately ≥4 logs for calicivirus and simian virus 40 and not effective against hepatitis A and porcine parvovirus [49]. Nevertheless, the MB system has shown a reduction of ≥5 logs of parvo virus B19 [52]. For the latter virus evidence exists that even MB without illumination showed a 1–2 log reduction power. Additional illumination enhances this by 3-4 log steps [53].
Fig. 4.
MB is a phenothiazine dye which intercalates into viral nucleic acid and subsequent illumination generates singlet oxygen leading to guanosine oxidation and destruction of the viral nucleic acid preventing viral replication.
Table 2.
Viral log reductions achieved by MB treatment
| Virus | Family | Model for | Reduction rate (log10) |
|---|---|---|---|
| Enveloped viruses | |||
| HIV-1 | Retro | HIV | ≥5.45* |
| WNV | Flavi | WNV | ≥5.78* |
| BVDV | Flavi | HCV | ≥5.44* |
| PRV | Herpes | HBV, CMV | ≥5.48* |
| Duck HBV | Hepadna | HBV | ≥6* |
| Influenza H3N2 | Orthomyxo | Influenza | ≥4.40* |
| CMV | Herpes | CMV | ≥4.08* |
| IBV | Corona | SARS | ≥4.90* |
| Hog cholera | Flavi | HCV | ≥5.92 |
| Herpes Simplex | Herpes | Herpes | ≥5.50 |
| Bovine herpes | Herpes | Herpes | ≥8.11 |
| Semliki Forest | Toga | Chikungunya | ≥7.00 |
| Sindbis | Toga | Chikungunya | ≥9.73 |
| Influenza | Orthomyxo | Influenza | ≥5.1 |
| Vesicular Stomatitis | Rhabdo | Rabies | ≥4.89 |
| Non-enveloped viruses | |||
| Had-5 | Adeno | Adeno | ≥5.33* |
| Calici | Calici | Noro | ≥3.9* |
| SV 40 | Polyoma | SV40 | ≥4 |
| Parvo B 19 | Parvo | Parvo B19 | ≥5 |
| Porcine parvo | Parvo | Parvo B19 | 0* |
| Polio | Picorna | Polio | >1 |
| Hepatitis A | Picorna | HAV | 0 * |
≥ Reduction below detection limit.
Tested under production conditions.
As for non-viral pathogens, a study on Trypanosoma cruzi using in vitro cultures and a mouse model shows that MB treatment provides a log reduction of >3.4 and 4.9–5.8 respectively, indicating that this pathogen reduction system could potentially prevent transfusion-transmitted Chagas disease [54]. Although the effect of MB system on malaria, an intracellular parasite, has not been characterised, leucocyte filtration and freeze-thawing process remove and disrupt parasite-containing cells, hence preventing its multiplication. These benefits are also applicable to cytomegalovirus and human T-lymphotropic virus I and II [55]. There have been reports that phenothiazines might destroy prions [56, 57] but the actual effect of the MB system on this pathogen remains to be investigated.
The MB system does not remove the viral nucleic acid which has been inactivated. As a result, assays which are based on nucleic acid amplification tests could remain positive even though the treated plasma is no longer infectious. Similarly, assays based on specific antibodies could also detect inactivated viral particles. Therefore, infectivity assays using cell cultures or animal models are most suitable for assessing the pathogen reduction capacity of the MB system. Such systems would also possibly reveal intracellular repair of insufficiently inactivated viral nucleic acid by the infected cultured or animal cells.
Adherance to Guidelines and Clinical Aspects
The effect of MB treatment on coagulation factors and anticoagulant proteins in plasma has been widely published and reviewed elsewhere [4, 38, 50, 58, 59, 60]. In brief, the loss of coagulation factors is mainly due to photo-oxidation of the proteins during the illumination process, and the loss caused by other steps, such as freeze-thawing, leucocyte filtration or MB removal, is negligible or limited [61], meeting phase 1 and 2 validation requirements. The loss of factor VIII and fibrinogen, the most commonly used parameters for quality monitoring of plasma, ranges from 10 to 35%. This large variation is possibly due to varying analytical procedures used in different laboratories. Moreover, slight changes in fibrin polymerisation similar to those seen in plasma from patients with dysfibrinogenaemia which are usually without clinical significance, have been observed [62]. A recent study has shown that MB treatment has no profound effect on the characteristics of thrombin generation as compared to the untreated counterpart [63]. The loss of other coagulation factors appears to be in lower ranges. MB treatment has little effect on anticoagulant or fibrinolytic proteins such as protein C, protein S, anti-thrombin III, and plasminogen.
Observational studies in Spain [64, 65] give the impression that MB plasma is possibly less effective than Q-plasma for plasma exchange in thrombotic thrombocytopenia purpura (TTP). Additionally, data from a prospective multicentre cohort report indicated a greater volume of plasma used and a higher frequency of recurrence [66]. In Spain both, the original Springe method and TMB are used in parallel, and no differentiation was made in this publication. In this respect such observation of reduced effectiveness of MB plasma is highly curious inasmuch as further studies indicate that MB plasma contains normal levels of ADAMTS-13 [67].
Castrillo et al. [68] reported data from quality control testing over 5 years in which more than 110,000 units of FFP from both random and aphaeresis donors were treated with MB. The data representing all ABO blood groups show that despite the loss of factor VIII during MB treatment, the level of factor VIII still met the criteria of the Council of Europe Guidelines for FFP, i.e. ≥70%. After the initial loss of plasma proteins, at <−30 °C storage, the levels of all plasma proteins tested remain stable for at least 27 [69] or even 39 months [70]. This data may prove useful to regulatory authorities to prolong the storage time of FFP, helping in the supply availability and management.
Cryoprecipitate prepared from MB FFP contains approximately 20-40% less fibrinogen and factor VIII compared with non-treated FFP based on a limited number of samples tested [59] but remain within the specification set by the Council of Europe Guidelines, i.e. factor VIII ≥70% of the value of the freshly collected plasma unit [71]. Experiments have shown that storing MB FFP units at 2–6°C for 4 or 8 h significantly increases fibrinogen recovery with a concomitant small loss of factor VIII recovery in the cryoprecipitate units [4,72, 73].
In clinical practice satisfactory results have been reported with this product, but there have been no full reports of large-scale randomised trials using relevant endpoints such as blood loss or exposure to other blood components.
State of Accreditation in Europe and Haemovigilance
Blood components for transfusion are classified differently by various European countries. In Germany they are considered pharmaceutical drugs and must meet pharmaceutical standards according to latest standards of research and development. For FFP production licenses are issued by local authorities as well as by marketing authorisations issued by competent federal authority. Major changes therefore require that the transfusion service reapply for a new licence. Similar systems are in place in other countries, e.g. in Switzerland and France, while in some other European countries blood components are considered as medicinal products, with equal stringent rules and regulations. Moreover, while European blood directives defines quality and safety standards for the testing, processing, storage, and distribution of human blood and components, individual member states are free to adopt higher standards than set in the EC directives. Therefore as one might expect states of accreditation as well as opinions on the scientific/clinical, regulatory and economic aspects vary widely within Europe.
A recent survey of established pathogen inactivation of 20 representative centres by Seifried et al. [12] revealed that 80% routinely used pathogen-inactivated FFP and 50% plans to introduce it for the first time or to expand their current portfolio in near future. From the future development point of view the outlook for pathogen reduction technology in routine production setting of Europe is multifaceted, as regulation varies and decision makers have different scientific, medical and political viewpoints. Nevertheless, in principle the regulatory framework, setting high standards for establishing pathogen inactivation and harmonising the procurement of safe blood in Europe, is in progress.
As for the current use of TMB system in European countries, one of the authors assembled some data from main users (summarised in table 3) when the TMB system has been registered or is in routine use in Europe.
Table 3.
Global overview of European countries where THERAFLEX MB-Plasma is in routine use or registered.
| Country | Country approvals | In routine use | % of therapeutic plasma |
|---|---|---|---|
| Austria | 2008 | 2008 | 5 |
| Belarus | 2009 | ||
| Belgium | 2004 | 2004 | 98 |
| Croatia | 2006 | ||
| Czech Republic | 2005 | ||
| France | 2005 | 2008 | 55 |
| Germany | 2007 | ||
| Greece | 2000 | 2000 | 10 |
| Italy | 2002 | 2002 | 18 |
| Lithuania | 2004 | ||
| Poland | 2006 | 2008 | 0.1 |
| Russia | 2006 | 2006 | 5 |
| Spain | 1999 | 1999 | 65 |
| Switzerland | 2007 | ||
| Ukraine | 2007 | ||
| UK | 2002 | 2002 | 4* |
Only used for children up to the age of 16.
During the last decade haemovigilance follow-up has become more and more adopted. National programs where established in several European countries which allow determining the extensiveness of different adverse events. With this information it is possible to evaluate the adverse event rate of TMB-treated plasma in the countries where this procedure is used. Unfortunately, in some countries where different safeguarding methods for plasma are used in parallel, adverse events are not always differentiated by the plasma type used, making it difficult to interpret and compare data with accuracy.
The French haemovigilance report from 2009 is now available [74]. During 2009 204,814 units of MB plasma have been issued. This is 55.1% of all plasmas produced. In total 371,658 plasma units were delivered. A relatively higher incidence of allergic events with MB-treated plasma when compared with quarantine FFP was reported; thus we are awaiting an explanation by a dedicated expert group whether it is a recipientor product-related reaction. The authors of a case report from France stated that they found some evidence for a potential cross-reactivity of Patent Blue V (PBV), a food colourant widely used in Europe, and MB which may explain such allergic reactions to MB plasma [75]. This is in contrast to previous publications. It has been shown that, in contrast to MB, PBV is binding to proteins and forms a complex [76]. An IgE-mediated mechanism was suggested [77]. In fact MB was recommended as a safe alternative to PBV for lymph node mapping [78,79].
The guidelines of clinical usage of pathogen-reduced plasmas in France are the same as those used for FFP. MB plasma should not be used in case of sensitisation to MB [80]. Recently, allergic reactions of patients receiving blood products became a special focus of interest in the French haemovigilance program. For this purpose a dedicated working party was inaugurated.
The available information from Belgium is most promising, indicating no different adverse event profile of MB-treated compared to non-treated plasma.
The most recent haemovigilance report for Belgium is summarising data from 2008 [81]. In total 2 severe transfusion reactions assigned to plasma were reported, both angio-oedema. Since in Belgium almost all therapeutic plasma (approximately 90,000 units/year) is MB-treated, this report gives the most valuable information on the frequency of adverse effects by the use of TMB. Comparative haemovigilance data before and after introduction of TMB plasma in Belgium is available [82]. The authors compared the incidence and the seriousness of adverse events from their hospital before mid of 2004, when they used solvent-detergent FFP (SD-FFP) with data after introduction of TMB plasma. They found no significant increase in adverse event incidence after MB FFP transfusion (odds ratio 1.2), and the seriousness of these events was comparable; in particular, allergic reactions did not increase from 2003 to 2004. The clinical efficacy of both FFP was similar.
The current legislation in the UK does not require single donation blood components to have a product license. Moreover, no independent formal pathogen reduction validation other than occasional studies on individual pathogen is required. Accordingly, it is important to rely on manufacturing data on viral safety provided for licensing or the parent product.
Nevertheless, in the UK there is a well established system in place, addressing transfusion-transmitted infection. As for the transfusion reactions the annual SHOT report indicates in total 44 acute transfusion reactions of FFP comprising 32 allergic/anaphylactic reactions in 2009 [83]. Two allergic/anaphylactic reactions were attributed to SD plasma and one febrile reaction to MB plasma. Additionally, one transfusion-associated circulatory overload was described with MB plasma. A 65-year-old woman with TTP was treated with 12 units of MB plasma (2,636 ml) without plasma exchange. She was successfully treated with furosemide. The impact of using only male donor plasma in reducing the TRALI awaits long-term clinical experience.
In Spain approximately 250,000 units of plasma are transfused annually 65% of which is MB-treated. Haemovigilance data from Spain suggest that the side-effects associated with the use of MB plasma are similar to those seen with standard FFP [84]. In the 2007 Spanish haemovigilance report FFP is involved in 34% of all allergic reactions reported [85], which is similar to Catalonia (32%) where all transfused plasma is MB-treated [86].
Castrillo et al. [68] have reported 5 years experience on more than 110,000 units of MB FFP with no adverse reactions observed. In addition, another group from Spain has reported their experience with MB FFP from 1996 to 2006 with the TMB system being used from 1999, producing more than over 88,000 units of TMB FFP with satisfactory outcomes [87].
In Italy it is not possible to attribute any adverse effect to plasma or MB-treated plasma because the first national haemovigilance report published in 2007 is not distinguishing adverse reactions by the different blood components [88].
A retrospective study on clinical efficacy of MB-treated plasma was published in Italy [89]. The authors concluded that MB-treated plasma allows an effective, safe and well tolerated treatment. No patient presented with early or late adverse effects during the administration when arresting any severe bleeding or coagulopathy, with no increased request of it.
Politis et al. [60] from Greece reported 5 years experience with MB FFP in which 8,500 units of MB FFP were compared with 54,435 units of untreated FFP given to patients with a similar range of clinical conditions. MB removal filter was used in 3,038 units of MB FFP. No seroconversion for HBV, HCV, HIV, or HTLV I/II was detected 6 months after MB FFP or untreated FFP transfusion. The incidence of adverse reactions was 1 in 8,500 with MB FFP and 1 in 2,177 with untreated FFP. Although the difference was not statistically significant, it is noteworthy that the only adverse reaction seen in the MB FFP group was non-serious allergic reaction. In the untreated FFP group, both non-leucodepleted and leu-codepleted products were used during the reported period, and the associated adverse reactions encompassed allergic reactions, anaphylactic shocks, bacterial infections, and non-haemolytic febrile transfusion reactions. The lower incidence of allergic reactions and non-haemolytic febrile transfusion reactions in the MB group was attributed to the early and effective removal of leucocytes as well as platelets by leucocyte filter [60].
Conclusion
Although today's blood components in Europe are considered to be safer than ever, research to further improve transfusion protection will continue with rigour, and the expansion of pathogen inactivation is a necessary task in the future plan. In this respect, despite some variable loss of some active components, the TMB plasma system for pathogen reduction meets the acceptance criteria of various national and European guidelines. Overall satisfactory results are generally obtained in many clinical experiences all over the world using MB FFP technology. Nevertheless, the controversial significance of small changes in potency on clinical outcomes, in particular in TTP, remains to be fully elucidated as MB treatment has little impact on the plasma content of ADAMTS-13. This highlights the current concept that the consistency in product efficacy is as important as products meeting the specification. An integrated national haemovigilance system is warranted to overcome the limitations of animal toxicity studies with a restricted amount of individuals. Observational studies or even multicentric, prospective cohort studies with patients might not reveal rare events.
In addition to clinical efficacy, one should also look at the risk/benefit ratio of MB plasma as compared to untreated plasma in specific clinical settings, taking into consideration the extent of donor exposure, the incidence of infections in donor population in a country as well as the risk of emerging pathogens. Finally, both financial and logistic factors have to be considered.
Achieving zero risk in haemotherapy remains the ultimate goal. This target has not been reached yet, but there are some promising developments. Pathogen reduction by TMB plasma is one additional piece to complete this ambition. Eventually it is expected that with increasing clinical data and further methodological improvement acceptance will rise, making pathogen reduction to become the standard routine procedure at least in Europe. Therefore TMB procedure for plasma is here to stay in Europe and would undoubtedly expand worldwide. The evolution in this direction has already begun.
Disclosure Statement
JS declares no conflict of interest. WGS and SR are employees of Maco-Pharma, the manufacturer of the described THERAFLEX MB-Plasma.
References
- 1.Allain JP, Bianco C, Blajchman MA, Brecher ME, Busch M, Leiby D, Lin L, Stramer S. Protecting the blood supply from emerging pathogens: the role of pathogen inactivation. Transfus Med Rev. 2005;19:110–126. doi: 10.1016/j.tmrv.2004.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambrecht B, Mohr H, Knuver-Hopf J, Schmitt H. Photoinactivation of viruses in human fresh plasma by phenothiazine dyes in combination with visible light. Vox Sang. 1991;60:207–213. doi: 10.1111/j.1423-0410.1991.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 3.Sandier SG. The status of pathogen-reduced plasma. Transfus Apher Sci. 2010;43:393–399. doi: 10.1016/j.transci.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Seghatchian J, Walker WH, Reichenberg S. Updates on pathogen inactivation of plasma using Theraflex methylene blue system. Transfus Apher Sci. 2008;38:271–280. doi: 10.1016/j.transci.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Stramer SL, Hollinger FB, Katz LM, Kleinman S, Metzel PS, Gregory KR, Dodd RY. Emerging infectious disease agents and their potential threat to transfusion safety. Transfusion. 2009;49(suppl 2):1S-29S. doi: 10.1111/j.1537-2995.2009.02279.x. [DOI] [PubMed] [Google Scholar]
- 6.Rock G. A comparison of methods of pathogen inactivation of FFP. Vox Sang. 2010 doi: 10.1111/j.1423-0410.2010.01374.x. DOI: 10.HH/j.1423-0410.2010.01374.x. [DOI] [PubMed] [Google Scholar]
- 7.Prowse C. Properties of pathogen-inactivated plasma components. Transfus Med Rev. 2009;23:124–133. doi: 10.1016/j.tmrv.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 8.AuBuchon JP. Current status of pathogen inactivation methods. ISBT Science Series. 2010:125–133. [Google Scholar]
- 9.McClaskey J, Xu M, Snyder EL, Tormey CA. Clinical trials for pathogen reduction in transfusion medicine: a review. Transfus Apher Sci. 2009;41:217–225. doi: 10.1016/j.transci.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Solheim B, Seghatchian J. Pathogen inactivation. In: Simon TL, Snyder EL, Solheim B, Stowell CP, Strauss RG, Petridis M, editors. Rossi's principles of transfusion medicine. Philadelphia: Blackwell; 2009. pp. 801–810. [Google Scholar]
- 11.Seghatchian J, de Sousa G. Pathogen-reduction systems for blood components: the current position and future trends. Transfus Apher Sci. 2006;35:189–196. doi: 10.1016/j.transci.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Seifried E, Henschler R, Müller MM. European perspectives on implementation. In: AuBuchon JP, Prowse C, editors. Pathogen Inactivation: The Penultimate Paradigm Shift. Bethesda: AABB Press; 2010. pp. 261–278. [Google Scholar]
- 13.Verfahren zur Darstellung blauer Farbstoffe aus Dimethylanilin und anderen tertiaren aromatischen Monaminen. Patent Number: DE000000001886A 15-12-1877,1-3.
- 14.Elkes J. Psychopharmacology: finding one's way. Neuropsychopharmacology. 1995;12:93–111. doi: 10.1016/0893-133X(93)00017-G. [DOI] [PubMed] [Google Scholar]
- 15.Guttmann P, Ehrlich P. Über die Wirkung des Methylenblau bei Malaria. Berlin Klin Wochenschr. 1891;28:953–956. [Google Scholar]
- 16.Zoungrana A, Coulibaly B, Sie A, Walter-Sack I, Mockenhaupt FP, Kouyate B, Schirmer RH, Klose C, Mansmann U, Meissner P, Müller O. Safety and efficacy of methylene blue combined with artesunate or amodiaquine for uncomplicated falciparum malaria: a randomized controlled trial from Burkina Faso. PLoS ONE. 2008;3:el630. doi: 10.1371/journal.pone.0001630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Commission: Resolution of the Council and of the Representatives of the Governments of the Member States, Meeting within the Council of 3 December 1990 on Improving the Prevention and Treatment of Acute Human Poisoning. 90/C 329/03 1990.
- 18.Teknos D, Ramcharan A, Oluwole SF. Pulmonary edema associated with methylene blue dye administration during sentinel lymph node biopsy. J Nati Med Assoc. 2008;100:1483–1484. doi: 10.1016/s0027-9684(15)31552-2. [DOI] [PubMed] [Google Scholar]
- 19.Ormeci N, Savas B, Coban S, Palabiyikoglu M, Ensari A, Kuzu I, Kursun N. The usefulness of chromoendoscopy with methylene blue in Barrett's metaplasia and early esophageal carcinoma. Surg Endose. 2008;22:693–700. doi: 10.1007/s00464-007-9463-x. [DOI] [PubMed] [Google Scholar]
- 20.Horwhat JD, May dono vitch CL, Ramos F, Colina R, Gaertner E, Lee H, Wong RK. A randomized comparison of methylene blue-directed biopsy versus conventional four-quadrant biopsy for the detection of intestinal metaplasia and dysplasia in patients with long-segment Barrett's esophagus. Am J Gastroenterol. 2008;103:546–554. doi: 10.1111/j.1572-0241.2007.01601.x. [DOI] [PubMed] [Google Scholar]
- 21.Juffermans NP, Vervloet MG, Daemen-Gubbels CR, Binnekade JM, de JM, Groeneveld AB. A dose-finding study of methylene blue to inhibit nitric oxide actions in the hemodynamics of human septic shock. Nitric Oxide. 2010;22:275–280. doi: 10.1016/j.niox.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Kwok ES, Howes D. Use of methylene blue in sepsis: a systematic review. J Intensive Care Med. 2006;21:359–363. doi: 10.1177/0885066606290671. [DOI] [PubMed] [Google Scholar]
- 23.Evora PR, Viaro F. The guanylyl cyclase inhibition by MB as vasoplegic circulatory shock therapeutical target. Curr Drug Targets. 2006;7:1195–1204. doi: 10.2174/138945006778226679. [DOI] [PubMed] [Google Scholar]
- 24.Evora PR, Simon MR. Role of nitric oxide production in anaphylaxis and its relevance for the treatment of anaphylactic hypotension with methylene blue. Ann Allergy Asthma Immunol. 2007;99:306–313. doi: 10.1016/S1081-1206(10)60545-5. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigues JM, Pazin FA, Rodrigues AJ, Vicente WV, Evora PR. Methylene blue for clinical anaphylaxis treatment: a case report. Sao Paulo Med J. 2007;125:60–62. doi: 10.1590/S1516-31802007000100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliveira Neto AM, Duarte NM, Vicente WV, Viaro F, Evora PR. Methylene blue: an effective treatment for contrast medium-induced anaphylaxis. Med Sci Monit. 2003;9:CS102–CS106. [PubMed] [Google Scholar]
- 27.Perdrau JR, Todd FRS. The photodynamic action of methylene blue and certain viruses. Proc R Soc Lond. 1933;112:288–298. [Google Scholar]
- 28.Felber TD, Smith EB, Knox JM, Wallis C, Melnick JL. Photodynamic inactivation of herpes simplex: report of a clinical trial. JAMA. 1973;223:289–292. [Google Scholar]
- 29.Heinmets F, Vinegar R, Taylor WW. Studies on the mechanism of the photosensitized inactivation of E. coli and reactivation phenomenon. J Gen Physiol. 1952;32:207–226. doi: 10.1085/jgp.36.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner GS, Kaplan C. Observations on photodynamic inactivation of vaccinia virus and its effect on immunogenicity. Epidemiol Infect. 1965;63:395–410. doi: 10.1017/s0022172400045289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chabanel A, Sensebé L, Masse M, Maurel JP, Plante J, Hivet D, Kannengjesser C, Naegeken C, Joussemet M, Marchesseau B, Rasongles P, Proust F, David C, Montembault AM, Bergeat P. Quality assessment of seven types of fresh-frozen plasma leucoreduced by specific plasma filtration. Vox Sang. 2003;84:308–317. doi: 10.1046/j.1423-0410.2003.00288.x. [DOI] [PubMed] [Google Scholar]
- 32.Verpoort T, Chollet S, Lebrun F, Goudaliez F, Mohr H, Walker WH. Elimination of methylene blue from photodynamically treated virus inactivated fresh frozen plasma: the Blueflex filter. Transfus Clin Biol. 2001;8:103s. [Google Scholar]
- 33.Bopp K-F, Morell A, Indrak K, Parkkinen J, Mertens H, Mohr H, Colamartino P, Stanescu I, Oyonarte S, Delaney FM, Padilla A. Pathogen inactivation of labile blood products. Transfus Med. 2001;11:149–175. doi: 10.1046/j.1365-3148.2001.00310.x. [DOI] [PubMed] [Google Scholar]
- 34.Naegelen C, Isola H, Dernis D, Maurel JP, Tardivel R, Bois S, Vignoli C, Cazenave JP. Evolution of techniques for preparation of labile blood products (LBP): pathogen inactivation in LBP (in French) Transfus Clin Biol. 2009;16:179–189. doi: 10.1016/j.tracli.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Begué S, Behague M, Verpoort T, Walicka I, Reichenberg S. THERAFLEX processed whole blood plasma quality using a new illumination device Macotronic B2. Transfusion. 2009;49:1A–304A. [Google Scholar]
- 36.Reichenberg S, Gravemann U, Behague M. Validation of virus inactivation of plasma by THERAFLEX MB-Plasma procedure using the illumination device Macotronic B2. Vox Sang. 2008;95:74–326. [Google Scholar]
- 37.Seghatchian J. What is happening? Are the current acceptance criteria for therapeutic plasma adequate? Transfus Apher Sci. 2004;31:67–73. doi: 10.1016/j.transci.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Seghatchian J, Krailadsiri P. What's happening? The quality of methylene blue treated FFP and cryo. Transfus Apher Sci. 2001;25:227–231. doi: 10.1016/s1473-0502(01)00109-4. [DOI] [PubMed] [Google Scholar]
- 39.Kumar V, Pinkoski L, Corash L, Chapman J. Preparation of fibrin sealant using methylene blue treated plasma using the Cryoseal FS-System. Vox Sang. 2007;93:54–274. [Google Scholar]
- 40.Pohler P, Leuschner J, Gravemann U, Walker WH, Reichenberg S, Mohr H, Müller TH. Methylene blue-treated plasma: toxicological profile of methylene blue and its photoproducts. Transfus Med. 2004;31:1–81. [Google Scholar]
- 41.Little AD. Executive summary of safety and toxicity information: methylene blue. 1990. http://ntp.niehs.nih.gov/?objectid=03DB4384-0364-AB0B-5C71EF4A37D6888A
- 42.Hejtmancik MR, Ryan MJ, Toft JD, Persing RL, Kurtz PJ, Chhabra RS. Hematological effects in F344 rats and B6C3F1 mice during the 13-week gavage toxicity study of methylene blue trihydrate. Toxicol Sci. 2002;65:126–134. doi: 10.1093/toxsci/65.1.126. [DOI] [PubMed] [Google Scholar]
- 43.Au W, Hsu TC. Studies on the clastogenic effects of biologic stains and dyes. Environ Mutagen. 1979;1:27–35. doi: 10.1002/em.2860010109. [DOI] [PubMed] [Google Scholar]
- 44.Guidelines for the Blood Transfusion Services in the United Kingdom 7 th ed., 2005, pp 1-358. www.official-documents.gov.uk/document/other/0117033715/0117033715.pdf/
- 45.Mohr H, Knuver-Hopf J, Lambrecht B, Scheidecker H, Schmitt H. No evidence for neoantigens in human plasma after photochemical virus inactivation. Ann Hematol. 1992;65:224–228. doi: 10.1007/BF01703949. [DOI] [PubMed] [Google Scholar]
- 46.Wagner SJ. Virus inactivation in blood components by photoactive phenothiazine dyes. Transfus Med Rev. 2002;16:61–66. doi: 10.1053/tmrv.2002.29405. [DOI] [PubMed] [Google Scholar]
- 47.Wainwright M, Mohr H, Walker WH. Phenothiazinium derivatives for pathogen inactivation in blood products. J Photochem Photobiol B. 2007;86:45–58. doi: 10.1016/j.jphotobiol.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Bachmann B, Knuver-Hopf J, Lambrecht B, Mohr H. Target structures for HIV-1 inactivation by methylene blue and light. J Med Virol. 1995;47:172–178. doi: 10.1002/jmv.1890470211. [DOI] [PubMed] [Google Scholar]
- 49.Mohr H, Lambrecht B, Selz A. Photodynamic virus inactivation of blood components. Immunol Invest. 1995;24:73–85. doi: 10.3109/08820139509062763. [DOI] [PubMed] [Google Scholar]
- 50.Williamson LM, Cardigan R, Prowse CV. Methylene blue-treated fresh-frozen plasma: what is its contribution to blood safety? Transfusion. 2003;43:1322–1329. doi: 10.1046/j.1537-2995.2003.00483.x. [DOI] [PubMed] [Google Scholar]
- 51.Mohr H, Knuver-Hopf J, Gravemann U, Redecker-Klein A, Muller TH. West Nile virus in plasma is highly sensitive to methylene blue-light treatment. Transfusion. 2004;44:886–890. doi: 10.1111/j.1537-2995.2004.03424.x. [DOI] [PubMed] [Google Scholar]
- 52.Knuver-Hopf J, Mohr H: Parvovirus B19 is sensitive to photodynamic and photochemical treatment. Abstract Book VIII European Congress of the ISBT.2003;94.
- 53.Knuver-Hopf J, Schaefer W, Groener A, Reichenberg S, Müller TH, Seltsam A. Human parvovirus in plasma is highly sensitive to methylene blue/light treatment. Vox Sang. 2010;99:1–516. [Google Scholar]
- 54.Girones N, Bueno JL, Carrion J, Fresno M, Castro E. The efficacy of photochemical treatment with methylene blue and light for the reduction of Trypanosoma cruzi in infected plasma. Vox Sang. 2006;91:285–291. doi: 10.1111/j.1423-0410.2006.00840.x. [DOI] [PubMed] [Google Scholar]
- 55.O'Shaughnessy DF, Atterbury C, Bolton MP, Murphy M, Thomas D, Yates S, Williamson LM. Guidelines for the use of fresh-frozen plasma, cryo-precipitate and cryosupematant. Br J Haematol. 2004;126:11–28. doi: 10.1111/j.1365-2141.2004.04972.x. [DOI] [PubMed] [Google Scholar]
- 56.Achour A. Phenothiazines and prion diseases: a potential mechanism of action towards oxidative stress. Int J Antimicrob Agents. 2002;20:305–306. doi: 10.1016/s0924-8579(02)00185-1. [DOI] [PubMed] [Google Scholar]
- 57.Amaral L, Kristiansen JE. Phenothiazines: potential management of Creutzfeldt-Jacob disease and its variants. Int J Antimicrob Agents. 2001;18:411–417. doi: 10.1016/s0924-8579(01)00432-0. [DOI] [PubMed] [Google Scholar]
- 58.Aznar JA, Bonanad S, Montoro JM, Hurtado C, Cid AR, Soler MA, De Miguel A. Influence of methylene blue photoinactivation treatment on coagulation factors from fresh frozen plasma, cryo-precipitates and cryosupernatants. Vox Sang. 2000;79:156–160. doi: 10.1159/000031234. [DOI] [PubMed] [Google Scholar]
- 59.Hornsey VS, Drummond O, Young D, Docherty A, Prowse CV. A potentially improved approach to methylene blue virus inactivation of plasma: the Maco Pharma Maco-Tronic system. Transfus Med. 2001;11:31–36. doi: 10.1046/j.1365-3148.2001.00282.x. [DOI] [PubMed] [Google Scholar]
- 60.Politis C, Kavallierou L, Hantziara S, Katsea P, Triantaphylou V, Richardson C, Tsoutsos D, An-agnostopoulos N, Gorgolidis G, Ziroyannis P. Quality and safety of fresh-frozen plasma inactivated and leucoreduced with the Theraflex methylene blue system including the Blueflex filter: 5 years' experience. Vox Sang. 2007;92:319–326. doi: 10.1111/j.1423-0410.2007.00898.x. [DOI] [PubMed] [Google Scholar]
- 61.Cardigan R, Allford S, Williamson L. Levels of von Willebrand factor-cleaving protease are normal in methylene blue-treated fresh-frozen plasma. Br J Haematol. 2002;117:253–254. doi: 10.1046/j.1365-2141.2002.3406_6.x. [DOI] [PubMed] [Google Scholar]
- 62.Depasse F, Sensebé L, Seghatchian J, Andreu G, Samama MM. The influence of methylene blue light treatment and methylene blue removal filter on fibrinogen activity states and fibrin polymerisation indices. Transfus Apher Sci. 2005;33:63–69. doi: 10.1016/j.transci.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 63.Gravemann U, Kusch M, Kónig H, Mohr H, Müller TH. Thrombin generation capacity of methylene blue-treated plasma prepared by the Theraflex MB Plasma System. Transfus Med Hemother. 2009;36:122–127. doi: 10.1159/000202413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alvarez-Larran A, Del Rio J, Ramirez C, Albo C, Pena F, Campos A, Cid J, Muncunill J, Sastre JL, Sanz C, Pereira A. Methylene blue-photoinactivated plasma vs. fresh-frozen plasma as replacement fluid for plasma exchange in thrombotic thrombo-cytopenic purpura. Vox Sang. 2004;86:246–251. doi: 10.1111/j.0042-9007.2004.00506.x. [DOI] [PubMed] [Google Scholar]
- 65.de la Rubia J, Arriaga F, Linares D, Larrea L, Carpió N, Marty ML, Sanz MA. Role of methylene blue-treated or fresh-frozen plasma in the response to plasma exchange in patients with thrombotic thrombocytopenic purpura. Br J Haematol. 2001;114:721–723. doi: 10.1046/j.1365-2141.2001.02991.x. [DOI] [PubMed] [Google Scholar]
- 66.del Rio-Garma J, Alvarez-Larran A, Martinez C, Muncunill J, Castella D, de la Rubia J, Zamora C, Corral M, Viejo A, Pena F, Rodriguez-Vicente P, Contreras E, Arbona C, Ramirez C, Garcia-Erce JA, Alegre A, Mateo J, Pereira A. Methylene blue-photoinactivated plasma versus quarantine fresh frozen plasma in thrombotic thrombocytopenic purpura: a multicentric, prospective cohort study. Br J Haematol. 2008;143:39–45. doi: 10.1111/j.1365-2141.2008.07292.x. [DOI] [PubMed] [Google Scholar]
- 67.del Rio-Garma J, Pereira A, Arroyo JL, Mateo J, Alvarez-Larran A, Martinez C, Muncunill J, Barbolla L. ADAMTS-13 activity and von Willebrand factor levels in methylene-blue photoinactivated plasma processed by either the Springe method or an 'in house' system. Vox Sang. 2008;95:101–105. doi: 10.1111/j.1423-0410.2008.01077.x. [DOI] [PubMed] [Google Scholar]
- 68.Castrillo A, Eiras A, Cid J, Castro A, Adelantado M, Abalo M, Areal C, Flores J, Cabrera J. Quality of methylene blue plasma over the last five years. Vox Sang. 2006;91(suppl 3):182. [Google Scholar]
- 69.Moog R, Reichenberg S, Hoburg A, Müller N. Quality of methylene blue-treated fresh-frozen plasma stored up to 27 months. Transfusion. 2010;50:516–518. doi: 10.1111/j.1537-2995.2009.02484.x. [DOI] [PubMed] [Google Scholar]
- 70.Gravemann U, Reichenberg S, Mohr H, Walker WH, Müller TH. Storage stability of methylene blue-treated plasma. Vox Sang. 2009;96:63–260. [Google Scholar]
- 71.European Committee (Partial Agreement) on Blood Transfusion (CD-P-TS): Guide to the Preparation, Use and Quality Assurance of Blood Components. Strasbourg Cedex: Council of Europe; 2009. [Google Scholar]
- 72.Garwood M, Cardigan RA, Drummond O, Hornsey VS, Turner CP, Young D, Williamson LM, Prowse CV. The effect of methylene blue photoinactivation and methylene blue removal on the quality of fresh-frozen plasma. Transfusion. 2003;43:1238–1247. doi: 10.1046/j.1537-2995.2003.00485.x. [DOI] [PubMed] [Google Scholar]
- 73.Hornsey VS, Young DA, Docherty A, Hughes W, Prowse CV. Cryoprecipitate prepared from plasma treated with methylene blue plus light: increasing the fibrinogen concentration. Transfus Med. 2004;14:369–374. doi: 10.1111/j.0958-7578.2004.00528.x. [DOI] [PubMed] [Google Scholar]
- 74.Carlier M, Ounnoughene N, Sandid I, Willaert B, Vo Mai, M-P, Trophilme C, Lienhart A, Breton P, Rebibo D, Worms B, Alpérovitch A.: Rapport annuel hémovigilance 2009. 2010. www.afssaps.fr/content/download/27068/359629/version/l/file/rapport-annuel-hemovigilance2009.pdf
- 75.Nubret K, Delhoume M, Orsel I, Laudy JS, Sellami M, Nathan N. Anaphylactic shock to fresh-frozen plasma inactivated with methylene blue. Transfusion. 2010 doi: 10.1111/j.1537-2995.2010.02800.x. DOI: 10.1111/j.l537-2995.2010.02800.x. [DOI] [PubMed] [Google Scholar]
- 76.Tsopelas C, Sutton R. Why certain dyes are useful for localizing the sentinel lymph node. J Nucl Med. 2002;43:1377–1382. [PubMed] [Google Scholar]
- 77.Wóhrl S, Focke M, Hinterhuber G, Stingl G, Binder M. Near-fatal anaphylaxis to patent blue V. Br J Dermatol. 2004;150:1037–1038. doi: 10.1111/j.1365-2133.2004.05931.x. [DOI] [PubMed] [Google Scholar]
- 78.Thevarajah S, Huston TL, Simmons RM. A comparison of the adverse reactions associated with isosulfan blue versus methylene blue dye in sentinel lymph node biopsy for breast cancer. Am J Surg. 2005;189:236–239. doi: 10.1016/j.amjsurg.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 79.van Zuuren E, Polderman MC, Kuijken I. Anaphylaxis to patent blue during sentinel lymph node identification. Contact Dermatitis. 2005;53:171. doi: 10.1111/j.0105-1873.2005.0407c.x. [DOI] [PubMed] [Google Scholar]
- 80.Schneider T, Hacquard M, Lecompte T. Indications des différents types de plasma dans es maladies hématologiques. Hématologie. 2009;15:356–363. [Google Scholar]
- 81.Muylle L, Roisin T. Hémovigilance en Belgjque: Rapport Annuel 2008. 2010. www.fagg-afmps.be/fr/binaries/HV%20Rapport%20annuel%202008%20201004_tcm291-99824.pdf
- 82.Deneys MC, Latinne D, Guerrieri C, Baele P. Three years haemovigilance of metyhlene blue-treated fresh frozen plasma: no increase in transfusion reaction incidence. Vox Sang. 2006;91(suppl 3):228. [Google Scholar]
- 83.Cohen H, Mold D, Jones H, Davies T, Mistry H., Ball J., Asher D, Cawley C, Chaffe B, Chapman C, Gray A, Jones J, Milkins C, New H, Norfolk D, Regan F, Still E, Tinegate H: The 2009 Annual SHOT Report. 2010. www.shotuk.org/wp-content/uploads/2010/07/SHOT2009.pdf
- 84.Lozano M, Cid J. Spain: methylene-blue plasma. In: AuBuchon JP, Prowse C, editors. Pathogen Inactivation: The Penultimate Paradigm Shift. Bethesda: AABB Press; 2010. pp. 181–188. [Google Scholar]
- 85.Informe hemovigilancia 2007 Ministerio de Sanidad y Politica Social, www.msps.es/profesionales/saludPublica/medicinaTransfusional/hemovigilan-cia/docs/informe_2007.pdf
- 86.L'Hemovigilancia a Catalunya. Informe 2007 Department de Salut. www.bancsang.net/ca/receptors/hemovigilancia.html
- 87.Larrea L, Calabuig M, Soler MA, Solves P, Mirabet V, Roig R. Our nine years experience in plasma inactivation. Vox Sang. 2007;93(suppl 1):167. [Google Scholar]
- 88.Giampaolo A, Piccinini V, Catalano L, Abbonizio F, Vulcano F, Hassan HJ. Primo programma di emovigilanza sulle reazioni avverse e gli errori trasfusionali in Italia: dati 2004-2005. www.issit/binary'/publ/cont/07-221189418036.pdf
- 89.Equitani F, Mistretta G, Mele L, Hortencio M, Filoni V, Pezone F, Moroni S, Menichella G. methylene blue-photoinactivated fresh frozen plasma is an effective and safe treatment for inherited and acquired coagulopathies and bleeding disorders. Blood. 2003;102(suppl 2):138b. [Google Scholar]