Abstract
Background
Two-component systems consisting of histidine kinases and their corresponding receivers are widespread in bacterial signal transduction. In the past few years, genes coding for homologues of two-component systems were also discovered in eukaryotic organisms. DokA, a homologue of bacterial histidine kinases, is an element of the osmoregulatory pathway in the amoeba Dictyostelium. The work described here addresses the question whether DokA is phosphorylated in vivo in response to osmotic stress.
Results
We have endogenously overexpressed individual domains of DokA to investigate post-translational modification of the protein in response to osmotic shock in vivo. Dictyostelium cells were labeled with [32P]-orthophosphate, exposed to osmotic stress and DokA fragments were subsequently isolated by immunoprecipitation. Thus, a stress-dependent phosphorylation could be demonstrated, with the site of phosphorylation being located in the kinase domain. We demonstrate biochemically that the phosphorylated amino acid is serine, and by mutational analysis that the phosphorylation reaction is not due to an autophosphorylation of DokA. Furthermore, mutation of the conserved histidine did not affect the osmostress-dependent phosphorylation reaction.
Conclusions
A stimulus-dependent serine phosphorylation of a eukaryotic histidine kinase homologue was demonstrated for the first time in vivo. That implies that DokA, although showing typical structural features of a bacterial two-component system, might be part of a eukaryotic signal transduction pathway that involves serine/threonine kinases.
Background
Two-component systems are central elements of the bacterial signaling circuitry [1]. Signal transduction by these systems usually involves autophosphorylation of a histidine kinase on a conserved histidine residue and subsequent transfer of the phosphoryl group to a conserved aspartate on a receiver domain. Until recently, two-component systems had only been found in bacteria. In the past few years, genes coding for histidine kinase homologues and their corresponding receivers have also been discovered in eukaryotic organisms [for a review see 2]. Most of the corresponding eukaryotic gene products are part of a phosphoryl relay, which consists of a hybrid histidine kinase with a kinase and a receiver domain on the same polypeptide, a histidine phosphotransfer protein and a second receiver as part of a response regulator [3]. The function of eukaryotic two-component systems as histidine kinases was questionable until Posas et al. showed that the Saccharomyces cerevisiae gene product Sln1 acts as a histidine kinase in vitro and in vivo [4]. More recently, histidine kinase activity of the ethylene receptor Etr1 from Arabidopsis was demonstrated in vitro [5]. Further studies showed, however, that eukaryotic two-component systems do not function as independent pathways, but are often connected to serine/threonine- and tyrosine kinase cascades. Thus, the yeast Sln1-Ypd1-Ssk1 phosphoryl relay acts as an osmosensor, which activates a MAP-kinase cascade when cells are exposed to high osmolarity [6]. The Dictyostelium discoideum protein RegA consists of a N-terminal receiver domain and a C-terminal phosphodiesterase domain [7]. Phosphorylation of the RegA response regulator via a two-component phosphoryl relay in turn activates the RegA phosphodiesterase thereby causing a decrease in the intracellular cAMP level. Eukaryotic phytochromes, another class of histidine kinase homologues, were shown to act as light-regulated serine/threonine kinases in vitro instead of acting according to the histidine kinase paradigm [8]. These results suggest that eukaryotic two-component systems, although being homologues of bacterial histidine kinases and receivers, might show post-translational modifications found in the already established eukaryotic signal transduction systems.
In the amoeba Dictyostelium discoideum, several genes coding for histidine kinases have been described [[9-13]]. Deletions of individual histidine kinase genes cause different developmental phenotypes such as rapid aggregation, disproportioned fruiting body and stalk ratios or impaired spore formation [13]. Moreover, cells lacking the histidine kinase gene dokA are osmosensitive, i.e. the viability of these cells is decreased when exposed to high osmolarity for up to two hours [9]. Given the evidence that DokA is part of the osmotic response system of Dictyostelium, we have examined whether DokA shows kinase activity in an osmolarity-dependent manner.
In this paper, we present evidence that the histidine kinase homologue DokA is phosphorylated on a serine residue in vivo when Dictyostelium cells are exposed to a high osmolarity medium. We further demonstrate that the phosphorylation site is located in a domain homologous to bacterial histidine kinases and that mutation of the conserved histidine does not affect the serine phosphorylation of DokA.
Results
Homologous expression of DokA domains
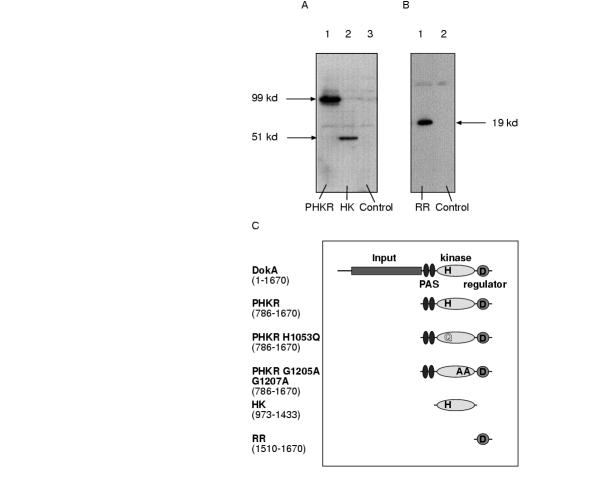
In order to investigate the DokA kinase activity in vivo, we have overexpressed the individual domains of DokA in Dictyostelium discoideum AX2 cells. In previous studies on two-component systems it was shown that the individual domains can be expressed separately, thereby maintaining their biochemical function [4, 5, 14]. Three fragments of DokA were expressed under the control of a constitutively active actin15 promoter by using the plasmid pDEX-RH [15]: a 99 kd C-terminal fragment of DokA consisting of two PAS domains [16], the kinase domain and the receiver domain (PHKR); the 51 kd kinase domain (HK) and the C-terminal 19 kd receiver domain (RR) (Fig. 1). Overexpression of these domains can be easily detected by immunostaining of a blotting membrane containing crude extracts from Dictyostelium cells which were transformed with the corresponding constructs (Fig. 1A and 1B). In contrast, wild type DokA, expressed under the control of the endogenous promoter, can not be detected by these methods, as it is only weakly expressed in vegetative cells and in the early stages of development [9]. The conserved residues of this class of signaling molecules are among others a histidine and an ATP binding motif in the kinase. We have therefore mutated the proposed site of histidine phosphorylation (H1053) in the PHKR (PHKR HQ) and two glycine residues (G 1205, G 1207) which are essential for ATP binding (PHKR GA GA) (Fig. 1C).
Figure 1.
Identification of overexpressed DokA fragments. Cells transformed with the pDEX-RH-dokA constructs were lysed in Laemmli sample buffer and subjected to SDS-PAGE. Proteins were blotted onto a PVDF membrane and DokA fragments were detected by immunostaining with the pAb PHKR antibody. The 99 kd fragment PHKR (Panel A, lane 1) and the 19 kd fragment RR (Panel B, lane 1) are expressed at comparable levels whereas the 51 kd fragment HK (Panel A, lane 2) is more weakly expressed. Wild-type DokA in AX2 cell extracts can not be detected (Panel A, lane 3; panel B, lane 2). All constructs used in this work are represented in a schematic drawing (Panel C).
In vivo phosphorylation of DokA
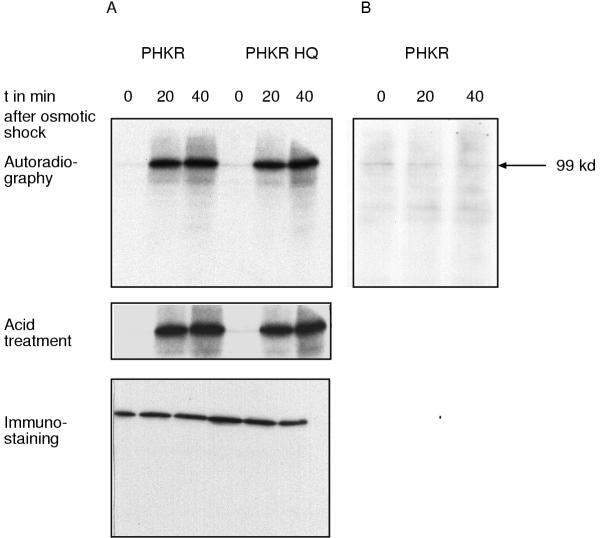
Because Dictyostelium cells lacking the dokA gene are sensitive to hyperosmotic stress, it was speculated that DokA is part of the osmotic response system of Dictyostelium [9]. It was, however, not clear whether DokA is phosphorylated in vivo in an osmotic stress-dependent manner. To address this question, we cultured Dictyostelium cells overexpressing the DokA fragment PHKR in a phosphate-depleted medium and labeled them with [32P]-orthophosphate. Labeled cells were osmotically shocked by adding 400 mM sorbitol [9] and subsequently lysed. Sorbitol was used as osmolyte because it is non-toxic and can not be metabolized by Dictyostelium cells. Other osmolytes (e. g. NaCl or glucose) have also been used in previous experiments to demonstrate osmosensitivity of dokA- cells under various hyperosmotic conditions (data not shown). The overexpressed DokA fragment was purified from the cell lysate by immunoprecipitation with a DokA-specific polyclonal antibody (Fig. 2A). The precipitate was subjected to SDS-PAGE and blotted onto a PVDF membrane. It was shown by autoradiography that PHKR is phosphorylated during hyperosmotic stress (Fig. 2A). The phosphorylation level is maximal 20 min after the onset of the osmotic shock and does not decrease until 40 min thereafter. In cells, which are not osmotically shocked, no phosphorylation of DokA was observed (Fig. 2B). We conclude from these results that DokA is phosphorylated in vivo in an osmotic stress-dependent manner.
Figure 2.
In vivo phosphorylation of DokA during osmotic stress. PHKR was immunoprecipitated from the cell lysate of radioactively labeled and osmotically shocked Dictyostelium PHKR cells. Purified proteins were separated by SDS-PAGE, blotted onto a PVDF membrane and subjected to autoradiography. Panel A: PHKR is phosphorylated in vivo during osmotic shock (t = 20 min, 40 min). Mutation of the conserved histidine in PHKR does not affect the phosphorylation of PHKR. Before the osmotic shock (t = 0 min), neither of the two DokA fragments are phosphorylated. The radioactive label can not be removed by treating the membrane with 1 N HCl for 1 h. It was shown by immunostaining with the mAb 426/HK antibody that equal amounts of protein were purified at each point in time. Panel B: PHKR precipitated from untreated cells is not radioactively labeled.
Since DokA is a homologue of bacterial histidine kinases, it was probable that the observed radioactive labeling was due to a histidine phosphorylation. We therefore overexpressed a mutated form of PHKR, which lacks the conserved histidine H1053 in AX2 cells. The PHKR H1053Q fragment was, however, also phosphorylated during osmotic shock (Fig. 2A). Furthermore, the radioactive label was not removed by acid treatment of the blotting membrane (Fig. 2A), indicating that PHKR is not phosphorylated at a histidine. Acid resistance is a typical feature of phosphorylated serine, threonine or tyrosine residues [17]. Tyrosine phosphorylation of PHKR was excluded by immunostaining with a monoclonal anti-phosphotyrosine antibody (data not shown) and phospho amino acid analysis (Fig. 3).
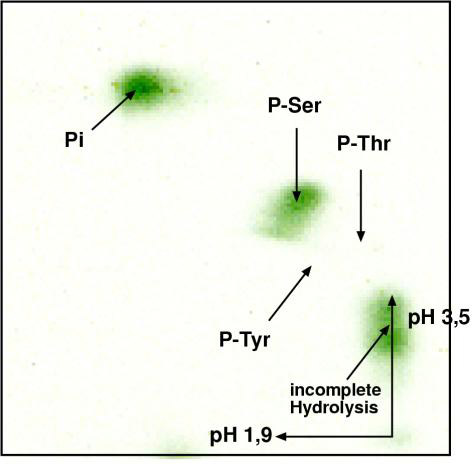
Figure 3.
Phospho-amino acid analysis. Radioactively labeled PHKR was purified by immunoprecipitation from osmotically shocked Dictyostelium cells. The protein was totally hydrolysed by boiling with 6 N HCl at 120°C. Two-dimensional thin-layer electrophoresis was carried out as described [17]. Phospho-serine was detected by autoradiography.
To distinguish between serine and threonine phosphorylation, we hydrolyzed radioactively labeled PHKR in boiling 6 N hydrochloric acid. The resulting mixture of amino acids was subjected to two-dimensional thin-layer electrophoresis [17]. Using autoradiography, we were able to show that PHKR is phosphorylated on a serine residue (Fig. 3).
Location of the phosphorylation site
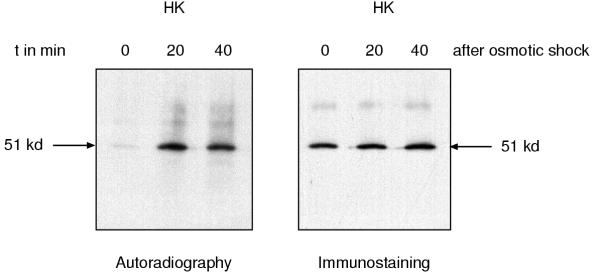
To determine the domain of DokA in which the phosphorylated serine residue is located, we purified the DokA fragments HK (kinase domain) and RR (receiver domain) from radioactively labeled and osmotically shocked cells overexpressing these fragments. The osmotic stress-dependent phosphorylation of PHKR was also found in case of the HK fragment (Fig. 4), whereas significant labeling of the RR fragment was not observed. Again, the phosphorylation of HK was acid-resistant (data not shown). We therefore conclude, that the observed phosphorylation of PHKR is occurring on a serine residue of the kinase domain.
Figure 4.
Location of the phosphorylation site. The DokA fragment HK (kinase domain) was purified from radioactively labeled and osmotically shocked Dictyostelium cells as described above. The HK fragment is strongly phosphorylated during osmotic shock (t = 20 min, 40 min), whereas no phosphorylation was observed before the shock (t = 0 min). It was shown by immunostaining with the mAb 426/HK antibody that equal amounts of protein were purified at each point in time.
Phosphorylation of mutated DokA fragments
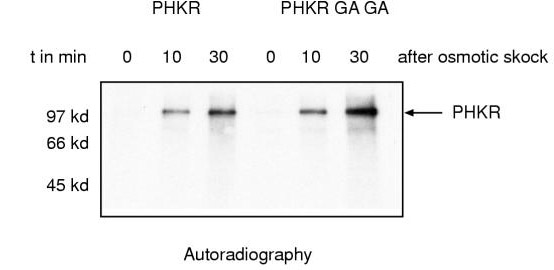
Phosphorylation of DokA during osmotic shock could be the result of an autophosphorylation reaction, as the kinase domain contains sequence motifs, which are homologous to domains of bacterial histidine kinases [9]. It was previously shown that mutation of two conserved glycine residues in the ATP-binding site of the eukaryotic histidine kinase homologue Etr1 suppresses ATP binding and thus prevents autophosphorylation of Etr1 on a histidine residue [5]. The two corresponding glycine residues in the DokA kinase domain are G1205 and G1207, respectively. To examine whether DokA is autophosphorylated during osmotic shock, we overexpressed a mutated form of PHKR, in which the conserved glycines were substituted against alanine. Interestingly, the PHKR GA GA fragment is also phosphorylated during osmotic shock (Fig. 5). Thus, the observed radioactive labeling of DokA is not the result of an autophosphorylation reaction of the catalytic domain.
Figure 5.
In vivo phosphorylation of a PHKR fragment with a disabled catalytic domain. Autoradiography of the PVDF membrane shows that a double mutation in the ATP binding site of PHKR (G1205A, G1207A) does not affect the in vivo phosphorylation of PHKR during osmotic shock (t = 10 min, 30 min).
Discussion
In the past few years, a number of eukaryotic genes coding for histidine kinase homologues have been cloned and characterized. Recent work showed that eukaryotic two-component systems are integrated in signal transduction pathways involving phosphodiesterases and MAP kinases [6, 7, 18]. The results presented here confirm this idea. By labeling Dictyostelium cells with [32P]-orthophosphate, we were able to show that overexpressed fragments of the Dictyostelium histidine kinase homologue DokA are phosphorylated in vivo on a serine residue. The phosphorylation was not present before and steadily increased after the osmotic shock reaching a plateau after 20 min. It should be noted that this phosphorylation reaction was only observed when cells were exposed to a hyperosmotic medium and not as a result of starvation or development.
The observed serine phosphorylation is in contrast to the classic two-component paradigm, which predicts only histidine and aspartate phosphorylation. However, several recent publications showed post-translational modification on serine and threonine residues, which are not in accordance with the above prediction. In particular, the stimulus-dependent serine/threonine phosphorylation of other eukaryotic histidine kinase homologues was recently demonstrated in vitro [8]. Phytochromes from oat and green algae contain histidine kinase-related domains, but lack the conserved histidine which is essential for autophosphorylation. However, they are able to autophosphorylate on serine/threonine residues when exposed to red light thereby acting as light-regulated protein kinases [8]. In contrast, the cyanobacterial phytochrome Cph1 acts as a light-regulated histidine kinase [19]. Considering these differences, oat and algal phytochromes have been designated as serine/threonine kinases with histidine kinase ancestry [8]. More recently, a sequence motif homologous to histidine kinases without the conserved histidine has been found in the adenylyl cyclase gene acrA in Dictyostelium [20].
It was previously suggested that DokA is part of the osmotic response system of Dictyostelium, as mutants lacking the dokA gene are sensitive against hyperosmotic stress [9]. Two-component systems are involved in osmoregulation in a variety of organisms [6]. In S. cerevisiae, the Sln1-Ypd1-Ssk1 phosphoryl relay acts as an osmosensor, which activates a MAP-kinase pathway when cells are exposed to hyperosmotic stress [6]. In hyperosmotically shocked mammalian cells, MAP-kinases are phosphorylated in vivo on threonine and tyrosine residues [21, 22]. In this respect, DokA resembles typical eukaryotic signal transduction proteins, as hyperosmotic stress causes serine phosphorylation of DokA. The observed osmotic stress-dependent phosphorylation supports the hypothesis that DokA is involved in an osmosensing signal transduction pathway in Dictyostelium [9, 23].
To address the question, whether DokA is phosphorylated in an autophosphorylation reaction, mutations of two conserved glycine residues in the ATP binding pocket of the catalytic domain of the kinase were introduced. The mutation was shown to completely inhibit ATP binding in histidine kinases [24]. Proteins carrying these mutations are not able to autophosphorylate on the conserved histidine residue [5]. In contrast, the serine phosphorylation of DokA is not affected by mutation of the conserved glycine residues G1205 and G1207, making an autophosphorylation reaction unlikely. Consistent with this finding is the fact that the mutation of the conserved histidine H1053 has no influence on the serine phosphorylation of DokA, indicating that no histidinyl-phosphate intermediate is necessary for this reaction. A physiologically relevant role of H1053 can, however, not be definitely precluded by this experimental approach as the DokA domains have been expressed in cells which contain a functional dokA allele. Interaction of the overexpressed peptides with wild type DokA might also be important for the detection of the osmotic stimulus as the DokA domains used in this work lack the N-terminal input domain [9]. Taken together, our results imply that DokA is a substrate for another serine/threonine kinase. To address this question, we analyzed the DokA amino acid sequence using the database PhosphoBase (http://www.cbs.dtu.dk/databases/PhosphoBase/) to search for putative phosphorylation sites. More than 50 serine phosphorylation sites were predicted by this algorithm. None of these sites was in the receiver domain, which is consistent with our finding that the phosphorylation site of DokA is located in the kinase domain. The results of the sequence analysis indicate that a number of protein kinases may interact and subsequently phosphorylate DokA. It was previously shown that DokA regulates the activity of the RdeA-RegA pathway, thereby causing a transient intracellular cAMP signal reaching a maximum after 2 min in response to an osmotic shock [23]. In contrast, the phosphorylation reaction described here shows a 10-fold slower kinetic. We therefore speculate that the serine/threonine kinase which phosphorylates DokA might act downstream of the RdeA-RegA pathway as part of a feedback mechanism. The exact localization of the phosphorylated serine residue is currently under investigation.
Conclusions
We have demonstrated an osmotic stress-dependent serine phosphorylation of the eukaryotic histidine kinase homologue DokA in vivo. The phosphorylation does not depend on the conserved histidine residue, which is essential for the function of two-component systems and is not due to an autophosphorylation reaction. This confirms the idea that eukaryotic homologues of bacterial signal transduction systems might be integrated in signaling pathways involving serine/threonine kinases.
Materials and Methods
Chemicals
Geneticin G 418 and Mes were obtained from Sigma, and sorbitol was from Merck. Yeast extract and peptone were from Oxoid. Radiochemicals were obtained from Amersham. "Complete" protease inhibitor cocktail was from Roche Biochemicals.
Cell culture
Amoebae of Dictyostelium discoideum strain AX2-214 were grown in AX medium [25] at 21°C up to a cell density of 3-5 × 106 cells/ml. Strains containing the G 418 resistance gene were cultivated in AX medium with 10 μg G 418/ml.
For in vivo phosphorylation experiments, cells were grown up to 5 × 106 cells/ml, diluted sevenfold in a phosphate-depleted medium (14.3 g peptone, 7.15 g yeast extract, 18 g maltose in 1 l 20 mM Mes, pH 6.5) and cultivated for 16 h.
Construction of cell lines
To create constructs of DokA fragments, the corresponding regions of the dokA gene were amplified by PCR using the plasmid pDIC3 [9]. Primers were constructed to introduce flanking EcoRI restriction sites into PCR products coding for the 99 kd fragment PHKR (bp 2356-5010 of the dokA gene), the 51 kd fragment HK (bp 2917-4299) and the 19 kd fragment RR (bp 4528-5010), respectively. The resulting DNA fragments were cloned into the EcoRI site of the overexpression vector pDEX-RH [15] and transformed into D. discoideum AX2 cells by electroporation [26]. The plasmid pDEX-RH bears the G 418 resistance gene that allows the selection of stable transformants. Point mutations in the DokA fragments were introduced into the plasmid before transformation into Dictyostelium cells using the QuikChange mutation kit (Stratagene). Selection was carried out with 10 μg G 418/ml and clones were tested for expression by Western blotting and immunostaining with the pAb PHKR antibody. Three independent clones were selected from each transformation.
Antibodies
Recombinant DokA fragments purified from E. coli M15 (PHKR and HK) were used to raise a polyclonal antibody pAb PHKR in rabbit and the monoclonal antibody mAb 426/HK in mouse. Antibodies were purified using Protein A sepharose (Pharmacia) according to the instructions of the manufacturer and stored at -20°C in phosphate-buffered saline with azide at 0.5 - 1 mg/ml.
In vivo phosphorylation of DokA fragments
Dictyostelium cells which were grown for 16 h in a phosphate-depleted medium were harvested and suspended in 20 mM Mes, pH 6.5 at 1 × 107 cells/ml. After 1 h of shaking (150 rpm at 21°C), [32P]-orthophosphate was added up to a specific radioactivity of 0.2 - 0.3 mCi/ml. The suspension was shaken for another 60 min and cells were then osmotically shocked by adding a 2 M sorbitol stock solution up to a final concentration of 400 mM. Samples of 1 × 107 cells were taken at different points in time before and after the shock. Cells were washed and subsequently frozen with lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1 % Triton X-100, 1 % sodium deoxycholate, 0.1 % SDS, 1 mM EDTA, 1 mM DTT, "Complete" protease inhibitor cocktail) in liquid nitrogen. After thawing and centrifugation (14,000 rpm at 4°C), the supernatant was mixed with 3 μg pAb PHKR antibody, incubated 30 min on ice and mixed with Pansorbin cells. After 30 min incubation, the precipitate was centrifuged, washed three times, incubated 3 min at 60°C with Laemmli sample buffer and subjected to SDS-PAGE.
SDS-PAGE and Western blot analysis
Polyacrylamide gel electrophoresis was performed according to Laemmli [27] at 4°C. Proteins on gels were stained with Coomassie Blue or transferred to a PVDF membrane (BioRad) by using a Trans-Blot SD semi-dry transfer system (BioRad). To prove the overexpression or immunoprecipitation of the desired DokA fragment, blots were immunostained with mAb 426/HK or pAb PHKR and an IgG anti-mouse peroxidase-conjugate or IgG anti-rabbit peroxidase-conjugate (Sigma). Stained proteins were visualized by using the Renaissance luminescence detection kit (NEN).
Phospho-amino acid analysis
Phospho-amino acid analysis was carried out using two-dimensional thin-layer electrophoresis as described elsewhere [17].
Acknowledgments
Acknowledgments
We would like to thank Dr. G. Gerisch for help producing the mono-clonal antibodies against HK, A. Ott for help producing the polyclonal sera against PHKR and RR, C. Breithaupt for her help in the course of the experiments and Dr. S. Dammeier for advice on phospho-amino acid analysis. This work was supported by grants (Schu778/3-1, Schu778/3-2) of the Deutsche Forschungsgemeinschaft to S.C.S..
Contributor Information
Felix Oehme, Email: felix.oehme.fo@bayer-ag.de.
Stephan C Schuster, Email: stephan.schuster@tuebingen.mpg.de.
References
- Hoch JA, Silhavy TJ. Two-Component Signal Transduction. Washington, DC: ASM press, 1995.
- Loomis WF, Shaulsky G, Wang N. Histidine kinases in signal transduction pathways of eukaryotes. J Cell Sci. 1997;110:1141–1145. doi: 10.1242/jcs.110.10.1141. [DOI] [PubMed] [Google Scholar]
- Appleby JL, Parkinson JS, Bourret RB. Signal transduction via the multi-step phosphorelay: not necessarily a road less traveled. Cell. 1996;86:845–848. doi: 10.1016/s0092-8674(00)80158-0. [DOI] [PubMed] [Google Scholar]
- Posas F, Wurgler-Murphy SM, Maeda T, Witten EA, Thai TC, Saito H. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 "two-component" osmosensor. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- Gamble RL, Coonfield ML, Schaller GE. Histidine kinase activity of the ETR1 ethylene receptor from Arabidopsis. Proc Natl Acad Sci USA. 1998;95:7825–7829. doi: 10.1073/pnas.95.13.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurgler-Murphy SM, Saito H. Two-component signal transducers and MAPK cascades. Trends Biochem Sci. 1997;22:172–176. doi: 10.1016/S0968-0004(97)01036-0. [DOI] [PubMed] [Google Scholar]
- Thomason PA, Traynor D, Cavet G, Chang WT, Harwood AJ, Kay RR. An intersection of the cAMP/PKA and two-component signal transduction systems in Dictyostelium. EMBO J. 1998;17:2838–2845. doi: 10.1093/emboj/17.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh KC, Lagarias JC. Eukaryotic phytochromes: Light-regulated serine/threonine protein kinases with histidine kinase ancestry. Proc Natl Acad Sci USA. 1998;95:13976–13981. doi: 10.1073/pnas.95.23.13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster SC, Noegel AA, Oehme F, Gerisch G, Simon MI. The hybrid histidine kinase DokA is part of the osmotic response system of Dictyostelium. EMBO J. 1996;15:3880–3889. [PMC free article] [PubMed] [Google Scholar]
- Wang N, Shaulsky G, Escalante R, Loomis WF. A two-component histidine kinase gene that functions in Dictyostelium development. EMBO J. 1996;15:3890–3898. [PMC free article] [PubMed] [Google Scholar]
- Zinda MJ, Singleton CK. The hybrid histidine kinase dhkB regulates spore germination in Dictyostelium discoideum. Dev Biol. 1998;196:171–183. doi: 10.1006/dbio.1998.8854. [DOI] [PubMed] [Google Scholar]
- Singleton CK, Zinda MJ, Mykytka B, Yang P. The histidine kinase dhkC regulates the choice between migrating slugs and terminal differentiation in Dictyostelium discoideum. Dev Biol. 1998;203:345–357. doi: 10.1006/dbio.1998.9049. [DOI] [PubMed] [Google Scholar]
- Thomason P, Traynor D, Kay RR. Taking the plunge-terminal differentiation in Dictyostelium. Trends Gen. 1999;15:15–19. doi: 10.1016/S0168-9525(98)01635-7. [DOI] [PubMed] [Google Scholar]
- Swanson RV, Schuster SC, Simon MI. Expression of CheA fragments which define domains encoding kinase, phosphotransfer and CheY binding activities. Biochemistry. 1993;32:7623–7629. doi: 10.1021/bi00081a004. [DOI] [PubMed] [Google Scholar]
- Faix J, Gerisch G, Noegel AA. Overexpression of the csA adhesion molecule under its own cAMP-regulated promoter impairs morphogenesis in Dictyostelium. J Cell Sci. 1992;102:203–214. doi: 10.1242/jcs.102.2.203. [DOI] [PubMed] [Google Scholar]
- Zhulin IB, Taylor BL. PAS domain S-boxes in archaea, bacteria and sensors for oxygen and redox. Trends Biochem Sci. 1997;22:331–333. doi: 10.1016/S0968-0004(97)01110-9. [DOI] [PubMed] [Google Scholar]
- Duclos B, Marcandier S, Cozzone SJ. Chemical properties and separation of phosphoamino acids by thin-layer chromatography and/or electrophoresis. Methods Enzymol. 1991;201:10–21. doi: 10.1016/0076-6879(91)01004-l. [DOI] [PubMed] [Google Scholar]
- Shaulsky G, Fuller D, Loomis WF. Developmental signal transduction pathways uncovered by genetic suppressors. Proc Natl Acad Sci USA. 1996;93:15260–15265. doi: 10.1073/pnas.93.26.15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh KC, Lagarias JC. A cyanobacterial phytochrome two-component light sensory system. Science. 1997;277:1505–1508. doi: 10.1126/science.277.5331.1505. [DOI] [PubMed] [Google Scholar]
- Soderbom F, Anjard C, Iranfar N, Fuller D, Loomis WF. An adenylyl cyclase that functions during late development of Dictyostelium. Development. 1999;126:5463–5471. doi: 10.1242/dev.126.23.5463. [DOI] [PubMed] [Google Scholar]
- Galcheva-Gargova Z, Derijard B, Wu IH, Davis RJ. An osmosensing signal transduction pathway in mammalian cells. Science. 1994;265:806–808. doi: 10.1126/science.8047888. [DOI] [PubMed] [Google Scholar]
- Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Ott A, Oehme F, Keller H, Schuster SC. Osmotic stress response in Dictyostelium is mediated by cAMP. EMBO J. 2000;19:5782–5792. doi: 10.1093/emboj/19.21.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Inouye M. Requirement of both kinase and phosphatase activities of an Escherichia coli receptor (Taz1) for ligand-dependent signal transduction. J Mol Biol. 1993;231:335–342. doi: 10.1006/jmbi.1993.1286. [DOI] [PubMed] [Google Scholar]
- Claviez M, Pagh K, Maruta H, Baltes W, Fisher P, Gerisch G. Electron microscopic mapping of monoclonal antibodies on the tail region of Dictyostelium myosin. EMBO J. 1982;1:1017–1022. doi: 10.1002/j.1460-2075.1982.tb01287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard PK, Ahern KG, Firtel RA. Establishment of a transient expression system for Dictyostelium discoideum. Nucleic Acids Res. 1988;16:2613–2623. doi: 10.1093/nar/16.6.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]