Abstract
In the family Streptococcaceae, the genes encoding zinc ABC uptake systems (called zit or adc) are regulated by a coencoded MarR family member (i.e., ZitR or AdcR), whereas in the great majority of bacteria, these genes are regulated by Zur, the Fur-like zinc-responsive repressor. We studied the zit operon from Lactococcus lactis and its regulation in response to Zn(II) in vivo. zit transcription is repressed by Zn(II) in a wide concentration range starting from nontoxic micromolar levels and is derepressed at nanomolar concentrations. The level of zit promoter downregulation by environmental Zn(II) is correlated with the intracellular zinc content. The helix-turn-helix domain of ZitR is required for downregulation. In vitro, the purified protein is a dimer that complexes up to two zinc ligands per monomer and specifically binds two intact palindromic operator sites overlapping the −35 and −10 boxes of the zit promoter. DNA binding is abolished by the chelator EDTA or TPEN and fully restored by Zn(II) addition, indicating that the active repressor complexes Zn(II) with high affinity. These results suggest that derepression under starvation conditions could be an essential emergency mechanism for preserving Zn(II) homeostasis by uptake; under Zn(II)-replete conditions, the function of ZitR repression could be to help save energy rather than to avoid Zn(II) toxicity. The characterization of a MarR family zinc-responsive repressor in this report gives insight into the way Streptococcaceae efficiently adapt to Zn(II) fluctuations in their diverse ecological niches.
INTRODUCTION
Zinc is a transition metal that plays key roles in major cellular functions, such as transcription, translation, replication, resistance to oxidative stress, and virulence, but zinc, like other metals, is both vital in trace amounts and toxic at high concentrations (15). Zinc homeostasis is achieved by the tight control of storage systems and transporters with broad diversity (5, 15, 30, 50). Bacterial responses to zinc have been studied by genome-wide transcriptional analyses in Escherichia coli (8, 26), Bacillus subtilis (31), and Streptococcus pneumoniae (24), showing altered expression of numerous genes implicated in tolerance for and efflux of zinc and other metals, and also in general stress resistance. Conversely, zinc depletion in E. coli regulates a small number of genes involved in high-affinity zinc binding and uptake or encoding zinc-independent ribosomal proteins (18).
The regulation of zinc transport is an essential cellular process achieved by zinc-responsive regulators (30), although some general regulators can be involved independently of zinc levels (17). Regulators specific to zinc resistance genes (encoding metallochaperones or efflux systems), including SmtB/ArsR and MerR family members, act as repressors in apoprotein form, whereas in the presence of zinc, the holoprotein form is either no longer active or becomes a true activator (9). In contrast, Zur repressors (Fur family) specific to zinc ABC uptake systems are active as holoproteins and switch off expression of transporters in the presence of zinc (20, 25). In E. coli, the prototypes of regulators for zinc efflux and uptake, ZntR and Zur, behave as efficient zinc sensor proteins whose affinity is in the femtomolar range (35), revealing the absence of free Zn(II) in the cytoplasm and the extraordinary chelation capacity of cytoplasmic proteins (35).
The Streptococcaceae (the genera Streptococcus and Lactococcus) differ from the great majority of other bacterial groups, including Gram-negative proteobacteria and most Gram-positive low-GC-percent bacteria, by their zinc uptake regulator. Rather than Zur proteins, Streptococcus and Lactococcus genomes encode homologous regulatory proteins named AdcR and ZitR, respectively (10, 30, 38), which do not belong to a well-known metalloregulator family. They form part of the MarR family (10, 38), whose members, in general, control diverse functions, including the transport of antimicrobials or drugs, but not of metals (51). adcR and zitR genes, in contrast to zur genes, belong to putative adc-zit operons (29, 38) that also encode an ABC uptake system (10, 13, 20) that is complete or incomplete (AdcA lipoprotein can be separately encoded) (29, 38). Although AdcA belongs to a cluster of binding proteins specific for either zinc or manganese, it was shown in S. pneumoniae that AdcABC/ZitSPQ proteins form an ABC uptake system specific for zinc that is widely conserved in bacteria (10, 20). adc-zit promoter regions display a conserved TTAACYRGTTAA palindrome (in one or two copies), proposed to be an AdcR/ZitR binding site (38). In pathogenic species, adcR is involved in biofilm formation (29), in the control of immunogenic surface proteins (1, 3, 34) and of zinc transport proteins (3), and in virulence (2). At the beginning of this work, little was known about AdcR/ZitR proteins (10, 30, 38). S. pneumoniae and Streptococcus suis AdcR proteins were then found to be DNA-binding proteins (1, 3), and S. suis AdcR was shown to be a metalloregulator specifically binding the TTAACYRGTTAA palindrome (1). While this work was submitted for publication, the S. pneumoniae AdcR protein was characterized in both apo- and Zn(II)-bound forms, and three of its Zn(II)-binding residues were identified (44).
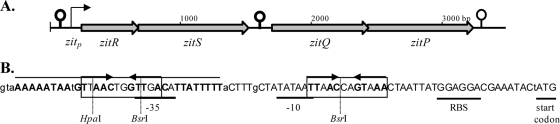
The lactic acid bacterium Lactococcus lactis has a conserved chromosomal zitRSPQ region (Fig. 1A) that is annotated as being involved in zinc uptake and regulation by homology to streptococcal adc operons. The zit promoter sequence contains two imperfect TTAACYRGTTAA palindromes (Fig. 1B) (38). In a previous study screening for exported fusions to a signal peptideless reporter in strain MG1363, the zitR gene was cocloned with the 5′ part of the zitS gene, and the latter was joined to the reporter open reading frame (ORF) (and referred to as nlp3, for new lipoprotein 3-encoding gene) (41). The zit promoter (zitp, previously referred to as PZn [28]), cloned together with zitR on a plasmid, was shown to be inducible by EDTA and repressed by zinc and proved to be a useful and efficient tool for heterologous expression in L. lactis (28, 32, 49).
Fig. 1.
L. lactis zit operon. (A) L. lactis zitRSQP genes (conserved in all four sequenced strains: MG1363, SK11 [both subsp. cremoris], IL1403, and KF147 [both subsp. lactis], although they are annotated as LACR_2420 to LACR_2417 in strain SK11) are represented as large gray arrows. A putative ZitR repressor, ZitS lipoprotein, ZitQ ATP-binding cassette protein, and ZitP permease are homologous to streptococcal AdcR, AdcA, AdcC, and AdcB proteins, respectively (even though the AdcA lipoprotein seems to be a fusion between two zinc-binding proteins, L. lactis ZitS [or E. coli ZnuA] and E. coli ZinT [38]). The predicted zitp promoter is shown, and ρ-independent terminators in strains MG1363, SK11, and IL1403 (http://bonsai.hgc.jp/∼mdehoon/terminators/NC_009004.trms) are indicated by hairpins. The strongest ρ-independent terminator, perfectly conserved in all three strains, is present between zitS and zitQ (although there is no consensus promoter sequence upstream of zitQ). Downstream of zit, ρ-independent terminators of different sequences are present in MG1363 and IL1403 only. (B) The zitp sequence from strain MG1363 (perfectly canonical −35 and −10 boxes are properly spaced at 17 bp) is shown, with nucleotides conserved in all four strains in uppercase letters. Putative signals for both transcription (−35 and −10 boxes) and translation (ribosome binding site [RBS] and start codon) are underlined. The two imperfect TTAACYRGTTAA palindromes (38) are boxed, with complementary nucleotides in boldface and marked by thick arrows. The upstream palindrome is embedded in a larger inverted repeat (thin lines) corresponding to the weak ρ-independent terminator for the upstream operon (http://bonsai.hgc.jp/∼mdehoon/terminators/NC_009004.trms). Relevant restriction sites are indicated (Fig. 7).
In this work, we studied the regulation of the L. lactis zit operon both in vivo and in vitro. In vivo, we determined the range of Zn(II) concentrations leading to repression and its low threshold. In vitro, a purified recombinant rZitR protein was analyzed: the conditions of its DNA-binding activity and the natures of its metal ligand and of its operator sites were determined. This study is the first biochemical characterization of a lactococcal ZitR/AdcR-type regulator. The L. lactis ZitR protein displays high affinity for zinc and is a repressor active in low, nontoxic zinc concentrations in vivo.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The strains used in this study were E. coli strain BL21(DE3) [F− ompT hsdSB(rB− mB−) gal dcm (DE3)] (Novagen) and Lactococcus lactis subsp. cremoris strain MG1363 (plasmid free). Plasmids (see Table S1 in the supplemental material) were constructed as follows. The zitp zitR region was amplified from pVE8064 (28) using MCPz (5′-GCTGATATCGAATTCGAGCTCGGTACCCGGGGATCCTTCATCGAAACTCTTCAGTTAGTACTG-3′) and M13 Reverse (5′-CAGGAAACAGCTATGACC-3′) primers. This PCR fragment was digested with BglII and EcoRI and cloned into pRC2, a medium-copy-number vector digested by BamHI and EcoRI (14), giving pVE8071. For pVE8072, Φ(uspSP-nucB)(Hyb), a gene fusion encoding a recombinant form of the staphylococcal nuclease reporter (SPUsp-NucB [NucB fused to the Usp45 signal peptide]), was excised from pVE8064 (28) with BamHI and EcoRI and inserted into pVE8071, leading to a zitR Φ(uspSP-nucB) operon under zitp control. To delete the zitR 3′ end (encoding the ZitR helix-turn-helix [HTH] domain), pVE8072 was digested with MluI and BamHI and filled in by T4 DNA polymerase, leading to pVE8074 bearing a zitRΔHTH Φ(uspSP-nucB) operon. For pVE8073, a zitRr allele encoding rZitR was amplified from the MG1363 chromosome using zitRNco2 (5′-AATACCATGGCTTTACGAAATCAAATCGAAC-3′) and Stop (5′-GCTCTAGAGCGGGATCCTTACTTCATCGAAACTCC-3′ (the BamHI restriction site is in boldface) primers, and NcoI-BamHI digestion allowed its cloning into pET-28b(+) (Novagen). The zitRr allele was subcloned into pVE8064 instead of zitR, leading to pVE8075. zitR was also entirely deleted from pVE8064 to produce pVE8076.
Growth conditions, metal deprivation, or supplementation.
E. coli was grown at 37°C with shaking in LB medium supplemented with kanamycin (50 μg/ml) where appropriate. L. lactis strains were grown at 30°C either in rich GM17 medium (48) or in chemically defined SA medium (10 nM ZnSO4, 80 nM MnCl2, 10 nM CuSO4, 30 nM CoCl2, and 10 μM FeSO4 [22]) supplemented with erythromycin (5 μg/ml) or chloramphenicol (10 μg/ml) where appropriate.
zitp regulation by a concentration gradient of Zn(II) was studied on plates as described previously (40). Highly concentrated ZnSO4 solutions (i.e., 1 mM or 1 M) were streaked on SA-agar medium, where lactococcal cells expressing a zitp-controlled reporter fusion were then cross-streaked and grown overnight (ON).
Northern blotting.
An overnight culture of MG1363 in SA medium was diluted in fresh medium, grown to the exponential phase (at an optical density at 600 nm [OD600] of 0.2), divided in two for addition or not of 2 μM ZnSO4, and then allowed to grow to stationary phase (OD600 = 0.8). Samples were taken from exponential-phase cultures prior to the addition and from stationary-phase cultures, and their volumes were standardized with respect to the OD600. Total RNAs were extracted as described previously (43), and after quantification (OD260), 70 μg was loaded on a 1.25% SeaKem GTG agarose gel in 10 mM phosphate buffer, using a 0.24- to 9.50-kb size standard (Promega). Electrophoresis was carried out at 100 V for 3 h (to allow specific zitRS mRNAs to migrate slightly faster than 16S rRNA), and ethidium bromide staining was performed. After being blotted on a Nytran-N nylon membrane (Schleicher and Schuell) and methylene blue stained (see Fig. S1 in the supplemental material), RNAs were hybridized at 42°C with an MG1363 zitRS′ probe (41). This probe was amplified from pVE8020 using the oligonucleotides 5′-AGTCGCAGGTTCTTTATG-3′ and 5′-CTAATGAGCGGGCTTTTT-3′. Labeling and detection were carried out using the ECL direct nucleic acid labeling and detection system (Amersham Pharmacia Biotech Europe GmbH).
Staphylococcal nuclease activity test.
Nuclease activity was detected as a pink halo on toluidine blue-agar medium (reference 41 and references therein) either overlaid on agar plates or used for dot-blotting of trichloroacetic acid (TCA)-precipitated cultures, using a culture volume standardized with respect to the OD600 (41), and the culture aliquot was diluted in SA medium when necessary to spot a constant volume.
Determination of metal content by ICP-MS.
Strain MG1363(pVE8072) grown overnight in SA plus chloramphenicol was diluted about 100-fold (according to the OD600) in 2.2 liters of SA. In early exponential phase (OD600 = 0.1), the culture was divided in two for 2 μM ZnSO4 addition or no treatment. Culture aliquots standardized according to the OD600 were taken from the subculture without ZnSO4 in the exponential phase (OD600 = 0.2) and from both subcultures after overnight treatment (OD600, ∼1), washed twice in water, and lyophilized. Inductively coupled plasma-mass spectrometry (ICP-MS) analyses of lyophilized cells or protein (see below) were performed by Service Central d'Analyze du CNRS (Vernaison, France) (http://www.sca.cnrs.fr).
rZitR overproduction and purification.
rZitR, a recombinant untagged form of MG1363 ZitR protein (UniProtKB/TrEMBL no. A2RNS2) was produced in E. coli after cloning of zitRr into the pET-28b(+) expression vector. rZitR bears three N-terminal substitutions (Ser2Ala, Ala4Arg, and Asp8Glu) at nonconserved positions in ZitR/AdcR proteins (Fig. 2) that have no effect on activity in vivo, as rZitR is as efficient as wild-type (WT) ZitR in repressing, in response to ZnSO4, a zitp-controlled and plasmid-borne reporter fusion (see Fig. S2 in the supplemental material). For overproduction in E. coli, an overnight culture of strain BL21(DE3)(pVE8073) was diluted 100-fold in prewarmed medium (1 liter), grown to an OD600 of 0.5, induced by 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside), and then grown for 3 h at 25°C to an OD600 of 2.0. Cells were centrifuged; washed twice in a 50 mM Tris-HCl, pH 6.5, 100 mM NaCl, and 1% saccharose buffer; frozen; resuspended in 40 ml of 20 mM Tris-HCl, pH 6.5, and 50 mM NaCl buffer; and disrupted by sonication on ice. The soluble fraction was recovered after 30 min of centrifugation at 20,000 × g and 4°C.
Fig. 2.
Streptococcal ZitR/AdcR proteins. (A) Sequences of the ZitR protein from L. lactis strain IL1403 (Lla; gb⊻AAK06214.1⊻AE006439_11) and of AdcR proteins from Streptococcus gordonii strain Challis (Sgo; gb|AAO43167.1|), S. pneumoniae strain R6 (Spn; gb|AAL00780.1|), S. pyogenes strain M1 group A streptococcus (GAS) (Spy; gb|AAK33210.1|), Streptococcus agalactiae strain NEM316 (Sag; emb|CAD45795.1|), and S. mutans strain UA159 (Smu; gb|AAN59599.1|AE015022_6) are aligned. (B) The sequence of the Zur protein from E. coli strain K-12 (Ec; P0AC51) is shown for comparison. Conserved residues are shown in boldface, with histidine and acidic residues boxed in black. Cysteine residues are indicated by arrows. Previously proposed Zn(II) (metal) ligands, i.e., a histidine- and aspartate-rich motif in ZitR/AdcR proteins (29) and the conserved cysteine residues of Zur proteins (39), are boxed in gray. The three recently identified Zn(II) ligands (all histidine residues) and the unique cysteine residue found not to be involved in Zn(II) binding in AdcR from S. pneumoniae strain D39 (44) are marked by + and −, respectively.
rZitR protein was then purified by a 2-step chromatography procedure: (i) anion-exchange chromatography (Q-Sepharose–Sepharose FF; Amersham) and (ii) heparin affinity chromatography (Heparin-Sepharose FF; Amersham) in the presence of 1 mM ZnCl2. The eluted sample (0.48 mg/ml protein, eluted by 50 mM Tris-HCl, pH 7, and 600 mM NaCl buffer) was >95% pure. A second batch purification was performed by essentially the same procedure, except that Heparin HyperDM20 (Biosepra) was used, and resulted in a 0.72-mg/ml protein yield (eluted by 20 mM Tris-HCl, pH 7, and 330 mM NaCl) that was >90% pure. The two purified samples (stored at 4°C) gave similar results in electrospray ionization-mass spectrometry (ESI-MS) and electrophoretic mobility shift assay (EMSA) experiments (see Fig. 6A for the first and Fig. 5, 6B, and 7 for the second).
Fig. 6.
rZitR DNA-binding activity and metal corepressor. A PCR product containing the zitp promoter was digested by AluI and incubated with purified rZitR protein. The sizes of restriction fragments according to a molecular size marker are indicated next to the gels. Only the 277-bp fragment containing intact zitp and the 248-bp and 115-bp fragments (negative controls) are shown (Fig. 7B shows smaller fragments). (A) DNA binding activity. ZnSO4 (0.1 mM) (left) or EDTA (1 mM) (right) was preincubated with purified rZitR protein before the addition of AluI-digested zitp-containing DNA (80 ng; 21 nM) and was also added to the electrophoresis buffer. rZitR protein was added in doubling amounts (in a dimer concentration range from 10.5 nM to 336 nM), and molar ratios between DNA and a protein dimer (rZitR2) are indicated. On the left, two shifted bands are marked by solid arrows and numbered (1 and 2). For the highest rZitR quantity (a ratio of 1:16), the observed smear starting from the second shifted band could be due to protein oligomerization. (B) rZitR corepressor. EMSA was essentially as for Fig. 6A, except that (i) ZnSO4 was not added during the incubation or electrophoresis step, (ii) less DNA (50 ng; 13 nM) was used, (iii) purified rZitR protein was added at a unique molar ratio between DNA and a protein dimer of 1:8 (i.e., 104 nM dimer), and (iv) slightly different migration conditions were used. Before being added to DNA for the binding assay, the purified rZitR protein was first pretreated or not with 1 mM EDTA (left) or 0.1 mM TPEN (right) to inactivate it; the presence (+) or absence (−) of rZitR protein and of the chelator agent in the binding reactions is indicated. Inactivated rZitR protein was then incubated or not (−) with the following metal cation salt: ZnSO4 (Zn(II)), CoCl2 (Co(II)), CuSO4 (Cu(II)), or NiCl2 (Ni(II)). The metal cation salts were added in slightly limiting amounts compared to the chelator agent, i.e., 0.9 mM and 90 μM for EDTA and TPEN, respectively (similar results were also obtained when metal cation salts were added at 0.8 mM and 80 μM, respectively). All shifted bands are marked by solid arrows and numbered (a third shifted band was observed here in contrast to panel A under slightly different migration conditions).
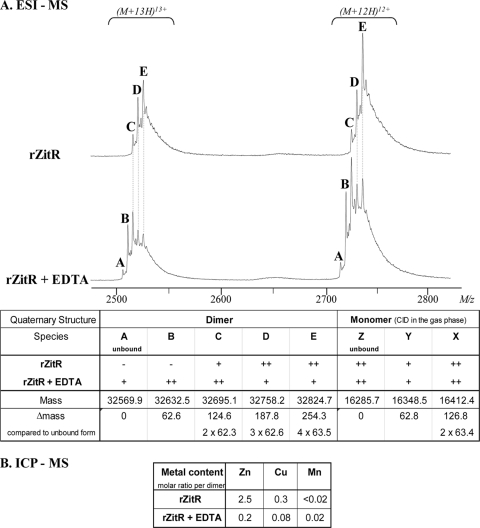
Fig. 5.
Purified rZitR protein analysis: oligomerization and ligand binding. (A) ESI-MS analysis. At the top, only a window on the mass spectra obtained under nondenaturing conditions is presented, showing the 12 [(M + 12H)12+] and 13 [(M + 13H)13+] charge states of both untreated and EDTA-treated rZitR dimers. Up to five species (A to E, with species A being the uncomplexed dimer) could be detected, two of which (the lightest species, A and B) were specific to the EDTA-treated rZitR protein. The table summarizes all ESI-MS measurements, including collision-induced dissociation (CID) in the gas phase that allowed the detection of three monomeric forms (species Z, Y, and X). According to their masses, species A and Z are the uncomplexed dimer and monomer, respectively. The mass of each species (the average of the masses measured for both EDTA-treated and untreated proteins) is indicated, together with its apparent abundance in the spectrum (−, absent; +, present; ++, major peak). (B) ICP-MS analysis. Zinc, copper, and manganese (as a negative control) contents were measured for both untreated and EDTA-treated proteins, and the molecular ratios of metal per dimer were calculated.
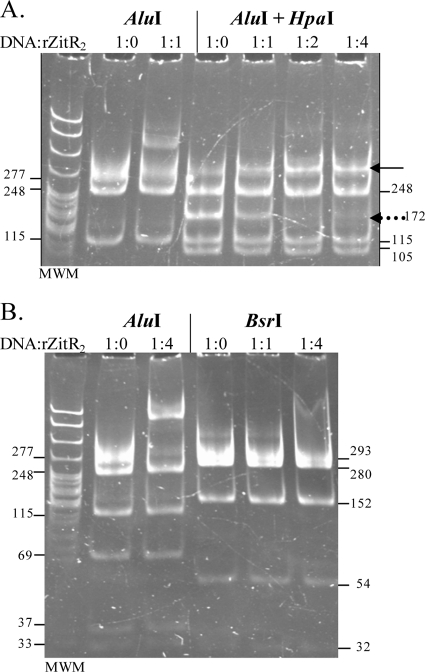
Fig. 7.
rZitR operator sites. The role of TTAACYRGTTAA palindromes was tested using HpaI and BsrI restriction enzymes that cut inside one or both of them, respectively (Fig. 1). zitp-containing DNA was digested by both HpaI and AluI (A) or by BsrI alone (B), using AluI simple digestion as a control. EMSA was essentially as for Fig. 6A, left, except that (i) ZnSO4 was not added during the incubation or electrophoresis step and (ii) 60 ng of DNA (15 nM) was used. On the right, the 172-bp AluI HpaI fragment is indicated by a dotted arrow, while the single shifted band is shown by a solid arrow. No shift was observed for the 105-bp AluI HpaI fragment and the BsrI fragments. MWM, molecular weight marker.
Gel filtration.
Five milliliters of rZitR (0.7 mg/ml) was injected on a HiLoad 16/60 (GE Healthcare) Superdex 75 equilibrated in the following buffer: 20 mM Tris-HCl, pH 7.5, 200 mM NaCl, 10 mM β-mercaptoethanol, and 100 μM ZnSO4 at 8°C. Apparent molecular weights were determined by comparison with a calibration curve using standard molecular weight markers. The two elution peaks were compatible with dimeric and tetrameric species of rZitR.
ESI-MS.
Two rZitR aliquots (15 nmol; 240 μg) were diluted in 15 ml of 10 mM ammonium acetate buffer with or without 3.5 mM EDTA. Cycles of protein desalting in 10 mM ammonium acetate buffer were performed using Centriplus YM-3 centrifugal filter devices (3 kDa) from Amicon (Millipore) to dilute initial buffer salts ∼106-fold. Lyophilized protein aliquots (2 nmol; 32 μg) were suspended either at 2 μM in a denaturing solvent (50% acetonitrile acidified with 0.1% formic acid) or at 10 μM in a nondenaturing buffer (10 mM ammonium acetate salt, pH 6.8). Mass measurements were performed on a hybrid quadrupole time-of-flight instrument (Q-TOF Ultima Global; Waters) equipped with an electrospray ion source. Under nondenaturing conditions, the instrument parameters (accelerating voltage and pressure in the interface of the mass spectrometer) were carefully controlled to preserve weak noncovalent interactions. In further experiments (collision-induced dissociation in the gas phase), kinetic energy was increased to produce gas phase dissociation of dimeric species into monomers (45, 47).
EMSA.
An 811-bp PCR fragment containing zitp (and zitR) was amplified from pVE8020 (41) or pVE8064 (28) using M13 Reverse and MUT (5′-GCTCTAGAGCGGGATCCTTCATCGAAACTCTTCAG-3′) primers (28) and purified with a QIAquick Gel Extraction Kit (Qiagen). Its digestion by (i) AluI or (ii) BsrI generates fragments of (i) 277, 248, 115, 69, 37, 33, 22, and 10 bp (HpaI cuts the 277-bp AluI fragment into two fragments of 172 and 105 bp) and (ii) 293, 280, 152, 54, and 32 bp. DNA-binding reactions (8 μl) were performed in 20 mM Tris-HCl, pH 8.0, 50 mM KCl, 20 μg/ml bovine serum albumin (BSA), 200 μM dithiothreitol (DTT), 200 μM MgCl2, and 4% glycerol with 50 to 80 ng (13 to 21 nM) of DNA, and the rZitR protein was added at the indicated molar DNA/protein ratios. rZitR was preincubated with ligands for 10 min at room temperature (see Fig. 6A). Binding reactions were carried out at room temperature for 30 min, and the reaction mixtures were then subjected to electrophoresis in 8% polyacrylamide gels using Tris-borate buffer (45 mM Tris, 45 mM boric acid, pH 8.0) that had been supplemented with 0.1 mM ZnSO4 or 1 mM EDTA in the experiment (see Fig. 6A). Electrophoresis was carried out at 70 V for 1.5 h at 4°C (see Fig. 6A) or at room temperature (see Fig. 7) and at 50V for 10 to 15 min and 80 V for 65 to 70 min at room temperature (see Fig. 6B). The gels were stained with ethidium bromide (5 μg/ml) for 30 min and rinsed with distilled water.
RESULTS
L. lactis ZitR protein.
L. lactis was chosen as a Streptococcaceae prototype to study the regulation of the zitRSQP genes (Fig. 1A) that encode a putative repressor (ZitR) and an ABC uptake system (ZitSQP). Lactococcal ZitR proteins (88% to 99% identity) and streptococcal AdcR proteins share 45% to 50% identity and are rich in acidic and histidine residues (Fig. 2A). Their C-terminal putative HTH domain is typical of the MarR family (smart00347) (10, 30) but is also related to the ArsR (smart00418) and distantly to the DtxR (smart00529) families dedicated to the regulation of metal homeostasis (9, 30). The predicted secondary structure of the L. lactis ZitR protein displays the αααββ motif (http://bioinf.cs.ucl.ac.uk/psipred/ and data not shown) shared by MarR regulators (51) and the majority of metalloregulators (30), suggesting that the ZitR protein could fold as a winged-helix protein. Finally, the L. lactis ZitR protein is a good model for AdcR regulators (MarR family) and could control both zinc uptake by ZitSQP and its own expression.
Repression of zit transcription.
The expression of the WT zit operon from L. lactis subsp. cremoris (strain MG1363) and its regulation in response to extracellular Zn(II) was explored by Northern blotting. To accurately test the specific effect of Zn(II), we used the chemically defined and metal-poor SA medium (22). An exponential-phase culture of MG1363 cells grown in SA medium was supplemented or not with a nontoxic concentration (2 μM) of ZnSO4, and mRNAs were extracted prior to the addition and after growth until the stationary phase. The exponential-phase extract revealed a major transcript corresponding in size to zitRS mRNA and a very weak band corresponding to a larger zitRSQ(P) mRNA (Fig. 3A and data not shown). zitRSQP genes thus seem to be cotranscribed, even though they are expressed at a low level compared to zitRS genes. The existence of zitRS mRNA supports the proposal that the strong and conserved stem-loop downstream of zitS (Fig. 1A) could be a ρ-independent terminator (12; http://bonsai.hgc.jp/∼mdehoon/terminators/NC_009004.trms), although mRNA processing cannot be excluded.
Fig. 3.

zitp regulation in response to extracellular Zn(II). (A) Regulation of a chromosomal zit operon in response to extracellular Zn(II). MG1363 cells were grown in SA medium to the exponential phase (Expo), and after the addition of nontoxic (2 μM) ZnSO4 (+) or not (−), growth was continued to the stationary phase (Stat). Equivalent amounts of RNA were loaded on a gel (as measured by the OD260). After electrophoresis, blotting, and methylene blue staining of the membrane, Northern blotting was performed with a probe covering the zitR gene and the zitS 5′ end. An mRNA corresponding in size to zitRS by comparison to a molecular weight marker and a weak and larger zit mRNA are indicated by arrows. (B) Correlation of the zitp expression level with the intracellular Zn content. MG1363(pVE8072) cells were grown in SA medium to exponential phase (Expo) or ON (i.e., late stationary phase) in the presence (+) or absence (−) of 2 μM ZnSO4, and culture aliquots (adjusted for equivalent OD600 values) from three independent experiments were lyophilized or not. Reporter activity was revealed by spotting fresh cells on toluidine blue-agar medium (one representative experiment with cells spotted on the same detection medium is shown). In parallel, ICP-MS was used to measure the Zn contents of all three lyophilized cell aliquots: average values (μg/g) and standard deviations are indicated. The Cu content, which was measured as a control, was not correlated with the zitp expression level, in contrast to the Zn content (not shown).
In cells grown to the exponential phase in Zn(II)-poor SA medium (10 nM) (22), zitRS(QP) genes were expressed, suggesting that zitp was turned on. In the stationary phase without Zn(II) addition, zitRS mRNA was clearly upregulated compared to the exponential phase. However, upregulation could also be achieved in the exponential phase by EDTA addition (data not shown): this result and other data (Fig. 3B) suggested that upregulation during growth could be an indirect effect of a decrease in the extracellular Zn(II) concentration rather than true growth phase-dependent regulation. After ZnSO4 addition in the stationary phase, no transcript was detectable, showing that zit operon transcription is repressed by Zn(II) at a moderate and nontoxic concentration. All these results demonstrate that the WT chromosomal zit operon is repressed in response to extracellular Zn(II) and that the repression threshold is low (<2 μM), i.e., well below the level of Zn(II) toxicity in L. lactis. These results are in keeping with our previous data using zitp-controlled reporter fusions in an operon with zitR (28), which showed that zitp regulation is preserved when zitp and zitR are cocloned on a plasmid. Using those reporter fusions, the zitp repression threshold was estimated at around 100 nM Zn(II) (reference 28 and data not shown), which fit the Northern blot results well.
Intracellular effector of zit regulation.
To gain insight into the intracellular signal for zitp regulation by extracellular Zn(II), the cellular metal content was determined by ICP-MS, while zitp expression was followed in parallel using a plasmid-borne reporter cassette. In pVE8072, a staphylococcal nuclease reporter fusion (28) is in an operon with the zitR gene and is expressed under the control of zitp. Strain MG1363(pVE8072) was grown in SA medium to early exponential phase for supplementation or not by ZnSO4 at 2 μM. Cells from the exponential phase (without addition) or overnight cultures were analyzed for both reporter activity and zinc content (Fig. 3B).
In overnight samples after ZnSO4 addition, reporter activity was nearly undetectable, as expected (Fig. 3A) (28), and the intracellular Zn content was high: it was increased by ∼4 fold compared to ON samples without addition (Fig. 3B). On the other hand, during growth in SA without addition (compare overnight and exponential samples in Fig. 3B), reporter activity was increased, consistent with chromosomal zit induction (Fig. 3A), while the intracellular Zn content was significantly decreased. These results were confirmed in another strain context (data not shown), indicating that the zitp expression level is inversely correlated with the cellular Zn content, suggesting that cytoplasmic zinc could mediate repression by extracellular Zn(II).
zitR with an intact HTH-encoding domain is necessary for zitp regulation in vivo.
The above-mentioned and previous expression results (28), together with sequence data, all point to ZitR as the likely repressor of zitp expression. We tested whether zitR could regulate a zitp-controlled reporter gene carried on a plasmid in an otherwise WT strain. When zitR was deleted from pVE8064 (28), resulting in the transcriptional fusion of the reporter gene to the zitp sequence, reporter expression was almost abolished, whatever the conditions (see Fig. S2 in the supplemental material), suggesting a major effect on transcription initiation or the mRNA 5′ structure and stability. A partial deletion specifically affecting the DNA-binding domain-encoding region was then designed, as in several other regulator genes (reference39, and reference 53 and references therein), and it resulted in loss of function (inactivation) without loss of expression.
The zitR 3′ end coding for the predicted ZitR HTH domain was deleted from pVE8072, leading to pVE8074, which carries the reporter gene in an operon with a truncated zitRΔHTH allele under the control of the zitp promoter. In an otherwise WT strain, the abilities of plasmid-borne intact zitR and truncated zitRΔHTH alleles to regulate reporter expression on plates in the presence of a Zn(II) concentration gradient were compared, as described previously (40). At high Zn(II) concentrations (millimolar range and below), whereas the reporter was repressed in the presence of intact zitR, it was derepressed at an intermediate level in the presence of the zitRΔHTH allele (see Fig. S3 in the supplemental material), indicating that in contrast to zitR, zitRΔHTH fails to fully repress zitp expression. At higher Zn(II) concentrations (molar range and below), the reporter in an operon with zitRΔHTH was fully induced (Fig. 4). zitp derepression in the presence of both plasmid-borne truncated zitRΔHTH and chromosomal WT zitR copies strongly suggests that zitRΔHTH in multicopy is a trans-dominant-negative allele. As proposed for other regulators (53), including the E. coli Zur protein (39), plasmid-encoded ZitRΔHTH could sequester chromosome-encoded WT ZitR into inactive heteromultimers (multimerization was indeed observed in vitro [see below]). These results suggest that in vivo, WT ZitR with an intact HTH domain is necessary for zitp repression by Zn(II).
Fig. 4.

Derepression of the zitp promoter in the presence of both plasmid-borne truncated zitRΔHTH and chromosomal WT zitR alleles at sublethal Zn(II) concentrations. The zitp expression level was followed using the staphylococcal nuclease reporter. WT intact zitR or truncated zitRΔHTH alleles are in an operon with the reporter gene on pVE8072 and pVE8074 plasmids, respectively, and their effects on reporter expression are compared in the otherwise WT strain MG1363 (carrying a WT zitR allele). Twenty microliters of ZnSO4 at 1 M (a lethal concentration) were streaked (vertical arrow) on chemically defined SA-agar medium to create a concentration gradient by diffusion (triangles). Lactococcal cells [MG1363(pVE8072) at the top and MG1363(pVE8074) at the bottom] were then cross-streaked on the same plate. After overnight growth, nuclease activity was revealed by a toluidine blue-agar overlay. In the presence of a truncated zitRΔHTH allele, the reporter gene is specifically derepressed near the deposit streak, and the full derepression area is indicated by arrows.
Purified rZitR protein is a dimer that complexes divalent cations.
We chose to purify a recombinant untagged ZitR form (rZitR), as the possibility that the His6 tags of most purified AdcR proteins (1, 3, 34) could interfere with metal binding cannot be excluded. After overexpression in E. coli and purification in the presence of ZnCl2, rZitR was first analyzed by gel filtration, revealing a major dimeric form and a minor tetrameric form (see Fig. S4 in the supplemental material). The rZitR protein was then subjected to ESI-MS analysis under denaturing or nondenaturing conditions (16, 42). Under denaturing conditions, the measured protein mass, 16,286.5 ± 0.5 Da, fit well with the predicted mass of the monomer (145 amino acids) after methionine deletion (16,285.6 Da). Under nondenaturing conditions, carefully controlled to preserve specific noncovalent interactions (i.e., by lowering the voltage of ion extraction and by increasing the pressure in the source-analyzer interface of the mass spectrometer to ∼400 Pa), untreated rZitR was observed mainly as a mixture of three main dimeric forms, species C, D, and, in the majority, E (Fig. 5A), and also as minor tetrameric forms (data not shown). EDTA treatment was found to shift the spectrum toward slightly lighter forms, comprising uncomplexed dimer (species A at 32,569.9 ± 1 Da) and species B, in addition to the previous species D, E, and, in the majority, C (Fig. 5A). These results indicated that divalent cations were noncovalently bound to purified rZitR protein and could be displaced by EDTA, leading to less coordinated complexes. Interestingly, the presence of the uncomplexed dimer (species A) showed that divalent cation binding is not required for protein dimerization, although it could help the process (as zitp derepression in the presence of both zitRΔHTH and WT zitR alleles was observed only at high Zn(II) concentrations).
Zinc cation is the metal ligand of rZitR protein.
The mass differences between uncomplexed dimer (species A) on one hand and species B, C, D, or E on the other hand correspond to 1, 2, 3, or 4 times ∼63 Da (Fig. 5A), suggesting that a unique divalent cation of ∼63 Da can bind to the rZitR protein in up to four cations per dimer. In further experiments, gas phase dissociation of dimeric species (collision-induced dissociation measurement [Fig. 5A]) (45, 47) generated three monomeric species from both treated and untreated rZitR; compared to uncomplexed monomer (Z species), species Y and X display increased masses of 1 or 2 times ∼63 Da, confirming the noncovalent binding of up to two divalent cations per monomer. The cation mass can be estimated from the average mass difference between all (dimeric and monomeric) consecutive species (62.9 Da). Taking into account measurement precision (0.005%, i.e., 1.6 Da for the dimer) and the possibility of proton loss upon metal binding, this value fits the atomic mass of either Zn (65.4 Da) or Cu (63.5 Da), whereas the nearest neighbors in the periodic table, Ni (58.7 Da) and Ga (69.7 Da), are clearly excluded. All these results indicate that the rZitR protein was purified as a mixture of dimeric forms noncovalently bound to two to four Zn(II) or Cu(II) cations.
ICP-MS analysis was performed to determine the rZitR ligand, and Zn, Cu, and Mn contents were measured in the protein samples (treated or not with EDTA) used for ESI-MS analysis. The results are shown as molar ratios per dimer of purified protein (Fig. 5B). The Mn content remained at a background level, and a low Cu content could be detected in untreated rZitR. Zn was clearly the most abundant metal atom of untreated rZitR, with more than two Zn atoms per untreated rZitR dimer, in agreement with the ESI-MS results (Fig. 5A), and its level was drastically (>12-fold) reduced by EDTA treatment. These results demonstrate that purified rZitR dimer is mainly bound to zinc atoms.
rZitR DNA-binding activity.
An EMSA was performed on zitp-containing PCR DNA digested by AluI (Fig. 6A). In the presence (Fig. 6A, left) or absence (Fig. 6B, second lane from the left in both gels) of added ZnSO4, rZitR specifically affected the migration of the 277-bp fragment containing zitp, in contrast to all other AluI fragments (Fig. 6 and 7). Thus, rZitR had been purified as an active DNA-binding protein able to specifically recognize the zitp-containing fragment, and as purified rZitR was shown to be a mixture of three dimeric forms complexing two to four Zn(II) [species C to E, i.e., rZitR2-Zn(II)2 to rZitR2-Zn(II)4], at least one of them should be endowed with DNA-binding activity.
A first faint shifted band appeared as soon as rZitR was added to zitp-containing DNA and increased in intensity as the quantity of rZitR dimer compared to DNA was progressively increased up to 4-fold (Fig. 6A, left). At that point, a second shifted band appeared. The presence of two shifted bands suggested that rZitR bound two operator sites in the zitp region. When the rZitR quantity was further doubled to reach an 8-fold excess compared to DNA, the second shifted band became dominant, and the 277-bp fragment almost completely disappeared. This profile suggested that 50% of zitp-containing DNA could be bound by the rZitR dimer when it was present in ∼3- to 4-fold excess, allowing the apparent dissociation constant for the DNA-binding reaction to be estimated at ∼50 to 70 nM. Finally, EDTA completely inactivated rZitR (Fig. 6A, right), showing that rZitR DNA-binding activity required divalent cations.
The metal ligand necessary for rZitR DNA-binding activity.
The nature of the metal ligand necessary for rZitR activity was examined. Four metal cations, Zn(II), Cu(II), Ni(II), and Co(II), were tested for the ability to restore the DNA-binding activity of rZitR that had been pretreated by EDTA or TPEN [N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine], another chelating agent (6) widely used in studies of zinc-binding regulators (36, 37), but not in the case of the S. pneumoniae AdcR protein (1). Both EDTA (Fig. 6B, left) and TPEN (at a lower concentration) (Fig. 6B, right) were able to abolish rZitR DNA-binding activity. Metal cations were subsequently added to inactivated rZitR. When the metal cations were added in limiting amounts compared to the chelator agent (Fig. 6B), full rZitR activity could be restored only in the case of Zn(II), confirming that Zn(II)-loaded rZitR, like untreated rZitR (Fig. 6B) that complexes Zn(II) (Fig. 5), is an active DNA-binding protein. This result showed that rZitR was able to compete with either EDTA or TPEN for Zn(II) binding and that its affinity for Zn(II) was higher than that of TPEN, i.e., more than 1015 M−1 (35). In the course of these experiments, a third shifted band appeared, possibly revealing a higher activity of rZitR (Fig. 6B). This suggested either that rZitR, when bound to both palindromes, was able to tetramerize (tetramerization was indeed observed during gel filtration and ESI-MS assays [see above]), thus bending DNA and affecting its migration, or, alternatively, that a third weak binding site might be present. Finally, the in vitro observation that, after inactivation by two chelator agents, Zn(II) is the only metal cation able to restore full rZitR activity, combined with our in vivo data, suggests that Zn(II) is the physiological corepressor of the ZitR protein.
rZitR operator sites.
In our EMSA experiments (Fig. 6), the appearance of two new bands when rZitR was added to a zitp-containing DNA was in agreement with the proposal that ZitR could bind the two TTAACYRGTTAA palindromes present in the zitp sequence (38). To test this hypothesis, The HpaI or BsrI enzyme was used to disrupt one or both palindromes (Fig. 1B) before EMSA. HpaI and AluI double digestion allowed a migration shift for only one of the two fragments (172 bp) obtained from the zitp-containing fragment of 277 bp (Fig. 7A). This indicated that, as expected, only one ZitR binding site was intact and that one palindrome was sufficient for rZitR binding. BsrI digestion impeded rZitR binding under conditions leading to a clear shift of the 277-bp fragment (compare the 6th and 3rd lanes from the left in Fig. 7B). These results demonstrate that the integrity of both palindromes is necessary for rZitR binding on zitp and confirm that they constitute the ZitR binding sites.
DISCUSSION
The physiological significance of zit regulation by extracellular zinc.
The L. lactis zit promoter, zitp, was shown here to be fully repressed by extracellular Zn(II) in a wide concentration range, from high (including sublethal) to moderate (above 2 μM) concentrations, and to be induced at very low concentrations (below 10 nM), indicating that the repression threshold is low. It was indeed estimated to be around (slightly above) 100 nM (reference 28 and data not shown). At 2 μM extracellular Zn(II) (and above), the high cellular Zn content suggests that Zn(II) uptake could be achieved by another, unidentified system (Fig. 8A), as is the case in E. coli (4, 19) and B. subtilis (17) (for reviews, see references 5 and 33). Thus, rather than ensuring resistance to Zn(II) toxicity, zit repression could allow energy to be saved by preventing the synthesis of useless Zit proteins under Zn(II) repletion (Fig. 8A). The main physiological significance of zit regulation could be an emergency response to zinc scarcity and starvation, as zinc scavenging by the induced high-affinity ZitSQP transporter could allow cell survival under Zn(II) depletion (Fig. 8B).
Fig. 8.
Model for zit regulation by Zn(II). Extracellular concentrations of soluble Zn(II) are shown at the top on a logarithmic scale (large horizontal arrow), and the estimated regulation threshold (100 nM) is indicated by a dashed vertical line. (A) Repression. In a wide concentration range above the threshold, from repletion to profusion and finally toxicity, repression is achieved by active holo-ZitR proteins able to bind the zitp promoter (active Holo-ZitR is shown as a Zn(II)4-ZitR2 complex, but other forms could also be active). As zit repression by Holo-ZitR is effective under repletion conditions, it could be a way for the cells to save energy. If another Zn(II) uptake system existed in L. lactis, as is the case in E. coli and B. subtilis (see Discussion), under toxic conditions, zit repression would not be sufficient to ensure resistance to Zn(II). (B) Derepression. Under Zn(II) deprivation, inactive ZitR forms that have lost their allosteric ligands appear. Under prolonged or extreme Zn(II) deprivation, or because of neosynthesis, apo-ZitR appears (as, in particular, the unbound ZitR2 dimer, although other forms could be inactive). Inactive ZitR forms could ensure continual sensing of intracellular Zn(II) and fast repression in response to a sudden Zn(II) increase. However, the main significance of zit regulation could be to allow efficient adaptation to zinc scarcity: zit induction under extreme Zn(II) deprivation could be an emergency response to allow zinc scavenging by the high-affinity Zit transporter and to ensure cell survival.
The low regulation threshold appears to be physiological, as L. lactis can encounter zinc-poor media. Milk, a natural ecological niche for lactococci, has a soluble zinc content of only 0.3 nM (52). Also, during growth, extracellular Zn(II) could be progressively depleted, as supported by the decrease in the cellular Zn content observed here in a synthetic medium (28). In streptococcal pathogens, a μM range threshold for adc regulation could also be physiological. In S. pneumoniae, an adc mutant cannot grow on defined medium unless 0.8 μM ZnSO4 is added (13). This suggests that adc genes for the Zn(II) uptake system are expressed only in less than 0.8 μM Zn(II), and their expression actually did not respond to Zn(II) addition in a rich medium initially containing 8 μM Zn(II) (24). Zinc exhaustion conditions can often be encountered in hosts, and the induction of Adc uptake systems could favor colonization. An increased risk of acute respiratory tract infections by S. pneumoniae is associated with zinc deficiency in children and can be reduced by zinc administration, and mice kept on a zinc-deficient diet show more extensive pneumococcal colonization in the nasal mucosa (46). Furthermore, as AdcR regulates genes encoding surface-exposed proteins involved in the invasion of Zn(II)-poor tissues (compared to blood), repression of these genes in response to elevated Zn(II) could allow their expression to be limited in the early stages of infection, when they are needed (7, 38). Finally, ZitR/AdcR regulators seem to play an important role in adaptation to the environment, including the host for pathogenic species.
Unbalanced expression of zit genes.
As the zit operon is weakly transcribed compared to zitRS genes, probably because of the stem-loop structure downstream of zitS, there could be an excess of ZitS lipoprotein compared to the ZitPQ proteins (Fig. 8B). Such a transcriptional regulation involving an internal stem-loop structure was first described for the mtsABC metal uptake operon in Streptococcus pyogenes (21). It was proposed that large amounts of the binding lipoprotein MtsA at the cell surface could serve as a reservoir of metal cations while awaiting contact with the less available MtsBC proteins embedded in the membrane (21). Another gene involved in Zn(II) binding, the E. coli znuA gene, which is adjacent to but divergent from the znuBC operon, is 2-fold more upregulated than the znuB and znuC genes under Zn(II) depletion conditions (18). Interestingly, the adcA genes in Streptococcus mutans and S. pyogenes are orphan genes (29, 38). It is conceivable that the lack of selective pressure to maintain all the genes involved in Zn(II) ABC uptake in an operon might be related to the need for higher expression of the gene encoding the binding protein to improve transport.
The L. lactis ZitR protein is a zinc-responsive repressor.
The HTH domain of the L. lactis ZitR protein was found to be required for zitp repression. In the case of the E. coli Zur repressor, the zurΔ46-91 allele was identified from a genomic library cloned on a plasmid by its ability to derepress a reporter fusion to znuA in an otherwise zur+ strain (39, 40). Purified ZurΔ46-91 protein failed to bind the znu operator site, thus defining amino acids 46 to 91 as part of the Zur DNA-binding domain (39). When mixed, WT and mutant proteins could form heterodimers, highly suggesting that the formation of inactive heterodimers was responsible for reporter derepression in vivo (39). Similarly, in the case of ZitR, reporter derepression in the presence of both plasmid-borne zitRΔHTH and chromosomal WT zitR alleles, and the fact that rZitR protein was purified as dimeric forms endowed with DNA-binding activity, supports the model that truncated ZitRΔHTH protein could titrate WT ZitR protein into inactive heterodimers. Furthermore, in the WT active homodimer, both C-terminal HTH domains should be necessary for stable DNA binding. The fact that full induction was observed only at high Zn(II) concentrations suggests both that heterodimer stability could be improved at high Zn(II) concentrations and that at moderate Zn(II) levels, some WT homodimers could lead to residual repression.
Here, ZitR protein was one of the first members of the AdcR metallorepressor group to be characterized in vitro: purified rZitR protein was found to be a dimer able to bind up to four Zn(II) cations. Only two AdcR proteins were previously purified (1, 3, 44), and the S. pneumoniae AdcR protein has only recently been characterized as a dimer bound to three to five Zn(II) cations (44). An even number of bound cations is generally observed for dimeric forms of metalloregulators, in agreement with symmetric binding of both monomers (30, 44). In the case of the rZitR protein, Zn(II) was shown to be required to restore full DNA-binding activity after protein inactivation by two chelator agents, suggesting that Zn(II) might exert an allosteric effect on DNA-binding activity. Although the activity of each individual dimeric form was not assessed here, we found that purified rZitR protein was active, meaning that among its three dimeric forms that complex two to four Zn(II), at least one is active [in Fig. 8A, active ZitR is shown as species E or Zn(II)4-ZitR2]. In the future, it would be interesting to test whether the ZitR protein, like the E. coli Zur protein, could bind two structural and two regulatory Zn(II) per dimer (37). If a similar model also applies to the ZitR protein, the dimeric forms that bind up to two Zn(II) per dimer [up to species C or Zn(II)2-ZitR2] could be inactive. In particular, ZitR proteins newly synthesized under Zn(II) deprivation should rapidly dimerize into an inactive apo-ZitR form (species A or ZitR2) that could ensure continual sensing of intracellular Zn(II) (Fig. 8B) and fast repression resetting once Zn(II) again became available (Fig. 8A). Our data, in particular the low regulation threshold, the correlation between the intracellular Zn content and the zitp repression level, and the high affinity of rZitR for Zn(II), suggest that ZitR, like Zur (35), could be a sensitive sensor of intracellular Zn(II) to efficiently respond to Zn(II) variations in the environment.
Why do Streptococcaceae use ZitR/AdcR metalloregulators rather than Zur proteins to control zinc uptake?
The L. lactis zinc-responsive ZitR repressor is a model of ZitR/AdcR metalloregulators that are specific to the Streptococcaceae group (10, 30, 38). Remarkably, in S. pneumoniae, Zn(II) efflux by the cation diffusion facilitator CzcD is not controlled by the CzrA regulator (ArsR-SmtB family), as in B. subtilis (31), but by SczA, the first described metalloregulator of the TetR family (23). It is quite interesting from an evolutionary point of view that Streptococcaceae seem to have peculiar metalloregulators to control all Zn(II) homeostasis aspects. The streptococcal specificity for ZitR/AdcR proteins is particularly intriguing, as they appear to be the functional analogs of Zur regulators, fulfilling the same physiological function and sharing the same biochemical properties. In vivo, both Zur (25, 39, 40) and the ZitR/AdcR proteins allow zinc acquisition when cells are starved for zinc. In vitro, for both E. coli Zur (37, 39) and L. lactis ZitR proteins, DNA-binding activity requires Zn(II) bound in up to two cations per monomer and the HTH domain, whereas Zn(II) is not required for protein dimerization.
Despite their similarities, there is a major difference between Zur and the ZitR/AdcR proteins: whereas Zur has four conserved cysteine residues, including two probably involved in Zn(II) binding, by homology to Fur proteins (Fig. 2B) (37, 39), the ZitR/AdcR proteins have a single one (30) (Fig. 2A), indicating that most, if not all, Zn(II)-binding sites [as the cysteine residue in S. pneumoniae AdcR protein was shown not to be required for Zn(II) binding (44)] involve only acidic or histidine residues. ZitR/AdcR proteins exemplify cysteine exclusion in the proteomes of aerobic Firmicutes (11) and, in particular, of lactococci and streptococci (see the supplemental material in reference 11; R. Daniels, personal communication). The difference in cysteine contents between Zur and the ZitR/AdcR proteins could be crucial for their folding and activity. In vitro, the E. coli Zur protein, which contains nine cysteine residues in total, is highly sensitive to oxidation that abolishes both Zn(II) and DNA binding, and the latter can be restored only by DTT treatment (39). On the other hand, rZitR DNA-binding activity was found to be insensitive to DTT (data not shown). In vivo, protein sensitivity to oxidation could be a problem for bacteria that encounter highly oxidative environments and/or whose cytoplasm is not reducing enough to deal with incorrect disulfide bonds, like Streptococcaceae. Pathogenic streptococci colonize and infect oxygen-exposed tissues, like the epidermis and the respiratory tract, and cysteine exclusion was proposed to provide such bacteria with a selective advantage (11). In L. lactis, most strains are not able to synthesize glutathione, one of the two major systems for disulfide bond reduction (27). Whether Streptococcaceae could need ZitR/AdcR instead of Zur as a Zn(II)-responsive repressor more resistant to oxidation and better suited either to a highly oxidative environment or to a poorly reducing cytoplasm deserves further investigation.
Supplementary Material
ACKNOWLEDGMENTS
We are very grateful to Marie-Dominique Roy, Johanna Kwiaton, and Sophie Longueville for participating to this study; to Benjamin Dray, Nicolas Leulliot, and Herman Van Tilbeurgh (IBBMC, Université Paris Sud, Orsay, France) for gel filtration assays; to Service Central d'Analyses (CNRS, Vernaison, France) for ICP-MS analyses; and to Alexander Bolotin, Alexander Sorokin, and Stanislas Dusko Ehrlich (Institut Micalis, INRA, Jouy-en-Josas, France) for providing unpublished sequence data. We thank Jean-Pierre Claverys (CNRS-Université Paul Sabatier, Toulouse, France), Agnès Rodrigue, and Marie-Andrée Mandrand-Berthelot (CNRS-INSA, Villeurbanne, France) for interesting discussions and Eric Devic and Hervé Ginesty (GTP-Technology, Toulouse, France) for their support. We thank the scientific committee of Biometals Congress (Santiago de Compostela, Spain, 2008) for giving us the opportunity to present this work as an oral communication. Special thanks are due to Alexandra Gruss, Marie-Agnès Petit, Pascale Serror, and Maarten van de Guchte (Institut Micalis, INRA, Jouy-en-Josas, France) for critical reading of the manuscript.
D.L. was the recipient of a Marie Curie Individual Fellowship from the European Commission and a grant from the Fondation pour la Recherche Médicale (France).
Footnotes
Supplemental material for this article may be found at http://jb.asm.org.
Published ahead of print on 11 February 2011.
REFERENCES
- 1. Aranda J., Garrido M. E., Cortes P., Llagostera M., Barbe J. 2008. Analysis of the protective capacity of three Streptococcus suis proteins induced under divalent-cation-limited conditions. Infect. Immun. 76:1590–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aranda J., et al. 2010. The cation-uptake regulators AdcR and Fur are necessary for full virulence of Streptococcus suis. Vet. Microbiol. 144:246–249 [DOI] [PubMed] [Google Scholar]
- 3. Aranda J., et al. 2009. Protective capacities of cell surface-associated proteins of Streptococcus suis mutants deficient in divalent cation-uptake regulators. Microbiology 155:1580–1587 [DOI] [PubMed] [Google Scholar]
- 4. Beard S. J., et al. 2000. Evidence for the transport of zinc(II) ions via the pit inorganic phosphate transport system in Escherichia coli. FEMS Microbiol. Lett. 184:231–235 [DOI] [PubMed] [Google Scholar]
- 5. Blencowe D. K., Morby A. P. 2003. Zn(II) metabolism in prokaryotes. FEMS Microbiol. Rev. 27:291–311 [DOI] [PubMed] [Google Scholar]
- 6. Blindauer C. A., Razi M. T., Parsons S., Sadler P. J. 2006. Metal complexes of N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN): Variable coordination numbers and geometries. Polyhedron 25:513–520 [Google Scholar]
- 7. Brenot A., Weston B. F., Caparon M. G. 2007. A PerR-regulated metal transporter (PmtA) is an interface between oxidative stress and metal homeostasis in Streptococcus pyogenes. Mol. Microbiol. 63:1185–1196 [DOI] [PubMed] [Google Scholar]
- 8. Brocklehurst K. R., Morby A. P. 2000. Metal-ion tolerance in Escherichia coli: analysis of transcriptional profiles by gene-array technology. Microbiology 146:2277–2282 [DOI] [PubMed] [Google Scholar]
- 9. Busenlehner L. S., Pennella M. A., Giedroc D. P. 2003. The SmtB/ArsR family of metalloregulatory transcriptional repressors: structural insights into prokaryotic metal resistance. FEMS Microbiol. Rev. 27:131–143 [DOI] [PubMed] [Google Scholar]
- 10. Claverys J. P. 2001. A new family of high-affinity ABC manganese and zinc permeases. Res. Microbiol. 152:231–243 [DOI] [PubMed] [Google Scholar]
- 11. Daniels R., et al. 2010. Disulfide bond formation and cysteine exclusion in gram-positive bacteria. J. Biol. Chem. 285:3300–3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Hoon M. J., Makita Y., Nakai K., Miyano S. 2005. Prediction of transcriptional terminators in Bacillus subtilis and related species. PLoS Comput. Biol. 1:e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dintilhac A., Alloing G., Granadel C., Claverys J. P. 1997. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol. Microbiol. 25:727–739 [DOI] [PubMed] [Google Scholar]
- 14. El Karoui M., et al. 2000. Orientation specificity of the Lactococcus lactis Chi site. Genes Cells 5:453–461 [DOI] [PubMed] [Google Scholar]
- 15. Finney L. A., O'Halloran T. V. 2003. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science 300:931–936 [DOI] [PubMed] [Google Scholar]
- 16. Fribourg S., et al. 2000. Structural characterization of the cysteine-rich domain of TFIIH p44 subunit. J. Biol. Chem. 275:31963–31971 [DOI] [PubMed] [Google Scholar]
- 17. Gaballa A., Helmann J. D. 2002. A peroxide-induced zinc uptake system plays an important role in protection against oxidative stress in Bacillus subtilis. Mol. Microbiol. 45:997–1005 [DOI] [PubMed] [Google Scholar]
- 18. Graham A. I., et al. 2009. Severe zinc depletion of Escherichia coli. J. Biol. Chem. 284:18377–18389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grass G., et al. 2005. The metal permease ZupT from Escherichia coli is a transporter with a broad substrate spectrum. J. Bacteriol. 187:1604–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hantke K. 2005. Bacterial zinc uptake and regulators. Curr. Opin. Microbiol. 8:196–202 [DOI] [PubMed] [Google Scholar]
- 21. Janulczyk R., Pallon J., Bjorck L. 1999. Identification and characterization of a Streptococcus pyogenes ABC transporter with multiple specificity for metal cations. Mol. Microbiol. 34:596–606 [DOI] [PubMed] [Google Scholar]
- 22. Jensen P. R., Hammer K. 1993. Minimal requirements for exponential growth of Lactococcus lactis. Appl. Environ. Microbiol. 59:4363–4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kloosterman T. G., van der Kooi-Pol M. M., Bijlsma J. J., Kuipers O. P. 2007. The novel transcriptional regulator SczA mediates protection against Zn2+ stress by activation of the Zn2+-resistance gene czcD in Streptococcus pneumoniae. Mol. Microbiol. 65:1049–1063 [DOI] [PubMed] [Google Scholar]
- 24. Kloosterman T. G., Witwicki R. M., van der Kooi-Pol M. M., Bijlsma J. J., Kuipers O. P. 2008. Opposite effects of Mn2+ and Zn2+ on PsaR-mediated expression of the virulence genes pcpA, prtA, and psaBCA of Streptococcus pneumoniae. J. Bacteriol. 190:5382–5393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee J. W., Helmann J. D. 2007. Functional specialization within the Fur family of metalloregulators. Biometals 20:485–499 [DOI] [PubMed] [Google Scholar]
- 26. Lee L. J., Barrett J. A., Poole R. K. 2005. Genome-wide transcriptional response of chemostat-cultured Escherichia coli to zinc. J. Bacteriol. 187:1124–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Y., Hugenholtz J., Abee T., Molenaar D. 2003. Glutathione protects Lactococcus lactis against oxidative stress. Appl. Environ. Microbiol. 69:5739–5745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Llull D., Poquet I. 2004. New expression system tightly controlled by zinc availability in Lactococcus lactis. Appl. Environ. Microbiol. 70:5398–5406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loo C. Y., Mitrakul K., Voss I. B., Hughes C. V., Ganeshkumar N. 2003. Involvement of the adc operon and manganese homeostasis in Streptococcus gordonii biofilm formation. J. Bacteriol. 185:2887–2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma Z., Jacobsen F. E., Giedroc D. P. 2009. Coordination chemistry of bacterial metal transport and sensing. Chem. Rev. 109:4644–4681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moore C. M., Gaballa A., Hui M., Ye R. W., Helmann J. D. 2005. Genetic and physiological responses of Bacillus subtilis to metal ion stress. Mol. Microbiol. 57:27–40 [DOI] [PubMed] [Google Scholar]
- 32. Morello E., et al. 2008. Lactococcus lactis, an efficient cell factory for recombinant protein production and secretion. J. Mol. Microbiol. Biotechnol. 14:48–58 [DOI] [PubMed] [Google Scholar]
- 33. Nies D. H., Grass G. 1 October 2009, posting date. Chapter 5.4.4.3 Transition metal homeostasis. In Böck A., Curtiss R., III, Kaper J. B., Karp P. D., Neidhardt F. C., Nyström T., Slauch J. M., Squires C. L., Ussery D. (ed.), EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC: doi:10.1128/ecosal.4.7.4 [Google Scholar]
- 34. Ogunniyi A. D., et al. 2009. Pneumococcal histidine triad proteins are regulated by the Zn2+-dependent repressor AdcR and inhibit complement deposition through the recruitment of complement factor H. FASEB J. 23:731–738 [DOI] [PubMed] [Google Scholar]
- 35. Outten C. E., O'Halloran T. V. 2001. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292:2488–2492 [DOI] [PubMed] [Google Scholar]
- 36. Outten C. E., Outten F. W., O'Halloran T. V. 1999. DNA distortion mechanism for transcriptional activation by ZntR, a Zn(II)-responsive MerR homologue in Escherichia coli. J. Biol. Chem. 274:37517–37524 [DOI] [PubMed] [Google Scholar]
- 37. Outten C. E., Tobin D. A., Penner-Hahn J. E., O'Halloran T. V. 2001. Characterization of the metal receptor sites in Escherichia coli Zur, an ultrasensitive zinc(II) metalloregulatory protein. Biochemistry 40:10417–10423 [DOI] [PubMed] [Google Scholar]
- 38. Panina E. M., Mironov A. A., Gelfand M. S. 2003. Comparative genomics of bacterial zinc regulons: enhanced ion transport, pathogenesis, and rearrangement of ribosomal proteins. Proc. Natl. Acad. Sci. U. S. A. 100:9912–9917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Patzer S. I., Hantke K. 2000. The zinc-responsive regulator Zur and its control of the znu gene cluster encoding the ZnuABC zinc uptake system in Escherichia coli. J. Biol. Chem. 275:24321–24332 [DOI] [PubMed] [Google Scholar]
- 40. Patzer S. I., Hantke K. 1998. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol. Microbiol. 28:1199–1210 [DOI] [PubMed] [Google Scholar]
- 41. Poquet I., Ehrlich S. D., Gruss A. 1998. An export-specific reporter designed for gram-positive bacteria: application to Lactococcus lactis. J. Bacteriol. 180:1904–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Potier N., Rogniaux H., Chevreux G., Van Dorsselaer A. 2005. Ligand-metal ion binding to proteins: investigation by ESI mass spectrometry. Methods Enzymol. 402:361–389 [DOI] [PubMed] [Google Scholar]
- 43. Raya R., Bardowski J., Andersen P. S., Ehrlich S. D., Chopin A. 1998. Multiple transcriptional control of the Lactococcus lactis trp operon. J. Bacteriol. 180:3174–3180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reyes-Caballero H., et al. 2010. The metalloregulatory zinc site in Streptococcus pneumoniae AdcR, a zinc-activated MarR family repressor. J. Mol. Biol. 403:197–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rogniaux H., et al. 1999. Binding of aldose reductase inhibitors: correlation of crystallographic and mass spectrometric studies. J. Am. Soc. Mass Spectrom. 10:635–647 [DOI] [PubMed] [Google Scholar]
- 46. Strand T. A., et al. 2003. Effects of zinc deficiency and pneumococcal surface protein A immunization on zinc status and the risk of severe infection in mice. Infect. Immun. 71:2009–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Strupat K., Rogniaux H., Van Dorsselaer A., Roth J., Vogl T. 2000. Calcium-induced noncovalently linked tetramers of MRP8 and MRP14 are confirmed by electrospray ionization-mass analysis. J. Am. Soc. Mass Spectrom. 11:780–788 [DOI] [PubMed] [Google Scholar]
- 48. Terzaghi B. E., Sandine W. E. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Trémillon N., et al. 2010. Production and purification of staphylococcal nuclease in Lactococcus lactis using a new expression-secretion system and a pH-regulated mini-reactor. Microb. Cell Fact. 9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Waldron K. J., Robinson N. J. 2009. How do bacterial cells ensure that metalloproteins get the correct metal? Nat. Rev. Microbiol. 7:25–35 [DOI] [PubMed] [Google Scholar]
- 51. Wilkinson S. P., Grove A. 2006. Ligand-responsive transcriptional regulation by members of the MarR family of winged helix proteins. Curr. Issues Mol. Biol. 8:51–62 [PubMed] [Google Scholar]
- 52. Zhang P., Allen J. C. 1995. A novel dialysis procedure measuring free Zn2+ in bovine milk and plasma. J. Nutr. 125:1904–1910 [DOI] [PubMed] [Google Scholar]
- 53. Zhu J., Winans S. C. 1998. Activity of the quorum-sensing regulator TraR of Agrobacterium tumefaciens is inhibited by a truncated, dominant defective TraR-like protein. Mol. Microbiol. 27:289–297 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.