Abstract
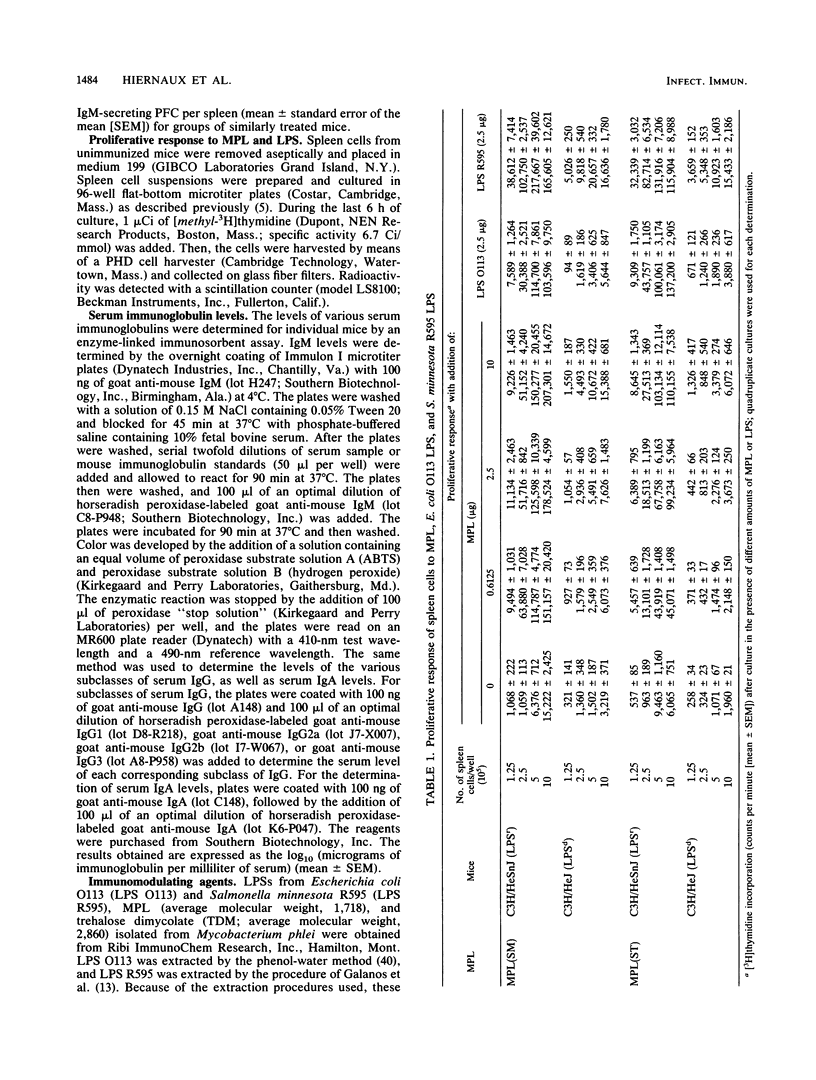
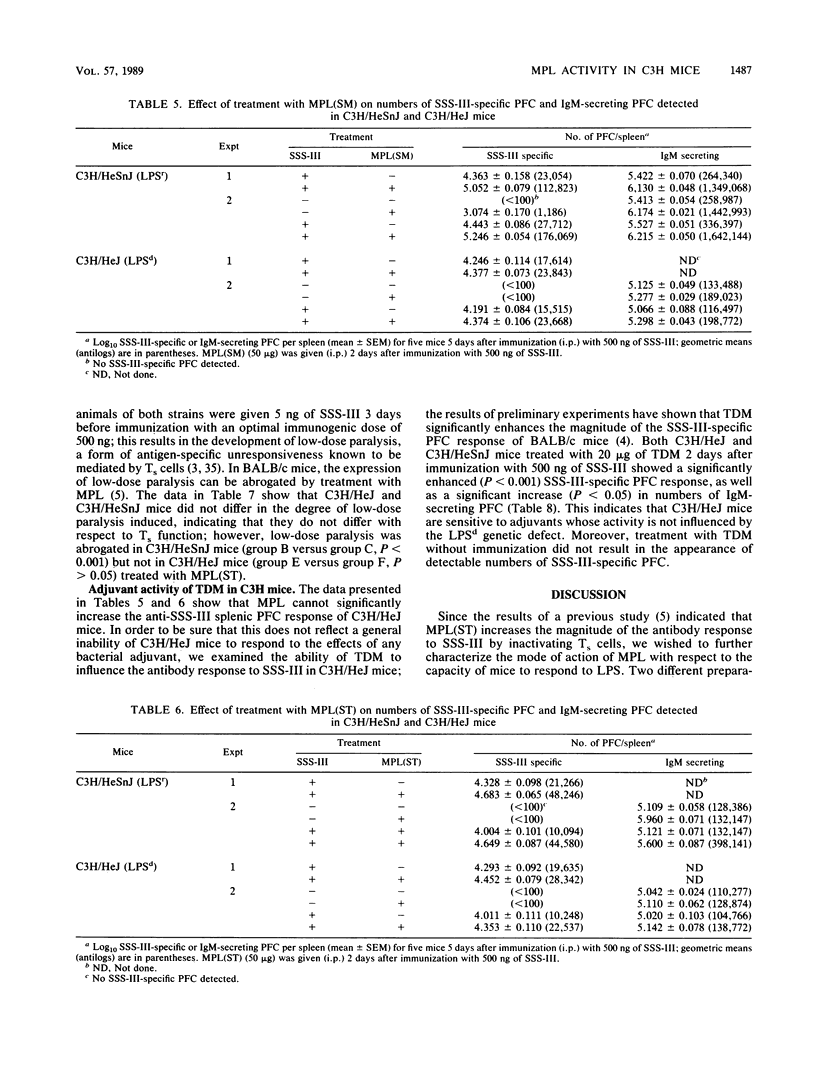
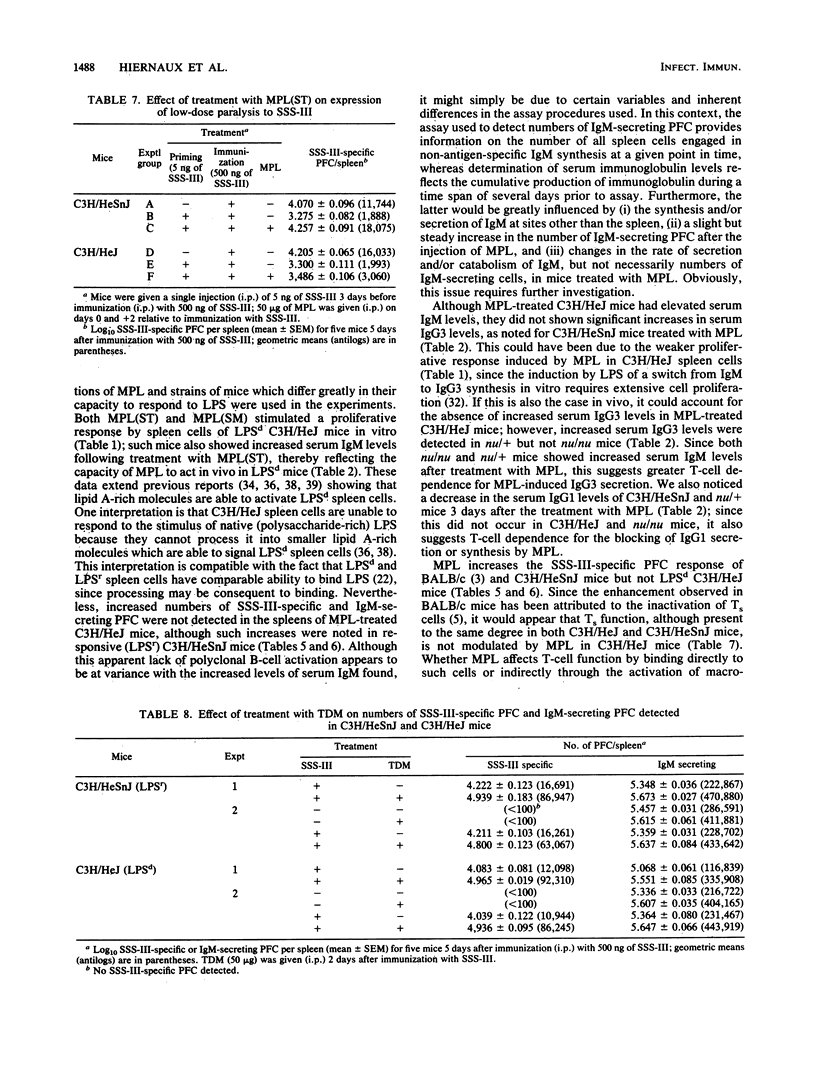
Treatment with nontoxic monophosphoryl lipid A (MPL) derived from a polysaccharide-deficient, heptoseless Re mutant of either Salmonella typhimurium or Salmonella minnesota R595 enhanced the immunoglobulin M (IgM) anti-type III pneumococcal polysaccharide (SSS-III) antibody response of C3H/HeSnJ mice. Such an adjuvant effect was not observed in lipopolysaccharide-nonresponder C3H/HeJ mice. Nevertheless, C3H/HeJ spleen cells produced a weak mitogenic response to both preparations of MPL in vitro, and C3H/HeJ mice showed a significant increase in serum IgM levels without an increase in numbers of splenic IgM-secreting plaque-forming cells after in vivo treatment with MPL. A significant increase in serum IgG3 levels was accompanied by a transient decrease in serum IgG1 levels in C3H/HeSnJ mice given MPL; such non-antigen-specific polyclonal effects were not observed in C3H/HeJ or in athymic nu/nu mice. Since the enhanced antibody response to SSS-III has been attributed to the inactivation of suppressor T cells by MPL and since suppressor-T-cell activity is demonstrable in both C3H/HeSnJ and C3H/HeJ mice, these findings imply that (i) the suppressor T cells of C3H/HeJ mice are refractory to inactivation by MPL and (ii) some of the polyclonal and mitogenic effects produced in C3H/HeJ mice are due to the direct action of MPL on B lymphocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander P., Evans R. Endotoxin and double stranded RNA render macrophages cytotoxic. Nat New Biol. 1971 Jul 21;232(29):76–78. doi: 10.1038/newbio232076a0. [DOI] [PubMed] [Google Scholar]
- Apte R. N., Galanos C., Pluznik D. H. Lipid A, the active part of bacterial endotoxins in inducing serum colony stimulating activity and proliferation of splenic granulocyte/macrophage progenitor cells. J Cell Physiol. 1976 Jan;87(1):71–78. doi: 10.1002/jcp.1040870110. [DOI] [PubMed] [Google Scholar]
- Baker P. H., Stashak P. W. Quantitative and qualitative studies on the primary antibody response to pneumococcal polysaccharides at ehe cellular level. J Immunol. 1969 Dec;103(6):1342–1348. [PubMed] [Google Scholar]
- Baker P. J., Amsbaugh D. F., Stashak P. W., Caldes G., Prescott B. Direct evidence for the involvement of T suppressor cells in the expression of low-dose paralysis to type III pneumococcal polysaccharide. J Immunol. 1982 Mar;128(3):1059–1062. [PubMed] [Google Scholar]
- Baker P. J., Fauntleroy M. B., Stashak P. W., Hiernaux J. R., Cantrell J. L., Rudbach J. A. Adjuvant effects of trehalose dimycolate on the antibody response to type III pneumococcal polysaccharide. Infect Immun. 1989 Mar;57(3):912–917. doi: 10.1128/iai.57.3.912-917.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Hiernaux J. R., Fauntleroy M. B., Prescott B., Cantrell J. L., Rudbach J. A. Inactivation of suppressor T-cell activity by nontoxic monophosphoryl lipid A. Infect Immun. 1988 May;56(5):1076–1083. doi: 10.1128/iai.56.5.1076-1083.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. I. Dose-response studies and the effect of prior immunization on the magnitude of the antibody response. Immunology. 1971 Apr;20(4):469–480. [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Prescott B. Use of erythrocytes sensitized with purified pneumococcal polysaccharides for the assay of antibody and antibody-producing cells. Appl Microbiol. 1969 Mar;17(3):422–426. doi: 10.1128/am.17.3.422-426.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A., Benner R., Björklund M., Forni L., Holmberg D., Ivars F., Martinez-A C., Pettersson S. A "trans" perspective on the control of immunoglobulin c gene expression. Immunol Rev. 1982;67:87–114. doi: 10.1111/j.1600-065x.1982.tb01057.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Gery I., Krüger J., Spiesel S. Z. Stimulation of B-lymphocytes by endotoxin. Reactions of thymus-deprived mice and karyotypic analysis of dividing cells in mice bearing T 6 T 6 thymus grafts. J Immunol. 1972 Apr;108(4):1088–1091. [PubMed] [Google Scholar]
- Gottlieb C. F. Application of transformations to normalize the distribution of plaque-forming cells. J Immunol. 1974 Jul;113(1):51–57. [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Hiernaux J. R., Jones J. M., Rudbach J. A., Rollwagen F., Baker P. J. Antibody response of immunodeficient (xid) CBA/N mice to Escherichia coli 0113 lipopolysaccharide, a thymus-independent antigen. J Exp Med. 1983 Apr 1;157(4):1197–1207. doi: 10.1084/jem.157.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson P. C., Puré E., Vitetta E. S., Krammer P. H. T cell-derived B cell differentiation factor(s). Effect on the isotype switch of murine B cells. J Exp Med. 1982 Mar 1;155(3):734–748. doi: 10.1084/jem.155.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izui S., Eisenberg R. A., Dixon F. J. Subclass-restricted IgG polyclonal antibody production in mice injected with lipid A-rich lipopolysaccharides. J Exp Med. 1981 Feb 1;153(2):324–338. doi: 10.1084/jem.153.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON A. G., GAINES S., LANDY M. Studies on the O antigen of Salmonella typhosa. V. Enhancement of antibody response to protein antigens by the purified lipopolysaccharide. J Exp Med. 1956 Feb 1;103(2):225–246. doi: 10.1084/jem.103.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. M., Amsbaugh D. F., Stashak P. W., Prescott B., Baker P. J., Alling D. W. Kinetics of the antibody response to type III pneumococcal polysaccharide. I. Evidence that suppressor cells function by inhibiting the recruitment and proliferation of antibody-producing cells. J Immunol. 1976 Mar;116(3):647–656. [PubMed] [Google Scholar]
- Lei M. G., Morrison D. C. Specific endotoxic lipopolysaccharide-binding proteins on murine splenocytes. I. Detection of lipopolysaccharide-binding sites on splenocytes and splenocyte subpopulations. J Immunol. 1988 Aug 1;141(3):996–1005. [PubMed] [Google Scholar]
- Lei M. G., Morrison D. C. Specific endotoxic lipopolysaccharide-binding proteins on murine splenocytes. II. Membrane localization and binding characteristics. J Immunol. 1988 Aug 1;141(3):1006–1011. [PubMed] [Google Scholar]
- Lüderitz O., Galanos C., Rietschel E. T. Endotoxins of Gram-negative bacteria. Pharmacol Ther. 1981;15(3):383–402. doi: 10.1016/0163-7258(81)90051-6. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosentreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by a macrophage cell line, P388D1. II. Biochemical characterization of LAF induced by activated T cells and LPS. J Immunol. 1978 May;120(5):1504–1508. [PubMed] [Google Scholar]
- Qureshi N., Mascagni P., Ribi E., Takayama K. Monophosphoryl lipid A obtained from lipopolysaccharides of Salmonella minnesota R595. Purification of the dimethyl derivative by high performance liquid chromatography and complete structural determination. J Biol Chem. 1985 May 10;260(9):5271–5278. [PubMed] [Google Scholar]
- Qureshi N., Takayama K., Ribi E. Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. J Biol Chem. 1982 Oct 10;257(19):11808–11815. [PubMed] [Google Scholar]
- Ribi E. Beneficial modification of the endotoxin molecule. J Biol Response Mod. 1984;3(1):1–9. [PubMed] [Google Scholar]
- Seppälä I. J., Mäkelä O. Adjuvant effect of bacterial LPS and/or alum precipitation in responses to polysaccharide and protein antigens. Immunology. 1984 Dec;53(4):827–836. [PMC free article] [PubMed] [Google Scholar]
- Severinson E., Bergstedt-Lindqvist S., van der Loo W., Fernandez C. Characterization of the IgG response induced by polyclonal B cell activators. Immunol Rev. 1982;67:73–85. doi: 10.1111/j.1600-065x.1982.tb01056.x. [DOI] [PubMed] [Google Scholar]
- Stevens T. L., Bossie A., Sanders V. M., Fernandez-Botran R., Coffman R. L., Mosmann T. R., Vitetta E. S. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988 Jul 21;334(6179):255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- Sultzer B. M., Castagna R. Inhibition of activated nonresponder C3H/HeJ lymphocytes by lipopolysaccharide endotoxin. Infect Immun. 1988 Dec;56(12):3040–3045. doi: 10.1128/iai.56.12.3040-3045.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. E., Amsbaugh D. F., Stashak P. W., Caldes G., Prescott B., Baker P. J. Cell surface antigens and other characteristics of T cells regulating the antibody response to type III pneumococcal polysaccharide. J Immunol. 1983 Jan;130(1):19–23. [PubMed] [Google Scholar]
- Tomai M. A., Johnson A. G., Ribi E. Glycolipid induced proliferation of lipopolysaccharide hyporesponsive C3H/HeJ splenocytes. J Leukoc Biol. 1988 Jan;43(1):11–17. doi: 10.1002/jlb.43.1.11. [DOI] [PubMed] [Google Scholar]
- Tomai M. A., Solem L. E., Johnson A. G., Ribi E. The adjuvant properties of a nontoxic monophosphoryl lipid A in hyporesponsive and aging mice. J Biol Response Mod. 1987 Apr;6(2):99–107. [PubMed] [Google Scholar]
- Vogel S. N., Madonna G. S., Wahl L. M., Rick P. D. In vitro stimulation of C3H/HeJ spleen cells and macrophages by a lipid A precursor molecule derived from Salmonella typhimurium. J Immunol. 1984 Jan;132(1):347–353. [PubMed] [Google Scholar]
- Vukajlovich S. W., Morrison D. C. Conversion of lipopolysaccharides to molecular aggregates with reduced subunit heterogeneity: demonstration of LPS-responsiveness in "endotoxin-unresponsive" C3H/HeJ splenocytes. J Immunol. 1983 Jun;130(6):2804–2808. [PubMed] [Google Scholar]



