Abstract
The filamentous cyanobacterium Anabaena (Nostoc) sp. strain PCC 7120 produces specialized cells for nitrogen fixation called heterocysts. Previous work showed that the group 2 sigma factor sigE (alr4249; previously called sigF) is upregulated in differentiating heterocysts 16 h after nitrogen step-down. We now show that the sigE gene is required for normal heterocyst development and normal expression levels of several heterocyst-specific genes. Mobility shift assays showed that the transcription factor NtcA binds to sites in the upstream region of sigE and that this binding is enhanced by 2-oxoglutarate (2-OG). Deletions of the region containing the NtcA binding sites in PsigE-gfp reporter plasmids showed that the sites contribute to normal developmental regulation but are not essential for upregulation in heterocysts. Northern RNA blot analysis of nifH mRNA revealed delayed and reduced transcript levels during heterocyst differentiation in a sigE mutant background. Quantitative reverse transcription-PCR (qRT-PCR) analyses of the sigE mutant showed lower levels of transcripts for nifH, fdxH, and hglE2 but normal levels for hupL. We developed a PnifHD-gfp reporter construct that showed strong heterocyst-specific expression. Time-lapse microscopy of the PnifHD-gfp reporter in a sigE mutant background showed delayed development and undetectable green fluorescent protein (GFP) fluorescence. Overexpression of sigE caused accelerated heterocyst development, an increased heterocyst frequency, and premature expression of GFP fluorescence from the PnifHD-gfp reporter.
INTRODUCTION
Bacteria can respond to environmental and intracellular conditions by regulation of gene expression, often via changes in transcriptional initiation of specific genes. Transcriptional regulation in bacteria is associated with two major families of sigma factors: sigma 70 (σ70), which is subdivided into four groups (19), and sigma 54 (σ54), which has no clear homologs in cyanobacteria or in Gram-positive bacteria with high GC contents (48). Sigma factors are often key regulators in complex responses such as bacterial development and biofilm formation. For example, in Bacillus subtilis, temporally and spatially regulated sigma factors control the expression of genes during starvation-induced sporulation (7, 28), and in the Gram-negative bacterium Pseudomonas aeruginosa, sigma factors control the expression of antibiotic and phagocyte resistance genes during biofilm formation (42).
In cyanobacteria, sigma factors have been shown to be involved in a variety of regulatory responses (24, 38). In this paper, we present data showing that the Anabaena (Nostoc) sp. strain PCC 7120 (here Anabaena PCC 7120) sigE gene (alr4249; previously called sigF), which encodes a group 2 σ70 family sigma factor, is required for the normal expression of some genes involved in heterocyst development. (We follow the revised nomenclature of Yoshimura et al. for cyanobacterial sigma factor names [3, 58].) The expression of sigE in the unicellular cyanobacterium Synechocystis sp. strain PCC 6803 increases after nitrogen depletion, and this response is dependent on the nitrogen regulator NtcA (35, 39); mobility shift assays using purified NtcA show an interaction with the sigE promoter region in vitro. Expression studies comparing a sigE null mutant with wild-type Synechocystis sp. strain PCC 6803 showed that the expression of one of the nitrogen assimilation genes, glnN, was impaired in the mutant strain (35). DNA microarray experiments with Synechocystis sp. strain PCC 6803 showed that the expression of genes involved in glycolysis, the oxidative pentose phosphate pathway, and glycogen catabolism was decreased in a sigE mutant strain, suggesting that sigE is involved in both nitrogen and sugar metabolism (41). Recent studies with Synechocystis sp. strain PCC 6803 showed that sigE is posttranslationally activated by the light-to-dark transition and that ChlH, the H subunit of Mg-chelatase, interacts with SigE in vivo and functions as an anti-sigma factor, transducing light signals to SigE in a process mediated by Mg2+ (40).
The multicellular cyanobacterium Anabaena PCC 7120 is a well-studied heterocyst-forming strain with tractable genetics and a fully sequenced genome. Heterocyst development is an established model for the study of bacterial differentiation, pattern formation, and nitrogen fixation (2, 17, 29). Anabaena PCC 7120 grows as long chains of photosynthetic vegetative cells in media containing combined nitrogen. In media lacking combined nitrogen, heterocysts differentiate from vegetative cells to form a semiregular pattern of single heterocysts every 10 to 20 vegetative cells along filaments. Heterocysts are capable of fixing nitrogen, which they supply to vegetative cells in the form of amino acids, and the vegetative cells supply fixed carbon to the heterocysts (17, 29). Heterocyst development can be synchronously induced by nitrogen step-down, i.e., the transfer of filaments to a medium lacking combined nitrogen.
Heterocysts are morphologically and physiologically differentiated from vegetative cells and exhibit large-scale changes in gene expression. One of the earliest steps in heterocyst differentiation is the increased expression of ntcA, which is an autoregulatory transcription factor belonging to the cyclic AMP (cAMP) receptor protein (CRP) family (22). NtcA together with HetR, a master regulator for heterocyst differentiation, directly or indirectly regulates the expression of many genes involved in heterocyst differentiation and the assimilation of atmospheric nitrogen (17, 29, 30, 36, 37, 54, 59). NtcA activity is modulated by 2-oxoglutarate (2-OG), which is an intermediate in the incomplete Krebs pathway present in cyanobacteria, and its increase constitutes the signal for nitrogen limitation.
During heterocyst differentiation, a polysaccharide layer is deposited around the proheterocyst, followed by the deposition of a glycolipid layer between the outer membrane and the polysaccharide layer (4). Synthesis and deposition of the polysaccharide layer requires the expression of a cluster of genes that constitute the HEP island (23). The expression of these genes is upregulated during the early stages of heterocyst differentiation. Synthesis of the glycolipid layer requires the expression of hglB, hglC, hglD, hglE (hglEA), hglK, and devBCA, which are all upregulated early during heterocyst differentiation (4, 15, 25). A second cluster of hgl genes includes the hglE2 gene (all1646), which shows devH-dependent upregulation after heterocyst induction (45). The morphologically differentiated heterocysts become micro-oxic partly because these layers limit oxygen diffusion into the heterocyst (16). In addition, heterocysts increase respiration to decrease oxygen levels (51).
Complete maturation of heterocysts involves the expression of genes in the late stages of heterocyst development that are necessary for nitrogen fixation and assimilation into amino acids. The nifHDK operon encodes the nitrogenase polypeptides and is upregulated 12 to 18 h after nitrogen step-down in heterocysts (14, 18). Other late heterocyst-specific genes include fdxH, which encodes a [2Fe-2S] ferredoxin (33), and hupL, which encodes the large subunit of uptake hydrogenase (9, 10). These genes are regulated during heterocyst development and potentially by oxygen or metabolite levels in heterocysts, but the factors required for the regulation of these late genes are not yet known.
The role of sigma factors in transcriptional regulation during heterocyst development has been studied for many years. Northern RNA blot analysis showed that two sigma factor genes, sigB and sigC, are upregulated 12 h after nitrogen step-down (6). However, single and double mutations of sigB and sigC showed that they are not essential for heterocyst differentiation or diazotrophic growth. In later work, insertional inactivation of sigC, sigF, sigB2, sigD, or sigE showed that the mutants form heterocysts and can grow on nitrate or diazotrophically but that sigB2 and sigD mutants are significantly slow to establish diazotrophic growth (27). A sigB2 sigD double mutant can form proheterocysts but is unable to grow diazotrophically, possibly due to extensive fragmentation of filaments upon nitrogen deprivation (27). The failure to find any single sigma factor that is essential for heterocyst development suggests that the cyanobacterial group 2 sigma factors have partially overlapping functions, which has hampered genetic analysis (3, 27, 32, 39, 58).
A more recent study of Anabaena PCC 7120 used gfp transcriptional reporter fusions to analyze the spatial and temporal patterns of expression for all sigma factor genes located on the chromosome except sigA (3). These studies found that three sigma factor genes (two group 2 sigma factor genes, sigC and sigE, and one representative of group 4, sigG) are upregulated in heterocysts after induction of heterocyst development (3). Expression of sigC is upregulated by 4 h after nitrogen step-down, suggesting that it may be involved in the early stages of heterocyst development. The sigG gene is expressed in vegetative cells grown in nitrogen-containing media, but after nitrogen step-down its expression in vegetative cells decreases, and by 10 h individual differentiating cells show increased expression. These results suggest that SigG may be involved in the expression of middle-stage heterocyst genes in addition to genes in vegetative cells grown on nitrate. The expression of sigE is localized to differentiating heterocysts at around 16 h after nitrogen step-down, suggesting that SigE might be important for the regulation of genes that are expressed in the late stages of heterocyst development.
In this work, we present evidence that sigE is required for normal heterocyst development and the normal expression of the heterocyst-specific nifH, fdxH, and hglE2 genes.
MATERIALS AND METHODS
Strains and culture conditions.
The strains and plasmids used in this study are listed in Table 1. Anabaena PCC 7120 and its derivatives were grown in BG-11 or BG-110, which lacks sodium nitrate, medium at 30°C with illumination of approximately 75 μmol photons m−2 s−1 from fluorescent lights as previously described (18, 46). Escherichia coli strains were grown in LB (Lennox L) medium containing appropriate antibiotics at 37°C. E. coli strain DH10B was used for all cloning experiments. Shuttle plasmids and suicide plasmids were transferred into E. coli donor strain AM1359 (56) by electroporation and transferred to Anabaena PCC 7120 strains by conjugation using standard protocols (13) with some modifications (27).
Table 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Anabaena strains | ||
| PCC 7120 | Wild-type Anabaena sp. strain PCC 7120 | R. Haselkorn |
| AMC649 | PCC 7120 sigE::luxAB-Smr/Spr cassette at ClaI site of sigE; Smr Spr | 27 |
| AMC1452 | PCC 7120 carrying PsigE-gfpmut2 on pAM3652; Kmr Nmr | 3 |
| AMC1773 | PCC 7120 carrying PpetE-sigE on pAM3961; Kmr Nmr | This study |
| AMC1774 | PCC 7120 with integrated pRL277-PnifHD-gfpmut2; Smr Spr | This study |
| AMC1775 | PCC 7120 with integrated pRL277-PnifHD-gfpmut2 and PpetE-sigE on pAM3961; Smr Spr Kmr Nmr | This study |
| AMC1776 | PCC 7120 with integrated pRL278-PnifHD-gfpmut2; Kmr Nmr | This study |
| AMC1777 | AMC649 carrying pRL278-PnifHD-gfpmut2; Kmr Nmr | This study |
| AMC1789 | PCC 7120 carrying PsigE-P1-gfpmut2 on pAM4469; Kmr Nmr | This study |
| AMC1790 | PCC 7120 carrying PsigE-P2-gfpmut2 on pAM4468; Kmr Nmr | This study |
| AMC1791 | PCC 7120 carrying PsigE-P3-gfpmut2 on pAM4467; Kmr Nmr | This study |
| AMC1792 | PCC 7120 carrying PsigE-P4-gfpmut2 on pAM4466; Kmr Nmr | This study |
| Plasmids | ||
| pAM1956 | Shuttle vector pAM505 containing promoterless gfpmut2 with unique upstream cloning sites SalI, SacI, KpnI, ACC651, and SmaI; Kmr Nmr | 56 |
| pAM2770 | Shuttle vector containing XhoI-PpetE-NdeI-lacZα-SapI(Cys)-6His (stop)-ClaI; Kmr Nmr | 31 |
| pAM3652 | pAM1956 containing 800-bp fragment upstream of sigE (alr4249), PsigE-gfpmut2 | 3 |
| pAM3929 | ntcA cloned into expression plasmid pET-28b+ in EcoRI and XhoI sites | This study |
| pAM3961 | sigE (ORF alr4249) cloned into pAM2770 containing PpetE in NdeI and XmaI sites; Kmr Nmr | This study |
| pAM4466 | pAM1956 containing 79-bp fragment upstream of sigE (alr4249), PsigE-P4-gfpmut2; Kmr Nmr | This study |
| pAM4467 | pAM1956 containing 260-bp fragment upstream of sigE (alr4249), PsigE-P3-gfpmut2; Kmr Nmr | This study |
| pAM4468 | pAM1956 containing 360-bp fragment upstream of sigE (alr4249), PsigE-P2-gfpmut2; Kmr Nmr | This study |
| pAM4469 | pAM1956 containing 500-bp fragment upstream of sigE (alr4249), PsigE-P1-gfpmut2; Kmr Nmr | This study |
| pRL277 | Conjugal suicide plasmid; Smr Spr | 13 |
| pRL278 | Conjugal suicide plasmid; Kmr Nmr | 13 |
The sigE mutant strain AMC649 was revived from a frozen stock and confirmed to show the previously reported phenotype (27). In addition, the sigE expression plasmid pAM3961 was transferred by conjugation into AMC649, and the resulting strain showed complementation of the delayed heterocyst differentiation phenotype.
DNA manipulations and plasmid constructions.
Standard protocols were used for cloning, E. coli transformation, and PCR. Primers used in this study are listed in Table 2. Total DNA from Anabaena PCC 7120 was isolated as previously described (27). DNA sequencing of plasmid inserts was performed by the Gene Technologies Laboratory (Texas A&M University) following the Big Dye sequencing protocol (Applied Biosystems) and by Genewiz (La Jolla, CA).
Table 2.
DNA primers used in this study
| Gene and expt | Forward primer |
Reverse primer |
||
|---|---|---|---|---|
| Name | Sequence (5′→3′) | Name | Sequence (5′→3′) | |
| sigE, probe | AMO-2222 | CCTATTCCCGGGCTCAAATCAACCAACC | AMO-2223 | AACAGTCATATGGAAATTATGTACCAAAC |
| sigE, upstream for EMSA | RAM-Fw | CTTGAATTCTAAAGATAAATATTCTTATTGCC | RAM-Rv | GTGGAATTCAAATATTTATTGCACGTAT |
| ntcA, overexpression | AMO-2026 | CCGGAATTCATCGTGACACAAGATAAG | AMO-2027 | TAACCTCGAGTTAAGTGAACTGTCTGCTG |
| PsigE-P1 | AMO-2558 | GTCGTCGACTTTTATACAAACAATTTTGTG | AMO-2554 | GGTGGTACCTTGAGGGATTCATGCTTT |
| PsigE-P2 | AMO-2557 | GTCGTCGACTTTATAAAAGTATATAATTATT | AMO-2554 | GGTGGTACCTTGAGGGATTCATGCTTT |
| PsigE-P3 | AMO-2556 | GTCGTCGACAGATTAAATGATTAATAAA | AMO-2554 | GGTGGTACCTTGAGGGATTCATGCTTT |
| PsigE-P4 | AMO-2555 | GTCGTCGACATGAATCTGAAAATTGCT | AMO-2554 | GGTGGTACCTTGAGGGATTCATGCTTT |
| nifH, probe | AMO-622 | TTCACGGTCAACCTTACGG | AMO-1038 | CGGTAAAGGCGG |
| Site-directed mutagenesis of PsigE-gfp | AMO-2217 | GTATTTTGAATGCAGATTTTATTC | AMO-2218 | GAATAAAATCTGCATTCAAAATAC |
| nifH, qRT-PCR | AMO-2185 | GCACAAGAAATCTACATC | AMO-2186 | TACGAAGTGAATCATTTG |
| hupL, qRT-PCR | AMO-2189 | TGCTTCTCACTTAACCTCTG | AMO-2190 | GTCAATGGCGAACAATCC |
| fdxH, qRT-PCR | AMO-2191 | TACCAAGTTAGATTGATC | AMO-2192 | AAGTAACACAAAGTAGAG |
| hglE2, qRT-PCR | AMO-2197 | TTGTTGAGGTGTATTGAG | AMO-2198 | CAGGAACATCAGTGATAC |
| rpoA, qRT-PCR | AMO-2204 | AGTTTGGACAAATGGTAG | AMO-2205 | GCTTGAGACAGTTATAGG |
A sigE overexpression plasmid was constructed by PCR amplification of sigE (alr4249) with primers containing an NdeI site at the 5′ end and an XmaI site at the 3′ end (AMO-2222 and AMO-2223) and then ligation of the fragment into the same sites in pAM2770, which contains a copper-inducible petE promoter.
Site-directed mutagenesis of the consensus NtcA binding site in the sigE promoter was conducted using standard protocols (26). Primers AMO-2217 and AMO-2218 were used to mutate the site in pAM3652 (PsigE-gfp).
Plasmids containing gfpmut2 transcriptional fusions with sigE upstream fragments (pAM3652, pAM4466, pAM4467, pAM4468, and pAM4469) were constructed in shuttle plasmid pAM1956 (Table 1). The sigE upstream fragments P1, P2, P3, and P4 were PCR amplified from total genomic DNA with primers shown in Table 2. The resulting fragments contained engineered SalI and Acc65I sites and were inserted at the same sites in pAM1956.
Electrophoretic mobility shift assay (EMSA).
A 6× His-tagged NtcA protein was overexpressed from the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible plasmid pET-28b+ and purified using a HisTrap HP 1-ml nickel column (GE Healthcare) by standard procedures. A 207-bp DNA fragment upstream from the sigE gene containing a putative consensus NtcA binding site was amplified by PCR using oligonucleotides RAM-Fw and RAM-Rv, each containing an engineered EcoRI site at the 5′ end. The PCR product was digested with EcoRI and labeled with [α-32P]dATP (3,000 Ci mmol−1) by fill-in using the DNA polymerase Klenow fragment. Binding assays were carried out in a final volume of 10 μl containing 10 mM Tris-HCl (pH 7.5), 50 mM KCl, 1 mM dithiothreitol, 5 ng μl−1 bovine serum albumin, 12.5% (vol/vol) glycerol, and 10 fmol of the DNA fragment; the reaction mixture was supplemented with 2-oxoglutarate (0.6 mM) where indicated. The labeled probe was incubated with 1 to 4 pmol of purified 6× His-NtcA protein for 30 min at room temperature. DNA fragments were separated by electrophoresis on a nondenaturing 5% polyacrylamide gel, vacuum dried, and visualized by phosphorimaging (Fujifilm). Negative controls for binding included DNA fragments from the hetR and sigC upstream regions (not shown).
RNA isolation.
A RiboPure-Bacteria kit (Ambion) was used to isolate total RNA from Anabaena PCC 7120 and its derivates. One flask was grown for each time point sample for each strain. Filaments from growing BG-11 cultures were inoculated into 250-ml flasks containing 100 ml of BG-11(NH4+), which is BG-110 supplemented with 2.5 mM ammonium chloride and 5 mM MOPS (morpholineethanesulfonic acid) (pH 8.0) to obtain a starting optical density at 750 nm (OD750) of 0.025 to 0.035. These cultures were grown on an orbital shaker under standard conditions overnight to an OD750 of 0.05 to 0.075, and then the cells were collected at room temperature by centrifugation in 50-ml conical tubes, washed twice with BG-110, and transferred to 100 ml of BG-110 in 250-ml flasks. The flasks were incubated under standard conditions for the appropriate time after nitrogen step-down. Each culture was then poured into a conical 250-ml centrifuge tube containing 100 g of crushed ice to rapidly chill the sample, and the filaments were collected by centrifugation at 5,000 × g for 10 min at 4°C. The cell pellet was immediately frozen at −80°C and stored until RNA was isolated.
Northern RNA blot analysis.
For each sample, 10 μg of total RNA was separated on a 1.5% agarose denaturing formaldehyde gel in MOPS buffer (47) and then transferred by capillary action to a Magnacharge nylon membrane (GE Osmonics) with 10× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]) (18). DNA probes were amplified by PCR with appropriate primers (Table 2), labeled with [α-32P]dCTP by random-primer labeling, and purified on Micro Bio-Spin P-30 columns (Bio-Rad). Blots were blocked, hybridized with radioactively labeled DNA probes, and washed using standard protocols (47) and then were exposed to a phosphorimager plate and scanned with a BAS-5000 phosphorimager (Fujifilm).
Time-lapse microscopy.
Time-lapse microscopy was used to record differential interference contrast (DIC), autofluorescence of photosynthetic pigments, and green fluorescent protein (GFP) fluorescence images during heterocyst development. Filaments of each reporter strain were grown in nitrate-containing BG-11 medium to an OD750 of 0.2 to 0.3, washed with BG-110, and resuspended in a small volume of BG-110 for heterocyst induction. Ten microliters of induced Anabaena filaments were placed into a single-chamber cover glass (Lab-Tek chamber slide system; Nalge Nunc International) and covered with a thin agarose pad supported by a dialysis membrane. The agarose pad was made by slicing open a dialysis tube (1.5 cm by 2.5 cm) (VWR Scientific; molecular mass cutoff, 3,500 kDa) that had been equilibrated in BG-110, laying the dialysis membrane on a glass slide, and then covering the membrane with 300 μl of warm filtered BG-110 containing 0.7% agarose. After the agarose cooled and solidified, small square sections (about 0.4 by 0.4 cm) of the agarose pad were cut and used to cover the induced filaments in the single-chamber cover glass. Excess liquid medium was removed, and the agarose pad was then carefully surrounded on all four sides by a total of about 2 ml of warm BG-110 containing 0.7% agarose in order to maintain moisture in the thin agarose pad. For time-lapse microscopy, the chamber was covered with its plastic lid.
Microscopy was performed with a DeltaVision Core system (Applied Precision) with a WeatherStation attached to an Olympus IX71 inverted microscope. The temperature was adjusted to 30°C. softWoRx software was used to acquire time-lapse images for 24 to 42 h with a 15-min time delay using a 40× objective. The time-lapse series was started approximately 10 to 20 min after nitrogen step-down. The Anabaena filaments were illuminated to support their growth during time-lapse microscopy with approximately 75 μmol photons m−2 s−1 from a fiber optic LED white light source (Schott IFC 60825) attached to the DeltaVision Core system and controlled by softWoRx software, which controlled switching between the external white light and the microscope white light and excitation light for taking images. For all images, a polychroic beam splitter was used. The DIC exposure time was 0.05 s. For autofluorescence images, a tetramethyl rhodamine isocyanate (TRITC) filter set was used (EX555/EM617) with an exposure time of 0.05 s, and for GFP images, a Chroma GFP filter set was used (HQ EX470/EM515) with a 0.5-s exposure time. Time-lapse images were processed using softWoRx software, and individual images from specific time points were exported as required.
Scoring of the heterocyst spacing pattern along filaments was performed essentially as previously described (57). Detached single heterocysts and aggregates of heterocysts were not scored. Because the strains AMC1774 and AMC1775 contained a nifHD-gfp reporter, GFP fluorescence was used as an additional character to identify proheterocysts. Strain AMC1777 was scored only by morphology because the nifHD-gfp reporter did not produce detectable GFP fluorescence in the sigE mutant background.
qRT-PCR analysis.
Quantitative real-time reverse transcription-PCR (qRT-PCR) analysis was carried out with a StepOnePlus 96-well qRT-PCR system (Applied Biosystems) and Power SYBR green PCR master mix (Applied Biosystems) mixed with 100 pmol/μl of each primer and 50 ng of total cDNA. All primers used in this study are listed in Table 2. Primers for nifH, fdxH, hupL, hglE2, and rpoA were designed with Vector NTI software (Invitrogen), and each primer was analyzed using the BioBike web interface for sequence similarity searches against the Anabaena PCC 7120 genome. cDNA synthesis from total RNA samples (grown on nitrate or at 12, 24, and 36 h after nitrogen step-down) was performed with an iScript cDNA kit (Bio-Rad). All samples were run in triplicate using the following amplification conditions: 1 cycle of 2 min at 52°C and 10 min at 95°C and then 40 cycles of 95°C for 15 s and 60°C for 30 s. Cycle threshold (CT) values for each gene were averaged and normalized against rpoA.
RESULTS
sigE transcripts.
Northern RNA blot analysis of sigE was performed to determine transcript size and abundance at different developmental times. The Northern RNA blot results showed that sigE was upregulated after nitrogen step-down and that transcript levels were highest at 12 and 24 h (Fig. 1). The sigE transcripts were present as a smear, suggesting a short half-life, with an upper edge at around 2.5 to 3.0 kb, which is consistent with a monocistronic transcript. sigE transcript levels were decreased at 30 and 48 h after nitrogen step-down. Several attempts to map the 5′ ends of sigE transcripts using rapid amplification of 5′ cDNA ends (RACE) were unsuccessful, possibly because the transcripts were unstable.
Fig. 1.
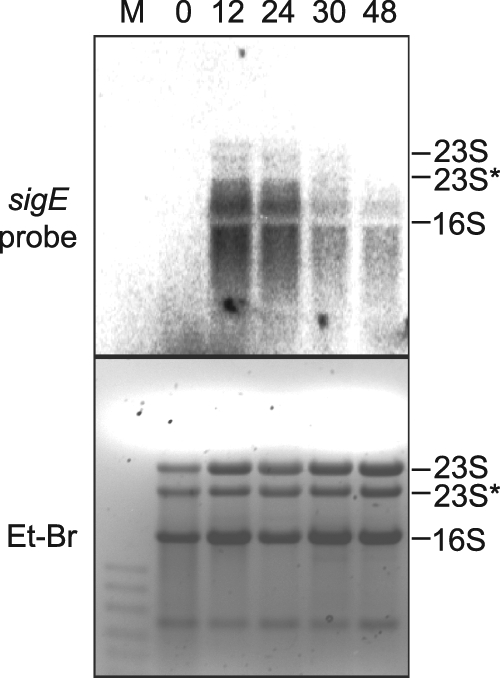
Northern RNA blot analysis of sigE transcripts during heterocyst development. Lanes are labeled with hours after nitrogen step-down from BG-11 to BG-110 medium. The upper panel shows a Northern RNA blot probed with a radioactive probe for sigE transcripts. The lower panel shows the ethidium bromide (Et-Br)-stained gel to show sample loading. Lane M, low-range ssRNA ladder (NEB) size marker.
RNA-Seq data from total RNA samples obtained at 0, 6, 12, and 21 h after nitrogen step-down were consistent with the Northern blot data, with RPKM (reads per kilobase of coding sequence [CDS] model per million mapped reads) values of 9.08, 8.90, 20.51, and 24.86, respectively (B. Flaherty, submitted for publication). The RNA-Seq data did not show a clear 5′ end for sigE transcripts, which is consistent with our RACE results. At all time points, staggered overlapping reads extended to about nucleotide −330 upstream of the first base of the open reading frame (ORF), with a gap in reads between about −60 and −140.
NtcA interacts with the upstream region of sigE in vitro.
It has been shown that some heterocyst-specific promoters carry an NtcA binding site with a consensus sequence of tGTAN8TACa, which is often located approximately 22 nucleotides upstream from a −10 TAN3T box (12, 21). Our bioinformatics analysis of the sigE promoter region identified a canonical NtcA binding site 22 nucleotides upstream of a TAN3T sequence, from position −683 to −698 relative to the sigE translational start site, which is in agreement with an independent analysis of NtcA binding sites in the Anabaena PCC 7120 genome (49). A second potential NtcA binding site that differs at one of the highly conserved bases is present at position −632 to −647. To determine if these sites interacted with NtcA, we performed electrophoretic mobility shift assays (EMSAs) using a radioactively labeled 207-bp PCR fragment containing both putative NtcA binding sites, purified 6× His-tagged NtcA protein, and the NtcA effector metabolite 2-oxoglutarate (2-OG). Figure 2 shows two band shifts of the labeled DNA fragment in the presence of increasing amounts of NtcA, indicating NtcA binding to this region at two sites. The affinity of NtcA for this promoter fragment increased in the presence of 2-OG, and the binding was significantly reduced in the presence of unlabeled competitor DNA.
Fig. 2.
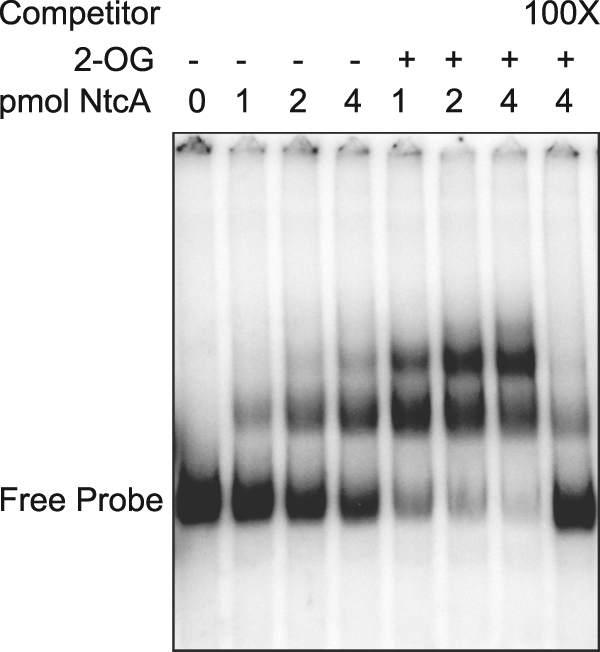
NtcA binds to the sigE upstream intergenic region. Binding of purified 6× His-tagged NtcA to a 207-bp fragment from the intergenic region upstream of sigE was assayed by electrophoretic mobility shift assay (EMSA). Sample mixtures contained 0.1 fmol of labeled DNA fragment with or without 0.6 mM 2-oxoglutarate (2-OG). The last lane contains a 100-fold molar excess of the unlabeled probe as a competitor.
To determine if the highly conserved NtcA binding site is required for developmentally regulated expression of sigE, the site was mutated in plasmid pAM3652, which carries PsigE-gfp. The NtcA binding site 5′-TGTA-N8-TAC-3′ in the sigE promoter region was replaced with 5′-GTGC-N8-TAC-3′, and the plasmid was introduced into wild-type Anabaena PCC 7120. The resulting strain, AMC1778, was analyzed by time-lapse microscopy after nitrogen step-down (data not shown). No significant differences in the pattern of GFP fluorescence were detected, showing that the conserved NtcA binding site is not essential for sigE expression in vivo, but this does not eliminate the possibility that the site contributes to normal expression.
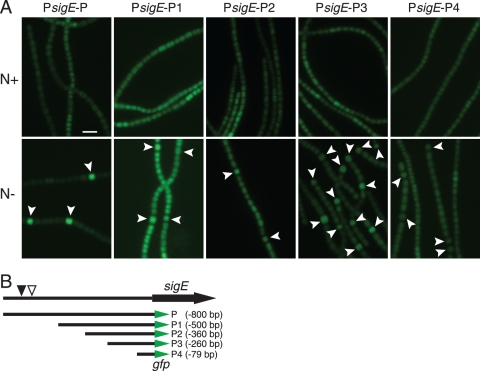
To identify the minimal upstream region required for heterocyst-specific sigE expression, a deletion series of four fragments was used to drive gfp reporter expression (Fig. 3). Reporter plasmids containing PsigE-P-gfp (−800 bp), PsigE-P1-gfp (−500 bp), PsigE-P2-gfp (−360 bp), PsigE-P3-gfp (−260 bp), and PsigE-P4-gfp (−79 bp) were transferred into wild-type Anabaena PCC 7120 to obtain reporter strains AMC1452, AMC1789, AMC1790, AMC1791, and AMC1792, respectively (Table 1). Observation of GFP fluorescence at 24 h after nitrogen step-down revealed that the strains containing PsigE-P-gfp, PsigE-P1-gfp,PsigE-P2-gfp, and PsigE-P3-gfp all showed higher levels of GFP fluorescence in heterocysts than in vegetative cells but that PsigE-P-gfp, which contained the entire upstream intergenic region, including the two NtcA binding sites, produced the strongest differential upregulation in heterocysts. The AMC1789 strain, containing PsigE-P1-gfp, showed an overall higher level of GFP fluorescence for unknown reasons, but overall, expression was higher in heterocysts than in vegetative cells. The strain containing PsigE-P4-gfp produced low-level expression in all cells. A strain carrying promoterless gfp on pAM1956 was dark. These data show that a 260-bp upstream region was sufficient to provide developmental regulation but that the entire upstream intergenic region, which includes the two NtcA binding sites, was required for robust regulation.
Fig. 3.
GFP reporter expression from different upstream regions of sigE. (A) GFP fluorescence micrographs of filaments for reporter strains grown with (N+, upper panels) and without (N−, lower panels) combined nitrogen for 24 h. (B) Map showing upstream regions of sigE driving the gfpmut2 reporter. Region P contains the entire 800-bp upstream intergenic region. The position of a conserved NtcA binding site is marked with a filled triangle, and a second potential binding site is marked with an open triangle. Heterocysts are indicated by arrowheads. Scale bar, 10 μm.
nifH expression is delayed and reduced in the sigE mutant strain.
To determine if SigE is required for expression of genes in the later stages of heterocyst development, we analyzed the expression of the nifH gene, which encodes the nitrogenase iron protein, in wild-type and sigE mutant backgrounds. Total RNAs from the wild-type strain and the sigE mutant strain AMC649 were isolated at different time points after nitrogen step-down and then analyzed by Northern RNA blotting. Figure 4 shows the expression of nifH in the wild type and the sigE mutant when grown in medium containing nitrate (labeled 0) and at 12, 24, 30, 36, and 48 h after nitrogen step-down. The nifH probe hybridizes with three transcripts of approximately 1.1, 2.8, and 4.7 kb, which contain the nifH, nifHD, and nifHDK open reading frames, respectively (18). Transcripts containing nifH in the wild-type background were detected at low levels at 12 h after nitrogen step-down and reached their highest level by 24 h. In the sigE mutant strain AMC649, nifH transcripts were detected only weakly at 24 h and 30 h after nitrogen step-down, increased at 36 h but to lower levels than the wild-type strain, and significantly decreased at 48 h. These results show that expression of nifH is delayed and reduced in the sigE mutant and, together with data showing that sigE is upregulated at later times in proheterocysts, suggest that SigE is required for normal levels of nifH expression.
Fig. 4.
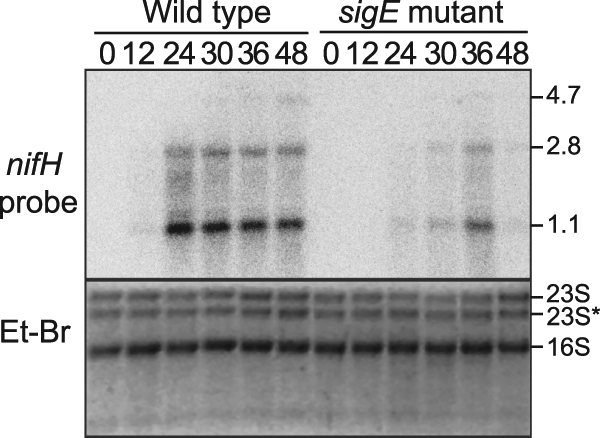
Northern RNA blot analysis of nifH transcripts from the wild-type strain and sigE mutant strain AMC649 during heterocyst development. Lanes are labeled with hours after nitrogen step-down from BG-11 to BG-110 medium. The upper panel shows a Northern RNA blot probed with a radioactive probe for the nifH transcript. The lower panel shows an ethidium bromide-stained gel to show sample loading. Three transcripts containing nifH were detected at approximately 1.1 kb, 2.8 kb, and 4.7 kb.
sigE influences the expression of a PnifHD-gfp reporter during heterocyst development.
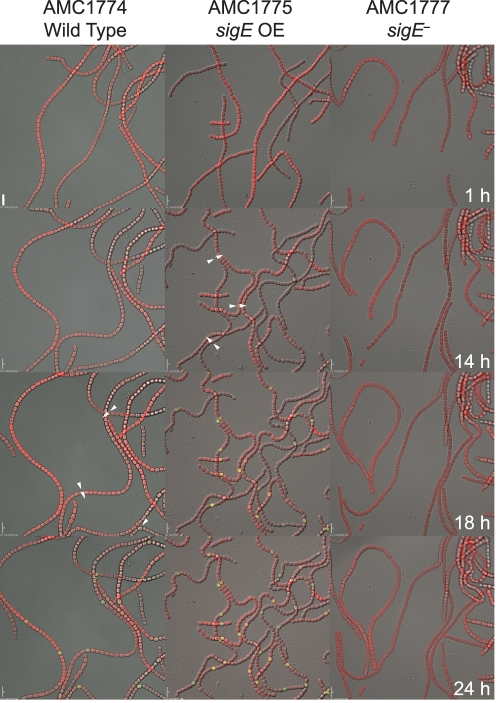
Time-lapse microscopy of heterocyst development was used to determine if sigE affects expression of a PnifHD-gfp reporter and/or the heterocyst pattern in vivo. Strains containing a PnifHD-gfp reporter integrated into the Anabaena PCC 7120 chromosome at the nifH locus were used for these studies. The PnifHD-gfp reporter strain was constructed and characterized by K. Kumar and will be published elsewhere; the strain shows normal diazotrophic growth, and the integrated plasmid contains the chromosome region from the start of the nifD ORF to the end of the nifS ORF. The PnifHD-gfp reporter was placed into three genetic backgrounds: wild type (AMC1774), a sigE knockout mutant (AMC1777), and a sigE overexpression strain (AMC1775). Heterocyst development was induced, and DIC, autofluorescence, and GFP fluorescence images were acquired every 15 min for 24 h for strains AMC1774 and AMC1775 and for 42 h for strain AMC1777. Figure 5 contains images corresponding to the indicated time points that were extracted from the time-lapse series (see Movies S1, S2, and S3 in the supplemental material).
Fig. 5.
Time-lapse microscopy images of a nifHD-gfp reporter construct integrated into the nifH locus on the chromosome in wild-type (AMC1774), sigE overexpression (OE) (AMC1775), and sigE mutant (AMC1777) backgrounds. Images are shown for 1, 14, 18, and 24 h after nitrogen step-down. Images are merged DIC (grayscale), autofluorescence (red), and GFP reporter fluorescence (green). Arrows indicate weak GFP fluorescence from proheterocysts. Scale bars, 10 μm.
At 1 h after nitrogen step-down, no GFP fluorescence or heterocyst morphological characteristics were evident in any of the three strains (AMC1774, AMC1775, and AMC1777). By 14 h, when morphological differentiation of proheterocysts was not yet obvious in the wild type, the sigE overexpression strain (AMC1775) showed GFP fluorescence in individual cells that were morphologically distinguishable as proheterocysts. AMC1774 and AMC1777 filaments did not show any GFP fluorescence or proheterocysts at 14 h after nitrogen step-down.
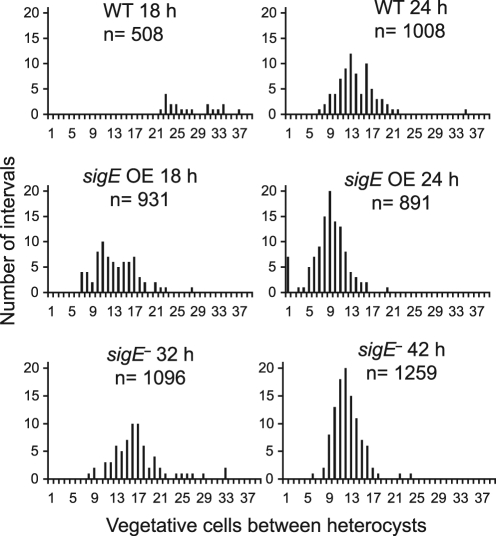
At 18 h after nitrogen step-down, the wild-type strain (AMC1774) showed GFP fluorescence in some individual cells, which is somewhat delayed compared to expression of nifH determined by Northern RNA blotting experiments (Fig. 4); this is likely due to differences in growth conditions between batch cultures and samples on the microscope and the time required to accumulate sufficient GFP to be detectable under our time-lapse microscopy conditions, which use low excitation intensity to avoid photobleaching or cell damage. For the wild-type strain at 18 h, slight morphological changes in the cells that showed GFP fluorescence were apparent, and the average number of vegetative cells in the interval between heterocysts or GFP-bright proheterocysts was 26.6 (Fig. 6). The sigE overexpression strain showed strong GFP fluorescence at 18 h and showed clear heterocyst morphological differentiation with an average interval of 12.4. In the sigE mutant strain (AMC1777), no GFP fluorescence or morphological differentiation was detected at 18 h after heterocyst induction.
Fig. 6.
The heterocyst pattern is influenced by sigE. The heterocyst pattern was determined for the strains shown in Fig. 5, which have a nifHD-gfp reporter in wild-type (AMC1774), sigE overexpression (AMC1775), and sigE mutant (AMC1777) backgrounds. For the wild-type (WT) and sigE overexpression (OE) backgrounds, the heterocyst pattern was scored by observing cell morphology and GFP fluorescence at 18 and 24 h after nitrogen step-down. For the sigE mutant background, which had delayed development and very low levels of nifHD-gfp reporter expression, the heterocyst pattern was scored by cell morphology at 32 and 42 h after nitrogen step-down. For each sample, the number of vegetative cells between heterocysts was scored, with adjacent heterocysts scored as an interval of zero. The x axis shows the number of intervals scored for each interval length. The average number of vegetative cells between heterocysts was 26.6 at 18 h and 13.1 at 24 h for the wild type, 12.4 at 18 h and 7.9 at 24 h for the sigE overexpression strain, and 15.8 at 32 h and 11.3 at 42 h for the sigE mutant.
At 24 h after nitrogen step-down, the PnifHD-gfp reporter in a wild-type background (AMC1774) showed GFP fluorescence in individual differentiated cells (Fig. 5) with an interval length of 13.1 vegetative cells (7.1% heterocysts) (Fig. 6). The sigE overexpression strain (AMC1775) showed a higher frequency of GFP-fluorescent heterocysts with an interval length of only 7.9 (11.2% heterocysts). These data show that extra copies of sigE caused accelerated heterocyst formation and expression of nifH and an increase in heterocyst frequency. At 24 h after nitrogen step-down, the sigE knockout mutant strain (AMC1777) did not show detectable GFP fluorescence or heterocyst morphological changes. By 32 h after nitrogen step-down, the sigE mutant showed morphological differentiation of some individual cells and an interval length of 15.8 (6.0% heterocysts), but no GFP fluorescence was evident. At 42 h, the sigE mutant had a pattern of mature heterocysts similar to that of the wild type (57) with an average interval length of 11.3 (8.1% heterocysts) (Fig. 6), but no GFP fluorescence was detectable even at this time.
The sigE gene is required for expression of other heterocyst-specific genes.
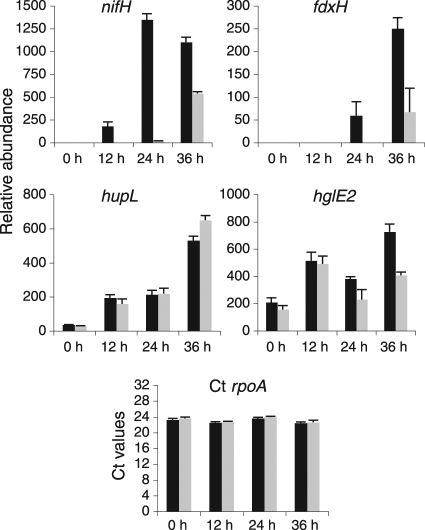
Previous observations of unsynchronized cultures of a sigE mutant strain (AMC649) found little effect on heterocyst development or diazotrophic growth (27), and in our current experiments, we observed no significant difference in the growth rates of sigE mutant strains compared to the wild type once diazotrophic growth was established. However, the sigE expression data (Fig. 1), the slight delay in heterocyst development (Fig. 5 and 6), and the delayed and reduced nifH expression (Fig. 4) observed in the sigE mutant suggest that SigE may be required for transcription of genes expressed in the middle to late stages of heterocyst differentiation. To determine if SigE is required for the expression of other heterocyst-specific genes, we used qRT-PCR to examine the expression levels of genes that are upregulated during middle and late stages of heterocyst development. We selected nifH, fdxH (encoding a heterocyst-specific ferredoxin), and hupL (encoding the large subunit of uptake hydrogenase) as genes that are expressed during late stages of heterocyst development (9, 52). For a gene expressed during the middle stages of heterocyst development, we selected hglE2 (all1646), which encodes a type I polyketide synthase potentially involved in heterocyst glycolipid synthesis (8, 15). For a baseline control mRNA, the expression of the housekeeping gene rpoA, which encodes the RNA polymerase alpha subunit, was analyzed by qRT-PCR during heterocyst development. rpoA message levels remained essentially invariant and at relatively low levels at all time points (Fig. 7); therefore, we used the rpoA message as an internal baseline control for our qRT-PCR experiments.
Fig. 7.
Quantitative reverse transcription-PCR (qRT-PCR) measurements of mRNA abundance in wild-type and sigE mutant strains. Relative mRNA abundance was determined for nifH, fdxH, hupL, and hglE2 using total RNA from wild-type Anabaena PCC 7120 (black bars) and the sigE mutant AMC649 (gray bars) at four time points after nitrogen step-down. The data represent average values and standard error measurements from three technical replicates, shown as percent relative abundance normalized to the RNA polymerase alpha subunit (rpoA) gene. The CT rpoA graph shows the average CT values for rpoA normalized against total RNA for wild-type Anabaena PCC 7120 and the sigE mutant AMC649.
Figure 7 shows the transcript levels of nifH, fdxH, hupL, and hglE2 relative to rpoA in wild-type and sigE mutant backgrounds during heterocyst induction. In medium containing nitrogen (0 h), nifH transcripts were not detected in either the wild type or the sigE mutant. At 12 h after nitrogen step-down, small amounts of nifH transcript were detected, but only in the wild-type strain. At 24 h a peak of nifH transcript was observed in the wild-type strain, while only trace amounts were detected in the sigE mutant strain. By 36 h, the levels of nifH transcript in the sigE mutant strain had increased to about half the wild-type transcript level. These results are in accordance with the data obtained by Northern RNA blot analysis (Fig. 4) and with the PnifHD-gfp reporter, which supports the hypothesis that SigE regulates the expression of nifH.
Expression of the fdxH gene was similar to that of nifH although somewhat delayed (Fig. 7). Message was first detected for wild-type filaments at 24 h and was abundant by 36 h, but for the sigE mutant strain, fdxH message was detectable at low levels in only the 36-h sample. These data suggest that SigE regulates the expression of fdxH during heterocyst development.
Although previous studies with Anabaena PCC 7120 showed expression of hupL only during the late stages of heterocyst development (9), hupL transcripts have been detected in ammonium-grown vegetative cells of Anabaena variabilis (5). Our present data show very low levels of hupL transcripts in samples from vegetative cells in nitrate-containing medium (0 h) and increasing levels at later stages of heterocyst development (Fig. 7). The hupL transcript levels were not significantly different for the sigE mutant strain and the wild type, indicating that SigE is not involved in the regulation of the hupSL operon.
For the hglE2 gene, transcripts were present in all samples, with the highest levels at 12 h and 36 h after heterocyst induction and a decrease at 24 h (Fig. 7). The hglE2 transcripts in the sigE mutant were significantly lower than those in the wild type at 24 and 36 h, with the levels at 36 h about half the wild-type levels. These data suggest that SigE may regulate hglE2 expression at late stages of heterocyst development but not in vegetative cells or at early stages of development. Together, these data indicate that SigE is involved with the regulation of some but not all genes at late stages of heterocyst development.
DISCUSSION
Previous studies had found that none of the Anabaena PCC 7120 group 2 sigma factors sigB, sigB2, sigC, sigD, and sigE is individually essential for heterocyst development or diazotrophic growth (6, 27). However, more recent results showed that sigC and sigE are upregulated in differentiating heterocysts (3). In this work, using a more detailed examination of gene expression and heterocyst development, we show that the sigE gene is required for normal heterocyst development and for normal expression of certain heterocyst genes.
Expression analysis of sigE by Northern RNA blotting showed developmental upregulation of transcripts beginning 12 h after nitrogen step-down, which is consistent with our previous observations using a PsigE-gfp reporter (3). The sigE transcripts showed significant degradation, and attempts to identify a specific sigE transcriptional start site were not successful, indicating a relatively short half-life, which is normal for most bacterial mRNAs.
Bioinformatic analysis identified a putative consensus NtcA binding site in the sigE upstream region, which could contribute to its developmental regulation. EMSA showed that NtcA binds to a DNA fragment containing this site and produced two shifted bands, indicating a second binding site. Binding was enhanced by the presence of 2-OG. Further analysis of the fragment sequence allowed the identification of another potential binding site that differs from the consensus at one position. Deletion of both binding sites resulted in a partial loss of developmental expression, indicating that NtcA plays a partial role in the regulation of sigE. Because ntcA mutants are defective for heterocyst development and highly pleiotropic, it is not possible to use ntcA mutants to establish a direct role for NtcA in the regulation of heterocyst-specific genes, especially those upregulated at the middle to late stages of development. Many Anabaena genes show complex regulation by multiple promoters, and it is likely that sigE expression is regulated by multiple transcription factors to provide for expression in vegetative cells and upregulation in differentiating cells (11, 12, 34). Further biochemical studies will be required to determine the function of the two NtcA binding sites and if they interact with each other or alter DNA topology to influence the expression of sigE or the adjacent gene all4248.
Because sigE is expressed during the later stages of heterocyst development, we hypothesized that sigE may be involved in the expression of the nitrogen fixation (nif) genes. The regulation of the Anabaena PCC 7120 nifHDK operon has been studied for over 25 years (14, 18, 20, 43, 44, 50, 53); however, little is known about the transcription components that regulate this operon. Our results suggest that sigE is involved in nifH expression. However, regulated nifH expression was not completely abolished, which means that other transcription factors must be involved and that sigma factors other than SigE must be able to provide transcription initiation. It is not clear if the residual expression is part of normal regulation or a consequence of cross talk among the multiple group 2 sigma factors in Anabaena PCC 7120. Demonstrating direct regulation of nifH expression by SigE will require in vitro transcription assays using purified components. However, these experiments are especially challenging because the cis-acting sequences that constitute the nifH promoter have not been identified in Anabaena PCC 7120, and recent data with Anabaena variabilis suggest that multiple distant promoters and RNA-processing events may be involved in expression of the nifHDK operon (50).
In addition to the delayed and reduced expression of nifH, the sigE mutant showed delayed heterocyst morphological differentiation; mature heterocysts were formed, but not until 32 h after nitrogen step-down (about 14 h longer than for the wild-type strain). This indicates that the SigE regulon includes genes required for normal heterocyst differentiation and morphogenesis but also that there must be some redundancy in the regulation. This redundancy may be a selective advantage, because it would provide robustness to the developmental process.
Overexpression of sigE caused premature expression of PnifHD-gfp and a higher heterocyst frequency than normal. The expression of PnifHD-gfp in the sigE overexpression strain is first detectable at 14 h after heterocyst induction (4 hours earlier than for the wild-type strain). Therefore, sigE is both necessary for normal nifHDK expression and sufficient to cause precocious expression of the operon. The simplest scenario is that SigE interacts directly with a promoter for the nifHDK operon; however, it is possible that the regulation is indirect. In addition, heterocyst morphogenesis and maturation occurred earlier and the final heterocyst frequency was higher in the sigE overexpression strain, indicating that the SigE regulon includes genes that influence the progression and timing of heterocyst differentiation.
Because sigE influenced the expression of nifH, we hypothesized that sigE might also regulate the expression of other late-stage heterocyst-specific genes such as fdxH and hupL (9, 52). qRT-PCR revealed that the expression levels of nifH and fdxH were both reduced in the sigE mutant strain. On the other hand, the expression of hupL was upregulated normally in the sigE mutant after nitrogen step-down. These results show that sigE is required for the expression of only a subset of late-stage heterocyst-specific genes and indicate that at least some factors required for hupL expression are different from those required for nifH and fdxH expression.
The hglE2 gene is necessary for the heterocyst glycolipid layer and is developmentally upregulated during the middle stage of heterocyst differentiation (1, 15, 55). In the sigE mutant strain, the hglE2 expression levels at 12 h were similar to those in the wild-type strain; however, by 36 h after nitrogen step-down, the transcript levels of hglE2 were only half of the wild-type levels. These data suggest that hglE2 is expressed in at least two ways: expression at early stages of heterocyst development that is SigE independent and SigE-dependent expression at a later stage.
The focus of this work was on gene regulation related to heterocyst development and nitrogen fixation, but it seems likely that SigE in Anabaena PCC 7120 could also be involved in the regulation of sugar catabolic genes, as has been demonstrated for other cyanobacteria (24, 38). Although gfp reporter experiments showed that the sigE gene was upregulated in differentiating cells after nitrogen step-down, it was also expressed at a low level in uninduced filaments and in the vegetative cells of induced filaments, which is consistent with SigE being involved in gene expression in those cells.
The results presented in this work indicate that SigE is involved in the expression of heterocyst-specific genes that are upregulated during the later stages of development. A sigE mutant, similarly to other cyanobacterial sigma factor mutants, showed incomplete genetic penetrance and expressivity. This has made the study of sigma factor function in cyanobacteria difficult. However, observations of quantitative changes in cellular and molecular phenotypes are beginning to tease out the roles played by sigma factors in cyanobacterial gene expression.
Supplementary Material
ACKNOWLEDGMENTS
We thank Britt Flaherty for her unpublished RNA-Seq data.
This work was supported by Department of Energy grant DE-FG03-ER020309 and National Science Foundation grant 0925126.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 11 February 2011.
REFERENCES
- 1. Adams D. G. 2000. Heterocyst formation in cyanobacteria. Curr. Opin. Microbiol. 3:618–624 [DOI] [PubMed] [Google Scholar]
- 2. Aldea M. R., Kumar K., Golden J. W. 2008. Heterocyst development and pattern formation, p. 75–90 In Winans S. C., Bassler B. L. (ed.), Chemical communication among bacteria. ASM Press, Washington, DC [Google Scholar]
- 3. Aldea M. R., Mella-Herrera R. A., Golden J. W. 2007. Sigma factor genes sigC, sigE, and sigG are upregulated in heterocysts of the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 189:8392–8396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Awai K., Lechno-Yossef S., Wolk C. P. 2009. Heterocyst envelope glycolipids, p. 179–202 In Wada H., Murata N. (ed.), Lipids in photosynthesis: essential and regulatory functions. Springer, New York, NY [Google Scholar]
- 5. Boison G., Bothe H., Schmitz O. 2000. Transcriptional analysis of hydrogenase genes in the cyanobacteria Anacystis nidulans and Anabaena variabilis monitored by RT-PCR. Curr. Microbiol. 40:315–321 [DOI] [PubMed] [Google Scholar]
- 6. Brahamsha B., Haselkorn R. 1992. Identification of multiple RNA polymerase sigma factor homologs in the cyanobacterium Anabaena sp. strain PCC 7120: cloning, expression, and inactivation of the sigB and sigC genes. J. Bacteriol. 174:7273–7282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campbell E. A., Westblade L. F., Darst S. A. 2008. Regulation of bacterial RNA polymerase sigma factor activity: a structural perspective. Curr. Opin. Microbiol. 11:121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Campbell E. L., Cohen M. F., Meeks J. C. 1997. A polyketide-synthase-like gene is involved in the synthesis of heterocyst glycolipids in Nostoc punctiforme strain ATCC 29133. Arch. Microbiol. 167:251–258 [DOI] [PubMed] [Google Scholar]
- 9. Carrasco C. D., Buettner J. A., Golden J. W. 1995. Programmed DNA rearrangement of a cyanobacterial hupL gene in heterocysts. Proc. Natl. Acad. Sci. U. S. A. 92:791–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carrasco C. D., Holliday S. D., Hansel A., Lindblad P., Golden J. W. 2005. Heterocyst-specific excision of the Anabaena sp. strain PCC 7120 hupL element requires xisC. J. Bacteriol. 187:6031–6038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ehira S., Ohmori M. 2006. NrrA directly regulates the expression of hetR during heterocyst differentiation in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 188:8520–8525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ehira S., Ohmori M. 2006. NrrA, a nitrogen-responsive response regulator facilitates heterocyst development in the cyanobacterium Anabaena sp. strain PCC 7120. Mol. Microbiol. 59:1692–1703 [DOI] [PubMed] [Google Scholar]
- 13. Elhai J., Vepritskiy A., Muro-Pastor A. M., Flores E., Wolk C. P. 1997. Reduction of conjugal transfer efficiency by three restriction activities of Anabaena sp. strain PCC 7120. J. Bacteriol. 179:1998–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elhai J., Wolk C. P. 1990. Developmental regulation and spatial pattern of expression of the structural genes for nitrogenase in the cyanobacterium Anabaena. EMBO J. 9:3379–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fan Q., et al. 2005. Clustered genes required for synthesis and deposition of envelope glycolipids in Anabaena sp. strain PCC 7120. Mol. Microbiol. 58:227–243 [DOI] [PubMed] [Google Scholar]
- 16. Fay P. 1992. Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol. Rev. 56:340–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Flores E., Herrero A. 2010. Compartmentalized function through cell differentiation in filamentous cyanobacteria. Nat. Rev. Microbiol. 8:39–50 [DOI] [PubMed] [Google Scholar]
- 18. Golden J. W., Whorff L. L., Wiest D. R. 1991. Independent regulation of nifHDK operon transcription and DNA rearrangement during heterocyst differentiation in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 173:7098–7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gruber T. M., Gross C. A. 2003. Multiple sigma subunits and the partitioning of bacterial transcription space. Annu. Rev. Microbiol. 57:441–466 [DOI] [PubMed] [Google Scholar]
- 20. Haselkorn R., Golden J. W., Lammers P. J., Mulligan M. E. 1986. Developmental rearrangement of cyanobacterial nitrogen-fixation genes. Trends Genet. 2:255–259 [Google Scholar]
- 21. Herrero A., Muro-Pastor A. M., Flores E. 2001. Nitrogen control in cyanobacteria. J. Bacteriol. 183:411–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herrero A., Muro-Pastor A. M., Valladares A., Flores E. 2004. Cellular differentiation and the NtcA transcription factor in filamentous cyanobacteria. FEMS Microbiol. Rev. 28:469–487 [DOI] [PubMed] [Google Scholar]
- 23. Huang G., et al. 2005. Clustered genes required for the synthesis of heterocyst envelope polysaccharide in Anabaena sp. strain PCC 7120. J. Bacteriol. 187:1114–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Imamura S., Asayama M. 2009. Sigma factors for cyanobacterial transcription. Gene Regul. Syst. Biol. 3:65–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jang J., Wang L., Jeanjean R., Zhang C. C. 2007. PrpJ, a PP2C-type protein phosphatase located on the plasma membrane, is involved in heterocyst maturation in the cyanobacterium Anabaena sp. PCC 7120. Mol. Microbiol. 64:347–358 [DOI] [PubMed] [Google Scholar]
- 26. Ke S. H., Madison E. L. 1997. Rapid and efficient site-directed mutagenesis by single-tube ‘megaprimer’ PCR method. Nucleic Acids Res. 25:3371–3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khudyakov I. Y., Golden J. W. 2001. Identification and inactivation of three group 2 sigma factor genes in Anabaena sp. strain PCC 7120. J. Bacteriol. 183:6667–6675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kroos L. 2007. The Bacillus and Myxococcus developmental networks and their transcriptional regulators. Annu. Rev. Genet. 41:13–39 [DOI] [PubMed] [Google Scholar]
- 29. Kumar K., Mella-Herrera R. A., Golden J. W. 2010. Cyanobacterial heterocysts. Cold Spring Harb. Perspect. Biol. 2:a000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Laurent S., et al. 2005. Nonmetabolizable analogue of 2-oxoglutarate elicits heterocyst differentiation under repressive conditions in Anabaena sp. PCC 7120. Proc. Natl. Acad. Sci. U. S. A. 102:9907–9912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee M. H., Scherer M., Rigali S., Golden J. W. 2003. PlmA, a new member of the GntR family, has plasmid maintenance functions in Anabaena sp. strain PCC 7120. J. Bacteriol. 185:4315–4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lopez-Gomollon S., et al. 2007. Cross-talk between iron and nitrogen regulatory networks in Anabaena (Nostoc) sp. PCC 7120: identification of overlapping genes in FurA and NtcA regulons. J. Mol. Biol. 374:267–281 [DOI] [PubMed] [Google Scholar]
- 33. Masepohl B., Scholisch K., Gorlitz K., Kutzki C., Bohme H. 1997. The heterocyst-specific fdxH gene product of the cyanobacterium Anabaena sp. PCC 7120 is important but not essential for nitrogen fixation. Mol. Gen. Genet. 253:770–776 [DOI] [PubMed] [Google Scholar]
- 34. Muro-Pastor A. M., Flores E., Herrero A. 2009. NtcA-regulated heterocyst differentiation genes hetC and devB from Anabaena sp. strain PCC 7120 exhibit a similar tandem promoter arrangement. J. Bacteriol. 191:5765–5774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Muro-Pastor A. M., Herrero A., Flores E. 2001. Nitrogen-regulated group 2 sigma factor from Synechocystis sp. strain PCC 6803 involved in survival under nitrogen stress. J. Bacteriol. 183:1090–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Muro-Pastor M. I., Reyes J. C., Florencio F. J. 2005. Ammonium assimilation in cyanobacteria. Photosynth. Res. 83:135–150 [DOI] [PubMed] [Google Scholar]
- 37. Muro-Pastor M. I., Reyes J. C., Florencio F. J. 2001. Cyanobacteria perceive nitrogen status by sensing intracellular 2-oxoglutarate levels. J. Biol. Chem. 276:38320–38328 [DOI] [PubMed] [Google Scholar]
- 38. Osanai T., Ikeuchi M., Tanaka K. 2008. Group 2 sigma factors in cyanobacteria. Physiol. Plant. 133:490–506 [DOI] [PubMed] [Google Scholar]
- 39. Osanai T., et al. 2006. Nitrogen induction of sugar catabolic gene expression in Synechocystis sp. PCC 6803. DNA Res. 13:185–195 [DOI] [PubMed] [Google Scholar]
- 40. Osanai T., et al. 2009. ChlH, the H subunit of the Mg-chelatase, is an anti-sigma factor for SigE in Synechocystis sp. PCC 6803. Proc. Natl. Acad. Sci. U. S. A. 106:6860–6865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Osanai T., et al. 2005. Positive regulation of sugar catabolic pathways in the cyanobacterium Synechocystis sp. PCC 6803 by the group 2 sigma factor sigE. J. Biol. Chem. 280:30653–30659 [DOI] [PubMed] [Google Scholar]
- 42. Potvin E., Sanschagrin F., Levesque R. C. 2008. Sigma factors in Pseudomonas aeruginosa. FEMS Microbiol. Rev. 32:38–55 [DOI] [PubMed] [Google Scholar]
- 43. Ramasubramanian T. S., Wei T. F., Golden J. W. 1994. Two Anabaena sp. strain PCC 7120 DNA-binding factors interact with vegetative cell- and heterocyst-specific genes. J. Bacteriol. 176:1214–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ramaswamy K. S., Carrasco C. D., Fatma T., Golden J. W. 1997. Cell-type specificity of the Anabaena fdxN-element rearrangement requires xisH and xisI. Mol. Microbiol. 23:1241–1249 [DOI] [PubMed] [Google Scholar]
- 45. Ramirez M. E., Hebbar P. B., Zhou R., Wolk C. P., Curtis S. E. 2005. Anabaena sp. strain PCC 7120 gene devH is required for synthesis of the heterocyst glycolipid layer. J. Bacteriol. 187:2326–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rippka R., Deruelles J., Waterbury J. B., Herdman M., Stanier R. Y. 1979. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111:1–61 [Google Scholar]
- 47. Sambrook J., Russell D. W. 2006. Northern hybridization. Cold Spring Harb. Protoc. doi:10.1101/pdb.prot3723 [DOI] [PubMed] [Google Scholar]
- 48. Studholme D. J., Buck M. 2000. The biology of enhancer-dependent transcriptional regulation in bacteria: insights from genome sequences. FEMS Microbiol. Lett. 186:1–9 [DOI] [PubMed] [Google Scholar]
- 49. Su Z., Olman V., Mao F., Xu Y. 2005. Comparative genomics analysis of NtcA regulons in cyanobacteria: regulation of nitrogen assimilation and its coupling to photosynthesis. Nucleic Acids Res. 33:5156–5171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ungerer J. L., Pratte B. S., Thiel T. 2010. RNA processing of nitrogenase transcripts in the cyanobacterium Anabaena variabilis. J. Bacteriol. 192:3311–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Valladares A., Herrero A., Pils D., Schmetterer G., Flores E. 2003. Cytochrome c oxidase genes required for nitrogenase activity and diazotrophic growth in Anabaena sp. PCC 7120. Mol. Microbiol. 47:1239–1249 [DOI] [PubMed] [Google Scholar]
- 52. Valladares A., Maldener I., Muro-Pastor A. M., Flores E., Herrero A. 2007. Heterocyst development and diazotrophic metabolism in terminal respiratory oxidases mutants of the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 189:4425–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Valladares A., Muro-Pastor A. M., Fillat M. F., Herrero A., Flores E. 1999. Constitutive and nitrogen-regulated promoters of the petH gene encoding ferredoxin:NADP+ reductase in the heterocyst-forming cyanobacterium Anabaena sp. FEBS Lett. 449:159–164 [DOI] [PubMed] [Google Scholar]
- 54. Vazquez-Bermudez M. F., Herrero A., Flores E. 2003. Carbon supply and 2-oxoglutarate effects on expression of nitrate reductase and nitrogen-regulated genes in Synechococcus sp. strain PCC 7942. FEMS Microbiol. Lett. 221:155–159 [DOI] [PubMed] [Google Scholar]
- 55. Wang Y., et al. 2007. Predicted glycosyl transferase genes located outside the HEP island are required for formation of heterocyst envelope polysaccharide in Anabaena sp. strain PCC 7120. J. Bacteriol. 189:5372–5378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yoon H. S., Golden J. W. 1998. Heterocyst pattern formation controlled by a diffusible peptide. Science 282:935–938 [DOI] [PubMed] [Google Scholar]
- 57. Yoon H. S., Golden J. W. 2001. PatS and products of nitrogen fixation control heterocyst pattern. J. Bacteriol. 183:2605–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yoshimura H., Okamoto S., Tsumuraya Y., Ohmori M. 2007. Group 3 sigma factor gene, sigJ, a key regulator of desiccation tolerance, regulates the synthesis of extracellular polysaccharide in cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 14:13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang C. C., Laurent S., Sakr S., Peng L., Bedu S. 2006. Heterocyst differentiation and pattern formation in cyanobacteria: a chorus of signals. Mol. Microbiol. 59:367–375 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.