Abstract
Indole has many, diverse roles in bacterial signaling. It regulates the transition from exponential to stationary phase, it is involved in the control of plasmid stability, and it influences biofilm formation, virulence, and stress responses (including antibiotic resistance). Its role is not restricted to bacteria, and recently it has been shown to include mutually beneficial signaling between enteric bacteria and their mammalian hosts. In many respects indole behaves like the signaling component of a quorum-sensing system. Indole synthesized within the producer bacterium is exported into the surroundings where its accumulation is detected by sensitive cells. A view often repeated in the literature is that in Escherichia coli the AcrEF-TolC and Mtr transporter proteins are involved in the export and import, respectively, of indole. However, the evidence for their involvement is indirect, and it has been known for a long time that indole can pass directly through a lipid bilayer. We have combined in vivo and in vitro approaches to examine the relative importance of protein-mediated transport and direct passage across the E. coli membrane. We conclude that the movement of indole across the E. coli membrane under normal physiological conditions is independent of AcrEF-TolC and Mtr. Furthermore, direct observation of individual liposomes shows that indole can rapidly cross an E. coli lipid membrane without the aid of any proteinaceous transporter. These observations not only enhance our understanding of indole signaling in bacteria but also provide a simple explanation for the ability of indole to signal between biological kingdoms.
INTRODUCTION
Indole is produced by a large number of Gram-positive and Gram-negative bacterial species, including Escherichia coli (17). It acts as an intercellular signal, influencing multiple aspects of bacterial physiology and has proved to be an important factor in the transition to stationary phase, activating the global regulator RpoS (15, 18). It also promotes resistance to a range of drugs and toxins through the induction of xenobiotic exporters (11, 16) and is involved in preventing plasmid instability associated with the accumulation of plasmid multimers (7).
Aspects of bacterial ecology and host-pathogen interactions that respond to indole include biofilm formation (8, 9) and the expression of virulence factors (12). It has even been proposed that indole is involved in interkingdom signaling. Intestinal epithelial cells respond to indole produced by enteric bacteria (4, 5), and recent evidence suggests that indole provides a link between the metabolism of the gut microflora and that of its mammalian host (26).
Knowledge of the mechanism by which indole crosses the bacterial membrane, and in some cases the eukaryotic cell membrane, is crucial to a full understanding of these diverse signaling roles. Although it has been known for more than 40 years that indole is capable of unaided diffusion through a lipid membrane (6), the past decade has seen many reports in the literature which suggest that protein-mediated indole transport may be important. The high-affinity tryptophan permease Mtr is often cited as the main conduit for indole import. The experiments leading to this conclusion (28) were performed in an E. coli mutant strain lacking the tryptophan biosynthetic pathway, which could be rescued on tryptophan-free medium by the addition of exogenous indole (indole being converted to tryptophan inside the cell by the enzyme tryptophanase). The discovery that indole could not rescue cells which also carried an mtr mutation led to the assignment of Mtr (already implicated in tryptophan import) as the main indole importer in E. coli. The AcrEF multidrug exporter is widely assumed to be at least partially responsible for indole export (13). This is based upon a report that indole accumulation in the culture supernatant of an E. coli acrEF deletion strain was reduced, while the intracellular indole concentration increased (13). However, these were small effects (≤2-fold) and were growth medium dependent. Subsequently, it was shown that the transcription of several multidrug exporter genes is increased upon exposure to indole (11), but none of these systems has thus far been shown to transport indole.
As newcomers to the field of indole signaling, we found the relative importance of direct and protein-mediated indole transport to be surprisingly ill defined. By omitting any mention of direct diffusion, many papers seemed implicitly to support the primacy of protein-mediated transport (see, for example, references 9, 25, and 27), while reports which addressed the diffusion issue directly did not relate the physical chemistry of the process to the role of indole in vivo (6, 10). In their recent review of indole signaling, Lee and Lee recognized the confusion over the relative importance of Mtr, AcrEF, and direct diffusion, concluding that “it is imperative to gain a clear understanding of how indole is imported and exported” (17).
In the light of these contradictory data, we decided to reexamine the transport of indole across biological membranes. We have studied the process in vivo, using E. coli as our model system, and in vitro, using fluorescence microscopy of isolated liposomes. We find that not only is indole transport independent of Mtr or AcrEF under normal growth conditions but that indole rapidly crosses E. coli membranes without the intervention of transporter proteins.
MATERIALS AND METHODS
Strains and growth conditions.
E. coli BW25113 was used as a parental strain, and deletion derivatives in the tnaA, mtr, acrE, acrF, and tolC genes were obtained from the Keio collection (3). All deletions were corroborated by PCR. (Although indole can be synthesized from chorismate in a TnaA-independent fashion, in practice indole production is undetectable even in a dense stationary phase culture of a tnaA mutant strain. This mutant strain was therefore used as our indole-nonproducing control.) The tnaA mtr double mutant was created by P1 phage transduction of the interrupted version of the tnaA gene into an mtr mutant background.
Unless otherwise stated, bacterial cultures were grown overnight in LB supplemented with kanamycin (30 μg ml−1) or ampicillin (100 μg ml−1) where appropriate.
Indole susceptibility of putative indole transport mutants.
Overnight cultures were adjusted to an optical density at 600 nm (OD600) of 0.03 in 20 ml of LB. After the cultures reached an OD600 of 0.1, the appropriate concentrations of indole were added. The OD600 was recorded every half an hour, and the generation times were calculated.
Microscopic observations were performed after 2 h of indole treatment. Cells were mounted on microscope slides on top of a thin film of 1% agarose-phosphate-buffered saline supplemented with appropriate concentrations of indole. Phase-contrast imaging was performed using a Nikon Eclipse 80i microscope equipped with a 100× CFI Plan Fluor objective lens. NIS-elements F 3.0 software (Nikon) was used for image acquisition, and image analysis was performed using ImageJ software (1).
Indigo assay for intracellular indole.
Indole was detected indirectly by measuring indigo produced by styrene mono-oxygenase encoded by the pSTYABB plasmid (15). Transformations of the plasmid were done following standard procedures (23).
To analyze the internalization of exogenously added indole, overnight cultures were diluted to an OD600 of 1 in medium containing an appropriate indole concentration. After 4 h of incubation at 37°C, 1 ml of culture was centrifuged and lysed by resuspension in 1 ml of N,N-dimethylformamide (DMF), which also dissolves indigo, and the mixture was incubated at room temperature for 1 h, with shaking, before centrifugation for 10 min to remove cell debris. The indigo present in the samples was quantified spectrophotometrically (OD610).
Mixed cultures of the putative transport mutants harboring pSTYABB, and indole-producing strains, were generated by diluting overnight cultures of appropriate strains 3-fold in LB and mixing in a 1:1 ratio. The mixed cultures were incubated in 12-well plates for 4 h at 37°C. Indigo quantification was performed as described above. Each condition and strain combination was tested in triplicate.
Kovac's assay for extracellular indole concentrations.
A portion (300 μl) of Kovacs reagent (10 g of p-dimethylamino-benzaldehyde dissolved in a mixture of 50 ml of HCl and 150 ml of amyl alcohol) was added to 1 ml of overnight culture. After 2 min, 30 μl of the upper phase was collected and diluted in 2 ml of HCl-amyl alcohol mixture (75 ml of HCl and 225 ml of amyl alcohol). The absorbance at 540 nm was measured, and indole concentrations were calculated by using a standard curve.
Fluorescence detection of indole transport across liposomes.
E. coli polar lipid extract was obtained from Avanti-Polar Lipids, Inc. It is a chloroform-methanol extract of E. coli B (ATCC 11303) grown in Kornberg minimal medium at 37°C. The composition of the lipid is as follows: phosphatidylethanolamine (PE), 67.0%; phosphatidylglycerol (PG), 23.2%; and cardiolipin (CA), 9.8%. Vesicles were formed by classical electroformation in a 50 mM sucrose solution (2). Then, 5 μl of the liposome suspension was mixed with 50 μl of 50 mM glucose and 4 mM indole or 0.5 mM indole-3-acetic acid (IAA) and then mixed for 5 s. The mixture was transferred into a microfluidic chip built from a 0.20-mm quartz coverslip (UQG Optics Ltd), on top, and a standard 0.12-mm glass coverslip at the bottom separated by an 80-μm layer of parafilm. The presence of indole inside and outside the sedimented liposomes was monitored.
The intrinsic fluorescence of indole (∼350 nm) and IAA (∼360 nm) was detected by using a custom-built UV microscope. A 100-W mercury arc lamp (LOT Oriel) was fitted with a monochromator (PhotoPhysics, f3.4) and a 1-mm core fused silica optical fiber (Ocean Optics). The emitted fluorescence light was collected by a 60× objective (UPLSAPO 60× W; Olympus), and a dichroic mirror for indole (Linos; DLHS UV, 351 to 355 nm) or a UV-enhanced aluminum mirror for IAA (Thorlabs; PFSQ10-03-F01) was used to reflect the light onto the camera. A 200-mm fused silica plan-convex lens (Thorlabs) was used to collimate the light coming out through the objective. Images were captured by using a charge-coupled device camera (Cool Snap HQ2, Photometrics). Images were analyzed by using ImageJ (1) and Origin 8 software (OriginLab, Northhampton, MA).
RESULTS
During preliminary studies in our laboratory we observed that after the addition of 2 to 10 mM indole to the culture medium of an indole-nonproducing (tnaA) mutant of E. coli, the concentration of indole inside the cells rose rapidly to match the external level. Furthermore, when the cells were resuspended in indole-free medium, the intracellular indole concentration declined rapidly, becoming undetectable after a few minutes (data not shown). This suggests that indole import and export are rapid processes and occur at similar rates, but it does not distinguish direct passage of indole through the membrane from protein-assisted transport. In an attempt to tease apart these processes, we examined the effect of indole on the growth and morphology of strains which lacked the putative transport proteins.
Indole import is independent of Mtr.
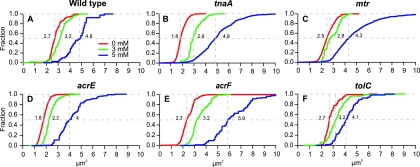
Indole addition to broth culture inhibits cell division and growth of wild-type E. coli (7). The addition of 3 mM indole increases the generation time by ca. 30%, while growth is abolished completely by 5 mM indole. Indole-treated cells also increase in size (Fig. 1 and Fig. 2A). Cell size data in Fig. 2 is displayed as a cumulative frequency plot of cross-sectional area with the median cell size given for each indole concentration. In the absence of indole, the median size of E. coli BW25113 (wild-type) cells was 2.7 μm2, increasing to 3.2 and 4.8 μm2 in response to 3 and 5 mM indole, respectively.
Fig. 1.
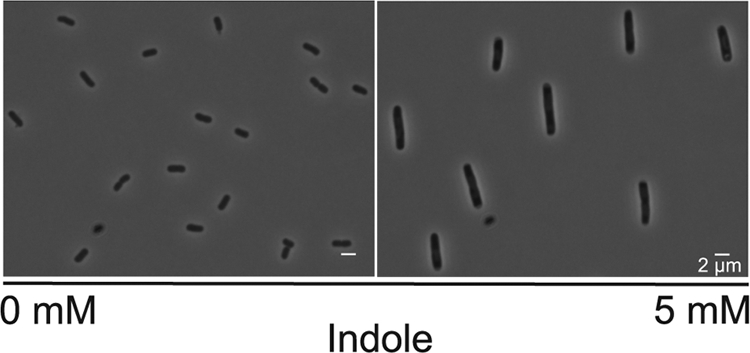
Effect of indole on the morphology of E. coli cells. Phase-contrast micrographs compare untreated BW25113 tnaA (indole negative) with cells exposed to indole (5 mM) for 2 h.
Fig. 2.
Indole susceptibility of putative transport mutants. The graphs show the cumulative fraction of cells at or below a particular size (specified on the x axis). E. coli BW25113 (A) and its tnaA (B), mtr (C), acrE (D), acrF (E), and tolC (F) mutant derivatives were exposed to 0, 3, or 5 mM indole for 2 h. The data represent the size distribution of 400 bacterial cells observed in at least three independent experiments for each strain and condition.
Assuming that the target of indole is internal to the cell, blocking indole import should make cells less susceptible to its effects. Since Mtr has been implicated in indole uptake, the impact of exogenous indole on the growth and morphology of tnaA and tnaA mtr strains was compared. (A tnaA background was used to avoid complications introduced by intracellular indole production.) In the absence of indole, cultures of the two strains had very similar generation times (∼28 min). In the presence of 3 mM indole, the generation times increased to 42 ± 4 min and 49 ± 3 min for tnaA and tnaA mtr, respectively (average of at least three repeats). Thus, the loss of Mtr seems slightly to increase indole sensitivity; the opposite of what would be expected if Mtr was an indole importer. In the presence of 3 or 5 mM indole the cell size distribution was very similar for the two strains so, once again, there was no evidence that the loss of Mtr made cells less responsive to indole (Fig. 2B and C). Interestingly, we noticed that in the absence of indole the tnaA mutant cells were smaller than either the tnaA mtr double mutant or the wild-type strain.
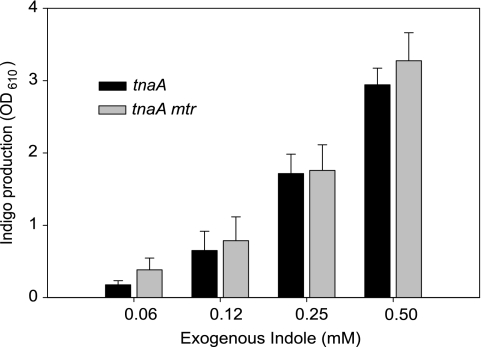
In order to make a direct assessment of indole import, we needed to assay its intracellular concentration. In the past this has been attempted by harvesting and washing cells, followed by lysis and assay of the released indole using the Kovacs method. However, our preliminary work had indicated that indole passes rapidly in and out of E. coli, resulting in an unavoidable loss of indole from cells during washing. To omit the washing step would introduce further inaccuracy since external indole associated with the wet cell pellet would be assayed together with intracellular indole. To overcome these problems, we used an indirect assay described by Lacour and Landini (15). In E. coli cells expressing the Pseudomonas putida styrene-monooxygenase from plasmid pSTYABB, intracellular indole is “trapped” by conversion to insoluble indigo, which can be quantified colorimetrically (OD610) after solubilization with DMF. To ensure that the indigo assay is specific for intracellular indole, we confirmed that no enzyme activity was detectable in the culture supernatant.
Using an indole-negative (tnaA) strain of E. coli BW25113 containing pSTYABB, we measured indole import by adding subinhibitory concentrations of indole (0 to 0.5 mM) to the culture medium and assaying the accumulated indigo after 4 h of incubation at 37°C. There was a clear quantitative relationship between the external indole concentration and the amount of indigo accumulated in the cells (Fig. 3). We repeated the experiment with a strain lacking the putative import protein (tnaA mtr) and found that the amount of indigo accumulated was indistinguishable from the tnaA strain. Thus, the rate of indole import is unaffected by the absence of Mtr.
Fig. 3.
Indole import is not impaired in the mtr mutant strain. The histogram compares indigo accumulation in tnaA and tnaA mtr mutants containing plasmid pSTYABB. Indole was added to the culture medium at the indicated concentration, and indigo accumulation was assayed spectrophotometrically (OD610) 4 h later. The results are the average of three independent experiments, and error bars represent the standard deviations.
In a second series of experiments, we established mixed cultures in which indole secreted into the medium by indole-positive producer cells was imported into indole-negative detector cells (tnaA pSTYABB), where it was converted to indigo. There was no addition of exogenous indole. We found that the amount of indigo accumulated by the detector cells was the same, irrespective of whether they were Mtr+ or Mtr−. As expected, when the producer cells were indole negative (tnaA mutant), no indigo was found in the detector cells (Table 1). Once again, the data point to indole import being independent of the Mtr transporter at physiological concentrations.
Table 1.
Measurement of indole import in mixed culture
| Producer cell | Indole status | Detector cell mean OD610 ± SDa |
|
|---|---|---|---|
| tnaA (indole-negative) mutant | tnaA mtr (indole-negative) mutant | ||
| Wild type | Positive | 2.88 ± 0.18 | 2.84 ± 0.41 |
| tnaA mutant | Negative | 0 | 0 |
Indigo produced by detector cells carrying the pSTYABB plasmid was measured. The data are the means of at least three independent repeats.
Indole export is independent of AcrEF-TolC.
If the target of indole is intracellular, mutants with functional import but defective export should be more sensitive to exogenous indole, since they will build up a higher-than-normal internal concentration. The tripartite efflux system AcrEF-TolC has been implicated in indole export (13). We compared the effect of indole on the growth rate and cell size of our wild-type strain with mutants carrying deletions of each of the genes encoding components of the efflux pump. If the pump plays an important role in indole export, all three mutants should display similar increases in indole sensitivity.
In the absence of indole no significant difference was observed between the generation times of wild-type BW25113 and its acrE, acrF, and tolC derivatives (all ∼28 min). In the presence of 3 mM indole the generation time of the wild type increased to 37 ± 3 min. The generation times for the acrE, acrF, and tolC mutants increased to 37 ± 4 min, 45 ± 3 min, and 40 ± 5 min, respectively. Thus, acrF was slightly more sensitive to indole, but no significant effect was seen for the acrE or tolC mutants.
We next compared the effect of 3 and 5 mM indole on the cell size of the wild-type (Fig. 2A) and its acrE, acrF, and tolC derivatives (Fig. 2D to F). In each case, 5 mM indole had a substantially greater effect than 3 mM, with the median cell size increasing by 1.5- to 2.6-fold over the no-indole control. The effect of the mutations upon the response to 5 mM indole was not consistent. The smallest increase was seen with the tolC mutant (1.5-fold), while the largest was associated with the acrF mutant (2.6-fold). Intermediate effects were seen with the wild type (1.8-fold) and the acrE mutant (2.3-fold).
A mixed-culture experiment was used to compare indole export by the BW25113 and the acrE, acrF, and tolC mutants. The mutant strains, along with their wild-type parent, were the indole-positive producer cells, while an indole-negative strain carrying the indigo plasmid (tnaA pSTYABB) was used as the detector (Table 2). There was no addition of exogenous indole, so the amount of indigo produced in the detector cells is a direct consequence of the export of indole into the culture supernatant by the producer cells. Our detector strain produced very similar amounts of indigo when grown in mixed culture with wild-type producer cells or with acrF or tolC mutants. However, a significantly lower amount of indigo was produced when the acrE strain was used as a donor. To verify this result, the Kovacs test was used to assay indole in the supernatants of conventional (unmixed) cultures of the strains from the mixed-culture experiment (Table 3). In 8-h cultures of the acrE strain, the indole concentration in the culture supernatant was ca. 60% of that in cultures of the wild type or the acrF or tolC mutant. However, after 24 h, the indole concentration in the acrE culture was not significantly different from that in the culture supernatants of the wild type or the acrF and tolC mutants.
Table 2.
Measurement of indole export in mixed culture
| Producer cell | Indole status | Detector cell mean OD610 ± SDa for tnaA (indole-negative) mutant |
|---|---|---|
| tnaA mutant | Negative | 0 |
| Wild type | Positive | 1.5 ± 0.04 |
| acrE mutant | Positive | 0.1 ± 0.001 |
| acrF mutant | Positive | 1.5 ± 0.05 |
| tolC mutant | Positive | 1.3 ± 0.07 |
Indigo produced by detector cells carrying the pSTYABB plasmid was measured. The data are the means of at least three independent repeats.
Table 3.
Direct measurement of supernatant indole
| Strain | Mean indole concn (mM) ± SDa at: |
|
|---|---|---|
| 8 h | 24 h | |
| BW25113 | 0.53 ± 0.07 | 0.55 ± 0.05 |
| tnaA mutant | 0 | 0 |
| acrE mutant | 0.29 ± 0.10 | 0.46 ± 0.04 |
| acrF mutant | 0.51 ± 0.06 | 0.54 ± 0.05 |
| tolC mutant | 0.53 ± 0.05 | 0.52 ± 0.02 |
The data are the means of at least three independent repeats.
The lower level of indole in acrE cultures is consistent with the report of Kawamura-Sato et al. (13) that the supernatant indole concentration was reduced in an acrEF double mutant. While Kawamura-Sato et al. saw this as evidence for reduced export, it could also be explained by a reduced rate of indole synthesis. In an attempt to distinguish between these possibilities, BW25113 and its acrE derivative were transformed with the pSTYABB plasmid. Since indigo is made from intracellular indole, a reduced rate of indole synthesis (with normal export) would lead to a corresponding reduction in indigo production. If, on the other hand, indole synthesis is normal but export is reduced, the intracellular indole concentration, and hence indigo production, should increase. We found that the acrE mutant accumulated only 29% of the indigo accumulated by the wild type in 16-h cultures. Although this result could in theory be explained by a simultaneous reduction in both production and export of indole, the simpler interpretation is that production is reduced but export is unaffected. Thus, although we cannot completely exclude the possibility of a role for AcrE, alone, in indole export, there is an absence of evidence in support of it. In any case, in the light of our observation that indole export is unaffected in tolC and acrF mutants, we can exclude an important role for the AcrEF-TolC pump in indole export.
Transport of indole through artificial lipid membranes.
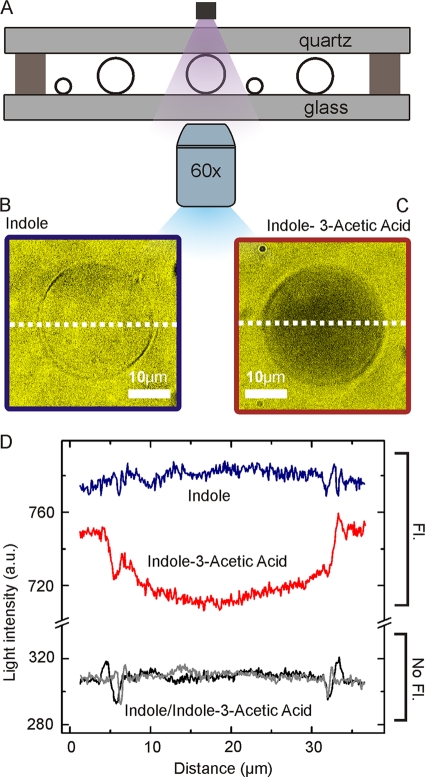
A simple explanation of our in vivo results is that indole passes through E. coli lipid membranes without the involvement of any protein-based transport system. To test this hypothesis, we examined the diffusion of indole, and the related compound IAA, through artificial membranes formed from polar lipid extract of E. coli by using a microfluidic chip (Fig. 4A).
Fig. 4.
Detection of the diffusion of indole and IAA through lipid membranes. (A) Schematic view of the microfluidic chip used to investigate transport across the liposome membrane. Circles represent individual liposomes. (B and C) Fluorescence images of E. coli lipid vesicles dipped in solutions of 3.6 mM indole or 0.4 mM IAA, respectively. (D) Spatial dependence of the fluorescence (Fl) following the equatorial line of the vesicles presented in Fig. 1B and C. The corresponding background measurements for the liposomes imaged with nonfluorescent illumination (No Fl) is presented as a control.
Indole or IAA was added to the solution surrounding giant lipid vesicles (liposomes) ∼30 μm in diameter. Both indole and IAA can be detected by their intrinsic fluorescence; when excited with deep UV light at 290 ± 5 nm, the compounds fluoresce in the UV region around 350 nm for indole and around 360 nm for IAA (24). Thus, using fluorescence microscopy, we were able to determine whether indole or IAA passes freely through the lipid membrane (Fig. 4B to D). A uniform level of fluorescence was detected between the inside and the outside of the vesicle when indole was added to the outside of the liposome, indicating that indole has passed into the interior of the vesicle (Fig. 4B). For IAA, the interior of the liposome appears darker than the outside, demonstrating a barrier to the diffusion of IAA into the vesicle (Fig. 4C). The vesicle illustrated in Fig. 4 is representative of at least 10 different vesicles in at least three separate experiments which always showed similar results. In the absence of indole or IAA there was no significant fluorescence. Our results clearly show that indole can pass through an intact and protein-free E. coli lipid membrane.
DISCUSSION
We have used a variety of in vivo and in vitro approaches to examine the transport of indole across the E. coli lipid membrane. None of our in vivo approaches has produced any convincing evidence for the involvement of the Mtr and AcrEF proteins in this process. In the light of this, and our demonstration that indole passes rapidly into the interior of protein-free liposomes comprised of E. coli polar lipid extract, we believe that the repeated implication in the scientific literature of the importance of protein-mediated indole transport is erroneous and misleading.
A rigorous exploration of indole transport requires a reliable method to assay its intracellular concentration. The most common indole assay is the Kovacs test, which is well suited to the measurement of supernatant indole. It is, however, less appropriate for the estimation of intracellular indole due to the leakage of indole from cells during their preparation. To avoid this, we adopted an assay in which intracellular indole is converted to water-insoluble indigo, which can be quantified by colorimetry after solubilization in DMF. The main disadvantage of the indigo assay is that, rather than reflecting the indole concentration at the time of sampling, indigo accumulates continuously in cells expressing the styrene-monooxygenase. Thus, the assay is only useful for making comparisons between strains if they are cultured under identical conditions. With this caveat, we found that the indigo assay provided a reliable quantitative estimate of intracellular indole.
In a previous study, Yanofsky et al. found that the tryptophan transporter Mtr was necessary for the growth of a tryptophan-requiring E. coli strain on indole-supplemented medium (28). Since indole, once imported into the cell, can be converted to tryptophan by the enzyme tryptophanase, they concluded that Mtr must be required for indole import. This conclusion is contradicted by our demonstration that indole can enter cells lacking the Mtr transporter. Although we cannot exclude the possibility that Mtr might have a role in scavenging very low concentrations of indole, its absence did not prevent indole internalization at concentrations normally found in late exponential or stationary phase E. coli cultures (9). The results of Yanofsky et al. may be explained by our observation that mtr mutants are more sensitive to growth inhibition by indole. It is therefore possible that the indole-supplemented medium used by Yanofsky et al. was inhibiting the growth of the tryptophan-requiring mtr mutant strain.
Efflux pumps of the resistance-nodulation-division (RND) family, including AcrEF, are nonspecific, inner-membrane transporters of structurally dissimilar, toxic compounds. The inner membrane transporter is associated with the outer membrane channel TolC (21). Kawamura-Sato et al. based their proposal that AcrEF-TolC plays a significant role in indole efflux on the observation that there was less indole present in the culture supernatant of an acrE acrF double mutant (13). However, we observed no difference in indole export between an acrF mutant strain and its wild-type counterpart. The possibility that other members of the RND family take over indole export in the acrF mutant can be excluded because a tolC mutant was not defective in indole export. Our data strongly suggest that no TolC-requiring pump, or combination of pumps, is essential for indole transport.
In order to discover whether proteins other than Mtr and AcrEF-TolC might be required for indole transport across a lipid membrane, we investigated indole transport into protein-free liposomes. Our experiments demonstrated that indole is capable of traversing the hydrophobic diffusion barrier imposed by E. coli lipid membranes in vitro. This is consistent with previous reports (6) in which unsubstituted indole was shown to be highly permeable through membranes of different compositions. However, we believe that this is the first study focused specifically on the permeability of bacterial membranes to indole.
Interestingly, although indole can pass through a lipid membrane without the assistance of a protein transporter, the passage of indole does not leave the membrane unaltered. It is well known that indole is capable of interacting with lipid membranes (10, 20, 22) and that the localization of indole within membranes may alter their physical structure (20) and hence their biological properties. In this respect the membrane itself should be seen as a target of indole signaling. Exposure to even small amounts of indole results in the induction of a number of transport systems in E. coli (13, 14), and it has been demonstrated that the indole induction of some efflux systems is mediated by the CpxA sensor kinase that monitors envelope stress (11). Since indole concentration is influenced by environmental factors such as population density, carbon source availability, temperature, and pH (14, 19), we believe it could act as a signal leading to a nonspecific export response that could improve the survival of E. coli in harsh environments. Consistent with this, it has recently been reported that, under antibiotic stress, resistant mutants will assist the survival of their antibiotic sensitive neighbors by the production of an elevated level of indole and the consequent induction of multiple stress responses (16).
In conclusion, the data presented in this report lead us to conclude that there is no significant role for either Mtr or AcrEF-TolC in the transport of indole in E. coli. Instead, we present evidence that indole passes rapidly and unassisted across the cell membrane. This, together with an increasing recognition that indole has important consequences for the biological function of lipid membranes, will have implications for understanding the rapidly growing list of roles for indole signaling, including the recent recognition of its role in interkingdom signaling.
ACKNOWLEDGMENTS
D.K.S. was the recipient of a research grant from the UK Biotechnology and Biological Sciences Research Council. C.C. and U.F.K. were supported by the Emmy Noether program of the Deutsche Forschungsgemeinschaft.
We thank Antonio Ciruela for carrying out the preliminary experiments on which this study was based.
Footnotes
Published ahead of print on 4 February 2011.
REFERENCES
- 1. Abramoff M. D., Magelhaes P. J., Ram S. J. 2004. Image processing with ImageJ. Biophotonics Int. 11:36–42 [Google Scholar]
- 2. Angelova M. I., Dimitrov D. S. 1986. Liposome electroformation. Faraday Discuss. Chem. Soc. 81:303 [Google Scholar]
- 3. Baba T., et al. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bansal T., Alaniz R. C., Wood T. K., Jayaraman A. 2010. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc. Natl. Acad. Sci. U. S. A. 107:228–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bansal T., et al. 2007. Differential effects of epinephrine, norepinephrine, and indole on Escherichia coli O157:H7 chemotaxis, colonization, and gene expression. Infect. Immun. 75:4597–4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bean R. C., Shepherd W. C., Chan H. 1968. Permeability of lipid bilayer membranes to organic solutes. J. Gen. Physiol. 52:495–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chant E. L., Summers D. K. 2007. Indole signalling contributes to the stable maintenance of Escherichia coli multicopy plasmids. Mol. Microbiol. 63:35–43 [DOI] [PubMed] [Google Scholar]
- 8. Di Martino P., Merieau A., Phillips R., Orange N., Hulen C. 2002. Isolation of an Escherichia coil strain mutant unable to form biofilm on polystyrene and to adhere to human pneumocyte cells: involvement of tryptophanase. Can. J. Microbiol. 48:132–137 [DOI] [PubMed] [Google Scholar]
- 9. Domka J., Lee J., Wood T. K. 2006. YliH (BssR) and YceP (BssS) regulate Escherichia coli K-12 biofilm formation by influencing cell signaling. Appl. Environ. Microbiol. 72:2449–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gaede H. C., Yau W.-M., Gawrisch K. 2005. Electrostatic contributions to indole-lipid interactions. J. Phys. Chem. 109:13014–13023 [DOI] [PubMed] [Google Scholar]
- 11. Hirakawa H., Inazumi Y., Masaki T., Hirata T., Yamaguchi A. 2005. Indole induces the expression of multidrug exporter genes in Escherichia coli. Mol. Microbiol. 55:1113–1126 [DOI] [PubMed] [Google Scholar]
- 12. Hirakawa H., Kodama T., Takumi-Kobayashi A., Honda T., Yamaguchi A. 2009. Secreted indole serves as a signal for expression of type III secretion system translocators in enterohaemorrhagic Escherichia coli O157:H7. Microbiology 155:541–550 [DOI] [PubMed] [Google Scholar]
- 13. Kawamura-Sato K., et al. 1999. Role of multiple efflux pumps in Escherichia coli in indole expulsion. FEMS Microbiol. Lett. 179:345–352 [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi A., Hirakawa H., Hirata T., Nishino K., Yamaguchi A. 2006. Growth phase-dependent expression of drug exporters in Escherichia coli and its contribution to drug tolerance. J. Bacteriol. 188:5693–5703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lacour S., Landini P. 2004. SigmaS-dependent gene expression at the onset of stationary phase in Escherichia coli: function of sigmaS-dependent genes and identification of their promoter sequences. J. Bacteriol. 186:7186–7195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee H. H., Molla M. N., Cantor C. R., Collins J. J. 2010. Bacterial charity work leads to population-wide resistance. Nature 467:82–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee J.-H., Lee J. 2009. Intercellular signal indole in microbial communities. FEMS Microbiol. Rev. 34:444. [DOI] [PubMed] [Google Scholar]
- 18. Lelong C., et al. 2007. The Crl-RpoS regulon of Escherichia coli. Mol. Cell Proteomics 6:648–659 [DOI] [PubMed] [Google Scholar]
- 19. Li Y., Cole K., Altman S. 2003. The effect of a single, temperature-sensitive mutation on global gene expression in Escherichia coli. RNA. 9:518–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mitchell S. A. 2009. Indole adsorption to a lipid monolayer studied by optical second harmonic generation. J. Phys. Chem. 113:10693–10707 [DOI] [PubMed] [Google Scholar]
- 21. Nikaido H., Takatsuka Y. 2009. Mechanisms of RND multidrug efflux pumps. Biochim. Biophys. Acta 1794:769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Norman K. E., Nymeyer H. 2006. Indole localization in lipid membranes revealed by molecular simulation. Biophys. J. 91:2046–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sambrook J. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 24. Van Duuren B. L. 1963. Effects of the environment on the fluorescence of aromatic compounds in solution. Chem. Rev. 63:325–354 [Google Scholar]
- 25. Wang D., Ding X., Rather P. N. 2001. Indole can act as an extracellular signal in Escherichia coli. J. Bacteriol. 183:4210–4216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wikoff W. R., et al. 2009. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. U. S. A. 106:3698–3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wood T. K. 2009. Insights on Escherichia coli biofilm formation and inhibition from whole-transcriptome profiling. Environ. Microbiol. 11:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yanofsky C., Horn V., Gollnick P. 1991. Physiological studies of tryptophan transport and tryptophanase operon induction in Escherichia coli. J. Bacteriol. 173:6009–6017 [DOI] [PMC free article] [PubMed] [Google Scholar]