Abstract
Through transposon mutagenesis and DNA sequence analysis, the main disease determinant of the entomopathogenic bacterium Yersinia entomophaga MH96 was localized to an ∼32-kb pathogenicity island (PAI) designated PAIYe96. Residing within PAIYe96 are seven open reading frames that encode an insecticidal toxin complex (TC), comprising not only the readily recognized toxin complex A (TCA), TCB, and TCC components but also two chitinase proteins that form a composite TC molecule. The central TC gene-associated region (∼19 kb) of PAIYe96 was deleted from the Y. entomophaga MH96 genome, and a subsequent bioassay of the ΔTC derivative toward Costelytra zealandica larvae showed it to be innocuous. Virulence of the ΔTC mutant strain could be restored by the introduction of a clone containing the entire PAIYe96 TC gene region. As much as 0.5 mg of the TC is released per 100 ml of Luria-Bertani broth at 25°C, while at 30 or 37°C, no TC could be detected in the culture supernatant. Filter-sterilized culture supernatants derived from Y. entomophaga MH96, but not from the ΔTC strain grown at temperatures of 25°C or less, were able to cause mortality. The 50% lethal doses (LD50s) of the TC toward diamondback moth Plutella xylostella and C. zealandica larvae were defined as 30 ng and 50 ng, respectively, at 5 days after ingestion. Histological analysis of the effect of the TC toward P. xylostella larva showed that within 48 h after ingestion of the TC, there was a general dissolution of the larval midgut.
INTRODUCTION
Toxin complexes (TCs) active on insects were first identified in the nematode-associated bacterium Photorhabdus luminescens and termed TCs, as three proteins combined to form a complex with insecticidal activity (5). TC toxins were subsequently identified in the genome of Serratia entomophila where they reside in the designated gene order sepA, sepB, and sepC (33); this toxin complex ABC designation defines the revised nomenclature of the TC proteins (25). The TC toxins derived from P. luminescens reside as multiple but dissimilar orthologues throughout the P. luminescens T011 genome (22), and different insecticidal activities may be attributed to a different TC cluster (32). The S. entomophila sepABC genes are plasmid borne, and their translated products are host specific, only causing amber disease in larvae of the New Zealand grass grub Costelytra zealandica (Coleoptera: Scarabaeidae) (33). TC-like toxins have since been identified in the genome of Xenorhabdus nematophilus (60), Pseudomonas syringae pv. tomato DC3000 (9), and some Yersinia species. The toxin complex A (TCA)-like (tcaB) gene of Yersinia pestis CO92 contains a frameshift mutation, and the toxin complex B (TCB)-like (tcaC) gene contains an internal deletion (51), indicative of a loss of function, while the corresponding TCA-like and TCB-like orthologues in Y. pestis KIM and 91001 do not (18, 62). Tennant et al. (66) showed that mutations in each of the Yersinia enterocolitica biotype 1A T83 genes, TCA-like (tcbA), TCB-like (tcaC), and TCC-like (tccC) genes, resulted in a reduced ability to colonize the intestinal tracts of BALB/c mice compared to the wild-type strain. This suggests that the TC proteins may enhance the persistence of the host bacterium, a scenario postulated by Erickson et al. (23), who found no Yersinia pseudotuberculosis TC-related toxicity toward the rat flea Xenopsylla cheopis even though expression of the TC genes is upregulated during the disease process. Bowen et al. (5) also identified insecticidal activity when the P. luminescens toxin complex (TCA) was injected into the hemocoel of Manduca sexta.
Although the mode of action of TC proteins has yet to be fully elucidated, the expression of individual TC genes has been reported to be sufficient to cause toxicity (45, 50). Full toxicity requires all three toxin complex ABC components, with the TCB and TCC components providing a potentiation of toxicity. If the TCB and TCC components are coexpressed in the same cell, they can be combined with TCA components from other species, or other TC clusters to cause an effect with altered host specificity, suggesting that the TCA component relates to target host range (32, 60, 69). Further evidence for this was provided by Lee et al. (43), who showed that the X. nematophila XptA1 TCA component is able to bind to brush border membrane vesicles of Pieris brassicae, and through electron microscopy and three-dimensional (3-D) structural analysis showed that XptA1 forms a tetramer-like structure with which the TCB and TCC components are theorized to interact. Lang et al. (42) proposed a model in which the P. luminescens TCA component (TcdA1) first makes contact with the target cell wall, whereby the TCC-type toxins TccC3 and TccC5 are internalized into the cytosol, inducing actin clustering, through ADP-ribosylation and RhoA GTPase activity, respectively.
Recently, changes in culture temperature have been found to influence TC gene expression. Through the use of Tn5lux Y. enterocolitica W22703 genome fusions, Bresolin et al. (8) found that temperatures below 30°C were optimal for the expression of the TC genes, TCA-like (tcaA, tcaB1, and tcaB2), TCB-like (tcaC), and TCC-like (tccC) genes, and that sonicated cell extracts, but not culture supernatants, derived from Y. enterocolitica W22703 grown at 30°C showed insecticidal activity toward the larva of M. sexta. However, sonicated cellular extracts derived from a strain containing a mutation in tcaB1 also exhibited activity toward M. sexta (8), suggesting that the activity may not be TC related. Hares et al. (31) demonstrated that the Y. pseudotuberculosis IP32953 strain secretes its TC derivative into the culture medium between 28 and 37°C, with a basal level of expression and secretion at 20°C but no expression at 15°C. However, the TC had no significant activity toward M. sexta larvae.
The bacterium Yersinia entomophaga MH96 was isolated from a diseased C. zealandica larva, and through host range testing, it was shown to have broad-host-range insect activity, affecting a number of coleopteran and lepidopteran species with the greatest biological activity against members of the Scarabaeidae family (36). After ingestion of this bacterium, the larvae change from a healthy gray color to a cream color and then shiny brown in a process that is accompanied by regurgitation, acute diarrhea, and then death of the insect within 72 h of infection (M. R. H. Hurst, unpublished data).
In this study, we describe the transposon mutagenesis, nucleotide sequence analysis, and identification of a unique cluster of TC genes that are the main virulence components of a 32-kb pathogenicity island termed PAIYe96 and represent a new member of the TC family. We demonstrate that at temperatures at or below 25°C, Y. entomophaga MH96 releases large amounts of the TC into Luria-Bertani (LB) broth, while at 37°C no TC is released into culture supernatant. By using ultracentrifugation, we could purify the TC. The TC could be visualized by electron microscopy. The host range activity toward a number of insect species of the TC could then be assessed.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. Bacteria were grown in Luria-Bertani (LB) broth or on LB agar, and the bacteria were grown at 37°C for Escherichia coli and at 30°C for Y. entomophaga MH96 and its derivatives. Cultures were incubated with shaking at 250 rpm in a Raytek orbital incubator. The antibiotics used for Y. entomophaga MH96 were ampicillin (400 μg ml−1), chloramphenicol (90 μg ml−1), kanamycin (100 μg ml−1), tetracycline (30 μg ml−1), and spectinomycin (100 μg ml−1). The antibiotics used for E. coli were ampicillin (100 μg ml−1), chloramphenicol (30 μg ml−1), kanamycin (50 μg ml−1), tetracycline (15 μg ml−1), and spectinomycin (100 μg ml−1).
Table 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| E. coli strains | ||
| DH10B | F−mcrA Δmrr-hsdRMS-mcrBCΔ80d lacZΔM15 ΔlacX74 endA1 recA1 deoR Δara leu-7697 araD139 galU galK nupG rpsL Δ− | 47 |
| S17-1 λpir | hsdR Pro ΔrecA RP4-2 Tc::Mu Kn::Tn7 integrated in the chromosome of the pir gene | 49 |
| XL1-BlueMRA | ΔmcrA183 ΔmcrCB-hsdSMR-mrr-173 endA1 supE44 thi-1 reA1 gyrA96 relA1 | Stratagene |
| Y. entomophaga strains | ||
| MH96 | Type strain; isolated from a diseased C. zealandica larva | 36 |
| MH96::1 | Tn5Km1 insertion 749 bp 3′ of the initiation codon of yenB; Knr | This study |
| MH96::2 | Tn5Km1 insertion 739 bp 3′ of the initiation codon of yenB; Knr | |
| MH96::3 | Tn5Km1 insertion 3,049 bp 3′ of the initiation codon of yenA2; Knr | |
| MH96::4 | Tn5Km1 insertion 2,946 bp 3′ of the initiation codon of yenA2; Knr | |
| MH96::5 | Tn5Km1 insertion 2,892 bp 3′ of the initiation codon of yenA2; Knr | |
| MH96::6 | Tn5Km1 insertion 1,649 bp 3′ of the initiation codon of yenA1; Knr | |
| MH96::7 | Tn5Km1 insertion 2,805 bp 3′ of the initiation codon of yenA1; Knr | |
| MH96::8 | Tn5Km1 insertion 2,945 bp 3′ of the initiation codon of yenA1; Knr | |
| MH96::9 | Tn5Km1 insertion 742 bp 3′ of the initiation codon of yenB; Knr | |
| MH96::10 | Tn5Km1 insertion 2,933 bp 3′ of the initiation codon of yenA1; Knr | |
| MH96::11 | Tn5Km1 insertion 2,061 bp 3′ of the initiation codon of yenA1; Knr | |
| MH96::12 | Tn5Km1 insertion 9,375 bp 3′ of the initiation codon of chi1; Knr | |
| MH96::13 | Tn5Km1 insertion 1,557 bp 3′ of the initiation codon of chi2; Knr | |
| MH96Sp | MH96 with a spectinomycin resistance cassette recombined into the SphI site of yen10; Spr | This study |
| ΔTC strain | MH96 with 19,067-bp BglII deletion and with yenA1yenA2 chi2yenByenC1 yenC2 genes; Cmr | This study |
| ΔC1-2 strain | MH96 with 2,649-bp EcoRV deletion and yenC1yenC2 genes; Spr | This study |
| ΔU-W strain | MH96 with 5,943-bp NcoI deletion and with yenU yenVyenTyenW genes; Knr | This study |
| Plasmids | ||
| pAYC177 | Amr Knr | 12 |
| pACYC184 | Cmr Tcr | 12 |
| pBRminicos2 | pBR322 containing pLAFR3-derived BglII cos site inserted into its BamHI site; Amr | 34 |
| pBRminicospac | pBRminicos2 with kanamycin resistance gene flanked by PacI- and EcoRI-flanked restriction enzyme sites inserted into the EcoRI site; Amr Knr | This study |
| pHP45 | Contains spectinomycin-resistant Ω fragment; Apr Spr | 56 |
| pJP5608 | R6K-based suicide plasmid; Tcr | 52 |
| pJPSK | pJP5608 containing the SphI kanamycin-resistant fragment from pPSH; Tcr Knr | This study |
| pJPRI | pJP5608 containing the EcoRI spectinomycin-resistant fragment from pPRISP; Tcr Spr | This study |
| pK3X | Genomic XbaI 18,243-bp clone; derived from strain MH96::6; Knr | This study |
| pKSAL | Genomic SalI 23,194-bp clone; derived from strain MH96::9; Knr | This study |
| pKSALH3 | pKSAL digested with HindIII and self-ligated; Amr | This study |
| pKSALSp | pKSALH3 digested with HindIII and SalI and ligated into the analogous sites of pVIK165; Knr Spr | This study |
| pLAFR3 | pRK290 with cos, lacZ, and multicloning site from pUC8; Tcr | 63 |
| pLAFR3P | pLAFR3 with kanamycin resistance cassette flanked by EcoRI and PacI restriction enzyme sites inserted into the EcoRI site of pLAFR3; Tcr | This study |
| Mini-Tn5Km1 | Mini-Tn5 derivative; Amr Knr | 17 |
| pPAC12 | MH96Sp 41,147-bp PacI fragment in the PacI site of pBRminicospac; clone with TC virulence genes; Amr Spr | This study |
| pPAC14 | Opposing PacI clone orientation to pPAC12; clone with TC virulence genes; Amr Spr | This study |
| pPAC14ΔBg | pPAC14 containing a chloramphenicol resistance cassette inserted into the BglII sites deleting a 19,067-bp BglII fragment; Amr Spr Cmr | This study |
| pPRI | pPAC14 with 6,565-bp EcoRI-derived fragment in pUCM19; Cmr | This study |
| pPRISP | pPRI containing an EcoRV-flanked spectinomycin cassette in the EcoRV site, encompassing yenC1-yenC2; Cmr Spr | This study |
| pPSH | pPAC14 13,667-bp SphI-derived fragment in pUCM19; Cmr | This study |
| pPSK | pPSH containing a kanamycin NcoI-flanked cassette in the NcoI site; Cmr Knr | This study |
| pUCM19 | Contains lacZα and multicloning site; Cmr | 64 |
| pUC19 | Contains lacZα and multicloning site; Apr | 71 |
| pVIK165 | Suicide plasmid; Knr | 38 |
| pVSALSp | pKSALSp, digested with HindIII and SalI and ligated into the analogous sites of pVIK165; Knr Spr | This study |
General DNA techniques.
Standard DNA techniques, including Southern blotting and colony hybridizations were performed as described by Sambrook et al. (59). Plasmids were electroporated into the appropriate Y. entomophaga MH96 strains using a Bio-Rad gene pulser (25 μF, 2.5 kV, and 200 Ω) (20). pBRminicos2 derivatives were packaged using a Gigapack IIIXL packaging extract (Stratagene). Primers and amplicons used in the study are listed in Table S1 in the supplemental material. PCR products and plasmid templates were purified using the high pure PCR product purification kit and the high pure plasmid isolation kit (Roche Diagnostics GmbH), respectively. The pKSAL and pKX3 clones (Table 1) were sequenced at the Australian Genome Research Facility (http://www.agrf.org.au/). Sequences were assembled using SEQMAN (DNASTAR Inc., Madison, WI). Databases at the National Center for Biotechnology Information (NCBI) were searched using BlastN, BlastX, and BlastP (1). Potential secretion signal sequences and associated cleavage sites were predicted by SignalP 3.0 server (http://www.cbs.dtu.dk/services/SignalP/).
Phylogenetic and similarity analyses of YenA2.
The protein YenA2 was chosen, as evidence suggests that the TCA protein specifies target host range (32, 60, 69). Conserved sequences were identified by carrying out a BlastP search against the nonredundant protein sequence database at the NCBI. Full-length, or nearly full-length, RefSeq sequences returning an E value of 0.0 were selected for analysis. Protein sequences were aligned with ClustalW (67) and then concatenated, trimmed, and back translated to nucleotides, and then multiple alignment was undertaken using MEGA4 (65). The alignment was cleaned with GBlocks (11), specifying a minimum block length of 5, the presence of an amino acid in 50% of sequences to qualify as conserved, and a maximum of 8 contiguous nonconserved sites. Saturation of the resulting sequence of 46 conserved nucleotide blocks was tested by DAMBE (70). Parameters for the best approximation of nucleotide substitution in this data set were identified by Modeltest 3.8 (55). Phylogenetic relationships were estimated with PHYML (28) using maximum likelihood with a heuristic search, based on a neighbor-joining starting tree. The robustness of the topology was estimated with 1,000 bootstrap replicates. Consensus trees were drawn with the PHYLIP (24) program consense using the extended majority rule.
Transposon mutagenesis.
Transposon mutagenesis was performed using the mini-Tn5 derivative Tn5 Km1 as described previously (17). To allow antibiotic selection of the recipient strain, the pACYC184 plasmid was transformed into Y. entomophaga MH96. The region including and flanking the transposon insertion point was cloned into pUC19 using Tn5 Km1-derived restriction enzyme sites, and the DNA was sequenced using the transposon-specific primers TnR1 and TnPst. Antibiotic markers were recombined into Y. entomophaga MH96 by either homologous recombination, mediated by the suicide vector pVIK165 as described previously (38), or through pLAFR3-based homologous recombination as previously described (33). Recombinants were validated by PCR and Southern analysis.
Cloning the virulence region of Y. entomophaga MH96.
Restriction enzyme analysis identified that the entire PAIYe96 TC-like cluster could be cloned using the restriction enzyme PacI (Fig. 1B). The spectinomycin resistance amplicon SPSP (see Table S1 in the supplemental material) was ligated into the SphI site of pKSALH3 in a region outside PAIYe96 (Fig. 1B). The formed construct (pKSALSp) was digested with HindIII and SalI ligated into the analogous site of pVIK165 to form pVSALSp (Table 1), allowing subsequent integration of the antibiotic cassette into the Y. entomophaga MH96 chromosome by homologous recombination to form Y. entomophaga MH96Sp. Y. entomophaga MH96Sp genomic DNA was purified, digested with PacI, ligated to the analogous site of pBRminicospac, and transduced into the E. coli strain XL1-BlueMRA. Several spectinomycin-resistant colonies were screened by restriction enzyme analysis, and two constructs designated pPAC12 and pPAC14 of opposing clone orientation were stored.
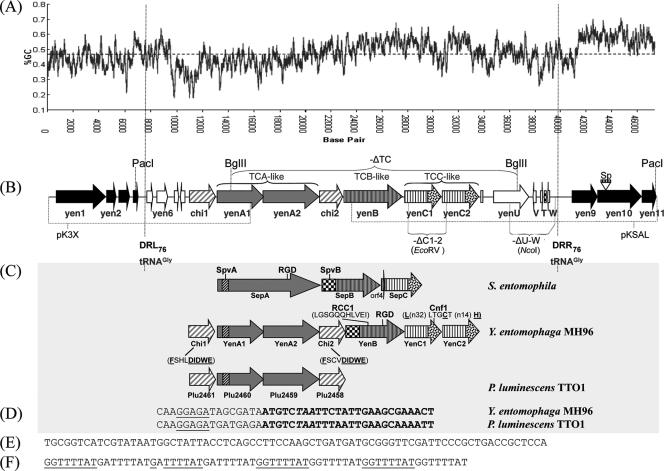
Fig. 1.
(A) G+C content of PAIYe96 and the associated genomic region (window size 300, window position shift). (B) Schematic of Y. entomophaga MH96 PAIYe96. Black arrows denote Y. entomophaga MH96 genomic DNA. The locations and designations of the PAIYe96 virulence determinants as defined in Table 2 are shown. The location of the SphI genomically integrated spectinomycin resistance cassette (Sp) to form Y. entomophaga MH96Sp is shown (Table 1; see text); the PacI restriction enzyme sites used to clone the region associated with the virulence genes are indicated. The BglII, EcoRV, and NcoI restriction enzyme sites used to delete the virulence gene-associated region in the ΔTC mutant strain and associated yenC1-yenC2 (-ΔC1-2) and yenUVTW (-ΔU-W) ORFs, respectively, are depicted (see text). The pKSAL and pKX3 sequencing clones (Table 1) are indicated. (C) Similarity of the toxin complex (TC) YenA1 and YenA2-chitinase associated region to its closest orthologues of P. luminescens TTO1 (Table 2) and comparison of the relative size of the YenA1 and YenA2 proteins to the toxin complex A (TCA) S. entomophila SepA orthologue. Regions of amino acid similarity to SpvA and SpvB are indicated. Predicted protein domains: YenA1 (SpvA, amino acids [aa] 56 to 185); YenB, (SpvB, aa 9 to 366; RCC1, aa 572 to 582; RGD, aa 836 to 838); Chi1 (Chi 18 hydrolytic site, aa 248 to 256); Chi2 (Chi 18 hydrolytic site, aa 431 to 349); YenC1 (necrotizing factor Cnf1, aa 815 to 819, and its associated Rho-activating domain and catalytic triad [10] [underlined] is indicated). The vertical lines denote the conserved amino terminus and the stippling in the arrows indicates the variable carboxyl region of the TCC-like Rhs elements (33). (D) Nucleotide sequence of the Y. entomophaga MH96 (yenA1-yenA2) and P. luminescens TTO1 (plu2460-plu2459) translational coupling DNA sequence. The termination codon of yenA1 or plu2460 is shown in italic type. The 5′ end of the yenA2 or plu2459 ORF is shown in bold type. The predicted yenA2 or plu2459 ribosomal binding site is underlined. (E) tRNAGly 76-bp direct repeats (DRL76 and DRR76). (F) Nucleotide sequence of the eight-copy 8-bp repeat located upstream of yenU.
Construction of a PAIYe96-TC and yenC1 yenC2 and yenUVTW deletion variants.
To construct the Y. entomophaga MH96 TC deletion derivative ΔTC strain, the chloramphenicol resistance amplicon BGCM was cloned into the BglII site of pPAC14, deleting 19,067 bp of DNA (Table 1 and Fig. 1B) to form pPAC14CM. The pPAC14CM construct was digested with PacI and ligated into the analogous site of pLAFRP (Table 1), and the chloramphenicol marker was recombined into the genome of Y. entomophaga MH96 by homologous recombination. To construct a double yenC1 yenC2 mutant, the 1,379-bp EcoRV-flanked spectinomycin resistance amplicon RVSP was ligated into the analogous site of the pPAC14 EcoRI subclone pPRI (Table 1) to form pPRISP. The EcoRI spectinomycin resistance-containing fragment was then cloned into the analogous site of the suicide vector pJP5608 to form pJPRI, which allowed the generation of the yenC1 yenC2 ΔC1-2 mutant by homologous recombination (Fig. 1B and Table 1). Deletion of yenUVTW was accomplished by cloning the NcoI-flanked kanamycin resistance amplicon NCKN into the analogous site of the pPAC14 SphI subclone (pPSH). The construct formed (pPSK) was digested with SphI, and the kanamycin resistance-bearing fragment was ligated into the SphI site of pJP5608. The correct construct, pJPSK (Table 1), was conjugated into Y. entomophaga MH96, allowing the deletion of yenUVTW by homologous recombination and the formation of the ΔU-W strain.
Purification of Y. entomophaga MH96 TC.
From a 50-ml overnight culture grown at 25°C, bacterial debris was removed by centrifugation (10 min, 10,000 × g, 4°C) followed by filter sterilization of the supernatant through a 0.2-μm Sartorius Minisart filter into a sterile tube. A 4-ml aliquot of the supernatant was centrifuged (2 h, 250,000 × g, 4°C) in a Sorvall RZ M120EX ultracentrifuge. The pellet was resuspended in 100 μl of 0.5× LB broth and further centrifuged (10 min, 12,000 × g, 4°C). The supernatant was applied to the surface of a 4.5-ml step gradient (1.0 ml of 5% glycerol layered on top of 3.5 ml of 40% glycerol in 0.5× LB broth) and centrifuged (2 h, 250,000 × g, 4°C). The pellet was finally resuspended in 0.25 ml of TM buffer (0.05 M Tris [pH 7.5], 0.1 M MgSO4·7H2O).
Protein analysis and chitinase assay.
Protein concentrations were determined based on the method of Bradford (6), using the Bio-Rad protein assay kit. Assessment of chitinase activity was undertaken using the Sigma Aldrich chitinase assay kit, which uses an assay based on the release of p-nitrophenol, from a chitinase substrate (the substrates for exochitinase activity were 4-nitrophenyl-N,N′-diacetyl-β-d-chitobioside and 4-nitrophenyl-N-acetyl-β-d-glucosaminide-β-N-acetylglucosaminidase; the substrate for endochitinase activity was 4-nitrophenyl β-d-N,N′,N″-triacetylchitotriose) which upon ionization in basic pH can be measured colorimetrically at 405 nm. Both assays were undertaken in accordance with the manufacturer's instructions, and measurements were recorded by a FLUOstar Optima (BMG Labtech) plate reader. Standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described previously (41). Proteins were visualized by silver staining by the method of Blum et al. (4). Western immunoblotting was performed by the method of Blake et al. (3), using New Zealand White rabbit polyclonal antibodies raised against peptides (Mimotopes, Pty Ltd.) based on the amino- and carboxyl-terminal amino acid sequences (YenA1, H-MDKYNN-C-GDSDNV–OH; YenB, MQNSQE-C-DTAALAI; YenC1, H-MNQFDS-C-TVVKLR-OH; YenC2, MDIQLFS-C-LKRRKSF) linked at the central cystine residue to keyhole limpet hemocyanin as the carrier.
Protein peptide analysis.
Protein bands were excised from Coomassie brilliant blue-stained SDS-polyacrylamide gels, submitted to trypsin digestion, and prepared for liquid chromatography-electrospray ionization ion trap-tandem mass spectrometry (LC-ESI-MS/MS) as previously described (53). LC-ESI-MS/MS was performed at the School of Biological Sciences, Victoria University, New Zealand (http://www.victoria.ac.nz/sbs/). Protein identities were assigned using Mascot (version 2.1.03; Matrix Science) using the NCBI nonredundant protein database (see Table S2 in the supplemental material for GenBank accession numbers). For N-terminal sequencing, bands containing 2 to 10 pmol of protein were analyzed by Edman degradation using an Applied Biosystems 494 Procise protein sequencing system and the “Pulsed Liquid PVDF” sequencing method at the Australian Proteome Analysis Facility (APAF Ltd.; http://www.proteome.org.au). Potential protein coiled-coil regions were predicted by Coiled-coil predictions server (http://npsa-pbil.ibcp.fr/cgi-bin/primanal_lupas.pl).
Assessment of the culture supernatants and sonicated filtrates for insect activity and assessment of the effect of temperature on TC stability.
Cultures were independently grown at 20, 25, 30, and 37°C to an optical density at 600 nm (OD600) of 0.6, and the cell pellets and culture supernatants were assessed by SDS-PAGE. For sonication, the cells were harvested by centrifugation (5 min, 8,000 × g, 4°C) and the pellet was resuspended in 1.2 ml of phosphate-buffered saline (10 mM sodium phosphate buffer [pH 7.4], 2.7 mM KCl, 137 mM NaCl). Two 0.7-ml samples were transferred to a 1.7-ml microcentrifuge tube and subjected to three 20-s rounds of sonication on wet ice using a Sanyo Soniprep 150 sonicator (18 Ω). The sonicated samples were centrifuged (3 min, 16,000 × g, 4°C), and the supernatant was filter sterilized by passage through a 0.2-μm Sartorius Minisart filter to a sterile tube. To determine the effect of temperature on the toxin, 500-μl aliquots of culture filtrate derived from a culture grown at 25°C were subjected independently to 25, 35, 42, 45, 50, 55, 60, and 70°C for 15 min and then assessed for loss of insecticidal activity by the standard bioassay against C. zealandica larvae.
Transmission electron microscopy.
To visualize the TC, 3 μl of the TC preparation obtained after ultracentrifugation was applied to Formvar-coated 300-mesh copper grids. The sample was left for 5 min, and excess fluid was drawn off with absorbent filter paper. A drop of 2% phosphotungstic acid was then added to each grid, and the excess fluid was drawn off with absorbent filter paper. The grids were air dried and examined with a Hitachi H-600 electron microscope (80 kV) at a magnification of ×150,000.
For assessment of outer membrane vesicle (OMV) formation by bacteria, bacteria (1.5 ml) from an overnight culture were pelleted by centrifugation (10 min, 4,000 × g, 4°C) and resuspended in 1.5 ml of sterile distilled water (dH2O). The sample was diluted 1:100 in a solution of 2% gluteraldehyde in 0.1 M phosphate buffer (0.65 mM K2HPO4, 0.35 mM KH2PO4) at pH 7.2, and the sample was left for 2 h. An aliquot of the solution was then added to an aluminum holder and dried under vacuum. The samples were sputter coated with gold-palladium and then examined using a JEOL JSM 7000F field emission gun scanning electron microscope (5 kV) at a working distance of 15 mm.
Bioassay and dose response.
Bioassays of the TC against the larvae of the New Zealand grass grub (Costelytra zealandica), redheaded cockchafer (Adoryphorus couloni), and the Tasmanian grass grub (Acrossidius tasmania) were performed. Healthy second- or third-instar larvae collected from the field were individually fed squares of carrot that had been rolled on a lawn of bacteria grown overnight on LB agar, resulting in approximately 107 cells per carrot cube. In the case of toxin, 5 μl (100 ng) of filtrate sample was applied to the surface of a 3-mm3 carrot piece, from which the larvae would feed. Twelve second- or third-instar larvae were used for each treatment. Inoculated larvae were maintained at 15°C in ice-cube trays or, for A. couloni larvae, in 12-well Corning Costar cell culture plates. The larvae were fed treated carrot for 5 days, and signs of disease were noted.
For neonate larvae of the codling moth (Cydia pomonella), light brown apple moth (Epiphyas postvittana), cotton bollworm (Helicoverpa armigera), and the cluster caterpillar (Spodoptera litura), 20 μl of TC (approximately 400 ng) was applied to an air-dried section (10- by 10- by 3-mm3) of general purpose laboratory diet mixture (61), allowing the solution to absorb and rehydrate the matrix. In the case of the diamondback moth (Plutella xylostella), filter-sterilized culture supernatant was diluted 50% in 0.04% Duwett (Elliot Chemicals Ltd.), and 15 μl of the dilutant was spread on a 2-cm-diameter cabbage leaf disc. The leaf was air dried for 2 h at ambient temperature, and the leaf disc was then placed on a piece of Whatman filter paper (grade no. 5) located on the bottom of a small plastic bottle. Six second- or third-instar P. xylostella larvae were added per treatment. Lepidopteran larvae were maintained on the laboratory bench at ambient temperature (approximately 20°C).
To determine the 50% lethal doses (LD50s) of the TC against C. zealandica and P. xylostella larvae, a 10-fold dilution series of filter-sterilized culture supernatant was set up in 0.1 M phosphate buffer. A 5-μl aliquot of each dilution was inoculated onto the diet the insect was being fed and assessed at day 5 for relative efficacy. To assess the ability of the TC to affect Galleria mellonella larva by hemocoelic injection, 10-fold serial dilutions of the filter-sterilized culture supernatant in 0.1 M phosphate buffer were made, and 10 μl of each dilution was injected into the leg of the third segment of the insect larva.
In all cases, the treatments were randomized, the bioassays were performed a total of three times, and the insects were observed daily for progression of disease. Negative controls had a similar treatment applied but using the TC deletion variant ΔTC strain. On day 3, any remaining food was removed and replenished with fresh untreated food. Sample sizes are shown below in tables.
Sectioning and staining.
Larvae from bioassays were chilled on ice (20 min) and then fixed in Bouin's fluid (24 h). Several incisions were made into the cuticle of first-instar larvae allowing penetration of the fixative. Following fixation, larvae were sent to Gribbles Veterinary Pathology New Zealand for sectioning. Mounted sections were examined by light microscopy with an Olympus BX50 light microscope.
Nucleotide sequence accession number.
The nucleotide sequences were deposited in GenBank under accession number DQ400808.
RESULTS
Identification of the Y. entomophaga MH96 virulence determinants.
In total, 1,400 independent Y. entomophaga MH96 mini-Tn5 Km1 mutants were screened for loss of virulence activity toward C. zealandica larvae by standard bioassay. Thirteen avirulent mutants were identified, and DNA sequence analysis of the cloned mini-Tn5 Km1 genomic DNA junction points identified that 11 of the mutations were located in TCA and TCB-like toxin complex genes, while the other two mutations were located in DNA with BlastX similarity to chitinase enzymes (Table 2). To complete DNA sequence analysis of the virulence determinants, two clones, pK3X and pKSAL, were constructed using Y. entomophaga MH96::6 and Y. entomophaga MH96::9 (Table 1) genomic DNA and the restriction enzymes XbaI and SalI, respectively (Fig. 1B) and their DNA was sequenced. In total, 47,476 bp was sequenced and deposited in GenBank under accession number DQ400808.
Table 2.
Similarities of products of putative ORFs to translated amino acid sequences in the NCBI database detected using BlastP
| Putative ORF product (size [no. of amino acids]) | No. of bp | Similarity, locus tag, protein domain (size [aa])a | Organism | Accession no. |
|---|---|---|---|---|
| Yen1 (1,522) | 84–4,652 | 85/91//1520 (1–1521), YintA_01003310, COG2202 (1,522) | Yersinia intermedia ATCC 29909 | ZP_04635957 |
| Yen2 (319) | 4,649–5,605 | 87/93//315 (1–315), YintA_01003311 COG3706; response regulator containing COG3706(319); CheY-like receiver domain and a GGDEF domain | Y. intermedia ATCC 29909 | ZP_04635956 |
| Yen3 (180) | 5,920–6,459 | 91/94//179 (1–179), hypothetical protein YPK_2500 (179) | Yersinia pseudotuberculosis YPIII | YP_001721230 |
| Yen4 (232) | 7,381–6,683 | 90/93//232 (1–232), pseudouridylate synthases, 23S RNA-specific COG0564 (232) | Yersinia mollaretii ATCC 43969 | ZP_04639205 |
| DRL76 | 7,877–7,952 | 97%b (489–504) tRNAGly | Escherichia coli | X04176 |
| Yen5 (122) | 7,978–8,343 | 69/79//119 (1–119), hypothetical protein yrohd0001_21470 Fels-1 prophage protein-like; pfam05666 | Yersinia rohdei ATCC 43380 | ZP_04611936 |
| Yen6 (278) | 8,767–9,600 | 82/91//276 (1–276), signal transduction histidine kinase (451) | Y. mollaretii ATCC 43969 | ZP_04640870 |
| Yen7 (121) | 9,962–10,324 | 55/71//119 (2–120), hypothetical protein UTI89_C4899 (122) | E. coli UTI89 | YP_543833 |
| Yen8 (138) | 10,935–10,519 | No similarity, transcriptional regulatory protein, C terminal, pfam00486 | ||
| Chi1 (543) | 11,709–13,337 | 55/71//551 (2–541), unnamed protein product Plu2461 (544); putative exochitinase, ChiA-like COG3325, pfam00704.13 | Photorhabdus luminescens subsp. laumondii TTO1 | CAE14835 |
| 40/57//517 (29–519), exochitinase CBM_5_12 (695) | Sodalis glossinidius | CAA72201 | ||
| 27/43//454 (62–503), ORF42; ChiA chitinase, (564) | Spodoptera litura nucleopolyhedrovirus | ABS70983 | ||
| 35/49//329 (251–560), ChiA, endochitinase (563) | Serratia marcescens | NP_258310 | ||
| 39/53//524 (30–518), Chi2 (634) | Y. entomophaga MH96 | ABG33867 | ||
| YenA1 (1,165) | 13,423–16,917 | 55/71//1183 (1–1164), insecticidal toxin complex protein TccA2 Plu2460 (1,173) | P. luminescens subsp. laumondii TTO1 | CAE14834 |
| 29/50//134 (187–316), virulence protein SpvA (187) | Salmonella enterica subsp. arizonae | AAD02678 | ||
| YenA2 (1,361) | 16,910–21,004 | 68/81//1376 (1–1363), insecticidal toxin complex protein TccB2 Plu2459 (1,362) | P. luminescens subsp. laumondii TTO1 | CAE14833 |
| Chi2 (634) | 21,102–23,003 | 72/82//627 (1–627), unnamed protein product Plu2458 (622), putative chitinase COG3325; pfam00704 | P. luminescens subsp. laumondii TTO1 | CAE14832 |
| 38/52//523 (110–616), Chi1 (543) | Xenorhabdus nematophila | ABG33870 | ||
| 54/67//655 (4–648), Chi (648) | Y. entomophaga MH96 | CAC38398 | ||
| YenB (1,488) | 23,198–27,661 | 59/74//1488 (1–1483), insecticidal toxin complex protein TcaC (1,485) | P. luminescens | AAL18451 |
| 46/60//358 (9–366), SpvB (591) | Salmonella enterica subsp. enterica serovar Choleraesuis | NP_073228 | ||
| YenC1 (975) | 27,757–30,681 | 59/73//679 (8–683), TccC1/XptB1 protein (1,016) | Xenorhabdus nematophila | AAS45281 |
| 31/46//260 (699–948), Pnf, Rho-activating domain of cytotoxic necrotizing factor (287) | P. luminescens | AAN64212 | ||
| YenC2 (971) | 30,903–33,815 | 70–79//690 (1–690), PTB2233, putative insecticidal toxin complex (994) | Y. pseudotuberculosis IP 32953 | CAH21471 |
| 48/63//1003 (2–956), insecticidal toxin complex protein TccC6 (965) | P. luminescens subsp. laumondii TTO1 | NP_931365 | ||
| 33,930–34,250 | 54–70//48,b remnant DNA sequence of SAM-dependent methyltransferases | Y. mollaretii ATCC 43969 | EEQ11137 | |
| YenU (857) | 34,810–37,380 | 27/467//287 (546–795) hypothetical protein Pfl_2644 (1,288) | Pseudomonas fluorescens PfO-1 | ABA74385 |
| 46/65//145 (140–283) hypothetical protein ETA_11720 | Erwinia tasmaniensis Et1/99 | YP_001907112 | ||
| YenV (151) | 38,263–37,811 | 79/88//134 (16–149) hypothetical protein ECA2267 (153), pfam08332.1 | Klebsiella pneumoniae | CAQ76722 |
| YenT (64) | 38,757–38,948 | 45/58//51 (1–51) Yersinia heat-stable enterotoxin type B (71) | Y. enterocolitica | P74977 |
| 38,890–38,934 | 90%b (1208–1248) insertion sequence IS911v4 | E. coli | AJ507493 | |
| YenW (95) | 39,412–39,125 | No similarity | ||
| 39,640–39,718 | 80%b (3102–3183) bacteriophage P27 tRNA-Ile-tRNA-Arg | E. coli | AJ249351 | |
| DRR76 | 39,850–39,925 | 97%b (489–504) tRNAGly | E. coli | X04176 |
| Yen9 (599) | 41,182–42,978 | 67/75//595 (4–598) ESA_01767 (604) | Enterobacter sakazakii ATCC BAA-894 | ABU77021 |
| Yen10 (1,211) | 42,988–46,620 | 76/84//1207 (1–1206) ESA_01768 (1,213) | E. sakazakii ATCC BAA-894 | ABU77022 |
| Yen11 (233) | 46,655–47,353 | 70/83//221 (8–228) ESA_01769 01769 (243) | E. sakazakii ATCC BAA-894 | ABU77023 |
Percent identities and percent similarities were calculated in relation to the deduced gene products of the sequenced ORF. The positions of amino acid similarity (percent identity/percent similarity over the indicated range of amino acid residue positions shown in parentheses) in relation to sequence generated in this study are shown; for example, 85/91//1520 (1–1521) means that there was 85% identity and 91% similarity over the indicated residues. aa, amino acids.
Percent DNA similarity compared to orthologue. See Fig. 1B for schematic of Y. entomophaga MH96 virulence-associated region.
Sequence analysis of the virulence region of PAIYe96.
Analysis of the completed DNA sequence of the Y. entomophaga MH96 virulence-associated region identified the presence of two TCA-like genes (yenA1 and yenA2), a TCB-like gene (yenB), and two TCC-like genes (yenC1 and yenC2) that reside on a 31,898-bp pathogenicity island (PAI) flanked by two tRNAGly 76-bp direct DNA repeats, designated DRL76 and DRR76 (Fig. 1B and E and Table 2). The PAI has a G+C content of 43.8%, which is lower than the predicted genome content of G+C, which is 49.3% (36), and has been designated PAIYe96. Located 118 bp 5′ of DRR76 is a region of DNA with similarity to tRNA-Lys and tRNA-Arg (Table 2). An eight-copy 8-bp repeat is located 440 bp upstream of yenU (Fig. 1F). Positioned between the yenC2 and yenU open reading frames (ORFs) is a region of DNA with disjointed BlastX similarity to an S-adenosylmethionine (SAM)-dependent methyltransferase (Table 2), which in Y. enterocolitica subsp. enterocolitica 8081 is located juxtaposed to tRNA-Pro. However, one region (bp 38890 to 38935) shares 90% DNA identity to the remnant transposase subunit B of transposon IS911 (Table 2).
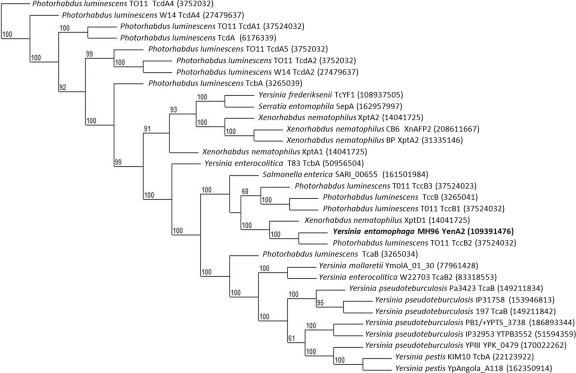
The TCA-like genes are divided into two components, yenA1 and yenA2 and, similar to the P. luminescens TTO1 gene cluster (plu2461-plu2458), are located juxtaposed to two chitinase-encoding genes. The translated products of yenA1 (130 kDa) and yenA2 (156 kDa) align successionally to span the entire region of their larger TCA orthologues such as the 262-kDa SepA protein (Fig. 1C). DNA sequence analysis identified that the initiation codon of yenA2 is positioned five nucleotides upstream of the termination codon of yenA1; a similar scenario was identified in the P. luminescens TTO1 (plu2460-plu2459) DNA sequence (Fig. 1D). Phylogenetic analysis of YenA2 showed that it was most similar to the TccB2 (Plu2459) and the X. nematophilus TC protein XptD1 (Fig. 2). The predicted Chi1 and Chi2 proteins have high similarity to chitinases from Serratia marcescens and the insect symbiont Sodalis glossinidius and contain a Chi18 hydrolytic motif (68) (Table 2).
Fig. 2.
Phylogenetic analysis consensus tree for YenA2 sequence to its closest orthologues. The tree is based on 3,515 trimmed nucleotides. The robustness of the topology was estimated with 1,000 bootstrap replicates. Bootstrap proportions are indicated at the branches. GenBank accession numbers are given in parentheses. The tree was drawn with Treedyn (15).
The yenT ORF located within PAIYe96 encodes a 64-amino-acid protein with high similarity to heat-stable enterotoxins of E. coli and Yersinia spp. (Table 2), some of which activate guanylate cyclase within the host intestinal epithelial cells, resulting in water loss (21). Amino acid alignment of these proteins identified a high degree of amino-terminal similarity preceding a predicted signal cleavage site at residue 23 of YenT. However, no similarity to the enterotoxin carboxyl-based “toxin domain” was identified (see Fig. S1 in the supplemental material). A putative two-component regulator Yen6 is positioned 5′ to the PAIYe96 TC gene cluster, while another putative regulator, Yen2, is located external to PAIYe96 5′ to DRL76 (Fig. 1B and Table 2). Six additional ORFs comprise the remainder of PAIYe96, four of which have no significant identity to protein sequences in the current database (Table 2).
Defining the compound active on insects.
Bioassays of the TC gene-bearing pPAC12 and pPAC14 clones in an E. coli background and the Y. entomophaga MH96 ΔTC strain (Table 1) toward C. zealandica larvae were performed, and the toxins were found to be innocuous, causing no symptoms of disease (Table 3). However, the ability to cause virulence could be restored to the ΔTC strain by transforming the bacterium with either of the TC gene-bearing pPAC12 or pPAC14 clones (Table 3). It is of interest that no Tn5 Km1-based insertions were identified in the yenC1, yenC2, and yenUVTW ORFs. To ascertain the roles of these genes in virulence, yenC1 yenC2 and yenUVTW deletion variants were constructed (as outlined in Materials and Methods). Bioassays of both Y. entomophaga MH96 ΔC1-2 and ΔU-W strains against C. zealandica larvae showed abolition and no alteration in virulence, respectively (Table 3).
Table 3.
Oral toxicity of Y. entomophaga and E. coli strains to C. zealandica larvae on day 5
| Straina | Mortality (n)b |
Significance level of the differencec | |
|---|---|---|---|
| Treated | Control | ||
| MH96 | 36 (36) | 0 (36) | <0.000 (2.2 × 10−16) |
| MH96Sp | 36 (36) | 0 (36) | <0.000 (2.2 × 10−16) |
| MH96::4 | 0 (36) | 0 (36) | NS (1.000) |
| MH96::6 | 3 (36) | 0 (36) | NS (0.2394) |
| MH96::9 | 0 (36) | 0 (36) | NS (1.000) |
| MH96::12 | 2 (36) | 0 (36) | NS (0.4930) |
| MH96::13 | 0 (36) | 0 (36) | NS (1.000) |
| ΔTC strain | 0 (36) | 2 (36) | NS (0.4930) |
| ΔTC(pPAC12) | 36 (36) | 0 (36) | <0.000 (2.2 × 10−16) |
| E. coli(pPAC12) | 0 (36) | 0 (36) | NS (1.000) |
| ΔTC(pPAC14) | 36 (36) | 0 (36) | <0.000 (2.2 × 10−16) |
| E. coli(pPAC14) | 0 (36) | 0 (36) | NS (1.000) |
| ΔC1-2 strain | 1 (36) | 0 (36) | NS (1.000) |
| ΔU-W strain | 36 (36) | 0 (36) | <0.000 (2.2 × 10−16) |
Y. entomophaga strains MH96 and derivatives (e.g., MH96::4), ΔTC strain, ΔC1-2 strain, and ΔU-W strain and E. coli carrying pPAC12 were studied.
Mortality is given as the number of larvae killed. n is the sample size in the treated group or in the negative-control group.
<0.000 significant difference at a 0.1% level (= 99.9% confidence level). NS, not significant.
The TC is released into LB broth at temperatures equal to or less than 25°C.
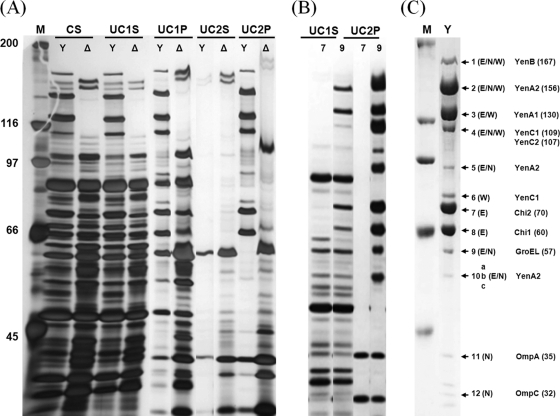
Assessment of the culture supernatants derived from Y. entomophaga MH96 grown in LB broth at temperatures of either 20, 25, 30, and 37°C, in conjunction with SDS-PAGE and bioassay analyses, identified that culture supernatants derived from cultures grown at 25°C or less contained large amounts of TC active on insects, with prominent YenA1 and YenA2 bands visualized by SDS-PAGE (Fig. 3). Analysis of the bacterial cell pellet by silver-stained SDS-PAGE detected significant levels of the TC components YenA1 and YenA2 at 25°C, but visually, only small amounts were seen for cultures grown at 30 and 37°C (Fig. 3). Assessment of the sonicated filtrates derived from cultures grown at either of these temperatures showed that they all had activity toward C. zealandica larvae, while sonicated filtrates derived from the ΔTC strain prepared under the same conditions were innocuous (Table 4).
Fig. 3.
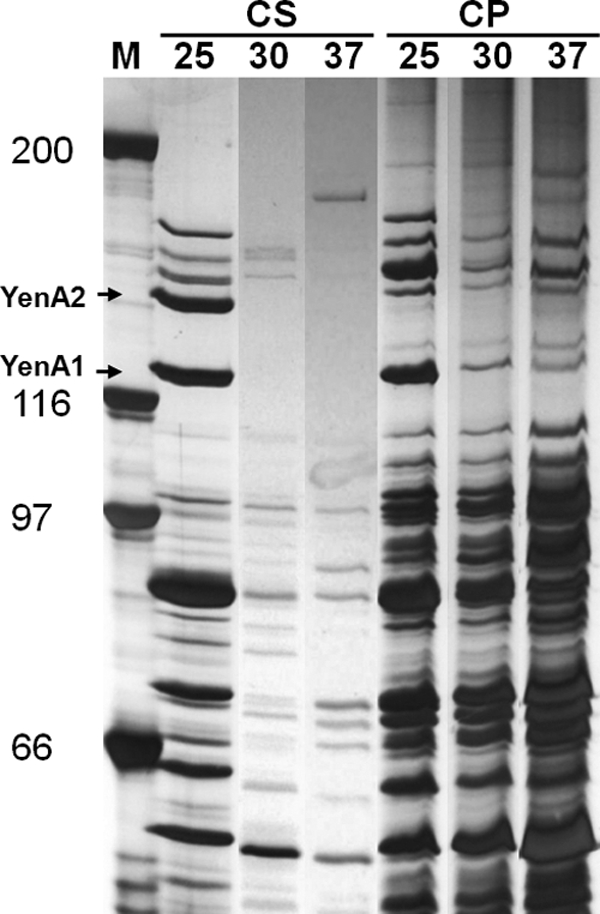
Silver-stained SDS-polyacrylamide gel of Y. entomophaga MH96 culture supernatant (CS) and cell pellets (CP) grown at 25, 30, and 37°C. The migration positions of the YenA1 and YenA2 proteins are indicated. Lane M contains Bio-Rad broad-range markers. The positions of molecular mass markers (in kilodaltons) are shown to the left of the gel.
Table 4.
Activity of Y. entomophaga MH96 culture or sonicated filtrates to C. zealandica larvae at various culture temperatures on day 5
| Culture or filtrate and strain | Culture temp (°C) | Mortality (n)a |
Significance level of the differenceb | |
|---|---|---|---|---|
| Treated | Control | |||
| Sonicated filtrate | ||||
| MH96 | 25 | 34 (36) | 0 (36) | <0.000 (0.4930) |
| 30 | 36 (36) | 0 (36) | <0.000 (2.2 × 10−16) | |
| 37 | 31 (36) | 0 (36) | <0.000 (3.4 × 10−15) | |
| ΔTC strain | 25 | 0 (36) | 0 (36) | NS (1.000) |
| 30 | 1 (36) | 0 (36) | NS (1.000) | |
| 37 | 0 (36) | 0 (36) | NS (1.000) | |
| Culture supernatant | ||||
| MH96 | 25 | 36 (36) | 0 (36) | <0.000 (2.2 × 10−16) |
| 30 | 1 (36) | 0 (36) | NS (1.000) | |
| 37 | 0 (36) | 0 (36) | 0 (36) | |
| ΔTC strain | 25 | 0 (36) | 0 (36) | NS (1.000) |
| 30 | 0 (36) | 0 (36) | NS (1.000) | |
| 37 | 0 (36) | 0 (36) | NS (1.000) | |
Mortality is given as the number of larvae killed. n is the sample size in the treated group or in the negative-control group. The negative Y. entomophaga ΔTC culture supernatant was prepared as Y. entomophaga MH96 was.
<0.000 significant difference at a 0.1% level (= 99.9% confidence level). NS, not significant.
Results of host range testing of the culture filtrate of Y. entomophaga MH96 grown at 25°C identified that 5 μl (100 ng) of culture filtrate had high insecticidal activity toward C. zealandica, A. couloni, A. tasmania, and P. xylostella larvae and that culture supernatants derived from the ΔTC strain prepared under similar conditions were innocuous (Table 5). Subjecting the culture supernatant to different temperatures showed that the culture filtrate lost insecticidal activity at a temperature equal to or greater than 60°C (Table 6). C. zealandica larvae fed with the purified TC became diarrhetic and were regurgitated from the C. zealandica larval mouth parts. The larvae exhibited reduced feeding activity and changed from a healthy gray color to a white-amber color within 12 to 18 h after ingestion; during this time, some of the larvae showed no clearing of the gut (see Fig. S2 in the supplemental material).
Table 5.
Activity of Y. entomophaga MH96 culture filtrates on various insect species on day 5
| Insect order and species | Mortality (n)a |
Significance level of the differenceb | |
|---|---|---|---|
| Treated | Control | ||
| Coleoptera | |||
| Costelytra zealandica | 34 (36) | 0 (36) | <0.000 (2.20 × 10−16) |
| Acrossidius tasmaniae | 36 (36) | 0 (36) | <0.000 (2.2 × 10−16) |
| Adoryphorus coulonic | 30 (36) | 0 (36) | <0.000 (2.37 × 10−14) |
| Lepidoptera | |||
| Epiphyas postvittana | 3 (36)d | 1 (36) | NS (0.6142) |
| Helicoverpa armigera | 2 (36)d | 3 (36) | NS (1.000) |
| Plutella xylostella | 36 (36) | 1 (36) | <0.000 (2.2 × 10−16) |
| Spodoptera litura | 2 (36)d | 1 (36) | NS (1.000) |
| Cydia pomonella | 31 (36)d | 2 (36) | <0.000 (3.39 × 10−15) |
Mortality is given as the number of larvae killed. n is the sample size in the treated group or in the negative-control group.
<0.000 significant difference at a 0.1% level (= 99.9% confidence level). NS, not significant.
A. couloni larvae were subjected to a second toxin dose on day 3.
The larvae exhibited reduced feeding and growth.
Table 6.
Effect of temperature on Y. entomophaga MH96 culture filtrates to C. zealandica larvae on day 5
| Temp (°C) | Mortality (n)a |
Significance level of the differenceb | |
|---|---|---|---|
| Treated | Control | ||
| 25 | 36 (36) | 0 (36) | <0.000 (2.2 × 10−16) |
| 35 | 36 (36) | 0 (36) | <0.000 (2.2 × 10−16) |
| 42 | 36 (36) | 1 (36) | <0.000 (2.2 × 10−16) |
| 45 | 31 (36) | 0 (36) | <0.000 (3.4 × 10−15) |
| 50 | 36 (36) | 0 (36) | <0.000 (2.2 × 10−16) |
| 55 | 28 (36) | 0 (36) | <0.000 (8.0 × 10−15) |
| 60 | 9 (36) | 1 (36) | NS (0.014) |
| 70 | 3 (36) | 0 (36) | NS (0.239) |
Mortality is given as the number of larvae killed. n is the sample size in the treated group or in the negative-control group.
<0.000 significant difference at a 0.1% level (= 99.9% confidence level). NS, not significant.
In assessment of P. xylostella larvae, these larvae also exhibited reduced feeding activity and the larvae changed to a lighter color and became turgid. The third-instar larvae of the redheaded cockchafer (A. couloni) underwent a characteristic amber color change when fed the TC. However, many of the larvae recovered and later appeared healthy. A repeated dose of the TC at day 3 was needed to induce mortality. Bioassays of the efficacy of the TC toward the Lepidoptera E. postvittana, C. pomonella, H. armigera, and S. litura showed that there was no mortality within 5 days of administering the TC to the larvae, although there was a significant reduction in growth and an absence of frass production (Table 5).
Assessment of the LD50 of the culture supernatant against larvae of P. xylostella and C. zealandica showed that as little as 30 or 50 ng, respectively, of toxin per larva was sufficient to cause mortality 5 days after ingestion. Some of the C. zealandica larvae that received a sublethal dose of the TC reverted from an amber phenotype to a healthy appearance (Table 7).
Table 7.
LD50s of semipurified TC on insects on day 5
| Insect | TC dose (ng) | Mortality (n)a |
Significance level of the differenceb | |
|---|---|---|---|---|
| Treated | Control | |||
| C. zealandica | 50 | 17 (36)c | 0 (36) | <0.000 (1.268 × 10−6) |
| P. xylostella | 30 | 18 (36) | 2 (36) | <0.000 (3.867 × 10−5) |
| G. mellonellad | 35 | 18 (36) | 2 (36) | <0.0002 |
Mortality is given as the number of larvae killed. n is the sample size in the treated group or in the negative-control group.
<0.000 significant difference at a 0.1% level (= 99.9% confidence level).
Some of the larvae reverted to healthy with resumption of feeding.
G. mellonella larvae were injected with semipurified TC into the hemocoel.
Purification and validation of the Y. entomophaga MH96 TC components.
Based on the premise that the TC had a combined mass of 2.6 million daltons, the Y. entomophaga MH96 culture supernatant was pelleted by ultracentrifugation through a glycerol step gradient. Assessment of the resuspended pellet by silver-stained SDS-PAGE enabled 10 prominent bands to be visualized (Fig. 4A, UC1P-Y). Six of these bands, as well as a less-intense high-molecular-weight band (YenB), correlated with the molecular masses of components of the TC (Fig. 4A, UC1P-Y). A similar analysis of the ultracentrifugated pellet of the ΔTC strain showed no other TC-related bands; however, two bands migrated with the predicted masses for Chi1 and Chi2. These bands were removed after a subsequent ultracentrifugation through a glycerol step gradient (Fig. 4A, UC2P-Δ). Four prominent bands of 75, 60, 35, and 32 kDa were present in both the ΔTC strain and Y. entomophaga strain MH96 (Fig. 4A, UC1P), the latter three proteins were subsequently identified by LC-ESI-MS/MS and/or N-terminal sequence analysis as the GroEL heat shock protein and two outer membrane-associated proteins, OmpA and OmpC (Table 8; see Table S2 in the supplemental material). Centrifugation of the resuspended ultracentrifuged pellet through a glycerol step gradient (Fig. 4A, UC2P-Y), removed three of the prominent bands, including the GroEL band, although a band with a mass similar to that of the GroEL protein remained in the ultracentrifuged pellet of the ΔTC strain (Fig. 4A, UC2P-Δ). With Y. entomophaga MH96, the TC, Chi1, and Chi2 proteins sedimented through the gradient (Fig. 4A, UC2P-Y).
Fig. 4.
(A) Silver-stained SDS-polyacrylamide gel of Y. entomophaga MH96 and ΔTC mutant strain. Y. entomophaga MH96 (Y) and ΔTC mutant strain (Δ) in culture supernatant (CS), ultracentrifuged supernatant (UC1S), ultracentrifuged pellet (UC1P), supernatant after ultracentrifugation and application to a step gradient (UC2S), and pellet after ultracentrifugation and application to a step gradient (UC2P). (B) Silver-stained SDS-polyacrylamide gel of Y. entomophaga MH96::7 (7) and Y. entomophaga MH96::9 (9), showing the absence of formation of the complex and a complex missing the YenB component, respectively. (C) SDS-polyacrylamide gel stained with Coomassie brilliant blue showing bands assessed by either LC-ESI-MS/MS (E), N-terminal sequence analysis (N) (Table 8), or Western immunoblot (W). The M lanes contain Bio-Rad broad-range markers. The positions of molecular mass markers (in kilodaltons) are indicated to the left of the gel. The locations of components of the TC, GroEL, OmpA, and OmpC proteins are indicated (Table 8; see text; also see Table S2 in the supplemental material).
Table 8.
Edman N-terminal sequencing data of proteins derived from the ultracentrifuged pellet
| Peptide no.a (band mass [kDa]) | NH2-terminal sequenceb |
Accession no. | |
|---|---|---|---|
| Obtained sequence | Predicted sequence, protein (kDa) | ||
| 1 (167) | MQN{S}QEM{A}I- | MQNSQEMAI, YenB (167) | ABG33866 |
| 2 (156) | {S}NSIEQKLQ | SNSIEAKLQ, YenA2 (156) | ABG33868 |
| 4 (107) | -N{Q}FL(F/S)ALT- | MNQFDSALH, YenC1 (107) | ABG33865 |
| 5 (88) | SNSIEAKLQ | SNSIEAKLQ, YenA2 (156) | ABG33868 |
| 9 (57) | AAKDVKFGN | GroEL (57) YE0354, Yersinia enterocolitica subsp. enterocolitica 8081 | YP001004730 |
| 10a (52) | -{Q}PLTF(E/L)PVV | QPLTFEPVV, YenA2 (156) | ABG33868 |
| 10b (51) | TFEPVVHDQ | TFEPVVHDQ, YenA2 (156) | ABG33868 |
| 10c (50) | DQTMSSAVDN | DQTMSAVDN, YenA2 (156) | ABG33868 |
| 11 (35) | APKDNTWYTc | OmpA (38) signal sequence cleaved is 36 kDa; YE1581; Y. enterocolitica subsp. enterocolitica 8081 (357) | Y_P001005874 |
| 12 (32) | AEIYNKDGNc | OmpC (39.6) signal sequence cleaved is 37.6 kDa; KPK_1516; Klebsiella pneumoniae (342) | Y_P002237369 |
See Fig. 4C for band number.
Braces around a letter indicate that it was a tentative call. -, no call determined due to cycle interference.
N-terminal sequence at the predicted signal sequence cleavage site of its protein orthologue.
LC-ESI-MS/MS analyses identified five of the seven predicted TC-associated proteins: Chi1, YenA1, YenA2, Chi2, and YenB, but not YenC1 or YenC2. Analysis of the ∼108-kDa band, which was expected to contain comigrating YenC1 (109-kDa) and YenC2 (107-kDa) proteins, was unsuccessful in identifying any peptides from either protein. The most-prominent peptides identified matched the amino acid sequences of the YenA1 and YenA2 proteins. LC-ESI-MS/MS analyses also identified peptides matching the YenA1 and YenA2 sequences in the 50- to 107-kDa range (Fig. 4C; see Table S2 in the supplemental material). The YenA1 antibody also cross-reacted with some of these bands (Fig. 5). Western immunoblotting was also able to detect YenB, YenC1, and the YenC2 protein (Fig. 5). The amino-terminal peptide of YenC1 was identified through N-terminal sequence analysis of the ∼108-kDa band (Table 8).
Fig. 5.
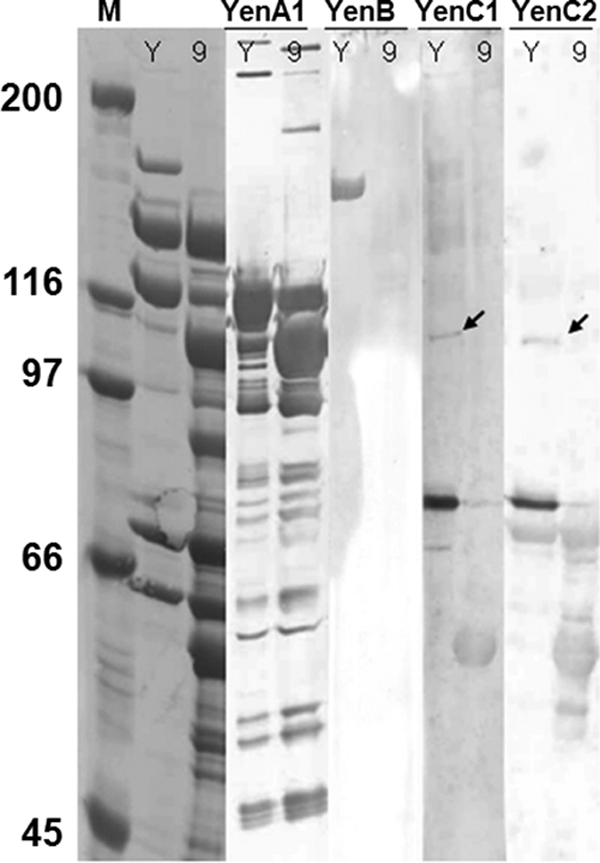
Western immunoblot of purified Y. entomophaga MH96 TC (Y) and the Y. entomophaga MH96::9 TC derivative (9). YenA1, YenB, YenC1, and YenC2 antibodies used as probes are listed at the top of the lanes in the gel (see Materials and Methods for antibody probes). The leftmost three lanes show a Coomassie brilliant blue-stained polyvinylidene difluoride (PVDF) membrane. The small black arrows point to the migration positions of the YenC1 and YenC2 proteins. Lane M, Bio-Rad broad-range marker.
Assessment of the N-terminal sequence of the 50-kDa band (Fig. 4C, band 10a,b,c), isolated from three independent Y. entomophaga MH96 cultures grown at 25°C identified three YenA2 peptide sequences initiating between amino acid residues 767 and 778 of YenA2 (Table 8). Amino acid alignment of YenA2 to the P. luminescens TCA proteins, TcaB, TcbA, and TcdA, identified a divergent region susceptible to cleavage in all three proteins and coincident with the putative cleavage site in YenA2 based on N-terminal sequencing (Table 8; see Fig. S3 in the supplemental material). The cleavage-susceptible region is flanked by conserved regions of protein similarity and resides approximately 200 amino acid residues prior to a predicted coiled-coil region located toward the C terminus (YenA2 amino acids [aa] 1025 to 1087). The predicted amino terminus of YenA2 was identified by N-terminal sequence analysis of the ∼90-kDa band (Table 8).
Analysis of the semipurified pellets derived from ultracentrifugation of the previously constructed Y. entomophaga MH96 TC mutants by SDS-PAGE showed that Y. entomophaga MH96::1, MH96::2, and MH96::9, containing a mutation in the 5′ region of yenB (Table 1), produced a TC variant containing only the YenA1, YenA2, Chi1, and Chi2 components (Fig. 4B, U2CP-9). No YenB or YenC1 and YenC2 protein could be visualized by SDS-PAGE or detected by Western immunoblotting (Fig. 5). Bioassay of the purified YenA1, YenA2, Chi1, and Chi2 complex from Y. entomophaga MH96::9 showed no activity toward C. zealandica larvae (Table 3). Assessment of the Y. entomophaga MH96 mutants, Y. entomophaga MH96::3-8 and Y. entomophaga MH96::10-13, containing mutations in yenA1, yenA2, chi1, and chi2 genes (Table 1) showed no complex or chitinases to pellet by ultracentrifugation (Fig. 4B, U2CP-7).
Transmission electron microscopy of the ultracentrifuged Y. entomophaga MH96 pellet revealed the presence of outer membrane vesicles (OMVs) ranging in size from 40 to 300 nm, as well as pyramidal structures of approximately 19 nm by 30 nm (Fig. 6A); the pyramidal structures were not present in the ΔTC strain. Scanning electron microscopy of Y. entomophaga MH96 identified the presence of small and large blebs protruding from the bacterial cell wall that typify OMV formation (Fig. 6B).
Fig. 6.
(A) Transmission electron micrograph of TC and associated OMVs. The white arrows point to TC particles, which appear as elongated pyramid-type structures. The electron micrograph was taken at a magnification of ×150,000 (bar = 50 nm). (B) Scanning electron micrograph of Y. entomophaga MH96 showing OMV blebs taken at a magnification of ×15,000 (bar = 1 μm).
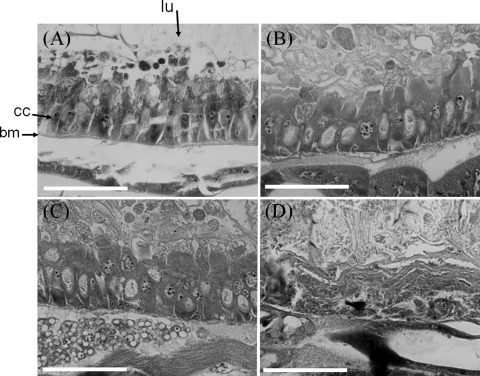
The semipurified Y. entomophaga MH96 TC was assessed for both exo- and endochitinase activity and found to have high endochitinase activity but no significant exochitinase (Table 9). Injection of the semipurified TC into the G. mellonella larval hemocoel showed that 35 ng of the TC was sufficient to give an LD50 within 5 days, while a similar preparation from the ΔTC strain was ineffective (Table 7). The larvae showed no initial change in color but instead became flaccid and eventually turned black 6 days after injection. To assess the effects of the TC on the insect gut ultrastructure, 100 ng of TC was fed to P. xylostella larvae, and histology was assessed over a 48-h period. Observations showed that within 24 h of feeding, the intestinal columnar cells swelled and cellular vesicles and debris appeared in the gut lumen (Fig. 7). Within 48 h, the columnar cells became dislodged, and the midgut epithelial cells became amorphous with no distinct cell forms being present (Fig. 7D).
Table 9.
Chitinase assay
| Chitinase | Substrate | Activity (μmol of p-nitrophenol released after 30 min at 37°C) |
||
|---|---|---|---|---|
| TC (0.1 μg/ml) | Standard chitinasea (0.05 μg/ml) | ΔTC strain | ||
| Exochitinase | 4-Nitrophenyl-N-acetyl-β-d-glucosaminide | 0.017 | 0.57 | 0.003 |
| 4-Nitrophenyl-N-N′-diacetyl-β-d-chitobioside | 0.0747 | 0.145 | 0.009 | |
| Endochitinase | 4-Nitrophenyl-N-acetyl-β-d-N,N′,N″-triacetylchitotriose | 0.1211 | 0.108 | 0.005 |
Chitinase from Trichoderma viride (catalog no. CS0980; Sigma Aldrich).
Fig. 7.
Histopathological effects of TC on the anterior midgut of P. xylostella epithelium. (A) Untreated larva, showing a regular arrangement of basal membrane (bm) and columnar cells (cc) in the midgut epithelium and the lumen (lu). (B to D) Larvae at 16 h (B), 24 h (C), and 40 h (D) after the larval ingestion of food dosed with 100 ng TC/cm2. Within 16 h, apical swelling of the columnar cells occurs with vesicle-like structures seen in the gut lumen. At 40 h, there is a complete dissolution of the larval gut lining and the presence of cellular debris in the lumen. Bars = 50 μm.
DISCUSSION
Through transposon mutagenesis we have defined a 31,898-bp pathogenicity island, termed PAIYe96, that contains a cluster of five TC toxin complex genes and two chitinase genes, the translated products of which are predicted to combine to form a large toxin complex (TC). Culture filtrates derived from only Y. entomophaga strain MH96 and not its ΔTC derivative were able to cause a pathotype similar to that of the wild-type bacterium.
In cultures where Y. entomophaga MH96 was grown at 25°C or less, large amounts of the TC were observed in LB broth. However, above 25°C, no toxin was detected in the culture supernatant. The effect of temperature on bacterial behavior has been documented in Yersinia pestis (30) and Yersinia enterocolitica (8, 14, 46), suggesting a shared phenomenon among the Yersinia. As little as 5 μl of filter-sterilized culture supernatant was able to cause lethality in C. zealandica, A. couloni, A. tasmaniae, and P. xylostella larvae (Table 5). The LD50s of the TC to P. xylostella and C. zealandica larvae were 30 ng and 50 ng, respectively. This is significantly lower than the LD50 of 875 ng of the P. luminescens-derived toxin A against M. sexta neonates (5). However, this amount correlates with the relative efficacy of E. coli-expressed Xenorhabdus nematophila XptA1, XptB, and XptC lysates against Pieris rapae (45 ng) or Pieris brassicae (3.2 ng) or the XptA2, XptB, and XptC TC against Heliothis virescens (5.3 ng); neither of these TCs was active against P. xylostella (60). Bioassay analysis of the Y. entomophaga MH96::9 lacking YenB, YenC1, and YenC2 identified no insecticidal activity when they were fed to C. zealandica larvae (Table 3), showing that unlike the P. luminescens TcdA (45) and X. nematophilus XptA1 (50) TCA components, the TC-associated chitinases (Chi1 and Chi2), or the TCA (YenA1 and YenA2) components are not active on insects.
Not all of the insect species tested were killed by the TC; E. postvittana, C. pomonella, H. armigera, and S. litura exhibited only reduced growth when subjected to the toxin. Reduced feeding activity was also identified by Bowen et al. (5), who used sublethal doses of the P. luminescens toxin complex (TCA) against M. sexta larvae. A reduction in growth was also used as a measure of toxicity of the E. coli-expressed Y. pseudotuberculosis IP32953-derived TC toxins, the TCA-like (tcaA tcaB), TCB-like (tcaC), and TCC-like (tccC) toxins, to larvae of M. sexta (54). Increasing the administered toxin dose or redosing the larvae of E. postvittana, C. pomonella, H. armigera, and S. litura was unable to cause mortality (Hurst, unpublished), even though the insects are susceptible to an oral dose of Y. entomophaga MH96 (36). The loss of toxicity could be due to a rapid degradation of the TC in the guts of the insects. The large third-instar larvae of the redheaded cockchafer required a repeated dose of the TC at day 3 to induce mortality. A sublethal dose of the TC to C. zealandica larvae resulted in a reversion effect, similar to that described for the S. entomophila-derived Sep proteins (35), which means that a continuous supply of the Y. entomophaga TC and TC toxins in general may be needed to exert an effect. Of further interest, these larvae underwent a transitional phase where a dark gut could be visualized through the cream color, indicating that the gut had not been voided and suggesting that factors other than gut clearance relate to the change in larval coloration.
Transposon mutagenesis identified 13 Y. entomophaga MH96 avirulent mutations that resided in the yenA1, yenA2, and yenB genes and in the chitinase-encoding genes (chi1 and chi2), confirming the roles of these proteins in insecticidal activity. However, no mutation was identified in either of the TCC-like (yenC1 or yenC2) components or in other regions of PAIYe96. Bioassay data of the yenC1 yenC2 double deletion variant (ΔC1-2) showed an abolition of virulence, indicating that YenC1 and YenC2 may be able to complement the loss of each other, a scenario that would explain the inability to detect transposon insertions in either of these genes by bioassay analysis. The mini-Tn5 Km1 insertions were nonpolar, as the translated products of TC genes located 3′ to the insertion point could be visualized by SDS-PAGE (see Fig. S4 in the supplemental material).
Although the pPAC12 and pPAC14 clones with TC genes were able to complement the loss of the ability to cause virulence in the ΔTC strain, the clones were unable to cause virulence in an E. coli background. Attempts to artificially induce the PAIYe96-associated TC cluster have also been unsuccessful (Hurst, unpublished). DNA sequence analysis identified a region of potential translational coupling located between yenA1 and yenA2 (Fig. 1D). A similar translational coupling has been documented between the lacL and lacM β-galactosidase genes from the bacterium Lactobacillus lactis (16), where it is suggested to enable the stalling and backward movement of the ribosomes, allowing the correct translation of the second ORF, i.e., yenA2. This could explain the inability of the clones bearing TC genes to cause virulence when in an E. coli background and may indicate that a factor encoded by Y. entomophaga MH96 is needed for correct TC expression.
Ultracentrifugation of the ΔTC strain or the Y. entomophaga MH96 strain containing mutations in either yenA1 or yenA2 showed no sedimentation of the Chi1 and Chi2 proteins. No sedimentation of either Chi1, YenA1, or YenA2 was observed with the Y. entomophaga MH96 chi2 mutant. SDS-PAGE of the ultracentrifuged pellets of Y. entomophaga MH96::9 (yenB mutant) showed that only the Chi1, Chi2, YenA1, and YenA2 proteins pelleted. Combined, these data indicate that these proteins form a subcomplex, similar to X. nematophila XptA1 TCA, a tetramer-like structure described by Lee et al. (43), in which the TCB and TCC components are theorized to interact. Further credence to the formation of a Chi1, Chi2, YenA1, and YenA2 complex is their juxtaposed gene locations, of chi1 and chi2 to yenA1 and yenA2, a gene order similar to their P. luminescens TTO1 orthologues (plu2461-plu2458) (Fig. 1C).
Western immunoblot and LC-ESI-MS/MS analysis identified several low-molecular-weight YenA1 and YenA2 bands indicative of proteolytic cleavage. N-terminal sequencing analysis identified YenA2 cleavage products that correlated with the relative positions of the predicted protease cleavage sites of the P. luminescens TC toxins TcaB, TcbA, and TcdA (5) (Table 8; see Fig. S3 in the supplemental material). The reason behind this cleavage is unknown but may relate to a bacterium-derived protease. N-terminal sequence analysis also identified the predicted signal sequence cleavage site of the OmpA and OmpC proteins, which are the main components of outer membrane vesicles (OMVs) (48). An additional protein that copurified with the TC was the GroEL protein, a bacterial chaperonin that facilitates the folding and transport of proteins (13, 57) and is often associated with OMVs (37). It is of interest that the ΔTC strain contained proteins with masses similar to those of Chi1 and Chi2 and an additional unidentified band located at ∼100 kDa (Fig. 4A, UC1P). This may indicate that in Y. entomophaga MH96, the TC predominantly associates with OMVs and that in the TC deletion variant other proteins with less affinity to OMVs are able to associate with the OMVs due to nonsteric hindrance by the TC.
Using C-terminal glycogen synthase kinase (GSK) epitope tags fused to the N-terminal amino acid residues of the Y. pestis KIM TC-like protein's TCA (YitA and YitB), TCB (YitC), and TCC (YipA and YipB), Gendlina et al. (27) identified that the GSK fusions are released by the Y. pestis type III system. The authors also found that under conditions optimal for type III secretion in Y. pseudotuberculosis, YitB could be detected in the culture supernatant. Bioassay analysis of sonicated filtrates derived from Y. entomophaga MH96 cultures grown at 25, 30, or 37°C showed that they were still active on insects, suggesting a temperature-dependent release of the TC from the cell. Electron microscopy identified OMVs in the ultracentrifuged pellet, and scanning electron microscopy showed large blebs resembling OMVs on the outer cell wall of Y. entomophaga MH96 (Fig. 6B). OMVs have been shown to be involved in the transport of degradative compounds, DNA (19), and toxins (39, 40), out of the bacteria. Along with the predicted chaperone function of the GroEL protein, it is tempting to speculate that GroEL and OMVs, respectively, are important in the assembly and transport of the TC out of Y. entomophaga MH96.
Through electron microscopy, pyramid-like structures were observed (Fig. 6A), and these structures were not seen in the ΔTC deletion variant. Given the size and purity of the fractions as assessed by SDS-PAGE, the pyramid-like structures are likely to be the TC. The semipurified TC was found to have high endochitinase activity and may therefore have an important role in the degradation of insect-based chitin such as that found in the peritrophic membrane (44). Regev et al. (58) demonstrated that the S. marcesens-derived ChiAII endochitinase is able to cause mortality alone and can act synergistically to enhance the Bacillus thuringiensis K26-21 δ-endotoxin crystal CryIC protein activity toward neonate Spodoptera littoralis. The estimated size of the X. nematophila TCA component (XptA1) is 16 by 19 nm (43), and the peritrophic membrane filtration cutoff point for type I and II peritrophic membranes is between 2 and 36 nm (44). The TC-associated chitinases may therefore enhance the activity of the core TC by facilitating the degradation of the chitin components of the insect intestinal tract and or other components of the insect. The TC was also able to cause lethality when injected into the larval hemocoelic cavity of G. mellonella, suggesting that the TC interacts with a generic conserved receptor, a scenario postulated by Bowen et al. (5), who identified both oral and hemocoel-based M. sexta insecticidal activity with the P. luminescens toxin complex (TCA).
Histological examination of P. xylostella after the larvae had been fed purified TC showed that within 48 h after ingestion, there was a general dissolution of the gut with expansion and dislodgement of the columnar cells (Fig. 7). Blackburn et al. (2) observed a similar phenomenon with P. luminescens TCA toxin toward the gut of M. sexta and drew parallels to the histological effects of the B. thuringiensis δ endotoxins, vegetative insecticidal protein Vip3A, and cholesterol oxidases.
Analysis of the PAIYe96 DNA sequence identified two 76-bp tRNAGly DNA sequences and a G+C content that were different from those of the bacterial genome. These are traits that typify a pathogenicity island (26, 29). Within PAIYe96, the G+C content of the DNA encompassing the TC gene cluster located 5′ of the remnant methyltransferase is 44.5%, while the region located 3′ encompassing yenUVTW is 40.1%, indicating that these regions may have been acquired at a different time points. Bioassay of the ΔU-W strain toward C. zealandica larvae showed no alteration in the ability to cause disease, suggesting that the yenUVTW genes may have some other role in the ecology of Y. entomophaga MH96. The overall G+C content of PAIYe96 (43.8%) is more similar to the genomic G+C content of P. luminescens subsp. laumondii TTO1 (42.8%), Photorhabdus asymbiotica (42.2%), and X. nematophilus (45%) than to Yersinia species (Y. pestis and Y. pseudotuberculosis, 47.6%) and Y. enterocolitica (47.3%). Phylogenetic analysis of YenA2 showed that its closest relatives were the TCA proteins, TccB2 (Plu2459) of P. luminescens subsp. laumondii TTO1 (22) and the X. nematophilus protein XptD1 (50). The target species of either of these proteins has yet to be determined. The YenA2 protein was phylogenetically distinct from other Yersinia species (Fig. 2). DNA comparison of the Y. entomophaga MH96 chi1 and chi2 genes identified 67 to 70% DNA identity to the chitinase genes of P. luminescens subsp. laumondii TTO1 (Fig. 1C) and the chi gene of X. nematophilus that is located juxtaposed to the TCA-like genes xptD1 and xptAI (50). This suggests that the TC gene cluster may have been acquired by horizontal gene transfer from one of these species. The absence of functional transposase, integrase, or other mobility genes within or flanking PAIYe96 indicates that the PAI is nonmobile and that PAIYe96 may therefore be in the final stages of integration with the host genome.
We have provided the first evidence of an orally active TC derivative from a Yersinia species. Its broad range of activity against a number of insect pest species indicates that it may prove useful as an alternative to B. thuringiensis toxins for use in insect control (7). To date, attempts to define parameters where toxin is not produced have proved unsuccessful (Hurst, unpublished). The ability to rapidly purify and visualize the TC will allow a detailed protein structural analysis and facilitate studies defining the TC insect-associated receptor.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Richard Townsend for the collection of larvae of the grass grub (C. zealandica), redheaded cockchafer (A. couloni), Tasmanian grass grub (A. tasmania) and chafer beetles, Odontria sp., and the rearing and provision of the white diamondback moth (P. xylostella). We thank Anette Becher for YenA2 phylogenetic analysis and Michael Landsberg of the Institute for Molecular Bioscience, University of Queensland for proofreading the manuscript.
This study was supported by contract C10X0804 from the New Zealand Foundation for Research, Science and Technology. We have no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 28 January 2011.
REFERENCES
- 1. Altschul S. F., et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blackburn M., Golubeva E., Bowen D., H. ffrench-Constant R. 1998. A novel insecticidal toxin from Photorhabdus luminescens, toxin complex a (Tca), and its histopathological effects on the midgut of Manduca sexta. Appl. Environ. Microbiol. 64:3036–3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. 1984. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal. Biochem. 136:175–179 [DOI] [PubMed] [Google Scholar]
- 4. Blum H., Beier H., Gross H. J. 1987. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93–99 [Google Scholar]
- 5. Bowen D., et al. 1998. Insecticidal toxins from the bacterium Photorhabdus luminescens. Science 280:2129–2132 [DOI] [PubMed] [Google Scholar]
- 6. Bradford M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 7. Bravo A., Soberón M. 2008. How to cope with insect resistance to Bt toxins? Trends Biotechnol. 26:573–579 [DOI] [PubMed] [Google Scholar]
- 8. Bresolin G., Morgan J. A., Ilgen D., Scherer S., Fuchs T. M. 2006. Low temperature-induced insecticidal activity of Yersinia enterocolitica. Mol. Microbiol. 59:503–512 [DOI] [PubMed] [Google Scholar]
- 9. Buell C. R., et al. 2003. The complete genome sequence of the Arabidopsis and tomato pathogen Pseudomonas syringae pv. tomato DC3000. Proc. Natl. Acad. Sci. U. S. A. 100:10181–10186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buetow L., Flatau G., Chiu K., Boquet P., Ghosh P. 2001. Structure of the Rho-activating domain of Escherichia coli cytotoxic necrotizing factor 1. Nat. Struct. Biol. 8:584–588 [DOI] [PubMed] [Google Scholar]
- 11. Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 17:540–552 [DOI] [PubMed] [Google Scholar]
- 12. Chang A. C. Y., Cohen S. N. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the p15A cryptic miniplasmid. J. Bacteriol. 134:1141–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chaudhuri T. K., Verma V. K., Maheshwari A. 2009. GroEL assisted folding of large polypeptide substrates in Escherichia coli: present scenario and assignments for the future. Prog. Biophys. Mol. Biol. 99:42–50 [DOI] [PubMed] [Google Scholar]
- 14. Chester B., Stotzky G. 1976. Temperature-dependent cultural and biochemical characteristics of rhamnose-positive Yersinia enterocolitica. J. Clin. Microbiol. 3:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chevenet F., Brun C., Banuls A. L., Jacq B., Christen R. 2006. TreeDyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinformatics 7:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. David S., Stevens H., van Riel M., Simons G., de Vos W. M. 1992. Leuconostoc lactis beta-galactosidase is encoded by two overlapping genes. J. Bacteriol. 174:4475–4481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Lorenzo V., Herrero M., Jakubzik U., Timmis K. N. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 172:6568–6572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deng W., et al. 2002. Genome sequence of Yersinia pestis KIM. J. Bacteriol. 184:4601–4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dorward D. W., Garon C. F., Judd R. C. 1989. Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J. Bacteriol. 171:2499–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dower W. J., Miller J. F., Ragsdale C. W. 1988. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 16:6127–6145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dubreuil J. D. 2008. Escherichia coli STb toxin and colibacillosis: knowing is half the battle. FEMS Microbiol. Lett. 278:137–145 [DOI] [PubMed] [Google Scholar]
- 22. Duchaud E., et al. 2003. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nat. Biotechnol. 21:1307–1313 [DOI] [PubMed] [Google Scholar]
- 23. Erickson D. L., et al. 2007. Acute oral toxicity of Yersinia pseudotuberculosis to fleas: implications for the evolution of vector-borne transmission of plague. Cell. Microbiol. 9:2658–2666 [DOI] [PubMed] [Google Scholar]
- 24. Felsenstein J. 2005. PHYLIP (Phylogeny Inference Package) version 3.6. Department of Genome Sciences, University of Washington, Seattle, WA [Google Scholar]
- 25. ffrench-Constant R., Waterfield N. 2005. An ABC guide to the bacterial toxin complexes. Adv. Appl. Microbiol. 58C:169–183 [DOI] [PubMed] [Google Scholar]
- 26. Gal-Mor O., Finlay B. B. 2006. Pathogenicity islands: a molecular toolbox for bacterial virulence. Cell. Microbiol. 11:1707–1719 [DOI] [PubMed] [Google Scholar]
- 27. Gendlina I., et al. 2007. Identification and type III-dependent secretion of the Yersinia pestis insecticidal-like proteins. Mol. Microbiol. 64:1214–1227 [DOI] [PubMed] [Google Scholar]
- 28. Guindon S., Gascuel O. 2003. PhyML-a simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52:696–704 [DOI] [PubMed] [Google Scholar]
- 29. Hacker J., Blum-Oehler G., Muhldorfer I., Tschape H. 1997. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol. Microbiol. 23:1089–1097 [DOI] [PubMed] [Google Scholar]
- 30. Hall P. J., Yang G. C., Little R. V., Brubaker R. R. 1974. Effect of Ca2+ on morphology and division of Yersinia pestis. Infect. Immun. 9:1105–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hares M., et al. 2008. The Yersinia pseudotuberculosis and Y. pestis toxin complex is active against cultured mammalian cells. Microbiology 154:3503–3517 [DOI] [PubMed] [Google Scholar]
- 32. Hey T. D., et al. July 2006. Insecticidal toxin complex fusion proteins. U.S. patent 2006/0168683
- 33. Hurst M. R., Glare T. R., Jackson T. A., Ronson C. W. 2000. Plasmid-located pathogenicity determinants of Serratia entomophila, the causal agent of amber disease of grass grub, show similarity to the insecticidal toxins of Photorhabdus luminescens. J. Bacteriol. 182:5127–5138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hurst M. R., Glare T. R., Jackson T. A. 2004. Cloning Serratia entomophila antifeeding genes—a putative defective prophage active against the grass grub Costelytra zealandica. J. Bacteriol. 186:5116–5128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hurst M. R., Jones S. M., Tan B. B., Jackson T. A. 2007. Induced expression of the Serratia entomophila Sep proteins shows activity towards the larvae of the New Zealand grass grub Costelytra zealandica. FEMS Microbiol. Lett. 275:160–167 [DOI] [PubMed] [Google Scholar]
- 36. Hurst M. R., Becher S. A., Young S. D., Glare T. R. 21 May 2010. Yersinia entomophaga sp. nov. isolated from the New Zealand grass grub Costelytra zealandica. Int. J. Syst. Evol. Microbiol. doi:10.1099/ijs.0.024406-0. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 37. Joshi M. C., et al. 2008. An insecticidal GroEL protein with chitin binding activity from Xenorhabdus nematophila. J. Biol. Chem. 283:28287–28296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kalogeraki V. S., Winans S. C. 1997. Suicide plasmids containing promoterless reporter genes can simultaneously disrupt and create fusions to target genes of diverse bacteria. Gene 188:69–75 [DOI] [PubMed] [Google Scholar]
- 39. Kuehn M. J., Kesty N. C. 2005. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 19:2645–2655 [DOI] [PubMed] [Google Scholar]
- 40. Kwon S. O., Gho Y. S., Lee J. C., Kim S. I. 2009. Proteome analysis of outer membrane vesicles from a clinical Acinetobacter baumannii isolate. FEMS Microbiol. Lett. 297:150–156 [DOI] [PubMed] [Google Scholar]
- 41. Laemmli U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 42. Lang A. E., et al. 2010. Photorhabdus luminescens toxins ADP-ribosylate actin and RhoA to force actin clustering. Science 327:1139–1142 [DOI] [PubMed] [Google Scholar]
- 43. Lee S. C., et al. 2007. Structural characterisation of the insecticidal toxin XptA1, reveals a 1.15 MDa tetramer with a cage-like structure. J. Mol. Biol. 366:1558–1568 [DOI] [PubMed] [Google Scholar]
- 44. Lehane M. J. 1997. Peritrophic matrix structure and function. Annu. Rev. Entomol. 42:525–550 [DOI] [PubMed] [Google Scholar]
- 45. Liu D., et al. 2003. Insect resistance conferred by 283-kDa Photorhabdus luminescens protein TcdA in Arabidopsis thaliana. Nat. Biotechnol. 21:1222–1228 [DOI] [PubMed] [Google Scholar]
- 46. Logue C. M., Sheridan J. J., McDowell D. A., Blair I. S., Hegarty T. 2000. The effect of temperature and selective agents on the growth of Yersinia enterocolitica serotype O:3 in pure culture. J. Appl. Microbiol. 88:1001–1008 [DOI] [PubMed] [Google Scholar]
- 47. Lorow D., Jessee J. 1990. Max efficiency DH10B™: a host for cloning methylated DNA. Focus 12:19 [Google Scholar]
- 48. Mashburn-Warren L. M., Whiteley M. 2006. Special delivery: vesicle trafficking in prokaryotes. Mol. Microbiol. 61:839–846 [DOI] [PubMed] [Google Scholar]
- 49. Miller J. H., Mekalanos J. J. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Morgan J. A., Sergeant M., Ellis D., Ousley M., Jarrett P. 2001. Sequence analysis of insecticidal genes from Xenorhabdus nematophilus PMFI296. Appl. Environ. Microbiol. 67:2062–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Parkhill J., et al. 2001. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413:523–527 [DOI] [PubMed] [Google Scholar]
- 52. Penfold R. J., Pemberton J. M. 1992. An improved suicide vector for construction of chromosomal insertion mutations in bacteria. Gene 118:145–146 [DOI] [PubMed] [Google Scholar]
- 53. Peng L., et al. 2008. Proteomic analysis of microsomes from lactating bovine mammary gland. J. Proteome Res. 7:1427–1432 [DOI] [PubMed] [Google Scholar]
- 54. Pinheiro V. B., Ellar D. J. 2007. Expression and insecticidal activity of Yersinia pseudotuberculosis and Photorhabdus luminescens toxin complex proteins. Cell. Microbiol. 9:2372–2380 [DOI] [PubMed] [Google Scholar]
- 55. Posada D., Crandall K. A. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817–818 [DOI] [PubMed] [Google Scholar]
- 56. Prentki P., Krisch H. M. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303–313 [DOI] [PubMed] [Google Scholar]
- 57. Radford S. E. 2006. GroEL: more than just a folding cage. Cell 125:831–833 [DOI] [PubMed] [Google Scholar]
- 58. Regev A., et al. 1996. Synergistic activity of a Bacillus thuringiensis delta-endotoxin and a bacterial endochitinase against Spodoptera littoralis larvae. Appl. Environ. Microbiol. 62:3581–3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 60. Sergeant M., Jarrett P., Ousley M., Morgan J. A. 2003. Interactions of insecticidal toxin gene products from Xenorhabdus nematophilus PMFI296. Appl. Environ. Microbiol. 69:3344–3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Singh P. 1983. A general purpose laboratory diet mixture for rearing insects. Insect Sci. Appl. 4:357–362 [Google Scholar]
- 62. Song Y., et al. 2004. Complete genome sequence of Yersinia pestis strain 91001, an isolate avirulent to humans. DNA Res. 11:179–197 [DOI] [PubMed] [Google Scholar]
- 63. Staskawicz B., Dahlbeck D., Keen N., Napoli C. 1987. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J. Bacteriol. 169:5789–5794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Takeshita S., Sato M., Toba M., Masahashi W., Hashimoto-Gotoh T. 1987. High-copy-number and low-copy-number plasmid vectors for lacZ alpha-complementation and chloramphenicol- or kanamycin-resistance selection. Gene 61:63–74 [DOI] [PubMed] [Google Scholar]
- 65. Tamura K., Dudley J., Nei M., Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 66. Tennant S. M., Skinner N. A., Joe A., Robins-Browne R. M. 2005. Homologues of insecticidal toxin complex genes in Yersinia enterocolitica biotype 1A and their contribution to virulence. Infect. Immun. 73:6860–6867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F., Higgins D. G. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang H., et al. 2004. Characterization and phylogenetic analysis of the chitinase gene from the Helicoverpa armigera single nucleocapsid nucleopolyhedrovirus. Virus Res. 100:179–189 [DOI] [PubMed] [Google Scholar]
- 69. Waterfield N., Hares M., Dowling A., ffrench-Constant R. 2005. Potentiation and cellular phenotypes of the insecticidal toxin complexes of Photorhabdus bacteria. Cell. Microbiol. 7:373–382 [DOI] [PubMed] [Google Scholar]
- 70. Xia X., Xie Z. 2001. DAMBE: data analysis in molecular biology and evolution. J. Hered. 92:371–373 [DOI] [PubMed] [Google Scholar]
- 71. Yanisch-Perron C., Vieira J., Messing J. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequence of M13mp18 and pUC19 vectors. Gene 33:103–119 [DOI] [PubMed] [Google Scholar]
- 72. Yoshimura S., et al. 1985. Essential structure for full enterotoxigenic activity of heat-stable enterotoxin produced by enterotoxigenic Escherichia coli. FEBS Lett. 181:138–142 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.