Abstract
Competence-stimulating-peptide (CSP)-mediated competence development in Streptococcus mutans is a transient and biphasic process, since only a subpopulation induces the expression of ComX in the presence of CSP, and the activation of the DNA uptake machinery in this fraction shuts down ∼3 to 4 h postinduction. Here, we combine for the first time, to our knowledge, the bacterial flow-cytometric sorting of cells and subpopulation-specific transcriptome analysis of both the competent and noncompetent fraction of CSP-treated S. mutans cells. Sorting was guided by a ComX-green fluorescent protein (ComX-GFP) reporter, and the transcriptome analysis demonstrated the successful combination of both methods, because a strong enrichment of transcripts for comX and its downstream genes was achieved. Three two-component systems were expressed in the competent fraction, and among them was ComDE. Moreover, the recently identified regulator system ComR/S was expressed exclusively in the competent fraction. In contrast, the expression of bacteriocin-related genes was at the same level in all cells. GFP reporter strains for ComE and CipB (mutacin V) confirmed this expression pattern on the single-cell level. Fluorescence microscopy revealed that some ComX-expressing cells committed autolysis in an early stage of competence initiation. In viable ComX-expressing cells, the uptake of DNA could be shown on the single-cell level. This study demonstrates that all cells in the population respond to CSP through the activation of bacteriocin-related genes. Some of these cells start to activate ComX expression but then segregate into two subpopulations, one becoming competent and another one that lyses, resulting in intrapopulation diversity.
INTRODUCTION
Competence development is a complex process involving complex regulatory networks that trigger the capacity to take up exogenous DNA from the environment (7, 8, 11, 16). This phenomenon occurs in Gram-negative and Gram-positive bacteria and frequently is encountered in bacteria of the oral cavity, e.g., Streptococcus mutans (39). S. mutans is considered the major etiological agent of dental caries, because it is associated with the initiation and progression of dental caries. So far, the development of genetic competence is best characterized in Bacillus subtilis and the human pathogen Streptococcus pneumoniae and differs significantly between these two species (8, 11, 16).
At the onset of the stationary phase, cells of B. subtilis differentiate into several distinct cell types; e.g., ∼10% of the cells initiate competence development. This phenomenon of phenotypic variation of a clonal population has been referred to as bistability due to the dual stable pattern of gene expression in genetically identical cells (12).
According to Ferrell, Jr., two mechanisms exist to manifest bistability in a bacterial population (13). One is the principle mechanism of competence initiation in B. subtilis and is described below. It requires the presence of a positively autoregulated transcriptional activator that responds to itself in a nonlinear manner. Specifically, above a certain threshold of the regulator a hypersensitive change in gene expression is induced due to the activation of a positive autofeedback loop. Consequently, this results in high levels of the regulator, and its controlled genes will be activated (2). Cells that do not exceed the threshold will remain inactive, resulting in the segregation of the clonal population. In B. subtilis, ComK fulfills these criteria and induces the expression of all genes in the competence pathway in a subpopulation.
Whether a cell exceeds the threshold of the regulator concentration to activate the positive feedback loop is determined by noise, or random fluctuations, in the expression of the comK gene. In B. subtilis, this can be artificially manipulated by inducing the expression of comK or by promoting the stabilization of ComK. Due to the nonlinear autoregulatory properties of ComK, bistability will remain but the number of competence-initiating cells can be varied in that way (2).
So far, there is no evidence for bistability in competence development in S. pneumoniae. With laboratory strains, routinely ∼100% of competent cells could be obtained (6).
During growth, S. pneumoniae releases the competence-stimulating peptide (CSP) (encoded by comC) by using the ComAB secretion apparatus. The interaction of CSP with the membrane-embedded histidine kinase ComD results in the activation of the cognate response regulator ComE through phosphoryl transfer. The activation of the ComDE two-component system (TCS) leads to the expression of ∼20 early competence genes (5, 15, 34, 42). The coordinated induction of competence in the whole population is achieved by a positive feedback loop, since ComE activates the expression of the comAB and comCDE operons. However, the feedback loop in this case does not lead to the bifurcation of the population. Martin et al. discovered that the transcriptional readthrough of the tRNAArg5 gene upstream of comCDE was crucial to maintain a sufficient level of ComDE, ensuring that all cells can initiate competence when the threshold of CSP is exceeded. The inactivation of this transcriptional readthrough resulted in the segregation of the culture, and only 25% started the expression of late competence genes (24). Among the early competence genes is the alternate sigma factor ComX. ComX is required for the induction of the late competence genes, which include, e.g., the genes necessary for DNA uptake and processing.
S. pneumoniae contains a paralogue of the ComABCDE system, designated BlpABCRH. BlpRH constitutes a two-component system that is activated by the autoinducing peptide BlpC (similarly to CSP and ComDE). BlpAB was shown to be necessary for the transport of BlpC. The BlpR regulon was shown to comprise several bacteriocin-like peptides (10, 37).
The ComCDE and ComAB system (transport of CSP) of S. mutans is more related to the Blp system of S. pneumoniae than to ComABCDE, based on genomic organization and Blast identity (25). Interestingly, in S. mutans the ComCDE system combines the action of the two orthologues in S. pneumoniae. The activation of ComE, through CSP and its receptor kinase ComD, leads to the induction of competence through the alternate sigma factor ComX, and at the same time ComE directly induces a set of bacteriocin-related genes (Fig. 1) (1, 17–19, 23, 41).
Fig. 1.
Current model of competence development in S. mutans. Five different systems were shown to influence competence development: the serine protease HtrA, the HdrRM and BsrRM regulatory systems, and the two-component systems CiaHR and ComDE. The comC gene product is the precursor of the CSP (competence-stimulating peptide), which is transported and processed via the CslAB (ComAB) transporter (not shown). The accumulation of CSP activates the histidine kinase ComD, which activates its response regulator ComE by phosphorylation. Activated ComE regulates the expression of several mutacin-related genes, including the self-acting bacteriocin CipB and its cognate immunity protein CipI. The HdrMR and BsrMR regulatory systems were shown to regulate each other. Furthermore, they were shown to be involved in the regulation of bacteriocin-regulated genes (not shown). The signals that are sensed by HtrA, CiaHR, HdrMR, and BsrMR are unknown. All signals, including that of CSP, are integrated by the transcriptional regulator ComR through as-yet unknown mechanisms. ComR activates the expression of comS. The gene product of comS is the precursor of the XIP (sigma factor X-inducing peptide). ComS is transported into the extracellular environment through an unknown transporter and is processed outside the cell (not shown). The mature XIP is transported back by the peptide transporter Opp/Ami. ComR, in conjunction with XIP, activates the expression of ComS, leading to an autocatalytic positive feedback, and of the alternate sigma factor ComX. ComX further activates gene expression from late competence promoters and thus drives the expression of DNA uptake and processing genes, leading to genetic competence. (Modified after Mashburn-Warren et al., Perry et al., and Xie et al. [26, 33, 44]).
Interestingly, the disruption of comE in S. pneumoniae completely abolishes transformation, suggesting that ComE is exclusively responsible for ComX activation. The deletion of comE from S. mutans affects only the CSP-inducible competence initiation; a basal level of competence remains (referred as to CSP-independent competence), suggesting that the CSP/ComDE system is one of several signaling pathways used to activate ComX (1).
Indeed, under conditions of biofilm growth the HdrRM system was shown to contribute to competence development through the activation of ComX by a still-unknown signal (Fig. 1) (29). Moreover, microarray analysis revealed that both regulators, ComE and HdrR, activate a large set of overlapping genes (29, 30). Recently, Xie et al. identified another regulatory system, designated BsrRM, that primarily regulates bacteriocin-related genes but also affects the HdrMR system and thus indirectly contributes to competence development (44) (Fig. 1).
In S. mutans, no binding motif for ComE is present in the promoter region of ComX, suggesting a missing link between both regulators and ComX. This gap was filled by a recent study, which identified a new peptide regulator system (ComS/R) that acts downstream of ComE and directly activates ComX (26). The deletion of ComR completely abolished competence, suggesting that signals from all regulatory circuits (CSP dependent and independent) are integrated at the level of ComR. ComR activates the expression of the peptide precursor ComS. ComS is secreted, processed, and internalized through the peptide transporter Opp. The processed peptide, designated XIP (for sigma X-inducing peptide), modulates the activity of ComR, which activates the expression of ComS and ComX (Fig. 1).
Using green fluorescent protein (GFP) reporter constructs, it was shown that in S. mutans after the addition of CSP, mutacin IV was expressed population wide, but the expression of ComX was restricted to some cells, suggesting a bistable switch downstream of bacteriocin induction (18, 33).
The scope of this study was to determine the CSP-dependent activation of competence on the single-cell level. Therefore, we used flow cytometry to separate competent from noncompetent cells, guided by a ComX-GFP reporter. RNA of both subpopulations was subjected to microarray analysis to disclose transcriptional changes. To our knowledge, such a combination of flow cytometry and microarray analysis has never been carried out before in bacteria. Data from GFP reporter strains for ComE and CipB (mutacin V) and for DNA uptake on the single-cell level indicate another bifurcation within the ComX-expressing subpopulation into cells committing autolysis and cells that develop competence. Our data provide a first view of the segregation of a CSP-induced clonal population of S. mutans into three phenotypically distinct subpopulations and show the level within the competence signaling cascade where it occurs and, thus, expand our knowledge of competence development in S. mutans.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Bacterial strains and plasmids and their relevant characteristics are listed in Table 1. Escherichia coli was cultured routinely in Luria-Bertani (LB; Carl-Roth, Karlsruhe, Germany) medium at 37°C. E. coli strains carrying plasmids were selected with 50 μg ml−1 spectinomycin, 200 μg ml−1 erythromycin, 20 μg ml−1 chloramphenicol, or 20 μg ml−1 tetracycline. All S. mutans strains were cultivated in Todd Hewitt broth medium supplemented with 0.5% (wt/vol) yeast extract (THBY; Becton Dickinson, Heidelberg, Germany). S. mutans strains were grown at 37°C, without agitation, aerobically (5% CO2 enriched). The selection of mutant strains was carried out with 10 or 4 μg ml−1 (pALSM derivates) erythromycin and 500 μg ml−1 spectinomycin.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH10β | General cloning strain | New England Biolabs |
| S. mutans | ||
| UA159 | Wild-type, Erms, Sps | ATCC 700610 |
| SMluccomE | UA159::φ(comEP-luc), Spr | This study |
| SMluccomX | UA159::φ(comXP-luc), Spr | This study |
| SMGFPcomX | UA159, carrying pALSM04 | This study |
| SMGFPcomE | UA159, carrying pALSM28 | This study |
| SMGFPcipB | UA159, carrying pALSM34a | This study |
| Plasmids | ||
| PKRC12 | Donor of EGFP | 38 |
| pFW5 | Suicide vector, Spr | 36 |
| pALEC15 | Derivate pFW5, luc, Spr | 21 |
| pALEC50 | pALEC15 + φ(comEP-luc), Spr | This study |
| pALEC52 | pALEC15 + φ(comXP-luc), Spr | 20 |
| pALSM01 | Modifed pAT18, Ermr | 28, 40 |
| pALSM02 | pALSM01, EGFP, Ermr | This study |
| pALSM03 | pALSM03, removal of lacp, Ermr | This study |
| pALSM04 | pALSM03 + φ(comXP-EGFP), Ermr | This study |
| PALSM28 | pALSM03 + φ(cipBP-EGFP), Ermr | This study |
| PALSM34a | pALSM03 + φ(comEP-EGFP), Ermr | This study |
Erm, erythromycin; Sp, spectinomycin; luc, luciferase.
The CSP peptide was synthesized in the department of Chemical Biology at the Helmholtz-Centre for Infection Research. The peptide was purified by high-performance liquid chromatography (HPLC) and dissolved in water to a concentration of 1 mM. The sequence is SGSLSTFFRLFNRSFTQALGK, and the peptide was synthesized with a free N terminus and a carboxylic group at the C terminus.
Construction of reporter plasmids and strains.
For the construction of pALEC50 and pALEC52, the suicide vector pALEC15 (21) was used as the backbone (Table 2). Upstream regions of comE (SMU.1917) and comX (SMU.1997) were amplified using primers PcomEF/R and PcomXF/R, digested with NcoI, and ligated into pALEC15. The plasmids were transformed into the wild type of UA159 to generate strains SMluccomE and SMluccomX by single-crossover recombination.
Table 2.
Primers used in this studya
| Primer | Sequence (5′→3′) | Purpose |
|---|---|---|
| PcomEF | TTTTTTCCATGGGGGTGTCGTCATTCTTCCTT | Construction of pALEC50 |
| PcomER | TTTTTTCCATGGTATTTCTCCTTTAATCTTCTAT | Construction of pALEC50 |
| PcomXF | AAAAAAACCATGGTCCAAAAATAAGTGACTAAGG | Construction of pALEC52 |
| PcomXR | AAAAAAACCATGGCTATTACGATGACCTCCTTT | Construction of pALEC52 |
| GFPF | AAAAAATCTAGAAAGATATCATGAGTAAAGGAGAAGAACTTTTCACTGG | eGFP |
| GFPR | AAAAAAGCTAGCCTATTTGTATAGTTCATCCATGCCATG | eGFP |
| PcomX2F | TCCAAAAATAAGTGACTAAGG | Promoter comX |
| PcomX2R | CTATTACGATGACCTCCTTT | Promoter comX |
| PcipBF | TTTATATCTCCTTTTTTTGATTATAATTAGTATACTA | Promoter cipB |
| PcipBR | ATGATAAATACCCCTTCCCCATTTTTAGTT | Promoter cipB |
| PcomE2F | TTATATAATCAATTGACAACGGCTGAT | Promoter comE |
| PcomE2R | TATTTCTCCTTTAATCTTCTAT | Promoter comE |
Restriction sites are in boldface.
For the construction of the GFP fluorescence reporter plasmids, a fragment containing the recA promoter from Streptococcus pyogenes and GFP was released from pVA-EGFP2 (28) by XbaI and religated, yielding pALSM01. The enhanced GFP (EGFP) from pKRC12 was amplified using primer GFPF/R, cut with XbaI/NheI, and ligated to pALSM01, giving pALSM02. Afterwards the vector was digested with PciI and EcoRI, blunted, and religated to release the lac promoter, giving pALSM03. Subsequently, the upstream regions of comX (PcomX2F/R), cipB (PcipBF/R), and comE (PcomE2F/R) were amplified and cloned into the EcoRV site. The plasmids were confirmed by sequencing and were transformed into the wild type of UA159.
Fluorescence microscopy.
Cells were pelleted, washed, and resuspended in phosphate-buffered saline (PBS). For chain disruption, cells were sonicated (20 cycles of 0.5-s pulse, 0.5-s break; MS72 probe with 10% power; Bandelin Sonoplus HD2200; Berlin, Germany). For imaging, an Olympus BX60 microscope, equipped with a colorview II camera and a 100x/1.3 oil immersion objective, was used. The filter U-MWIBA3 (excitation, 460 to 495 nm; emission, 510 to 550 nm; dichromatic filter, 505 nm) from Olympus (Seelze, Germany) was used to visualize EGFP with an exposure time of 5 s. The filter 20HE (excitation, 546 ± 12 nm; emission, 607 ± 80 nm; dichromatic filter, 560 nm) from Carl-Zeiss (Zaventum, Belgium) was used to visualize Cy3, with an exposure time of 10 s. The filter U-MWIY2 (excitation, 545 to 580 nm; emission, 610IF [interference type]; dichromatic filter, 600 nm) from Olympus was used to visualize PI (propidium iodide), with an exposure time of 1 s. Images were recorded under the same conditions using the cellB software from Olympus. For better visualizations on printouts, brightness and contrast were modified equally for all images using Adobe Photoshop.
Visualization of DNA uptake.
Overnight cultures were diluted 1:10 and incubated as described above. CSP (0.2 μM) was added after 1 h, and cultures were incubated further for 2 h. One hundred μl of cells was withdrawn, and an ∼2-kb DNA fragment (PCR amplified), labeled with Cy3 (ULS labeling; see below), was added at a final concentration of 1 μg/ml and incubated for 15 min at 37°C. Afterwards, 30 U of DNase I (Roche, Mannheim, Germany) was added and incubated for 5 min at 37°C. Cells were washed, resuspended in PBS, and visualized using fluorescence microscopy.
Luciferase assay.
Luciferase assays were performed by withdrawing 100 μl culture for the measurement of the optical density using a Victor3 Wallac plate reader and luciferase measurement. Samples were mixed with 3× assay buffer (75 mM tricine, 15 mM MgSO4, 1.5 mM EDTA, 1.5 mM dithiothreitol [DTT], 900 μM ATP, 3 mg/ml bovine serum albumin [BSA], and 3% [wt/vol] d-glucose, pH 7.8) and incubated 10 min prior to the injection of 100 μl d-luciferin (120 μM final concentration) solved in 20 mM tricine (pH 7.8). d-Luciferin (Carl-Roth, Karlsruhe, Germany) was resuspended in 20 mM tricine (pH 7.8, 1 mg/ml), aliquoted, and stored at −70°C until use. Luminescence was recorded for 30 s (Victor Wallac;uminescence reader; Perkin Elmer Life Sciences) and normalized against the optical density at 620 nm (OD620) to calculate the relative light units (RLU). All measurements were done for at least two biological replicates.
Fluorescence-activated cell sorting (FACS) analysis and subpopulation sorting.
All media used for flow cytometry were filtered through a 0.22-μm filter. For subpopulation sorting, an overnight culture of S. mutans SMGFPcomX was diluted 1:10 into fresh medium and incubated at 37°C. After 1 h, induction with 0.2 μM CSP was carried out. Cells were further incubated for 2 h and sonicated as described above for disrupting cell chains for fluorescence microscopy prior to flow cytometry. This procedure was repeated every half hour, and every sample was subjected to sorting for 30 min. In total, 10 individual samples were sorted, yielding a single biological sample. Control cells of a mixed induced and not induced culture were sonicated and kept in RNA protect until the sorting was finished.
Flow-cytometric sorting was performed on a FACSAria II cell sorter system (Becton Dickinson). Filtered PBS was used as the sheath fluid. Cells were kept at 37°C during sorting and were collected directly in RNA protect. Forward and side scatter (FSC and SSC, respectively) were set to log with a threshold of 200 on both parameters. The detection of GFP fluorescence was through a 525-nm/50-nm bandpass filter. Photomultiplier (PMT) voltage was 370 for FSC, 205 for SSC, and 467 for GFP. Despite sonication, chains of two or more cells still occurred in the culture. We used pulse width (-W) and −area (-A) of FSC and SSC to identify these doublets and chains. When a cell/particle passes through the laser beam, it will generate a signal pulse (signal over time), which has a height (−H), width (−W), and integrated area (-A). Single cells of different sizes will show a constant pulse width and strongly correlated height-to-area ratio. Doublets and other aggregates will show a similar pulse height, but they can be identified by their increased pulse width and area. According to the FSC-W and SSC-W plots, gates were set more stringently to allow the sorting of single cells. Noninduced cells reached a fluorescence intensity of approximately 800 (arbitrary units); thus, cells with a higher value were sorted as GFP+ and cells with a lower value as GFP−. Before cells were collected in RNA protect, the efficiency of sorting was determined and both subpopulations again were subjected to analysis. The whole experiment was done in duplicate, and each replicate was used separately for transcriptome analysis. To measure the GFP intensity of all reporter strains, cells were induced and treated as described above, and 100,000 cells were analyzed. The median values of GFP-positive cells (intensity, >400 U) from two biological replicates was determined.
RNA extraction.
RNA protect samples (Qiagen, Hilden, Germany) containing sorted or mixed cells were filtered through a 0.22-μm filter (Millipore, Cork, Ireland). The filter was stored at −70°C until RNA extraction. The filter was covered with 1 ml Tris-EDTA (TE) buffer (pH 8) containing 2.5 mg lysozyme and 50 U mutanolysin and incubated at room temperature for 25 min. RLT buffer (RNeasy kit; Qiagen) containing sterile, acid-washed glass beads (diameter, 106 μm) was added and vortexed for 3 min. Subsequent RNA extraction was carried out using the RNeasy microkit (Qiagen) according to the manufacturer's instructions. Genomic DNA was removed using DNase I (Qiagen) on a column digestion protocol. The absence of genomic DNA was controlled by a standard PCR. The quality of the total RNA was controlled on a denaturing formaldehyde agarose gel.
Microarray.
A customized whole-genome microarray of S. mutans UA159 was used to carry out microarray experiments and was described previously (45). Briefly, the array contained probes for all open reading frames (ORF) and noncoding regions of S. mutans. Each ORF and intergenic region was covered with three antisense probes (60 bp in length) in duplicate. Positive and negative controls from Agilent were included in the design.
RNA extraction was carried out as described above, and labeling was achieved using the ULS fluorescent labeling kit (Kreatech, Germany). Reference RNA from the non-CSP-induced mixed culture was labeled using Cy3. The three remaining RNA populations (CSP-induced mixed and sorted by flow cytometry) were labeled with Cy5. The degree of labeling was calculated using a web form on the manufacturer's homepage (http://www.kreatech.com/Portals/kreatech/downloads/labeling/27_DoL%20calculator_28082007.xls). For each condition, RNA of two individual biological samples was used. Four hundred fifty ng of reference RNA was mixed with 450 ng of each Cy5-RNA and was subjected to fragmentation according to the protocols provided by Agilent. Hybridization was carried out at 65°C for 17 h using the Agilent hybridization chamber. Scanning was achieved using the Agilent DNA microarray scanner. Background correction was performed using the Agilent algorithms, and raw data were extracted using the Agilent feature extraction software. Data processing was carried out with the Bioconducter-Linear Models for Microarray analysis (LIMMA) package (43) using the R language (http://www.r-project.org). We compared the expression data of the three CSP-induced RNA populations with RNA from the non-CSP-induced mixed culture (fold mixed refers to induced mixed culture versus noninduced mixed culture, GFP+ refers to the induced, sorted, competent subpopulation versus noninduced mixed culture, and GFP− refers to the induced, sorted, noncompetent subpopulation versus noninduced mixed culture). Moreover, we compared directly the transcription of the CSP-induced sorted competent and noncompetent subpopulations, referred to as GFP+/GFP−. The P values for differential expression were adjusted for the false discovery rate using the method by Benjamini and Hochberg (BH) (4). Genes with a log2-fold change of >0.85 and a P value of <0.05 were selected for further analysis.
Real-time RT-qPCR.
The synthesis of cDNA was carried out with 500 ng of total RNA using the Quantitect reverse transcription kit (Qiagen) according to the manufacturer's protocols. Quantitative real-time reverse transcription-PCR (RT-qPCR) was performed using the LightCycler 480 system (Roche, Mannheim, Germany), and the reaction mixtures were prepared using the Quantitect SYBR green PCR kit (Qiagen). Changes in the level of gene expression were calculated automatically by the LightCycler 480 software using the ΔΔ threshold cycle (ΔΔCT) method. The gyrase A gene (SMU.1114) was used as the housekeeping reference gene. All steps were performed according to the manufacturers' protocols. RNA extraction and reverse transcription were carried out from two independent biological samples. All quantitative real-time PCR measurements were done in duplicate.
Microarray data accession number.
Microarray data have been deposited at NCBI-GEO under the accession number GSE25284.
RESULTS
Kinetics of gene expression of key competence genes in response to CSP.
To obtain a population-wide overview of the gene expression of key competence genes, we performed a time series experiment to determine the response in gene expression to the addition of CSP using quantitative real-time PCR. Table 3 lists the genes that were chosen for analysis. The induction of the ComE direct target genes cipB and cipI (mutacin V and its cognate immunity protein CipI, respectively) reached almost maximal levels 15 min after the addition of CSP, showing that low levels of ComE at the time of induction are sufficient to achieve the maximal induction in response to CSP. The regulatory genes (comE, comS, and comR) acting upstream of ComX were induced 15 min after induction and achieved their highest expression 2 h after CSP addition. In contrast, the induction of the alternate sigma factor ComX occurred with a delay first being induced 30 min after CSP addition, confirming the observations of Ahn et al. (1). Similarly to its upstream regulators, the expression of comX was highest 2 h postinduction.
Table 3.
Fold change of gene expression compared to levels at time zero in response to 0.2 μM CSP
| Gene | Fold change at time pointa (min): |
||||
|---|---|---|---|---|---|
| 15 | 30 | 60 | 120 | 240 | |
| comE | 1.75 ± 0.14 | 2.91 ± 0.35 | 8.54 ± 1.28 | 18.58 ± 2.23 | 1.98 ± 0.22 |
| comR | 1.38 ± 0.12 | 2.68 ± 0.27 | 2.24 ± 0.29 | 2.97 ± 0.48 | 0.97 ± 0.05 |
| comS | 2.17 ± 0.13 | 20.88 ± 2.71 | 68.3 ± 12.98 | 144.08 ± 30.26 | 8.97 ± 1.17 |
| comX | 0.88 ± 0.03 | 1.99 ± 0.12 | 5.29 ± 0.58 | 12.48 ± 1.75 | 0.15 ± 0.02 |
| cipB | 27.49 ± 3.02 | 33.86 ± 4.74 | 34.91 ± 5.24 | 41.02 ± 6.56 | 94.99 ± 16.15 |
| cipI | 8.45 ± 0.59 | 11.44 ± 1.94 | 10.83 ± 1.63 | 15.52 ± 2.48 | 14.22 ± 1.99 |
Average gene expression and standard deviations from two biological samples. Data were determined by quantitative RT-PCR.
Expression of comE and comX was transiently activated and showed separation into two subpopulations.
To determine the induction pattern of the comE and comX genes upon CSP addition, we constructed firefly luciferase reporter plasmids for both genes. Figure 2 shows that both genes were expressed transiently, reaching their highest values around 2 to 2.5 h postinduction, confirming the qPCR data. The delayed induction of ComX compared to that of ComE cannot be seen, because the first sample for luciferase measurement was taken 30 min after CSP addition, where both genes already were induced. Using a ComX-GFP fusion, we showed that the expression of ComX was restricted to a part of the culture. This phenotypic variation in ComX expression and the averaged GFP intensity of the whole culture is shown in Fig. 3. Throughout all of the experiments, we observed that approximately 30 to 50% of the cells were expressing GFP. Compared to the time frame for the luciferase reporter, it took approximately 30 min longer (1 h postinduction) until the first cells became detectable in the fluorescence microscope after the addition of exogenous CSP. An additional 30 min for chromophore maturation was needed to obtain a detectable signal in a fluorescence microtiter plate reader. Since mRNA levels were highest after 2 h postinduction and a suitable amount of detectable GFP had been produced, we decided to separate both subpopulations using flow cytometry between 2 and 2.5 h after the addition of 0.2 μM CSP.
Fig. 2.
Transient induction of comE and comX by CSP. Shown are the growth characteristics and luminescence development of the comE (A) and comX (B) promoter-luciferase fusion in the absence and presence of 0.2 μM CSP. CSP was added after 1 h of incubation. Figures show the means and standard deviations from at least two independent experiments.
Fig. 3.
Subpopulation-specific induction of SMGFPcomX in the presence of CSP. (A) Growth curves and the development of GFP fluorescence. CSP (0.2 μM) was added 1 h after inoculation. Figures show the means and standard deviations from two independent experiments. Also shown is the fluorescence microscopy of CSP-induced (sonicated) cells 2 (B) and 6 h (C) postinduction.
Both subpopulations could be successfully separated using flow cytometry.
Prior to flow cytometry, the chains of S. mutans were disrupted using a sonication treatment. In pre-experiments we tested the ability to disrupt chains by sonication and checked for membrane integrity by staining the cells with propidium iodide (a fluorescence dye that stains DNA and that does not penetrate intact membranes; therefore, it is used for the detection of damaged/dead cells). No influence of sonication was noticed, e.g., there was no increased number of PI-stained cells. Using sonication, it was possible to break down the majority of chains to singlet or doublet cells (see Fig. S1 in the supplemental material).
For the calibration of the FACSAria II, we first analyzed sterile uninoculated media to determine the particle background of the media (Fig. 4A). Afterwards, media containing bacteria were used to determine cells/particles of inoculated media and for setting the voltages and gates (Fig. 4B). For the discrimination of chains or other aggregates, FSC-W and SSC-W were enabled and gating was carried out more stringently. Noninduced cells reached a GFP fluorescence intensity of approximately 800 U (Fig. 4C). To ensure that we did not collect GFP− cells with high autofluorescence, we set the gate for GFP+ cells at this threshold. The GFP− population was gated below 300 GFP fluorescence units. The fraction between gates P4 and P5 was not collected.
Fig. 4.
Scatter plots and GFP fluorescence distribution of S. mutans cells analyzed by flow cytometry. (A) Side- and forward-scatter plots of uninoculated medium, with the corresponding gates for cell sorting. (B) Side- and forward-scatter plots of inoculated medium, with the corresponding gates for cell sorting. (C) GFP intensity from medium and not induced cells of the gate P3. Left, intensity of uninoculated medium; right, intensity of uninduced SMGFPcomX cells. (D) GFP intensity of induced SMGFPcomX cells of gate P3 and reanalysis after sorting.
The histogram in Fig. 4D represents the GFP intensity of all analyzed cells of gate P3. A bimodular distribution of dark and GFP-expressing cells became clearly apparent. However, with respect to the amount of cells in both fractions, this pattern was quite dynamic, depending on the fitness of the preculture (see Fig. S2 in the supplemental material). The analysis of both separated subpopulations is shown in Fig. 4D. The majority of dark or GFP-expressing cells could be successfully separated from each other.
Microarray analysis showed an enrichment and depletion pattern of comX transcripts in the subpopulations compared to that of the mixed population.
The aim of the ComX-GFP-guided separation of both subpopulations was to compare their individual gene expression levels to those of the transcriptome of induced and noninduced mixed populations, respectively. GFP-expressing cells were designated GFP+ or competent fraction/subpopulation, and non-GFP-expressing cells were referred to as GFP− or noncompetent fractions.
Sorting resulted in an enrichment (13.8-fold) of comX transcription in the GFP+ population and a depletion (2-fold) in the GFP− cells compared to that of the mixed population (3.6-fold) (Table 4; also see Table S1 in the supplemental material). The 2-fold induction of comX in the GFP− population may reflect a carryover of GFP+ cells during the sorting process; alternatively, it might have been caused by naturally competent cells (independently of CSP) whose comX transcription level was insufficient for detectable GFP fluorescence. Another possibility would be stress-triggered competence induction through the process of sorting, which cannot occur in the mixed populations because they were not subjected to flow cytometry.
Table 4.
Selected genes that were significantly changed after cytometric sorting
| Classification and locus tag SMU no. | Description | Gene name | Cytometry resultsa |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Induced mixed culture |
GFP− |
GFP+ |
GFP+/GFP− |
|||||||
| Fold change | P | Fold change | P | Fold change | P | Fold change | P | |||
| Regulatory functions | ||||||||||
| 61 | ComR | comR | 1.4 | 1.70E-02 | 1.1 | 2.27E-01 | 1.8 | 1.31E-03 | 1.4 | 4.9E-01 |
| Shp61 | ComS within intergenic region 51 | comS | 15.37 | 1.11E-04 | 6.59 | 5.86E-04 | 81.03 | 1.26E-05 | 11.76 | 7.48E-04 |
| 1916 | Histidine kinase of the competence regulon, ComD | comD | 3.95 | 9.95E-04 | 2.62 | 4.45E-03 | 6.99 | 2.13E-04 | 2.09 | 2.36E-02 |
| 1917 | Response regulator of the competence regulon, ComE | comE | 3.87 | 4.23E-04 | 2.54 | 2.16E-03 | 7.31 | 7.47E-05 | 2.31 | 7.37E-03 |
| 1997 | ComX1, transcriptional regulator of competence-specific genes | comX1 | 3.56 | 2.58E-02 | 2.01 | 1.40E-01 | 13.79 | 1.50E-03 | 4.96 | 2.48E-02 |
| Transformasome related | ||||||||||
| 498 | Late competence protein | comF | 9.37 | 3.86E-04 | 3.60 | 4.25E-03 | 31.81 | 5.38E-05 | 6.93 | 2.28E-03 |
| 499 | Late competence protein | 12.60 | 3.65E-04 | 4.82 | 2.88E-03 | 47.99 | 5.36E-05 | 8.35 | 8.07E-03 | |
| 539c | Signal peptidase type IV | 2.85 | 4.14E-03 | 1.77 | 3.91E-02 | 8.75 | 1.75E-04 | 4.32 | 3.73E-03 | |
| 625 | Competence protein | comEA | 20.29 | 2.10E-04 | 7.12 | 1.38E-03 | 70.87 | 4.32E-05 | 8.26 | 4.83E-03 |
| 626 | Competence protein | 18.62 | 1.64E-04 | 6.64 | 1.12E-03 | 55.17 | 3.88E-05 | 6.63 | 5.23E-03 | |
| 836 | Hypothetical protein | 9.93 | 7.66E-04 | 3.83 | 7.22E-03 | 34.45 | 1.11E-04 | 6.61 | 3.26E-03 | |
| 1979c | Hypothetical protein | 16.28 | 1.38E-04 | 5.69 | 1.13E-03 | 44.87 | 3.35E-05 | 6.83 | 3.61E-03 | |
| 1980c | Hypothetical protein | 37.48 | 1.92E-05 | 11.83 | 1.11E-04 | 111.95 | 5.66E-06 | 8.30 | 1.50E-03 | |
| 1981c | Hypothetical protein | 41.42 | 1.52E-05 | 13.06 | 8.33E-05 | 134.85 | 4.25E-06 | 8.47 | 7.13E-04 | |
| 1982c | Hypothetical protein | 35.24 | 1.53E-05 | 11.54 | 8.57E-05 | 116.79 | 3.99E-06 | 8.52 | 6.48E-04 | |
| 1983 | Putative competence protein ComYD | comYD | 23.54 | 1.14E-04 | 7.52 | 8.42E-04 | 74.40 | 2.75E-05 | 8.08 | 3.90E-03 |
| 1984 | Putative competence protein ComYC | comYC | 40.28 | 1.58E-05 | 12.21 | 9.46E-05 | 140.37 | 4.12E-06 | 9.17 | 5.54E-04 |
| 1985 | ABC transporter ComYB | comYB | 30.59 | 2.92E-05 | 9.49 | 1.97E-04 | 91.35 | 8.13E-06 | 7.93 | 5.71E-04 |
| 1987 | ComYA; late competence gene | comYA | 44.78 | 1.51E-05 | 13.28 | 8.81E-05 | 164.36 | 3.86E-06 | 9.63 | 7.10E-04 |
| Putative bacteriocins, immunity proteins, bacteriocin island | ||||||||||
| 150 | Hypothetical protein | nlmA | 6.53 | 3.84E-04 | 5.65 | 5.49E-04 | 7.29 | 2.98E-04 | 1.10 | 7.16E-01 |
| 151 | Hypothetical protein | nlmB | 6.45 | 5.51E-04 | 5.67 | 7.56E-04 | 7.01 | 4.55E-04 | 1.04 | 8.81E-01 |
| 423 | Hypothetical protein | 6.66 | 2.34E-04 | 5.92 | 3.11E-04 | 7.25 | 1.92E-04 | 1.04 | 8.58E-01 | |
| 925 | Hypothetical protein | cipI | 6.18 | 5.92E-05 | 5.20 | 9.33E-05 | 8.75 | 2.66E-05 | 1.38 | 1.10E-01 |
| 1892c | Hypothetical protein | 0.87 | 6.18E-01 | 0.55 | 7.56E-02 | 0.46 | 3.52E-02 | 0.71 | 1.82E-01 | |
| 1902c | Hypothetical protein | 2.83 | 7.64E-04 | 2.80 | 8.09E-04 | 3.13 | 5.09E-04 | 0.96 | 8.32E-01 | |
| 1903c | Hypothetical protein | 4.90 | 1.04E-04 | 4.83 | 1.08E-04 | 5.69 | 6.90E-05 | 1.00 | 9.90E-01 | |
| 1904c | Hypothetical protein | 4.52 | 3.74E-04 | 4.35 | 4.19E-04 | 5.04 | 2.73E-04 | 0.99 | 9.47E-01 | |
| 1905c | Putative bacteriocin secretion protein | 4.83 | 2.74E-04 | 4.75 | 2.86E-04 | 5.40 | 2.01E-04 | 0.96 | 8.53E-01 | |
| 1906c | Hypothetical protein | 6.63 | 1.91E-04 | 6.47 | 2.03E-04 | 6.37 | 2.10E-04 | 0.84 | 5.51E-01 | |
| 1908c | Hypothetical protein | 6.38 | 1.91E-04 | 6.13 | 2.11E-04 | 6.40 | 1.90E-04 | 0.88 | 5.48E-01 | |
| 1909c | Hypothetical protein | 6.23 | 2.40E-04 | 5.93 | 2.72E-04 | 6.03 | 2.61E-04 | 0.88 | 6.32E-01 | |
| 1910c | Hypothetical protein | 6.78 | 2.47E-04 | 6.60 | 2.64E-04 | 6.60 | 2.64E-04 | 0.87 | 6.56E-01 | |
| 1912c | Hypothetical protein | 6.07 | 1.52E-04 | 5.61 | 1.86E-04 | 6.06 | 1.53E-04 | 0.92 | 6.98E-01 | |
| 1913c | Putative immunity protein | 5.69 | 2.78E-04 | 5.49 | 3.06E-04 | 5.86 | 2.58E-04 | 0.89 | 6.10E-01 | |
| 1914c | Hypothetical protein | cipB | 8.61 | 1.02E-04 | 8.60 | 1.02E-04 | 8.24 | 1.12E-04 | 0.78 | 4.58E-01 |
Genes that are significantly changed (log2 fold change, >0.85; and P < 0.05) are in boldface.
Genes that were significantly changed in at least one of the populations are listed in Table S1 in the supplemental material, and a selection of genes is presented in Table 4. Highlights are discussed below. Additionally, a direct comparison of the GFP+ and GFP− subpopulations was carried out. The direct comparison of both fractions did not reflect the large increase of gene expression in response to CSP in the competent (GFP+) fraction in a proper way due to the weak enrichment of comX, its downstream genes, and other known regulators (see below) in the GFP− subpopulation. Thus, the fold change values are considerably smaller than those of the non-CSP-induced control, and smaller changes may be overlooked. For better comparison, the values of all four conditions are listed together. Genes that were significantly changed (fold change, ±1.8; P < 0.05) are marked in boldface. The results for some genes were confirmed using quantitative real-time PCR and are listed in Table S1 in the supplemental material. The enrichment or depletion of transcripts in the GFP+ or GFP− population could be confirmed by qPCR. However, there was a considerable difference in the amount of transcripts in both biological samples, yielding high standard deviations. For a better comparison, we inserted the individual results of the qPCR for both biological samples in Table S1.
Three two-component systems showed an enrichment/depletion pattern similar to that of comX.
Our microarray analysis revealed that transcripts for the two-component system comDE were enriched in the GFP+ fraction similarly to comX. Moreover, we observed an enrichment of two additional two-component systems, namely, HK/RR 4 (SMU.927-928) and HK/RR 9 (SMU.1964-1965). The induction of these three TCS upon CSP addition is in agreement with a population-wide transcriptome analysis carried out by Perry et al. (33). Interestingly, a deletion of HK9 resulted in a decreased transformation efficiency, whereas the inactivation of HK4 showed no effect (22). However, the new finding that these TCS are induced in the competent cell fraction suggests that they take part in competence development, but their exact role needs further experimental exploration. Using qPCR, we confirmed the microarray data for comE and comX (see Table S1 in the supplemental material).
Interestingly, we found several intergenic regions (IGR) that were differentially regulated in both subpopulations independently of their adjacent genes. Table 5 lists all of the IGRs that were differentially expressed in the mixed population, GFP+ subpopulation, or GFP− subpopulation compared to the expression of the noninduced control (cutoff criteria fold change, >2; P < 0.05).
Table 5.
Intergenic regions that were significantly changed after cytometric sorting and whose adjacent genes were not affected
| Genomic position | IGR between ORFs (SMU no.) | Cytometry resultsa |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Induced mixed culture |
GFP− |
GFP+ |
GFP+/GFP− |
|||||||
| Fold change | P | Fold change | P | Fold change | P | Fold change | P | |||
| 136886-137007 | 132 | 133c | 1.73 | 2.73E-01 | 3.43 | 4.15E-02 | 1.28 | 6.00E-01 | 0.24 | 1.31E-01 |
| 179653-179873 | 181 | 182 | 1.69 | 7.64E-02 | 2.68 | 9.38E-03 | 1.97 | 3.50E-02 | 0.77 | 4.63E-01 |
| 367013-367066 | 390 | 391c | 1.68 | 1.33E-01 | 2.63 | 2.22E-02 | 1.97 | 6.80E-02 | 0.76 | 4.52E-01 |
| 375868-376028 | 401c | 402 | 1.55 | 5.32E-02 | 1.74 | 2.46E-02 | 2.26 | 5.85E-03 | 1.25 | 4.79E-01 |
| 435807-435921 | 466 | 467 | 1.59 | 2.09E-01 | 2.53 | 3.67E-02 | 2.06 | 7.75E-02 | 0.84 | 7.41E-01 |
| 596121-596312 | 636 | 637c | 1.77 | 1.13E-01 | 2.15 | 5.11E-02 | 2.19 | 4.74E-02 | 0.95 | 8.98E-01 |
| 715015-715084 | 765 | 766 | 2.34 | 9.05E-02 | 2.99 | 4.37E-02 | 1.46 | 3.92E-01 | 0.50 | 2.15E-01 |
| 718674-719212 | 770c | 771c | 4.53 | 4.54E-03 | 3.03 | 1.51E-02 | 17.74 | 2.85E-04 | 4.85 | 3.61E-02 |
| 745624-745833 | 799c | 800 | 1.46 | 1.28E-01 | 2.14 | 1.52E-02 | 1.37 | 1.85E-01 | 0.69 | 2.24E-01 |
| 755296-755491 | 807 | 809 | 2.42 | 1.11E-01 | 3.18 | 5.39E-02 | 3.61 | 3.90E-02 | 1.11 | 8.80E-01 |
| 759342-759398 | 813 | 814 | 1.67 | 5.56E-02 | 2.08 | 1.68E-02 | 1.92 | 2.52E-02 | 0.95 | 8.74E-01 |
| 837975-838127 | 883 | 885 | 3.64 | 3.47E-02 | 3.71 | 3.29E-02 | 1.86 | 2.20E-01 | 0.52 | 2.20E-01 |
| 844419-844621 | 889 | 890 | 2.06 | 4.32E-02 | 2.50 | 1.95E-02 | 1.60 | 1.37E-01 | 0.66 | 2.64E-01 |
| 845165-845295 | 890 | 891 | 1.67 | 5.46E-02 | 2.01 | 1.96E-02 | 1.48 | 1.13E-01 | 0.76 | 3.30E-01 |
| 878575-878770 | 923 | 924 | 1.94 | 2.86E-02 | 2.59 | 7.54E-03 | 2.08 | 2.00E-02 | 0.82 | 5.52E-01 |
| 896354-896469 | 944 | 946 | 1.66 | 1.18E-01 | 2.07 | 4.36E-02 | 1.43 | 2.40E-01 | 0.74 | 4.15E-01 |
| 938355-938638 | 991 | 992 | 2.15 | 1.06E-01 | 4.15 | 1.54E-02 | 2.61 | 5.73E-02 | 0.68 | 4.76E-01 |
| 996212-996313 | 1048 | 1050 | 2.13 | 6.85E-02 | 2.95 | 2.21E-02 | 1.91 | 1.05E-01 | 0.71 | 4.59E-01 |
| 998766-999060 | 1052 | 1053 | 1.51 | 2.21E-01 | 2.25 | 4.24E-02 | 1.69 | 1.37E-01 | 0.76 | 5.59E-01 |
| 1008295-1008566 | 1063 | 1064c | 2.37 | 4.82E-02 | 3.70 | 1.14E-02 | 3.90 | 9.84E-03 | 1.05 | 9.05E-01 |
| 1019708-1019801 | 1076 | 1077 | 2.03 | 5.05E-02 | 2.05 | 4.91E-02 | 1.56 | 1.68E-01 | 0.76 | 4.76E-01 |
| 1021518-1021647 | 1077 | 1078c | 1.99 | 3.50E-02 | 2.29 | 1.88E-02 | 2.00 | 3.47E-02 | 0.86 | 6.68E-01 |
| 1138619-1138670 | 1195 | 1196c | 2.31 | 7.34E-02 | 3.46 | 2.12E-02 | 3.40 | 2.22E-02 | 1.03 | 9.59E-01 |
| 1139685-1139763 | R0092 | 1197 | 2.50 | 1.02E-02 | 2.73 | 7.10E-03 | 2.73 | 7.20E-03 | 1.06 | 8.36E-01 |
| 1307188-1307342 | 1378 | 1379 | 2.07 | 6.15E-02 | 2.20 | 4.77E-02 | 1.61 | 1.74E-01 | 0.77 | 4.93E-01 |
| 1381695-1381764 | 1450 | 1451 | 1.76 | 1.39E-01 | 2.57 | 3.34E-02 | 2.95 | 2.10E-02 | 1.00 | 9.95E-01 |
| 1759753-1759939 | 1865 | 1867c | 2.13 | 6.08E-02 | 3.12 | 1.57E-02 | 1.82 | 1.13E-01 | 0.68 | 3.41E-01 |
| 1889423-1889587 | 2016 | 2017 | 1.29 | 3.09E-01 | 2.04 | 2.78E-02 | 1.66 | 7.95E-02 | 0.79 | 4.01E-01 |
| 1892786-1893019 | 2025 | 2026c | 1.51 | 5.55E-02 | 1.40 | 9.90E-02 | 2.14 | 6.56E-03 | 1.96 | 1.59E-02 |
| 1906075-1906218 | 2035 | 2036 | 1.74 | 1.98E-01 | 2.76 | 4.33E-02 | 2.20 | 8.96E-02 | 0.78 | 5.86E-01 |
IGRs that are significantly changed (fold change, >2.0; P < 0.05) are in boldface.
Since their neighboring genes were not affected, these intergenic regions may represent regulatory RNA molecules, and a detailed analysis is under way. In addition, Table S2 in the supplemental material lists all differentially expressed intergenic regions that were changed, comparing the CSP-induced samples to the noninduced control (cutoff criteria fold change, ±1.8; P < 0.05).
Induction of ComS and ComR was restricted to the competent fraction.
In accordance with the recent finding that XIP(ComS)/ComR is the proximal regulator for comX induction, both genes showed the same pattern of enrichment and depletion in the two subpopulations (Table 4) (26). The transcription of ComR has been shown to be 2- to 3-fold increased due to the presence of CSP, loss of hdrM, or overexpression of hdrR (30, 33), confirming the weak induction we observed in our transcriptome analysis. Since comS is a newly annotated open reading frame, it was not directly included in our array design, but the intergenic region harboring comS was induced 81-fold in the GFP+ fraction and only 6.6-fold in the GFP− fraction.
Transformasome genes were highly enriched in the competent subpopulation.
The alternative sigma factor ComX is responsible for the transcriptional activation of DNA uptake and processing genes and is called the transformasome (8). As a consequence of comX enrichment in the GFP+ subpopulation, the gene transcripts responsible for building and assembling the transformasome were highly enriched (up to 164-fold) in the GFP+ fraction compared to that of the noninduced mixed population. Due to the weak enrichment of comX transcripts in the GFP− fraction, we observed a weak induction of ComX downstream genes in this fraction, too. However, by comparing the ratio of both separated subpopulations, we obtained a transcriptional enrichment of up to ∼10 fold for the ComX downstream target genes, demonstrating the success of the applied approaches.
Table 4 lists the genes for DNA uptake and processing based on their orthologues in S. pneumoniae. Recently, Okinaga et al. identified a gene (SMU.836) that is not part of the transformation machinery of S. pneumoniae but was shown to be essential for DNA uptake in S. mutans (30). This gene also was enriched in the competent population.
Besides other DNA-processing proteins, the five genes that are essential for the processing of transformed DNA in S. pneumoniae (CoiA, DprA, RecA, SsbB, and RadA) followed the same pattern of enrichment and depletion. (radA [SMU.327] was only slightly induced [1.7 fold, GFP+] and thus did not fulfill the criteria of the microarray data analysis).
Bacteriocin-related genes were transcribed similarly in both subpopulations.
In S. mutans, bacteriocin production is connected with competence development. The (putative) nonlantibiotic bacteriocins SMU.423, SMU.1906c, mutacin IV (nlmAB), CipB (mutacin V), and its cognate immunity protein (CipI) (33) were shown to be directly regulated by ComE due to the presence of a ComE binding motif in the promoter regions (17, 41).
Since comDE was enriched in the GFP+ subpopulation, it was expected that its direct target genes would be enriched as well. Table 4 lists the putative bacteriocin-related genes (according to the Oralgen database at http://www.oralgen.lanl.gov/) that were induced by CSP in our study. Surprisingly, in contrast to the transformasome and DNA-processing genes, there was no clear enrichment of bacteriocins in the competent fraction. The ratio of both subpopulations shows that there was only a little enrichment for the mutacin V immunity protein CipI (cipI; SMU.925). In contrast, the self-acting mutacin CipB (mutacin V), as well as the other bacteriocin-related genes, was equally induced in both subpopulations. To increase the sensitivity of detection, we used qPCR to determine the transcriptional level and confirmed the data for nlmA, cipB, and cipI (see Table S1 in the supplemental material). This finding was surprising to us, since the majority of these genes are directly controlled by ComE because of the presence of a ComE binding motif. Due to the enrichment of comE transcripts, one would expect a similar pattern for its bacteriocin target genes.
The addition of CSP reduces the growth rate of S. mutans. Perry et al. demonstrated that this decrease in growth was due to autolysis caused by the action of CipB. Furthermore, they showed that the majority of cells expressing ComX (visualized with a GFP reporter) were susceptible to propidium iodide staining, presumably due to an increased expression of CipB or to an imbalance of CipB/CipI (33). According to their observations, the results from our transcriptional analysis were unexpected. We did not perform a time series experiment to determine the transcriptional pattern of both genes. However, at least for the time point under the conditions analyzed here, it seems very unlikely that cells undergoing competence development triggered their cell death at the same time.
CSP-induced autolysis is correlated with weak ComX expression.
To evaluate the correlation of ComX expression and PI staining in more depth, we counterstained CSP-induced cells (SMGFPcomX) with PI and visualized them using fluorescence microscopy.
The overlay image in Fig. 5 represents a typical experiment. When we first analyzed the fluorescence images, we did not see a correlation between green and red cells, which confirmed our transcriptome data. However, when we increased the green fluorescence intensity for the whole image, weakly fluorescing green cells became visible. The important observation was that the majority of PI-stained cells was weakly fluorescing green and thus expressed GFP to a small extent. In addition to the data shown in Fig. 5, we tested four different conditions: the addition of 0.2 or 2 μM CSP, immediately and 1 h after inoculation, respectively. Under all tested conditions, we did observe this correlation of weak GFP and PI fluorescence (data not shown). Several large-field images can be found in the supplementary data (see Fig. S3 in the supplemental material).
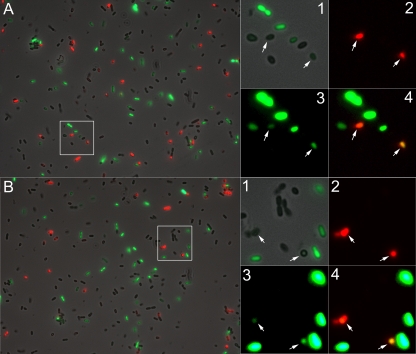
Fig. 5.
Propidium iodide (PI) counterstaining of CSP-induced SMGFPcomX cells. Cells were diluted 1:10 in fresh medium and incubated for 1 h before the addition of 0.2 (A) or 2 μM (B) CSP. Images were recorded 2 h postinduction. The large images on the left are overlays of phase-contrast, GFP, and PI fluorescence. The highlighted area represents cells for every phenotype and is magnified at the right (image 1, overlay of phase contrast and GFP; image 2, PI channel; image 3, increased brightness of the GFP channel; image 4, overlay of bright GFP and PI). White arrows mark cells with a very weak GFP expression that became visible when the brightness of GFP was increased. Importantly, the majority of dark cells did not show enhanced green fluorescence after brightness modification, confirming that the weak green fluorescence was due to GFP expression and not to autofluorescence. The majority of red cells showed an enhanced green fluorescence after increasing the brightness.
Autolysis through CipB presumably is mediated through an overexpression of CipB or an imbalance of CipB/CipI. If some cells produce CipB to a greater extent, one would expect a bimodal distribution of cipB expression when single cells are analyzed. Using flow cytometry, we observed a unimodal distribution of CipB expression after CSP addition (Fig. 6B). Consequently, autolysis must occur due to a different amount of the cognate immunity protein CipI. This conclusion is supported by our transcriptome data, which show an enrichment of CipI in the ComX-induced subpopulation but an equal expression of CipB in both subpopulations. This is in full accordance with our microscopic observations, showing that moderate to strong GFP expression did not correlate with cell death, because these cells were protected by CipI. The data suggest the presence of an additional subpopulation among the ComX-induced cells. During the competence development of the CSP-responsive subpopulation, some cells apparently were selected to undergo autolysis. This would explain the small amount of GFP, because these cells already started the competence cascade and were expressing ComX/GFP. The possibility that some of the cells expressing GFP only weakly were collected in the GFP− fraction would represent an additional explanation for the weak induction of the competence genes in this fraction.
Fig. 6.
Single-cell analysis of comE, cipB, and comX expression. (A) Microscopic pictures of CSP-induced cells of SMGFPcomE (left), SMGFPcipB (middle), and SMGFPcomX (right). Images of phase-contrast, GFP (green, gene expression), and Cy3 (yellow, DNA uptake) fluorescence were merged. (B) GFP intensity distribution from 100,000 cells of strains SMGFPcomE (left), SMGFPcipB (middle), and SMGFPcomX (right) as determined by flow cytometry. The mean GFP intensity and standard deviations from two biological replicates of the GFP+ fraction are shown. (C) Gene expression analysis with quantitative real-time PCR using both subpopulations of FACS-separated, CSP-induced SMGFPcomX cells and the noninduced mixed population.
Fluorescence microscopy confirmed that CipB is expressed in all cells, but ComE is expressed in only a subpopulation.
The analysis of the subpopulation-specific transcription data had indicated that phenotypic variation occurred upstream of ComX, presumably at the step of ComE induction. To confirm these results, we constructed GFP reporter strains for comE and cipB (mutacin V), which provide a tool to measure protein synthesis rather than gene transcription. Fluorescence microscopy confirmed the transcriptional data, showing that ComE-GFP was expressed only in a subpopulation, whereas CipB-GFP was induced in all cells (Fig. 6a). Without the addition of CSP, no GFP fluorescence could be observed, showing that the synthesis of CipB and ComE under these conditions was due to CSP addition, not to other stimuli. To confirm that cells expressing ComE-GFP or ComX-GFP actually were competent, we visualized the uptake of Cy3-labeled DNA. As expected, only cells expressing GFP were able to internalize DNA (yellow cells in Fig. 6A), thus confirming their competent state.
Synthesis of GFP from the cipB promoter was weak compared to that of comE and comX.
During microscopy, we observed that cells carrying pALSM04 (PcomX-GFP) and pALSM34a (PcomE-GFP) produced more GFP than cells carrying pALSM28 (PcipB-GFP). To further confirm this observation, we analyzed cells of CSP-induced cultures of these strains by flow cytometry.
For measuring the GFP intensity, we set the gate for GFP+ at >400 fluorescence units to obtain the smallest overlap between small amounts of GFP and high autofluorescence.
As expected, all cells expressing CipB-GFP exhibited higher fluorescence upon induction by CSP and shifted to the right on the x axis (Fig. 6B). In contrast, only a part of the total population of the ComE-GFP and ComX-GFP cells showed an increased fluorescence and shifted to the right upon CSP addition. The median intensities for the gated cells (>400 U) were 734 ± 75 for CipB-GFP, 911 ± 33 for ComE-GFP, and 1,328 ± 196 for ComX-GFP, confirming the microscopic observation that ComE-GFP and ComX-GFP produced larger amounts of GFP than CipB-GFP and that these genes are induced exclusively in a subpopulation.
The finding that relatively little GFP was produced from the cipB-gfp mRNA was surprising, considering the relative amounts of transcripts for each gene. Figure 6c shows the relative cDNA abundance, determined by qPCR, for both biological replicates of noninduced cells and the two induced subpopulations separated by flow cytometry. The relative amount of cipB cDNA was ∼385-fold higher than that of gyrA (in both subpopulations). The level of comE in the GFP+ fraction was approximately 18-fold compared to that of gyrA, and that of comX was even lower. Therefore, the strength of the comE promoter under these conditions apparently was moderate, and that of cipB was very high. It is worth mentioning that the amount of cipB mRNA/cDNA also was very high in the absence of CSP.
Based on the high level of transcription of cipB, one would expect that the cipB reporter strain would produce more GFP than the other two.
For each reporter strain, the coding sequence of each gene was replaced by GFP, but the promoter sequence and ribosomal binding site remained unchanged (except for an addition of ATC in front of the start codon). The translational efficiency of any mRNA is highly dependent on the nucleotide composition in the translation initiation region (30 bp upstream of the start codon), which determines mRNA conformation and ribosome affinity. Apparently the transcript of cipB had a low translational efficiency compared to those of comE and comX in our reporter construct, but we cannot currently infer the translational strength of the native cipB mRNA.
Alternatively, posttranscriptional regulatory events could have taken place and been, e.g., mediated by small regulatory RNAs.
DISCUSSION
Competence development in S. mutans is a bistable system and results in intrapopulation diversity. The analysis of differential gene expression to obtain detailed insights into the different regulation of the subpopulations requires the use of single-cell and RNA techniques.
Kreth et al. and Perry et al., for the first time, used GFP reporter strains to visualize gene expression on the single-cell level in S. mutans to study competence development (18, 33). However, microarray analysis was averaged over the entire population.
To our knowledge, the combination of flow cytometry and transcriptome analysis of the separated subpopulations has never been done in bacteria. Many efforts have been carried out to improve the existing fluorescence proteins, but a remaining problem for coupling reporter gene expression with cellular transcriptome analysis is the delay between the synthesis of mRNA, translation, and subsequent chromophore maturation. For the GFP used here, it was shown that under the control of the arabinose-utilizing system at full derepression, the onset of detectable fluorescence was ∼16 ± 2.5 min postinduction. However, with decreasing inducer concentrations the time until fluorescence became detectable and increased up to ∼34 ± 10 min (27).
Thus, the time course of CSP-mediated competence development in S. mutans allowed the combination of both techniques. Competence development in S. mutans is a slow process, since the highest levels of mRNA for the key regulators comE and comX are obtained approximately 2 h postinduction and competence is maintained for another 2 h, while competence in S. pneumoniae is observed only in a time window of about 40 min after CSP induction (42). Thus, there is enough time for GFP maturation in S. mutans. We demonstrate here the applicability and success of combining both approaches to compare the differential gene expression of a competent and noncompetent fraction derived from the same population, since mRNA levels for the key regulators ComDE, ComR, ComS, ComX, and the transformasome genes were highly enriched in the GFP+ fraction.
We show that all bacteriocin-related genes were similarly expressed in both populations upon the addition of CSP, particularly CipB (mutacin V). Perry and coworkers demonstrated that CipB is a self-acting bacteriocin, and that the protein CipI confers immunity against it. Moreover, they showed that the majority of the ComX-expressing cells underwent autolysis (33). In contrast, we did not see a correlation of strong ComX expression and autolysis. However, under all tested conditions, we found that autolysis was indeed correlated with ComX expression, but these cells did not produce high levels of GFP.
Perry and coworkers showed that the immunity protein CipI is regulated by the LiaSR two-component system in a cell density-dependent manner. Furthermore, they demonstrated that the time of CSP addition to planktonically growing cells was very important, because it influenced the amount of cells that underwent autolysis (32). Different experimental conditions may explain why they observed that nearly all CSP-responsive cells committed autolysis. Nevertheless, our data strongly indicate that the CSP-responsive subpopulation itself bifurcates into two subpopulations, one that triggers autolysis and another that develops the full competence response.
The altruistic behavior of autolysis would not be favored by evolution without providing benefits to the remaining bacterial population. One advantage of autolysis is the release of DNA, which provides building material for the biofilm matrix and thus increases the ability to form biofilms, which has been shown already for S. mutans (32, 35). Competence is measured by determining the number of cells that integrate added DNA into the chromosome. In S. mutans a transformation frequency of not more than 1 to 2% can be obtained, regardless of the employed conditions or the source of donor DNA. We show here that the amount of ComX-expressing cells in the total population was roughly 30 to 50%. Our transcriptome data indicate that the DNA uptake machinery in these cells was highly expressed, but nevertheless transformation efficiencies in these dimensions could not be achieved. Possibly, internalized DNA was not used for recombination but for repair, as is the case for S. pneumoniae (9), or as a source of nutrients. The latter possibility seems unlikely, because natural competence in S. mutans develops in the exponential growth phase, where nutrients are not exhausted. Another role for the DNA uptake machinery apart from internalizing DNA has been demonstrated by Petersen and coworkers (35). They showed that the pilin-like proteins of the DNA uptake protein complex, which are evolutionarily related to type IV pili, are necessary for biofilm formation.
Similar observations also were reported for other bacteria, e.g., Pseudomonas aeruginosa, where type IV pili determined the shape of the biofilm architecture (3). The transformasome protein ComYB of S. mutans is needed for the correct assembly of the pilin-like proteins. The deletion of ComYB not only resulted in decreased DNA uptake of 88% but also severely affected the formation of the biofilm, since the remaining components of the multiprotein complex could not be assembled correctly (35).
The two-component system ComDE represents the major signal transduction system for CSP-mediated competence development in S. mutans. The deletion of either comD or comE resulted in no competence induction through the addition of CSP, therefore the activation and induction of ComDE is the first step in CSP-mediated competence development (1). The CSP signal is further transmitted to the ComR regulator, which directly activates the expression of ComX/SigX (26). Our study demonstrates for the first time that bifurcation into competent and noncompetent cells occurred before the induction of ComX, since the ComE-GFP reporter was expressed only in a subpopulation. It has to remain open which mechanism is responsible for the bifurcation of the population at the level of ComE. It is not known how the transcription of comE is regulated, except that it is induced by CSP. The action of ComR can be precluded due to the lack of the highly conserved consensus ComR recognition site in the promoter region of comE. Similarly, ComX can be excluded, since there is no binding motif for ComX in the promoter region of comE and the knockout of ComX does not affect CSP-induced comE expression (33).
We present a model, adapted from Mashburn-Warren et al. (26), that summarizes the current knowledge of CSP-mediated competence development (Fig. 7), and it is described below.
Fig. 7.
Working model for bifurcation steps during CSP-mediated competence development in S. mutans. The CSP pheromone activates the histidine kinase ComD (not shown), which phosphorylates its cognate response regulator, ComE. Phosphorylated ComE induces population-wide bacteriocin gene expression. Cells having a high level of ComE, probably due to noise in ComE expression, induce a positive feedback loop for ComE through an unknown mechanism and activate further ComR and the alternate sigma factor ComX. The population segregates into autolysing cells and cells that become competent due to a different expression of the immunity protein CipI. Finally, at least three different subpopulations have developed from the clonal culture due to the presence of a high CSP concentration.
Before the natural accumulation or exogenous addition of CSP, the ComDE system is expressed at a basal level but is not activated. Above the threshold of CSP, ComE molecules become phosphorylated and activate the expression of the bacteriocin-related genes. We assume that, due to the presence of the ComE binding box in their promoter regions, a small amount of activated ComE protein is sufficient to drive full expression. Because ComDE is present in all cells at a low concentration, bacteriocins are expressed in the whole population at a high rate. The CSP-mediated induction of comDE transcription, which occurs only in a subpopulation, may require an unknown factor, possibly in combination with population-wide variability in the expression of comE. Due to the lack of the known ComE binding site in the comE promoter region, the presence of a currently unknown factor that is needed for comE induction is likely. We suggest that competence-initiating cells are selected by the noise in ComE expression, and that cells exceeding a certain threshold activate a positive feedback by interaction with an unknown factor. A similar mechanism has been found to control competence in B. subtilis, except that ComK directly activates a positive autoregulatory loop (12).
We show here that despite a moderate amount of comE transcripts, a high level of GFP was obtained from the comE-gfp promoter fusion. Ozbudak et al. demonstrated that a high translational efficiency coupled to a low transcriptional rate resulted in the largest amount of noise (31). According to their observations and calculations, high levels of noise in ComE expression would have to be expected, supporting the hypothesis that cells were selected due to noise in ComE expression.
We suggest that two levels of ComE expression exist in the whole population. Expression below the threshold induces bacteriocin synthesis, while that above the threshold triggers the induction of comDE and comR and selects the cells for the subsequent activation of ComX. We further suggest that cells that establish a positive feedback for ComE activate (directly or indirectly) ComR, which then induces the expression of comS and comX and subsequently the expression of the late competence genes.
Our data indicate a second bifurcation, inducing autolysis in some cells through the action of CipB. According to the studies of Perry et al., the immunity determinant CipI, regulated by LiaR, was shown to dictate the cell fate of autolysis (32, 33), which is in accordance with the enrichment of cipI transcripts in the ComX-expressing subpopulation. Beside its autolytic properties, CipB is a potent mutacin, targeting mainly nonstreptococcal species (14). Thus, CSP-mediated competence initiation liberates DNA from competitors in the oral cavity, providing a source either for genetic diversity or for the replenishment of the nucleotide pool that can be used for DNA repair. Finally, the liberated DNA represents a building material for the biofilm matrix. The additional DNA binding capacity of the pseudo-type IV pili of the DNA uptake complex further promotes the formation of biofilm, which encases non-CSP-responsive cells as well. In case the surrounding competitors are not sensitive to the released mutacins, autolysis represents a safe mechanism for liberating DNA that can be used as biofilm building material. Thus, the phenotypic diversity and the altruistic behavior of lytic cells provides a benefit to the whole population.
In summary, we performed a subpopulation-specific transcriptome analysis comparing CSP-induced competent and noncompetent cells. The results show that flow cytometry and the subsequent transcriptome analysis of S. mutans cells were successfully combined, resulting in a strong enrichment of mRNA of comX and its downstream targets, e.g., the transformasome-related genes. We demonstrated, for the first time, that bifurcation into competent and noncompetent cells occurs at the level of ComE and not ComX, but that the expression of bacteriocin-related genes occurs similarly in all cells, particularly the self-acting mutacin CipB. We suggest that, due to noise in the basal level of ComE expression, some cells exceed a threshold of CSP-activated ComE protein molecules, enabling a positive feedback loop and entering the competent state. Moreover, our data indicate a second bifurcation within the competent fraction, where some cells undergo autolysis, presumably due to a decreased amount of CipI. Intrapopulation diversity during competence development apparently has several advantages. It enables S. mutans to cope with competitors, produces membrane-embedded DNA binding proteins, and liberates DNA that can be used for genetic diversity or repair or as biofilm building material, and thus it reflects the successful adaptation to its ecological niche.
Supplementary Material
ACKNOWLEDGMENT
We thank Andreas Podbielski for providing the pFW5 plasmid.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 11 February 2011.
REFERENCES
- 1. Ahn S. J., Wen Z. T., Burne R. A. 2006. Multilevel control of competence development and stress tolerance in Streptococcus mutans UA159. Infect. Immun. 74:1631–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Avery S. V. 2005. Cell individuality: the bistability of competence development. Trends Microbiol. 13:459–462 [DOI] [PubMed] [Google Scholar]
- 3. Barken K. B., et al. 2008. Roles of type IV pili, flagellum-mediated motility and extracellular DNA in the formation of mature multicellular structures in Pseudomonas aeruginosa biofilms. Environ. Microbiol. 10:2331–2343 [DOI] [PubMed] [Google Scholar]
- 4. Benjamini Y., Hochberg Y. 1995. Controlling the false discovery rate-a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 57:289–300 [Google Scholar]
- 5. Cheng Q., Campbell E. A., Naughton A. M., Johnson S., Masure H. R. 1997. The com locus controls genetic transformation in Streptococcus pneumoniae. Mol. Microbiol. 23:683–692 [DOI] [PubMed] [Google Scholar]
- 6. Claverys J. P., Havarstein L. S. 2007. Cannibalism and fratricide: mechanisms and raisons d'etre. Nat. Rev. Microbiol. 5:219–229 [DOI] [PubMed] [Google Scholar]
- 7. Claverys J. P., Martin B., Havarstein L. S. 2007. Competence-induced fratricide in streptococci. Mol. Microbiol. 64:1423–1433 [DOI] [PubMed] [Google Scholar]
- 8. Claverys J. P., Martin B., Polard P. 2009. The genetic transformation machinery: composition, localization, and mechanism. FEMS Microbiol. Rev. 33:643–656 [DOI] [PubMed] [Google Scholar]
- 9. Claverys J. P., Prudhomme M., Martin B. 2006. Induction of competence regulons as a general response to stress in gram-positive bacteria. Annu. Rev. Microbiol. 60:451–475 [DOI] [PubMed] [Google Scholar]
- 10. de Saizieu A., et al. 2000. Microarray-based identification of a novel Streptococcus pneumoniae regulon controlled by an autoinduced peptide. J. Bacteriol. 182:4696–4703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dubnau D. 1991. Genetic competence in Bacillus subtilis. Microbiol. Rev. 55:395–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dubnau D., Losick R. 2006. Bistability in bacteria. Mol. Microbiol. 61:564–572 [DOI] [PubMed] [Google Scholar]
- 13. Ferrell J. E., Jr 2002. Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr. Opin. Cell Biol. 14:140–148 [DOI] [PubMed] [Google Scholar]
- 14. Hale J. D., Ting Y. T., Jack R. W., Tagg J. R., Heng N. C. 2005. Bacteriocin (mutacin) production by Streptococcus mutans genome sequence reference strain UA159: elucidation of the antimicrobial repertoire by genetic dissection. Appl. Environ. Microbiol. 71:7613–7617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Håvarstein L. S., Gaustad P., Nes I. F., Morrison D. A. 1996. Identification of the streptococcal competence-pheromone receptor. Mol. Microbiol. 21:863–869 [DOI] [PubMed] [Google Scholar]
- 16. Johnsborg O., Havarstein L. S. 2009. Regulation of natural genetic transformation and acquisition of transforming DNA in Streptococcus pneumoniae. FEMS Microbiol. Rev. 33:627–642 [DOI] [PubMed] [Google Scholar]
- 17. Kreth J., et al. 2007. The response regulator ComE in Streptococcus mutans functions both as a transcription activator of mutacin production and repressor of CSP biosynthesis. Microbiology 153:1799–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kreth J., Merritt J., Shi W., Qi F. 2005. Co-ordinated bacteriocin production and competence development: a possible mechanism for taking up DNA from neighbouring species. Mol. Microbiol. 57:392–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kreth J., Merritt J., Zhu L., Shi W., Qi F. 2006. Cell density- and ComE-dependent expression of a group of mutacin and mutacin-like genes in Streptococcus mutans. FEMS Microbiol. Lett. 265:11–17 [DOI] [PubMed] [Google Scholar]
- 20. Kunze B., et al. 2010. Damage of Streptococcus mutans biofilms by carolacton, a secondary metabolite from the myxobacterium Sorangium cellulosum. BMC Microbiol. 10:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lemme A., Sztajer H., Wagner-Dobler I. 2010. Characterization of mleR, a positive regulator of malolactic fermentation and part of the acid tolerance response in Streptococcus mutans. BMC Microbiol. 10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lévesque C. M., et al. 2007. Systemic inactivation and phenotypic characterization of two-component systems in expression of Streptococcus mutans virulence properties. Lett. Appl. Microbiol. 45:398–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Y. H., et al. 2002. A quorum-sensing signaling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation. J. Bacteriol. 184:2699–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martin B., et al. 2010. Expression and maintenance of ComD-ComE, the two-component signal-transduction system that controls competence of Streptococcus pneumoniae. Mol. Microbiol. 75:1513–1528 [DOI] [PubMed] [Google Scholar]
- 25. Martin B., Quentin Y., Fichant G., Claverys J. P. 2006. Independent evolution of competence regulatory cascades in streptococci? Trends Microbiol. 14:339–345 [DOI] [PubMed] [Google Scholar]
- 26. Mashburn-Warren L., Morrison D. A., Federle M. J. 2010. A novel double-tryptophan peptide pheromone controls competence in Streptococcus spp. via an Rgg regulator. Mol. Microbiol. 78:589–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Megerle J. A., Fritz G., Gerland U., Jung K., Radler J. O. 2008. Timing and dynamics of single cell gene expression in the arabinose utilization system. Biophys. J. 95:2103–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakagawa I., Nakata M., Kawabata S., Hamada S. 2001. Cytochrome c-mediated caspase-9 activation triggers apoptosis in Streptococcus pyogenes-infected epithelial cells. Cell Microbiol. 3:395–405 [DOI] [PubMed] [Google Scholar]
- 29. Okinaga T., Niu G., Xie Z., Qi F., Merritt J. 2010. The hdrRM operon of Streptococcus mutans encodes a novel regulatory system for coordinated competence development and bacteriocin production. J. Bacteriol. 192:1844–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Okinaga T., Xie Z., Niu G., Qi F., Merritt J. 2010. Examination of the hdrRM regulon yields insight into the competence system of Streptococcus mutans. Mol. Oral Microbiol. 25:165–177 [DOI] [PubMed] [Google Scholar]
- 31. Ozbudak E. M., Thattai M., Kurtser I., Grossman A. D., van Oudenaarden A. 2002. Regulation of noise in the expression of a single gene. Nat. Genet. 31:69–73 [DOI] [PubMed] [Google Scholar]
- 32. Perry J. A., Cvitkovitch D. G., Levesque C. M. 2009. Cell death in Streptococcus mutans biofilms: a link between CSP and extracellular DNA. FEMS Microbiol. Lett. 299:261–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perry J. A., Jones M. B., Peterson S. N., Cvitkovitch D. G., Levesque C. M. 2009. Peptide alarmone signalling triggers an auto-active bacteriocin necessary for genetic competence. Mol. Microbiol. 72:905–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pestova E. V., Havarstein L. S., Morrison D. A. 1996. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol. Microbiol. 21:853–862 [DOI] [PubMed] [Google Scholar]
- 35. Petersen F. C., Tao L., Scheie A. A. 2005. DNA binding-uptake system: a link between cell-to-cell communication and biofilm formation. J. Bacteriol. 187:4392–4400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Podbielski A., Spellerberg B., Woischnik M., Pohl B., Lutticken R. 1996. Novel series of plasmid vectors for gene inactivation and expression analysis in group A streptococci (GAS). Gene 177:137–147 [DOI] [PubMed] [Google Scholar]
- 37. Reichmann P., Hakenbeck R. 2000. Allelic variation in a peptide-inducible two-component system of Streptococcus pneumoniae. FEMS Microbiol. Lett. 190:231–236 [DOI] [PubMed] [Google Scholar]
- 38. Riedel K., et al. 2001. N-acylhomoserine-lactone-mediated communication between Pseudomonas aeruginosa and Burkholderia cepacia in mixed biofilms. Microbiology 147:3249–3262 [DOI] [PubMed] [Google Scholar]
- 39. Tanzer J. M., Livingston J., Thompson A. M. 2001. The microbiology of primary dental caries in humans. J. Dent. Educ. 65:1028–1037 [PubMed] [Google Scholar]
- 40. Trieu-Cuot P., Carlier C., Poyart-Salmeron C., Courvalin P. 1991. Shuttle vectors containing a multiple cloning site and a lacZ alpha gene for conjugal transfer of DNA from Escherichia coli to gram-positive bacteria. Gene 102:99–104 [DOI] [PubMed] [Google Scholar]
- 41. van der Ploeg J. R. 2005. Regulation of bacteriocin production in Streptococcus mutans by the quorum-sensing system required for development of genetic competence. J. Bacteriol. 187:3980–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ween O., Gaustad P., Havarstein L. S. 1999. Identification of DNA binding sites for ComE, a key regulator of natural competence in Streptococcus pneumoniae. Mol. Microbiol. 33:817–827 [DOI] [PubMed] [Google Scholar]
- 43. Wettenhall J. M., Smyth G. K. 2004. limmaGUI: a graphical user interface for linear modeling of microarray data. Bioinformatics 20:3705–3706 [DOI] [PubMed] [Google Scholar]
- 44. Xie Z., Okinaga T., Niu G., Qi F., Merritt J. 2010. Identification of a novel bacteriocin regulatory system in Streptococcus mutans. Mol. Microbiol. 78:1431–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xue X., Tomasch J., Sztajer H., Wagner-Dobler I. 2010. The delta subunit of RNA polymerase, RpoE, is a global modulator of Streptococcus mutans environmental adaptation. J. Bacteriol. 192:5081–5092 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.