Abstract
The type II secretion system (T2SS) functions as a transport mechanism to translocate proteins from the periplasm to the extracellular environment. The ExeA homologue in Aeromonas hydrophila, GspAAh, is an ATPase that interacts with peptidoglycan and forms an inner membrane complex with the ExeB homologue (GspBAh). The complex may be required to generate space in the peptidoglycan mesh that is necessary for the transport and assembly of the megadalton-sized ExeD homologue (GspDAh) secretin multimer in the outer membrane. In this study, the requirement for GspAB in the assembly of the T2SS secretin in Aeromonas and Vibrio species was investigated. We have demonstrated a requirement for GspAB in T2SS assembly in Aeromonas salmonicida, similar to that previously observed in A. hydrophila. In the Vibrionaceae species Vibrio cholerae, Vibrio vulnificus, and Vibrio parahaemolyticus, gspA mutations significantly decreased assembly of the secretin multimer but had minimal effects on the secretion of T2SS substrates. The lack of effect on secretion of the mutant of gspA of V. cholerae (gspAVc) was explained by the finding that native secretin expression greatly exceeds the level needed for efficient secretion in V. cholerae. In cross-complementation experiments, secretin assembly and secretion in an A. hydrophila gspA mutant were partially restored by the expression of GspAB from V. cholerae in trans, further suggesting that GspABVc performs the same role in Vibrio species as GspABAh does in the aeromonads. These results indicate that the GspAB complex is functional in the assembly of the secretin in Vibrio species but that a redundancy of GspAB function may exist in this genus.
INTRODUCTION
The type II secretion system (T2SS), also known as the main terminal branch of the general secretory pathway (GSP), is utilized by many Gram-negative bacteria to translocate folded proteins across the outer membrane from the periplasm to the extracellular milieu. This system is a major virulence factor, responsible for the translocation of a variety of proteins that mediate pathogenic effects, including the pore-forming toxin aerolysin of Aeromonas hydrophila (15, 16), cholera toxin of Vibrio cholerae (43), and heat-labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC) (53).
Proteins destined for T2SS secretion are transported across the inner membrane via the Sec (38) and Tat (54) pathways into the periplasm. Once in the periplasm, the T2SS, a complex composed of 12 to 16 proteins that spans the periplasmic space, functions in extrusion of the folded protein substrate through the megadalton-sized outer membrane complex (the secretin), which is composed of 12 to 14 GspD monomers (4). Transport of the protein through the pore created by the secretin could involve extension and retraction of a pseudopilus structure composed of GspG to GspK that is anchored to an inner membrane platform complex composed of GspE, -F, -L, and -M (2, 19, 23, 37, 39). In this way, the pseudopilus may act as a piston to push folded proteins located in the periplasm through the pore of the secretin multimer. The energy required for this process is presumably provided by the ATPase function of GspE (7), and in some species, including the aeromonads, energy derived from the proton motive force is also required (24). Other members of the T2SS include the substrate specificity selector protein GspC (10), the small outer membrane pilotin lipoprotein GspS that is required for secretin assembly in some bacteria and functions in protecting the secretin from proteolytic degradation (13, 48), and a prepilin peptidase (GspO or provided by a type IV pilin assembly system) involved in proteolytic processing of prepilin subunits during assembly of the pseudopilus (51).
Members of the Aeromonas and Vibrio genera are significant human and animal pathogens. These species, classified as part of the same clade of gammaproteobacteria (55), include Vibrio cholerae, which is the causative agent of the gastrointestinal disease cholera, and the fish, amphibian, and opportunistic human pathogen A. hydrophila. The T2SS encoded within these species is composed of Gsp proteins GspC to -N, the prepilin peptidase TapD, and an additional set of proteins, GspA and GspB, but no identifiable GspS homologue (20, 21, 35). The GspA and GspB proteins encoded in Aeromonas hydrophila (GspAAh and GspBAh) and Vibrio cholerae (GspAVc and GspBVc) are alike, with GspA proteins being 40% identical and 54% similar and GspB proteins 27% identical and 44% similar. Interestingly, Vibrio vulnificus encodes a 718-amino-acid (aa) protein that contains a GspA domain within the N-terminal 530 aa of the protein and a GspB domain within the C-terminal 188 aa (see Fig. S1 and S2 in the supplemental material). The identification of a GspAB fusion protein is perhaps not surprising, since according to the “Rosetta Stone” hypothesis for evolution of protein interactions, a propensity exists for interacting pairs of proteins to evolve into one protein because fusion greatly increases the affinity of each protein for the other and is therefore thermodynamically favorable (27).
Previous studies of the 60-kDa ExeA homologue (GspAAh) and 25-kDa ExeB homologue (GspBAh) in A. hydrophila demonstrated the requirement for coordinated expression of both GspAAh and GspBAh such that deletion of either protein prevented detection of the other (20). GspAAh and GspBAh span the inner membrane once and form a large heteromultimeric complex (18, 20) that interacts with peptidoglycan (PG) (25). The GspABAh complex has been demonstrated to be required for the localization and multimerization of the GspD secretin multimer in the outer membrane of A. hydrophila (3). We have also shown that the GspAAh cytoplasmic domain is a novel ATPase (46) and have confirmed the function of a peptidoglycan-binding site in the GspAAh periplasmic domain as being required for the assembly of the secretin multimer (17, 25). Thus, our working hypothesis is that the GspAB complex in some way modifies or organizes the peptidoglycan to allow assembly of the GspD secretin, a function presumably necessitated by the 50-kDa size constraint imposed by the peptidoglycan mesh (9).
As described above, GspABAh is required for the transport and assembly of the secretin in A. hydrophila. In other bacteria with a functional T2SS, however, the role of the GspAB complex remains unclear. In some bacteria, no identifiable GspAB homologue is present; in other bacteria, a GspB but no GspA is present (8); and in others, GspAB homologs have been identified in silico but have not been studied with respect to their role in T2SS secretion or secretin assembly.
In this study, we sought to ascertain the involvement of the GspAB complex in the assembly of the T2SS secretin multimer in Aeromonas salmonicida and several Vibrio species. Previous studies have shown strong similarities between the T2SS systems of this genus, such that A. hydrophila and V. cholerae hybrid T2SS component proteins are often functional (42), and the genomes of all sequenced Vibrio species contain homologous gspAB genes. Similar to the results of previous studies of A. hydrophila, we found that GspAB was absolutely required in A. salmonicida for both assembly of the secretin multimer and secretion of T2SS substrates. Conversely, the presence of GspAB was not an absolute requirement for function of the T2SS in V. cholerae, V. vulnificus, and Vibrio parahaemolyticus. Insertional inactivation of gspA in these bacteria resulted in minor decreases in the secretion of T2SS substrates. However, a significant decrease in the amount of secretin multimer formed was observed for each of the gspA mutants, suggesting that the GspAB complex is involved in the assembly of the secretin in members of the Vibrionaceae. The minimal effect on secretion despite the clear role of GspAB in secretin assembly in V. cholerae was explained when the expression of gspDVc in trans in a gspDVc mutant demonstrated that native levels of secretin expression in this bacterium substantially exceed the required capacity for secretion. The expression of the V. cholerae gspAB genes in trans in A. hydrophila gspAB mutants resulted in partial complementation of both secretin assembly and secretion of the T2SS substrate aerolysin, whereas the expression of V. cholerae gspAB in wild-type A. hydrophila cells inhibited aerolysin secretion. These results suggest that, similar to the T2SS of aeromonads, GspAB of vibrios facilitates the assembly of the secretin; however, unlike the aeromonads, other unidentified factors may exist that provide redundancy for secretin assembly in the vibrios.
MATERIALS AND METHODS
Bacterial strains, plasmids and growth conditions.
The strains and plasmids used in this study are shown in Table 1. A. salmonicida was grown in Luria-Bertani (LB) medium with Davis salts (28) at 22°C until the mid-exponential phase of growth. A. hydrophila was grown in LB medium supplemented with 30 mM Na2HPO4, 30 mM K2HPO4, 16.5 mM NaH2PO4, 16.5 mM KH2PO4, 0.75 mM (NH4)2SO4, 0.4 mM MgSO4, pH 7.0, at 30°C until mid-log phase. Vibrio species were grown in LB at a temperature of 37°C for V. cholerae and 30°C for V. vulnificus and V. parahaemolyticus until the mid- to late logarithmic phase of growth. E. coli strains S17-1 and XL1-Blue were cultured in brain heart infusion (BHI) and LB, respectively. Antibiotics were used at the following concentrations: rifampin, 50 μg/ml; kanamycin (Kan), 50 μg/ml; chloramphenicol, 1.25 μg/ml; and streptomycin, 20 μg/ml.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype/phenotype | Source |
|---|---|---|
| A. hydrophila strains | ||
| Ah65 | Wild type | Howard laboratory |
| C5.84 | Ah65 gspA::Tn5-751 | 20 |
| V. cholerae strains | ||
| Bah2 | E7946 derivative, Δtlc ΔattRS ΔctxΦ Δrtx | 34 |
| Bah2-R | Rifr mutant of Bah2 | This work |
| Bah2-gspA | gspA mutant of Bah2-R | This work |
| TRH7000 | El Tor O1 biotype, thy HgrctxAB | 32 |
| TRH7000 gspA | gspA mutant of TRH7000 | This work |
| TRH7000 gspD | gspD mutant of TRH7000, Kanr | 26 |
| V. vulnificus strains | ||
| V. vul. 67181283 | Patient isolate | Provincial laboratory |
| VvR | Rifr mutant of V. vulnificus 67181283 | This work |
| Vv-gspA | gspA::Kan mutant of VvR | This work |
| V. parahaemolyticus strains | ||
| V.para US32052027 | Patient isolate | Provincial laboratory |
| VpR | Rifr mutant of V. parahaemolyticus US32052027 | This work |
| Vp-gspA | gspA::Kan mutant of VpR | This work |
| A. salmonicida strains | ||
| As449 | Wild type | 22 |
| AsR | As449 Rifr | This work |
| As-gspA | AsR gspA::Kan | This work |
| E. coli strains | ||
| SM10λpir | thi-1 thr leu tonA lacY supE recA::RP4-2-Tc::Mu Kanr | 29 |
| S17-1 | recA thi pro hsdR, RP4::2-Tc::Mu::Kan Tn7 λpir | 49 |
| XL1-Blue | recA1 lac endA1 gyrA96 thi-1 hsdR17 supE44 relA1[F′ proAB lacIqZΔM15 Tn10] | 6 |
| Plasmids | ||
| pMMB207 | tac promoter, wide-host-range vector; Cmr | 30 |
| pRJ31.1 | 2.5-kb BstXI fragment containing gspABAh in SmaI of pMMB207; Cmr | 20 |
| pMMB/gspABVc | 5.6-kb EcoRI fragment containing gspABVc in EcoRI of pMMB207; Cmr | This work |
| pMMB/gspAVc | Deletion of C-terminal half of gspBVc from pMMB/gspABVc; Cmr | This work |
| pMMB/gspDVc | gspDVc cloned into pMMB67; Apr | 26 |
| pMMB68 | etxB under Ptac control; Ptac mob+ Apr | 41 |
| pMRS101 | oriR6K sacBR pir Apr Str | 44 |
| pUC4K | lacZα Apr Kmr | Amersham |
| pBluescript SK | Plac lacZα f1 ColE1 Apr | Stratagene |
Rifampin-resistant variants of A. salmonicida and each Vibrio strain were selected by spreading a 10-times-concentrated cell suspension from 1 ml of overnight saturated culture onto a BHI plate containing rifampin (100 μg/ml). Following overnight incubation at an appropriate temperature, rifampin-resistant colonies were selected and restreaked to confirm the resistant phenotype.
Enzyme assay of culture supernatants.
Samples of whole-cell broth culture were taken and added to an equal volume of 2× sample buffer (125 mM Tris-HCl, pH 6.8, 20% glycerol, 4% SDS, 0.01% bromophenol blue, 10% β-mercaptoethanol) and heated for 5 min at 95°C. The remaining culture was centrifuged at 31,000 × g for 15 min, and 10 ml of supernatant was taken, filter sterilized, and stored at −20°C before being used to assay culture supernatant enzyme activity. When required, supernatant was concentrated approximately 10-fold by use of a Microcon-10 concentrator (10-kDa molecular-mass cutoff).
Lipase activity in culture supernatants was determined by assaying the release of p-nitrophenol (pNP) from p-nitrophenol caprylate (pNPC) (1), for which 100 μl of culture supernatant was added to 900 μl of substrate containing 100 mM Tris, pH 8.0, 0.2% Triton X-100, and 1 mM pNPC. The reaction mixture was incubated at room temperature for 30 min while the absorbance at 410 nm was measured at 5-min intervals. A unit of lipase activity equals 1 nmol pNPC hydrolyzed per minute. All enzymatic activities are expressed as units/ml supernatant per unit of optical density at 600 nm (OD600) in the culture.
The amylase activity in supernatants from cultures of Vibrio species was determined according to the protocol that Jiang and Howard (21) used to assay the T2SS-dependent amylase activity in A. hydrophila culture supernatants. A 100-μl volume of supernatant was added to 250 μl 5% starch azure in 20 mM Na2HPO4 (pH 7), 50 mM NaCl, pH 7.0, and incubated at 37°C with vigorous shaking for 16 h. The reaction was stopped by the addition of 50 μl 2.5 M sodium acetate, the supernatant was collected by 5 min of centrifugation at 21,000 × g, and the absorbance at 595 nm was determined. Units are defined as the change in absorbance at 595 nm/hour.
Protease activity in culture supernatants was determined using azocasein as the substrate. A 100-μl volume of supernatant was incubated with 300 μl of 1% azocasein in 60 mM phosphate buffer (pH 7.2). The reaction mixture was incubated at 37°C with shaking for 2 h, stopped by the addition of 133 μl 30% trichloroacetic acid, and incubated on ice for 30 min. The sample was then centrifuged at 21,000 × g for 5 min, and 400 μl of supernatant was recovered. An equal volume of 1 M NaOH was added to the supernatant, and the absorbance at 450 nm was determined. Units of protease activity are expressed as the change in absorbance at 450 nm/hour.
To conduct the toxin B subunit secretion assay, the etxB-expressing plasmid pMMB68 that encodes the B-subunit of the heat-labile enterotoxin of E. coli was introduced into V. cholerae Bah2R and Bah2-gspA strains by conjugation. Strains were grown in M9 medium (28) supplemented with 0.2% glucose and 2% Casamino Acids. Isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 0.05 mM to induce the expression of EtxB, and the culture was grown for 1.5 h at 37°C. The amount of EtxB in culture supernatant and whole-cell extract disrupted by sonication was determined by GM1 enzyme-linked immunosorbent assay (52) as described previously (32).
Aerolysin was assayed as described previously (47).
Marker exchange mutagenesis and complementation assays.
Alleles gspAAh::Kan and gspAVc::Kan were introduced into Aeromonas and Vibrio species by marker exchange mutagenesis using the suicide vector pMRS101 (44). A 1,429-bp fragment of the A. salmonicida gspA gene (gspAAs) was amplified by PCR utilizing the primers US42 (ATCTCAACTACGGGTTGCAG) and US43 (CATCACATTGAGACGCAGCAG), blunt-end ligated into the EcoRV site of pBluescript II SK(+), and electroporated into E. coli XL-Blue. The aminoglycoside 3′-phosphotransferase gene conferring kanamycin resistance was excised from plasmid pUC4K via a HincII digestion and inserted into an EcoRV site located within gspA (nucleotide position 780) of a recombinant plasmid clone. The gspAAh::Kan gene was transferred from pSK into the suicide vector pMRS101 by ApaI/XbaI digestion. Recombinant plasmids were digested with NotI to remove the pBR322 origin of replication of the plasmid, self-ligated, and electroporated into E. coli SM10λpir by selecting for streptomycin and kanamycin resistance. The donor (SM10λpir containing pMRS101/exeA::Kan) and the recipient strain AsR were grown overnight, subcultured 1:10 in BHI broth without antibiotics, and incubated at 22°C for 1 h prior to conjugation. Five-hundred-microliter volumes of donor and recipient cells were mixed and collected by centrifugation. The pellet was resuspended in 100 μl BHI, applied to a prewarmed BHI plate, and incubated for 3.5 h at 30°C. Half of the conjugation pool plate was scraped off, streaked onto LB containing kanamycin, rifampin, and sucrose (10%), and incubated at 22°C to directly screen for recombinant recipient colonies. The exchange of alleles was verified by PCR.
Marker exchange mutagenesis of Vibrio species was conducted as described above, except that 2,100- to 3,300-bp fragments containing all or part of gspAB were amplified from the following species using the indicated specific primer sets: UR70 (GAATTTGAGGTCAGCTATCCGA) and UR71 (GCATAAGCGGAATTCATCGCA) for V. cholerae, US30 (GTACAGCTGGCCGCTATCAT) and US31 (TGGTTGGGTTCAAAGCAAGT) for V. parahaemolyticus strain US32052027, and US32 (GCTGGGTTCAAGCAAAATTC) and US33 (TGACACATGGCGCAAAATAC) for V. vulnificus strain 67181283. The aminoglycoside phosphotransferase gene conferring kanamycin resistance was inserted into an EcoRV site located at positions 1106 and 1088 of the V. vulnificus and V. parahaemolyticus gspA genes, respectively, and into a SphI site located at position 1040 of V. cholerae gspA. gspA::Kan fragments were transferred into pMRS101 from pSK using BamHI/ApaI sites for V. vulnificus and V. parahaemolyticus gspA::Kan and BamHI/SalI for V. cholerae gspA::Kan.
The A. hydrophila gspAB clone pRJ31.1 was described previously (20). A 5.6-kb EcoRI fragment containing the entire gspAB operon of V. cholerae, including the presumed promoter, was cloned into pMMB207 to create plasmid pMMB/gspABVc. The plasmids were conjugated into V. cholerae and A. hydrophila strains from E. coli S17-1 as described above for complementation assays.
Immunoblotting.
GspD multimeric and monomeric protein forms were detected by immunoblot analysis of whole-cell samples taken from liquid culture. Samples were electrophoresed in a 3 to 8% SDS gradient PAGE gel (Bio-Rad) until an 84-kDa protein standard was approximately 1 cm from the bottom of the gel and then transferred to polyvinylidene difluoride membrane. The amount of sample loaded was normalized such that 5 μl of a sample consisting of equal parts of 2× sample buffer and culture grown to an OD600 of 2.0 was loaded for electrophoresis, with the exception of the experiment whose results are shown in Fig. 2, in which samples were normalized according to the OD600 shown. Proteins were visualized using primary rabbit anti-GspDAh (18) or rabbit anti-GspDVc antiserum, a peroxidase-conjugated anti-rabbit IgG secondary antibody (Sigma), and a chemiluminescent substrate (GE Healthcare) detected with Hyperfilm (GE Healthcare). GspDVc antibodies were raised in New Zealand White rabbits by using purified EpsD-His6 monomers according to the method of Harlow and Lane (14).
Fig. 2.
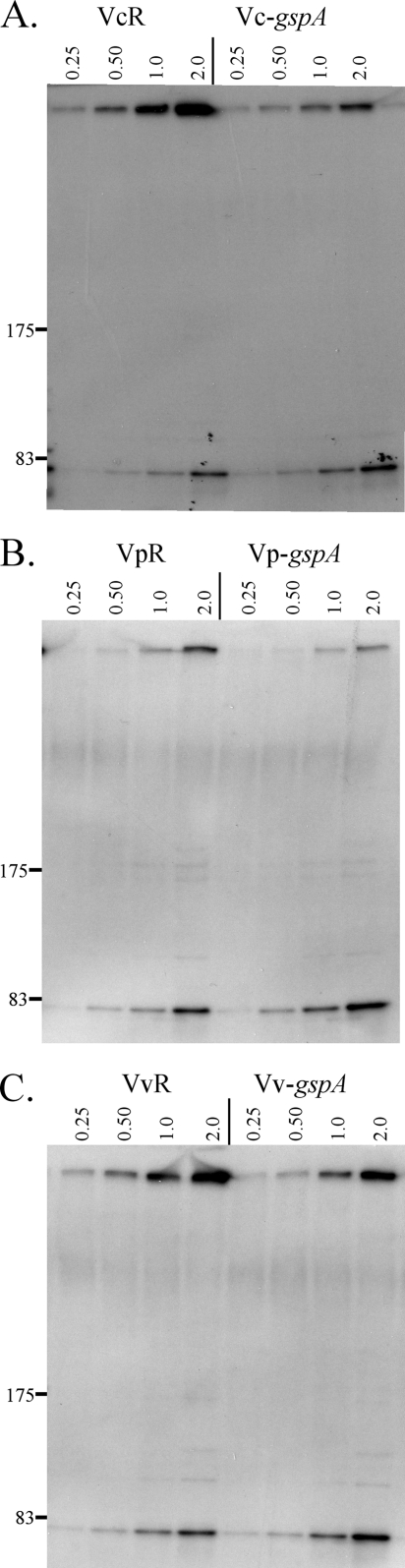
Effect of gspA mutation on assembly of the GspD secretin in Vibrio species. Anti-GspDVc immunoblot analysis of whole-cell samples taken from liquid culture at various stages of growth from early exponential phase to late exponential phase (OD600 is shown at the top of each lane) of wild-type (Bah2-R) and gspA V. cholerae (Bah2-gspA) (A), wild-type (VpR) and gspA V. parahaemolyticus (Vp-gspA) (B), and wild-type (VvR) and gspA V. vulnificus (Vv-gspA) (C) is shown. Locations of prestained standard protein markers (in kDa) are given.
Statistical analysis.
The unpaired two-sided Student t test was used for all statistical analysis. Values were considered significantly different at P values of <0.05.
RESULTS
T2SS function and secretin assembly are abrogated in A. salmonicida gspA mutants.
To investigate the importance of GspAB in the assembly of the secretin in A. salmonicida, we constructed a gspA mutant of As449 by marker exchange mutagenesis and determined the assembly and function of the T2SS by immunoblot detection of the GspD secretin and assay of culture supernatants for glycerophospholipid:cholesterol acyl transferase (GCAT) activity using the lipase assay. GCAT is a protein known to be secreted by the T2SS (5).
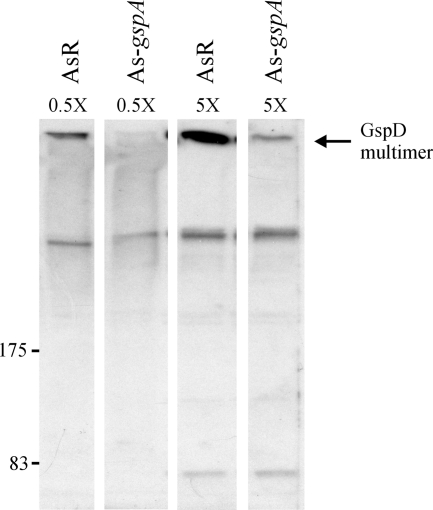
The GCAT assay results indicated that secretion was severely affected by the loss of GspAAs, as evidenced by the low lipase activity in culture supernatants from strain As-gspA in comparison to the lipase activity for strain AsR (Table 2). As expected from previous results in A. hydrophila, the loss of T2SS function in the A. salmonicida gspA mutant was accompanied and presumably caused by the failure to assemble the GspD secretin. The amount of secretin multimer from whole-cell samples was almost nonexistent in the gspA mutant compared to the amount in the wild type (Fig. 1). Although a small amount of multimer was evident in concentrated samples of the gspA strain, the amount was negligible in comparison to the amount in the wild type (Fig. 1, 3rd and 4th lanes). It should be noted that the failure to assemble the secretin multimer in A. hydrophila gspA mutants is accompanied by the accumulation of monomer GspD in the inner membrane (3), although in A. salmonicida, no accumulation of the monomer could be observed in the gspA mutant. This may result from degradation of the monomer when it cannot be assembled into the multimer in A. salmonicida. These results clearly demonstrate the essential requirement for GspAB in the assembly of the secretin in A. salmonicida and suggest that the absolute requirement for GspAB in secretin assembly is not specific to A. hydrophila but is a characteristic of the aeromonads.
Table 2.
Enzymatic activities detected in culture supernatants of wild-type and gspAB Aeromonas and Vibrio species
| Organism | Strain | Mean activity ± SD (%)a |
||
|---|---|---|---|---|
| Lipaseb | Proteasec | Amylased | ||
| A. salmonicida | AsR | 0.980 ± 0.072 (100) | NA | NA |
| As-gspA | 0.243 ± 0.051 (24.8)* | NA | NA | |
| V. cholerae | Bah2-R | 3.71 ± 0.042 (100) | 3.08 ± 0.287 (100) | NA |
| Bah2-gspA | 3.74 ± 0.378 (101)† | 2.28 ± 0.269 (74)* | NA | |
| V. vulnificus | VvR | 61.3 ± 6.74 (100) | 0.221 ± 0.019 (100) | 22.6 ± 2.20 (100) |
| Vv-gspA | 53.7 ± 6.97 (87.6)† | 0.186 ± 0.011 (84.2)† | 19.9 ± 1.51 (88.1)† | |
| V. parahaemolyticus | VpR | 0.276 ± 0.031 (100) | NA | 19.8 ± 1.10 (100) |
| Vp-gspA | 0.211 ± 0.001 (76.4)* | NA | 18.8 ± 1.03 (94.9)† | |
The wild-type activity for each species is set as 100%. NA, no detectable activity;
, significantly different;
, not significantly different.
Activity is expressed in μM/min/OD600 unit.
Activity is expressed in A450/hr/OD600 unit.
Activity is expressed in A595/hr/OD600 unit × 103.
Fig. 1.
In A. salmonicida, loss of GspAAs abrogates assembly of the GspD secretin multimer. Anti-GspDAh immunoblot analysis of 0.5- and 5-fold-concentrated whole-cell samples taken from AsR and As-gspA strains grown in liquid culture to an OD600 of 2.0 is shown. Locations of prestained standard protein markers (in kDa) and the GspDAs secretin multimer are given.
Mutation of gspA has a minor effect on secretion of T2SS substrates in Vibrio species but significantly decreases GspD multimer formation.
In order to examine the involvement of GspAB in secretin assembly in Vibrio species, the gspAB genes were cloned and gspA mutant strains constructed for each of V. cholerae, V. parahaemolyticus and V. vulnificus by marker exchange mutagenesis. To investigate T2SS function in the V. cholerae ctxAB strain, we introduced a plasmid-encoded copy of the structurally similar cholera toxin B-subunit (CtxB) homologue heat-labile E. coli enterotoxin B subunit (EtxB) (50) into wild-type and gspA strains. EtxB has been extensively studied and has previously been shown to be secreted by the T2SS in V. cholerae (33).
In marked contrast to the requirement for GspAB in assembly of the secretin in A. hydrophila (3) and A. salmonicida (Fig. 1), mutation of gspA had a modest effect on T2SS secretion in Vibrio strains. The V. cholerae gspA mutant (Bah2-gspA) and the parent strain secreted equivalent amounts of the EtxB subunit (73% ± 8% [mean ± standard deviation] and 70% ± 4%, respectively; no significant difference). Similarly, as shown by the results in Table 2, the Bah2-gspA secreted equivalent amounts of lipase activity and 26% lower levels of protease activity compared to the wild type. Similar results were obtained for V. cholerae TRH7000 and TRH7000 gspA strains (data not shown).
Lipase, protease, and amylase activities in culture supernatants of the V. vulnificus gspA mutant (Vv-gspA) were not significantly different from those observed in supernatant from the wild-type culture (Table 2). Lipase and amylase activities were detected in V. parahaemolyticus culture supernatants, and the gspA mutant (Vp-gspA) secreted 24% less lipase activity than the wild type and an equivalent amount of amylase activity in comparison to the wild type.
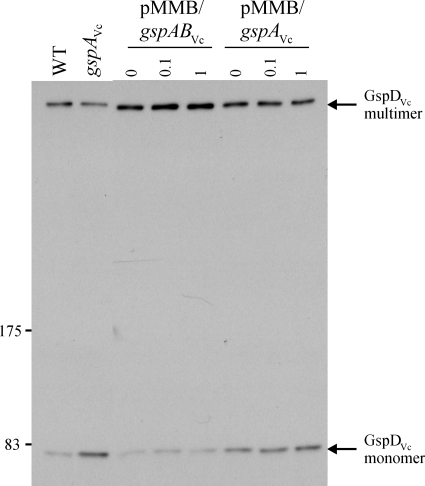
Although mutation of gspA had a minimal effect on the secretion of T2SS substrates, a substantial reduction in the amount of assembled gspD secretin was observed in the gspA mutants of V. cholerae (Fig. 2A), V. parahaemolyticus (Fig. 2B), and V. vulnificus (Fig. 2C) compared to the amount in the wild type. Due to the difficulty in quantifying the relative differences in the amounts of protein by immunoblotting techniques, we provided multiple points for comparison by repeatedly sampling cultures of the gspA mutant and the wild type over the entire growth curve. In a manner similar to the secretin of A. hydrophila and A. salmonicida, the vast majority of the approximately 1-MDa assembled Vibrio secretin in wild-type cells is stable when heated in SDS-PAGE sample buffer. At each point in the growth curve, a smaller amount of assembled secretin and a greater amount of unassembled GspD monomer were observed in samples taken from the gspA mutant than in samples from the wild type (Fig. 2). This phenotype could be complemented in V. cholerae, since secretin levels increased in response to expression of gspAVc in trans, but only if the complementing plasmid also encoded gspBVc (Fig. 3). Apparently the gspA mutation also decreased the level of GspB in the cell, either through a direct polarity effect or, possibly, because GspB is unstable in the absence of GspA, as was previously found in A. hydrophila (18). In any case, this result confirmed the involvement of both GspA and GspB in secretin assembly.
Fig. 3.
Complementation of the partial secretin-negative phenotype of V. cholerae Bah2-gspA by expression of GspABVc or GspAVc in trans from plasmids pMMB207/gspABVc and pMMB207/gspAVc. The cultures were grown to an OD600 of 2.0 in the absence or presence of IPTG (0.1 and 1.0 mM), and whole-cell samples were electrophoresed and immunoblotted with anti-GspDVc. Locations of standard protein markers (in kDa) are given. WT, wild type.
The V. cholerae T2SS exhibits excess secretion capacity.
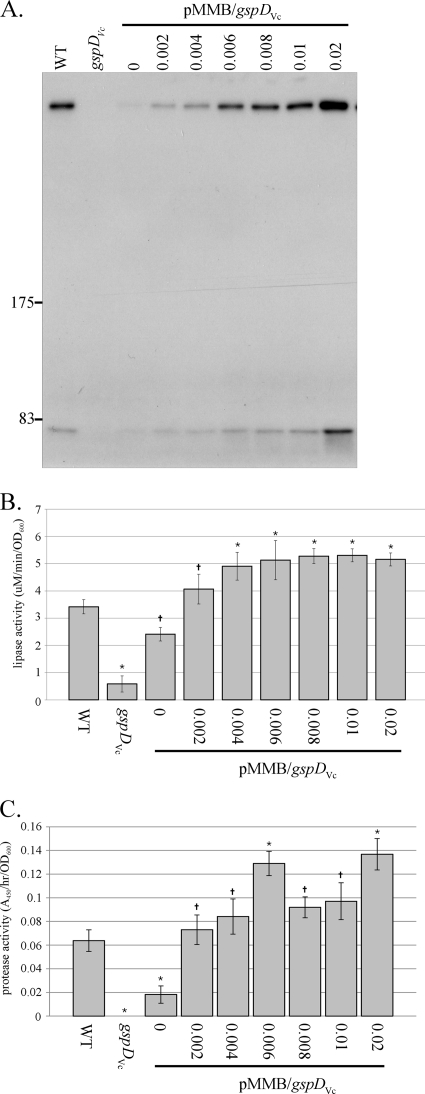
The absence of GspABVc in V. cholerae resulted in a substantial decrease in secretin assembly (Fig. 2A) that was not accompanied by a concomitant decrease in lipase and protease activities in culture supernatants (Table 2). This result suggested that either the lipase and protease assayed in culture supernatant are not substrates of the T2SS or that wild-type levels of secretin are not required for efficient secretion of lipase and protease from the cell. The lipase and protease are indeed substrates of the T2SS, because these activities were nearly absent in supernatants from a culture of V. cholerae TRH7000 gspD (Fig. 4). In order to assay secretion capacity, the same strain was complemented by the expression of gspD in trans at various levels of induction. The results, shown in Fig. 4, revealed that a relatively low level of secretin in comparison to the amount expressed in wild-type cells is sufficient for full secretion, since wild-type levels of lipase were present in supernatants from cells without induction (Fig. 4B) and wild-type levels of protease were present in supernatant from cells induced with 0.002 mM IPTG (Fig. 4C). In the absence of induction and at an induction level of 0.002 mM IPTG, much less secretin was observed in the complemented gspD strain than in wild-type cells (Fig. 4A). These data demonstrate that, at least under the growth conditions used in these experiments, there is a natural overexpression of the secretin relative to the amount of extracellular secretion, such that more secretin is assembled than is required for the secretion of substrates into the medium, a situation that may exist due to differential expression of genes that encode the T2SS and the substrates of the system.
Fig. 4.
Wild-type amount of GspDVc secretin is not required for secretion of lipase and protease to wild-type levels. (A) The amounts of secretin multimer assembled in Vibrio cholerae TRH7000, the gspDVc strain, and the gspDVc strain expressing gspDVc in trans (induced from plasmid pMMB/gspDVc with 0 to 0.02 mM IPTG as indicated above the immunoblot) were assessed by immunoblot analysis with anti-GspDVc antibody. Locations of standard protein markers (in kDa) are given. (B and C) The results of triplicate assays of lipase (B) and protease (C) activities in supernatant taken from cultures of TRH7000, the gspDVc strain, and the gspDVc strain expressing gspDVc in trans (induced with 0 to 0.02 mM IPTG) are shown. Significant difference ( , nonsignificant; *, significant [P < 0.05]) was determined based on comparison of each value against activity observed in supernatant from wild-type culture. WT, wild type.
, nonsignificant; *, significant [P < 0.05]) was determined based on comparison of each value against activity observed in supernatant from wild-type culture. WT, wild type.
V. cholerae GspAB partially complements the secretin assembly and secretion defects of A. hydrophila gspA mutants.
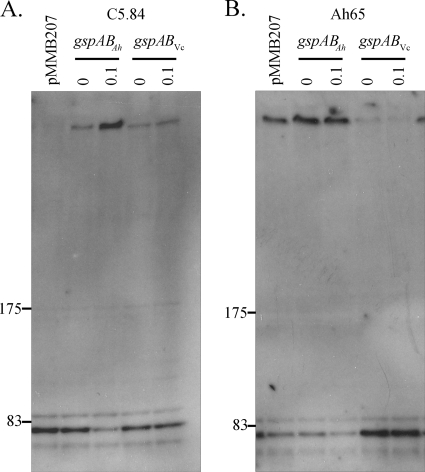
The secretion and secretin assembly results suggested that although Vibrio GspAB is not essential for the function of the T2SS, it is nevertheless involved in the assembly of the secretin. Another approach to elucidate the function of GspAB in Vibrio species would be to determine if GspABVc could complement A. hydrophila gspA mutants or otherwise interact with the T2SS of A. hydrophila. We investigated this possibility by introducing pMMB/gspABVc into Ah65 and C5.84 and analyzed the T2SS function and assembly. The recombinant plasmid contained the presumed gspABVc promoter and a vector-encoded tac promoter to allow increased expression. The expression of gspABVc in trans partially complemented the secretin assembly defect of C5.84, as indicated by the reestablishment of secretin multimer assembly in C5.84 (pMMB/gspABVc) (Fig. 5A). The amount of secretin multimer assembled was somewhat dependent upon the level of gspABVc expression, since induction with 0.1 mM IPTG resulted in a slightly greater amount of ExeD multimer formed. As previously demonstrated in the control experiment, the expression of gspABAh carried on plasmid pRJ31.1 in strain C5.84 completely restored secretin assembly (Fig. 5A) (3).
Fig. 5.
Secretin assembly in A. hydrophila gspAB and the wild type upon expression of V. cholerae gspAB in trans. The amount of secretin multimer assembled upon induced expression (0.1 mM IPTG) of gspABAh, gspABVc, or neither (pMMB207) in C5.84 (A) and Ah65 (B) was assessed by anti-GspDAh immunoblot analysis. Locations of prestained standard protein markers (in kDa) are given.
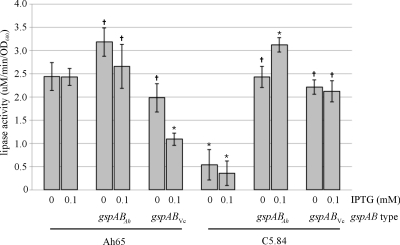
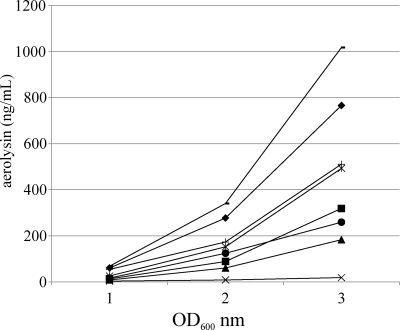
The C5.84 secretion-negative phenotype was nearly fully complemented by the expression of gspABVc in trans. As shown in Fig. 6, the lipase activity in supernatants from the uninduced C5.84(pMMB/gspABVc) culture was 90% of the wild-type amount and did not increase with further induction. In the control experiment, wild-type gspABAh completely complemented the lipase secretion defect of the gspA mutant even without induction. Likewise, aerolysin secretion was partially restored in C5.84(pMMB/gspABVc) and did not substantially increase with induction, as shown in Fig. 7. In the control experiment, the expression of gspABAh almost fully complemented the aerolysin secretion defect of C5.84 even without induction and, with induction, generated levels of aerolysin secretion greater than that observed in the wild type.
Fig. 6.
Lipase activities in supernatants from cultures of wild-type and gspA A. hydrophila upon expression of gspABAh and gspABVc in trans. Lipase activities were assayed in supernatants taken from wild-type (Ah65) and gspA (C5.84) A. hydrophila cultures expressing gspABAh (from plasmid pRJ31.1) or gspABVc (from plasmid pMMB/gspABVc) with or without induction with IPTG. Assays of culture supernatants were performed in triplicate, and the standard deviations are indicated. The results shown are representative of those obtained in multiple experiments. Significant difference ( , nonsignificant; *, significant [P < 0.05]) was calculated based on comparison of each value against lipase activity observed in wild-type (Ah65) culture.
, nonsignificant; *, significant [P < 0.05]) was calculated based on comparison of each value against lipase activity observed in wild-type (Ah65) culture.
Fig. 7.
The aerolysin secretion-negative phenotype of A. hydrophila gspA is partially complemented by expression of V. cholerae gspAB in trans. The amount of aerolysin secreted upon expression of gspABVc and gspABAh in wild-type (Ah65) and gspA (C5.84) A. hydrophila was determined at various stages of growth (indicated by the OD600). The strains used were Ah65 (♦), Ah65 gspABVc (■), Ah65 gspABVc induced with 0.1 mM IPTG (▴), C5.84 ( ), C5.84 gspABVc (
), C5.84 gspABVc ( ), C5.84 gspABVc induced with 0.1 mM IPTG (●), C5.84 gspABAh (
), C5.84 gspABVc induced with 0.1 mM IPTG (●), C5.84 gspABAh ( ), and C5.84 gspABAh induced with 0.1 mM IPTG (
), and C5.84 gspABAh induced with 0.1 mM IPTG ( ). The results shown are representative of those obtained in multiple experiments.
). The results shown are representative of those obtained in multiple experiments.
GspABVc confers a dominant negative effect on secretin multimer formation and secretion of T2SS substrates in wild-type A. hydrophila.
Interestingly, the expression of gspABVc in wild-type A. hydrophila decreased the amount of assembled secretin multimer and increased the amount of GspD monomer as visualized by immunoblotting compared to the results for cells containing the vector only (Fig. 5B). This effect was not observed for Ah65(pRJ31.1) cells overexpressing the gspABAh genes, since the expression of gspABAh resulted in more secretin multimer and less unassembled monomer than were observed in the wild type. The expression of gspABVc in wild-type A. hydrophila also decreased the amount of aerolysin secreted, with a greater effect generated upon increased induction of gspABVc expression (Fig. 7). Likewise, the amount of lipase activity in the Ah65(pMMB/gspABVc) culture supernatant was significantly decreased upon the induction of gspABVc with 0.1 mM IPTG, to 45% (Fig. 6) of the lipase activity in the Ah65 culture supernatant.
DISCUSSION
In Gram-negative bacteria that contain a T2SS, the presence of the gspAB operon in addition to the set of “core” gsp genes is not well conserved, therefore suggesting that the role of GspAB is not integral to the assembly of the T2SS. However, in some bacteria that contain gspAB, such as A. hydrophila, the GspAB complex is absolutely required for the construction of the T2SS (3), characterized by assembly of the megadalton-sized GspD secretin multimer in the outer membrane. The function of the GspABAh complex (46) may involve reorganization of the peptidoglycan meshwork to allow monomeric GspD subunits or the secretin multimer itself to pass through to reach the outer membrane or may possibly provide a scaffold for the assembly of the secretin. This activity is mediated at least in part by the C-terminal peptidoglycan-binding motif of GspAAh (17, 25). In this study, we sought to determine if the requirement for the GspAB complex in the assembly of the secretin in A. hydrophila also applies to another species of Aeromonas, A. salmonicida, as well as to three Vibrio species, V. cholerae, V. parahaemolyticus, and V. vulnificus, by detecting the amount of assembled GspD secretin and by assaying the activity of T2SS substrates in culture supernatants isolated from wild-type strains and those without the GspAB complex.
We demonstrated a requirement for GspAAs in the assembly of the secretin in A. salmonicida. A significant reduction in lipase activity (75%) was detected in the gspA mutant in comparison to the lipase activity in the wild type (Table 2), presumably caused by the lack of assembled GspD secretin multimer observed in the gspA strain (Fig. 1). This finding is consistent with that described by Ast et al. (3), where loss of the GspABAh complex abrogated both the secretion of aerolysin and the assembly of the secretin multimer in A. hydrophila. Together, these data suggest that GspAB is generally required for the assembly of the GspD secretin in Aeromonas species.
Surprisingly, we found that, contrary to the essential role of GspAB in the assembly of the secretin in the Aeromonas species A. hydrophila (3) and A. salmonicida (Fig. 1), GspAB is not an absolute requirement for the function of the T2SS in vibrios, because mutation of gspA (which also apparently inactivated gspB; see Fig. 3) did not prevent the secretion of T2SS substrates, including lipase, amylase, protease (Table 2), and EtxB (see Results) in V. cholerae, V. parahaemolyticus, and V. vulnificus. However, although the secretion of T2SS substrates remained largely unchanged in the gspA strain compared to the levels of secretion in wild-type strains, we did find significant reductions in the amounts of assembled secretin multimer in the absence of GspAB in V. cholerae, V. parahaemolyticus, and V. vulnificus (Fig. 2). The dichotomy of the finding that substantial decreases in secretin multimer formation had only minimal effects on secretion can be explained by an apparent excess in T2SS secretion capacity at normal GspD levels, since minimal expression of gspD was shown to fully complement secretion in the V. cholerae gspD mutant (Fig. 4). In any case, the significant reduction in assembled secretin multimer in the absence of GspA suggested that the GspAB complexes of the Vibrio strains perform an important role in the assembly of their respective secretins.
In order to more definitively determine if GspAB performs the same role in vibrios as in aeromonads, we expressed a plasmid-carried copy of gspABVc in the A. hydrophila gspAB strain C5.84 and asked if GspABVc could complement the secretin assembly-negative and secretion-negative phenotypes of this strain. The expression of GspABVc reestablished the assembly of the GspDAh secretin multimer (Fig. 5A) and the secretion of the T2SS substrates aerolysin (Fig. 7) and lipase (Fig. 6) in C5.84. Although complementation was not complete, this is perhaps not surprising given that GspABAh and GspABVc are 36% identical and 51% similar. Therefore, although GspABVc did not completely complement the gspAB phenotype of C5.84, its partial complementation did show that GspABVc and GspABAh likely perform the same function in their respective species.
Although similar enough for partial complementation of the gspABAh secretion- and secretin assembly-negative phenotypes of C5.84, GspABVc and GspABAh are apparently not sufficiently identical to function together as a heteromultimer composed of both complexes. When expressed in wild-type strain Ah65, GspABVc decreased the amount of assembled secretin (Fig. 5B) and the secretion of T2SS substrates (Fig. 6) in a dose-dependent manner. Since GspAB functions as a heteromultimeric complex (46), this phenotype was likely caused by a dominant negative effect of GspABVc whereby the assembly of an inactive heteromultimeric complex composed of GspABAh and GspABVc reduces the ability of the strain to assemble a functional secretin multimer and, thus, secrete T2SS substrates into the growth medium. The dominant negative effect of GspABVc expression is consistent with the ability to inactivate the function of a multimeric protein by the expression of a mutant subunit in trans, a hallmark of multisubunit proteins.
The difference in the requirement for GspAB in the assembly of the T2SS secretin in Vibrio and Aeromonas species suggests that a redundant GspAB function exists in the Vibrio genus that is absent in the Aeromonas genus. Although amino acid similarity studies of the V. cholerae proteome did not identify other obvious GspAB candidates, it is possible that a protein involved in peptidoglycan metabolism that is characterized as part of another periplasm-spanning system could perform the redundant function of GspABVc. Alternatively, Vibrio species may encode a specific outer membrane determinant, such as lipopolysaccharide, or an outer membrane protein, such as a GspS pilot protein, that enables secretin assembly to exist in the absence of the PG-binding function of GspAB. For instance, a pilot protein could stabilize and localize the secretin to the outer membrane, thereby allowing partial secretin assembly in the outer membrane under conditions (absence of GspAB) that normally result in the degradation of GspD in other species, such as aeromonads.
The reason for the apparent lack of GspAB in a number of other species that contain a T2SS remains unclear but could involve one or a combination of factors, including an alternative peptidoglycan physiology or the presence of additional peptidoglycan-spanning systems. First, the difference between GspAB-containing Gram-negative bacteria and those that do not have the gspAB operon could be the nature of their peptidoglycan layer. Previous studies have suggested that the composition and organization of the peptidoglycan layer is identical in all Gram-negative bacteria (45); however, modifications of the glycan or amino acid components of the peptidoglycan of Gram-negative bacteria are species specific, particularly in the frequency of cross-links in peptidoglycan (11). Second, the involvement of peptidoglycan-binding proteins in the assembly of structures that traverse the peptidoglycan is well documented, with examples including FlgJ in the flagellar assembly of Salmonella enterica serovar Typhimurium (31), PtlE in type IV secretion of Bordetella pertussis, and IpgF in type III secretion in Shigella sonnei (56). A redundancy of function might exist in some species whereby a lytic transglycosylase or peptidoglycan-binding protein involved in remodeling the peptidoglycan layer for the assembly of one system could serve in that capacity for the assembly of the T2SS.
Identification of the putative GspAB-redundant function in Vibrio species may define the involvement of other proteins that perform roles in the assembly of the secretin and may also help to further elucidate the role of GspAB in the assembly of the T2SS.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (to S.P.H.) and grant AI49294 from the National Institutes of Health (to M.S.).
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 4 March 2011.
REFERENCES
- 1. Aragon V., Kurtz S., Fleiger A., Neumeister B., Cianciotto N. P. 2000. Secreted enzymatic activities of wild-type and pilD-deficient Legionella pneumophila. Infect. Immun. 68:1855–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arts J., et al. 2007. Interaction domains in the Pseudomonas aeruginosa type II secretory apparatus component XcpS (GspF). Microbiology 153:1582–1592 [DOI] [PubMed] [Google Scholar]
- 3. Ast V. M., et al. 2002. Expression of the ExeAB complex of Aeromonas hydrophila is required for the localization and assembly of the ExeD secretion port multimer. Mol. Microbiol. 44:217–231 [DOI] [PubMed] [Google Scholar]
- 4. Bitter W., Koster M., Latjinhouwers M., de Cock H., Tommassen J. 1998. Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol. Microbiol. 27:209–219 [DOI] [PubMed] [Google Scholar]
- 5. Brumlik M. J., van der Goot F. G., Wong K. R., Buckley J. T. 1997. The disulfide bond in the Aeromonas hydrophila lipase/acyltransferase stabilizes the structure but is not required for secretion or activity. J. Bacteriol. 179:3116–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bullock W. O., Fernandez J. M., Short J. M. 1987. XL1-Blue: a high efficiency plasmid transforming recA Escherichia coli strain with β-galactosidase selection. Biotechniques 5:376–379 [Google Scholar]
- 7. Camberg J. L., Sandkvist M. 2005. Molecular analysis of the Vibrio cholerae type II secretion ATPase EpsE. J. Bacteriol. 187:249–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Condemine G., Dorel C., Hugovieux-Cotte-Pattat N., Robert-Baudouy J. 1992. Some of the out genes involved in the secretion of pectate lyases in Erwinia chrysanthemi are regulated by KdgR. Mol. Microbiol. 6:3199–3211 [DOI] [PubMed] [Google Scholar]
- 9. Demchick P., Kock A. L. 1996. The permeability of the wall fabric of Escherichia coli and Bacillus subtilis. J. Bacteriol. 178:768–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gerard-Vincent M., et al. 2002. Identification of XcpP domains that confer functionality and specificity to the Pseudomonas aeruginosa type II secretion apparatus. Mol. Microbiol. 44:1651–1665 [DOI] [PubMed] [Google Scholar]
- 11. Glauner B. 1988. Separation and quantification of muropeptides with high-performance liquid chromatography. Anal. Biochem. 172:451–464 [DOI] [PubMed] [Google Scholar]
- 12. Reference deleted.
- 13. Hardie K. R., Seydel A., Guilvout I., Pugsley A. P. 1996. The secretin-specific, chaperone-like protein of the general secretory pathway: separation of proteolytic protection and piloting functions. Mol. Microbiol. 22:967–976 [DOI] [PubMed] [Google Scholar]
- 14. Harlow E., Lane D. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 15. Howard S. P., Buckley J. T. 1982. Membrane glycoprotein receptor and hole-forming properties of a cytolytic toxin. Biochemistry 21:1662–1667 [DOI] [PubMed] [Google Scholar]
- 16. Howard S. P., Buckley J. T. 1985. Protein export by a gram-negative bacterium: production of aerolysin by Aeromonas hydrophila. J. Bacteriol. 161:1118–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Howard S. P., Gebhart C., Langen G. R., Li G., Strozen T. G. 2006. Interactions between peptidoglycan and the ExeAB complex during assembly of the type II secretin of Aeromonas hydrophila. Mol. Microbiol. 59:1062–1072 [DOI] [PubMed] [Google Scholar]
- 18. Howard S. P., Meiklejohn H. G., Shivak D., Jahagirdar R. 1996. A TonB-like protein and a novel membrane protein containing an ATP-binding cassette function together in exotoxin secretion. Mol. Microbiol. 22:595–604 [DOI] [PubMed] [Google Scholar]
- 19. Hu N. T., et al. 2002. XpsG, the major pseudopilin in Xanthomonas campestris pv. campestris, forms a pilus-like structure between cytoplasmic and outer membranes. Biochem. J. 365:205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jahagirdar R., Howard S. P. 1994. Isolation and characterization of a second exe operon required for extracellular protein secretion in Aeromonas hydrophila. J. Bacteriol. 176:6819–6826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang B., Howard S. P. 1991. Mutagenesis and isolation of Aeromonas hydrophila genes which are required for extracellular secretion. J. Bacteriol. 173:1241–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kay W. W., et al. 1981. Purification and disposition of a surface protein associated with virulence of Aeromonas salmonicida. J. Bacteriol. 147:1077–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Korotkov K. V., Hol W. G. 2008. Structure of the GspK-GspI-GspJ complex from the enterotoxigenic Escherichia coli type II secretion system. Nat. Struct. Mol. Biol. 15:462–468 [DOI] [PubMed] [Google Scholar]
- 24. Letellier L., Howard S. P., Buckley J. T. 1997. Studies on the energetics of proaerolysin secretion across the outer membrane of Aeromonas species. Evidence for a requirement for both the protonmotive force and ATP. J. Biol. Chem. 272:11109–11113 [DOI] [PubMed] [Google Scholar]
- 25. Li G., Howard S. P. 2010. ExeA binds to peptidoglycan and forms a multimer for assembly of the type II secretion apparatus in Aeromonas hydrophila. Mol. Microbiol. 76:772–781 [DOI] [PubMed] [Google Scholar]
- 26. Lybarger S. R., Johnson T. L., Gray M. D., Sikora A. E., Sandkvist M. 2009. Docking and assembly of the type II secretion complex of Vibrio cholerae. J. Bacteriol. 191:3149–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marcotte E. M., et al. 1999. Detecting protein function and protein-protein interactions from genome sequences. Science 285:751–753 [DOI] [PubMed] [Google Scholar]
- 28. Miller J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 29. Miller V. L., Mekalanos J. J. 1988. A novel suicide vector and its use in construction of insertion mutants: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morales V. M., Backman A., Bagdasarian M. 1991. A series of wide-host range low-copy-number vectors that allow direct screening of recombinants. Gene 7:39–47 [DOI] [PubMed] [Google Scholar]
- 31. Nambu T., Minamino T., Macnab R. M., Kutdukake K. 1999. Peptidoglycan-hydrolyzing activity of the FlgJ protein, essential for flagellar rod formation in Salmonella typhimurium. J. Bacteriol. 181:1555–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Overbye L. J., Sandkvist M., Bagdasarian M. 1993. Genes required for extracellular secretion of enterotoxin are clustered in Vibrio cholerae. Gene 132:101–106 [DOI] [PubMed] [Google Scholar]
- 33. Overbye Michel L., Sandkvist M., Bagdasarian M. 1995. Specificity of the protein secretory apparatus: secretion of the heat-labile enterotoxin B subunit pentamers by different species of gram negative bacteria. Gene 152:41–45 [DOI] [PubMed] [Google Scholar]
- 34. Pearson G. D., Woods A., Chiang S. L., Mekalanos J. J. 1993. CTX genetic element encodes a site-specific recombination system and an intestinal colonization factor. Proc. Natl. Acad. Sci. U. S. A. 90:3750–3754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pepe C. M., Eklund M. W., Strom M. S. 1996. Cloning of an Aeromonas hydrophila type IV pilus biogenesis gene cluster: complementation of pilus assembly functions and characterization of a type IV leader peptidase/N-methyltransferase required for extracellular protein secretion. Mol. Microbiol. 19:857–869 [DOI] [PubMed] [Google Scholar]
- 36. Reference deleted.
- 37. Possot O. M., Vignon G., Bomchil N., Ebel F., Pugsley A. P. 2000. Multiple interactions between pullulanase secreton components involved in stabilization and cytoplasmic membrane association of PulE. J. Bacteriol. 182:2142–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pugsley A. P., Kornacker M. G., Poquet I. 1991. The general secretion pathway is directly required for extracellular pullulanase secretion in Escherichia coli. Mol. Microbiol. 5:343–352 [DOI] [PubMed] [Google Scholar]
- 39. Py B., Loiseau L., Barras F. 2001. An inner membrane platform in the type II secretion machinery of gram-negative bacteria. EMBO Rep. 2:244–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reference deleted.
- 41. Sandkvist M., Hirst T. R., Bagdasarian M. 1987. Alterations at the carboxy terminus change assembly and secretion properties of the B subunit of Escherichia coli heat-labile enterotoxin. J. Bacteriol. 169:4570–4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sandkvist M., Keith J. M., Bagdasarian M., Howard S. P. 2000. Two regions of EpsL involved in species-specific protein-protein interactions with EpsE and EpsM of the general secretion pathway in Vibrio cholerae. J. Bacteriol. 182:742–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sandkvist M., Morales V., Bagdasarian M. 1993. A protein required for secretion of cholera toxin through the outer membrane of Vibrio cholerae. Gene 123:81–86 [DOI] [PubMed] [Google Scholar]
- 44. Sarker M. R., Cornelis G. R. 1997. An improved version of suicide vector pKNG101 for gene replacement in gram-negative bacteria. Mol. Microbiol. 23:410–411 [DOI] [PubMed] [Google Scholar]
- 45. Schleifer K. H., Kandler O. 1972. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol. Rev. 36:407–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schoenhofen I. C., Li G., Strozen T. G., Howard S. P. 2005. Purification and characterization of the N-terminal domain of ExeA: a novel ATPase involved in the type II secretion pathway of Aeromonas hydrophila. J. Bacteriol. 187:6370–6378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schoenhofen I. C., Stratilo C., Howard S. P. 1998. An ExeAB complex in the type II secretion pathway of Aeromonas hydrophila: effect of ATP-binding cassette mutations on complex formation and function. Mol. Microbiol. 29:1237–1247 [DOI] [PubMed] [Google Scholar]
- 48. Shevchik V. E., Condemine G. 1998. Functional characterization of the Erwinia chrysanthemi OutS protein, an element of a type II secretion system. Microbiology 144:3219–3228 [DOI] [PubMed] [Google Scholar]
- 49. Simon R., Prifer U., Puhler A. 1983. A broad range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Biotechnology 1:784–791 [Google Scholar]
- 50. Sixma T. K., et al. 1993. Refined structure of E. coli heat labile enterotoxin, a close relative of cholera toxin. J. Mol. Biol. 230:890–918 [DOI] [PubMed] [Google Scholar]
- 51. Strom M. S., Nunn D., Lory S. 1991. Multiple roles of the pilus biogenesis protein pilD: involvement of pilD in excretion of enzymes from Pseudomonas aeruginosa. J. Bacteriol. 173:1175–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Svennerholm A. M., Holmgren J. 1978. Identification of Escherichia coli heat-labile enterotoxin by means of a ganglioside immunosorbent assay (GM1-ELISA) procedure. Curr. Microbiol. 1:19–23 [Google Scholar]
- 53. Tauschek M., Gorrell R. J., Strugnell R. A., Robins-Browne R. M. 2002. Identification of a protein secretory pathway for the secretion of heat-labile enterotoxin by an enterotoxigenic strain of Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 99:7066–7071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Voulhoux R., et al. 2001. Involvement of the twin-arginine translocation system in protein secretion via the type II pathway. EMBO J. 20:6735–6741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Williams K. P., et al. 2010. Phylogeny of Gammaproteobacteria. J. Bacteriol. 192:2305–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zahrl D., et al. 2005. Peptidoglycan degradation by specialized lytic transglycosylases associated with type III and type IV secretion systems. Microbiology 151:3455–3467 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.