Abstract
Release of Ca2+ with dipicolinic acid (CaDPA) was monitored by Raman spectroscopy and differential interference contrast microscopy during germination of individual spores of Bacillus subtilis strains with alterations in GerD and SpoVA proteins. Notable conclusions about germination after the addition of nutrient were as follows. (i) Following l-alanine addition, wild-type and gerD spores and spores with elevated SpoVA protein levels (↑SpoVA spores) slowly released ∼10% of their CaDPA during a variable (6- to 55-min) period ending at Tlag, the time when faster CaDPA release began. (ii) Tlag times were lower for ↑SpoVA spores than for wild-type spores and were higher for gerD spores. (iii) The long Tlag times of gerD spores were partially due to slow commitment to germinate. (iv) The intervals between the commitment to germinate and CaDPA release were similar for wild-type and ↑SpoVA spores but longer for gerD spores. (v) The times for rapid CaDPA release, ΔTrelease = Trelease − Tlag (with Trelease being the time at which CaDPA release was complete), were similar for wild-type, gerD, and ↑SpoVA spores. (vi) Spores with either one of two point mutations in the spoVA operon (spoVA1 and spoVA2 spores) exhibited a more rapid rate of CaDPA release beginning immediately after l-alanine addition leading to ∼65% CaDPA release prior to Tlag. (vii) Tlag times for spoVA1 and spoVA2 spores were longer than for wild-type spores. (viii) The intervals between spoVA1 and spoVA2 spores' commitment and CaDPA release were similar to those for wild-type spores, but commitment occurred later. In contrast to germination after the addition of nutrient, Tlag and ΔTrelease times were relatively similar during dodecylamine germination of spores of the five strains. These findings suggest the following. (i) GerD plays no role in CaDPA release during spore germination. (ii) SpoVA proteins are involved in CaDPA release during germination with nutrients, and probably with dodecylamine. (iii) Spores release significant CaDPA before commitment. (iv) CaDPA release during Tlag and ΔTrelease may signal subsequent germination events.
INTRODUCTION
Spores of various Bacillus species can remain dormant for long periods in the absence of nutrients, but when specific nutrients return, spores can rapidly return to life in the process of germination followed by outgrowth (6, 11). Germination is important not only to spores but also to the food and medical product industries, since spores of a number of species are major agents of food spoilage and food-borne disease (12). In contrast to dormant spores that are hard to kill, germinated spores have lost most dormant spore resistance properties and are relatively easily killed (11, 12). Consequently, there is much interest in mechanisms of spore germination, since preventing this process entirely or promoting it efficiently could have significant applied applications.
Spore germination in nature is likely triggered by low-molecular-weight nutrients, with l-alanine being a well-characterized nutrient germinant of Bacillus subtilis spores (6, 11, 12). Nutrient germinants bind to specific germinant receptors (GRs) located in the spore's inner membrane, and this triggers the release of monovalent cations and the spore core's large depot of pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) in a 1:1 chelate with divalent metal ions, predominantly Ca2+ (CaDPA) (∼25% of core weight [dry weight]) and replacement of these compounds with water. The GerD protein is also involved in GR-mediated events that lead to CaDPA release during germination, but the mechanism of this involvement is unknown (7, 11). In spores of Bacillus species, CaDPA release then triggers subsequent events in germination, in particular the hydrolysis of the spore's large peptidoglycan cortex by either one of two redundant cortex-lytic enzymes (CLEs), CwlJ and SleB. CaDPA release appears to directly activate CwlJ, although activation of SleB may be via effects of CaDPA release on core water content. Cortex hydrolysis in turn allows core expansion and further core water uptake, giving a fully germinated spore with a protoplast that is ∼80% water (wet weight), in contrast to the much lower water content in the dormant spore core (11).
In many ways, the most dramatic event in spore germination is the release of CaDPA. This release is triggered not only by nutrients but also by nonnutrient agents, such as the cationic surfactant dodecylamine, although dodecylamine germination requires neither GRs nor the GerD protein (7, 9, 11). The mechanism for release of the spore's huge CaDPA depot through the spore's generally quite impermeable inner membrane is not clear (5, 11, 12). However, there are data consistent with proteins encoded by the spoVA operon being involved in this CaDPA release (6, 11, 13–16). (i) The spoVA operon is expressed only during sporulation in the developing spore and just prior to CaDPA uptake by developing spores. (ii) spoVA deletion mutants do not accumulate CaDPA, although they synthesize it normally in the mother cell compartment of the sporulating cell. (iii) At least one SpoVA protein, SpoVAD, is present in the spore's inner membrane, and it seems likely that all six SpoVA proteins are also there (6, 14). (iv) Populations of spores with increased levels of SpoVA proteins (↑SpoVA spores) release CaDPA faster during germination than do wild-type spores (16). (v) Populations of spores of strains with either one of two point mutations in the spoVA operon that can drastically alter CaDPA accumulation during sporulation can also exhibit slow CaDPA release during germination after the addition of nutrient (13).
The evidence for the involvement of GerD and SpoVA proteins in CaDPA release during spore germination has come from measurements on spore populations. However, the rates of germination of individual spores in populations are extremely heterogenous, and in measurements of populations, this heterogeneity in the behavior of individual spores is masked (2, 18, 19). In addition, analysis of the germination of individual Bacillus spores has given much new insight into the events in spore germination, in particular CaDPA release (2, 18, 19). Consequently, we have analyzed the l-alanine and dodecylamine germination of multiple individual spores of B. subtilis strains with and without the GerD protein and with either point mutation in the spoVA operon or with an increased level of SpoVA proteins, using both differential interference contrast (DIC) microscopy and Raman spectroscopy to monitor germination events in individual spores, in particular CaDPA release.
MATERIALS AND METHODS
B. subtilis strains and spore preparation.
The B. subtilis strains used in this work are isogenic derivatives of strain PS832, a prototrophic strain of strain 168 and are as follows. B. subtilis PS533 (wild type) is strain PS832 carrying plasmid pUB110, which has a gene encoding resistance to kanamycin (10). In strain FB62 (gerD mutant), almost all of the gerD coding region has been deleted (7). In strain PS3411, the strong forespore-specific sspB promoter expressed at the same time in sporulation as the weaker spoVA promoter drives the transcription of the spoVA operon, elevating SpoVA protein levels in spores ∼4-fold (↑SpoVA spores) (14). (iv) Strain PS3640 (spoVA1) has a single-base change (T to A) in the ribosome binding site of the spoVAC gene (13); this mutant is temperature sensitive (TS) for CaDPA accumulation in the spore during sporulation, and the mutant spores have a defect in DPA release in l-alanine spore germination. Strain PS3642 (spoVA2) has a C-to-T change in bp 1267 of the spoVAC gene. The sporulation phenotype of this strain is similar to that of strain PS3640, but PS3640 spore populations are somewhat TS for l-alanine germination (13). Since the results on the germination of individual spores of the two strains with point mutations in the spoVA operon were relatively similar (see Results), these two strains are referred to together as spoVA1 and spoVA2 strains.
Spores of the various strains were prepared on 2× SG medium agar plates (3, 4). The plates were incubated at 30°C for 2 to 4 days. In some cases, the plates were incubated at 23°C for an additional 2 to 4 days to allow lysis of the remaining growing or sporulating cells. The spores were scraped from the plates and washed extensively with cold distilled water with intermittent sonication treatment (3). The final spores were stored in water at 4°C protected from light and were >98% free of growing or sporulating cells and germinated spores as observed by phase-contrast microscopy.
Spore germination and measurement of the germination of spore populations or individual spores.
Spores of various B. subtilis strains germinated in 25 mM Tris-HCl buffer (pH 8.3) with 10 mM l-alanine or in 25 mM HEPES buffer (pH 7.4) with 0.8 mM dodecylamine at 25°C and 45°C. These temperatures were chosen for the following two reasons: (i) to use 45°C to facilitate data collection from individual spores of strain FB62 spores (gerD spores) in a reasonable observation period, because these spores germinated much better at 45°C; and (ii) to assess the reported TS germination behavior of spoVA1 spores (13). Spores were heat activated before germination after the addition of nutrient by incubating the spores in a water bath at 70°C for 30 min and then cooling them on ice for at least 15 min. In experiments measuring spores' commitment to germinate, germination was at 30°C with 10 mM l-valine in 25 mM HEPES buffer (pH 7.4) plus 50 μM TbCl3, as described previously (17). Spores do not need to be heat activated for dodecylamine germination (9).
Germination of multiple individual spores was monitored by differential interference contrast (DIC) microscopy as described previously (16a, 18, 19). Briefly, heat-activated spores (1 μl; ∼108 spores/ml in water) were spread on the surface of a microscope coverslip that was dried in a vacuum desiccator for ∼10 min, and the coverslips were mounted on and sealed to a microscope sample holder kept at a constant temperature. The DIC images of multiple spores adhered on coverslips were recorded at a rate of 1 frame per 15 s for 60 to 120 min by a digital charge-coupled device camera (16 bits; 1,600 by 1,200 pixels) following the addition of preheated germination solution to the spores on the coverslips. The averaged pixel intensity of an area of 40 by 40 pixels that covered each individual spore (the whole spore) on the DIC image was calculated, the DIC image intensity of each individual spore was plotted as a function of the incubation time with a resolution of 15 s, and the initial intensity at the first time of measurement, T0, was normalized to 1, and the intensity at the end of the measurement was set at zero. Invariably, the DIC image intensity had been constant for ≥10 min at the end of measurements. The kinetics of Ca2+ with dipicolinic acid (CaDPA) release during spore germination were measured simultaneously by DIC microscopy and Raman spectroscopy on individual adhered spores as described previously (16a, 18) (see below). The CaDPA levels in individual spores were determined from the intensity of the CaDPA-specific Raman band at 1,017 cm−1. As found previously (Wang et al., submitted), the end of the most rapid fall in DIC image intensity during wild-type spore germination with nutrients corresponded to the point at which release of CaDPA was complete, and this time point was defined as Trelease. At this time, the DIC image intensity was 30 to 35% of that at T0 when the DIC image intensity (I) at the end of measurements with wild-type spores was set at zero (Wang et al., submitted). Consequently, the CaDPA content of wild-type spores at any time t relative to that at T0 could be calculated as (It − 32.5%)/68% (16a) (see Results).
In addition to Trelease, a number of other parameters were used to describe wild-type spore germination, including Tlag, Ilag, T1, I1, Irelease, and ΔTrelease. These germination parameters were defined as follows. Tlag is the time following T0 at which more rapid CaDPA release began. Ilag is the DIC image intensity at Tlag. T1 is the time between Tlag and Trelease when CaDPA release became even faster. I1 is the DIC image intensity at T1. Irelease is the DIC image intensity at Trelease. ΔTrelease is Trelease − Tlag. However, with spoVA1 and spoVA2 spores, it was very difficult to distinguish a T1 point (see Results). Consequently, for spoVA1 and spoVA2 spores, T1 and I1 values were not determined.
The degree of germination of spore populations was measured by simultaneously monitoring the germination of ≥300 individual spores by DIC microscopy, and at various times, the percentage of these spores that had released their CaDPA was determined as described above. The commitment of a spore to germinate is defined as the earliest point after the addition of a germinant when the spore will continue on through the germination process even if the germinant is removed or its action is blocked (17). The commitment of heat-activated spores to germinate with l-valine was determined at 30°C in liquid germination medium (25 mM HEPES buffer [pH 7.4] with 10 mM l-valine) by blocking further commitment by d-alanine addition at various times and measuring CaDPA release fluorometrically with Tb3+ using a multiwell plate fluorescence reader as described previously (17). The kinetics of the germination and commitment process in spore populations were described by the following parameters. C25, C50, and C75 are the times for 25%, 50%, and 75%, respectively, of the spore population to become committed to germinate (note also that spores that have released CaDPA have by definition already committed to germinate). D25, D50, and D75 are the times at which the population has released 25%, 50%, and 75%, respectively, of their total CaDPA. Finally, Δ25, Δ50, and Δ75 are the differences between the respective C and D values, as described previously (17).
RESULTS
l-Alanine germination of individual B. subtilis spores and spore populations.
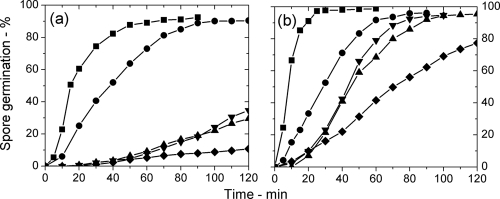
DIC microscopic analysis of the l-alanine germination of populations of B. subtilis spores of various strains at 25°C and 45°C showed that wild-type spore populations germinated slower than spores with elevated SpoVA protein levels (↑SpoVA spores) at both temperatures (Fig. 1a and b), as expected (14). In contrast, gerD spores germinated slower than wild-type spores at both temperatures, with ≤10% of gerD spores germinating in 2 h at 25°C. Spores of strains with either one of two single-base changes in the spoVA operon (spoVA1 and spoVA2 spores) that altered DPA uptake in sporulation (13) also germinated slower than wild-type spores, in particular at 25°C. While DIC microscopy was used here to measure the completion of spore germination, Raman spectroscopy of individual germinating spores has shown that CaDPA release generally parallels a decrease of 60 to 70% in the DIC image intensity of the spores during germination (2, 18; also see below).
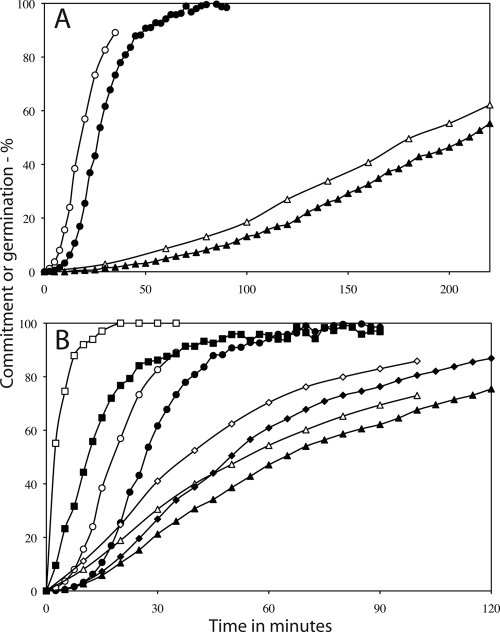
Fig. 1.
(a and b) Germination of populations of spores of various B. subtilis strains at 25°C (a) and 45°C (b) in response to the addition of l-alanine. Spores of B. subtilis PS533 (wild-type) (●), FB62 (gerD) (♦), PS3411 (↑SpoVA) (■), PS3640 (spoVA1) (▴), and PS3642 (spoVA2) (▾) germinated in response to the addition of l-alanine, and the percent germination was determined as described in Materials and Methods.
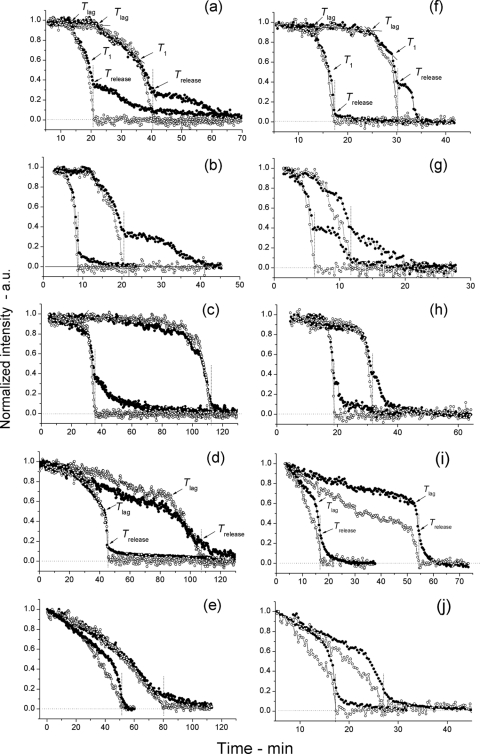
While the results noted above indicated that GerD and SpoVA proteins play a significant role in spore germination, as suggested previously (6, 7, 13–16), the heterogeneity in the germination behavior of individual spores can be masked when only the germination of spore populations is measured (2, 18, 19). Consequently, we used DIC microscopy and Raman spectroscopy to monitor the germination of individual spores of the various strains. Analysis of individual wild-type, gerD, and ↑SpoVA spores indicated there were generally four different phases in the l-alanine germination of these spores as measured by DIC microscopy and Raman spectroscopy (Fig. 2a to c and f to h; Fig. 3; Table 1). After the spores were mixed with l-alanine, there was a period of slow albeit variable decrease of ∼10% in the spores' DIC image intensity until Tlag, when the DIC image intensity began to decrease more rapidly. The slow initial decrease in DIC image intensity was not seen when the spores were incubated in germination buffer alone (data not shown). Subsequently, at T1, when an additional ∼35% in DIC image intensity had been lost, the rate of decrease in the DIC image intensity became even more rapid, then slowed again at Trelease, and eventually stabilized at a low value that was set at zero. Using Raman spectroscopy to simultaneously monitor individual spore levels of CaDPA during germination showed that ∼10% of spores' CaDPA was released in the period prior to Tlag, with the remainder between Tlag and Trelease (Fig. 2a to c and f to h). Previous work has also shown that for wild-type spores the slow decrease of ∼30% in the DIC image intensity beginning approximately at Trelease is due to the hydrolysis of the spore's peptidoglycan cortex with attendant core swelling and water uptake (2). The overall patterns of changes in DIC image intensity were largely similar for multiple individual wild-type, gerD, and ↑SpoVA spores during l-alanine germination at both 25°C and 45°C (Fig. 3). However, the actual timing of the various points in germination varied significantly between individual spores, in particular the Tlag time, as seen previously, and there were also differences between individual spores in the amount of decrease in DIC image intensity following Trelease, as well as significant variation in the time period for this final decrease (Fig. 2a to c and f to h; Fig. 3; Table 1) (2, 18, 19).
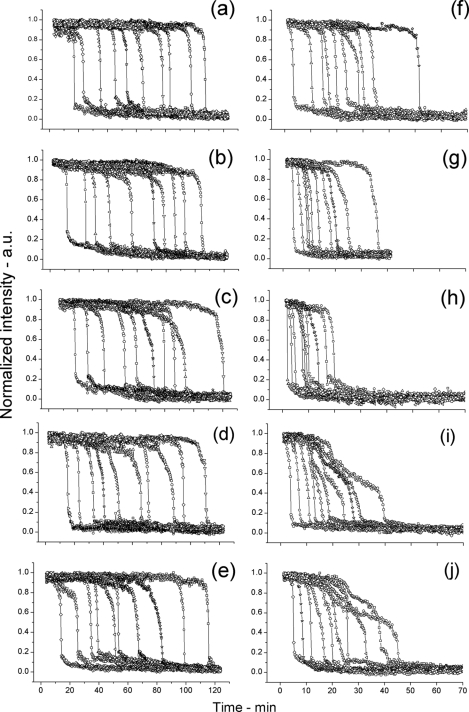
Fig. 2.
Germination of individual spores of various B. subtilis strains in response to l-alanine measured simultaneously by Raman spectroscopy and differential interference contrast (DIC) microscopy. (a to j) Spores of various strains of B. subtilis germinated at 25°C (a to e) or 45°C (f to j) in response to the addition of l-alanine. The dynamics of the germination of individual spores were monitored by both Raman spectroscopy (○) and DIC microscopy (●), and the times of various points in germination were determined as described in Materials and Methods. In each graph, time (in minutes) is shown on the x axis, and normalized intensity are shown in arbitrary units (a.u.) on the y axis. Note that data from only two individual spores are shown in panels a to j and that the two most adjacent curves with filled and open circles in all panels are from the same individual spore. The spores from strains PS533 (wild-type) (a and f), PS3411 (↑SpoVA) (b and g), FB62 (gerD) (c and h), PS3640 (spoVA1) (d and i), and PS3642 (spoVA2) (e and j) were analyzed and are shown in the various panels. In panels a and f, the times of Tlag, T1, and Trelease are indicated by arrows, but in panels d and i, only Tlag and Trelease are indicated, since T1 points could not be identified in most spoVA1 and spoVA2 spores germinating in response to l-alanine as described in Materials and Methods. The Trelease points in all panels are also noted by vertical dotted lines, and the horizontal dotted line at the bottom of the panels is the zero level as described in Materials and Methods.
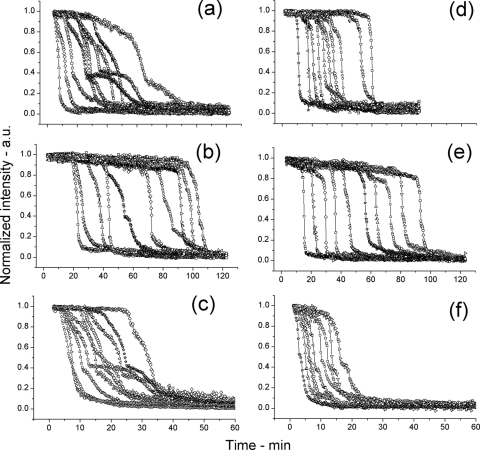
Fig. 3.
Germination of multiple individual spores of various B. subtilis strains in response to l-alanine measured by DIC microscopy. (a to f) Spores of various strains of B. subtilis at 25°C (a to c) or 45°C (d to f) germinated in response to l-alanine, and each panel in this figure shows curves of the dynamics of the germination of 10 individual spores monitored by DIC microscopy as described in Materials and Methods. The spores analyzed were from strains PS533 (wild-type) (a and d), FB62 (gerD) (b and e), and PS3411 (↑SpoVA) (c and f).
Table 1.
Parameters of the germination of spores of various B. subtilis strains at 25°C and 45°C in response to the addition of l-alaninea
| Germination temp (°C) | Strain | Tlag (min) | T1 (min) | Trelease (min) | ΔTrelease (min) | Δ(T1 − Tlag) (min) | Δ(Trelease − T1) (min) | Ilag (%) | I1 (%) | Irelease (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 25 | PS533 (wild type) | 22.2 ± 17.2 | 33.4 ± 21.8 | 34.9 ± 22.0 | 12.7 ± 7.0 | 11.2 ± 6.8 | 1.5 ± 0.4 | 94 ± 3 | 55 ± 11 | 33 ± 12 |
| FB62 (gerD) | 52.4 ± 29.4 | 56.5 ± 29.4 | 58.1 ± 29.5 | 5.7 ± 2.8 | 4.5 ± 2.7 | 1.3 ± 0.3 | 91 ± 4 | 58 ± 10 | 29 ± 9 | |
| PS3411 (↑SpoVA) | 9.4 ± 8.8 | 14.9 ± 10.2 | 16.1 ± 10.2 | 6.7 ± 2.9 | 5.5 ± 2.7 | 1.2 ± 0.2 | 90 ± 11 | 54 ± 13 | 32 ± 9 | |
| PS3640 (spoVA1) | 71.7 ± 23.9 | NDb | 73.6 ± 24.3 | 1.9 ± 0.7 | ND | ND | 39 ± 9 | ND | 23 ± 4 | |
| PS3642 (spoVA2) | 65.4 ± 27.4 | ND | 66.9 ± 27.6 | 1.5 ± 0.5 | ND | ND | 37 ± 8 | ND | 21 ± 5 | |
| 45 | PS533 (wild type) | 23.5 ± 13.2 | 25.9 ± 13.5 | 27.2 ± 13.6 | 3.6 ± 1.6 | 2.4 ± 1.4 | 1.2 ± 0.3 | 90 ± 5 | 75 ± 7 | 32 ± 11 |
| FB62 (gerD) | 53.0 ± 26.8 | 55.1 ± 26.6 | 56.1 ± 26.6 | 3.0 ± 1.4 | 2.0 ± 1.4 | 1.0 ± 0.3 | 88 ± 4 | 73 ± 9 | 32 ± 11 | |
| PS3411 (↑SpoVA) | 5.7 ± 5.6 | 7.9 ± 5.4 | 8.9 ± 5.5 | 3.2 ± 1.2 | 2.2 ± 1.1 | 1.0 ± 0.2 | 94 ± 5 | 72 ± 9 | 37 ± 11 | |
| PS3640 (spoVA1) | 44.8 ± 18.7 | ND | 45.7 ± 18.7 | 1.0 ± 0.2 | ND | ND | 43 ± 7 | ND | 26 ± 5 | |
| PS3642 (spoVA2) | 43.4 ± 15.0 | ND | 44.4 ± 15.0 | 1.0 ± 0.2 | ND | ND | 33 ± 11 | ND | 17 ± 5 |
Spores of various B. subtilis strains germinated at 25°C or 45°C in response to l-alanine. The parameters of the germination process were determined for 30 individual spores for each strain by differential interference contrast (DIC) microscopy as described in Materials and Methods. The values were averaged, and standard deviations were calculated. The various kinetic parameters of spore germination are defined as follows. Tlag is the time at which rapid CaDPA release began. T1 is the time at which the rate of CaDPA release following Tlag shifted to an even faster rate. Trelease is the time at which CaDPA release was complete. ΔTrelease is Trelease − Tlag. Ilag is the normalized DIC intensity at Tlag. I1 is the normalized DIC intensity at T1. Irelease is the normalized DIC intensity at Trelease.
ND, not determined since T1 values could not be determined during the l-alanine germination of spores from these two strains.
Analysis of average values of various parameters of l-alanine germination of multiple individual wild-type, gerD, and ↑SpoVA spores (Fig. 2a to c and f to h; Table 1) revealed a number of notable findings including the following. (i) The values for Tlag, T1, and Trelease were very heterogenous in populations of spores of these strains at both temperatures tested, with much of the heterogeneity likely due to initial variability in Tlag. (ii) The average Tlag values were much higher for gerD spores than for wild-type spores at both 25 and 45°C. Note that many gerD spores did not germinate in the measurement period, and only data from spores that germinated were included in the average values of germination parameters. (iii) Values for ΔTrelease for gerD spores were actually lower than for wild-type spores at 25°C. (iv) The values for Tlag, T1, and Trelease were all lower for ↑SpoVA spores than for wild-type spores. (v) The values for the DIC image intensities at various key times in germination (Ilag, I1, and Irelease) were similar for wild-type, gerD, and ↑SpoVA spores. (vi) The period of slower CaDPA release following Tlag, Δ(T1 − Tlag), took longer in wild-type spores than in ↑SpoVA spores at 25°C, but these times were essentially the same at 45°C. The times for the most rapid period of CaDPA release, Δ(Trelease − T1), were also very similar for wild-type, gerD, and ↑SpoVA spores at both temperatures. Overall, the findings when these spores germinated in l-alanine in HEPES buffer (pH 7.4) at 25°C and 45°C were similar to those listed above, and germination of several different preparations of spores of these strains in l-alanine with Tris-HCl buffer also gave similar findings (data not shown).
Spores of the spoVA1 and spoVA2 strains with point mutations in the spoVA operon exhibited a number of differences in the changes in DIC intensity and CaDPA release during l-alanine germination in Tris-HCl buffer at pH 8.3 or HEPES buffer at pH 7.4 compared to wild-type, gerD, or ↑SpoVA spores (Fig. 2d, e, i, and j; Fig. 4; Table 1; also data not shown). In particular, the following differences were found. (i) There was a steady and more rapid rate of decrease in the DIC image intensity of these spores, and the CaDPA level began to decrease at T0, such that at Tlag, their DIC image intensity and CaDPA level had fallen 40 to 75% (average decrease of ∼60%). (ii) The Tlag times for spoVA1 and spoVA2 spores were larger than for wild-type spores, and Ilag values were much lower undoubtedly because of the much larger amount of CaDPA released prior to Tlag. (iii) A T1 point usually could not be identified for the spoVA1 and spoVA2 spores, although it could be identified for a few spores. (iv) Values for ΔTrelease were lower than for wild-type spores, perhaps because so little CaDPA was actually released in this period. (v) Irelease values were lower than for wild-type, gerD, and ↑SpoVA spores, suggesting there was significant cortex hydrolysis in the spoVA1 and spoVA2 spores prior to Trelease; as a consequence, the CaDPA content of germinating spoVA1 and spoVA2 spores was underestimated somewhat by the formula used for wild-type, gerD, and ↑SpoVA spores. (vi) The kinetic parameters of spore germination did not indicate that CaDPA release during germination of individual spoVA1 and spoVA2 spores was significantly TS.
Fig. 4.
Germination of multiple individual spoVA1 and spoVA2 spores in response to l-alanine measured by DIC microscopy. (a to d) Spores of spoVA1 and spoVA2 strains germinated at 25°C (a and b) or 45°C (c and d) in response to l-alanine, and each panel in this figure shows curves of the dynamics of the germination of 10 individual spores followed by DIC microscopy as described in Materials and Methods. The spores analyzed were from strains PS3640 (spoVA1) (a and c) and PS3642 (spoVA2) (b and d).
Commitment to germination of spores of various B. subtilis strains after the addition of nutrient.
One of the biggest differences in the germination of individual wild-type spores and those with alterations in GerD or SpoVA proteins after the addition of nutrient was in Tlag, both its length and the amounts of CaDPA released during this period. Thus, it was of obvious interest to examine other events in Tlag that might be altered in spores with changes in GerD or SpoVA proteins. Currently, the only event other than slow CaDPA release and release of some monovalent cations that is known to take place prior to the Tlag point is commitment, whereby a spore becomes irreversibly committed to germinate after the addition of a nutrient germinant, including complete release of CaDPA, even if the triggering action of the nutrient germinant is then blocked (5, 11, 17). Thus, B. subtilis spores germinating via the GerA GR with l-alanine or l-valine exhibit no CaDPA release if an excess of the competitive inhibitor d-alanine is added together with the l-amino acid germinant (17). However, if d-alanine is added after mixing of the l-amino acid germinant with spores, CaDPA release will often continue well after d-alanine addition, with up to 25% of spores releasing CaDPA following d-alanine addition. These latter spores are said to be committed to germinate, although the actual mechanism of commitment is not known. Attainment of various levels of commitment during germination of wild-type spore populations at 37°C after the addition of nutrient takes place ∼5 min prior to comparable extents of release of the spores' CaDPA (17).
The kinetics of commitment and CaDPA release of spore populations germinating in response to the addition of l-valine were measured at 30°C to slow the germination of ↑SpoVA spore populations sufficiently to allow accurate measurement of commitment. This analysis showed that for wild-type spores, achievement of various levels of commitment was 8 to 10 min earlier than comparable extents of CaDPA release (Fig. 5A; Table 2), slightly longer than at 37°C (17). However, for gerD spores, commitment was 22 to 25 min earlier than CaDPA release, although commitment generally took place much later than for wild-type spores. In contrast, for ↑SpoVA and spoVA1 and spoVA2 spore populations, the time intervals between the same extents of commitment and CaDPA release were similar to those for the wild-type spore population at several extents of completion of commitment and CaDPA release (Fig. 5B; Table 2). However, commitment generally occurred later for spoVA1 and spoVA2 spores than for wild-type spores, and this finding indicates that there can be significant CaDPA release from individual germinating spores before commitment occurs. Note also that invariably the intervals between the same extents of commitment and CaDPA release were greater, as the percentage of the spore population achieving commitment increased, as seen previously (17). The reason for this phenomenon is not clear, but it may be due to the heterogeneity in GR levels in spore populations, with the spores that commit first being those with the highest GR levels, as suggested previously (17).
Fig. 5.
Commitment of spores of various B. subtilis strains to germinate. (A and B) Heat-activated spores of strains PS533 (wild-type) and FB62 (gerD) (A) and PS3411 (↑SpoVA), PS3640 (spoVA1), and PS3642 (spoVA2) (B) germinated at 30°C in response to l-valine, and the kinetics of commitment (open symbols) and CaDPA release (filled symbols) were measured as described in Materials and Methods. (A) Kinetics of commitment (white symbols) and release of CaDPA (black symbols) in B. subtilis PS533 (wild-type) spores (circles) and FB62 (gerD) spores (triangles). (B) Kinetics of commitment (white symbols) and release of CaDPA (black symbols) in B. subtilis PS533 (wild-type) spores (circles), PS3411 (↑SpoVA) spores (squares), PS3640 (spoVA1) spores (triangles), and PS3642 (spoVA2) spores (diamonds).
Table 2.
Times for commitment and CaDPA release and the intervals between these times during germination of populations of spores of various B. subtilis strains in response to l-valinea
| Strain | C25 (min) | D25 (min) | Δ25 (min) | C50 (min) | D50 (min) | Δ50 (min) | C75 (min) | D75 (min) | Δ75 (min) |
|---|---|---|---|---|---|---|---|---|---|
| PS533 (wild type) | 12.5 | 20 | 8 | 18 | 27 | 9 | 26 | 36 | 10 |
| FB62 (gerD) | 115 | 137 | 22 | 180 | 205 | 25 | NDb | ND | ND |
| PS3411 (↑SpoVA) | 1 | 6 | 5 | 4 | 13 | 9 | 8 | 20 | 12 |
| PS3640 (spoVA1) | 26 | 34 | 8 | 56 | 65 | 9 | 107 | 119 | 12 |
| PS3642 (spoVA2) | 21 | 29 | 8 | 40 | 50 | 11 | 69 | 83 | 14 |
Spores at 30°C germinated in response to 10 mM l-valine, and the times to reach various levels of commitment and CaDPA release and Δ values at these levels of commitment (C values) and CaDPA release (D values) were determined from the data in Fig. 5A and B as described in Materials and Methods.
ND, not determined, as the gerD spores did not reach 75% commitment and CaDPA release in the measurement period.
Germination of spores with variations in GerD and SpoVA proteins in response to dodecylamine.
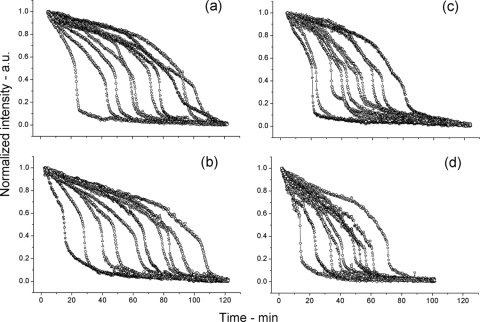
In addition to nutrients such as l-alanine, spore germination can also be triggered by nonnutrients such as dodecylamine (9, 11, 12). However, germination in response to dodecylamine does not proceed via activation of GRs, and GerD is not involved in spore germination with dodecylamine. Rather, dodecylamine triggers CaDPA release either by directly damaging the spore's inner membrane leading to CaDPA release or by triggering the opening of channels used normally for CaDPA release during spore germination. Analysis of the germination of multiple individual wild-type and gerD spores and spores with alterations in SpoVA proteins in response to dodecylamine indicated that unlike germination in response to l-alanine, the kinetics of germination for spore populations of all five strains in response to dodecylamine were rather similar at 25°C (Fig. 6a to e). Thus, there was only a small amount of CaDPA release prior to Tlag as shown by the decrease in DIC intensity of only ∼10% at Tlag, the T1 values could be readily determined for spoVA1 and spoVA2 spores, the Irelease values were similar for spores of all 5 strains, and the amounts of CaDPA released between Tlag and T1 were similar for spores of the five strains (Table 3). In addition, the average kinetic parameters, in particular various time points in the germination of wild-type spores and spores with alterations in GerD or SpoVA proteins in response to dodecylamine were very similar at 25°C (Table 3). The patterns of changes in DIC image intensity were also very similar during germination of wild-type, gerD, and ↑SpoVA spores in response to dodecylamine at 45°C, and the ΔTrelease values for the spores from these three strains were all essentially identical, although the gerD and ↑SpoVA spores had shorter Tlag times than the wild-type spores (Table 3). However, a few of the spoVA1 and spoVA2 spores exhibited different kinetic patterns of DIC image intensity during germination in response to dodecylamine at 45°C, in particular more complex kinetics (Fig. 6i and j), and this difference resulted in a longer average ΔTrelease time for the spoVA1 and spoVA2 spores, with most of this difference in the Δ(T1 − Tlag) period (Table 3).
Fig. 6.
Germination of multiple individual spores of various B. subtilis strains in response to dodecylamine as measured by DIC microscopy. (a to j) Spores of various strains of B. subtilis at 25°C (a to e) or 45°C (f to j) germinated in response to dodecylamine, and each panel in this figure shows curves of the dynamics of the germination of 10 individual spores followed by DIC microscopy as described in Materials and Methods. The spores analyzed in the various panels were from strains PS533 (wild type) (a and f), FB62 (gerD) (b and g), PS3411 (↑SpoVA) (c and h), PS3640 (spoVA1) (d and i), and PS3642 (spoVA2) (e and j).
Table 3.
Parameters of the germination of spores of various B. subtilis strains at 25°C and 45°C in response to the addition of dodecylaminea
| Germination temp (°C) | Strain | Tlag (min) | T1 (min) | Trelease (min) | ΔTrelease (min) | Δ(T1 − Tlag) (min) | Δ(Trel − T1) (min) | Ilag (%) | I1 (%) | Irelease (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 25 | PS533 (wild type) | 48 ± 30.4 | 50.4 ± 30.9 | 51.3 ± 30.9 | 3.3 ± 1.6 | 2.4 ± 1.6 | 0.9 ± 0.2 | 90 ± 3 | 69 ± 1 | 18 ± 6 |
| FB62 (gerD) | 48.0 ± 32.0 | 50.9 ± 32.5 | 51.8 ± 32.6 | 3.8 ± 1.9 | 2.9 ± 1.8 | 0.9 ± 0.3 | 90 ± 3 | 69 ± 8 | 17 ± 4 | |
| PS3411 (↑SpoVA) | 43.6 ± 26.5 | 46.2 ± 27.1 | 47 ± 27.1 | 3.4 ± 1.0 | 2.5 ± 1.7 | 0.9 ± 0.2 | 89 ± 6 | 62 ± 11 | 17 ± 4 | |
| PS3640 (spoVA1) | 53.1 ± 31.7 | 56.1 ± 32.5 | 57 ± 32.6 | 3.9 ± 2.0 | 3.0 ± 1.9 | 0.9 ± 0.2 | 90 ± 4 | 65 ± 17 | 15 ± 4 | |
| PS3642 (spoVA2) | 49 ± 32.6 | 52.5 ± 33.4 | 53.7 ± 33.4 | 4.4 ± 3.1 | 3.5 ± 3.0 | 0.9 ± 0.3 | 89 ± 4 | 64 ± 14 | 18 ± 6 | |
| 45 | PS533 (wild type) | 16.3 ± 15.3 | 18.6 ± 15.59 | 17.8 ± 15.6 | 2.3 ± 1.3 | 1.5 ± 1.3 | 0.8 ± 0.2 | 90 ± 4 | 66 ± 13 | 16 ± 5 |
| FB62 (gerD) | 9.9 ± 7.0 | 11.4 ± 7.7 | 12.3 ± 7.7 | 2.4 ± 1.1 | 1.6 ± 1.0 | 0.9 ± 0.2 | 93 ± 4 | 66 ± 17 | 12 ± 3 | |
| PS3411 (↑SpoVA) | 7.26 ± 5.3 | 8.6 ± 6 | 9.4 ± 6.1 | 2.2 ± 1.2 | 1.4 ± 1.1 | 0.8 ± 0.2 | 92 ± 4 | 70 ± 13 | 19 ± 6 | |
| PS3640 (spoVA1) | 15.7 ± 15.0 | 19.3 ± 15.3 | 20.3 ± 15.3 | 4.6 ± 2.8 | 3.6 ± 2.7 | 0.9 ± 0.2 | 91 ± 4 | 54 ± 12 | 16 ± 5 | |
| PS3642 (spoVA2) | 14.7 ± 9.8 | 19.1 ± 12.1 | 20.2 ± 12.2 | 5.5 ± 4.5 | 4.4 ± 4.5 | 1.1 ± 0.4 | 90 ± 9 | 61 ± 19 | 16 ± 5 |
Spores of various B. subtilis strains germinated at 25°C or 45°C in response to dodecylamine. The parameters of the germination process were determined for 30 individual spores for each strain by DIC microscopy as described in Materials and Methods, and the values were averaged and standard deviations were calculated. The various kinetic parameters of spore germination are defined as follows. Tlag is the time at which rapid CaDPA release began. T1 is the time at which the rate of CaDPA release following Tlag shifted to an even faster rate. Trelease is the time at which CaDPA release was complete. ΔTrelease is Trelease − Tlag. Ilag is the normalized DIC intensity at Tlag. I1 is the normalized DIC intensity at T1. Irelease is the normalized DIC intensity at Trelease.
DISCUSSION
As seen in the current work and previously (2, 18, 19; Wang et al., submitted), during germination of individual wild-type B. subtilis spores in response to the addition of a nutrient, there was a minimal and generally slow decrease of ∼10% in the level of CaDPA prior to Tlag, and this was followed by two periods of much more rapid CaDPA release, with both of these events generally paralleled by comparable changes in the spore's DIC image intensity. Following full CaDPA release, there was usually a further decrease in the DIC image intensity caused by spore cortex hydrolysis and the attendant core water uptake and swelling. The kinetic pattern of these events in spores with elevated SpoVA protein levels was generally similar to the pattern in wild-type spores, although at 25°C and 45°C, the values for Tlag were significantly lower than for wild-type spores, as were values for ΔTrelease, at least at 25°C. This finding is consistent with SpoVA proteins being important components of the mechanism that determines the length of Tlag. The times between various extents of commitment and CaDPA release during germination in response to a nutrient were very similar for both ↑SpoVA and wild-type spores, suggesting that SpoVA proteins play little, if any, role in the period between commitment and germination, although they must play some role prior to commitment as noted above.
The kinetic pattern of events during germination of individual gerD spores in response to a nutrient was also similar to that for wild-type spores. However, Tlag values for gerD spores were much higher than for wild-type spores at both 25°C and 45°C. These data indicate that GerD probably plays no direct role in CaDPA release during spore germination but acts in some fashion only to decrease the Tlag period. The intervals between 25% and 50% commitment and CaDPA release were 2.5- to 4-fold higher for gerD spores than for wild-type or ↑SpoVA spores, respectively, suggesting that GerD also plays some role between commitment and CaDPA release. Surprisingly, the ΔTrelease times for gerD spores were also lower than for wild-type spores during l-alanine germination at 25°C, although not at 45°C. The reason for this difference is not clear, but at 25°C, <10% of the gerD spores germinated in the measurement period, and perhaps these spores had particularly low ΔTrelease times, while ∼75% of the gerD spores germinated at 45°C.
In contrast to the similar kinetic pattern of events during germination of individual wild-type, gerD, and ↑SpoVA spores in response to the addition of a nutrient, the spoVA1 and spoVA2 spores showed a rather different pattern. In particular, there was more rapid CaDPA release prior to Tlag with the spoVA1 and spoVA2 spores, and this resulted in much lower values for Ilag and CaDPA levels at Tlag. In addition, following Tlag, most remaining CaDPA appeared to be released faster than by wild-type spores, since the ΔTrelease times for spoVA1 and spoVA2 spores were significantly lower than those of wild-type spores. However, the latter similarity may only be apparent, since spoVA1 and spoVA2 spores released much less CaDPA during ΔTrelease than wild-type spores. The times between various extents of commitment and CaDPA release for the spoVA1 and spoVA2 spores were also almost identical to those for wild-type spores. These data indicate the following during germination in response to a nutrient. SpoVA proteins do play a role in determining Tlag values, as suggested above. SpoVA proteins also play a role in determining the actual rate at which CaDPA is released during germination in response to a nutrient, as suggested previously by analyses of the behavior of spoVA1 and spoVA2 spore populations (13, 16). Note, however, that while individual spoVA1 and spoVA2 spores germinating in response to nutrients clearly exhibited different kinetics of CaDPA release than wild-type spores, the spoVA1 spores did not exhibit any TS germination parameters. The reason for this difference from previous results (13, 16) is not known.
There are a number of other notable observations from analysis of the germination parameters of multiple individual spores of these five B. subtilis strains in response to a nutrient. First, approximately 70% of the CaDPA in spoVA1 and spoVA2 spores was released prior to Tlag, in contrast to the release of only ∼10% CaDPA in this period by wild-type, gerD, and ↑SpoVA spores. This larger amount of CaDPA release prior to Tlag by spoVA1 and spoVA2 spores was not simply because of the long Tlag times of these spores, since gerD spores also released only ∼10% of their CaDPA prior to Tlag yet had Tlag times as long as or longer than those of wild-type spores. It appears that for some reason, the initial CaDPA release by spoVA1 and spoVA2 spores is faster than that by wild-type, gerD, and ↑SpoVA spores and does not readily progress to more rapid CaDPA release as it does for wild-type, gerD, and ↑SpoVA spores. However, the reason that alterations in SpoVA proteins should cause these effects is not clear. Second, the amounts of CaDPA released prior to Tlag by wild-type and gerD spores were almost identical and quite similar to the amount released in this period by ↑SpoVA spores, even though the spores of these three strains have Tlag values that differ up to 9-fold. Thus, it seems likely that it is the amount of CaDPA released prior to Tlag that is linked somehow to the subsequent faster CaDPA release, rather than the precise rate of CaDPA release during Tlag. This further suggests that at least one defect in spoVA1 and spoVA2 spores is in response of the spores to the initial decrease in CaDPA levels, such that a ≥2-fold decrease in the CaDPA level is needed in Tlag before faster CaDPA release is triggered during the germination of these spores in response to a nutrient. Third, the values for Irelease were consistently lower during germination of spoVA1 and spoVA2 spores than the values for wild-type, gerD, or ↑SpoVA spores. Previous work has indicated that the great majority of the DIC image intensity remaining at Trelease in wild-type spores germinating in response to nutrients is likely because significant cortex hydrolysis does not begin until around Trelease (2, 16a, 18, 19). This suggests that significant cortex hydrolysis must take place prior to Trelease with most spoVA1 and spoVA2 spores, such that these spores have begun to take up water and swell well before full CaDPA release. Presumably, the large amount of CaDPA released prior to Tlag during germination of spoVA1 and spoVA2 spores in response to a nutrient has at least partially activated one of the spores' cortex-lytic enzymes (CLEs), CwlJ or SleB. This also appears to be the case with a few spores of the other three strains. Perhaps analysis of the uptake of fluorescent nucleic acid stains during germination of individual spoVA1 and spoVA2 spores might test the validity of this suggestion, since such uptake appears to require cortex hydrolysis (1).
In contrast to the differences in both the general kinetic patterns and parameters of germination of spores of the five strains in response to a nutrient studied in this work, there were only minimal differences seen when germination of these spores in response to dodecylamine was monitored. The only notable differences were slightly lower Tlag values for gerD and ↑SpoVA spores at 45°C and slightly larger Trelease and Δ(T1 − Tlag) values for spoVA1 and spoVA2 spores at 45°C. Perhaps these larger Trelease and Δ(T1 − Tlag) values for spoVA1 and spoVA2 spores and the numbers of these spores that exhibit complex changes in the kinetics of change in DIC image intensity during germination in response to dodecylamine at 45°C are a reflection of some TS behavior of these spores. Since germination in response to dodecylamine does not involve the spore's GRs (7), this suggests that it is only in germination via the GRs that the SpoVA proteins altered in spoVA1 and spoVA2 spores have major effects on the kinetics of CaDPA release. Unfortunately, the precise mechanism whereby dodecylamine triggers spore germination is not known, although recent work suggests that dodecylamine may trigger spore germination by causing the opening of CaDPA channels in the spore's inner membrane, perhaps by activating SpoVA proteins (17). Why alterations in SpoVA proteins would thus not affect CaDPA release kinetics during germination in response to dodecylamine is not clear. Another difference between germination in response to dodecylamine and germination in response to a nutrient was that Irelease values were notably lower for germination in response to dodecylamine. One possible explanation for this result is that there is significant cortex hydrolysis of the spore cortex prior to Trelease during germination. However, it is also possible that this is a direct effect of the dodecylamine itself, which will kill germinated spores and cause release of all small molecules and perhaps even proteins if the dodecylamine is not neutralized following its triggering of spore germination (8, 9).
While the many observations made in this work represent new information about the events taking place in spore germination, we still cannot explain some of these observations, since there is so much we do not know about events in spore germination such as commitment and CaDPA release or the specific roles that germination proteins play in these events. However, the results obtained in this work do allow a number of new conclusions, in particular about the germination of individual spores. (i) Both the GerD and SpoVA proteins play a major role in the timing of Tlag during germination in response to a nutrient. (ii) Significant CaDPA release takes place before the commitment of spores to germinate, but not because the CaDPA released has triggered commitment by activating cortex hydrolysis, as the intervals between T0 and commitment are the same in wild-type and cwlJ sleB spores (17). (iii) Alterations in SpoVA proteins can drastically alter the rate of CaDPA release during germination in response to a nutrient. (iv) There are clearly multiple phases of CaDPA release during germination in response to a nutrient. (v) Neither GerD nor SpoVA proteins play a major role in determining the kinetic parameters of spores germinating in response to dodecylamine. (vi) The GerD protein appears to be important in some event or events that take place between commitment and rapid CaDPA release. These conclusions and other observations reported in this communication will likely serve as the basis for much further work on the mechanism of the various events during germination of spores of Bacillus species.
One interesting possibility is also suggested by the findings presented in this work, which is that the various phases of CaDPA release in spore germination have some signaling function that coordinates other germination events. Thus, CaDPA release from an individual spore is known to activate the CLE CwlJ in this spore, and as a consequence of this regulation, the ΔTrelease times are ∼10-fold longer in cwlJ spores germinating in response to nutrients than in wild-type spores, and the intervals between commitment and CaDPA release are up to 5-fold longer (1, 17). In contrast, ΔTrelease values with sleB spores are identical to those in wild-type spores. Thus, it is possible that the increased rate of CaDPA release between Tlag and T1 is what triggers CwlJ action, leading to even more rapid CaDPA release between T1 and Trelease. Perhaps comparison of the kinetics of CaDPA release during nutrient-triggered germination of wild-type, cwlJ, sleB, and cwlJ sleB spores at 25°C to slow CaDPA release between Tlag and T1 would help decide whether this scenario is correct.
In some ways, the most interesting observation made in this work is the low to moderate rates of CaDPA release beginning at T0 and continuing until Tlag observed for spores of all five strains used in this work, the low rates for wild-type, gerD, and ↑SpoVA spores, and the higher rates for spoVA1 and spoVA2 spores. What does this initial CaDPA release mean mechanistically, how is this triggered by the addition of germinant, and at Tlag how and why do these rates transition to the more rapid rates leading to complete CaDPA release? Is the transition at Tlag due to activation of CaDPA channels because of the release in the Tlag period of some measured amount of CaDPA or does the CaDPA release in the Tlag period cause some change in the spore's inner membrane or cortex that now allows faster CaDPA release? We have no answers to these questions at this time. However, the answers will likely be crucial in order to have a detailed understanding of the signal transduction pathways that operate during spore germination.
ACKNOWLEDGMENTS
This work was supported by a Multidisciplinary University Research Initiative (MURI) award from the U.S. Department of Defense (P.S. and Y.-Q.L.).
Footnotes
Published ahead of print on 11 March 2011.
REFERENCES
- 1. Kong L., Zhang P., Setlow P., Li Y.-Q. 2010. Monitoring the kinetics of uptake of a nucleic acid dye during the germination of single spores of Bacillus species. Anal. Chem. 82:8717–8724 [DOI] [PubMed] [Google Scholar]
- 2. Kong L., et al. Phase contrast microscopy, fluorescence microscopy, Raman spectroscopy and optical tweezers to characterize the germination of individual bacterial spores. Nat. Protoc., in press [DOI] [PubMed] [Google Scholar]
- 3. Nicholson W. L., Setlow P. 1990. Sporulation, germination and outgrowth, p. 391–450 In Harwood C. R., Cutting S. M. (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom [Google Scholar]
- 4. Paidhungat M., Setlow B., Driks A., Setlow P. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182:5505–5512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paidhungat M., Setlow P. 2002. Spore germination and outgrowth, p. 537–548 In Sonenshein A. L., Hoch J. A., Losick R. (ed.), Bacillus subtilis and its relatives: from genes to cells. American Society for Microbiology, Washington, DC [Google Scholar]
- 6. Paredes-Sabja D., Setlow P., Sarker M. R. 2011. Germination of spores of Bacillales and Clostridiales species: mechanisms and proteins involved. Trends Microbiol. 19:85–94 [DOI] [PubMed] [Google Scholar]
- 7. Pelczar P. L., Igarashi T., Setlow B., Setlow P. 2007. Role of GerD in germination of Bacillus subtilis spores. J. Bacteriol. 189:1090–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rode L. J., Foster J. W. 1961. Germination of bacterial spores with alkyl primary amines. J. Bacteriol. 81:768–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Setlow B., Cowan A. E., Setlow P. 2003. Germination of spores of Bacillus subtilis with dodecylamine. J. Appl. Microbiol. 95:637–648 [DOI] [PubMed] [Google Scholar]
- 10. Setlow B., Setlow P. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 178:3486–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Setlow P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550–556 [DOI] [PubMed] [Google Scholar]
- 12. Setlow P., Johnson E. A. 2007. Spores and their significance, p. 35–67 In Doyle M. P., Beuchat L. R., Montville T. J. (ed.), Food microbiology: fundamentals and frontiers, 3rd ed. ASM Press, Washington, DC [Google Scholar]
- 13. Vepachedu V. R., Setlow P. 2004. Analysis of the germination of spores of Bacillus subtilis with temperature sensitive spo mutations in the spoVA operon. FEMS Microbiol. Lett. 239:71–77 [DOI] [PubMed] [Google Scholar]
- 14. Vepachedu V. R., Setlow P. 2005. Localization of SpoVAD to the inner membrane of spores of Bacillus subtilis. J. Bacteriol. 187:5677–5682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vepachedu V. R., Setlow P. 2007. Analysis of interactions between nutrient germinant receptors and SpoVA proteins of Bacillus subtilis spores. FEMS Microbiol. Lett. 274:42–47 [DOI] [PubMed] [Google Scholar]
- 16. Vepachedu V. R., Setlow P. 2007. Role of SpoVA proteins in the release of dipicolinic acid during germination of Bacillus subtilis spores triggered by dodecylamine or lysozyme. J. Bacteriol. 189:1565–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a. Wang G., Zhang P., Setlow P., Li Y.-Q. Kinetics of germination of wet-heat-treated individual spores of Bacillus species, monitored by Raman spectroscopy and differential interference contrast microscopy. Appl. Environ. Microbiol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yi X., Setlow P. 2010. Studies of the commitment step in the germination of spores of Bacillus species. J. Bacteriol. 192:3424–3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang P., et al. 2010. Factors affecting the variability in the time between addition of nutrient germinants and rapid DPA release during germination of spores of Bacillus species. J. Bacteriol. 192:3608–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang P., Kong L., Wang G., Setlow P., Li Y.-Q. 2010. Combination of Raman tweezers and quantitative differential interference microscopy for measurement of dynamics and heterogeneity during the germination of individual bacterial spores. J. Biomed. Opt. 15:056010 [DOI] [PubMed] [Google Scholar]