Abstract
Purines can be used as the sole source of nitrogen by several strains of K. pneumoniae under aerobic conditions. The genes responsible for the assimilation of purine nitrogens are distributed in three separated clusters in the K. pneumoniae genome. Here, we characterize the cluster encompassing genes KPN_01787 to KPN_01791, which is involved in the conversion of allantoin into allantoate and in the deamination of guanine to xanthine. These genes are organized in three transcriptional units, hpxSAB, hpxC, and guaD. Gene hpxS encodes a regulatory protein of the GntR family that mediates regulation of this system by growth on allantoin. Proteins encoded by hpxB and guaD display allantoinase and guanine deaminase activity, respectively. In this cluster, hpxSAB is the most tightly regulated unit. This operon was activated by growth on allantoin as a nitrogen source; however, addition of allantoin to nitrogen excess cultures did not result in hpxSAB induction. Neither guaD nor hpxC was induced by allantoin. Expression of guaD is mainly regulated by nitrogen availability through the action of NtrC. Full induction of hpxSAB by allantoin requires both HpxS and NAC. HpxS may have a dual role, acting as a repressor in the absence of allantoin and as an activator in its presence. HpxS binds to tandem sites, S1 and S2, overlapping the −10 and −35 sequences of the hpxSAB promoter, respectively. The NAC binding site is located between S1 and S2 and partially overlaps S2. In the presence of allantoin, interplay between NAC and HpxS is proposed.
INTRODUCTION
Purines are nitrogen-rich compounds that are widespread in the biosphere. When ammonia, the preferred nitrogen source, is limiting, many microorganisms can obtain nitrogen from purines. The first step in the assimilation of adenine or guanine as a nitrogen source is a deamination reaction catalyzed by specific enzymes, yielding one molecule of ammonia and one molecule of hypoxanthine or xanthine, respectively. The catabolic pathway for hypoxanthine and xanthine assimilation occurs in two stages. In the first stage, both compounds are oxidized by the action of xanthine dehydrogenase to uric acid, which is then converted to allantoate via allantoin by two sequential ring-opening steps (Fig. 1A). In the second stage, allantoate is transformed to CO2 plus ammonia. Although the first part of this pathway is common to all species studied so far, the degradation of allantoate can follow different routes depending on the microbial species (8, 28, 42, 43, 44).
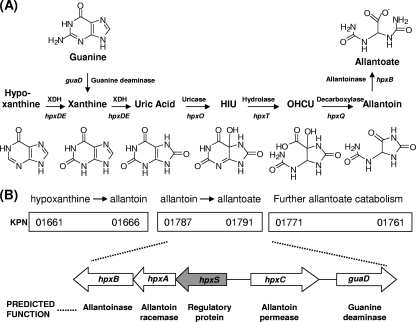
Fig. 1.
(A) Metabolic map for guanine and hypoxanthine assimilation to allantoate. For each enzymatic step, the associated gene in K. pneumoniae is indicated. (B) Gene organization of the three clusters involved in the assimilation of purine nitrogens in K. pneumoniae as described by Pope et al. (28). A more detailed scheme of the gene organization of the cluster studied in this work is presented below. The arrows show the extents and directions of transcription of the genes. The predicted function of the hpx-encoded proteins is indicated below each gene. XDH, xanthine dehydrogenase; HIU, 5-hydroxyisourate; OHCU, 2-oxo-4-hydroxy-4-carboxy-5-ureidoimidazoline.
Among enterobacteria, purine catabolism has been characterized for Escherichia coli and some species of Klebsiella. In E. coli, nitrogen assimilation from adenine is incomplete, since allantoin is not further metabolized in the presence of oxygen (43). Nitrogen assimilation from allantoin involves allantoate amidohydrolase and ureidoglycolate dehydrogenase, enzymes encoded by genes of the all regulon, whose expression takes place only under anaerobic conditions in E. coli (8, 32). In contrast, in Klebsiella, the purine catabolic pathway is complete and proceeds past allantoin since all nitrogens are assimilated under aerobic conditions (10, 28).
In Klebsiella pneumoniae strain KC2653, the hpx genetic system responsible for the oxidation of hypoxanthine to allantoin has been characterized by de la Riva et al. (10). This cluster of seven genes is organized in four transcriptional units, hpxDE, hpxR, hpxO, and hpxPQT. Gene hpxP encodes hypoxanthine permease; genes hpxDE encode the enzyme involved in the oxidation of hypoxanthine to uric acid, which is a two-component oxygenase structurally related to the class of IB dioxygenases described by Batie et al. (3) involved in aromatic ring hydroxylations; gene hpxO encodes an FAD-dependent monooxygenase involved in the oxidation of uric acid to 5′-hydroxyisourate (HIU) that has been characterized by O'Leary et al. (27); and gene hpxT encodes the HIU hydrolase involved in the transformation of HIU to 2-oxo-4-hydroxy-4-carboxy-5-ureidoimidazoline (OHCU). The latter compound undergoes stereoselective decarboxylation to produce CO2 and S-allantoin by the action of OHCU decarboxylase, encoded by gene hpxQ. Expression of this system is activated by nitrogen limitation and by the presence of specific substrates, with hpxDE and hpxPQT controlled by both signals. Induction of hpxPQT requires uric acid formation, whereas expression of hpxDE is induced by the presence of hypoxanthine through the HpxR regulatory protein, encoded by the hpxR gene (10).
The metabolism of purines has also been studied by Pope et al. (28) for Klebsiella oxytoca M5a1. In this strain, a cluster of 23 genes responsible for the utilization of purines as the sole nitrogen source has been identified. The function of some genes of this cluster was assigned by growth, complementation tests, and sequence similarity. Comparison with the K. pneumoniae MGH78578 genome revealed that these genes are organized in three separated clusters in K. pneumoniae. Genes KPN_01787 to KPN_01790 have been proposed to be involved in the metabolism of allantoin to allantoate, and gene KPN_01791 has been proposed to be involved in guanine deamination (Fig. 1B).
In the presence of ammonia, there is a strong repression of many systems that allow enterobacterial species such as E. coli or K. pneumoniae to use alternative nitrogen sources such as amino acids or purines. When ammonia is limiting, the ability of cells to obtain nitrogen from these compounds usually requires a two-component system in which phosphorylated NtrC binds to an enhancer and interacts with RNA polymerase bearing the sigma factor σ54. The Ntr (nitrogen-regulated) system activates a set of genes involved in the catabolism of nitrogenous compounds whose degradation products include ammonia or glutamate (30). The Ntr system also activates the transcription of the nitrogen assimilation control protein (NAC) that regulates a subset of genes that are dependent on RNA polymerase bearing σ70 for their transcription (11, 18, 24, 38). Thus, NAC couples the σ54-dependent transcription of the Ntr system with that of the σ70-dependent catabolic genes. Recently, more than 90 new NAC target genes have been identified by chromatin immunoprecipitation experiments with K. pneumoniae. The KPN_01789/01790 intergenic region was among the promoter sequences that were coimmunoprecipitated with NAC (13).
In this study, we characterize the KPN_01787/01791 gene cluster of K. pneumoniae KC2563, which contains the genes responsible for allantoin-to-allantoate metabolism and gene guaD, involved in the deamination of guanine to xanthine. Regulation analysis of the different transcriptional units by nitrogen limitation or by the HpxS-specific regulator is approached. In addition, evidence for the catalytic activity of GuaD as guanine deaminase and HpxB as allantoinase is provided.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The genotypes and sources of the bacterial strains, plasmids, and promoter fusions are given in Table 1. All K. pneumoniae strains are derived from strain W70 (25). Genetic crosses were performed by P1-mediated transduction (15).
Table 1.
Strains and plasmids used in this study
| Strain, fusion, or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| K. pneumoniae strains | ||
| KC2653 | hutC515 Δ[bla]-2 dadA1 str-6 | 22 |
| KC5249 | hutC515 Δ[bla]-2 nac-2 | R. A. Bender |
| KC2738 | hutC515 ntrC::Tn5-131 | 6 |
| KB17K | KC2653 hpxS::kan | This study |
| E. coli strains | ||
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10(Tcr)] | Stratagene |
| S17(λ pir) | Tpr SmrrecA thi pro hsdR hsdM+ RP4::2-Tc::Mu::Km Tn7 λ | Biomedal |
| EB6193 | RP4-2 tet Mu-1 Kan::Tn7 integrant; leu-63::IS10 recA1 creC510 hsdR17 endA1 zbf-5 uidA(ΔMuI)::pir+thi Spr Smr | R. A. Bender |
| DH5αF′ | φ80dlacZΔM15 recA1 endA1 λ−gyrA96 thi-1 hsdR17 (rK− mK+) phoA supE44 relA1 deoR Δ(lacZYA-argF)U169 | Gibco BRL |
| EB4335 | DH5α/pCB1083; pCB1026 | 33 |
| Fusionsa | ||
| Φ(hpxS-lacZ) | hpxS (positions −263 to +92) fused to lacZ | This study |
| Φ(hpxC-lacZ) | hpxC (positions −189 to +170) fused to lacZ | This study |
| Φ(guaD-lacZ) | guaD (positions −156 to +83) fused to lacZ | This study |
| Plasmids | ||
| pGEMT | Apr; cloning vector for PCR products | Promega |
| pKAS32 | Apr; pGP704; rpsL | 40 |
| pRS415 | Apr; promoterless lacZYA reporter for operon fusions | 39 |
| pCB1583 | Apr Kmr; promoterless lacZ reporter for integration of operon fusions into host genome with oriR6K replication origin; rpsL | R. A. Bender |
| pMAL-c2x | Apr; vector for cytoplasmic expression of maltose binding protein fusions | New England Biolabs |
| pKD4 | Apr Kmr | 9 |
| pCB1083 | lacIq cloned into pACYC184 | 33 |
| pCB1026 | Wild-type nac cloned in pQE70 | 33 |
Nucleotide sequences are given in the 5′-to-3′ direction for the coding strand of each gene and are numbered relative to the gene transcription initiation nucleotide at position +1.
Growth conditions and preparation of cell extracts.
Cultures were grown at 30°C with aeration in Luria broth (LB) (7) or in W4 minimal medium (41) supplemented with glucose at 0.4% as the sole carbon source. NaCl was removed from LB plaques for selection of directed mutants. For limiting-nitrogen conditions, freshly made glutamine (Gln) was used at 0.04%. Ammonium sulfate and Gln (NGln), both at 0.2%, were used for nitrogen excess (4, 5). Hypoxanthine and uric acid were prepared as described by Rouf and Lomprey (34). Allantoin, allantoate, guanosine, and adenosine were used at 0.05%. The selection medium in the conjugation experiments consisted of W4 minimal medium supplemented with sodium citrate at 0.4% as the carbon source and ammonium sulfate at 0.2% as the nitrogen source, supplemented with the appropriate antibiotics. When required, the following antibiotics were used at the indicated concentrations: ampicillin (Ap), 100 μg/ml; kanamycin (Km), 50 μg/ml; streptomycin (Sm), 50 μg/ml; and tetracycline (Tet), 30 μg/ml. 5-Bromo-4chloro-3-indolyl β-d-galactoside (X-Gal), and isopropyl-β-d-thiogalactoside (IPTG) were used at 30 and 10 μg/ml, respectively.
Cell extracts were obtained by sonic disruption of bacterial cells collected by centrifugation at the end of the exponential phase and resuspended in the appropriate buffer.
Enzyme activities.
For β-galactosidase activity, cultures were grown to an optical density at 600 nm (OD600) of 0.5. Cells were collected by centrifugation, washed in 1% KCl, and suspended at a concentration that involved 1 to 1.5 mg of protein per ml (2). β-Galactosidase activity was assayed in detergent-treated whole cells, using o-nitrophenyl-β-d-galactopyranoside (ONPG) as the substrate, and was expressed in U/mg of cell protein (26). One unit of β-galactosidase activity corresponds to the amount of enzyme that hydrolyzes 1 nmol of ONPG per min. The data reported are the averages from at least four separate experiments performed in triplicate.
Allantoinase was assayed as described previously (8). The assay was based on the colorimetric method described by Lee and Roush (21) applied to the reaction of the allantoate product with phenylhydrazine-HCl and potassium ferricyanide.
Guanine deaminase was determined as described elsewhere (35). Assays were performed with a final volume of 1 ml containing 20 mM potassium phosphate buffer (pH 7.4), 60 μM guanine, and 0.01 U commercial xanthine oxidase (Sigma-Aldrich, Germany). Uric acid produced in the reaction was monitored by its specific absorbance at 293 nm (ε = 12 mM/cm). For kinetic parameter determination, guanine concentrations ranging from 5 to 250 μM were used.
Protein concentration was determined by the method of Lowry et al. (23), with bovine serum as a standard.
DNA manipulation and site-directed mutagenesis.
Bacterial genomic DNA was obtained using a Wizard genomic DNA purification kit (Promega), and plasmid DNA was prepared using the Wizard Plus SV Midipreps DNA purification system (Promega). DNA manipulations were performed essentially as described by Sambrook and Russell (36). DNA fragments were amplified by PCR, using chromosomal DNA as a template. When necessary, specific restriction sites were incorporated at the 5′ ends of the primers to facilitate the cloning of the fragments in the appropriate vector. PCRs were performed with Pfu DNA polymerase under standard conditions. DNA was sequenced using an automated ABI 377 DNA sequencer and fluorescent-dye termination methods.
Site-directed mutagenesis of HpxS or NAC binding sites was performed by PCR using primers containing the desired mutations. All primers used are listed in Table 2.
Table 2.
Oligonucleotides used in this study
| Primer | Sequencea | Experiment |
|---|---|---|
| Psc(1)_EcoRI.fw | CGGAATTCTGGTAAATGCTTT | Construction of Φ(hpxC-lacZ) |
| Psc(1)_Sma1.rv | TCCCCCGGGTGTGCACGTCCGAC | |
| Psc(2)_EcoRI.fw | CGGAATTCTGTGCACGTCC | Construction of Φ(hpxSAB-lacZ) |
| Psc(2)_Sma1.rv | TCCCCCGGGTGGTAAATGCTTTC | |
| PguaD_EcoRI.fw | CGGAATTCCCACCTACCGC | Construction of Φ(guaD-lacZ) |
| PguaD_BamHI.rv | CGCGGATCCACGCCGGCGATA | |
| HpxS_BamHI.rv | CGGGATCCTCACGACTCCTT | Cloning of hpxS in pMAL-c2x |
| HpxS_EcoRI.fw | CGGAATTCATGAATAATGAACATCGTCTCCAGG | |
| PmalregguaD1.fw | CGCGAATTCATGATGGATTACCAGACCGC | Cloning of guaD in pMAL-c2x |
| pmalguaD.rv | AATTCTAGATCAATCCTGACACCACACCCGCTC | |
| pmalregpuuE1.fw | CGCGAATTCATGGGAGAGAACCAGGAACAC | Cloning of hpxB in pMAL-c2x |
| pmalpuuE.rv | AATTCTAGACTACCCGCGATACGGATGGGTTTCG | |
| hpxC_sp1 | GAACCTGCCAGCTGGCCAGC | Mapping of the 5′ end of the hpxC transcript |
| hpxC_sp3 | TGGTCGCGGGTCGGCGCCAG | |
| hpxS_sp1 | TGACCAGTTGCACAGCGGCG | Mapping of the 5′ end of the hpxSAB transcript |
| hpxS_sp2 | CGGCTCACGGCGAACACCTC | |
| hpxS_sp3 | CGATAGCCGTCATCAGCGCC | |
| guaD_sp1 | GATCGACATAGCCCTTCAGG | Mapping of the 5′ end of the guaD transcript |
| guaD_sp2 | AGCAGGGCGATAATTTTCCC | |
| guaD_sp3 | CCTCATCCGGGGTCTCCGCC | |
| HpxSmut2 | GCCGAATTCGACCATCGTCGCCATATGCATTGC | Directed mutagenesis of the hpxS gene |
| HpxSmut1_EcoRI | AAACCGCCGGACCAGCGAATTCCGG | |
| KanXho.fw | GACATACTCGAGGTGTAGGCTGGAGCTGCTTC | Directed mutagenesis of the hpxS gene |
| KanXho.rv | AGTATCCTCGAGCATATGAATATCCTCCTTAG | |
| Psc(2)_Sma1.rv | TCCCCCGGGTGGTAAATGCTTTC | Probe P1 |
| Psc(2)_del1.rv | TCCTCTGAATGAAAACCCTTTG | |
| Psc(2)_Sma1.rv | TCCCCCGGGTGGTAAATGCTTTC | Probe P2 |
| Psc(2)_del2.rv | GAATGATGAATCTTGTATAC | |
| Psc(2)_Sma1.rv | TCCCCCGGGTGGTAAATGCTTTC | Probe P4 |
| Psc(2)_del3.rv | GATCTGCTATCGATAAGTG | |
| Psc(1)_Sma1.rv | TCCCCCGGGTGTGCACGTCCGAC | Probe P6 |
| Psc(1)_del1.fw | GGTTTCACTTATCGATAGCAG | |
| Psc(1)_Sma1.rv | TCCCCCGGGTGTGCACGTCCGAC | Probe P7 |
| Psc(1)_del2.fw | GTATACAAGATTCATCATTC | |
| Psc(1)_Sma1.rv | TCCCCCGGGTGTGCACGTCCGAC | Probe P8 |
| Psc(1)_del3.fw | GCCTCAGCAATCCATATCAAAGG | |
| Psc(1)_EcoR1.fw | CGGAATTCTGGTAAATGCTTT | Probe P3 |
| ShpxS1.rv | TTAAATTTTATGTATACGATCTGCTATCG | |
| Psc(1)_EcoR1.fw | CGGAATTCTGGTAAATGCTTT | Probe P3* (mutated S1) |
| ShpxSmut1.rv | TTAAATTTTATTCATGGGATCTGCTATCGATAAGTG | |
| ShpxS2.fw | ATTTAATTTTTGTATACAAGATTCATCATTCTGGC | Probe P5 |
| Psc(2)_del1.rv | TCCTCTGAATGAAAACCCTTTG | |
| ShpxSmut2.fw | ATTTAATTTTTCCATGAAAGATTCATCATTCTGGC | Probe P5* (mutated S2) |
| Psc(2)_del1.rv | TCCTCTGAATGAAAACCCTTTG | |
| NACboxmut.fw | CGATAGCAGATCGTATACATAAAGGCATAATTTTTGGCAACAAGA | Probe m-NAC site |
| NACboxmut.rv | CCAGGCCAGAATGATGAATCTTGTTGCCAAAAATTATGCTTTATG |
Restriction sites incorporated at the 5′ end are in bold. Nucleotides changed to obtain mutated probes are underlined.
Mapping of the 5′ end of the hpxC, hpxSAB, and guaD transcripts.
The 5′ regions of the hpxC, hpxSAB, and guaD transcripts were determined by rapid amplification of cDNA 5′ ends (5′-RACE) (36) using a commercial 5′-RACE kit (Roche Diagnostics, GmbH). Total RNA was isolated from KC2653 cells grown aerobically to an OD600 between 0.5 and 1 in glucose minimal medium with glutamine at 0.04% as a nitrogen source (nitrogen-limiting conditions) using a Qiagen RNeasy total RNA kit and then treated with RNase-free DNase (Ambion). For mapping the 5′ end of hpxSAB, RNA was also obtained from cells grown in glucose minimal medium with allantoin as the sole nitrogen source. The cDNAs were transcribed from RNA with specific hpxC, hpxSAB, or guaD antisense oligonucleotides. A homopolymeric (dA) tail was added (via terminal transferase) to the 3′ termini of the corresponding cDNAs. Amplification of reverse transcription products was performed with nested gene-specific primers and an oligo(dT) anchor primer. The double-stranded cDNAs obtained were cloned into pGEMT vector for sequencing.
Directed mutagenesis of the K. pneumoniae hpxS gene.
An hpxS knockout mutant of K. pneumoniae KC2653 was generated by antibiotic marker exchange using the suicide plasmid pKAS32. This vector contains the R6K origin of replication, which functions only in bacteria that produce the replication protein π. In addition, this vector expresses the E. coli rpsL gene, encoding ribosomal protein S12, which provides a positive selection for bacteria that have exchanged cloned plasmid sequences with the corresponding chromosomal sequences.
To clone the hpxS gene, primers were designed to amplify the corresponding open reading frames plus their flanking regions by PCR. A Km cassette was obtained by inserting the Km resistance gene kan, which was obtained by PCR amplification from plasmid pKD4 (9), into the corresponding coding region. In the Km cassette, the kan gene was flanked by K. pneumoniae-specific genomic sequences of at least 500 bp. Mutagenesis of the chromosomal gene was carried out by homologous recombination between the Km cassette and the wild-type gene after E. coli S17.1 (λ pir) harboring the recombinant pKAS32 derivative containing the Km cassette was mated with K. pneumoniae KC2653 as the recipient.
To construct the hpxS knockout mutant, the Km cassette was obtained as follows. A 1,787-bp fragment encompassing hpxS and its flanking regions was amplified by PCR from genomic DNA of strain KC2653. Restriction sites for EcoRV or EcoRI were incorporated at the 5′ ends of the primers to facilitate directed cloning of the amplified fragment into pKAS32. Disruption of the cloned hpxS gene was performed by insertion of the kan gene into the XhoI site located 291 bp downstream of the ATG codon.
The obtained pKAS32 recombinant plasmid was propagated into strain EB6193 and introduced into E. coli S17.1(λ pir) for mating with the recipient Smr strain KC2653 as described previously. Transconjugants were selected for resistance to Km on citrate plates and further purified in this medium. Isolated colonies were then grown on LB plates without NaCl containing Km and Sm (1 mg/ml) to facilitate homologous recombination. After several rounds of growth at 30°C in this medium, colonies were screened for sensitivity to Ap in order to identify which transconjugants had undergone allelic exchange and therefore did not carry the plasmid integrated (merodiploids of the target gene). Gene disruption of the correct target gene was verified by PCR and subsequent sequencing. In this way, strain KB17K was selected for further studies.
Construction of lacZ transcriptional fusions.
Transcriptional fusions were constructed by inserting the promoter fragments into plasmid pRS415 (39). This plasmid carries a cryptic lacZ operon and confers resistance to ampicillin. To construct the hpxSAB-lacZ fusion, a 360-bp fragment encompassing the hpxSAB-hpxC intergenic region was amplified by PCR, cloned into plasmid pRS415 using the EcoRI-BamHI sites. The same promoter fragment was cloned in the opposite direction to construct the hpxC-lacZ fusion. For all constructs, plasmid DNA was sequenced to ensure that that fragment was inserted in the correct orientation and that no mutations had been introduced during the amplification reaction.
To transfer the lacZ fusions into K. pneumoniae chromosome as a single copy, the recombinant plasmid was first digested with EcoRI and SacI, and the fragment containing the promoter fusion was subcloned into plasmid pCB1583 (22). This is a λ pir-dependent plasmid whose lacZ gene is flanked by genes of the K. pneumoniae d-ribose operon, thus allowing the integration of the cloned fusion by homologous recombination into the d-ribose operon of the K. pneumoniae recipient strain. The recombinant plasmids containing Φ(hpxSAB-lacZ) or Φ(hpxC-lacZ) were selected after transformation of strain EB6193 as blue colonies on LB–X-Gal–Km plates and then introduced into E. coli S17.1(λ pir) by electroporation. After several rounds of selection in different growth media, stable recombinants in the rbs locus were isolated as those displaying a Kms Smr d-ribose-negative phenotype. To transfer the fusions in the K. pneumoniae chromosome, conjugation was performed with the double-rifampin-streptomycin-resistant derivative of K. pneumoniae strain KC2653. After several rounds of selection in different growth media, stable recombinants were isolated as those displaying a Kms Smr d-ribose negative phenotype.
Expression and purification of recombinant proteins.
GuaD, HpxB, and HpxS were purified using the malE gene fusion system. For this purpose, the corresponding genes (guaD, hpxB, and hpxS) were amplified by PCR and cloned into the plasmid pMal-c2x. The restriction sites used for hpxS cloning were BamHI and EcoRI, whereas those used for guaD and hpxB cloning were EcoRI and XbaI. In all cases, the forward primer was designed to fuse the ATG start codon of each gene in-frame with malE. Overproduction of MalE-fused proteins was achieved in strain XL1-Blue carrying the recombinant plasmid. The induction conditions were 0.5 mM IPTG for 16 h at 30°C for MalE-HpxS and 0.3 mM IPTG for 3 h at 37°C for MalE-GuaD and MalE-HpxB. The fusion proteins were then purified by affinity chromatography with amylose resin (New England BioLabs) in accordance with the manufacturer's instructions. For protein purification, the cell pellet from 100 ml culture of strain XL1-Blue bearing the recombinant plasmid was suspended in 2 ml of column buffer (20 mM Tris-HCl, pH 7.4, containing 0.2 M NaCl and 1 mM EDTA) and sonicated on ice. The cell lysate was centrifuged at 15,000 × g, and the supernatant was loaded onto the amylose resin column. After the mixture was loaded onto the column, the resin was washed with column buffer. Elution was performed with column buffer containing 10 mM maltose, and the fusion protein was digested with factor Xa by incubation at room temperature for 12 h. The cleaved HpxS protein was used in gel shift experiments. However, MalE-HpxB and MalE-GuaD folding made difficult the cleavage of the fusion protein in solution (digestion was less than 50%). Recombinant MalE-HpxB and MalE-GuaD were used in enzyme activity assays since no changes in activity were observed for these fused proteins with respect to HpxB or GuaD obtained after cleavage of the recombinant MalE-fused proteins inside the amylose column.
His-tagged NAC was purified using nickel affinity resin as described elsewhere (33). One-liter cultures (LB supplemented with 100 μg/ml of ampicillin and 10 μg/ml of tetracycline) of strain EB4335 harboring plasmids pCB1083 and pCB1026 were grown to an OD600 of 0.6. At this point, IPTG was added to give a concentration of 1 mM to induce the production of the protein. After 4 h of incubation, the cell pellet was suspended in 5 ml of column buffer (100 mM sodium phosphate [pH 7.0], 250 mM NaCl, 2.5 mM MgCl2, 10% glycerol, 10 mM imidazole, and 1 mM 2-mercaptoethanol) and sonicated on ice. The cell lysate was centrifuged at 15,000 × g, and the supernatant was incubated with 1 ml of nickel resin with agitation for 1 h at 4°C. This mixture was poured to a column, and the resin was first washed with 20 to 30 ml of wash buffer (100 mM sodium phosphate [pH 7.0], 500 mM NaCl, 2.5 mM MgCl2, 10% glycerol, 90 mM imidazole, and 1 mM 2-mercaptoethanol). Elution was performed with column buffer containing 250 mM imidazole. Fractions were pooled and dialyzed overnight to remove imidazole.
Purified proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) performed according to the standard procedure (19) and were either used immediately or stored at −20°C in glycerol to 20%.
DNA binding studies.
The nonradioactive digoxigenin (DIG) gel shift kit for 3′-end labeling of DNA fragments (Roche Applied Science, Indianapolis, IN) was used for protein-DNA binding assays. The fragments obtained by PCR were labeled with terminal transferase and digoxigenin-ddUTP according to the manufacturer's instructions (Roche).
Labeled DNA fragments were incubated either with purified HpxS or NAC in 10 mM Tris-HCl (pH 7.4), 100 mM KCl, 10 mM MgCl2, 10% glycerol, and 2 mM dithiothreitol in a total volume of 20 μl. Poly(dI-dC) was used as a nonspecific competitor. When indicated, a 10-fold excess of noncompetitive or competitive nonlabeled DNA was added to the reaction mixtures. HpxS binding mixtures were incubated for 20 min at 37°C, whereas NAC binding mixtures were incubated at room temperature for 20 min (14) and then loaded onto a prerun gel of 5% native polyacrylamide, containing 10% glycerol in 1× TBE (Tris-borate-EDTA buffer). Blotting was performed using a Bio-Rad electroblotting system (model Trans blot) according to the manufacturer's instructions. Chemiluminescence detection of DIG-labeled DNA-protein complexes on the nylon membranes was detected using Hyperfilm ECL (Amersham Pharmacia).
RESULTS AND DISCUSSION
Transcriptional organization of the hpxBASC-guaD gene cluster.
By similarity to K. oxytoca, the gene cluster encompassing genes KPN_01787 to KPN_01791 in K. pneumoniae has been proposed to encode allantoinase (hpxB), allantoin racemase (hpxA), a regulatory protein of the GntR familiy (hpxS), allantoin permease (hpxC), and guanine deaminase (guaD) (Fig. 1) (28). However, no experimental evidence for the transcriptional regulation of these genes is available.
An in silico analysis of the intergenic region between hpxSAB and hpxC and the guaD 5′-flanking region using the Footprint and Promscan programs (http://www.promscan.uklinux.nrt/home.html) identified a putative σ70 promoter at the 5′ end of hpxSAB and putative σ54 promoters at the 5′ ends of the hpxC and guaD genes. In addition, putative sites for NtrC were also visualized (Fig. 2), suggesting that the expression of these genes may be regulated by nitrogen availability.
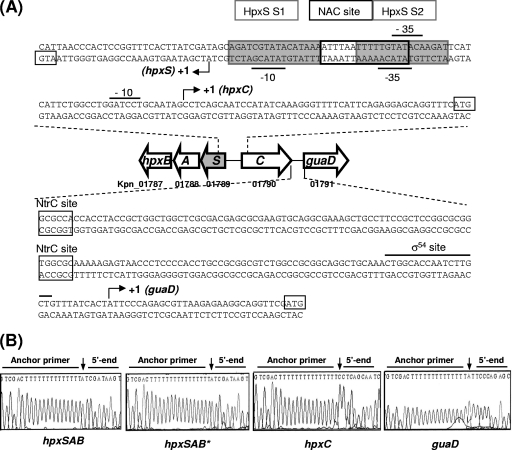
Fig. 2.
(A) Promoter sequences of the hpxBASC-guaD gene cluster. For each gene, the ATG initiation codon is boxed, the consensus sequence for RNA polymerase (the −10 and −35 sequences for σ70 recognition in hpxSAB and hpxC or the σ54 recognition sequence in guaD) is indicated, and the transcriptional start site is shown by a black arrowhead labeled +1. Putative NtrC binding sites identified using the Promscan and Virtual Footprint programs are indicated. Proposed binding sites for NAC and HpxS (S1 and S2) are also indicated (sites were proposed according to EMSA results and similarity to the consensus sequence of each regulatory protein). (B) Identification of the 5′ ends of the indicated genes by sequencing across ligation sites of 5′-RACE products. Chromatograms display the sequences at ligation sites of typical cloned 5′-RACE products derived from transcripts obtained from strain KC2653 cells grown in low-ammonia medium (GGln). For hpxSAB, this analysis was also performed with cells grown in the presence of allantoin (hpxSAB*). Arrows indicate the transcription initiation site.
The 5′ ends of the hpxSAB, hpxC, and guaD transcripts were experimentally determined by the 5′-RACE method (Fig. 2B). Total RNA was obtained from aerobic cultures of strain KC2653 on glucose-glutamine. For hpxSAB, the transcriptional start site was identified 26 nucleotides upstream of the ATG codon of the hpxS gene. The same 5′ end was identified in cells grown in the presence of allantoin. Inspection of the sequences upstream of nucleotide +1 revealed the presence of the −10 (TATACG) and −35 (TATACA) sequences, similar to the σ70 consensus sequence separated by 16 bp (positions matching the consensus are underlined) (Fig. 2A). For guaD, a sequence matching the consensus sequence reported for σ54 RNA polymerase was identified upstream of the corresponding nucleotide at position +1 (Fig. 2A). However, for hpxC, the putative σ54 recognition sequence identified in silico (CTGGCCTGGATCCTGCAA) was located between positions −20 and −3 with respect to the experimentally determined nucleotide at position +1. Therefore, this sequence does not fit the optimal location for σ54 promoters, and it is unlikely to be functional. Inspection of this region revealed the presence of the −10 (GATCCT) and −35 (TATACA) sequences, similar to the σ70 consensus sequences, which are separated by an unusually long stretch of 20 bp (Fig. 2A). Although this spacer does not fit the optimal 17 ± 1-bp length, spacers from 15 to 21 bp have been described (17). For σ70-dependent E. coli promoters, the spacing of promoter elements can regulate the basal expression of the corresponding gene (16).
Thus, experimental identification of the transcriptional start sites confirmed the organization of this cluster in three transcriptional units. As expected, transcription of guaD depends on the σ54 RNA polymerase subunit whereas transcription of hpxSAB depends on σ70. Regarding hpxC, our results suggest that its transcription may be driven by σ70 RNA polymerase.
The protein encoded by guaD displays guanine deaminase activity.
Sequence analysis of K. pneumoniae GuaD using a BLAST search showed that this protein has 86% identity with the proposed guanine deaminase of K. oxytoca and 58% identity with E. coli GuaD. The sequence includes the motif PGFVDAHVH between positions 73 and 81, which matches the consensus sequence implicated in metal binding (Zn2+) in prokaryotic proteins of the cyclic amidohydrolase family (12). This motif is also present in K. oxytoca GuaD (28).
To confirm GuaD function, the corresponding gene of strain KC2653 was cloned in plasmid pMal-c2X, and the protein was purified as described in Materials and Methods. The activity of the purified enzyme was determined using guanine or adenine as a substrate. The results showed only activity toward guanine. The kinetic parameters for guanine were determined under standard reaction conditions (pH 7.4; 37°C) from the double-reciprocal Lineweaver-Burk plot. This analysis yielded a Km value of 8.9 μM and a Vmax value of 1.13 μmol min−1 mg−1.
The protein encoded by hpxB displays allantoinase activity.
The protein encoded by gene hpxB (KPN_ 01787) in K. pneumoniae displayed 89% identity with HpxB of K. oxytoca (28) and 58% identity with the metal-independent allantoinase encoded by puuE in Pseudomonas fluorescens (29). In K. oxytoca, the ortholog hpxB gene was proposed to encode allantoinase since hpxB insertion mutants failed to grow with allantoin as the sole nitrogen source but displayed normal growth with allantoate (28).
To confirm HpxB function in K. pneumoniae, the gene hpxB of strain KC2653 was cloned in plasmid pMal-c2X, and the protein was purified. The activity of the purified enzyme was determined using allantoin as a substrate, as described in Materials and Methods. The specific enzyme activity was 390 U/mg. Purified HpxB displayed allantoinase activity in the absence of any bivalent metal.
HpxB does not display similarity to metal-dependent allantoinases belonging to the amidohydrolase superfamily (20). Instead, this protein displays high similarity to PuuE, a novel allantoinase of Pseudomonas fluorescens that is annotated in structure and sequence databases as polysaccharide deacetylase on the basis of its similarity to enzymes that remove N-linked or O-linked acetyl groups from cell wall polysaccharides (29). As for HpxB, other enzymes of the purine catabolic pathway encoded in K. pneumoniae, such as xanthine oxidase (hpxDE) and uricase (hpxO), do not display similarity to other reported enzymes known to catalyze these reactions (10). The lack of similarity to proteins with equivalent activity in other bacteria seems to be a general feature for enzymes of the purine assimilation pathway in K. pneumoniae. This finding, together with the distribution of these genes in separate clusters, suggests that the purine assimilation pathway in K. pneumoniae may have an evolutionary origin different from that of other enterobacteriaceae.
Since allantoinases such as PuuE display stereospecificity for the S enantiomer of allantoin and the product generated by OHCU decarboxylases is S-allantoin, the presence of a gene encoding a putative racemase (hpxA) in the hpxSAB operon seems unnecessary. In fact, Pope et al. (28) showed that in K. oxytoca the hpxA function is dispensable for growth on racemic allantoin. HpxA may be important for efficient scavenging of external allantoin present at low concentration in the environment.
Effect of the nitrogen source on the expression of the hpxSAB, hpxC, and guaD.
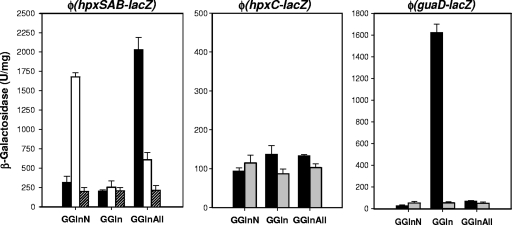
To study whether the expression of the gene cluster encompassing hpxSAB, hpxC, and guaD is regulated by nitrogen availability, the expression of the promoter fusions Φ(hpxSAB-lacZ), Φ(hpxC-lacZ), and Φ(guaD-lacZ) in the genetic background of strain KC2653 grown with different nitrogen sources was analyzed (Fig. 3, black bars). β-Galactosidase activity in glucose cultures with nitrogen excess (GNGln), nitrogen limitation (GGln), or nitrogen limitation in the presence of hypoxanthine (GGlnHx), guanosine (GGlnGns), or allantoin (GGlnAll) was measured.
Fig. 3.
Expression analysis of transcriptional fusions of the hpxBASC-guaD gene cluster in different genomic backgrounds and under different growth conditions. Cells of the parental strain KC2653 (black bars) and the derived hpxS (white bars), nac (dashed bars), or ntrC (gray bars) mutant strain bearing the promoter fusion Φ(hpxSAB-lacZ), Φ(hpxC-lacZ), or Φ(guaD-lacZ) were grown in the indicated culture media. GNGln is a nitrogen excess medium that contains 0.4% glucose and 0.2% each ammonium sulfate and Gln, GGln is a nitrogen-limiting medium that contains 0.4% glucose and 0.04% Gln, and GGlnAll is the nitrogen-limiting medium containing 0.05% allantoin. β-Galactosidase activity is expressed in units/mg. Error bars indicate standard deviations.
The expression of Φ(guaD-lacZ) was repressed by nitrogen excess and induced approximately 100-fold under nitrogen-limiting conditions (GGln) (Fig. 3). Addition of hypoxanthine or guanosine to nitrogen-limiting cultures did not result in an increase of β-galactosidase activity with respect to the level for cultures grown in low-glutamine-concentration medium (GGln) (1,625 U/mg), indicating that guaD is mainly regulated by nitrogen limitation (not shown). Under these conditions, deamination of guanine would provide ammonia for growth. When allantoin was used as the nitrogen source or added to nitrogen-limiting cultures, repression of Φ(guaD-lacZ) was observed at levels close to those displayed by cells grown in high-ammonia medium (GNGln). In Bacillus subtilis, even though addition of allantoin to nitrogen-limiting cultures induces most of the genes of the purine catabolic pathway, the expression of those encoding the subunits of xanthine dehydrogenase is repressed (37). This repression may be important for reducing purine utilization as the nitrogen source when allantoin is present in the medium.
Regarding Φ(hpxC-lacZ), analysis of β-galactosidase activity showed that hpxC, which encodes the putative allantoin permease, is not regulated by nitrogen limitation. In this case, activity levels under limiting-nitrogen conditions (GGln) were scarcely 1.5-fold higher than those displayed by cells grown in high-ammonia medium (GNGln). Addition of allantoin to limiting-nitrogen cultures did not significantly modify β-galactosidase levels (Fig. 3).
Analysis of Φ(hpxSAB-lacZ) showed a different pattern of expression. This operon was slightly repressed under nitrogen-limiting conditions (GGln). Maximal induction was achieved by growth in GGln medium plus allantoin or with allantoin as the sole nitrogen source (Fig. 3). These results suggest additional control of Φ(hpxSAB-lacZ) by allantoin or derived metabolites. However, neither guanosine nor hypoxanthine, whose catabolism yields allantoin, nor allantoate, the next intermediate metabolite in the allantoin catabolic pathway, induced Φ(hpxSAB-lacZ) expression (β-galactosidase values were around 200 U/mg). The basal level of expression in guanosine or hypoxanthine medium may be sufficient to provide allantoinase activity to metabolize endogenous allantoin generated from uric acid oxidation and, hence, support growth on these compounds as the nitrogen source. However, in this case intracellular levels of allantoin or derived metabolites may not be high enough to trigger hpxSAB induction. We hypothesize that when allantoin is used as a nitrogen source, a higher intracellular level is achieved. In this case, allantoin itself or a derived metabolite not yet identified may act as the inducer molecule for hpxSAB expression.
We next examined whether this specific regulation is influenced by nitrogen availability. To this end, allantoin was added to high-ammonia medium (GNGlnAll). In these nitrogen excess conditions, allantoin did not induce hpxSAB expression (not shown). Thus, taken together, these results indicate that both signals, allantoin (or an allantoin-derived metabolite) and limiting nitrogen, are required for the transcriptional activation of Φ(hpxSAB-lacZ).
Expression of hpxSAB, hpxC, and guaD in ntrC and nac mutants.
To further examine whether the regulation of hpxSAB, hpxC, and guaD transcriptional units is mediated by components of the Ntr system, the nac (strain KC5249) and ntrC (strain KC2738) mutations were introduced by P1 transduction into the genetic background of strain KC2653, bearing Φ(hpxSAB-lacZ), Φ(hpxC-lacZ), or Φ(guaD-lacZ). The expression of these transcriptional fusions in the derived mutants in cultures grown under different nitrogen conditions (GNGln, GGln, GGlnAll) was analyzed.
As expected, expression of Φ(guaD-lacZ) under nitrogen-limiting conditions was strongly reduced in the ntrC mutant (Fig. 3, gray bars). In this mutant, β-galactosidase levels were close to those obtained under nitrogen excess conditions. In contrast, no significant changes in the expression pattern of Φ(guaD-lacZ) were observed in the nac mutant background (not shown). These results are consistent with the presence of conserved NtrC binding sites upstream from the σ54 sequences in the guaD promoter (Fig. 2) and strongly support the involvement of NtrC in the regulation of this gene.
Expression of Φ(hpxC-lacZ) was not modified in the genomic background of the ntrC (Fig. 3) or nac (not shown) mutants. This is in accordance with the lack of regulation by nitrogen availability and reinforces the idea that transcription of this gene may be driven by σ70-RNA polymerase.
Induction by allantoin of Φ(hpxSAB-lacZ) was abolished both in the ntrC (not shown) and the nac (Fig. 3, dashed bars) mutants. No significant differences in β-galactosidase levels were observed in cultures of these mutants performed under high- or limiting-nitrogen conditions (Fig. 3). Since hpxSAB is transcribed by a σ70-dependent RNA polymerase, the NtrC effect must be indirect, through the action of NAC. In fact, a recent report on chromatin immunoprecipitation analysis revealed the 5′ upstream region of hpxSAB as a putative target of NAC (13). These results strongly suggest that NAC is one of the regulators required for full induction of Φ(hpxSAB-lacZ) by allantoin.
Role of HpxS in the regulation of the hpxBASC-guaD gene cluster.
As described for K. oxytoca (28), the hpxS-encoded protein is a member of the large GntR family of transcriptional regulators which is formed by four subfamilies. In silico analysis indicated that HpxS belongs to the FadR subfamily. The regulators belonging to this subfamily share a similar amino-terminal helix-turn-helix domain, which recognizes a consensus sequence, 5′-NNNTNGTANTACNANNN-3′ (conserved nucleotides defining the consensus are in bold). In contrast, these regulators display high heterogeneity in the C-terminal effector and oligomerization domain (31).
To evaluate the role of HpxS as a transcriptional regulator of this gene cluster, a knockout mutant in which the gene encoding HpxS was disrupted by a Km cassette (strain KB17K) was generated. Phenotypic analysis of this mutant revealed that HpxS deficiency did not abolish allantoin utilization as a nitrogen source. To confirm its role as a regulator, the expression of Φ(hpxSAB-lacZ), Φ(hpxC-lacZ), and Φ(guaD-lacZ) in the hpxS mutant grown under different nitrogen conditions was analyzed (Fig. 3, white bars). Induction of Φ(hpxSAB-lacZ) by allantoin was reduced 3-fold in this mutant, suggesting that the function of HpxS in the presence of allantoin is the transcriptional activation of the hpxSAB operon. In a high-ammonia concentration (GNGln), β-galactosidase values were 4-fold higher than that of the parental strain (Fig. 3), which suggests that HpxS could act as a repressor in the absence of allantoin, whereas in a low-ammonia concentration (GGln), no increase in β-galactosidase activity was observed (Fig. 3). These results suggest that, in the absence of HpxS, NAC can act as a repressor of hpxSAB transcription. The intermediate levels of β-galactosidase observed in the hpxS mutant grown in low-ammonia medium plus allantoin (GGlnAll) may be attributed to parallel intermediate NAC intracellular levels under this condition.
Regarding Φ(hpxC-lacZ) and Φ(guaD-lacZ), the results showed no significant changes in their expression pattern in the genetic background of the hpxS mutant with respect to the levels for the parental strain KC2653 (not shown), indicating that HpxS is not involved in the transcriptional control of hpxC and guaD.
This set of results indicates that in the hpxBASC-guaD gene cluster, only the hpxSAB operon is regulated by HpxS. This protein may have a dual role, acting as a repressor in the absence of allantoin and as an activator in its presence. However, full activation of hpxSAB by allantoin also requires the participation of NAC.
Analysis of binding of HpxS to promoter regions of the hpxBASC-guaD gene cluster.
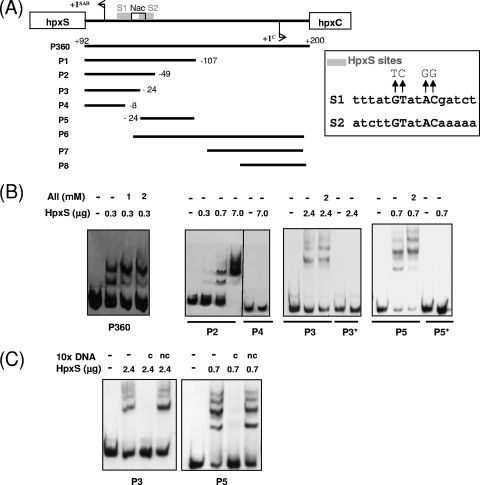
Binding of HpxS to the intergenic region of hpxSAB-hpxC was studied by electrophoretic mobility shift assays (EMSA). The interactions with the complete hpxSAB-hpxC intergenic region were assessed using HpxS protein, purified as described in Materials and Methods, and probe P360 (Fig. 4B, left panel). The results showed binding of HpxS to this probe. This is consistent with the proposed role for HpxS in the regulation of hpxSAB. Binding experiments were also performed in the presence of allantoin and other metabolic intermediates of the purine degradation pathway, such as guanine or uric acid (upstream pathway metabolites) and allantoate, ureidoglycolate, or oxamate (downstream pathway metabolites). None of these compounds abolished binding of HpxS to probe P360 (not shown). Only allantoin seemed to modify the pattern of complexes formed, diminishing the amount of the less retarded complex (Fig. 4B, left panel).
Fig. 4.
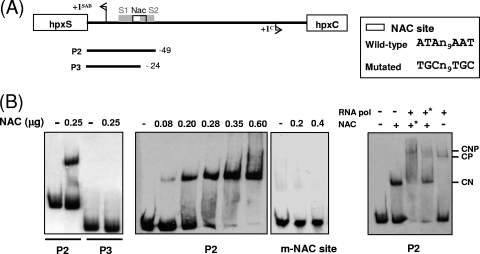
Binding of HpxS to promoter fragments of the hpxSAB operon. (A) Diagram of the hpxS-hpxC intergenic region showing the transcription start site for each gene. The promoter fragments used as probes and their end terminus positions with respect to position +1 of hpxS are shown below. Position +200 in P360 is given according to position +1 of hpxC. Mutations introduced by site-directed mutagenesis in the HpxS binding sites S1 and S2 (gray boxes) are shown above the corresponding wild-type sequences. The Nac binding site is indicated by a white box. (B) EMSA performed with recombinant HpxS and the indicated probes. The digoxigenin-labeled probes covering different parts of the hpxS-hpxC intergenic region were added to binding mixtures containing HpxS in the absence or presence of allantoin at the indicated concentrations. Probes P3* and P5* contained the indicated mutations in the HpxS S1 and S2 binding sites, respectively. All mixtures contained 500-fold molar excesses of poly(dI-dC). Reaction mixtures were incubated at 30°C for 15 min and directly subjected to PAGE. (C) EMSA of the HpxS-S1 and HpxS-S2 complexes in the presence of competing or noncompeting DNA. As competing DNA (c), a 10-fold excess of the same nonlabeled probe (P3 or P5) was used. In both cases, nonlabeled probe P4 was added to binding mixtures as noncompeting DNA (nc).
Regarding guaD, no retarded complexes were observed in EMSA performed with HpxS and a probe encompassing the 5′ upstream region of this gene (positions −156 to +83) (not shown). This is consistent with the results obtained in the expression analysis of guaD in the genetic background of an hpxS mutant and clearly rules out a direct role for HpxS in guaD control.
To locate the HpxS binding site more precisely in the hpxSAB-hpxC intergenic region, eight additional fragments, corresponding to deletions of this intergenic region, were tested as probes in subsequent electrophoretic mobility shift experiments (Fig. 4A and B). HpxS bound to P1, P2, P3, P5, and P6 but not to P4, P7, or P8. Binding of HpxS to the nonoverlapping fragments P3 and P5 (Fig. 4B) indicated the presence of two binding sites for this protein in the hpxS promoter region. An in silico analysis of this region allowed us to identify the palindromic sequences (5′-TTTATGTATACGATCT-3′ and 5′-ATCTTGTATACAAAAA-3′) almost matching the consensus sequence for the GntR regulators, although in both cases only two positions are present between the conserved GT and AC (consensus nucleotides are in bold, and other matching nucleotides are underlined) (Fig. 2). One binding site (site S1) was located between positions −4 and −19 with respect to the hpxS transcriptional start site and the other (site S2) between positions −26 and −41 (Fig. 2). Thus, these tandem S1 and S2 sites are orientated in the same helical phase. Specificity of binding of HpxS to these sites was assessed in control experiments performed with nonlabeled competing DNA (Fig. 4C). Notice that approximately 3-fold-larger amounts of HpxS were used in EMSA performed with probe P3 with respect to probe P5 (Fig. 4B and C), suggesting that this protein may display greater affinity for S2 than for S1. Addition of allantoin to binding mixtures did not modify the pattern of HpxS complexes with site S1 (probe P3) but significantly reduced the amount of the less retarded complex in favor of the more retarded one with site S2 (probe P5) (Fig. 4B). This suggests that allantoin may induce a conformational change in HpxS bound to S2 that modifies its oligomerization state, thus reinforcing the idea that allantoin can act as an effector molecule of HpxS function.
To confirm that HpxS binds to these sequences, site-directed mutagenesis of the two conserved nucleotides in each half-site of both palindromes was performed (Fig. 4A). Gel shift experiments showed that these mutations abolished HpxS binding (Fig. 4B).
Other regulators belonging to the FadR subfamily bind to more than one operator site in the target promoter and have been described to have a dual role. For instance, in E. coli, LldR acts as a repressor of lldPRD expression in the absence of l-lactate, but in the presence of l-lactate, LldR acts as an activator. In this model, the regulatory protein binds to two operator sequences in the lldPRD operon, leading to DNA looping and the repression of transcription. Binding of the inducer l-lactate to LldR promotes a conformational change that disrupts the DNA loop, allowing the formation of the transcription open complex (1). Concerning HpxS, the short distance between the two HpxS binding sites, S1 and S2, does not allow DNA looping. Since both HpxS sites are orientated in the same helical phase, side-by-side interaction of HpxS molecules bound to tandem S1 and S2 sites can mediate hpxSAB repression. The activator role of HpxS in the presence of allantoin may depend on conformational changes induced by this effector molecule. However, allantoin itself is not sufficient to relieve hpxSAB repression. The results of the transcriptional fusion analysis indicated that NAC is required for activation of hpxSAB transcription.
Analysis of binding of NAC to the hpxSAB-hpxC intergenic region.
Our results pointed to NAC as a regulator of hpxSAB expression. In addition, as stated above, the hpxSAB-hpxC intergenic region has recently been identified among the promoter sequences coimmunoprecipitated with NAC in K. pneumoniae (13).
Binding of NAC to the hpxSAB-hpxC intergenic region was analyzed by EMSA with purified NAC protein and probes encompassing different sequences of this region (Fig. 5). Purified NAC bound to P1 and P2 but not to P3, P4, and P5.
Fig. 5.
Binding of NAC to promoter fragments of the hpxSAB operon. (A) Diagram of the hpxS-hpxC intergenic region showing the transcription start site for each gene. The promoter fragments used as probes and their end terminus positions with respect to position +1 of hpxS are shown below. Mutations introduced by site-directed mutagenesis in the NAC binding site (white box) are shown above the corresponding wild-type sequences. (B) EMSA performed with recombinant NAC. The indicated digoxigenin-labeled probes were added to binding mixtures containing the indicated amounts of NAC. Probe m-NAC site corresponds to the mutated NAC binding site probe. Binding of NAC in the presence of RNA polymerase is shown in the right panel. In this experiment, NAC was added at 0.25 μg and RNA polymerase at 0.28 μg. Where both proteins were present, the asterisk indicates the first protein added to the binding mixtures. CN, retarded complex with NAC; CP, retarded complex with RNA polymerase; and CNP, retarded complex with both proteins. All mixtures contained 500-fold molar excesses of poly(dI-dC). Reaction mixtures were incubated at 20°C for 15 min and directly subjected to PAGE.
Regarding guaD, as expected for a σ54-dependent gene, no retarded complexes were observed in EMSA performed with NAC and a probe encompassing the 5′ upstream region of guaD (positions −156 to +83) (not shown).
To further characterize binding of NAC to the hpxSAB promoter, different amounts of NAC were used in gel shift experiments with probe P2 (Fig. 5B). Binding of NAC to this probe was observed at concentrations similar to those described for other promoters, which are in the range of 1 to 5 pmol (33). An in silico analysis of this region allowed us to identify a conserved NAC binding site (5′-ATAN9AAT-3′) between positions −20 and −34 with respect to the transcriptional start site of hpxSAB (Fig. 2). Indeed, site-directed mutagenesis of conserved residues in this palindrome (5′-TGCN9TGC-3′) abolished NAC binding (Fig. 5B).
Since this NAC site partially overlaps the −35 σ70 recognition sequence for hpxSAB transcription, EMSA were performed to analyze the effect of NAC on RNA polymerase binding. To this end, binding of E. coli K-12 RNA polymerase (Sigma-Aldrich, Germany) to probe P2 was analyzed in the absence or presence of NAC (Fig. 5B). Preincubation of probe P2 with NAC before the addition of RNA polymerase resulted in the formation of a new retarded complex (CNP) of higher molecular mass than the complex obtained with RNA polymerase alone (CP) or NAC alone (CN). These results indicated that both proteins bind to this probe simultaneously. In addition, preincubation with NAC also increased the intensity of the band corresponding to the CNP complex in comparison with the level for binding reactions in which RNA polymerase was added before NAC. These results indicated that in vitro NAC has a positive effect on binding of RNA polymerase to the hpxSAB promoter. However, transcriptional fusion analysis showed that in the absence of HpxS (hpxS mutant), NAC, expressed under limiting-nitrogen conditions (GGln), represses hpxSAB transcription (Fig. 3, white bars).
Taken together, these results suggest that the activator function of NAC in vivo depends on its interplay with HpxS in the presence of allantoin. In the hpxSAB promoter, the NAC binding site (centered at position −27) is located between S1 (centered at position −12) and S2 (centered at position −33) and partially overlaps S2 (Fig. 2). As NAC will bind opposite to HpxS, it is tempting to speculate that NAC may contact HpxS bound to S2, leading to the disruption of the interactions between the HpxS molecules bound to S1 and S2. Although NAC can help the recruitment of RNA polymerase to the hpxSAB promoter, transcriptional activation of this operon requires the presence of allantoin. This effector molecule binds to HpxS, probably to its C-terminal domain as described for other members of the GntR family, and would promote a conformational change that modifies HpxS function. In this context, it is conceivable that the conformational change induced in HpxS bound to S2 may modify NAC conformation and its interaction with RNA polymerase, leading to the formation of a transcription open complex and the activation of hpxSAB transcription. More work would be needed to define the interactions of NAC with HpxS.
Thus, the presence in the hpxSAB promoter of two HpxS binding sites (S1 and S2) and a NAC binding site located between S1 and S2 and partially overlapping S2 led us to propose that the activity of this promoter is modulated depending on the differential binding of HpxS and NAC to these cis-acting elements in response to different metabolic conditions (allantoin and nitrogen availability).
ACKNOWLEDGMENTS
This research was supported by grants BFU 2007-63090/BMC and BFU2010-22260-C02-01 from the Ministerio de Educación y Ciencia, Spain, to L.B. K.G. received a predoctoral fellowship from the Generalitat de Catalunya, Spain.
We thank Robert A. Bender for providing strain EB4335 and for critical reading of the manuscript.
Footnotes
Published ahead of print on 25 February 2011.
REFERENCES
- 1. Aguilera L., et al. 2008. Dual role of LldR in regulation of lldPRD operon, involved in L-lactate metabolism in Escherichia coli. J. Bacteriol. 190:2997–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baldauf S. L., Cardani M. A., Bender R. A. 1988. Regulation of the galactose-inducible lac operon and the histidine utilization operons in pts mutants of Klebsiella aerogenes. J. Bacteriol. 170:5588–5593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Batie C. J., Ballou D. P., Correll C. C. 1992. Phthalate dioxygenase reductase and related flavin-iron-sulfur containing electron transferases, p. 543–556 In Müller F. (ed.), Chemistry and biochemistry of flavoenzymes. CRC Press, Boca Raton, FL [Google Scholar]
- 4. Bender R. A., et al. 1977. Biochemical parameters of glutamine synthetase from Klebsiella aerogenes. J. Bacteriol. 129:1001–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bender R. A., Snyder P. M., Bueno R., Quinto M., Magasanik B. 1983. Nitrogen regulation system of Klebsiella aerogenes: the nac gene. J. Bacteriol. 156:444–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bender R. A., Friederich B. 1990. Regulation of assimilatory nitrate reductase formation in Klebsiella aerogenes W70. J. Bacteriol. 172:7256–7259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bertani G. 2004. Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J. Bacteriol. 186:595–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cusa E., Obradors N., Baldoma L., Badia J., Aguilar J. 1999. Genetic analysis of a chromosomal region containing genes required for assimilation of allantoin nitrogen and linked glyoxylate metabolism in Escherichia coli. J. Bacteriol. 181:7479–7748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Datsenko K. A., Wanner B. L. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de la Riva L., Badia J., Aguilar J., Baldoma L. 2008. The hpx genetic system for hypoxanthine assimilation as a nitrogen source in Klebsiella pneumoniae: gene organization and transcriptional regulation. J. Bacteriol. 190:7892–7903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feng J., Goss T. J., Bender R. A., Ninfa A. J. 1995. Repression of the Klebsiella aerogenes nac promoter. J. Bacteriol. 177:5535–5538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fernández J. R., Byrne B., Firestein B. L. 2009. Phylogenetic analysis and molecular evolution of guanine deaminases: from guanine to dendrites. J. Mol. Evol. 68:227–235 [DOI] [PubMed] [Google Scholar]
- 13. Frisch R. L., Bender R. A. 2010. Expanded role for the nitrogen assimilation control protein (NAC) in the response of Klebsiella pneumoniae to nitrogen stress. J. Bacteriol. 192:4812–4820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frisch R. L., Bender R. A. 2010. Properties of the NAC-binding site within the ureD promoter of Klebsiella pneumoniae. J. Bacteriol. 192:4821–4826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goldberg R. B., Bender R. A., Streicher S. L. 1974. Direct selection for P1-sensitive mutants of enteric bacteria. J. Bacteriol. 118:810–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hidalgo E., Demple B. 1997. Spacing of promoter elements regulates the basal expression of the soxS gene and converts SoxR from a transcriptional activator into a repressor. EMBO J. 16:1056–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huerta A. M., Francino M. P., Morett E., Collado-Vides J. 2006. Selection for unequal densities of σ70 promoter-like signals in different regions of large bacterial genomes. PLoS Genet. 2:1740–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Janes B. K., Bender R. A. 1998. Alanine catabolism in Klebsiella aerogenes: molecular characterization of the dadAB operon and its regulation by the nitrogen assimilation control protein. J. Bacteriol. 180:563–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laemmli U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriphage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 20. LaPointe G., Viau S., LeBlanc D., Robert N., Morin A. 1994. Cloning, sequencing, and expression in Escherichia coli of the D-hydantoinase gene from Pseudomonas putida and distribution of homologous genes in other microorganisms. Appl. Environ. Microbiol. 60:888–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee K. W., Roush A. H. 1964. Allantoinase assays and their application to yeast and soybean allantoinases. Arch. Biochem. Biophys. 108:460–467 [DOI] [PubMed] [Google Scholar]
- 22. Liu Q., Bender R. A. 2007. Complex regulation of urease formation from the two promoters of the ure operon of Klebsiella pneumoniae. J. Bacteriol. 189:7593–7599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. 1951. Protein measurement with the Folin Phenol reagent. J. Biol. Chem. 193:265–273 [PubMed] [Google Scholar]
- 24. Macaluso A., Best E. A., Bender R. A. 1990. Role of nac gene product in the nitrogen regulation of some NTR-regulated operons of Klebsiella aerogenes. J. Bacteriol. 172:7249–7255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. MacPhee D. G., Sutherland I. W., Wilkinson J. F. 1969. Transduction in Klebsiella. Nature 221:475–476 [DOI] [PubMed] [Google Scholar]
- 26. Miller J. H. 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 27. O'Leary S. E., Hicks K. A., Ealick S. E., Begley T. P. 2009. Biochemical characterization of the HpxO enzyme from Klebsiella pneumoniae, a novel FAD-dependent urate oxidase. Biochemistry 48:3033–3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pope S. C., Chen L. L., Stewart V. 2009. Purine utilization by Klebsiella oxytoca M5a1: genes for ring-oxidizing and -opening enzymes. J. Bacteriol. 191:1006–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramazzina I., et al. 2008. Logical identification of an allantoinase analog (puuE) recruited from polysaccharide deacetylases. J. Biol. Chem. 283:23295–23304 [DOI] [PubMed] [Google Scholar]
- 30. Reitzer L. 2003. Nitrogen assimilation and global regulation in Escherichia coli. Annu. Rev. Microbiol. 57:155–176 [DOI] [PubMed] [Google Scholar]
- 31. Rigali S., Derouaux A., Giannotta F., Dusart J. 2002. Subdivision of the helix-turn-helix GntR family of bacterial regulators in the FadR, HutC, MocR, and YtrA subfamilies. J. Biol. Chem. 277:12507–12515 [DOI] [PubMed] [Google Scholar]
- 32. Rintoul M. R., et al. 2002. Regulation of the Escherichia coli allantoin regulon: coordinated function of the repressor AllR and activator AllS. J. Mol. Biol. 324:599–610 [DOI] [PubMed] [Google Scholar]
- 33. Rosario C. J., Bender R. A. 2005. Importance of a tetramer formation by the nitrogen assimilation control protein for strong repression of glutamate dehydrogenase formation in Klebsiella pneumoniae. J. Bacteriol. 187:8291–8299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rouf M. A., Lomprey R. F. 1968. Degradation of uric acid by certain aerobic bacteria. J. Bacteriol. 96:617–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saint-Marc C., et al. 2009. Phenotypic consequences of purine nucleotide imbalance in Saccharomyces cerevisiae. Genetics 183:529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sambrook J., Russell D. W. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 37. Schultz A. C., Nygaard P., Saxild H. H. 2001. Functional analysis of 14 genes that constitute the purine catabolic pathway in Bacillus subtilis and evidence for a novel regulon controlled by the PucR transcription activator. J. Bacteriol. 183:3293–3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schwacha A., Bender R. A. 1993. The product of the Klebsiella aerogenes nac (nitrogen assimilation control) gene is sufficient for activation of the hut operons and repression of the gdh operon. J. Bacteriol. 175:2116–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Simons R. W., Houmanand F. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85–96 [DOI] [PubMed] [Google Scholar]
- 40. Skorupski K., Taylor R. K. 1996. Positive selection vectors for allelic exchange. Gene 169:47–52 [DOI] [PubMed] [Google Scholar]
- 41. Smith G. R., Yeheskel S. H., Magasanik B. 1971. Genetic and metabolic control of enzymes responsible for histidine degradation in Salmonella typhimurium. J. Biol. Chem. 246:3320–3329 [PubMed] [Google Scholar]
- 42. Vogels G. D., Van der Drift C. 1976. Degradation of purines and pyrimidines by microorganisms. Bacteriol. Rev. 40:403–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xi H., Schneider B. L., Reitzer L. 2000. Purine catabolism in Escherichia coli and function of xanthine dehydrogenase in purine salvage. J. Bacteriol. 182:5332–5341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yoo H. S., Genbauffe F. S., Cooper T. G. 1985. Identification of the ureidoglycolate hydrolase gene in the DAL gene cluster of Saccharomyces cerevisiae. Mol. Cell. Biol. 5:2279–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]