Abstract
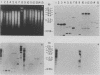
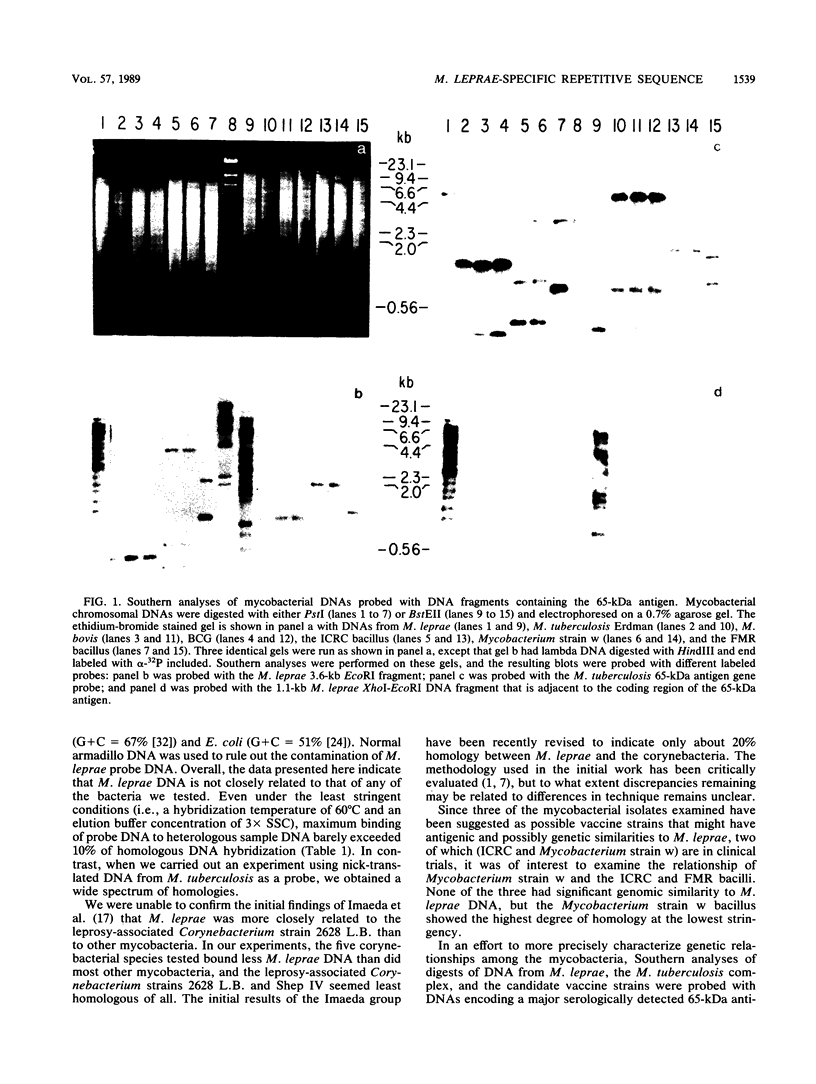
Comparative DNA hybridization studies of genomic DNA indicated that, while different isolates of armadillo-derived Mycobacterium leprae have a high degree of homology, binding of M. leprae genomic DNA to DNA of other species of mycobacteria or to corynebacteria was low, establishing that M. leprae is only remotely genetically related to any of the species examined. Several candidate leprosy vaccine mycobacterial strains were similarly found to have little genetic similarity to M. leprae. In contrast, the DNAs of the slow-growing mycobacteria M. tuberculosis, M. africanum, M. bovis, and M. microti were found to be very closely related. In the course of these studies, an M. leprae-specific repetitive sequence, greater than 15-fold per genome equivalent, was identified that might be useful for diagnostic and epidemiological studies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botstein D., White R. L., Skolnick M., Davis R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980 May;32(3):314–331. [PMC free article] [PubMed] [Google Scholar]
- Bradley S. G. Relationships among mycobacteria and nocardiae based upon deoxyribonucleic acid reassociation. J Bacteriol. 1973 Feb;113(2):645–651. doi: 10.1128/jb.113.2.645-651.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri S., Fotedar A., Talwar G. P. Lepromin conversion in repeatedly lepromin negative BL/LL patients after immunization with autoclaved Mycobacterium w. Int J Lepr Other Mycobact Dis. 1983 Jun;51(2):159–168. [PubMed] [Google Scholar]
- Clark-Curtiss J. E., Docherty M. A. A species-specific repetitive sequence in Mycobacterium leprae DNA. J Infect Dis. 1989 Jan;159(1):7–15. doi: 10.1093/infdis/159.1.7. [DOI] [PubMed] [Google Scholar]
- Clark-Curtiss J. E., Jacobs W. R., Docherty M. A., Ritchie L. R., Curtiss R., 3rd Molecular analysis of DNA and construction of genomic libraries of Mycobacterium leprae. J Bacteriol. 1985 Mar;161(3):1093–1102. doi: 10.1128/jb.161.3.1093-1102.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. E., Brownell G. H. Genophore homologies among compatible nocardiae. J Bacteriol. 1972 Feb;109(2):720–729. doi: 10.1128/jb.109.2.720-729.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia F. F., Inouye S., Inouye M. A 26-base-pair repetitive sequence specific for Neisseria gonorrhoeae and Neisseria meningitidis genomic DNA. J Bacteriol. 1986 Sep;167(3):1009–1015. doi: 10.1128/jb.167.3.1009-1015.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deo M. G., Bapat C. V., Chullawalla R. G., Bhatki W. S. Potential anti-leprosy vaccine from killed ICRC bacilli--a clinicopathological study. Indian J Med Res. 1981 Aug;74:164–177. [PubMed] [Google Scholar]
- Draper P. Cell walls of Mycobacterium leprae. Int J Lepr Other Mycobact Dis. 1976 Jan-Jun;44(1-2):95–98. [PubMed] [Google Scholar]
- Gilson E., Perrin D., Saurin W., Hofnung M. Species specificity of bacterial palindromic units. J Mol Evol. 1987;25(4):371–373. doi: 10.1007/BF02603122. [DOI] [PubMed] [Google Scholar]
- Groman N., Cianciotto N., Bjorn M., Rabin M. Detection and expression of DNA homologous to the tox gene in nontoxinogenic isolates of Corynebacterium diphtheriae. Infect Immun. 1983 Oct;42(1):48–56. doi: 10.1128/iai.42.1.48-56.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross W. M., Wayne L. G. Nucleic acid homology in the genus Mycobacterium. J Bacteriol. 1970 Nov;104(2):630–634. doi: 10.1128/jb.104.2.630-634.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Closs O., Bjorvatn B., Kronvall G., Axelsen N. H. Antibody response in rabbits to immunization with Mycobacterium leprae. Infect Immun. 1977 Dec;18(3):792–805. doi: 10.1128/iai.18.3.792-805.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Fujiwara T., Brennan P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J Biol Chem. 1982 Dec 25;257(24):15072–15078. [PubMed] [Google Scholar]
- Husson R. N., Young R. A. Genes for the major protein antigens of Mycobacterium tuberculosis: the etiologic agents of tuberculosis and leprosy share an immunodominant antigen. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1679–1683. doi: 10.1073/pnas.84.6.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaeda T., Kirchheimer W. F., Barksdale L. DNA isolated from Mycobacterium leprae: genome size, base ratio, and homology with other related bacteria as determined by optical DNA-DNA reassociation. J Bacteriol. 1982 Apr;150(1):414–417. doi: 10.1128/jb.150.1.414-417.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchheimer W. F., Storrs E. E. Attempts to establish the armadillo (Dasypus novemcinctus Linn.) as a model for the study of leprosy. I. Report of lepromatoid leprosy in an experimentally infected armadillo. Int J Lepr Other Mycobact Dis. 1971 Jul-Sep;39(3):693–702. [PubMed] [Google Scholar]
- McLafferty M. A., Harcus D. R., Hewlett E. L. Nucleotide sequence and characterization of a repetitive DNA element from the genome of Bordetella pertussis with characteristics of an insertion sequence. J Gen Microbiol. 1988 Aug;134(8):2297–2306. doi: 10.1099/00221287-134-8-2297. [DOI] [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Chen H. Y., Brinster R. L. Differential regulation of metallothionein-thymidine kinase fusion genes in transgenic mice and their offspring. Cell. 1982 Jun;29(2):701–710. doi: 10.1016/0092-8674(82)90186-6. [DOI] [PubMed] [Google Scholar]
- Portaels F. Unclassified mycobacterial strains susceptible to dapsone isolated from the environment in Central Africa. Int J Lepr Other Mycobact Dis. 1980 Sep;48(3):330–330. [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Schiller J., Strom M., Groman N., Coyle M. Relationship between pNG2, an Emr plasmid in Corynebacterium diphtheriae, and plasmids in aerobic skin coryneforms. Antimicrob Agents Chemother. 1983 Dec;24(6):892–901. doi: 10.1128/aac.24.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanford J. L., Rook G. A., Convit J., Godal T., Kronvall G., Rees R. J., Walsh G. P. Preliminary taxonomic studies on the leprosy bacillus. Br J Exp Pathol. 1975 Dec;56(6):579–585. [PMC free article] [PubMed] [Google Scholar]
- Storrs E. E. The nine-banded armadillo: a model for leprosy and other biomedical research. Int J Lepr Other Mycobact Dis. 1971 Jul-Sep;39(3):703–714. [PubMed] [Google Scholar]
- Wayne L. G., Gross W. M. Base composition of deoxyribonucleic acid isolated from mycobacteria. J Bacteriol. 1968 Dec;96(6):1915–1919. doi: 10.1128/jb.96.6.1915-1919.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G. On the relationship of members of the tuberculosis complex to other species of mycobacteria. Indian J Chest Dis Allied Sci. 1982 Apr-Sep;24(2-3):118–126. [PubMed] [Google Scholar]
- Wood N. B., Rake A. V., Shapiro L. Structure of Caulobacter deoxyribonucleic acid. J Bacteriol. 1976 Jun;126(3):1305–1315. doi: 10.1128/jb.126.3.1305-1315.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Mehra V., Sweetser D., Buchanan T., Clark-Curtiss J., Davis R. W., Bloom B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature. 1985 Aug 1;316(6027):450–452. doi: 10.1038/316450a0. [DOI] [PubMed] [Google Scholar]