Abstract
The bacterial cell envelope is of critical importance to the function and survival of the cell; it acts as a barrier against harmful toxins while allowing the flow of nutrients into the cell. It also serves as a point of physical contact between a bacterial cell and its host. Hence, the cell envelope of Rhizobium leguminosarum is critical to cell survival under both free-living and symbiotic conditions. Transposon mutagenesis of R. leguminosarum strain 3841 followed by a screen to isolate mutants with defective cell envelopes led to the identification of a novel conserved operon (RL3499-RL3502) consisting of a putative moxR-like AAA+ ATPase, a hypothetical protein with a domain of unknown function (designated domain of unknown function 58), and two hypothetical transmembrane proteins. Mutation of genes within this operon resulted in increased sensitivity to membrane-disruptive agents such as detergents, hydrophobic antibiotics, and alkaline pH. On minimal media, the mutants retain their rod shape but are roughly 3 times larger than the wild type. On media containing glycine or peptides such as yeast extract, the mutants form large, distorted spheres and are incapable of sustained growth under these culture conditions. Expression of the operon is maximal during the stationary phase of growth and is reduced in a chvG mutant, indicating a role for this sensor kinase in regulation of the operon. Our findings provide the first functional insight into these genes of unknown function, suggesting a possible role in cell envelope development in Rhizobium leguminosarum. Given the broad conservation of these genes among the Alphaproteobacteria, the results of this study may also provide insight into the physiological role of these genes in other Alphaproteobacteria, including the animal pathogen Brucella.
INTRODUCTION
Rhizobium leguminosarum is a Gram-negative, rod-shaped, soil-dwelling bacterium that forms a mutualistic association with leguminous plants (20). R. leguminosarum must be able to adapt to diverse environmental conditions, both in the soil and during its transition into a host plant. R. leguminosarum, and rhizobia in general, has a complex cell envelope consisting of the cytoplasmic membrane, the peptidoglycan cell wall, the outer membrane (with several lipopolysaccharide [LPS] modifications), and a number of excreted surface polysaccharides (21, 48). Numerous studies have investigated the importance of the cell envelope for survival of rhizobia both under free-living conditions and during symbiosis (11, 13, 24, 58, 61). For example, the outer membranes of Rhizobium spp. are critical for resisting the harmful effects of detergents, hydrophobic antibiotics, and cationic peptides (11, 24, 58). In addition, loss of both the O antigen and exopolysaccharides in Rhizobium etli biovar phaseoli CE3 causes increased sensitivity to plant-derived toxic compounds such as coumestrol, suggesting that the cell envelope plays an important role in protecting rhizobia from toxins encountered in the rhizosphere (13). Hyper- and hypo-osmotic stress tolerances have also been associated with cell envelope components. Periplasmic cyclic β-1,2-glucans and very-long-chain fatty acid (VLCFA)-modified lipid A are required for hypo-osmotic stress tolerance in R. leguminosarum and Sinorhizobium meliloti (11, 24, 58), while an intact outer membrane and exopolysaccharides are necessary for tolerance to hyperosmotic stress (61). In addition, several surface polysaccharides, LPS, and many membrane proteins have all been shown to play essential roles in the development of a successful symbiosis (7, 16, 25, 41, 48, 59).
Recently, rhizobial mutants harboring mutations in genes required for cell envelope development that are unable to grow on complex media have been identified (21, 58), such as the routinely used tryptone-yeast extract medium (TY), a peptide-rich medium composed of 5 g of tryptone, 3 g of yeast extract, and 0.5 g of calcium chloride per liter of H2O (6). Mutation of genes involved in synthesis of a very-long-chain fatty acid which is a component of the lipid A of LPS resulted in loss of growth on standard complex media in R. leguminosarum and S. meliloti (15, 58). Homologs of the ChvI/ChvG two-component signal transduction system which regulates several key components of the cell envelope in rhizobia, are required for growth of R. leguminosarum, S. meliloti, and Agrobacterium spp. on complex media (4, 17, 32). A mutation in a periplasmic protease gene, ctpA, which has been implicated in cell envelope development results in an inability of R. leguminosarum to grow on standard complex media, and the mutant forms large, spherical cells on solid TY after incubation for 24 h (22). All of these studies suggest a strong correlation between a fully functional cell envelope and growth of rhizobia on complex media.
To identify novel genes important for cell envelope structure and function in R. leguminosarum, we used Tn5 transposon mutagenesis and screened for mutants unable to grow on solid complex TY. The transposon mutagenesis screen led to the isolation of a mutant in which the Tn5 insertion had disrupted the third gene of an operon consisting of genes annotated as encoding a moxR-like AAA+ ATPase (RL3499), a conserved hypothetical protein (RL3500), and two large conserved transmembrane proteins (RL3501 and RL3502) (66). These genes are conserved among several alphaproteobacterial orders; however, to date they remain uncharacterized. Using a variety of mutagenesis approaches, including single-gene in-frame deletion mutants and insertional polar mutants, we have demonstrated that the genes in this operon are important for cell envelope integrity and cell morphology.
MATERIALS AND METHODS
Strains, media, and growth conditions.
Table 1 gives a list of strains and plasmids used in this study. Primer sequences are listed in Table S1 in the supplemental material. Escherichia coli strains were cultured using Luria-Bertani (LB) medium (45), which was supplemented as necessary with antibiotics at the following concentrations (μg ml−1): gentamicin, 15; ampicillin, 100; spectinomycin, 100; and tetracycline, 10. R. leguminosarum cells were cultured using tryptone-yeast extract medium (TY) (6) or Vincent's minimal medium with 10 mM mannitol (VMM) (60), supplemented as required with antibiotics at the following concentrations (μg ml−1): gentamicin, 30; neomycin, 100; tetracycline, 5; and streptomycin, 500.
Table 1.
Strains, plasmids, and primers used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| E. coli strains | ||
| TOP 10 | F−mcrA Δ(mrr hsdRMS mcrBC) φ80lacZΔM15 ΔlacX74recA1araΔ139 Δ(ara-leu)7697galUgalKrpsL (Smr) endA1nupG | Invitrogen |
| S17-1 | RP4 tra region, mobilizer strain recA derivative of MM294A with RP4-2 (Tc::Mu::Km::Tn7) integrated into the chromosome | 49 |
| DH5α | endA1 hsdR17 supE44 thi-1 recA1 gyrA96 relA1 Δ(argF-lacZYA) ΔlacU169 φ80lacZΔM15 | Invitrogen |
| R. leguminosarum strains | ||
| 3841 | Spontaneous streptomycin-resistant derivative of R. leguminosarum bv. viciae strain 300 | 28 |
| VF39SM | R. leguminosarum bv. viciae, spontaneous streptomycin-resistant mutant of VF39, Smr | 42 |
| DF20 | VF39, chvGΩKm mutant, unable to grow on complex media, Smr Nmr | 17 |
| SM1 | 3841, RL3501 RL3502 polar TGN Tn5 mutant, TYs Gmr Smr Nmr | This study |
| 38EV84 | 3841 RL3501 RL3502 polar mutant, Smr Gmr TYs | This study |
| 38EV99 | 3841, RL3499 nonpolar deletion mutant, TYs Smr | This study |
| 38EV00 | 3841, RL3500 nonpolar deletion mutant, TYs Smr | This study |
| 38EV01 | 3841, RL3501 nonpolar deletion mutant, TYs Smr | This study |
| 38EV02 | 3841, RL3502 mutant, TYs Smr Gmr | This study |
| 38EV87 | 3841, RL3499 insertional polar mutant, TYs Smr Nmr | This study |
| 38DF39 | 3841, ropBΩKm mutant, Smr Nmr | This study |
| 38EV03 | 3841, RL3499 nonpolar deletion mutant from background 38EV99, ropBΩKm mutant, Smr Nmr | This study |
| Plasmids | ||
| pCR2.1 Topo | TOPO TA cloning vector, 4.0 kb, Kmr Apr | Invitrogen |
| pFUS1par | Broad-host-range vector with promoterless gusA for transcriptional fusions, par stabilized, Tcr | 63 |
| pTGN | Tn5 derivative, Gmr Ampr promoter-less Nmr, gfp | 55 |
| pJQ200SK+ | Suicide vector,P15a ori mob sacB Gmr | 43 |
| pCRS530 | Vector containing CAS-GNm cassette, Apr Kmr | 44 |
| pDG71 | Broad-host-range derivative of pHC41, constitutively expressed tryptophan promoter and gfp(mut3), Tcr | 18 |
| pDF4 | pFus1par with ropB::gusA transcriptional fusion, Tcr | 17 |
| pSM4 | pFUS1par, Rl3499::gusA, Tcr, par stabilized | This study |
| pEV85 | Broad-host-range vector pDG71 containing functional copy of RL3499 downstream of tryptophan promoter, Tcr | This study |
| pEV88 | Broad-host-range vector pDG71 containing functional copy of RL3500 downstream of tryptophan promoter, Tcr | This study |
| pEV90 | Broad-host-range vector pDG71 containing functional copy of RL3502 downstream of tryptophan promoter, Tcr | This study |
| pEV91 | Broad-host-range vector pDG71 containing functional copy of ropB downstream of tryptophan promoter, Tcr | This study |
Transposon mutagenesis.
Mutagenesis was performed using a mini-Tn5 derivative carried on plasmid pTGN (55). Biparental matings of the E. coli mobilizer strain S17-1 containing the pTGN vector and R. leguminosarum 3841 were performed at 30°C for 24 h on TY plates. The mating mixture was plated on VMM with streptomycin and neomycin, and isolated colonies were subsequently screened for inability to grow on the solid complex medium TY (TY−). Genomic DNA was isolated from the TY− isolates, and the transposon insertion site was identified using the arbitrary PCR protocol as described by Miller-Williams et al. (37). Briefly, the specific primer GmTAIL-1 (SigmaGenosys Canada, Oakville, Ontario, Canada), which binds within the gentamicin cassette of the transposon, and the arbitrary degenerate primer DGEN1 were used in the primary reaction, which was followed by a secondary reaction with the primers GmTAIL-2 and DGEN2 and 1 μl of primary reaction product as the template.
Sequence analysis.
The specific site of transposon insertion was identified using a BLASTN search (1) and the Rhizobase database (Kazusa DNA Research Institute [http://bacteria.kazusa.or.jp/rhizobase/]). Primers were designed using Oligo 4.0 software (National Biosciences, Plymouth, MN). DNA sequencing was performed by Bio Basic Inc. (Markham, Ontario, Canada). DNA sequence data were analyzed using 4Peaks software (version 1.7.2; Mekentosj, Alsmeer, Netherlands). The number and arrangement of transmembrane domains were predicted using ConPred II (3). Putative signal peptide cleavage sites were predicted using the SignalP 3.0 server (5). Alignment of the R. leguminosarum 3841 genome with those of other Alphaproteobacteria was performed using Mauve multiple-genome alignment software (9). Sequence alignments were performed using ClustalW (56), and phylogenetic trees were constructed using MEGA 4 software (29).
Standard molecular techniques.
Plasmid isolation was performed using the alkaline lysis method (45). Restriction endonucleases were purchased from Invitrogen (Burlington, Ontario, Canada) and used according to the manufacturer's instructions. When necessary, PCR products were isolated from agarose gels using reagents and protocols from the QIAex II gel extraction kit (Qiagen, Missisuaga, Ontario, Canada).
RNA extraction.
RNA was extracted using a modification of the method supplied with TRIzol reagent (Invitrogen). Briefly, 3 ml of overnight culture was pelleted and resuspended in 1 ml of TRIzol. The solution was transferred to a tube containing FastPrep RNA matrix (Qbiogene Inc., CA) and vortexed at full speed for 10 min using a 1.5- to 2-ml vortex adaptor (MoBio Laboratories Inc., CA). Tubes were then centrifuged at 12,000 × g for 5 min and the supernatant recovered to a new 1.5-ml microcentrifuge tube. The remainder of the extraction procedure followed the protocol supplied with the TRIzol reagent (Invitrogen).
RT-PCR.
Primer sequences used for reverse transcription-PCR (RT-PCR) are listed in Table S1 in the supplemental material. Reverse transcription reactions were carried out according to the protocol of Manzon et al. (34), as modified by Vanderlinde et al. (58). Briefly, 10 μl of RNA was treated with 1 U of DNase I (Fermentas, Burlington, Ontario, Canada) in a total volume of 16 μl for 30 min at 37°C. DNase I was heat inactivated at 75°C for 5 min, and 1.2 μl of the appropriate reverse primer (2 μM) was then added, followed by incubation at 70°C for 10 min. Samples were snap cooled on ice and 2 μl of 10 mM deoxynucleoside triphosphates (dNTPs) was added, followed by incubation at 37°C for 5 min. One microliter of RevertAid Moloney murine leukemia virus (M-MuLV) reverse transcriptase (Fermentas) was added, and samples were incubated at 42°C for 50 min, followed by heat inactivation at 70°C for 15 min. PCR mixtures contained 1× reaction buffer, 2 mM MgSO4, 0.2 mM dNTPs, 2 μM forward primer, 0.12 μM reverse primer, and 1 U Taq DNA polymerase (UBI, Calgary, Alberta, Canada). Two microliters from the reverse transcription reaction product was used as the template. PCR amplification was performed with a Techne TC312 thermocycler (Techne, Staffordshire, United Kingdom) at 94°C for 5 min, followed by 30 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 1 min and a final extension at 72°C for 5 min. PCR products were subsequently analyzed by agarose gel electrophoresis.
Construction of nonpolar mutations in RL3499, RL3500, RL3501, and RL3502.
A crossover PCR-based approach described by Sukdeo and Charles (53) was used to create nonpolar mutations in RL3499, RL3500, and RL3501. Briefly, crossover PCR was used to amplify fragments of RL3499, RL3500, and RL3501 that contained a short synthetic in-frame fragment of DNA in place of 828-bp, 741-bp, and 1,905-bp fragments of each gene, respectively. The PCR fragments were then cloned into the pGEM-T vector using reagents and protocols provided by the manufacturer (Promega, Nepean, Ontario, Canada). Fragments were subsequently cloned into the suicide vector pJQ200SK+ by an ApaI and PstI digest (RL3499 and RL3500) or an ApaI and SpeI digest (RL3501). Plasmids were then transformed into the E. coli mobilizer strain S17-1 for conjugation into R. leguminosarum.
Following conjugation, mutants were isolated by first selecting for single crossovers based on gentamicin resistance. Single-crossover strains with confirmed sucrose sensitivity were then plated on VMM with 5% sucrose to select for double-crossover mutants based on loss of the plasmid-borne sacB gene from the chromosome. Replacement of the wild-type gene with the in-frame-deleted gene construct was confirmed by PCR cloning the regions surrounding each deletion and DNA sequencing to verify that the proper in-frame fragment was introduced during homologous recombination.
A mutation in RL3502 was made by amplifying a 867-bp internal gene fragment using the primers SM1RTF3 and SM1RTR3. The PCR product for mutagenesis of RL3502 was cloned into the pCR2.1 TOPO vector, creating the plasmid pEV85. The fragment was subsequently ligated into the suicide vector pJQ200SK+ following a SstI-XbaI double digest, creating the plasmid pEV86, which was then used for mutagenesis. The plasmid pEV86 was conjugated into 3841, and putative insertional mutants were selected based on gentamicin resistance. Mutation of RL3502 was confirmed by PCR.
Complementation of RL3499, RL3500, RL3501, and RL3502.
Primers RL3499F and RL3499R, RL3500F and RL3500R, and RL3502F and RL3502R were used to amplify 1,210-bp, 990-bp, and 2,462-bp fragments of RL3499, RL3500, and RL3502, respectively. The PCR products were then cloned into the pCR2.1 TOPO vector as per the manufacturer's instructions. The vectors for complementation were then constructed by excising the fragments from pCR2.1 TOPO with BamHI and ligating into the BamHI site of the vector pDG71. The fragments were cloned immediately downstream and in the same direction as a constitutively expressed trp promoter from pDG71. Orientation of the fragments was confirmed by restriction mapping and DNA sequencing. Tetracycline resistance was used to select for the presence of the plasmids following conjugation.
In lieu of a complementing plasmid for RL3501, an independent mutant was made by amplifying a 429-bp internal gene fragment using the primers SM1RTF2 and SM1RTR2. The PCR product for mutagenesis of RL3501 was cloned into the pCR2.1 TOPO vector, creating plasmid pEV83. The fragment was subsequently ligated into the suicide vector pJQ200SK+ following a SstI-XbaI double digest, creating the plasmid pEV84, which was then used for mutagenesis. The plasmid pEV84 was conjugated into 3841, and putative insertional mutants were selected based on gentamicin resistance. Mutants were confirmed by PCR.
Construction of an RL3499-RL3502 inactivation mutant.
A 1,210-bp fragment of RL3499 was amplified using primers RL3499F and RL3499R and cloned into the pCR2.1 TOPO vector. The fragment was then excised with SstI and XbaI and cloned into the suicide vector pJQ200SK+. To disrupt the RL3499 gene, the GUSNm cassette from pCRS530 was cloned into a SalI site at bp 762 of the RL3499 PCR product. The resulting plasmid, pEV87, was transformed into the E. coli mobilizer strain S17-1 and then conjugated into 3841 for mutagenesis. Mutants were initially selected on the basis of neomycin and sucrose resistance and gentamicin sensitivity. Replacement of the wild-type gene with the mutated gene was then confirmed by PCR. The GUSNm cassette contains a transcriptional terminator, and therefore replacement of the wild-type gene with the GUSNm-disrupted allele created a polar mutation and eliminated expression of all four genes of the operon.
Creation of a vector constitutively expressing ropB.
To determine the role of ropB downregulation in the phenotypes observed for the RL3499-RL3502 mutants, the RL3499 nonpolar mutant was provided a plasmid constitutively expressing ropB. To construct this plasmid, a 691-bp fragment containing the entire ropB open reading frame (ORF) was amplified with primers RopBconF and RopBconR. The fragment was first cloned into pGEM-T and subsequently cloned into the BamHI site of pDG71. The orientation of the fragment was confirmed by restriction digest and sequencing. The functionality of the constitutively expressed ropB was confirmed by successful complementation of a ropB mutant.
Construction of an RL3499 ropB double mutant, strain 38EV03.
To construct the double mutant, the plasmid pDF39 containing ropB disrupted with an ΩKm cassette in the suicide vector pJQ200SK was conjugated into the RL3499 nonpolar mutant 38EV99. Double-crossover mutants were selected on the basis of neomycin and sucrose resistance and gentamicin sensitivity. Putative mutants were confirmed by PCR.
Construction of an RL3499::gusA transcriptional fusion.
A 619-bp fragment upstream of RL3499 was amplified with the primers PFSM1F and PFSM1R under standard PCR conditions. The fragment was subsequently cloned into the pCR2.1 TOPO vector as per the manufacturer's instructions. The promoter fragment was then excised using KpnI and XhoI and ligated into the pFUS1P vector containing the promoterless gusA reporter gene in a par-stabilized plasmid. The resulting plasmid, pSM4, was then conjugated into the R. leguminosarum wild-type strains 3841 and VF39 and the VF39 chvG mutant DF20 to measure gene expression as described below.
β-Glucuronidase (gusA) reporter gene assays.
The enzyme assays for β-glucuronidase activity were carried out based on the β-galactosidase activity method of Miller (35), with modifications described by Yost et al. (64). Except where indicated, all assays were carried out on cultures in late log phase.
Growth and sensitivity assays.
The log reductions in growth of the various operon-related mutants were determined on VMM supplemented with different peptide sources, amino acids, detergents, or salts. Briefly, a suspension of cells from a VMM plate was diluted and the CFU ml−1 determined by the spot plate technique for both VMM and VMM plus supplement, as indicated (20). The log growth reduction was calculated as the difference between the log CFU ml−1 on VMM and the log CFU ml−1 on VMM plus supplement. Except where specified, all media was buffered to a pH of 6.8 to 7.0. Antibiotic sensitivity assays were carried out using disk diffusion assays and measuring zones of inhibition after 2 days of growth on solid VMM. Disk diffusion assays were also used to determine the sensitivity of the SM1 mutant to all 20 amino acids. Briefly, 10 μl of an amino acid solution at a concentration of either 500 mM (A, C, D, E, F, G, H, I, K, L, N, P, Q, R, S, T, and W) or 250 mM (M, V, and Y) was applied to a 7-mm disk. Amino acids shown in bold were dissolved in 1 M HCl; all other solutions were made up in distilled water. Zones of inhibition for the wild type and the SM1 mutant were then measured after 2 days of growth on solid VMM at 30°C.
Microscopy.
Cellular morphology was observed using both optical and electron microscopy techniques. Cells viewed using optical microscopy were stained with crystal violet, and the stained cells were visualized with a 1,000× oil immersion objective using an Olympus BX51 light microscope (Olympus, PA). Electron microscopy was performed by slightly modifying the procedure of Miller et al. (36), as described by Tambalo et al. (54). Briefly, strains were grown on VMM plates at 30°C for 48 h. Freshly grown cultures were then transferred to a TY plate and incubated at 30°C for 18 to 24 h. A culture suspension was prepared from TY or VMM plates using sterile double-distilled water. Resuspension in water had no apparent adverse affect on the cell morphology. A Formvar-carbon-coated grid was placed on top of a cell suspension drop for 3 min, and excess liquid was removed. Staining was performed using 1% uranyl acetate for 30 s. Samples were observed using a Hitachi 7650 transmission electron microscope (TEM), and images were taken using the AMT Image Capture Engine. Cells were measured using AMT Image software. For thin sectioning prior to viewing under TEM, cells from the heavily inoculated area of TY agar plates, incubated at 30°C for 18 to 24 h, were scraped directly into 1 ml of fixative (2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer). Samples were fixed for 90 min, washed in cacodylate buffer, fixed with 1% osmium tetroxide, and enrobed in agar. After fixation in 2% uranyl acetate, cells were dehydrated in an ethanol series and embedded in Epon resin. Thin sections were cut and stained with uranyl acetate and lead citrate. Specimens were examined with a Philips EM410 instrument operating at 60 kV.
Plant assays.
Nodulation assays were carried out with peas (Pisum sativum cv. Trapper) as the host legume. Seeds were germinated and planted as described by Yost et al. (65). Following germination, peas were inoculated with approximately 1 × 109 cells of either wild-type 3841 or the RL3499-RL3502 polar mutant 38EV87. Plants were grown at ambient temperature with a 16-h photoperiod for 3 weeks prior to harvesting. The number of nodules per plant was determined by direct counting. The plant shoot dry weights were determined by removing the roots just above the cotyledon and drying the shoots overnight at 60°C prior to weighing.
RESULTS
Mutagenesis of an operon from R. leguminosarum bv. viciae 3841 containing previously uncharacterized conserved genes.
An R. leguminosarum mutant (SM1) was isolated during a transposon mutagenesis screen to identify mutants unable to grow on complex media. Eight mutants unable to grow on the complex medium TY were identified from a screen of 1,750 individual transposants (Table 2). Of the eight mutants isolated, three mutants had insertion sites that mapped to genes related to the cell envelope. The mutant SM1 was chosen for further study because the mutation occurred within a previously uncharacterized gene with broad conservation among the Alphaproteobacteria.
Table 2.
Summary of results from transposon mutagenesis screen for mutants impaired in growth on TY medium
| Mutant | Transposon insertion site, bpa | Annotated function | Reference |
|---|---|---|---|
| DLF | fabF2 (RL2815), 999 | 3-Oxoacyl acyl carrier protein synthase | 58 |
| 8A | ctpA (RL4693) | Putative carboxy-terminal processing protease precursor | 22; this study |
| 12A | pRL80079, 227 | Putative transcriptional regulator | This study |
| 12D | pRL80117, 211 | Hypothetical protein | This study |
| 17B | RL2975, 750 | ABC transporter involved in desiccation tolerance and biofilm formation | 57 |
| 18B | phaD2 (RL1375), 1439 | Putative Na+/H+ antiporter subunit D | This study |
| 18D | RL4077, 3 | Conserved hypothetical protein | This study |
| SM1 | RL3501, 281 | Conserved hypothetical membrane protein | This study |
The base pair indicated refers to the nucleotide within the open reading frame of the gene directly upstream of the transposon insertion site.
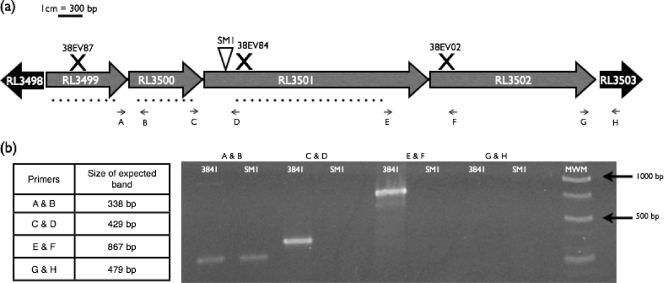
The Tn5 insertion site of SM1 was mapped to base pair 281 of RL3501, a gene annotated as conserved with unknown function (66). The predicted start and stop codons of the neighboring genes suggested that RL3501 is the third gene in an operon that includes genes RL3499 to RL3502 (Fig. 1a). RT-PCR was used to confirm the operon structure (Fig. 1b). The gene RL3499 shares homology with genes encoding ATPases that have been associated with various cellular activities (AAA+ ATPases), and gene RL3500 contains a domain of unknown function designated domain of unknown function 58 (DUF58). RL3502, the gene immediately downstream of RL3501, is a conserved gene annotated as coding for a protein with DUF1355. Based on the RT-PCR data, the original transposon insertion resulted in a polar mutation preventing gene expression of both RL3501 and RL3502. To study the individual contributions of each gene in the operon, in-frame, nonpolar deletion mutants for each gene were constructed.
Fig. 1.
(a) Schematic of the RL3499-RL3502 operon region. Gene annotations were obtained from the Rhizobase database (Kazusa DNA Research Institute; http://bacteria.kazusa.or.jp/rhizobase/). The triangle indicates the transposon insertion site, X's indicate the locations of disruption of insertional mutants, and dotted lines indicate regions deleted in the nonpolar mutants. Arrows indicate primer binding sites. (b) RT-PCR of the wild-type strain and the SM1 mutant to determine operon structure. MWM, molecular weight marker (GeneRuler 1-kb DNA ladder; Fermentas). Controls with no reverse transcriptase did not yield amplification products, indicating that no contaminating DNA was present in the RNA samples, and all sets of primers were confirmed to amplify the amplicon of the appropriate size by using standard PCR and genomic DNA from wild-type strain 3841 as the template (data not shown).
Mutation of the RL3499-RL3502 operon inhibits growth on media containing peptides or glycine.
The polar mutants SM1 and 38EV87 and all of the nonpolar mutants were capable of growth on minimal defined media with mannitol as the sole carbon source but were unable to grow on the complex medium, TY (Table 3). When a high concentration of the SM1 mutant is plated on TY, it is possible to isolate colonies with suppressor mutations that restore growth of the mutant on TY. Growth of the SM1 and 38EV87 mutants on VMM containing either tryptone (0.5%), yeast extract (0.3%), or both resulted in at least 5-log-unit reductions in growth, whereas growth of the wild type was unaffected (data not shown). Nonpolar mutation of RL3499, RL3500, or RL3501 resulted in a 2-log-unit reduction in growth on VMM containing 0.3% yeast extract, whereas growth of the RL3502 mutant was unaffected under these conditions. Furthermore, growth of all the mutants was negatively affected on VMM containing both tryptone and yeast extract, with average log unit reductions ranging from 4.9 to >7.0 compared to growth on standard VMM.
Table 3.
Sensitivity of RL3499-RL3502 mutant strains to glycine, detergents, and alkaline pH
| Strain | Log growth reduction (mean ± SD)a |
||||
|---|---|---|---|---|---|
| TY | VMM | VMM + Gly | VMM + SDS | VMM, pH 9.0 | |
| 3841 | −0.09 ± 0.13 | 0 | −0.11 ± 0.14 | 0.79 ± 0.19 | 0.06 ± 0.25 |
| SM1 | >7.0 | 0 | 4.8 ± 0.19 | 5.9 ± 0.03 | 3.7 ± 1.0 |
| 38EV99 | >7.0 | 0 | 4.0 ± 0.36 | 3.9 ± 0.15 | 2.5 ± 1.3 |
| 38EV00 | >7.0 | 0 | 4.4 ± 0.23 | 4.0 ± 0.12 | 3.2 ± 0.30 |
| 38EV01 | >7.0 | 0 | 4.4 ± 0.28 | 4.0 ± 0.19 | 3.0 ± 0.53 |
| 38EV02 | >7.0 | 0 | 4.5 ± 0.11 | 4.4 ± 0.16 | 3.1 ± 0.42 |
| 38EV87 | >7.0 | 0 | 5.0 ± 0.30 | 5.3 ± 0.14 | 6.1 ± 0.50 |
Difference in log CFU/ml between VMM and VMM with 1 mM glycine (Gly) or 0.35 mM SDS or difference in log CFU/ml between VMM at pH 7.0 and VMM at pH 9.0 (see Materials and Methods). All nonzero values for the mutants represent statistically significant differences between the wild type and mutant strains at a P value of <0.005 (Student's t test).
Earlier research studying rhizobial growth has documented sensitivity to yeast extract and peptide sources such as hydrolyzed casein (46, 47). In some reports this sensitivity was attributed to the presence of the amino acid glycine (46). To determine whether our mutants were also sensitive to glycine, growth of the mutants was tested on VMM with 1 mM glycine. The SM1 and 38EV87 mutants and all of the nonpolar mutants were significantly sensitive to glycine, with at least 4.0 log-unit reductions in growth compared to that on VMM (Table 3). Growth of the wild type was unaffected by the addition of the glycine to the medium. Titrating the concentration of glycine below 1 mM did not elicit an effect in the SM1 mutant, suggesting a structural rather than regulatory effect. Based on disk diffusion assays, growth of the SM1 mutant was inhibited only by glycine, and there were no adverse growth effects observed in the presence of the other 19 amino acids (data not shown). Notably, the 1 mM glycine required to inhibit growth of the SM1 mutant on VMM is significantly higher than the concentration of free glycine found in tryptone or yeast extract, suggesting that there may be additional growth-inhibiting factors present in these medium components.
Complementation of the nonpolar mutations in genes RL3499, RL3500, and RL3502 by heterologous expression of these genes from the constitutive trp promoter in plasmid pDG71 successfully restored growth on TY. Attempts to PCR amplify the entire RL3501 ORF were unsuccessful. In lieu of complementation, an independent polar mutation in RL3501 was constructed by insertional mutagenesis, and this mutant was confirmed to have the same phenotypes as the polar SM1 mutant. Therefore, it is unlikely that the phenotypes observed in the SM1 mutant are caused by a secondary-site mutation.
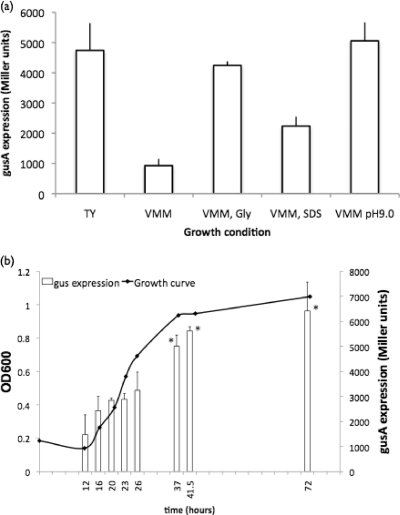
The requirement for the RL3499-RL3502 operon for growth in the presence of peptides was supported further with data from gene expression studies. The expression of the operon was 5.1-fold higher in cells grown in TY than in those grown in VMM (Fig. 2a). Addition of tryptone, yeast extract, or glycine to VMM increased the level of expression 2.7-fold, 3.1-fold, and 4.5-fold, respectively. Transcription of the operon was also induced by addition of other peptide sources to VMM, including soytone and beef extract (data not shown). Furthermore, expression of the operon in TY was maximal during the stationary phase of growth (Fig. 2b).
Fig. 2.
(a) Gene expression of the RL3499-RL3502 operon under various growth conditions as described in Materials and Methods. Statistically significant difference in gusA activity compared to VMM at a P value of <0.001 (Student's t test) occurred with all treatments. (b) Gene expression of the RL3499-RL3502 operon in TY broth over a 72-h period. *, statistically significant difference in gene expression compared to 12 h of growth at a P value of <0.05 (Student's t test).
Mutants defective in RL3499-RL3502 are sensitive to stressors that target the cell envelope, including detergents, hydrophobic antibiotics, and alkaline pH.
All of the mutants tested displayed increased sensitivity to the detergent SDS (Table 3). The RL3499-RL3502 mutants were also sensitive to the hydrophobic antibiotic erythromycin (data not shown). In rhizobia, sensitivity to these compounds has been linked to alterations in outer membrane integrity, suggesting that mutations in RL3499-RL3502 may cause structural defects in the outer membrane in R. leguminosarum. Growth on medium containing SDS did not result in any observable differences in cellular morphology (data not shown).
The mutants were also sensitive to growth on VMM at alkaline pH (Table 3). At pH 8.0, the mutants had an average log reduction in growth of at least 1.5 compared to growth at neutral pH. The effect was further magnified at pH 9.0, with average log unit reductions of at least 2.5 compared to an average reduction of 0.06 log unit for the wild type (Table 3). There were no adverse affects on growth of the mutants on VMM at pH 5.0 or 6.0 (data not shown). Gene expression of the RL3499::gusA fusion measured in VMM at pH 8.0 and pH 9.0 provided further support that the RL3499 operon is important for growth at alkaline pH (Fig. 2a).
Mutations affecting the cell envelope often result in sensitivity to osmotic and desiccation stresses (37, 38, 58). However, the mutants were not sensitive to either hyper- or hypo-osmotic stress (data not shown).
Divalent cations rescue growth of the RL3499-RL3502 mutants on media containing peptides, glycine, or SDS.
Addition of calcium (3.4 mM) to VMM supplemented with tryptone, yeast extract, glycine, or SDS restored growth of all the mutants to wild-type levels. Addition of calcium to VMM containing both tryptone and yeast extract resulted in a roughly 2-log-unit increase in growth of the SM1 and 38EV87 polar mutants and completely restored growth of the nonpolar mutants to wild-type levels. However, supplementing TY with additional calcium (5 mM) did not rescue growth of any of the mutants. A similar effect was seen with medium supplemented with 3.4 mM MgCl2, or MgSO4, indicating that Mg2+ can substitute for the divalent cation and that the growth restoration effect is not related to the presence of a specific anion. Growth of the SM1 mutant was unaffected by the addition of EDTA to the medium, and therefore it is unlikely that the sensitivity to tryptone, yeast extract, and glycine is due to chelation of divalent cations from components in the media. Additional calcium did not rescue growth of the mutants on VMM at alkaline pH.
Gene expression studies suggest a linkage between the RL3499-RL3502 operon and the outer membrane protein RopB.
Many of the phenotypes described for the operon mutants are similar to those described for a mutant strain no longer expressing the outer membrane protein gene ropB in R. leguminosarum bv. viciae VF39 (17), a strain that is closely related to R. leguminosarum 3841(17, 63). We used pDF4, a ropB::gusA transcriptional fusion, to determine whether the sensitivity of these mutants to outer membrane stressors could be, in part, due to diminished expression of ropB. The expression of pDF4 from 3841, SM1, and 38EV99 grown on VMM containing tryptone and CaCl2 was 1.1E04 ± 939 Miller units, 307 ± 2.83 Miller units, and 111 ± 9.9 Miller units, respectively. Effectively, the expression of ropB in the SM1 and 38EV99 mutants was similar to the background level for the empty-vector control, which was 250 ± 27.2 Miller units. Similar results were found previously for expression of ropB in a fabF2 fabF1 mutant, which lacks the very-long-chain fatty acid (VLCFA) modification characteristic of rhizobial lipid A (58). To determine whether the downregulation of ropB in the SM1 mutant was related to alterations in the VLCFAs, the expression of the fab VLCFA biosynthetic genes in a wild-type background was compared to that in the SM1 mutant. No difference in expression levels was found between the two strains (data not shown). As well, no differences were observed between the LPS of the RL3499-RL3502 mutants and wild-type LPS when analyzed by deoxycholate (DOC)-PAGE (data not shown). Taken together, these results suggest that the lack of ropB expression in the SM1 mutant is not directly related to gross changes in the structure of the lipopolysaccharide.
To further investigate the role of ropB downregulation in the observed phenotypes of the RL3499-RL3502 mutants, an RL3499 mutant strain (38EV99) constitutively expressing ropB (pEV91), as well as a double mutant with mutations in both RL3499 (nonpolar) and ropB, was analyzed (Table 4). As stated above, mutation of RL3499 resulted in loss of growth on the complex medium TY. Mutation of ropB does not affect growth on TY, and constitutive expression of ropB in the 38EV99 mutant did not restore growth on TY. The ropB mutant (38DF39), the RL3499 mutant (38EV99), and the double mutant (38EV03) were all sensitive to 0.35 mM SDS, with log unit reductions of 3.8, 3.9, and 4.3, respectively. Constitutive expression of ropB in either the ropB or RL3499 mutant background completely restored growth of these mutants on SDS to wild-type levels (Table 4). The ropB and RL3499 mutants are also both sensitive to the hydrophobic antibiotic erythromycin. Zone-of-inhibition assays were used to compare the sensitivities of the different mutant and complemented strains to erythromycin. The ropB mutant had a 2-fold increase in sensitivity, and this sensitivity was reduced slightly to 1.5-fold in the ropB-complemented strain. The RL3499 mutant had a 1.5-fold increase in sensitivity compared to the wild type. The sensitivity of this mutant, however, was not diminished by complementation with ropB. The RL3499 ropB double mutant had a zone of inhibition of 22.6 mm ± 1.15 mm, which is similar to the zone found for the ropB single mutant.
Table 4.
Comparison of phenotypes using various ropB and RL3499 mutant backgrounds
| Strain | Genotype | Growth on TY (mean ± SD)a | SDS sensitivity (mean ± SD)a |
|---|---|---|---|
| 3841 | Wild type | −0.09 ± 0.13 | 0.79 ± 0.19 |
| 38DF39 | ropB | 0.141 ± 0.08 | 3.81 ± 0.32 |
| 38DF39(pEV91) | ropB ropB(Con)b | 0.18 ± 0.35 | 0.51 ± 0.58 |
| 38EV99 | RL3499 | >7.0 | 3.9 ± 0.15 |
| 38EV03 | RL3499 ropB | >7.0 | 4.3 ± 0.16 |
| 38EV99(pEV91) | RL3499 ropB(Con) | >7.0 | 0.24 ± 0.20 |
Difference in log CFU/ml between VMM and TY or between VMM and VMM with 0.35 mM SDS (see Materials and Methods). Values in bold represent statistically significant differences between the wild-type and mutant strains at a P value of <0.005 (Student's t test).
ropB constitutively expressed from the trp promoter in pDG71.
The ropB mutant was not sensitive to glycine or alkaline pH, and constitutive expression of ropB in the RL3499 mutant had no effect on the mutant's sensitivity to these growth conditions (data not shown).
Regulation of RL3499-RL3502 by the sensor kinase ChvG.
We previously reported that the sensor kinase ChvG regulates expression of the outer membrane protein gene ropB in the closely related strain R. leguminosarum VF39 (17). ChvG is also important in the expression of the homologous ropB in S. meliloti (8). Because of the diminished ropB expression in the SM1 mutant, we investigated the involvement of ChvG in regulating gene expression of the RL3499-RL3502 operon. We were unable to create a chvG mutant of R. leguminosarum 3841. Therefore, we transferred the RL3499-RL3502 gusA fusion, pSM4, into a VF39 background and found the pattern of expression to be identical to the pattern observed in 3841. In both 3841 and VF39, gusA expression from pSM4 is induced approximately 4-fold on VMM with tryptone and calcium chloride relative to expression on VMM with calcium (Table 5). In the chvG mutant, expression of gusA is similarly 3.5-fold higher on VMM with tryptone and calcium than on VMM with calcium; however, the overall level of expression is 2.5-fold lower in the chvG mutant than in 3841 or VF39 (P < 0.004 by Student's t test) (Table 5). Expressions of the genes sodB and oxyR were unaffected in the chvG mutant, suggesting that the downregulation of the pSM4 fusion is not caused by a general effect of the chvG mutation. These results suggest that chvG is required for maximal expression of the RL3499-RL3502 operon but that an unknown additional factor is involved in the upregulation of the genes in the presence of peptide-containing medium. Chen et al. (8) combined gene microarray data from gain-of-function and reduced-function chvI mutants to identify direct transcriptional targets of ExoS/ChvI in S. meliloti. However, the RL3499-RL3502 homolog in S. meliloti was not identified as a direct target by that study. These results could suggest that ChvG and by extension ChvI may have an indirect role in the regulation of the RL3499-RL3502 operon, or it could be that the reduced-function chvI mutant used in the microarray retained enough functionality to partially modulate expression of the operon, thereby excluding it from their further analysis. Further experiments will be required to determine if the RL3499-RL3502 operon is, in fact, a direct target of the ChvG/ChvI two-component system.
Table 5.
Expression of the RL3499-RL3502 operon is reduced in a chvG mutant
| Medium | Rl3499-Rl3502 operon expression (mean ± SD) in: |
||
|---|---|---|---|
| 38SM4 | VFSM4 | DF20SM4 | |
| VMM + Ca | 1,773 ± 346 | 1,627 ± 100 | 500 ± 32a |
| VMM + Tryp + Ca | 4,303 ± 940 | 4,162 ± 548 | 1,130 ± 190a |
The difference in gusA expression between the wild-type and chvG mutant backgrounds is statistically significant at a P value of <0.003 (Student's t test).
Mutation of the RL3499-RL3502 operon affects cell morphology.
Alterations in the cell morphology of R. leguminosarum when grown in the presence of yeast extract and glycine have been previously observed (46, 47). Therefore, the cell morphologies of the SM1 and nonpolar mutants were examined using light microscopy. When observed using oil immersion light microscopy, mutant cells incubated on solid TY were enlarged, circular, and distorted. Similar cell shapes were observed for the mutants incubated on VMM with tryptone, yeast extract, or glycine. Inclusion of an additional 3.4 mM calcium chloride in the supplemented VMM restored the typical rod-shaped morphology to the mutant cells (data not shown).
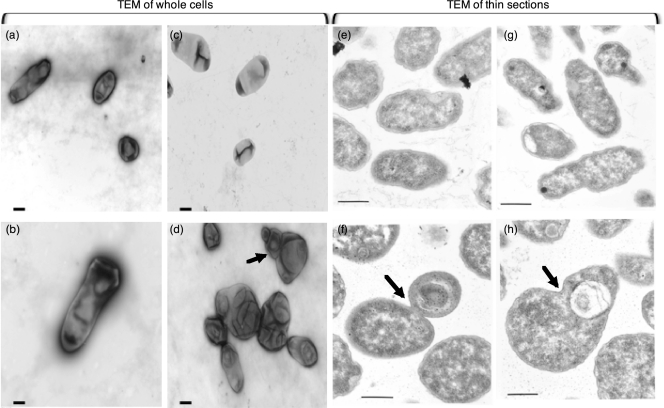
Electron microscopy was used to define further the morphological defects using the SM1 mutant as the example. Observed at a magnification of ×1,000,000, the SM1 mutant grown on VMM is an enlarged rod compared to the wild-type strain (Fig. 3). The average cell length of the wild-type strain, 3841, is 1.84 μm ± 0.608 (n = 20), while that of the SM1 mutant is 3.43 μm ± 0.881 (n = 23). This difference is statistically significant at a P value of <0.0001 (Student's t test). On TY the wild-type cells were predominantly small rod-shaped cells, whereas the SM1 mutant cells were distorted spheres of variable size (Fig. 3).
Fig. 3.
Transmission electron microscopy of wild-type strain 3841 (top panels) and the SM1 mutant (bottom panels). Cells were grown on VMM (a and b) or incubated on TY (c to h) for 24 h prior to imaging. Staining and thin sectioning were performed as described in Materials and Methods. Scale bars represent 500 nm. The arrows point to examples of the unusual cell structures that were frequently observed.
Thin sectioning was used to determine if the mutants displayed gross defects in membrane structure. Although no obvious defects in the outer membrane or inner membrane were observed, the cell morphologies observed in the thin sections corroborated the abnormal cell morphologies and enlarged cells observed under whole-cell TEM imaging (Fig. 3).
Inactivation of the RL3499-RL3502 operon affects symbiosis with pea (Pisum sativum).
To determine the effect of an RL3499-RL3502 mutation on symbiosis, a polar mutant of RL3499 (38EV87) that lacked expression of the entire operon was tested on pea plants. Plants infected by the mutant strain had approximately twice as many nodules as plants infected with the wild type, producing 300 ± 16 nodules, and 153 ± 23 nodules, respectively. The dry weights of the plants infected by wild-type 3841 and the 38EV87 mutant were 0.541 g ± 0.036 g and 0.578 g ± 0.070 g, respectively. The dry weight of the uninfected control was 0.302 g ± 0.015 g. Pea plants and other legumes tightly control the number of nodules on their roots in response to their nitrogen requirements (14, 52). The greater abundance of nodules in plants infected with the 38EV87 mutant may suggest that this mutant is less efficient at nitrogen fixation and therefore the pea plant requires more nodules to fulfill its nitrogen demands.
The coding regions of RL3499-RL3502 contain a number of highly conserved domains.
RL3499 is predicted to encode a putative MoxR-like ATPase (E value, 1.1e−67). The MoxR family of AAA+ ATPases is represented in all major lineages of bacteria and archaea (51). The exact function of these proteins is unknown; however, the research available to date suggests that they act as novel molecular chaperones (51). The second gene in the operon encodes a conserved hypothetical protein with a domain of unknown function designated DUF58 (E value, 1.1e−14) encoded from bp 54 to 151. Proteins containing DUF58 are often found immediately downstream of MoxR ATPases. Snider and Houry (51) divided all of the available MoxR-like protein sequences into seven distinct subfamilies. The arrangement of the genes in the R. leguminosarum RL3499-RL3502 operon suggests that they belong within the MoxR proper subfamily, which is the largest and most diverse group.
RL3501 and RL3502 are predicted to code for transmembrane proteins, with four and three transmembrane domains, respectively. The predicted locations and orientations of the TM domains in the cytoplasmic membrane suggest that the majority of the amino acids of both proteins are located in the periplasm. RL3501 codes for a particularly large, 937-amino-acid protein and contains a number of conserved domains, including an N-terminal double-transmembrane domain from amino acid 6 to 86 (E value, 1e−08), a possible von Willebrand factor type A domain from amino acid 92 to 263 (P value, 0.016), and a class I GATase domain from amino acid 299 to 547 (E value, 7e−11). The N-terminal double-transmembrane domain is often found in membrane proteins of 600 to 1,000 amino acids and is predicted to have the N terminus in the periplasm, followed by two transmembrane regions with approximately 25 amino acids between them in the cytoplasm. The von Willebrand factor type A domain contains a metal ion adhesion site (MIDAS) for incorporation of a metal ligand and is often found in proteins associated with moxR ATPases similar to that RL3499. These domains have been characterized extensively in eukaryotes and have roles in a number of processes, including transcription, DNA repair, transport, and the proteasome. The class I GATase domain catalyzes the transfer of ammonia from glutamine to an acceptor molecule. These domains are often present in biosynthetic enzymes, as well as some peptidases. Although this domain is suggested to be present in RL3501, the predicted conserved catalytic triad consisting of cysteine, histidine, and glutamate is not present, and therefore this domain may not be suggestive of functional activity. RL3502 is 689 amino acids and contains DUF1355 (E value, 3e−24) from amino acid 279 to 451.
The operon RL3499-RL3500 is highly conserved within the Alphaproteobacteria.
To determine the distribution of the operon, we compared the protein sequence of the entire operon to the genome sequences currently available in the GenBank database. Presently, the operon is confined to the Alphaproteobacteria and is conserved in all genome-sequenced members of the Rhizobiales, Parvularcula, Rhodobacterales, and Rhodospiralles, with the notable exception of Magnetospirillum spp. Williams et al. (62) have reported a robust species tree for the Alphaproteobacteria. Based on their analysis, the Rickettsiales are the earliest branching order. The distribution of the operon throughout the other alphaproteobacterial orders suggests that this operon was acquired by a common ancestor following the divergence of the Rickettsiales and subsequently was lost from the Caulobacterales and Sphingomonadales. The distribution is narrower than that of the ChvG/ChvI two-component regulatory system, which is found in almost all alphaproteobacterial orders, with the exception of the Rickettsiales.
Individual neighbor-joining trees were constructed for each of the four genes in the operon (data not shown). All of these trees were consistent with the branching observed for the alphaproteobacterial species tree, indicating that the operon has likely been inherited vertically through speciation events. Furthermore, the gene trees for the individual genes are all consistent with each other, suggesting that no recombination or reassortment of the operon has taken place throughout its evolution, providing some evidence that an intact operon is important to the Alphaproteobacteria in which the operon is found.
The orthologous nature of the operon in all of these diverse species is further confirmed by the shared synteny of the surrounding gene region, in particular in the region upstream of the moxR-like gene (RL3499). In all species the gene immediately upstream of the moxR-like gene encodes a DUF1285-containing conserved hypothetical protein and is transcribed in the opposite direction. Within the Rhizobiales, the genes immediately downstream of the operon are highly conserved as well and include genes encoding an acetyltransferase and a glutathione transferase.
DISCUSSION
Hypothetical predicted proteins of unknown function, many of which are conserved, typically represent 30 to 40% of the annotated proteins in a sequenced genome. Consequently, these so-called “conserved hypothetical proteins” have been identified as an important priority for biological and biochemical characterization (19). The RL3499-RL3502 operon is a broadly conserved operon from R. leguminosarum which has not been previously characterized. While the genes within this operon contain several predicted conserved domains, there is currently no specific functional information for these domains that can assist in assigning a biological role to these genes. Therefore, this study provides the first insight into the possible function of the proteins encoded by these genes in R. leguminosarum and, by extension, other Alphaproteobacteria.
The phenotypes observed for mutants within the operon suggest an important role for these proteins in the proper functioning of the cell envelope in R. leguminosarum. However, the pleiotropic nature of the phenotypes makes it difficult to pinpoint the specific function of these genes. The mutants are sensitive to the amino acid glycine, indicating that the cell wall may be one structure affected by mutation of these genes. Glycine toxicity in bacteria is a well-documented phenomenon and has been linked to a 20 to 40% decrease in the extent of cross-linking of the peptidoglycan and significant changes in cell morphology, including elongation, spheroplast formation, and rounding of rod-shaped cells (26). Yeast extract or glycine induces significant changes in cell morphology in some strains of Rhizobium spp., resulting in alteration of the typical rod-shaped cells into enlarged, distorted, pleomorphic shapes and spheroplasts that sometimes resemble bacteroid cells (46, 47). The RL3499-RL3502 mutants had similarly altered cell shape in the presence of glycine, providing further evidence for cell wall alterations in these mutants. Additionally, the RL3499-RL3502 mutants were sensitive to alkaline pH. Alkaline pH sensitivity has been linked to cell wall structure in Bacillus sp. strain C-125, where cells with lower rates of peptidoglycan cross-linking were more sensitive to alkaline pH than cells with higher rates of cross-linking (2). Glycine has also been shown to increase the permeability of the inner and outer membranes (26, 33), suggesting that glycine may have a pleiotropic effect on the structure of the bacterial cell envelope. Therefore, some of the observed mutant phenotypes may also be due to alterations in the outer membrane.
Operon mutants were sensitive to erythromycin, polymyxin B, and SDS. These compounds have been associated with defects in outer membrane integrity in rhizobia, suggesting that mutation of the operon disrupts the stability of the outer membrane. A link between cell wall structure and outer membrane integrity in these mutants is possible. Recently, Goley et al. (23) described DipM, a putative LytM endopeptidase from Caulobacter crescentus. Mutation of dipM results in a thickening of the peptidoglycan and outer membrane blebbing, which the authors attributed to loss of interactions between proteins in the Tol-Pal complex, which links the peptidoglycan and outer membrane in Gram-negative bacteria (23). However, in the SM1 mutant no obvious defects in the outer membrane, such as separating from the cell, were observed in the thin sections of the TEM images.
The effects of peptides, glycine, and SDS on the operon mutants could be overcome by supplementing the medium with 3.4 mM calcium or magnesium ions. Divalent cations, and calcium in particular, stabilize the cell envelope through a number of mechanisms (10, 39, 50). For example, calcium may have a role in regulating the level of the key cell division protein FtsZ, which is often reduced in cell wall-defective mutants (40). Calcium and magnesium also help to stabilize the outer membrane through hydrostatic interactions with lipopolysaccharides (27, 30, 39, 50). Calcium may also have a role in controlling the autolytic process by suppressing the action of peptidoglycan hydrolases (30, 31). The mode of action of SDS and other detergents on bacterial cells is through destabilization of the outer membrane. Therefore, it is possible that the SDS sensitivity of the operon mutants was rescued due to increased stabilization of the outer membrane by the divalent cations. The role of the divalent cations in rescuing the glycine growth and morphology phenotypes, however, is less apparent. The RL3499-RL3502 mutants were not sensitive to the chelating agent EDTA, indicating that chelation of extracellular cations by peptides or glycine is likely not responsible for the observed sensitivity of the mutants to these compounds. However, since EDTA would not cross the cell envelope, it is possible that chelation of intracellular cations by glycine (12) is playing a role in the observed phenotypes of the operon mutants. Due to the complex multifunctional activity of calcium, it will be a daunting task to identify the specific mechanisms whereby calcium restores wild-type phenotypes to the mutants.
A number of the phenotypes described for the RL3499-RL3502 operon mutants are similar to those described for a mutant with a mutation in the outer membrane protein RopB. We found that there is virtually no expression of ropB in the SM1 mutant. To better clarify which phenotypes might be related to this decrease in ropB expression, a strain constitutively expressing ropB and a ropB RL3499 double mutant were analyzed. The constitutively expressed ropB was able to fully restore SDS tolerance to wild-type levels and partially complement erythromycin sensitivity in a ropB mutant, confirming that the constitutively expressed ropB produces a functional version of RopB in vivo. Constitutive expression of ropB in the RL3499 mutant fully restored growth on medium containing SDS, suggesting that the SDS sensitivity phenotype is directly related to downregulation of ropB in the RL3499-RL3502 mutants. However, constitutive expression of ropB did not restore growth of the RL3499 mutant on TY, nor did it increase resistance of the mutant to erythromycin. These results indicate that while ropB downregulation in the RL3499-RL3502 mutants is contributing to some of the observed cell envelope-related phenotypes, mutation of these genes is also affecting other components of the cell envelope of R. leguminosarum. The pleiotropic nature of the phenotypes resulting from mutation of the RL3499-RL3502 operon suggests that mutation may affect regulation of other genes. However, global gene expression studies (e.g., transcriptomics) are required prior to reflecting on the mechanisms causing the altered expression of other genes within the operon mutants.
The two-component regulatory system containing the sensor kinase ChvG has been implicated in regulation of a number of cell envelope components in Rhizobium spp. (8, 17). The expression data for the RL3499::gusA fusion in the chvG mutant background suggests that ChvG does play a role in RL399-RL3502 operon expression; however, another factor is required for the induction of the RL3499-RL3502 operon in the presence of peptides. Future research will focus on identifying additional transcription factors required for induction of the RL3499-RL3502 operon in the presence of peptide-rich growth media as well as elucidating the mechanisms involved in the downregulation of ropB in strains defective in the RL3499-RL3502 operon.
The defective phenotypes observed in the operon mutants may have implications for symbiosis. Pea plants infected by the mutant require twice as many nodules to attain shoot dry weights similar to those of plants inoculated with the wild type. Although beyond the scope of the present study, these results suggest that further study is warranted, and future work will focus on characterizing the bacteroid physiology of the operon mutants.
In conclusion, the data point to a general role for the operon in cell envelope physiology. However, the pleiotropic nature of the mutant phenotypes suggests that it will be particularly difficult to assign a primary function to these genes. Notably, the occurrence of spontaneous suppressor mutants on TY is encouraging, as mapping the locations of suppressor mutations to identify gene partners of the operon may provide an opportunity to gain further insight into the mechanistic function of the operon. Future experimentation will focus on using a proteomics approach that targets membrane and periplasmic cellular lysate fractions to further characterize the nature of the cell envelope in the operon mutants.
Supplementary Material
ACKNOWLEDGMENTS
We thank John Stavrinides for invaluable assistance with the bioinformatics analysis. We gratefully acknowledge Judy Sholdice for technical assistance with electron microscopy.
This research was supported by Natural Sciences and Engineering Research Council (NSERC) grants to C.K.Y. and S.F.K. E.M.V. was supported by a Canada Graduate Scholarship from the NSERC.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 25 February 2011.
REFERENCES
- 1. Altschul S. F., et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aono R., Sanada T. 1994. Hyper-autolysis of the facultative alkaliphile Bacillus sp. C-125 cells grown at neutral pH: culture-pH dpendent cross-linking of the peptide moieties of the peptidoglycan. Biosci. Biotechnol. Biochem. 58:2015–2019 [Google Scholar]
- 3. Arai M., et al. 2004. ConPred II: a consensus prediction method for obtaining transmembrane topology models with high reliability. Nucleic Acids Res. 32:W390–W393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bélanger L., Dimmick K., Fleming J., Charles T. 2009. Null mutations in Sinorhizobium meliloti exoS and chvI demonstrate the importance of this two-component regulatory system for symbiosis. Mol. Microbiol. 74:1223–1237 [DOI] [PubMed] [Google Scholar]
- 5. Bendtsen J. D., Nielsen H., von Heijne G., Brunak S. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783–795 [DOI] [PubMed] [Google Scholar]
- 6. Beringer J. E. 1974. R factor transfer in Rhizobium leguminosarum. J. Gen. Microbiol. 84:189–198 [DOI] [PubMed] [Google Scholar]
- 7. Campbell G. R. O., Reuhst B. L., Walker G. C. 2002. Chronic intracellular infection of alfalfa nodules by Sinorhizobium meliloti requires correct lipopolysaccharide core. Proc. Natl. Acad. Sci. U. S. A. 99:3938–3943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen E. J., Fisher R. F., Perovich V. M., Sabio E. A., Long S. R. 2009. Identification of direct transcriptional target genes of ExoS/ChvI two-component signaling in Sinorhizobium meliloti. J. Bacteriol. 191:6833–6842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Darling A. C., Mau B., Blattner F. R., Perna N. T. 2004. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 14:1394–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dominguez D. C. 2004. Calcium signaling in bacteria. Mol. Microbiol. 54:291–297 [DOI] [PubMed] [Google Scholar]
- 11. Dylan T., Helinski D. R., Ditta G. S. 1990. Hypoosmotic adaptation in Rhizobium meliloti requires β-(1→2)-glucan. J. Bacteriol. 172:1400–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eisenhut M., Bauwe H., Hagemann M. 2007. Glycine accumulation is toxic for the cyanobacterium Synechocystis sp. strain PCC 6803, but can be compensated by supplementation with magnesium ions. FEMS Microbiol. Lett. 277:232–237 [DOI] [PubMed] [Google Scholar]
- 13. Eisenschenk L., et al. 1994. Inhibition of Rhizobium etli polysaccharide mutants by Phaseolus vulgaris root compounds. Appl. Environ. Microbiol. 60:3315–3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferguson B. J., et al. 2010. Molecular analysis of legume nodule development and autoregulation. J. Integr. Plant Biol. 52:61–76 [DOI] [PubMed] [Google Scholar]
- 15. Ferguson G. P., Datta A., Carlson R. W., Walker G. C. 2005. Importance of unusually modified lipid A in Sinorhizobium stress resistance and legume symbiosis. Mol. Microbiol. 56:68–80 [DOI] [PubMed] [Google Scholar]
- 16. Ferguson G. P., Roop R. M. I. I., Walker G. C. 2002. Deficiency of a Sinorhizobium meliloti bacA mutant in alfalfa symbiosis correlates with alteration of the cell envelope. J. Bacteriol. 184:5625–5632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Foreman D. L., Vanderlinde E. M., Bay D. C., Yost C. K. 2010. Characterization of a gene family of outer membrane proteins (ropB) in Rhizobium leguminosarum bv. viciae VF39SM and the role of the sensor kinase ChvG in their regulation. J. Bacteriol. 192:975–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gage D. J. 2002. Analysis of infection thread development using gfp- and DsRed-expressing Sinorhizobium meliloti. J. Bacteriol. 184:7042–7046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Galperin M. Y., Koonin E. V. 2010. From complete genome sequence to ‘complete’ understanding? Trends Biotechnol. 28:398–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gaudy A. F., Jr., Abu-Niajj F., Gaudy E. T. 1963. Statistical study of the spot-plate technique for viable-cell counts. Appl. Microbiol. 11:305–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gibson K. E., Kobayashi H., Walker G. C. 2008. Molecular determinants of a symbiotic chronic infection. Annu. Rev. Genet. 42:413–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gilbert K. B., Vanderlinde E. M., Yost C. K. 2007. Mutagenesis of the carboxy terminal protease CtpA decreases desiccation tolerance in Rhizobium leguminosarum. FEMS Microbiol. Lett. 272:65–74 [DOI] [PubMed] [Google Scholar]
- 23. Goley E. D., Comolli L. R., Fero K. E., Downing K. H., Shapiro L. 24 May 2010. DipM links peptidoglycan remodeling to outer membrane organization in Caulobacter. Mol. Microbiol. doi:10.1111/j.1365–2958.2010.07222.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Griffitts J. S., Long S. R. 2008. A symbiotic mutant of Sinorhizobium meliloti reveals a novel genetic pathway involving succinoglycan biosynthetic functions. Mol. Microbiol. 67:1292–1306 [DOI] [PubMed] [Google Scholar]
- 25. Haag A. F., et al. 2009. The Sinorhizobium meliloti LpxXL and AcpXL proteins play important roles in bacteroid development within alfalfa. J. Bacteriol. 191:4681–4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hammes W., Schleifer K. H., Kandler O. 1973. Mode of action of glycine on the biosynthesis of peptidoglycan. J. Bacteriol. 116:1029–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hartmann R., Bock-Hennig S. B., Schwarz U. 1974. Murein hydrolases in the envelope of Escherichia coli. Properties in situ and solubilization from the envelope. Eur. J. Biochem. 41:203–208 [DOI] [PubMed] [Google Scholar]
- 28. Johnston A. W., Beringer J. E. 1975. Identification of the rhizobium strains in pea root nodules using genetic markers. J. Gen. Microbiol. 87:343–350 [DOI] [PubMed] [Google Scholar]
- 29. Kumar S., Nei M., Dudley J., Tamura K. 2008. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinformatics 9:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leduc M., Kasra R., van Heijenoort J. 1982. Induction and control of the autolytic system of Escherichia coli. J. Bacteriol. 152:26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leduc M., van Heijenoort J. 1980. Autolysis of Escherichia coli. J. Bacteriol. 142:52–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li L., et al. 2002. A global pH sensor: Agrobacterium sensor protein ChvG regulates acid-inducible genes on its two chromosomes and Ti plasmid. Proc. Natl. Acad. Sci. U. S. A. 99:12369–12374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li Z., et al. 2009. Calcium leads to further increase in glycine-enhanced extracellular secretion of recombinant alpha-cyclodextrin glycosyltransferase in Escherichia coli. J. Agric. Food Chem. 57:6231–6237 [DOI] [PubMed] [Google Scholar]
- 34. Manzon R. G., Neuls T. M., Manzon L. A. 2007. Molecular cloning, tissue distribution, and developmental expression of lamprey transthyretins. Gen. Comp. Endocrinol. 151:55–65 [DOI] [PubMed] [Google Scholar]
- 35. Miller J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 36. Miller L. D., Yost C. K., Hynes M. F., Alexandre G. 2007. The major chemotaxis gene cluster of Rhizobium leguminosarum bv. viciae is essential for competitive nodulation. Mol. Microbiol. 63:348–362 [DOI] [PubMed] [Google Scholar]
- 37. Miller-Williams M., Loewen P. C., Oresnik I. J. 2006. Isolation of salt-sensitive mutants of Sinorhizobium meliloti strain Rm1021. Microbiology 152:2049–2059 [DOI] [PubMed] [Google Scholar]
- 38. Nagarajan T., Vanderleyden J., Tripathi A. K. 2007. Identification of salt stress inducible genes that control cell envelope related functions in Azospirillum brasilense Sp7. Mol. Genet. Genomics 278:43–51 [DOI] [PubMed] [Google Scholar]
- 39. Norris V., et al. 1991. Calcium in bacteria: a solution to which problem? Mol. Microbiol. 5:775–778 [DOI] [PubMed] [Google Scholar]
- 40. Onoda T., et al. 2000. Effects of calcium and calcium chelators on growth and morphology of Escherichia coli L-form NC-7. J. Bacteriol. 182:1419–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parada M., et al. 2006. Sinorhizobium fredii HH103 mutants affected in capsular polysaccharide (KPS) are impaired for nodulation with soybean and Cajanus cajan. Mol. Plant Microbe Interact. 19:43–52 [DOI] [PubMed] [Google Scholar]
- 42. Priefer U. 1989. Genes involved in lipopolysaccharide production and symbiosis are clustered on the chromosome of Rhizobium leguminosarum biovar viciae VF39. J. Bacteriol. 171:6161–6168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Quandt J., Hynes M. F. 1993. Versatile suicide vectors which allow direct selection for gene replacement in Gram-negative bacteria. Gene 127:15–21 [DOI] [PubMed] [Google Scholar]
- 44. Reeve W. G., et al. 1999. Constructs for insertional mutagenesis, transcriptional signal localization and gene regulation studies in root nodule and other bacteria. Microbiology 145:1307–1316 [DOI] [PubMed] [Google Scholar]
- 45. Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 46. Sherwood M. 1972. Inhibition of Rhizobium trifolii by yeast extracts or glycine is prevented by calcium. Microbiology 71:351 [Google Scholar]
- 47. Skinner F. A., Roughley R. J., Chandler M. R. 1977. Effects of yeast extraction concentration on viability and cell distortion in Rhizobium spp. J. Appl. Bacteriol. 43:287–297 [Google Scholar]
- 48. Skorupska A., Janczarik M., Marczak M., Mazur A., Król J. 2006. Rhizobial exopolysaccharides: genetic control and symbiotic functions. Microb. Cell Fact. 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Simon R., Priefer U., Puhler A. 1983. A broad host range mobilization system for in vivo genetic engineering-transposon mutagenesis in Gram negative bacteria. Biotechnology 1:784–791 [Google Scholar]
- 50. Smith R. J. 1995. Calcium signaling in bacteria. Adv. Microb. Physiol. 37:83–133 [DOI] [PubMed] [Google Scholar]
- 51. Snider J., Houry W. A. 2006. MoxR AAA+ ATPases: a novel family of molecular chaperones? J. Struct. Biol. 156:200–209 [DOI] [PubMed] [Google Scholar]
- 52. Stougaard J. 2000. Regulators and regulation of legume root nodule development. Plant Physiol. 124:531–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sukdeo N., Charles T. C. 2003. Application of crossover-PCR-mediated deletion-insertion mutagenesis to analysis of the bdhA-xdhA2-xdhB2 mixed-function operon of Sinorhizobium meliloti. Arch. Microbiol. 179:301–304 [DOI] [PubMed] [Google Scholar]
- 54. Tambalo D. D., et al. 2010. Regulation of flagellar, motility and chemotaxis genes in Rhizobium leguminosarum by the VisN/R-Rem cascade. Microbiology 156:1673–1685 [DOI] [PubMed] [Google Scholar]
- 55. Tang X., Lu B. F., Pan S. Q. 1999. A bifunctional transposon mini-Tn5gfp-km which can be used to select for promoter fusions and report gene expression levels in Agrobacterium tumefaciens. FEMS Microbiol. Lett. 179:37–42 [DOI] [PubMed] [Google Scholar]
- 56. Thompson J. D., Higgins D. G., Gibson T. J. 1994. Clustal-W—improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vanderlinde E. M., et al. 2010. Identification of a novel ABC transporter required for desiccation tolerance, and biofilm formation in Rhizobium leguminosarum bv. viciae 3841. FEMS Microbiol. Ecol. 71:327–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vanderlinde E. M., et al. 2009. Rhizobium leguminosarum biovar viciae 3841, deficient in 27-hydroxyoctacosanoate-modified lipopolysaccharide, is impaired in desiccation tolerance, biofilm formation and motility. Microbiology 155:3055–3069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vedam V., Haynes J. G., Kannenberg E. L., Carlson R. W., Sherrier D. J. 2004. A Rhizobium leguminosarum lipopolysaccharide lipid-A mutant induces nitrogen-fixing nodules with delayed and defective bacteroid formation. Mol. Plant Microbe Interact. 17:283–291 [DOI] [PubMed] [Google Scholar]
- 60. Vincent V. M. 1970. A manual for the practical study of root-nodule bacteria. Lippincott Williams & Wilkins, Hagerstown, MD [Google Scholar]
- 61. Vriezen J. A. C., de Bruijn F. J., Nüsslein K. 2007. Responses of rhizobia to desiccation in relation to osmotic stress, oxygen and temperature. Appl. Environ. Microbiol. 73:3451–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Williams K. P., Sobral B. W., Dickerman A. W. 2007. A robust species tree for the Alphaproteobacteria. J. Bacteriol. 189:4578–4586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yost C. K., Rath A. M., Noel T. C., Hynes M. F. 2006. Characterization of genes involved in erythritol catabolism in Rhizobium leguminosarum bv. viciae. Microbiology 152:2061–2074 [DOI] [PubMed] [Google Scholar]
- 64. Yost C. K., Del Bel K. L., Quandt J., Hynes M. F. 2004. Rhizobium leguminosarum methyl-accepting chemotaxis protein genes are down-regulated in the pea nodule. Arch. Microbiol. 182:505–513 [DOI] [PubMed] [Google Scholar]
- 65. Yost C. K., Rochepeau P., Hynes M. F. 1998. Rhizobium leguminosarum contains a group of genes that appear to code for methyl-accepting chemotaxis proteins. Microbiology 144:1945–1956 [DOI] [PubMed] [Google Scholar]
- 66. Young J. P., et al. 2006. The genome of Rhizobium leguminosarum has recognizable core and accessory components. Genome Biol. 7:R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.