Abstract
The obligate aceticlastic methanogen Methanosaeta thermophila uses a membrane-bound ferredoxin:heterodisulfide oxidoreductase system for energy conservation. We propose that the system is composed of a truncated form of the F420H2 dehydrogenase, methanophenazine, and the heterodisulfide reductase. Hence, the electron transport chain is distinct from those of well-studied Methanosarcina species.
TEXT
Biogenic methane production is dominated by methanoarchaea of the genera Methanosarcina (Ms.) and Methanosaeta (Mt.) that grow on acetate (6). Interestingly, Methanosaeta species can use only acetate as a substrate and are therefore obligate aceticlastic methanogens. Members of this genus are of special importance for the productivity of biogas plants, especially for reactor performance and stability at low acetate concentrations. To optimize biomethanation, it is necessary to acquire a comprehensive understanding of the biochemistry of acetate-dependent methanogenesis. Energy conservation in Methanosaeta species is not well understood, and even the sequencing of the Methanosaeta thermophila genome (13) did not unravel its mechanism. Comparative genomics indicated that the core methanogenic pathway, the breakdown of acetyl-coenzyme A (CoA) to methane, is obviously well conserved in Mt. thermophila. It can be concluded that acetate is activated by acetyl-CoA synthetases and the resulting acetyl-CoA serves as a substrate for a CO dehydrogenase/acetyl-CoA synthase (CODH/ACS) that oxidizes the carbonyl group to CO2 and reduces ferredoxin. The methyl group is first transferred to tetrahydromethanopterin and then to coenzyme M (CoM) (2-mercaptoethanesulfonate) by the action of a membrane-bound Na+ translocating methyltransferase. Methyl-CoM is oxidatively coupled to coenzyme B (CoB) (N-7-mercaptoheptanoyl-l-threonine phosphate) with the heterodisulfide CoM-S-S-CoB and methane as end products (5, 15). In contrast, the composition of the Mt. thermophila respiratory chain and the mode of energy conservation have remained largely unknown. Evidence was found only for the presence of the reduced ferredoxin (Fdred) forming CODH/ACS and the heterodisulfide reductase (1, 13, 14). In Methanosarcina species, a ferredoxin:heterodisulfide oxidoreductase is used for energy conservation in acetate metabolism. Methanosarcina mazei and Methanosarcina barkeri employ the Ech hydrogenase for H2 production from Fdred and the H2 uptake hydrogenase (Vho) that finally reduces methanophenazine, the electron donor for the heterodisulfide reductase (HdrDE). In Methanosarcina acetivorans, Ech hydrogenase is absent, and instead the Rnf complex is proposed to be responsible for Fdred oxidation (6). Surprisingly, the Mt. thermophila genome does not contain genes coding for either hydrogenases or an Rnf complex (13). If Fdred serves as an electron donor for the respiratory chain, the presence of a novel oxidoreductase must be postulated.
To investigate the electron transport processes in Mt. thermophila, we isolated cytoplasmic membranes from Mt. thermophila DSM6194 as described previously (17) with cell disruption by French pressure treatment (1,000 lb/in2). Enzyme assays were carried out at 55°C (optimal growth temperature) (3, 17). Benzyl viologen-dependent heterodisulfide reductase activity was high, with 878 ± 90 mU mg−1 membrane protein (Table 1), and was comparable to activities found in Methanosarcina species (3) (Fig. 1). Hence, it is tempting to speculate that a membrane-bound heterodisulfide oxidoreductase system is used for energy conservation in Mt. thermophila, with a so-far-unidentified enzyme system that channels electrons into the respiratory chain. Genes encoding the F420H2 dehydrogenase (Fpo) were identified in the genome of Mt. thermophila, and this protein is therefore a candidate for electron input into the respiratory chain. Fpo is usually involved in methylotrophic methanogenesis and oxidizes F420H2 that is formed in the methanogenic pathway of Methanosarcina species. The Methanosarcina core enzyme FpoA to -O is highly homologous to NADH dehydrogenase I from bacteria and eukarya. However, the reduced cofactor oxidizing subunits from F420H2 dehydrogenases and NADH dehydrogenases are not homologous. The corresponding module of the bacterial and eukaryotic enzymes is made from the subunits NuoEFG. In contrast, the oxidation of reduced cofactor F420 is catalyzed by subunit FpoF of the F420H2 dehydrogenase (4). Interestingly, the Mt. thermophila genome codes only for an incomplete F420H2 dehydrogenase (FpoA to -N) that lacks FpoF and thus should be unable to oxidize F420H2, as shown for the Ms. mazei ΔfpoF mutant (16). Nevertheless, substantial quantities of F420 can be found in Mt. thermophila cells (9), so the F420H2 oxidizing reactivity of the membranes was determined. As expected, we could not detect either F420H2:heterodisulfide oxidoreductase activity or F420H2 dehydrogenase activity (Table 1). These findings show that energy conservation is not dependent on F420. Also, NAD(P)H did not serve as an electron donor for heterodisulfide reduction in Mt. thermophila (Table 1). Many methanogens rely on hydrogen as an electron donor and/or obligate intermediate in the oxidation of other reducing equivalents (Fdred/F420H2). For this purpose, some methanogens make use of a cytoplasmic F420 reducing hydrogenase (Frh) that can oxidize F420H2 with concomitant H2 production and then use the membrane-bound Vho hydrogenase:heterodisulfide oxidoreductase to conserve energy (10, 16). Mt. thermophila does not possess genes coding for Frh or Vho, and indeed there was no hydrogenase activity or hydrogen:heterodisulfide oxidoreductase activity in Mt. thermophila membranes (Table 1). Therefore, an involvement of hydrogen or a hydrogen cycling mechanism for energy conservation in Mt. thermophila can be excluded. For the investigation of the Fd:heterodisulfide oxidoreductase in Mt. thermophila, an Ms. mazei ferredoxin, MM1619, was employed. The gene mm1619 was cloned into pPR-IBA1 using BsaI restriction sites (primers 5′-ATGGTAGGTCTCAAATGCCAGCAATAGTTAACGCAGATGAA-3′ and 5′-ATGGTAGGTCTCAGCGCTTTCCGTTACTTTAATTGCCTGGTTC-3′), and the recombinant protein was produced in Escherichia coli BL21(DE3) (8, 12) and purified anaerobically (16). When ferredoxin MM1619 was reduced with the thermophilic organism Moorella thermoacetica CODH/ACS and incubated with Mt. thermophila membranes, heterodisulfide reduction was observed. This reaction was strictly dependent on ferredoxin and proceeded with a velocity of 470 ± 44 mU mg−1 membrane protein (Table 1). In comparison to experiments performed with membranes isolated from acetate-grown Ms. mazei (unpublished results), this reaction was 2- to 3-fold faster in Mt. thermophila than in Ms. mazei. These experiments elucidate the identity of the electron donor to the Methanosaeta respiratory chain as ferredoxin (Fig. 1). Our current working hypothesis is that in Mt. thermophila, the energy-conserving system is an Fd:heterodisulfide oxidoreductase that comprises the Fpo complex (without FpoF) and the heterodisulfide reductase, both of which are probably able to translocate H+ or Na+ across the cytoplasmic membrane (2, 7). The “headless” Fpo complex does not interact with F420H2, but it is tempting to speculate that iron-sulfur clusters in the FpoB or FpoI subunits directly accept electrons from Fdred. In addition, it is evident that the membrane-bound methyltransferase contributes to the maintenance of the electrochemical ion gradient. An A1AO ATP synthase finally takes advantage of the ion motive force and produces ATP from ADP + Pi (11).
Table 1.
Activities of membrane-bound oxidoreductases in Mt. thermophila
| Enzymea (system) | Electron donor | Electron acceptor | Reduction rate of electron acceptor (nmol min−1 mg−1) |
|---|---|---|---|
| F420H2 dehydrogenase | F420H2 | Metronidazole | <1 |
| Hydrogenase | H2 | Methyl viologen | <1 |
| Heterodisulfide reductase | Benzyl viologen | CoM-S-S-CoB | 878 ± 90 |
| F420H2: heterodisulfide oxidoreductase | F420H2 | CoM-S-S-CoB | <1 |
| H2:heterodisulfide oxidoreductase | H2 | CoM-S-S-CoB | <1 |
| Fd:heterodisulfide oxidoreductase | Fdred | CoM-S-S-CoB | 470 ± 44 |
| NADH:heterodisulfide oxidoreductase | NADH | CoM-S-S-CoB | <1 |
| NADPH:heterodisulfide oxidoreductase | NADPH | CoM-S-S-CoB | <1 |
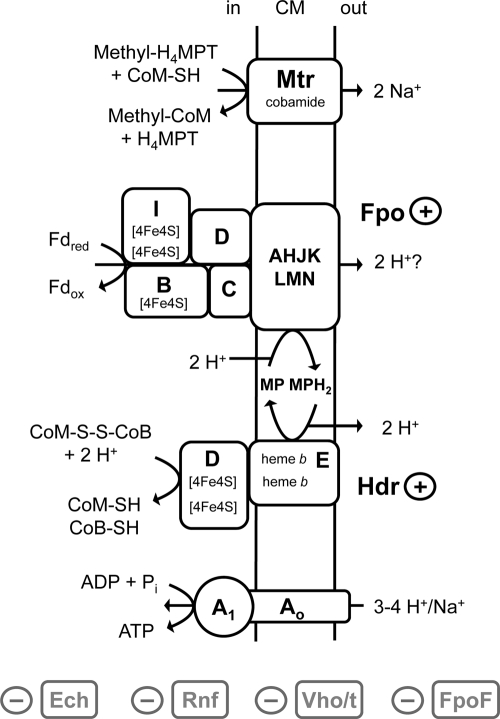
Fig. 1.
Putative model of energy-conserving electron transfer reactions in Mt. thermophila. A1AO, A1AO ATP synthase; CM, cytoplasmic membrane; Ech, Ech hydrogenase; Fdred, reduced ferredoxin; Fdox, oxidized ferredoxin; FpoABCDHIJKLMN, subunits A to N of the F420H2 dehydrogenase; FpoF, F subunit of the F420H2 dehydrogenase; H4MPT, tetrahydromethanopterin; HdrDE, heterodisulfide reductase subunits D and E; Mtr, methyltransferase; MP, methanophenazine; MPH2, reduced methanophenazine; Rnf, Rnf complex; Vho/t, viologen-reducing hydrogenase one/two; (+), present in Mt. thermophila; (−), not present in Mt. thermophila.
In light of the discussion about energy-conserving systems in aceticlastic methanogens, it is important to note that an Fd:heterodisulfide oxidoreductase activity was also found in the membrane fraction of an Ms. mazei Δech mutant (17). The organism also contains the Fpo complex, and it was shown that subunit FpoF is in part located in the cytoplasm, indicating that the Fpo complex is not always completely covered by FpoF. This situation resembles the electron transport system of Mt. thermophila, and it is tempting to speculate that the Fpo complex (without FpoF) of Ms. mazei is also able to catalyze the oxidation of Fdred, thereby channelling electrons directly into the respiratory chain.
Acknowledgments
We thank Elisabeth Schwab for technical assistance and Paul Schweiger for critical reading of the manuscript. Many thanks also go to Gunes Bender and Steve Ragsdale, Department of Biological Chemistry, University of Michigan Medical School, for providing the CODH/ACS from Moorella thermoacetica.
This work was supported by the Deutsche Forschungsgemeinschaft (grant De488/9-1).
Footnotes
Published ahead of print on 8 April 2011.
REFERENCES
- 1. Allen G. W. J., Zinder S. H. 1996. Methanogenesis from acetate by cell-free extracts of the thermophilic acetotrophic methanogen Methanothrix thermophila CALS-1. Arch. Microbiol. 166:275–281 [DOI] [PubMed] [Google Scholar]
- 2. Bäumer S., et al. 2000. The F420H2 dehydrogenase from Methanosarcina mazei is a redox-driven proton pump closely related to NADH dehydrogenases. J. Biol. Chem. 275:17968–17973 [DOI] [PubMed] [Google Scholar]
- 3. Brodersen J., Bäumer S., Abken H. J., Gottschalk G., Deppenmeier U. 1999. Inhibition of membrane-bound electron transport of the methanogenic archaeon Methanosarcina mazei Gö1 by diphenyleneiodonium. Eur. J. Biochem. 259:218–224 [DOI] [PubMed] [Google Scholar]
- 4. Brüggemann H., Falinski F., Deppenmeier U. 2000. Structure of the F420H2:quinone oxidoreductase of Archaeoglobus fulgidus–-Identification and overproduction of the F420H2-oxidizing subunit. Eur. J. Biochem. 267:5810–5814 [DOI] [PubMed] [Google Scholar]
- 5. Deppenmeier U. 2002. The unique biochemistry of methanogenesis. Prog. Nucleic Acid Res. Mol. Biol. 71:223–283 [DOI] [PubMed] [Google Scholar]
- 6. Ferry J. G., Lessner D. J. 2008. Methanogenesis in marine sediments. Ann. N. Y. Acad. Sci. 1125:147–157 [DOI] [PubMed] [Google Scholar]
- 7. Ide T., Bäumer S., Deppenmeier U. 1999. Energy conservation by the H2:heterodisulfide oxidoreductase from Methanosarcina mazei Gö1: identification of two proton-translocating segments. J. Bacteriol. 181:4076–4080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jeoung J. H., Dobbek H. 2007. Carbon dioxide activation at the Ni,Fe-cluster of anaerobic carbon monoxide dehydrogenase. Science 318:1461–1464 [DOI] [PubMed] [Google Scholar]
- 9. Kamagata Y., et al. 1992. Characterization of 3 thermophilic strains of Methanothrix (Methanosaeta) thermophila sp. nov. and rejection of Methanothrix (Methanosaeta) thermoacetophila. Int. J. Syst. Evol. Microbiol. 42:463–468 [DOI] [PubMed] [Google Scholar]
- 10. Kulkarni G., Kridelbaugh D. M., Guss A. M., Metcalf W. W. 2009. Hydrogen is a preferred intermediate in the energy-conserving electron transport chain of Methanosarcina barkeri. Proc. Natl. Acad. Sci. U. S. A. 106:15915–15920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lewalter K., Müller V. 2006. Bioenergetics of archaea: ancient energy conserving mechanisms developed in the early history of life. Biochim. Biophys. Acta 1757:437–445 [DOI] [PubMed] [Google Scholar]
- 12. Nakamura M., Saeki K., Takahashi Y. 1999. Hyperproduction of recombinant ferredoxins in Escherichia coli by coexpression of the ORF1-ORF2-iscS-iscU-iscA-hscB-hscA-fdx-ORF3 gene cluster. J. Biochem. 126:10–18 [DOI] [PubMed] [Google Scholar]
- 13. Smith K. S., Ingram-Smith C. 2007. Methanosaeta, the forgotten methanogen? Trends Microbiol. 15:150–155 [DOI] [PubMed] [Google Scholar]
- 14. Terlesky K. C., Ferry J. G. 1988. Ferredoxin requirement for electron transport from the carbon monoxide dehydrogenase complex to a membrane-bound hydrogenase in acetate-grown Methanosarcina thermophila. J. Biol. Chem. 263:4075–4079 [PubMed] [Google Scholar]
- 15. Thauer R. K., Kaster A. K., Seedorf H., Buckel W., Hedderich R. 2008. Methanogenic archaea: ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 6:579–591 [DOI] [PubMed] [Google Scholar]
- 16. Welte C., Deppenmeier U. 2011. Re-evaluation of the function of the F420 dehydrogenase in electron transport in Methanosarcina mazei. FEBS J. 278:1277–1287 [DOI] [PubMed] [Google Scholar]
- 17. Welte C., et al. 2010. Function of Ech hydrogenase in ferredoxin-dependent, membrane-bound electron transport in Methanosarcina mazei. J. Bacteriol. 192:674–678 [DOI] [PMC free article] [PubMed] [Google Scholar]