Abstract
The asparaginyl-tRNA synthetase-like SlgZ and methyltransferase SlgM enzymes are involved in the biosynthesis of the tetramic acid streptolydigin in Streptomyces lydicus. Inactivation of slgZ led to a novel streptolydigin derivative. Overexpression of slgZ, slgM, or both in S. lydicus led to a considerable increase in streptolydigin production.
TEXT
Hybrid polyketide-nonribosomal peptide compound streptolydigin (Fig. 1 A), produced by Streptomyces lydicus, is a member of the tetramic acid family (21). It is a potent inhibitor of bacterial RNA polymerase (RNAP) (20, 22, 23). In addition, streptolydigin has been shown to inhibit terminal deoxynucleotidyltransferase, an enzyme found in large amounts in leukocytes from patients with acute lymphoblastic leukemia or with rare cases of acute and chronic myelocytic leukemia (6, 7). The streptolydigin biosynthesis gene cluster from S. lydicus NRRL2433 has been recently characterized, and the involvement of a hybrid polyketide synthase (PKS)/nonribosomal peptide synthetase (NRPS) system has been confirmed. In addition, genes encoding proteins involved in secretion, pathway regulation, tailoring modification, and precursor supply were identified (16). Among the precursor supply-associated genes, slgZ and slgM, encoding an asparaginyl-tRNA synthetase like enzyme and a methyltransferase, respectively, have been proposed to be involved in the tailoring modification of precursor 3-methyl-aspartate (16). In this communication, we provide further experimental evidence of the role of these two genes in precursor supply for streptolydigin biosynthesis.
Fig. 1.
(A) Structures of streptolydigin (compound 1) and novel derivative streptolydigin B (compound 2). (B) Schematic representation of SlgZ (S. lydicus), AsnRS2, and AsnRS (P. abyssi). A sequence comparison of three characteristic motifs present in the catalytic core is shown. The invariant residues from each motif are indicated by black circles. (C) Supply of amino acid precursor for the biosynthesis of streptolydigin. A′, SlgN1 adenylation domain; A, SlgN2 adenylation domain; C, condensation domain; PCP, peptidyl carrier protein.
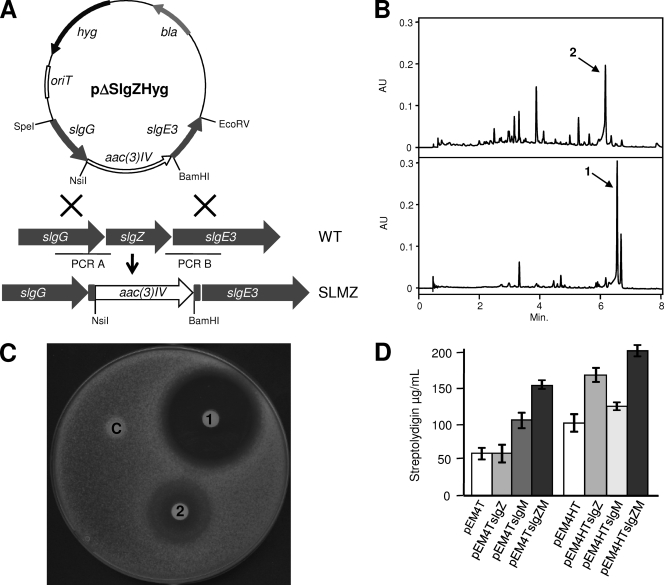
SlgZ shows similarity to class IIb aminoacyl-tRNA synthetases. However, this was a surprising finding, since, apparently, no aminoacyl-tRNA synthetase was anticipated to be necessary for streptolydigin biosynthesis. To the best of our knowledge, few aminoacyl-tRNA synthetases have been demonstrated to be involved in antibiotic biosynthesis; such synthetases include seryl-tRNA synthetase VlmL from Streptomyces viridifaciens MG456-hF10 (8) and aminoacyl-tRNA synthetase-like cyclodipeptide synthases such as AlbC, which uses aminoacyl-tRNAs as substrates to catalyze the formation of the diketopiperazine peptide bonds (10). AlbC participates in the biosynthesis of valinomycin, catalyzing the transfer of the seryl residue from seryl-tRNA to the hydroxyl group of isobutylhydroxylamine (9). Nevertheless, SlgZ also shows similarity to archaeal asparaginyl-tRNA synthetases and, in particular, to a kind of such enzymes that contains the catalytic core of asparaginyl-tRNA synthetases but lacks the N-terminal anticodon-binding site (Fig. 1B). This kind of truncated form of asparaginyl-tRNA synthetases has been shown to produce asparagine by amidation of aspartic acid. In particular, this activity has been demonstrated in the case of AsnRS2 from Pyrococcus abyssi. This proved that AsnRS2 is the archaeal orthologue of the bacterial ammonia-dependent asparagine synthetase A (19). The role of SlgZ in streptolydigin biosynthesis was therefore further investigated by deleting slgZ from S. lydicus by intergeneric conjugation from Escherichia coli ET12567(pUB307) and using pΔslgZHyg (Table 1). A single-crossover strain, which was apramycin and hygromycin resistant, was grown in the absence of antibiotic selection, and then colonies were screened for hygromycin sensitivity and apramycin resistance as a consequence of a double recombination event. This approach led to the mutant SLMZ (Fig. 2 A).
Table 1.
Bacterial strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Description or relevant characteristicsa | Reference, source, or restriction enzyme |
|---|---|---|
| Strains | ||
| S. lydicus | ||
| NRRL2433 | Streptolydigin producer | 16 |
| SLMZ | S. lydicus with slgZ deletion; streptolydigin B producer | This study |
| SLMM | S. lydicus with slgM deletion | This study |
| Escherichia coli | ||
| DH10B | For subcloning | Invitrogen |
| ET12567(pUB307) | For S. lydicus conjugation | 12 |
| Plasmids | ||
| Slg4A8 | Source of slgZ and slgM | 16 |
| pOJPM | Source of slgM | 16 |
| pCR-BLUNT | For initial cloning and sequencing of PCR products | Invitrogen |
| pOJ260 | Source of oriT RK2 region | 2 |
| pOJ260P | For gene replacement in S. lydicus | 15 |
| pEM4T | For gene expression in S. lydicus | 14 |
| pLHyg | Source of hygromycin resistance gene hyg | 15 |
| pEFBA | Source of apramycin resistance gene aac(3)IV | 13 |
| pEFBAoriT | pEFBA containing oriT region from pOJ260 as an XbaI-SpeI fragment | This study |
| pΔslgZHyg | pEFBAoriT containing hyg as an SpeI-NheI fragment and PCR products HEI19/HEI20 and HEI21/HEI22 flanking aac(3)IV; used to generate mutant SLMZ | This study |
| pOJM | pOJ260P containing PCR product CRIS15/CRIS16; used to generate mutant SLMM | This study |
| pEM4TslgZ | pEM4T containing slgZ PCR product HEI23/HEI24 | This study |
| pEM4TslgM | pEM4T containing slgM as a BamHI-EcoRI fragment from pOJPM | This study |
| pEM4TslgZM | pEM4TslgM containing slgZ as a BamHI fragment | This study |
| pEM4HT | pEM4T containing hyg as an SpeI-NheI fragment | |
| pEM4HTslgZ | pEM4TslgZ containing hyg as an SpeI-NheI fragment | This study |
| pEM4HTslgM | pEM4TslgM containing hyg as an SpeI-NheI fragment | This study |
| pEM4HTslgZM | pEM4TslgZM containing hyg as an SpeI-NheI fragment | This study |
| Primers | ||
| HEI19 | AACTAGTCCTTCACCGCCCTGGCCC | SpeI |
| HEI20 | AAAATGCATGTGAGGGCGGCAGCTGGC | NsiI |
| HEI21 | AAGGATCCCGAAGATCCCCGGCGTGG | BamHI |
| HEI22 | AAGATATCGTCGAGGAACGCGTGCGG | EcoRV |
| CRIS15 | AATCTAGAACGTGGGCGACGACACGG | XbaI |
| CRIS16 | AGAATTCCTGGTAGCCGCCGTCCG | EcoRI |
| HEI23 | AAGGATCCGGCAAGTACCGCGGCGCC | BamHI |
| HEI24 | AAGGATCCGGCAGACAGCCCGCTGCC | BamHI |
Underlining in the primer sequences indicates restriction sites.
Fig. 2.
(A) Scheme representing the replacement of slgZ in the chromosome of S. lydicus (wild type [WT]) with the apramycin resistance cassette. aac(3)IV, apramycin resistance gene; hyg, hygromycin resistance gene; bla, β-lactamase gene. (B) UPLC analysis of mutant SLMZ (upper panel) and mutant SLMZ complemented by plasmid pEM4TslgZ (lower panel). AU, arbitrary units. (C) Antibiotic activity of streptolydigin (1) and streptolydigin B (2) against S. albus. Each paper disk was soaked with 2 μg of the corresponding compound. Control (C), without antibiotic. (D) Effect of slgZ, slgM, and slgZ-slgM overexpression in S. lydicus on streptolydigin production. Cultures were performed on R5A solid media, and streptolydigin production was determined by high-performance liquid chromatography (HPLC) analysis. Experiments were run in triplicate.
Analysis of the products accumulated by mutant SLMZ (Fig. 2B) showed a novel compound (3) with an ultrahigh-performance liquid chromatography (UPLC) retention time of 6.1 min. Upon mass spectrometry (MS) analysis, this peak showed two ions with m/z 587 and 473 ([M + H]+), corresponding to the unfragmented compound and the aglycon fragment ions, respectively. The structural elucidation of this compound was carried out using one-dimensional (1D) 1H, two-dimensional (2D) 1H cooler synchrotron (COSY), 1H, 13C heteronuclear single-quantum coherence (HSQC)-edited, and heteronuclear multiple-bond correlation (HMBC) nuclear magnetic resonance (NMR) experiments. This allowed us to identify the compound as streptolydigin B, a derivative of streptolydigin with a tetramic acid lateral side chain derived from glutamate instead of 3-methyl-aspartate (Fig. 1A). Production of streptolydigin in mutant SLMZ was restored by introduction of plasmid pEM4TslgZ, containing slgZ under the control of the ermE* promoter (Fig. 2B). All these experiments demonstrate the utilization of glutamate instead of 3-methyl-aspartate as the substrate by the streptolydigin NRPS complex and consequently show a certain degree of substrate flexibility. This incorporation might be sustained by the 2-fold increase in the glutamate pool of SLMZ compared with that of the S. lydicus wild type, observed when the intracellular amino acid pools were determined by growing the strains in a minimal medium with NH4Cl as the nitrogen source, medium that sustains the production of streptolydigin by S. lydicus. The analysis of glutamate pools was performed by amino acid derivatization with phenylisothiocyanate and UPLC analysis as described previously (1).
The fact that SLMZ incorporates glutamate is a clear suggestion that the SlgZ protein acts as an aminotransferase, converting 3-methyl-aspartate into 3-methyl-asparagine, in a manner similar to that of AsnR2 of P. abyssi (19). Therefore, we propose that SlgZ is not a classical asparaginyl-tRNA synthetase but a 3-methyl-asparagine synthetase.
The antibiotic activity of streptolydigin B was tested and compared with that of streptolydigin by a bioassay as previously described (16). Streptolydigin B showed less activity against Streptomyces albus than streptolydigin (Fig. 2C). This indicates that the modification of the tetramic acid lateral side chain, and in particular the replacement of the acetamide moiety by a carboxyl group, reduces the antibacterial activity of this compound. This effect is supported by the observation of the nitrogen of this moiety interacting with the Thermus thermophilus RNAP β′ subunit Asn729 and the fact that the RNAP is less sensitive to tirandamycin (22), which also lacks the acetamide group.
The effect of SlgZ on the production of streptolydigin was assessed by overexpressing the corresponding gene in the S. lydicus wild-type strain. We used two plasmids, pEM4TslgZ and pEM4HTslgZ (Table 1), both carrying slgZ under the control of the ermE* promoter but each carrying a different antibiotic marker, that for apramycin in pEM4TslgZ and that for hygromycin in pEM4HTslgZ. Expression of slgZ by using pEM4TslgZ had no apparent effect on streptolydigin production, while its expression by using pEM4HTslgZ led to a 1.5-fold increase in streptolydigin production (Fig. 2D).
SlgM is a putative methyltransferase that contains the methyltransferase-11 signature domain present in S-adenosylmethionine (SAM)-dependent methyltransferases of this family of enzymes (pfam 08241). An slgM mutant was generated by gene disruption using pOJM (Table 1). Transconjugants were selected for resistance to apramycin. Analysis of the products accumulated by mutant SLMM showed no production of streptolydigin or any other derived compound (data not shown). This was an unexpected result, since, as previously reported, demethyl-streptolydiginone was isolated from mutant SLM7H13 (16), with l-rhodinose biosynthesis genes slgS3 to slgS7 deleted, and it was expected to be accumulated by SLMM. Accumulation of a streptolydigin intermediate or derivative compound would confirm the participation of SlgM in tailoring modification, as has been the case with other methyltransferases involved in the biosynthesis of polyketides and nonribosomal peptide compounds (17, 18). However, the lack of antibiotic production in mutant SLMM is in agreement with the results obtained by inactivating lipMt in the tetramic acid α-lipomycin biosynthesis gene cluster from Streptomyces aureofaciens Tü117 (3). In that case, the lack of LipMt abolished α-lipomycin production and led to the authors of that study to propose that the methylation event occurs on an NRPS-bound glutamic acid substrate (3). Curiously, in the α-lipomycin biosynthesis gene cluster lipMt, lipX2, and lipNrps are organized in the same manner as slgM, slgL, and slgN2 are in the streptolydigin gene cluster. The proteins encoded by these genes in each pathway are homologues and are proposed to perform equivalent activities (3, 16).
The absence of demethyl-streptolydiginone in cultures of SLMM led us to propose also a bound 3-methyl-asparagine-NRPS as the substrate for the SlgM methylation event during streptolydigin biosynthesis (Fig. 1C). This N-methylation step might be required for the correct condensation of the amino acid-NRPS bound to the already formed polyketide backbone. The absence of SlgM might imply the presence of a bound 3-methyl-asparagine-NRPS (with a free amino group), thus blocking the biosynthetic pathway (or, less probably, generating an unstable intermediate). Otherwise, if not blocked, the streptolydigin NRPS should incorporate glutamate, thus generating streptolydigin B (as in mutant SLMZ), a compound that is not produced by mutant SLMM. The bound 3-methyl-asparagine-NRPS substrate might arise from the incorporation of 3-methyl-aspartate into the NRPS, followed by its amidation by SlgZ (Fig. 1C), since purified SlgZ retains a slight level of aspartate amidation activity but is unable to amidate free 3-methyl-aspartate (data not shown). Production of streptolydigin in mutant SLMM was restored by introduction of plasmid pEM4TslgM, containing slgM under the control of the ermE* promoter.
Considering the positive effect of expressing slgZ in the S. lydicus wild-type strain on streptolydigin production, the effects of expressing slgM and both slgZ and slgM in this strain were analyzed. The plasmids used to carry on these experiments were pEM4TslgM, pEM4HTslgM, pEM4TslgZM, and pEM4HTslgZM (Table 1). The use of pEM4TslgM and pEM4HTslgM led to moderate increases in streptolydigin production (2- and 1.1-fold, respectively) (Fig. 2D). However, a cooperative effect was observed by expressing slgZ and slgM by using pEM4TslgZM and pEM4HTslgZM. These coexpression experiments led to 3- and 2.1-fold increases in production, respectively (Fig. 2D).
One issue remains to be clarified: the origin of demethyl-streptolydiginone isolated and characterized from mutant SLM7H13 (16). In this mutant, two nonglycosylated compounds were isolated: demethyl-streptolydiginone and streptolydiginone. The initial proposal for the streptolydigin pathway assumed that demethyl-streptolydiginone was the precursor of streptolydiginone to be N methylated by SlgM. The results obtained in this work with mutant SLMM prove that demethyl-streptolydiginone is not the substrate for SlgM. However, demethyl-streptolydiginone could be derived from streptolydiginone by an S. lydicus demethylase. Demethylation activity, performed by family 51 of cytochrome P450s, of different structural types of bioactive compounds is widely distributed among actinomycetes and has been reported from several Streptomyces and Mycobacterium species. In particular, drug N demethylases have been reported from Streptomyces griseus ATCC 13273 (5), Streptomyces platensis NRRL2364 (4), and other Streptomyces spp. (11).
Acknowledgments
This research was supported by a grant of the Spanish Ministry of Science and Innovation (BFU2006-00404 to J.A.S.) and by Red Temática de Investigación Cooperativa de Centros de Cáncer (Ministry of Health grant ISCIII-RETIC RD06/0020/0026). We thank Obra Social Cajastur for financial support to C.O. and the Spanish Ministry of Science and Innovation for a Ph.D. student fellowship (Formación de Personal Investigator [FPI]) for C.G. We thank the Spanish Ministerio de Ciencia e Innovación of Spain (MICINN, SAF2008-01845) and the Centro de Investigación Príncipe Felipe for their economic support.
Footnotes
Published ahead of print on 11 March 2011.
REFERENCES
- 1. Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. 1984. Rapid analysis of amino acids using pre-column derivatization. J. Chromatogr. 336:93–104 [DOI] [PubMed] [Google Scholar]
- 2. Bierman M., et al. 1992. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43–49 [DOI] [PubMed] [Google Scholar]
- 3. Bihlmaier C., et al. 2006. Biosynthetic gene cluster for the polyenoyltetramic acid alpha-lipomycin. Antimicrob. Agents Chemother. 50:2113–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davis P. J., Glade J. C., Clark A. M., Smith R. V. 1979. N-demethylation of lergotrile by Streptomyces platensis. Appl. Environ. Microbiol. 38:891–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis B. L., Liu M. S., Hanson R. L., Parker W. L., Patel R. N. 2009. Microbial N-demethylation: biotransformation and recovery of a drug metabolite. Biotechnol. Appl. Biochem. 53:133–137 [DOI] [PubMed] [Google Scholar]
- 6. DiCioccio R. A., Srivastava B. I. 1976. Selective inhibition of terminal deoxynucleotidyl transferase from leukemic cells by streptolydigin. Biochem. Biophys. Res. Commun. 72:1343–1349 [DOI] [PubMed] [Google Scholar]
- 7. DiCioccio R. A., et al. 1980. Structure-activity relationship, selectivity and mode of inhibition of terminal deoxyribonucleotidyltransferase by streptolydigin analogs. Biochem. Pharmacol. 29:2001–2008 [DOI] [PubMed] [Google Scholar]
- 8. Garg R. P., González J. M., Parry R. J. 2006. Biochemical characterization of VlmL, a seryl-tRNA synthetase encoded by the valanimycin biosynthetic gene cluster. J. Biol. Chem. 281:26785–26791 [DOI] [PubMed] [Google Scholar]
- 9. Garg R. P., Qian X. L., Alemany L. B., Moran S., Parry R. J. 2008. Investigations of valanimycin biosynthesis: elucidation of the role of seryl-tRNA. Proc. Natl. Acad. Sci. U. S. A. 105:6543–6547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gondry M., et al. 2009. Cyclodipeptide synthases are a family of tRNA-dependent peptide bond-forming enzymes. Nat. Chem. Biol. 5:414–420 [DOI] [PubMed] [Google Scholar]
- 11. Griffiths D. A., Best D. J., Jezequel S. G. 1991. The screening of selected microorganisms for use as models of mammalian drug metabolism. Appl. Microbiol. Biotechnol. 35:373–381 [DOI] [PubMed] [Google Scholar]
- 12. Kieser T., Bibb M. J., Buttner M. J., Chater K. F., Hopwood D. A. 2000. Practical Streptomyces genetics. The John Innes Foundation, Norwich, United Kingdom [Google Scholar]
- 13. Lozano M. J., et al. 2000. Characterization of two polyketide methyltransferases involved in the biosynthesis of the antitumor drug mithramycin by Streptomyces argillaceus. J. Biol. Chem. 275:3065–3074 [DOI] [PubMed] [Google Scholar]
- 14. Menéndez N., et al. 2006. Deoxysugar transfer during chromomycin A3 biosynthesis in Streptomyces griseus subsp. griseus: new derivatives with antitumor activity. Appl. Environ. Microbiol. 72:167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Olano C., et al. 2004. Biosynthesis of the angiogenesis inhibitor borrelidin by Streptomyces parvulus Tü4055: cluster analysis and assignment of functions. Chem. Biol. 11:87–97 [DOI] [PubMed] [Google Scholar]
- 16. Olano C., et al. 2009. Deciphering biosynthesis of the RNA polymerase inhibitor streptolydigin and generation of glycosylated derivatives. Chem. Biol. 16:1031–1044 [DOI] [PubMed] [Google Scholar]
- 17. Olano C., Méndez C., Salas J. A. 2009. Antitumor compounds from actinomycetes: from gene clusters to new derivatives by combinatorial biosynthesis. Nat. Prod. Rep. 26:628–660 [DOI] [PubMed] [Google Scholar]
- 18. Olano C., Méndez C., Salas J. A. 2010. Post-PKS tailoring steps in natural products-producing actinomycetes from the perspective of combinatorial biosynthesis. Nat. Prod. Rep. 27:571–616 [DOI] [PubMed] [Google Scholar]
- 19. Roy H., Becker H. D., Reinbolt J., Kern D. 2003. When contemporary aminoacyl-tRNA synthetases invent their cognate amino acid metabolism. Proc. Natl. Acad. Sci. U. S. A. 100:9837–9842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sánchez-Hidalgo M., Núñez L. E., Méndez C., Salas J. A. 2010. Involvement of the beta subunit of RNA polymerase in resistance to streptolydigin and streptovaricin in the producer organisms Streptomyces lydicus and Streptomyces spectabilis. Antimicrob. Agents Chemother. 54:1684–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schobert R., Schlenk A. 2008. Tetramic and tetronic acids: an update on new derivatives and biological aspects. Bioorg. Med. Chem. 16:4203–4221 [DOI] [PubMed] [Google Scholar]
- 22. Temiakov D., et al. 2005. Structural basis of transcription inhibition by antibiotic streptolydigin. Mol. Cell 19:655–666 [DOI] [PubMed] [Google Scholar]
- 23. Tuske S., et al. 2005. Inhibition of bacterial RNA polymerase by streptolydigin: stabilization of a straight-bridge-helix active-center conformation. Cell 122:541–552 [DOI] [PMC free article] [PubMed] [Google Scholar]