Abstract
Members of the CarA/LitR family are MerR-type transcriptional regulators that contain a C-terminal cobalamin-binding domain. They are thought to be involved in light-induced transcriptional regulation in a wide variety of nonphototrophic bacteria. Based on the distribution of this kind of regulator, the current study examined carotenoid production in Thermus thermophilus, and it was found to occur in a light-induced manner. litR and carotenoid and cobalamin biosynthesis genes were all located on the large plasmid of this organism. litR or cobalamin biosynthesis gene knockout mutants were unable to switch off carotenoid production under dark conditions, while a mutant with a mutation in the downstream gene adjacent to litR (TT_P0055), which encodes a CRP/FNR family transcriptional regulator, was unable to produce carotenoids, irrespective of light conditions. Overall, genetic and biochemical evidence indicates that LitR is bound by cobalamin and associates with the intergenic promoter region between litR and crtB (phytoene synthase gene), repressing the bidirectional transcription of litR and crtB. It is probable that derepression of LitR caused by some photodependent mechanism induces the expression of TT_P0055 protein, which serves as a transcriptional activator for the crtB operon and hence causes the expression of carotenoid biosynthesis and the DNA repair system under light condition.
INTRODUCTION
Light is an environmental stimulus that affects many living organisms, including prokaryotes. In recent years, various kinds of photoreceptors have been discovered across a wide range of bacterial taxa (24). Interestingly, genome sequencing studies have revealed the presence of many putative light-sensing proteins in nonphotosynthetic bacteria (30). Such findings provide new insight into the physiology and ecology of bacteria, although photodependent bacterial phenotypes have not yet been extensively characterized.
A well-known photodependent physiological feature of nonphototrophic bacteria is their production of carotenoids, the yellow, orange, or red pigments that protect cells from harmful oxygen radicals, including photooxidative stress. It has been widely observed that bacterial cells accumulate this kind of pigment when they are illuminated. This means that many bacteria have retained a certain genetic regulatory system that transmits an illumination signal to the genes that govern the expression of carotenoid biosynthesis, but the details of such a regulatory system are poorly known. The only exception to this is the knowledge of light-induced carotenoid production in Myxococcus xanthus, a Gram-negative gliding bacterium. The complex signaling network of this organism involves the function of multiple regulators, including the MerR-type transcriptional regulators CarA and CarH (see reference 23 and references cited therein).
Recently, we discovered that carotenoid production in Streptomyces coelicolor A3(2), a Gram-positive filamentous bacterium, also occurred in a light-induced manner, and we identified the regulatory proteins responsible for its control (29). We also studied the transcriptional regulation of its carotenoid biosynthetic gene (crt) cluster and found that litR (light-induced transcription, regulator) and litS (light-induced transcription, sigma factor) were responsible for the photodependent transcription of the crt cluster. Evidence indicated that the LitR protein, a MerR-type transcriptional regulator, is involved in the light-induced transcription of litS, which encodes an extracytoplasmic function (ECF) sigma factor that directs the transcription of crt operons (29). A subsequent study involving a heterologous host expression experiment raised the possibility that LitR is used exclusively in the light-dependent transcriptional control of the litS promoter (28).
LitR belongs to the MerR family, whose regulatory activity depends on the binding of a ligand (3, 12). Usually, the ligand has a direct correlation with the role of genes whose expression is controlled by the MerR family regulator. For example, the MerR of Gram-negative bacteria is bound by mercury ions and induces the transcription of the mercury resistance (mer) operon; the operator-bound MerR represses the transcription of the mer operon in the absence of mercury ions but in turn activates transcription when mercury ions bind to the C-terminal ligand-binding site of MerR (3).
LitR, as well as CarA and CarH, of M. xanthus contains a cobalamin (Cbl) (vitamin B12)-binding motif in its C-terminal region. Our previous observations suggest that blue-light absorption by Cbl plays a crucial role in the LitR function (28). Blue light is responsible for light-induced carotenoid production in S. coelicolor. The LitR of S. coelicolor A3(2), however, could not be obtained as a soluble recombinant protein in an Escherichia coli expression system; in addition, its coding sequence could not be disrupted in our repeated experiments (29). These difficulties have hampered our detailed characterization of the roles of LitR in S. coelicolor.
Interestingly, CarA/LitR homologs are found in a wide variety of nonphototrophic bacteria, including not only Actinobacteria but also Gram-negative bacteria such as Thermus, Pseudomonas, Shewanella, and Vibrio spp. (28). In these divergent organisms, the litR homologs commonly cluster with illumination-related genes, such as those encoding carotenoid biosynthesis enzymes, DNA photolyase, and PAS domain proteins. These findings have prompted us to speculate that these genes are expressed in a light-dependent manner, based on the function of the CarA/LitR homologs.
In the current study, we examined the role of the CarA/LitR homolog of Thermus thermophilus, an extremely thermophilic bacterium. The chemical structure of the carotenoids of this organism has been studied (31, 32), but its light dependence has not been elucidated. The evidence obtained via this study indicates that LitR and an additional transcriptional regulator are crucial for the light-dependent transcriptional control of carotenoid biosynthesis in this organism.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture media.
The wild-type (WT) strain of T. thermophilus used in this study was HB27 TH104 (proC, pTT8) (14). Escherichia coli JM109 and BL21(DE3)/pLysS (Takara-Shuzo, Kyoto, Japan) were used as hosts for DNA manipulation and protein expression, respectively. pUC19 (Takara-Shuzo) was used for general DNA manipulation. pT7Blue (Takara-Shuzo) was used for TA cloning of PCR-generated DNA fragments. pGEX-6P-2 (GE Healthcare Bio-Sciences KK, Tokyo, Japan) was used for the expression of LitR and TT_P0055 (here designated TTP55) protein in E. coli. Enzymes used for DNA manipulation were purchased from Takara-Shuzo. The conditions for the culture and genetic manipulation for E. coli and Thermus were as described by Maniatis et al. (19) and Koyama et al. (18), respectively. The E. coli-Thermus shuttle vector pTEV (carrying hygromycin B resistance) and pUC18-pJHK3 (carrying ampicillin and kanamycin resistance) (13) were used for the genetic manipulation of T. thermophilus. These plasmids had a copy number of eight per genome (11). T. thermophilus was grown at 60°C in TM medium (containing, per liter, 2 g yeast extract [Difco Laboratories, Detroit, MI], 4 g polypeptone, 1 g NaCl, and 10 ml Castenholz basal salt solution) (all chemicals were purchased from Wako Pure Chemicals, Osaka, Japan, unless otherwise indicated). Castenholz basal salt solution contained, per liter, 1 g nitrilotriacetic acid, 0.6 g CaSO4·2H2O, 1 g MgSO4·7H2O, 0.08 g NaCl, 1.03 g KNO3, 6.89 g NaNO3, 1.11 g Na2HPO4, 10 ml aqueous 0.03% FeCl3 solution, and 10 ml Nitsch's trace elements (pH 8.2) (containing, per liter, 5 ml H2SO4, 2.2 g MgSO4·5H2O, 0.5 g ZnSO4·7H2O, 0.5 g H3BO3, 0.016 g CuSO4, 0.025 g Na2MoO4·2H2O, and 0.046 g CoCl2·6H2O). E. coli was grown in Luria-Bertani (LB) medium (19), and 1.0 to 1.5% agar (Kokusan, Tokyo, Japan) was added to prepare solid media. To enable the selection of transformants of E. coli and T. thermophilus, ampicillin, kanamycin, and hygromycin B were added at 50 μg/ml.
Carotenoid production.
To observe light-dependent carotenoid production, T. thermophilus HB27 was cultured at 60°C for 2 days on solid TM medium under dark and light conditions, using an illuminating incubator (BR-180LF; Taitech, Saitama, Japan) equipped with white-light fluorescent lamps (20 W; Toshiba, Tokyo, Japan). Under light conditions, the solid culture was illuminated with white light at approximately 2.4 μmol s−1 m−2. The same lamp, covered with a blue or red light filter, was used to generate blue (400- to 460-nm) or red (600- to 700-nm) light, respectively. The method of extracting carotenoids was described previously (31). An absorption spectrum of the carotenoid fraction was recorded by using a UV spectrometer (UVmini-1240; Shimadzu, Kyoto, Japan).
Gene disruption.
Kanamycin-resistant mutants of T. thermophilus were generated by the standard homologous recombination technique, using disruption plasmids. Each disruption plasmid contained a thermostable kanamycin resistance gene (htk) cassette (13). To construct disruption plasmids for litR, TTP55, and the cob cluster, two flanking fragments were amplified by PCR using the primer sets R-F/R-MR and R-MF/R-R (litR), 55F/55MR and 55MF/55R (TTP55), and cobF/cobMR and cobMF/cobR (cob cluster) (oligonucleotide primers used in this study are shown in Table S1 in the supplemental material) and cloned onto pUC19 by three-fragment ligation. Each resulting plasmid was digested with BglII or BamHI and ligated with a promoterless htk cassette amplified by PCR using the primer sets HTKF1/HTKR to generate the disruption plasmid. Disruption plasmids were linearized by digestion with DraI and introduced into T. thermophilus WT cells (9). Subsequently, kanamycin-resistant mutants were screened and assessed for true recombination by performing PCR with appropriate primers. For genetic complementation, the coding sequence cassette for litR and TTP55 was prepared by PCR using the primer sets RcomF/RcomR and 55comF/55comR, respectively, and cloned between the NdeI and SphI sites of pTEV. The resultant plasmid carried each coding sequence downstream from the slp promoter (7), which directed the constitutive expression of each coding sequence.
RNA isolation.
To isolate total RNA, T. thermophilus strains were cultured in TM liquid medium. A preculture (60°C, 17 h, with rotary shaking at 135 rpm) was inoculated at 1% into 100 ml of TM medium prepared in a 500-ml baffled Erlenmeyer flask and incubated at 60°C with rotary shaking at 135 rpm, using the same illuminating incubator as described above. Cells were harvested by centrifugation and suspended in modified Kirby mix (containing 1% [wt/vol] N-lauroylsarcosine, 6% [wt/vol] p-aminosalicylic acid sodium salt, and 6% [vol/vol] phenol in 50 mM Tris-HCl, pH 8.3) (17); 2-mm glass beads were added, and the mixture was vigorously beaten using a Shake Master (Biomedical Science, Tokyo, Japan). Contaminating proteins were removed by a three-iteration extraction with acid-phenol-chloroform. The total nucleic acid volume thus obtained was precipitated first by 2-propanol treatment and then via a sodium acetate treatment. The precipitant was then treated with DNase I, extracted with acid-phenol-chloroform, and ethanol precipitated to obtain the RNA fraction. Approximately 200 to 300 μg of total RNA was obtained from the 40-ml culture.
S1 nuclease mapping.
The transcriptional activities of promoters preceding TTP55 (P55), litR (PlitR), and crtB (PcrtB) were studied by S1 protection analysis. Hybridization probes were first generated by PCR, using the primers 55SF/55SR (P55) and RSF/RSR (PlitR and PcrtB) (see Table S1 in the supplemental material), and cloned onto pT7Blue by TA cloning. The probes were then reamplified by PCR using the primers M13-RV/55SR* (P55), M13-RV/RSR* (PlitR), and M13-M4/RSF* (PcrtB) (primers labeled at their 5′ ends with [γ-32P]ATP using T4 polynucleotide are denoted with asterisks). The primers M13-M4 and M13-RV (M13 sequencing primers) were purchased from Takara-Shuzo. The resulting probes contained a 5′-terminal mismatch region that distinguished the full-size mRNA-DNA hybrid (371, 395, and 355 bp for P55, PlitR, and PcrtB, respectively) from the unhybridized probe DNA (264, 288, and 288 bp for P55, PlitR, and PcrtB, respectively). For the high-resolution analysis of PlitR and PcrtB, probes were prepared by PCR using the primer sets RSFH/RSRH* and RSFH*/RSRH, respectively.
The 32P-labeled probe (30,000 cpm) was mixed with 40 μg of total RNA extracted from T. thermophilus in 20 μl of sodium trichloroacetate (NaTCA) hybridization buffer (containing 3 M NaTCA, 50 mM PIPES, and 5 mM EDTA, pH 7.0), incubated at 65°C for 15 min, and gradually cooled to 45°C within 12 to 15 h. To this mixture was added 300 μl of chilled 5× S1 digestion buffer containing 100 units S1 nuclease; the mixture was then incubated at 37°C for 1 h. The S1 reaction was terminated by adding 75 μl of stop solution (containing 2.5 M ammonium acetate and 50 mM EDTA). After ethanol precipitation using 10 μg yeast tRNA as a carrier, the DNA-RNA hybrid was dissolved in 4 μl of formamide dye and applied to a 6% polyacrylamide gel. Radioactivity detection was carried out by exposing dried gels to a Fuji imaging plate (Fuji Film, Tokyo, Japan) and scanning with a Typhoon 9410 image analyzer (GE Healthcare, Tokyo, Japan). Marker 10 (Nippon Gene, Tokyo, Japan) labeled with [γ-32P]ATP using T4 polynucleotide kinase was used as a standard to estimate the transcript sizes in the low-resolution assay. To determine the transcription start sites in the high-resolution analysis, Maxam-Gilbert sequencing ladders (G+A and T+C reactions) derived from the 32P-labeled probe DNA were used as a reference. The quality of the RNA was verified via a control assay for sigA, encoding the major sigma factor (22). The probe for sigA was amplified by PCR using the primers ASF/ASR* (see Table S1 in the supplemental material).
Preparation of recombinant proteins.
LitR and TTP55 were expressed and purified via a standard system, using E. coli as a host. The coding sequences of litR and TTP55 were amplified by PCR using the primers RexF/RexR (WT LitR), RexF/RexR2 (C-terminally truncated mutant; ΔLitR), and 55exF/55exR (TTP55) and cloned between the BamHI and EcoRI sites of pGEX-6P-2 to generate the expression plasmid. A preculture (28°C, 16 h, LB medium) of E. coli harboring this plasmid was inoculated at 1% into 150 ml LB medium prepared in a 500-ml baffled Erlenmeyer flask and cultured for 6 h (1 mM isopropyl-β-d-thiogalactopyranoside [IPTG] was added at 3 h) at 28°C with rotary shaking (135 rpm). Cells were harvested by centrifugation, suspended in phosphate-buffered saline (PBS) (19), and disrupted with a mechanical cell presser. The cell extract was centrifuged at 70,000 × g for 30 min; the resulting supernatant was used in glutathione S-transferase (GST) affinity chromatography. The supernatant was applied to a 5-ml GSTrap FF column, using an ÄKTA fast protein liquid chromatography (FPLC) system (GE Healthcare). The GST tag was removed by treatment with Precision protease (GE Healthcare). The protein concentration was measured with a protein assay kit (Bio-Rad). The molecular size of LitR was estimated by gel filtration of 0.3 mg of the purified recombinant protein. Gel filtration was performed by using a Superdex 200 HR 10/30 column on an ÄAKTA FPLC system (GE Healthcare) according to the manufacturer's recommendation. The column was developed with PBS (containing 140 mM NaCl, 2.7 mM KCl, 10 mM Na2PO4, and 1.8 mM KH2PO4) at a flow rate of 0.25 ml/min. Molecular size standards (aldolase, albumin, ovalbumin, RNase A, and blue dextran, indicating 158, 67, 43, 13.7, and 2 kDa, respectively) were contained in a gel filtration calibration kit (GE Healthcare). To prepare Cbl-treated recombinant protein, the GST-tagged LitR (WT LitR and ΔLitR) was added to a 10-fold molar excess of methylcobalamin (MeCbl) (Sigma-Aldrich, Tokyo, Japan) and incubated for 1 h at 37°C. The mixture was then applied to a GSTrap FF column and eluted by treatment with Precision protease as described above. Absorption spectra of the resultant LitR recombinants were recorded by using a UVmini-1240 spectrometer (Shimadzu).
DNA-binding assay.
The DNA-binding activity of LitR was first studied with a gel mobility shift assay. Probe DNA was generated by PCR using primer sets RSF/RSRH (PlitR) and ASF/ASR (PsigA) (control) (see Table S1 in the supplemental material) and labeled at the 5′ end with [γ-32P]ATP, using T4 polynucleotide kinase. A total of 0.5 to 5.0 ng of 32P-labeled probe (10,000 to 20,000 cpm) was mixed with 30 to 120 pmol of recombinant LitR, prepared as discussed above; it was then incubated at 30°C for 30 min in 50 μl of binding buffer [containing 10 mM Tris-HCl (pH 7.0), 50 mM KCl, 1 mM EDTA, 1 mM dithiothreitol, 10% (vol/vol) glycerol, 1 μg poly(dI-dC), and 50 μg/ml of bovine serum albumin]. Following incubation, the DNA-protein complex and free DNA were resolved on nondenaturing polyacrylamide gels containing 6% acrylamide.
To determine the binding site of LitR, a DNase I footprint analysis was carried out. 32P-labeled DNA fragments were prepared by PCR using the primers FPA/FPB* for the antisense strand and FPA*/FPB for the sense strand. The reaction mixture (50 μl) contained 10 kcpm 32P-labeled DNA probe, 10 to 320 pmol LitR, 25 mM HEPES-KOH (pH 7.9), 0.5 mM EDTA-NaOH (pH 8.0), 50 mM KCl, and 10% glycerol. After incubation at 55°C for 30 min, DNase I was added at a final concentration of 20 μg/ml, and the mixture was further incubated for 1 min at 25°C. The reaction was terminated by adding 100 μl of stop solution (containing 100 mM Tris-HCl [pH 8.0], 100 mM NaCl, 1% sodium N-lauroyl sarcosinate, 10 mM EDTA-NaOH [pH 8.0], and 25 mg/ml salmon sperm DNA) and 300 μl of phenol-chloroform (1:1). After ethanol precipitation, the pellet was washed with 80% ethanol, dissolved in a 6-μl formamide-dye mixture, and run on a 6% polyacrylamide gel. To determine the LitR-binding site in the high-resolution analysis, Maxam-Gilbert sequencing ladders (G+A and T+C reactions) generated from the 32P-labeled probe DNA fragment were used as a reference.
In vitro runoff transcription.
The in vitro runoff transcription assay was performed using a previously described method (26). DNA templates containing the transcriptional start sites of PlitR and PcrtB were generated by PCR using the primers PLA/PLR for template A (313 bp), PLB/PLR for template B (213 bp), PLC/PLR for template C (172 bp), PLD/PLR for template D (173 bp), and PLE/PLR for template E (174 bp) (see Table S1 in the supplemental material). The mutated template (template A′) was prepared by PCR using primers PLA/PLR from the mixture of two amplicons generated by PCR with primers PLA/PMR and PMF/PLR. A total of 0.5 pmol of template DNA was mixed with 2 pmol RNA polymerase holoenzyme of T. thermophilus (AR Brown, Tokyo, Japan), 100 nmol of ribonucleotides (including [α-32P]CTP), 0 to 20 pmol of LitR, and 0 to 10 pmol TTP55. Transcripts were analyzed by polyacrylamide gel electrophoresis. Marker 10 (Nippon Gene, Tokyo, Japan) labeled with [γ-32P]ATP was used as a standard.
RESULTS
Gene organization of the litR-crt locus on the large plasmid of T. thermophilus.
The complete genome sequence of T. thermophilus is available for two strains, HB8 and HB27, which exhibit marked similarity to each other (5, 10). Each genome consists of a 1.85-Mb chromosome and a large (0.26-Mb) plasmid; HB8, but not HB27, harbors a small (9.32-kb) plasmid (10). We used the HB27 strain for genetic characterization; the numbering used here is for that strain.
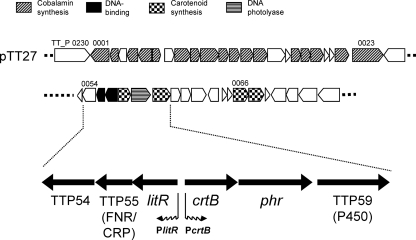
The locus containing the coding sequence for CarA/LitR homolog (here the coding sequence will be referred as litR based on its involvement in the light-induced transcription of not only carotenoid biosynthesis genes but other protective genes) was located on the large plasmid, pTT27 (Fig. 1). The corresponding region was known to be a locus involved in DNA damage repair (5). litR (TT_P0056) comprised a possible operon structure with two downstream coding sequences that encode a cyclic AMP (cAMP) receptor protein (CRP)/fumarate and nitrate reduction regulator (FNR) family transcriptional regulator (TT_P0055; designated TTP55 here) and a subunit of NADH:ubiquinone oxidoreductase (TT_P0054). The litR operon was flanked by the crtB operon, which contained coding sequences for phytoene synthase (crtB) (TT_P0057), DNA photolyase (phr) (TT_P0058), and cytochrome P450 monooxygenase. The P450 monooxygenase is known for its involvement in the hydroxylation of β-carotene (2) (TT_P0059). Additional genes involved in carotenoid biosynthesis (TT_P0066 and TT_P0067) existed downstream of the crtB operon. pTT27 also contained a Cbl biosynthetic gene cluster (TT_P0001-0023) (Fig. 1). No litR homolog or Cbl biosynthetic genes were found on the chromosome.
Fig. 1.
Schematic representation of the litR locus of T. thermophilus. This locus was carried by the large plasmid pTT27 in a region known to contain genes involved in DNA damage repair (5). The genes flanking litR (TT_P0056) encode a cAMP receptor protein homolog (TT_P0055), NADH-ubiquinone oxidoreductase (TT_P0054), phytoene synthase (crtB) (TT_P0057), DNA photolyase (phr) (TT_P0058), and cytochrome P450 monooxygenase (TT_P0059). The two downstream carotenoid biosynthesis genes, TT_P0066 and TT_P0067, encode phytoene dehydrogenase and isopentenyl pyrophosphate isomerase, respectively. pTT27 also carries a Cbl biosynthesis gene (cob) cluster that encompasses the 20-kb region corresponding to TT_P0001 to TT_P0023.
Light-induced carotenoid production in T. thermophilus.
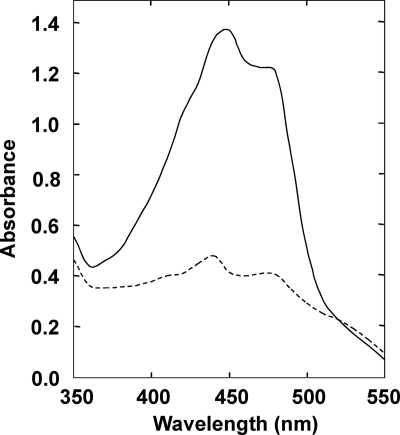
Although carotenoid production in T. thermophilus has not been known to be affected by illumination, the proximity of litR and crtB suggested that it occurs in a photodependent manner. To assess this possibility, T. thermophilus was grown under light and dark conditions. As shown in Fig. 2 A (panels corresponding to WT/pTEV), the WT strain produced a marked yellow pigment under light conditions; in contrast, the colonies of this strain appeared white or pale yellow under dark conditions. The induction of pigment production was observed when white or blue light was used and was not observed when red light was used (data not shown). The UV-visible absorption spectrum of a methanol extract of the illuminated cells showed a typical carotenoid profile, exhibiting multiple absorption peaks around 450 nm (Fig. 3). It is known that T. thermophilus produces zeaxanthin derivatives, including a novel glucosylated form termed thermozeaxanthin (λmax, 452 and 477 nm) (32). The yellow pigments produced under light conditions are likely to contain these known carotenoids.
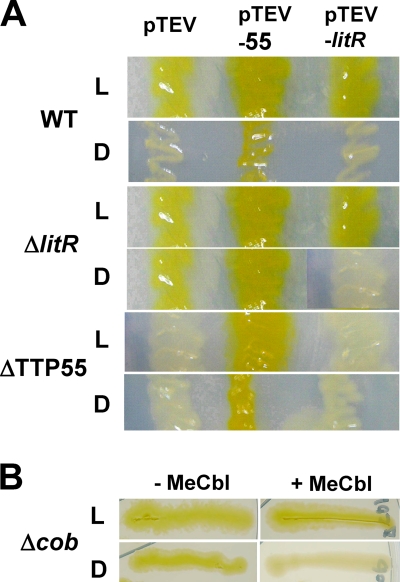
Fig. 2.
Light-induced carotenoid production in T. thermophilus. (A) Colonies of the wild-type (WT) strain, the litR mutant (ΔlitR), and the CRP/FNR family transcriptional regulator gene mutant (ΔTTP55), each harboring the plasmids pTEV (empty vector; control), pTEV-55 (pTEV carrying TTP55), and pTEV-litR (pTEV carrying litR). (B) Colonies of the mutant lacking Cbl biosynthesis genes (Δcob) grown in the absence (−MeCbl) and presence (+MeCbl) of 0.1 mM MeCbl. All colonies were developed under light (L) and dark (D) conditions at 60°C for 2 days on solid TM medium.
Fig. 3.
UV-visible absorption spectrum of carotenoid extracted from illuminated cells of T. thermophilus (solid line). The spectrum for cells grown under dark conditions is also shown (dashed line).
To study the role of litR and the downstream regulatory gene that encodes a CRP/FNR family protein (TTP55), kanamycin-resistant knockout mutants were generated through a homologous recombination technique (see Materials and Methods). The resulting litR mutant produced carotenoids under both light and dark conditions (Fig. 2A, ΔlitR/pTEV); in contrast, the TTP55 mutant was defective in carotenoid production (Fig. 2A, ΔTTP55/pTEV). This suggests that litR and TTP55 encode negative and positive regulators for carotenoid production, respectively.
The introduction of a plasmid carrying an intact litR gene (pTEV-litR) restored the light-dependent carotenoid production in the litR mutant (Fig. 2A, ΔlitR/pTEV-litR) but did not affect the carotenoid-deficient phenotype of the TTP55 mutant (ΔTTP55/pTEV-litR). On the other hand, the introduction of a plasmid carrying an intact TTP55 gene (pTEV-55) caused constitutive carotenoid production in both mutants as well as in the WT strain, probably due to the constitutive expression of the plasmid-borne TTP55 (Fig. 2A, pTEV-55).
Transcriptional analysis by S1 nuclease mapping.
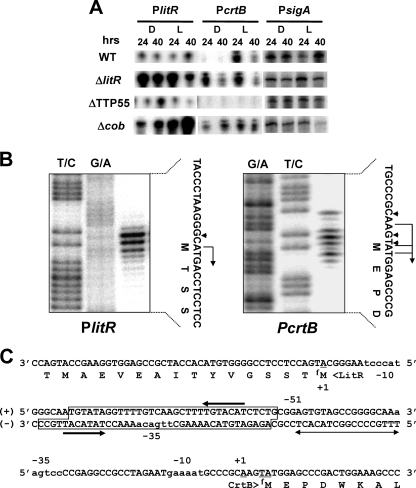
To study the details of light-dependent genetic control, transcription activities were studied by low-resolution S1 nuclease protection analysis (Fig. 4A). Preliminary analyses with respect to the intergenic regions indicated that the two divergent promoters, PlitR and PcrtB (Fig. 1), were responsible for the major transcription of the litR and crtB operons, respectively (data not shown). In the WT strain, the activity of PcrtB was induced by light, while the activity of PlitR was largely not affected by illumination (Fig. 4A). In the litR mutant, PcrtB was active under both light and dark conditions; PlitR activity was remarkably upregulated compared to that in the WT strain under either condition. In the TTP55 mutant, PcrtB activity was abolished, irrespective of illumination, while PlitR activity was at the same level as in the WT strain.
Fig. 4.
S1 protection analyses of the bidirectional promoter. (A) Low-resolution analysis. Activities of promoters preceding litR (PlitR), crtB (PcrtB), and sigA (the housekeeping sigma factor gene; control) (PsigA) were estimated by the signal intensities of protected fragments. Since the litR mutant (ΔlitR) retains the N-terminal portion of the coding sequence, the same S1 probe was used for both the WT and ΔlitR strains. RNA was isolated from cells grown for 24 and 40 h at 60°C. Strain designations are the same as those in Fig. 2. (B) High-resolution analysis for the determination of transcriptional start sites of PlitR and PcrtB. Maxam-Gilbert sequencing ladders (T+C and G+A reactions) were generated using the same 32P-labeled fragment as the probe DNA. The positions of the S1-protected fragments are denoted by arrowheads, and the transcriptional start sites assigned to the residues are denoted by bent arrows. It is known that the fragments generated by the chemical-sequencing reactions migrate 1.5 nucleotides (nt) further than the corresponding fragments generated by the S1 nuclease digestion of the DNA-RNA hybrids (half a residue from the presence of the 3′-terminal phosphate group and one residue from the elimination of the 3′-terminal nucleotide) (27). The RNA prepared from WT cells grown under light conditions for 40 h on solid TM medium was used for hybridization. (C) Nucleotide sequence of the intergenic promoter region between litR and crtB; partial amino acid sequences of LitR and CrtB are also shown. The translation initiation codon of CrtB is located 18 bp downstream from that assigned in the genome sequence database (http://www.genome.ad.jp). The transcriptional start positions determined by high-resolution analysis are designated +1. The potential −35 and −10 sequences are shown in lowercase. The region protected in the DNase I footprint experiment (Fig. 7) and an inverted repeat contained in this region are denoted by a box and convergent arrows, respectively. The region essential for transcriptional activation by TTP55 is indicated by a divergent arrow.
The transcriptional start sites of PlitR and PcrtB were determined by high-resolution S1 analysis (Fig. 4B and C). The results showed that transcription in PlitR started at the A nucleotide that is the first nucleotide of the translational initiation codon (ATG) of LitR and that transcription in PcrtB started at the A nucleotide 4 bp upstream from the translational initiation codon (ATG) of CrtB. The evidence indicates that both LitR and CrtB are translated by a leaderless mechanism (20). We previously observed a similar situation with respect to the transcriptional start site of litR in S. coelicolor A3(2) (29). The putative −35 and −10 sequences were TTGACA…TACCCT (PlitR) and AAGTCC…GAAAAT (PcrtB); the former was identical to the consensus of σA-dependent promoters of T. thermophilus (TTGACA...TANCCT) described by Sevostyanova et al. (25).
Binding of Cbl to LitR and of LitR to DNA.
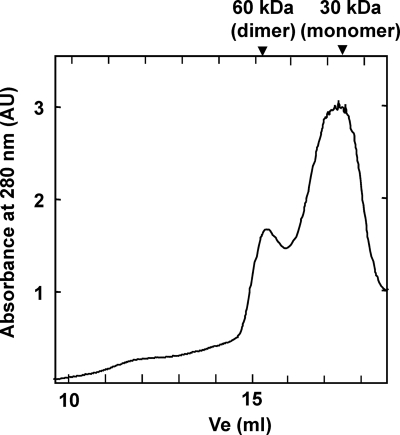
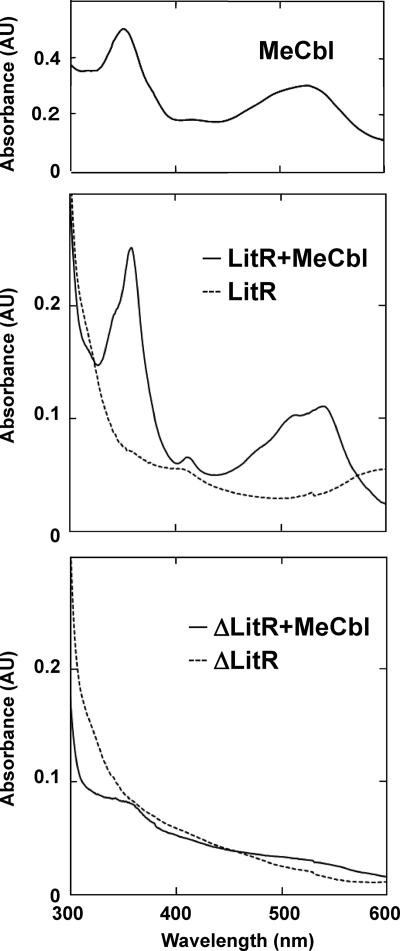
LitR belongs to the MerR family, whose regulatory activity depends on the binding of a ligand (3, 12). The C-terminal region of LitR exhibits similarity with a Cbl-binding motif (corresponding to amino acids [aa]165 to 273 of T. thermophilus LitR). Cbl is a cobalt (Co)-containing tetrapyrolle that absorbs blue light; hence, it seems likely that Cbl serves as a ligand of LitR and plays some role in photosensing. To study the actual binding of Cbl, recombinant LitR was prepared by the standard method, using an E. coli expression system (see Materials and Methods). Gel filtration analysis of the recombinant LitR, purified by standard affinity chromatography, showed the presence of its dimer form (Fig. 5). The LitR recombinant protein was then incubated with MeCbl, and its absorption spectrum was studied (see Materials and Methods). The MeCbl-treated LitR recombinant exhibited a spectrum similar to that of free MeCbl (Fig. 6). On the other hand, the similarly treated LitR mutant lacking the C-terminal Cbl-binding domain (corresponding to aa 151 to 285, containing the whole Cbl-binding domain) did not exhibit the characteristic absorption profile. These results indicate that Cbl binds the C-terminal region of LitR.
Fig. 5.
Gel filtration chromatogram of LitR recombinant protein.
Fig. 6.
Absorption spectrum of MeCbl-treated LitR recombinant. Spectra of free MeCbl (upper panel) and the MeCbl-treated WT LitR (LitR+MeCbl) (solid line in the middle panel) and truncated mutant LitR (ΔLitR+MeCbl) (solid line in the lower panel) are shown. Spectra of untreated recombinants are also shown (dashed lines in the middle and lower panels).
Furthermore, to study the involvement of Cbl in photodependent regulation, a mutant with a knockout of the Cbl biosynthesis gene (cob) cluster was generated. The null mutant for the coding sequences corresponding to TT_P0001-0005 (Fig. 1A) was viable and produced carotenoids under both light and dark conditions (Fig. 2B, left panels). The ability of this mutant to repress carotenoid production under dark conditions was restored by supplying MeCbl (Fig. 2B, right panels) or hydroxycobalamin or cyanocobalamin (data not shown). The supply of MeCbl affected neither the constitutive carotenoid production in the litR mutant nor the carotenoid-deficient phenotype of the TTP55 mutant (data not shown). The transcriptional activities of PcrtB and PlitR in the cob mutant exhibited a profile similar to that in the litR mutant (Fig. 4A, bottom); PcrtB was active under both light and dark conditions, and PlitR activity was markedly upregulated compared to that in the WT strain. These results indicate that Cbl is essential for the light dependence of the transcription at PcrtB and hence that of carotenoid production.
The LitR recombinant was then studied for its DNA-binding activity. First, it was subjected to a gel mobility shift assay; the result showed that LitR caused a specific mobility shift of a probe DNA that contained the intergenic region between litR and crtB (see Fig. S1 in the supplemental material). This DNA-binding was observed with LitR in both monomeric and dimeric fractions in the gel filtration (Fig. 5) irrespectively of the treatment with illumination or MeCbl (data not shown).
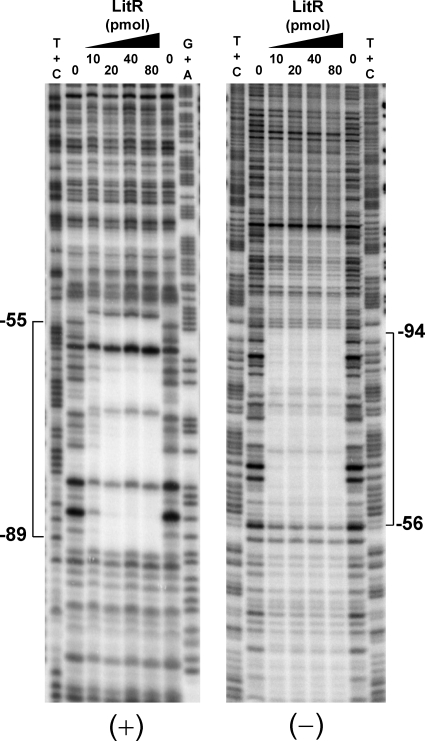
We then carried out a DNase I footprint analysis to determine the binding site. As shown in Fig. 7, the region corresponding to positions −55 to −94 with respect to the transcriptional start point of crtB was protected from digestion by DNase I, due to the binding of LitR (see also Fig. 4C). The protected region contained the −35 region of PlitR and an inverted repeat, ATGTATA…(16 bp)…TGTACAT. The protection profile was not affected by treatment of LitR protein with illumination or MeCbl (data not shown).
Fig. 7.
DNase I footprinting for determining the LitR binding site. The assay was performed on the sense (+) and antisense (−) strands. The amounts of recombinant LitR protein added to the reaction are shown. The position numbering is based on that for PcrtB (the transcriptional start point of PcrtB is numbered +1). The DNase I digests were run with the same probes that were chemically cleaved (G+A and T+C lanes).
In vitro transcription.
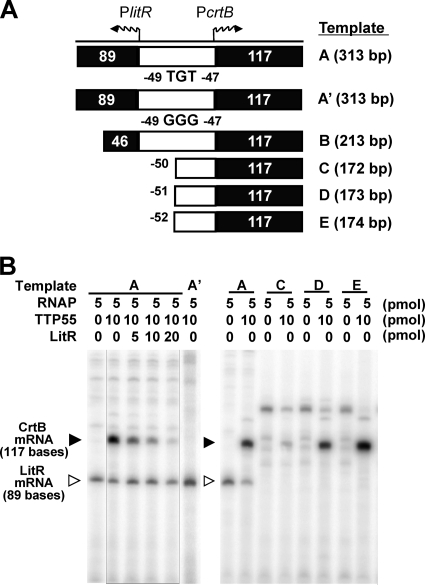
Overall, the results supported the view that the LitR protein negatively controls PlitR activity via its binding to the intergenic region and that the TTP55 protein serves as a positive regulator for PcrtB. To verify this, an in vitro transcription analysis was carried out (Fig. 8). The commercial RNA polymerase holocomplex successfully generated LitR mRNA (89 bases for template A), but the transcription was not affected by the addition of LitR or TTP55 recombinant protein. We tested various conditions but could not observe repression of PlitR activity by LitR.
Fig. 8.
In vitro runoff transcription assay. A schematic representation of the locations and sizes of the template DNA fragments (A) and a representative result of the assay (B) are shown. The commercial RNA polymerase holocomplex (RNAP) of T. thermophilus, the purified TTP55 recombinant, and the LitR recombinant were added to the reaction mixture in the indicated amounts. Closed and open triangles denote the positions of the expected transcripts for PcrtB and PlitR, respectively.
In contrast, the formation of CrtB mRNA (117 bases for templates A and B) (the results for template B are shown in Fig. S2 in the supplemental material) depended on the supply of TTP55 protein. This TTP55-dependent transcription for crtB was inhibited by the dose of LitR recombinant. The assay using the trimmed templates (templates C to E) showed that TTP55-dependent transcription occurs on templates D and E but not on template C, indicating that the transcription at PcrtB based on the function of TTP55 requires the region encompassing positions −51 to +1 (see also Fig. 4C). This region contains a potential binding sequence (AGTGT[N7]GCAAAA) that exhibits similarity with the consensus [WWGTGA(N5-7)ACACWW] for the cAMP-independent CRP/FNR regulator of T. thermophilus (1). Based on this observation, a mutant template (A′) was generated by introducing transversion mutations (from T to G) at positions −47 and −49 (corresponding to the underlined bases in the potential binding sequence given above) and used as a template for in vitro runoff analysis. The result showed that the reaction using this mutated fragment did not form CrtB mRNA (Fig. 8B). This supported the view that the corresponding region is involved in the recognition by TTP55; however, our gel shift analysis has not yet successfully detected the binding of TTP55 protein to any DNA fragment containing this region. The transcription efficiency in any assay was not affected by exposure to light or supply of Cbl and cAMP (data not shown).
DISCUSSION
As inferred from the distribution of the CarA/LitR homolog (28), T. thermophilus was found to produce carotenoids in a light-dependent manner. Although some observations have suggested a certain correlation between illuminated environments and the occurrence of carotenoid production in this bacterial genus (6), the existence of a light-dependent regulatory mechanism has not been known. Perhaps the ability to switch off the expression of protective functions under dark conditions benefits the organism by allowing it to save energy and prevent DNA repair errors. We have also studied pigment production in related organisms and found that carotenoids also occur in a photodependent manner in several other species, including Thermus aquaticus, Thermus oshimai, Thermus igniterrae, and Thermus filiformis (our unpublished observations).
Figure 9 shows the current working hypothesis for the transcriptional control of the crtB promoter. The genetic evidence indicates that the two tandem transcriptional regulators LitR and TTP55 are essential for the light-dependent transcriptional control of carotenoid production in T. thermophilus. A simple explanation for the roles of the two regulators is that LitR represses PlitR and PcrtB activities through its binding at a unique site located close to these promoters, and its derepression allows the production of TTP55, which in turn activates PcrtB to direct the expression of carotenoid biosynthesis and photolyase. This model is largely supported by the results obtained in this study, except that the repression of PlitR activity by LitR was not reproduced in the in vitro transcription assay (Fig. 8). This makes us think of the possibility that an additional element is required in order to fully reproduce the transcriptional control at PlitR in vitro. We can also find an inconsistency in the negative feedback loop formed by LitR; the regulatory circuit in the current model keeps just a low transcription level of the litR operon. Some posttranscriptional inactivation mechanism for LitR should be involved in the effective expression of TTP55 and hence that of the crtB operon.
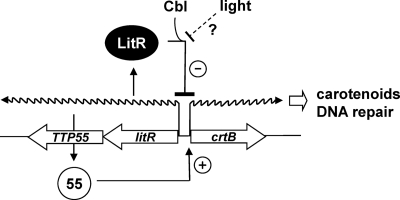
Fig. 9.
Hypothetical model for light-dependent transcriptional control at the intergenic region between litR and crtB of T. thermophilus. LitR binds the unique site overlapping the −35 region of PlitR, and it negatively regulates the PlitR activity. Derepression of LitR due to an unknown mechanism causes the expression of TTP55, which in turn activates PcrtB. PcrtB directs the transcription of genes that encode carotenoid biosynthesis enzymes and DNA photolyase. Illumination may induce PcrtB activity via the inactivation of LitR.
The absorption spectrum of the MeCbl-treated LitR recombinant (Fig. 6) demonstrated that LitR is bound by MeCbl. The localization of Cbl biosynthesis on the large plasmid also suggests its correlation with LitR function. Further, the characteristics of the cob mutant indicated that Cbl is essential to the photodependence of the transcription of crt genes. Although in vitro experiments have not yet shown the dependence of LitR function on Cbl, the evidence strongly suggests that Cbl binding is crucial for the function of LitR.
LitR belongs to the MerR family, but it differs from that kind of regulator in terms of the structure of the promoter controlled. The typical MerR family regulator binds a palindrome structure localized between the −10 and −35 regions. It is known that the unusual distance between −10 and −35 regions of the MerR family-dependent promoter (19 bp) is longer than the normal promoter (17 bp) and prevents the RNA polymerase holocomplex from initiating transcription. The binding of the ligand-free MerR regulator to the operator site also inhibits the initiation of transcription. However, the ligand-bound form, in turn, causes DNA to unwind through its conformation change and allows RNA polymerase to reach the −10 region and initiate transcription (3). Unlike this feature of the MerR-type promoter, the PlitR region bound by LitR exhibited a normal structure (i.e., a 17-bp spacer between the −10 and −35 regions); this suggests that the function of LitR has diverged from that of the MerR family regulator. A similar feature is known for the CarA-dependent promoter of M. xanthus (21).
TTP55 is one of the four CRP/FNR family proteins of T. thermophilus. Although TTP55 has a putative cAMP-binding domain in its N-terminal region, cAMP was not required for its activity to induce PcrtB. Agari et al. (1) recently reported that SdrP, another CRP/FNR family protein of T. thermophilus, does not require cAMP binding for its activity; in fact, the three-dimensional (3D) structure of this protein does not afford sufficient space for the incorporation of cAMP. A similar feature has been observed with respect to the 3D structure of TTP55 (Y. Agari and A. Shinkai, personal communication). Therefore, currently we assume that the crtB operon is not included in the cAMP regulon of T. thermophilus.
The major question now is how the illumination signal is transmitted to the activity of PcrtB. There is the simple hypothesis that blue-light absorption by Cbl causes the aforementioned derepression of LitR and induces the expression of TTP55 to activate PcrtB. It is known that blue-light absorption affects the activity of MetH, a Cbl-dependent methionine synthase; the catalytic reaction of this enzyme, involving methyl transfer from MeCbl to homocysteine, is known to suffer a photolytic conversion of cob(I)alamin to cob(II)alamin, which in turn prompts the inactivation of the enzyme (15, 16). Although the hypothesis is attractive, in vitro study has not yet reproduced the photodependent activation of PcrtB or shown any effect of Cbl on LitR function. This again makes us think of the involvement of a regulatory element(s) other than LitR and TTP55 in the light-dependent transcriptional control in T. thermophilus. In M. xanthus, light-induced carotenoid production is regulated by a complex system involving two Cbl-binding MerR family proteins (i.e., CarA and CarH), as well as related signal transducers, including a specific sigma factor and antagonists. It is assumed that a membrane-bound protoporphyrin and CarF are involved in photosensing (4, 8), but the exact mechanism is not yet known. Currently, we believe the photodependent regulatory mechanism in T. thermophilus to be different from that in M. xanthus, since the regulators identified in M. xanthus do not have distinct homologs in T. thermophilus; however, it is still possible that an illumination-dependent element affects the transcriptional control by LitR and TTP55. A detailed biochemical characterization of the function of the LitR-Cbl complex will help in understanding the exact mechanism of light-dependent transcriptional control in T. thermophilus.
Supplementary Material
ACKNOWLEDGMENTS
We thank Seiki Kuramitsu for providing pUC18-pJHK3 and Satoko Yoshizawa for helpful discussion.
This study was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (High-Tech Research Center Project and Grant-in-Aid for Scientific Research no. 19614011), the Noda Institute for Scientific Research, Araki Memorial Foundation, and the Japan Bioindustry Association.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 18 March 2011.
REFERENCES
- 1. Agari Y., Kashihara A., Yokoyama S., Kuramitsu S., Shinkai A. 2008. Global gene expression mediated by Thermus thermophilus SdrP, a CRP/FNR family transcriptional regulator. Mol. Microbiol. 70:60–75 [DOI] [PubMed] [Google Scholar]
- 2. Blasco F., Kauffmann I., Schmid R. D. 2004. CYP175A1 from Thermus thermophilus HB27, the first beta-carotene hydroxylase of the P450 superfamily. Appl. Microbiol. Biotechnol. 64:671–674 [DOI] [PubMed] [Google Scholar]
- 3. Brown N. L., Stoyanov J. V., Kidd S. P., Hobman J. L. 2003. The MerR family of transcriptional regulators. FEMS Microbiol. Rev. 27:145–163 [DOI] [PubMed] [Google Scholar]
- 4. Browning D. F., Whitworth D. E., Hodgson D. A. 2003. Light-induced carotenogenesis in Myxococcus xanthus: functional characterization of the ECF sigma factor CarQ and antisigma factor CarR. Mol. Microbiol. 48:237–251 [DOI] [PubMed] [Google Scholar]
- 5. Bruggemann H., Chen C. 2006. Comparative genomics of Thermus thermophilus: plasticity of the megaplasmid and its contribution to a thermophilic lifestyle. J. Biotechnol. 124:654–661 [DOI] [PubMed] [Google Scholar]
- 6. da Costa M. S., Nobre M. F., Rainey F. A. 2001. The genus Thermus, p. 404–414In Garrity G. M., et al. (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 1 Springer, New York, NY [Google Scholar]
- 7. Faraldo M. M., de Pedro M. A., Berenguer J. 1992. Sequence of the S-layer gene of Thermus thermophilus HB8 and functionality of its promoter in Escherichia coli. J. Bacteriol. 174:7458–7462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fontes M., Galbis-Martinez L., Murillo F. J. 2003. A novel regulatory gene for light-induced carotenoid synthesis in the bacterium Myxococcus xanthus. Mol. Microbiol. 47:561–571 [DOI] [PubMed] [Google Scholar]
- 9. Hashimoto Y., Yano T., Kuramitsu S., Kagamiyama H. 2001. Disruption of Thermus thermophilus genes by homologous recombination using a thermostable kanamycin-resistant marker. FEBS Lett. 506:231–234 [DOI] [PubMed] [Google Scholar]
- 10. Henne A., et al. 2004. The genome sequence of the extreme thermophile Thermus thermophilus. Nat. Biotechnol. 22:547–553 [DOI] [PubMed] [Google Scholar]
- 11. Hishinuma F., Tanaka T., Sakaguchi K. 1978. Isolation of extrachromosomal deoxyribonucleic acids from extremely thermophilic bacteria. J. Gen. Microbiol. 104:193–199 [DOI] [PubMed] [Google Scholar]
- 12. Hobman J. L. 2007. MerR family transcription activators: similar designs, different specificities. Mol. Microbiol. 63:1275–1278 [DOI] [PubMed] [Google Scholar]
- 13. Hoseki J., Yano T., Koyama Y., Kuramitsu S., Kagamiyama H. 1999. Directed evolution of thermostable kanamycin-resistance gene: a convenient selection marker for Thermus thermophilus. J. Biochem. 126:951–956 [DOI] [PubMed] [Google Scholar]
- 14. Hoshino T., Kosuge T., Hidaka Y., Tabata K., Nakahara T. 1994. Molecular cloning and sequence analysis of the proC gene encoding delta 1-pyrroline-5-carboxylate reductase from an extremely thermophilic eubacterium Thermus thermophilus. Biochem. Biophys. Res. Commun. 199:410–417 [DOI] [PubMed] [Google Scholar]
- 15. Jarrett J. T., et al. 1996. Mutations in the B12-binding region of methionine synthase: how the protein controls methylcobalamin reactivity. Biochemistry 35:2464–2475 [DOI] [PubMed] [Google Scholar]
- 16. Jarrett J. T., Goulding C. W., Fluhr K., Huang S., Matthews R. G. 1997. Purification and assay of cobalamin-dependent methionine synthase from Escherichia coli. Methods Enzymol. 281:196–213 [DOI] [PubMed] [Google Scholar]
- 17. Kirby K. S., Fox-Carter E., Guest M. 1967. Isolation of deoxyribonucleic acid and ribosomal ribonucleic acid from bacteria. Biochem. J. 104:258–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koyama Y., Hoshino T., Tomizuka N., Furukawa K. 1986. Genetic transformation of the extreme thermophile Thermus thermophilus and of other Thermus spp. J. Bacteriol. 166:338–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maniatis T., Fritsch E. F., Sambrook J. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 20. Moll I., Grill S., Gualerzi C. O., Blasi U. 2002. Leaderless mRNAs in bacteria: surprises in ribosomal recruitment and translational control. Mol. Microbiol. 43:239–246 [DOI] [PubMed] [Google Scholar]
- 21. Navarro-Aviles G., et al. 2007. Structural basis for operator and antirepressor recognition by Myxococcus xanthus CarA repressor. Mol. Microbiol. 63:980–994 [DOI] [PubMed] [Google Scholar]
- 22. Nishiyama M., Kobashi N., Tanaka K., Takahashi H., Tanokura M. 1999. Cloning and characterization in Escherichia coli of the gene encoding the principal sigma factor of an extreme thermophile, Thermus thermophilus. FEMS Microbiol. Lett. 172:179–186 [DOI] [PubMed] [Google Scholar]
- 23. Perez-Marin M. C., Padmanabhan S., Polanco M. C., Murillo F. J., Elias-Arnanz M. 2008. Vitamin B12 partners the CarH repressor to downregulate a photoinducible promoter in Myxococcus xanthus. Mol. Microbiol. 67:804–819 [DOI] [PubMed] [Google Scholar]
- 24. Purcell E. B., Crosson S. 2008. Photoregulation in prokaryotes. Curr. Opin. Microbiol. 11:168–178 [DOI] [PubMed] [Google Scholar]
- 25. Sevostyanova A., et al. 2007. Temporal regulation of viral transcription during development of Thermus thermophilus bacteriophage phiYS40. J. Mol. Biol. 366:420–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shinkai A., et al. 2007. Transcription activation mediated by a cyclic AMP receptor protein from Thermus thermophilus HB8. J. Bacteriol. 189:3891–3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sollner-Webb B., Reeder R. H. 1979. The nucleotide sequence of the initiation and termination sites for ribosomal RNA transcription in X. laevis. Cell 18:485–499 [DOI] [PubMed] [Google Scholar]
- 28. Takano H., Beppu T., Ueda K. 2006. The CarA/LitR-family transcriptional regulator: its possible role as a photosensor and wide distribution in non-phototrophic bacteria. Biosci. Biotechnol. Biochem. 70:2320–2324 [DOI] [PubMed] [Google Scholar]
- 29. Takano H., Obitsu S., Beppu T., Ueda K. 2005. Light-induced carotenogenesis in Streptomyces coelicolor A3(2): identification of an extracytoplasmic function sigma factor that directs photodependent transcription of the carotenoid biosynthesis gene cluster. J. Bacteriol. 187:1825–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van der Horst M. A., Key J., Hellingwerf K. J. 2007. Photosensing in chemotrophic, non-phototrophic bacteria: let there be light sensing too. Trends Microbiol. 15:554–562 [DOI] [PubMed] [Google Scholar]
- 31. Yokoyama A., et al. 1995. Thermozeaxanthins, new carotenoid-glycoside-esters from thermophilic eubacterium Thermus thermophilus. Tetrahedron Lett. 36:4901–4904 [Google Scholar]
- 32. Yokoyama A., Shizuri Y., Hoshino T., Sandmann G. 1996. Thermocryptoxanthins: novel intermediates in the carotenoid biosynthetic pathway of Thermus thermophilus. Arch. Microbiol. 165:342–345 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.