Abstract
Expression of lysP, which encodes the lysine-specific transporter LysP in Escherichia coli, is regulated by the concentration of exogenous available lysine. In this study, the LysR-type transcriptional regulator ArgP was identified as the activator of lysP expression. At lysine concentrations higher than 25 μM, lysP expression was shut off and phenocopied an argP deletion mutant. Purified ArgP-His6 bound to the lysP promoter/control region at a sequence containing a conserved T-N11-A motif. Its affinity increased in the presence of lysine but not in the presence of the other known coeffector, arginine. In vivo data suggest that lysine-loaded ArgP and arginine-loaded ArgP compete at the lysP promoter. We propose that lysine-loaded ArgP prevents lysP transcription at the promoter clearance step, as described for the lysine-dependent regulation of argO (R. S. Laishram and J. Gowrishankar, Genes Dev. 21:1258-1272, 2007). The global regulator Lrp also bound to the lysP promoter/control region. An lrp mutant exhibited reduced lysP expression in the absence of external lysine. These results indicate that ArgP is a major regulator of lysP expression but that Lrp modulates lysP transcription under lysine-limiting conditions.
INTRODUCTION
Amino acid transporters play several important roles in bacteria. Besides their function in nutrient supply, these systems are also involved in osmoregulation, pH homeostasis, signal transduction, and detoxification. More than one uptake system normally exists for the transport of a single amino acid, allowing bacteria to adapt to different environmental conditions. Escherichia coli has three different transport systems for the uptake of the amino acid l-lysine that differ in transport mechanism, substrate specificity, apparent Michaelis constant (Km), and regulation of their synthesis. The lysine-arginine-ornithine (LAO) system is encoded by argT-hisJQMP. HisQMP2 forms the ABC-transporter; HisJ and ArgT are periplasmic binding proteins that are specific for histidine and lysine, arginine, or ornithine, respectively (40). The cadaverine-lysine antiporter CadB imports lysine and excretes cadaverine (48) but is produced only under conditions of low pH. Last, but not least, LysP is a specific transporter for l-lysine that belongs to the amino acid, polyamine, and organocation (APC) transporter family (8, 49). Considering the important role of lysine and LysP in amino acid metabolism and pH homeostasis in E. coli, the aim of this work was to investigate the factors and mechanisms involved in the transcriptional regulation of lysP.
The lysP gene was originally named cadR because its mutants exhibit a pleiotropic phenotype including derepressed levels of lysine decarboxylase CadA, in addition to a reduction in lysine transport (37). The Cad system, which plays a role in pH homeostasis in Enterobacteria, comprises the membrane-integrated transcriptional activator CadC and the cadBA operon, encoding the lysine decarboxylase CadA and the lysine-cadaverine antiporter CadB (26, 27, 58). This system is induced under conditions of low external pH and the simultaneous presence of exogenous lysine. As a result of the lysine decarboxylation reaction, which consumes one cytoplasmic H+, cadaverine is produced and subsequently excreted, leading to an increase of the external pH. For a long time it, was unclear how the function of LysP was linked to the regulation of the Cad system. Tetsch et al. (2008) demonstrated that LysP is able to modulate the activity of the membrane-integrated protein CadC (55). According to the proposed model, LysP and CadC interact via their transmembrane domains in the absence of lysine. This interaction blocks CadC-dependent expression of the cadBA operon. In the presence of lysine, LysP releases CadC and CadC becomes susceptible to activation by low pH. These findings suggest that LysP has an additional regulatory function, which is typical for so-called trigger transporters (54). Thus, in addition to transport activity, LysP senses lysine availability and transduces the signal to CadC.
Neely and Olson (1996) demonstrated that a high external lysine concentration downregulates lysP expression (32). Expression of most of the genes belonging to the lysine biosynthesis pathway in E. coli is repressed by lysine, but there are multiple modes of regulation known (2, 34, 50). In E. coli and Bacillus subtilis, expression of lysC, which encodes one of the isoenzymes that catalyze the first step in the lysine biosynthesis pathway, is controlled by direct binding of lysine to a conserved leader sequence in its mRNA (34, 52). Orthologs of lysP in Gram-positive bacteria are controlled by lysine-dependent riboswitches, named LYS or L-box elements (43). However, lysine riboswitches are not found in the 5′ untranslated region of lysP mRNA of Gram-negative bacteria (43). Here, we show that lysP transcription is subject to two different types of control. The LysR-type transcriptional regulator (LTTR) ArgP was identified as a major regulator of lysP transcription. In addition, lysP expression is fine-tuned by the global regulator Lrp (leucine-responsive protein). Lrp was also found to be involved in the regulation of cadBA expression.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains used in this study are listed in Table 1. E. coli JM109 (60) was used as a carrier for all plasmids. For the construction of the reporter strains, MG-LR and MG-CR, a method based on rpsL counterselection in combination with the Red/ET recombination system was employed (19) according to the protocol recommended by the technical manual of the Quick and Easy E. coli deletion kit (Gene Bridges). Briefly, the coding sequence of the target gene (lysP or cadBA) was replaced by an rpsL-neo cassette (Gene Bridges) by Red/ET recombination in strain MG16R (Table 1) to give strains MG16R4 and MG16R12. Afterwards, the rpsL-neo cassette was replaced by promoterless lacZ using the Red/ET recombination technique, according to the following procedure. Strain MG16R4 or MG16R12 carrying plasmid pRedET (Gene Bridges) was transformed with a linear DNA fragment comprising the promoterless lacZ gene flanked with homology sequences for the target genes, and subsequently, clones of interest were selected on LB agar plates (PlysP::lacZ fusion) or LB (pH 5.8) agar plates (PcadBA::lacZ fusion) containing 50 μg ml−1 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) and 50 μg ml−1 streptomycin. Blue colonies were tested for kanamycin sensitivity. The Kms clones were verified by colony PCR followed by DNA sequencing.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or description | Reference or source |
|---|---|---|
| E. coli strains | ||
| MG1655 | F− λ−ilvGrfb50rph-1 | 1 |
| MG1655-ΔlacZ | MG1655 ΔlacZ::Tetr | K. Jahreis (personal gift) |
| MC4100 | F−araD139 Δ(argF-lacZ)U169 rpsL150 relA flb-530 Strr | 7 |
| MG16R | MG1655 ΔlacZ::TetrrpsL150 Strr | This work |
| MG16R4 | MG1655 ΔlacZ::TetrrpsL150 ΔlysP::rpsL-neo Kmr Strs | This work |
| MG-LR | MG1655 ΔlacZ::TetrrpsL150 ΔlysPPlysP::lacZ Strr | This work |
| MG-LR9 | MG-LR ΔlysR::Kmr Strr | This work |
| MG-LR10 | MG-LR ΔyeiE::Kmr Strr | This work |
| JCP95 | pop3125 (dapBp [−118/+35]-lacZ) ΔargP::Camr | 2 |
| MG-LR17 | MG-LR ΔargP::Camr Strr | This work |
| MG-LR15 | MG-LR Δlrp::Kmr Strr | This work |
| MG-LR4 | MG-LR PlysPΔTN11A::lacZ (deletion from position −83 to position −52 in the lysP promoter) | This work |
| MG16R12 | MG1655 ΔlacZ::TetrrpsL150 ΔcadBA::rpsL-neo Kmr Strs | S. Ude (unpublished results) |
| MG-CR | MG1655 ΔlacZ::TetrrpsL150 ΔcadBAPcadBA::lacZ Strr | S. Ude (unpublished results) |
| MG-CR15 | MG-CR Δlrp::Kmr Strr | This work |
| BL21(DE3) pLysS | F−ompT r−B m−B | 51 |
| Plasmids | ||
| pBAD33 | Arabinose-inducible PBAD promoter; pACYC184 ori; Ampr | 18 |
| pBADlysP | lysP in pBAD33; Ampr | 55 |
| pBAD24 | Arabinose-inducible PBAD promoter, pBR322 ori; Ampr | 18 |
| pBADargP | argP cloned in the EcoRI and HindIII sites of pBAD24; Ampr | This work |
| pBADlrp | lrp cloned in the EcoRI and HindIII sites of pBAD24; Ampr | This work |
| pET21a | T7 promoter based expression vector with His tag; Ampr | Novagen |
| pET21argP | argP cloned in the NdeI and XhoI sites of pET21a; Ampr | This work |
| pET16b | T7 promoter based expression vector with His tag; Ampr | Novagen |
| pET16lrp | lrp cloned in the NdeI and BamHI sites of pET16b; Ampr | This work |
| pRS415 | Operon fusion vector | 47 |
| pRSlysP | pRS415::lysP promoter (positions −218 to +28) | This work |
| pRSlysP0 | pRS415::lysP promoter with a deletion from position −88 to position −53 | This work |
| pRSlysP1 | pRS415::lysP promoter with replacement of A/C at position −53 | This work |
| pRSlysP2 | pRS415::lysP promoter with replacement of T/G at position −65 | This work |
| pRSlysP3 | pRS415::lysP promoter with replacements of A/C and T/G | This work |
E. coli strains MG-LR4, MG-LR9, MG-LR10, and MG-LR15 were constructed using the Quick and Easy E. coli deletion kit (Gene Bridges) according to the instructions of the manufacturer. Introduction of the argP::Camr allele into MG-LR and lrp::Kmr into MG-CR was performed by P1vir-mediated phage transduction (56), using JCP95 and MG-LR15, respectively, as donor strains. Strain BL21(DE3)pLysS was cultivated in tryptone-phosphate medium (29) and used as a host for pET plasmids (Novagen) for protein overproduction. For determination of lysP expression levels, cells were grown in minimal medium (15) supplemented with glucose or fructose at a final concentration of 0.4% (wt/vol). For determination of cadBA expression levels, cells were cultivated in glucose minimal medium; the phosphate buffer of the medium was adjusted to either pH 5.8 or pH 7.6. Antibiotics were used at the following concentrations: ampicillin, 100 μg ml−1; kanamycin, 50 μg ml−1; chloramphenicol, 34 μg ml−1; tetracycline, 12.5 μg ml−1; and streptomycin, 50 μg ml−1.
Construction and analysis of lysP promoter variants.
A sequence of 246 bp encompassing the whole lysP promoter/control region (positions −218 to +28) (see Fig. 2) was amplified by PCR with primers lysPupEcoRI (5′G GAA TTC CGC TTT CTG GAC TAT TGC GAT C 3′) and lysPprBamHI (5′ CGG GAT CCA CAA AAA TGC TAT CCA TCT TAA 3′) and cloned upstream of the promoterless lacZ gene in vector pRS415 (47). Introduction of deletions or point mutations of the conserved T-N11-A motif was achieved by purchasing the corresponding synthetic DNA fragments (Mr. Gene, Regensburg, Germany), which were subcloned into the EcoRI and BamHI sites of pRS415. E. coli strain MG1655-ΔlacZ was transformed with the resulting plasmids to test lysP expression as described below.
Fig. 2.
Nucleotide sequence of the lysP regulatory region (positions −218 to +58). Predicted −35 and −10 promoter motifs (BProm; http://linux1.softberry.com/berry.phtml?topic=bprom&group=help&subgroup=gfindb) and the start of transcription (position +1) previously identified by primer extension analysis (32) are indicated. Gray-shaded nucleotides show the potential LTTR binding site identified in silico with the conserved T-N11-A motif. Zones protected against DNase I digestion are boxed (see Fig. 5). The start codon is marked in bold letters.
In vivo lysP and cadBA expression studies.
Expression of lysP and cadBA in vivo was determined by means of β-galactosidase assays. For the analysis of PlysP::lacZ expression, cells of an overnight culture grown in minimal medium were inoculated into fresh medium (supplemented with amino acids where indicated), resulting in an optical density at 600 nm (OD600) of 0.05. Cultures were grown aerobically in Erlenmeyer flasks at 37°C. To determine the expression of PcadBA::lacZ, cells were precultured in minimal medium at pH 7.6 and then inoculated into fresh minimal medium at pH 5.8 or pH 7.6, supplemented with lysine and/or arginine where indicated. Cultures were incubated under microaerobic conditions at 37°C to mid-logarithmic growth phase. β-Galactosidase activity measurements were performed as previously described (55) for at least three independent experiments. Values are given in Miller units (MU), which were calculated according to Miller (28).
Molecular biology techniques.
Plasmid DNA and genomic DNA were isolated by using a HiYield plasmid minikit (Sued-Laborbedarf Gauting) and a DNeasy blood and tissue kit (Qiagen), respectively. DNA fragments were purified from agarose gels using a Hi-Yield PCR cleanup and gel extraction kit (Sued-Laborbedarf Gauting). Phusion high-fidelity DNA polymerase or Phire hot-start DNA polymerase (Finnzymes) was used according to the supplier's instructions. Restriction enzymes were purchased from New England Biolabs and used according to the manufacturer's directions.
Purification of Lrp and ArgP.
E. coli BL21(DE3)pLysS harboring plasmid pET16lrp or pET21argP (Table 1) was grown to exponential phase at 30°C, and expression of genes encoding N-terminally His-tagged Lrp (His6-Lrp) or C-terminally His-tagged ArgP (ArgP-His6) was induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG). After 3 h of induction, cells were harvested and washed with 100 mM Na-K-phosphate buffer (pH 7.5) at 4°C. The cell pellet was frozen in liquid nitrogen and stored at −80°C until use. Cells were lysed by passage through a high-pressure cell disrupter (Constant Systems). After centrifugation of the disrupted cells, the supernatant containing the His6 protein was incubated with Ni2+-nitrilotriacetic acid (NTA) resin (Qiagen) preequilibrated with lysis buffer (20 mM imidazole, 50 mM NaH2PO4, 300 mM NaCl, 10% [vol/vol] glycerol, pH 8.0). After 1 h of incubation, the protein-resin complex was washed twice with washing buffer (50 mM imidazole, 50 mM NaH2PO4, 300 mM NaCl, 10% [vol/vol] glycerol, pH 8.0). Finally, the His-tagged protein was eluted in several fractions with buffer containing 250 mM imidazole, 50 mM NaH2PO4, 300 mM NaCl, and 10% (vol/vol) glycerol, pH 8.0. His6-Lrp was dialyzed against Lrp binding buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 0.1 mM dithiothreitol [DTT], 0.1 mM EDTA, 10% [vol/vol] glycerol) and ArgP-His6 against ArgP-binding buffer (24) at 4°C. The purified proteins were stored in the corresponding binding buffers in the presence of 1 mM phenylmethylsulfonyl fluoride (PMSF) at 4°C and used within 1 week or stored at −20°C. Protein concentration was determined according to Bradford (3).
EMSAs.
Probes for the electrophoretic mobility shift assay (EMSA) were amplified by PCR using primers labeled at their 5′ ends with the 6-isomer of carboxyfluorescein (6-FAM) and genomic DNA from E. coli MG1655 as a template unless indicated otherwise. To analyze the binding of ArgP-His6 to the lysP promoter/control region, three different fragments were used: (i) a fragment of 276 bp comprising the sequence from position −218 to position +58 (PlysP fragment) (see Fig. 2), which was obtained by PCR using primers lysPup (5′-CGCTTTCTGGACTATTGCGATC-3′) and lysPprlow (5′-CGCTTCTGTGGTTTTAGTTTCG-3′); (ii) a fragment of 142 bp comprising the sequence from position −84 to position +58 (T-N11-A fragment), which was amplified with primers TN11A (5′-TATAATCCCTGGGCGATCATG-3′) and lysPprlow; and (iii) a fragment of 93 bp (−35-10 fragment, comprising positions −35 to +58) obtained by amplification with primers −35 (5′-CGGAAGGATTGCCAATCGT-3′) and lysPprlow. To evaluate the binding of His6-Lrp to the lysP promoter/control region, only the PlysP fragment was used. To determine the binding of His6-Lrp to the cadBA promoter/control region, a fragment comprising positions −150 to +72 upstream of the cadB gene was cloned into the EcoRI and BamHI sites of the pUC19 plasmid (60) and amplified with primers 6-FAM uni-24 (5′-ACGACGTTGTAAAACGACGGCCAG-3′) and rev-24 (5′-TTCACACAGGAAACAGCTATGACC-3′). As a control for nonspecific binding, a DNA fragment of 258 bp within the lysP coding sequence (control fragment) obtained by amplification with lysPcup (5′-ACATCAGCGTTAGTCCGT-3′) and lysPclow (5′-ATGGAGGTCAGGAAGCACA-3′) was used. After PCR amplification, the obtained DNA fragments were purified by 7% (wt/vol) polyacrylamide gel electrophoresis according to the protocol of the GenElute gel extraction kit (Sigma). ArgP-DNA binding assays were performed by incubating 30 fmol of a DNA fragment with increasing concentrations of ArgP-His6 in 25 μl ArgP binding buffer supplemented with 12 μg ml−1 sonicated salmon sperm DNA as a nonspecific competitor, 50 μg ml−1 bovine serum albumin, and, where indicated, 0.1 mM lysine or arginine. Binding of His6-Lrp to DNA was performed with 30 fmol of a DNA fragment and increasing concentrations of His6-Lrp in 25 μl Lrp binding buffer supplemented with 20 μg ml−1 salmon sperm DNA and 0.1 mM lysine where indicated. After incubation at 25°C for 30 min, complexes were resolved by electrophoresis in a 6.5% (wt/vol) polyacrylamide gel under a constant voltage of 10 V/cm at room temperature for 1.5 h. Gels were scanned with a Typhoon Trio imager (Amersham Biosciences) under an excitation wavelength of 488 nm. Quantification of free DNA and protein-bound DNA was performed by densitometry using the ImageQuant 5.0 analysis software program (Molecular Dynamics). The quantified data were plotted versus the protein concentration to obtain the binding profile. The apparent dissociation constants (KD) of ArgP-DNA binding assays were determined to be the protein concentration at which the fraction of bound DNA equals 0.5. The binding profiles obtained from the Lrp binding assays were fitted to the Hill equation to determine the KD value and the Hill coefficient (n).
DNase I footprinting assays.
DNase I footprinting analysis was performed according to the method described by Sandaltzopoulos and Becker (44). One hundred fifty nanograms of a PlysP DNA fragment (bases −218 to +58) labeled at its 5′end with 6-FAM as described above was incubated with different concentrations of ArgP-His6 in ArgP binding buffer supplemented with 12 μg ml−1 sonicated salmon sperm DNA as a nonspecific competitor, 50 μg ml−1 bovine serum albumin, and, where indicated, 0.1 mM lysine in a final volume of 50 μl. After incubation at 25°C for 30 min, 50 μl of a 5 mM CaCl2 solution was added, and incubation was prolonged for 1 min. Subsequently, 0.25 U of DNase I was added, and after 5 min, the reaction was stopped by adding 0.5 ml DF buffer from the Hi-Yield PCR cleanup and gel extraction kit (Sued-Laborbedarf Gauting). After purification, the DNA fragments were analyzed with an ABI PRISM 377 DNA sequencer, and the data were evaluated with the Peak Scanner software program (Applied Biosystems).
DNA affinity purification assay for the identification of DNA-binding proteins.
To isolate putative transcriptional regulators of lysP, a DNA affinity purification protocol was applied (17). For this purpose, a biotinylated PlysP fragment was generated by PCR using primers lysPup, labeled with biotin at the 5′end, and lysPprlow. As a control, a biotinylated DNA fragment located within the lysP coding sequence (obtained by amplification with biotin-labeled lysPcup and lysPclow) was used. About 600 pmol of the biotin-labeled DNA fragments was immobilized with streptavidin-coated magnetic particles (Chemagen Biopolymer-Technologies) according to the manufacturer's instructions. For the preparation of the cytoplasmic protein extract, E. coli MG1655 was cultivated in 800 ml of glucose minimal medium to an OD600 of 0.8. Cells were harvested at 4°C, washed with cold protein binding buffer B (PBB) (41), resuspended in 8 ml of the same buffer, and broken with a French press. After centrifugation to remove the cellular debris, the supernatant extract was incubated with DNA-coated magnetic beads (previously equilibrated with PBB) at room temperature for 30 min. Washing to remove unspecific bound proteins and elution of tightly bound proteins was performed as described by Rey et al. (41). Eluted fractions were collected, subjected to sodium dodecyl sulfate-polyacrylamide electrophoresis (SDS-PAGE) (23), and stained with Coomassie blue. Proteins were identified by peptide fingerprint analysis (20) using a matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry (MS) system (Voyager DE STR; Applied Biosystems). Samples were prepared and identified as described previously (59).
RESULTS
lysP transcription is negatively regulated by lysine.
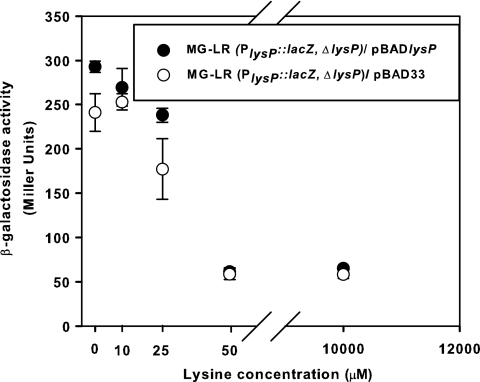
Previous work showed that lysP mRNA declines within 4 min after addition of 10 mM lysine (32). To analyze the concentration-dependent effect of external lysine on lysP transcription in more detail, E. coli strain MG-LR, an MG1655 derivative that carries a PlysP::lacZ fusion, was constructed. In this strain, the coding sequence of the lysP gene was replaced by the reporter gene lacZ, so that the lacZ gene is located exactly in the same genetic context as the lysP gene. Since the MG-LR strain is ΔlysP, an expression plasmid carrying the lysP gene (pBADlysP) (Table 1) was introduced into this strain to determine whether the expression from the lysP promoter (PlysP) was affected by the presence of LysP. β-Galactosidase activities indicated that lysP expression was completely repressed by external lysine concentrations of 50 μM and higher (Fig. 1). The availability of LysP did not alter the lysine-mediated repression (Fig. 1).
Fig. 1.
Effect of the external lysine concentration and LysP on lysP expression. Cultures of E. coli strain MG-LR (PlysP::lacZ ΔlysP) with plasmid pBAD33 or pBADlysP were grown in fructose minimal medium supplemented with different concentrations of external lysine and 0.006% (wt/vol) arabinose to induce the expression of the lysP gene cloned in the pBAD33 plasmid. When cultures reached an OD600 of ∼0.5, samples were collected and β-galactosidase activities were determined. The experiment was performed in triplicate, and error bars indicate standard deviations from the means.
Identification of ArgP as a regulator of lysP expression.
Analysis of the lysP promoter revealed a conserved T-N11-A motif, a typical binding site of LysR-type transcriptional regulators (LTTRs) that is in close proximity to a sequence with strong similarity to the consensus sequence for σ70-dependent E. coli promoters (Fig. 2). Based on this motif and previous data from the literature (2, 8, 16, 50), three LTTRs were selected as putative transcriptional regulators of lysP: LysR, YeiE, and ArgP. LysR is the activator protein required for expression of lysA, which encodes the enzyme that catalyzes the last step in lysine biosynthesis, the decarboxylation of diaminopimelate (DAP), into lysine (50). LysR is responsive to the intracellular concentration of DAP and lysine. YeiE is encoded by a gene located immediately upstream of lysP, and the induction of yeiE expression increased the expression of lysP (16). Unexpectedly, under the conditions tested (minimal medium at pH 7.6 and 5.8 with or without the addition of external lysine and rich medium), neither a deletion of lysR nor a deletion of yeiE affected the expression of lysP (data not shown). It has been reported that mutations in argP affect the uptake of arginine, ornithine, and lysine (8). However, the direct implication of ArgP in the regulation of genes encoding the corresponding transport proteins responsible for the uptake of these amino acids has never been analyzed.
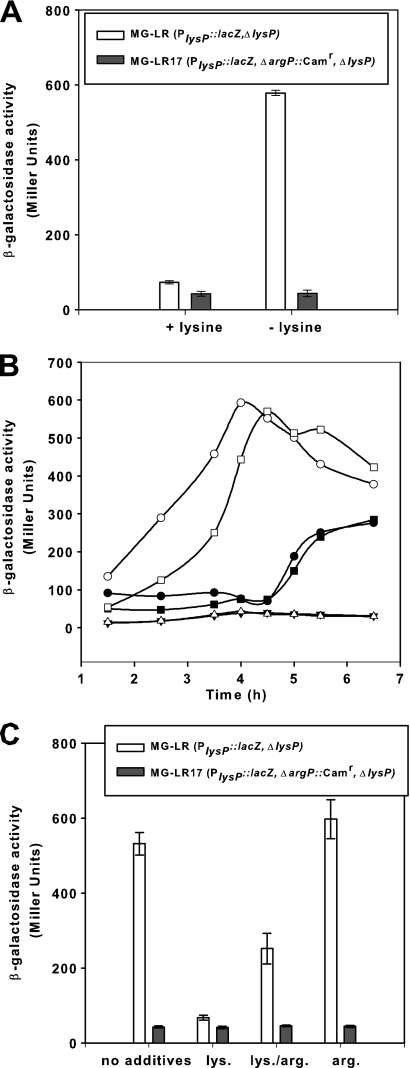
To evaluate whether ArgP was involved in the transcriptional regulation of lysP, a nonfunctional argP allele (ΔargP::Camr) was transduced into strain MG-LR carrying the chromosomal PlysP::lacZ fusion. The resulting mutant, named MG-LR17, and the argP+ parent strain were grown in glucose minimal medium with or without the addition of 0.1 mM lysine and analyzed for β-galactosidase activities (Fig. 3A). The results clearly showed that expression of lysP did not occur in the argP mutant, either in the absence or in the presence of lysine.
Fig. 3.
Regulation of lysP expression by lysine, arginine, and ArgP. (A) Effect of the argP deletion on lysP expression. Parent strain MG-LR (PlysP::lacZ ΔlysP) and the argP mutant MG-LR17 (PlysP::lacZ ΔargP::Camr ΔlysP) were grown aerobically in glucose minimal medium with or without the addition of 0.1 mM lysine. When cultures reached an OD600 of ∼0.8, samples were analyzed for β-galactosidase activity. (B) Complementation of the argP mutant with pBADargP. Strains MG-LR/pBAD24 (○, •), MG-LR17/pBAD24 (▵, ▾), and MG-LR17/pBADargP (□, ▪) were grown in glucose minimal medium without lysine (open symbols) or with the addition of 0.1 mM lysine (closed symbols). Arabinose at a final concentration of 0.2% (wt/vol) was added to all cultures after 2.5 h of growth. β-Galactosidase activities were determined at different time points during growth. (C) Effect of basic amino acids on lysP expression. Strains MG-LR and MG-LR17 were cultivated as described for panel A in glucose minimal medium without supplementation or supplemented with 10 mM lysine (lys.) and/or arginine (arg.) for determination of β-galactosidase activity. All experiments were performed at least three times, and where indicated, error bars represent standard deviations from the means.
To confirm the role of ArgP in the regulation of lysP transcription, the argP gene was cloned into plasmid pBAD24 under the control of the arabinose-inducible promoter (18). The argP mutant MG-LR17 was transformed with the resulting plasmid, named pBADargP (Table 1). Expression of lysP was monitored in cells of the argP+ strain (MG-LR) bearing the pBAD24 vector and the argP mutant with pBAD24 or pBADargP grown in glucose minimal medium plus arabinose, with and without the addition of lysine. As shown in Fig. 3B, plasmid-carried argP fully restored the lysP expression pattern in the MG-LR17 mutant. In the absence of lysine, transcription of lysP in strain MG-LR increased immediately. In the presence of lysine, induction was prevented within the first hours of growth and slowly increased after prolonged growth, presumably due to lysine limitation. In the argP mutant, induction of lysP did not occur. However, plasmid-carried argP rescued lysP expression in mutant MG-LR17. In the absence of lysine, induction of lysP occurred after argP expression was induced (2.5 h). In the presence of lysine, the expression pattern of this complemented mutant was dependent on the growth phase and probably on lysine availability. These results indicated that ArgP is responsible for the transcriptional activation of lysP in the absence of lysine.
Previously, ArgP was identified as a lysine-dependent regulator of argO, which encodes an arginine exporter (24, 30), and dapB, which encodes an enzyme of the lysine biosynthesis pathway (2). Transcription of these genes was also affected by the presence of arginine in the culture medium, indicating that both lysine and arginine are coeffectors of ArgP (2, 24). Therefore, the effect of arginine on the expression of the PlysP::lacZ fusion in strains MG-LR and MG-LR17 was tested. Figure 3C shows the β-galactosidase activities of cells cultivated in glucose minimal medium without amino acids or supplemented with lysine, arginine, or lysine plus arginine. When arginine was added to the growth medium, lysP expression in the MG-LR strain was induced to the same extent as in cells that were grown in the absence of amino acids. Importantly, the presence of arginine partially relieved the repressive effect of lysine, as indicated by the >3-fold-higher β-galactosidase activities (251 ± 41.1 Miller units [MU]) of cells that were cultivated in arginine plus lysine medium than of those that were grown in lysine medium (β-galactosidase activity, 67 ± 6.3 MU). As expected, there was no lysP expression in the ΔargP mutant under all tested conditions.
ArgP binds to the lysP promoter/control region at a T-N11-A motif in the presence and absence of lysine.
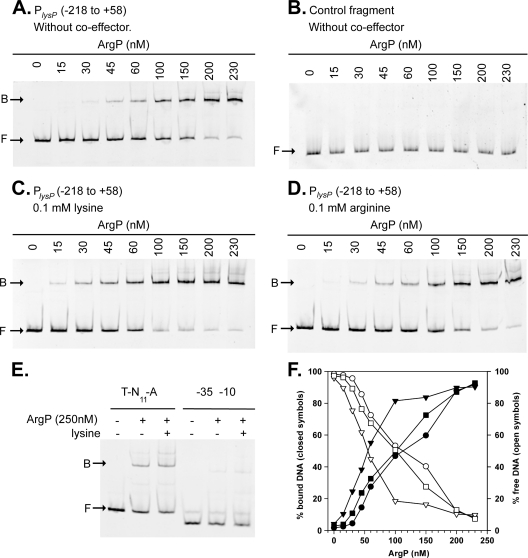
To determine whether ArgP directly regulates lysP transcription, we tested binding of ArgP to the lysP promoter/control region. For this purpose, ArgP with a C-terminal hexahistidine tag (ArgP-His6) was purified. In a control experiment, this ArgP derivative complemented strain MG-LR17, indicating that the His6 tag did not alter the function of ArgP (data not shown). A fluorescently labeled DNA fragment encompassing the lysP promoter/control region from position −218 to position +58 (PlysP fragment) was incubated with increasing concentrations of purified ArgP-His6 in the presence of salmon sperm DNA as a nonspecific competitor (Fig. 4A). A DNA fragment of similar size within the lysP coding sequence was used as a control for nonspecific binding (Fig. 4B). ArgP-His6 specifically bound to the PlysP fragment with an apparent KD of 125 ± 13 nM (Fig. 4A and F).
Fig. 4.
Binding of ArgP to the lysP control region. (A, C, and D) Electrophoretic mobility shift assays (EMSAs) of a fluorescently labeled DNA fragment from bp −218 to bp +58 encompassing the lysP promoter/control region (PlysP) with increasing concentrations of purified ArgP-His6 in the absence of coeffector (A) or in the presence of 0.1 mM lysine (C) or 0.1 mM arginine (D). The positions of free DNA (F) and ArgP-DNA complexes (B) are marked with arrows. (B) A DNA fragment within the lysP coding sequence was used as a control for unspecific binding. (E) Binding of ArgP-His6 to a fragment from position −84 to position +58 bearing the potential ArgP-binding site (T-N11-A) and to a fragment from position −35 to position +58 (−35-10) in the presence or absence of lysine. (F) Binding curves obtained after the quantification of free DNA (open symbols) and ArgP-bound DNA (closed symbols) in EMSA gels without coeffector (○, •) in the presence of lysine (▿, ▾) or arginine (□, ▪).
To determine whether lysine or arginine affected binding of ArgP to PlysP, these amino acids were added to the binding assay at a final concentration of 0.1 mM (Fig. 4C and D). Whereas arginine did not affect the binding of ArgP to the lysP promoter/control region, the presence of lysine increased the binding affinity approximately 2-fold (KD, 63 ± 9 nM) (Fig. 4C and F). This differential effect of lysine and arginine on DNA affinity of ArgP has already been reported for the argO control region (24).
As already mentioned, a potential ArgP-binding site, ATGAAGGTGTCTTAT, is centered at position −59 in the lysP promoter/control region (Fig. 2). To evaluate the importance of this sequence for binding of ArgP to the lysP control region, a DNA fragment from position −84 to position +58 containing the T-N11-A conserved motif (T-N11-A fragment) and another, from position −35 to position +58, without this T-N11-A sequence (−35-10 fragment) were incubated with ArgP in the presence and absence of lysine (Fig. 4E). A retarded band was apparent when ArgP was incubated with the T-N11-A fragment, whereas in the presence of the DNA fragment without the T-N11-A motif (−35-10 fragment), only a faint retarded band was seen, indicating that the T-N11-A sequence is required for proper binding of ArgP to the lysP promoter/control region. Moreover, the affinity of ArgP for the T-N11-A fragment was found to be slightly higher in the presence of lysine (Fig. 4E).
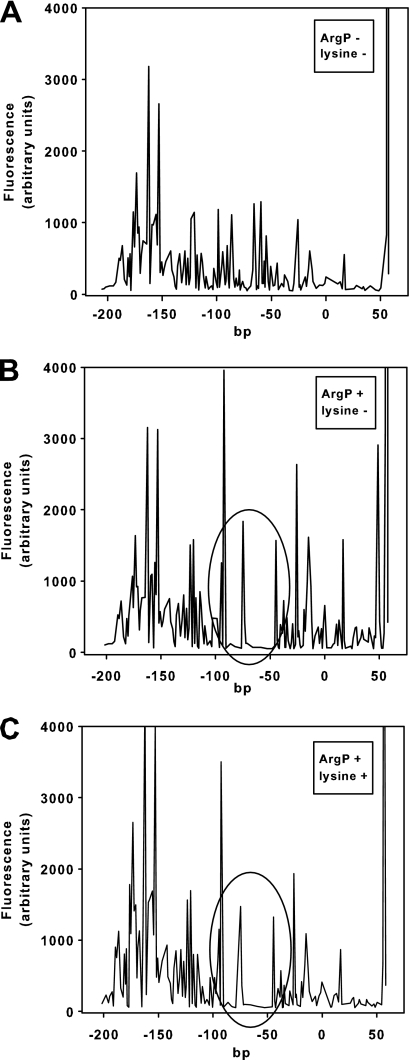
To study the ArgP-binding site in the lysP promoter/control region in more detail, DNase I footprinting analysis was performed in the absence and presence of lysine (Fig. 5). ArgP-His6 protected the stretch from position −91 to position −47, with an intervening unprotected region between positions −76 and −71 (Fig. 2 and 5). Importantly, ArgP bound to the same sites in the presence and absence of its coeffector lysine (Fig. 5B and C).
Fig. 5.
Determination of the ArgP-binding site within the lysP control region. DNase I digestion patterns were determined for a DNA fragment from position −218 to position +58 of the lysP control region labeled with fluorescein at the 5′ end of the top strand. Panel A shows the restriction pattern obtained in the absence of purified ArgP-His6, and panels B and C show the pattern obtained in the presence of 4.3 μM purified protein in the absence (B) or presence (C) of 0.1 mM lysine. Regions protected by ArgP (position −91 to −77 and −70 to −47) are encircled.
Elimination or modification of the T-N11-A motif affected lysP expression in vivo.
To evaluate the importance of the T-N11-A motif within the lysP promoter/control region for in vivo expression of lysP, a fragment encompassing this sequence (nucleotides −83 to −52) (Fig. 2) was deleted in the MG-LR strain, resulting in strain MG-LR4. Elimination of this motif completely abolished lysP expression under all conditions tested (Fig. 6), indicating its importance for ArgP-mediated transcriptional regulation. To analyze the effect of point mutations within this motif, the whole lysP promoter/control region was fused to a promoterless lacZ gene in vector pRS415 (Table 2). The deletion of the motif (pRSlysP0) or the replacement of both conserved T and A nucleotides (pRSlysP3) prevented lysP induction under lysine-limiting conditions. The replacements of A (pRSlysP1) or T (pRSlysP2) did not abolish lysP induction but significantly reduced it (Table 2).
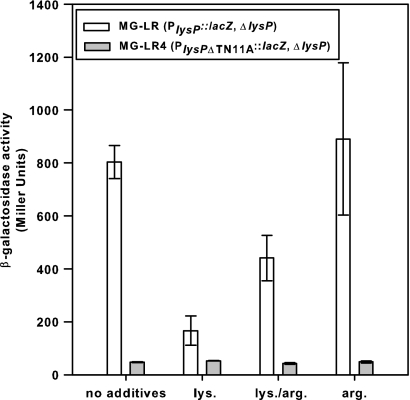
Fig. 6.
Effect of elimination of the T-N11-A motif in the lysP control region on lysP expression. Parent strain MG-LR (PlysP::lacZ ΔlysP) and strain MG-LR4 (PlysPΔTN11A::lacZ ΔlysP) were grown aerobically to an OD600 of ∼0.8 in glucose minimal medium without supplementation or supplemented with 10 mM lysine (lys.) and/or arginine (arg.) for determination of β-galactosidase activity. All experiments were performed at least three times. Error bars represent standard deviations from the means.
Table 2.
Effects of modifications within the lysP promoter/control region on lysP expressiona
| E. coli strain or strain carrying plasmid | Description of modification within the lysP promoter/control region | Induction of lysP upon lysine limitation (lysPno lysine/lysP10 mM lysine)b |
|---|---|---|
| MG-LR | None | 7.90 |
| MG1655-ΔlacZ/pRSlysP | None | 3.43 |
| MG-LR4 | Deletion of T-N11-A motif (nucleotides −83 to −52) | 1.03 |
| MG1655-ΔlacZ/pRSlysP0 | Deletion of T-N11-A motif (nucleotides −88 to −53) | 1.28 |
| MG1655-ΔlacZ/pRSlysP1 | Replacement of A/C at position −53 | 2.77 |
| MG1655-ΔlacZ/pRSlysP2 | Replacement of T/G at position −65 | 1.85 |
| MG1655-ΔlacZ/pRSlysP3 | Replacement of A/C (position −53) and T/G (position −65) | 1.24 |
Strains were grown aerobically in glucose minimal medium in the absence or presence of 10 mM lysine to an OD600 of ∼0.8. β-Galactosidase activity was determined and served as a measurement for lysP expression.
The ratio between lysP expression levels in the absence and presence of lysine indicates the inducibility of the lysP promoter. Data were obtained from at least three independent experiments, and average values (the standard deviation was about 15%) were used for calculating the ratios.
Isolation and identification of other proteins that specifically bind to the lysP promoter/control region.
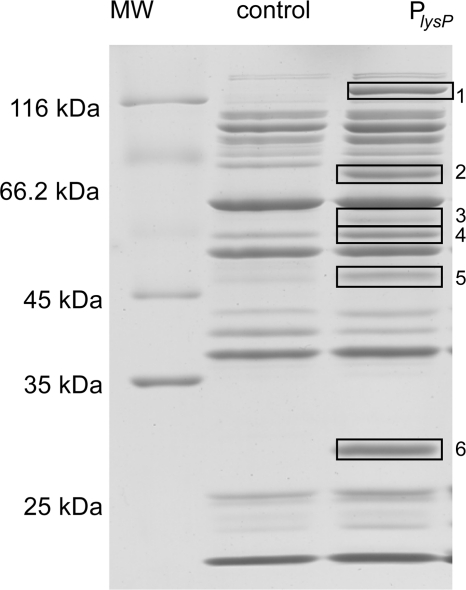
So far, our results demonstrate that ArgP directly binds to the lysP promoter/control region and that the T-N11-A sequence located close to the RNA polymerase binding site is important for binding and crucial for lysP transcription. According to the in vivo analyses, ArgP is a transcriptional activator of lysP in the absence of lysine. The in vitro experiments demonstrated that ArgP binds more avidly to the lysP control region in the presence of lysine, which might underlie the lysine-mediated prevention of lysP expression similarly to the previously described shutoff of argO expression by lysine-loaded ArgP (24). Alternatively, another protein might coregulate lysP repression. This scenario is plausible, because ArgP and Lrp competitively activate argO (35). To search for other proteins that might bind to the lysP promoter, a DNA affinity purification approach was used. The 276-bp fragment encompassing the lysP promoter/control region (positions −218 to +58) was biotinylated, linked to streptavidin-coated magnetic beads, and incubated with a concentrated soluble protein extract from E. coli MG1655 grown in glucose minimal medium. The same was done with a fragment of 258 bp within the lysP coding sequence, which served as a control. Proteins tightly bound to the DNA fragments were eluted as described in Materials and Methods and analyzed by SDS-PAGE. As shown in Fig. 7, several proteins that specifically bound to the lysP promoter/control region and not to the control fragment were detected.
Fig. 7.
SDS-PAGE of proteins after DNA affinity purification. A biotin-labeled DNA fragment from position −218 to position +58 encompassing the lysP promoter/control region (PlysP) and a DNA fragment within the lysP coding region (control) were bound to streptavidin-coated magnetic beads and subsequently incubated with a soluble extract of E. coli MG1655 grown in glucose minimal medium. Tightly bound proteins were eluted with a high-ionic-strength buffer and separated by SDS-PAGE. Boxed bands correspond to proteins that specifically bind to the lysP promoter/control region (Table 3). MW, molecular mass marker.
The binding proteins were identified by MALDI-TOF mass spectrometry. With the exception of one, we were able to identify the eluted proteins (Table 3). All of them turned out to be DNA-binding proteins. The most abundant protein was the leucine-responsive-protein, Lrp. As Lrp is a global transcriptional regulator that controls the expression of numerous genes in response to the availability of amino acids and nitrogen bases (6, 9, 33), the role of this protein in lysP expression was analyzed in more detail.
Table 3.
Identification of proteins after DNA affinity purification by peptide fingerprint analysis
| Banda | Identified protein |
|---|---|
| 1 | HsdR; host restriction endonuclease R. Subunit of EcoKI restriction-modification system |
| 2 | HsdM; modification methyltransferase component of the EcoKI restriction-modification system |
| 3 | HsdS; specificity-determinant component of EcoKI restriction-modification system |
| 4 | NadR; transcriptional regulator |
| 5 | Nonidentified protein |
| 6 | Lrp; leucine-responsive protein |
The band numbers correspond to those shown in Fig. 7.
Lrp stimulates transcription of lysP by direct binding to its control region.
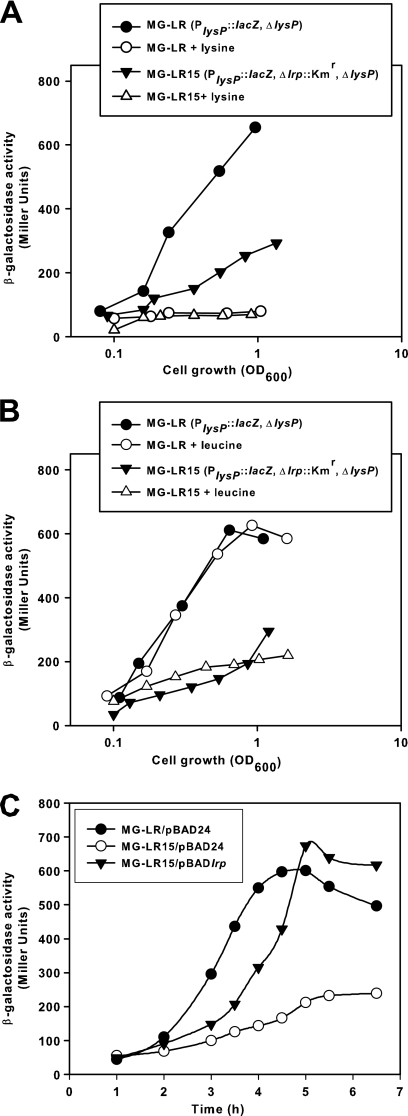
To determine whether Lrp influences lysP transcription, the lrp gene was inactivated in E. coli MG-LR, resulting in strain MG-LR15 (Δlrp::Kmr). Expression of the PlysP::lacZ fusion in strains MG-LR and MG-LR15 was monitored during growth in glucose minimal medium with and without the addition of lysine. Figure 8A shows that the PlysP activity of the Δlrp::Kmr mutant in the absence of lysine is lower than that of the parent strain. At the late exponential growth phase (optical density at 600 nm [OD600] of about 1.0), the expression level of lysP in the MG-LR15 strain was about 50% lower than that in the parent strain MG-LR. The presence of 0.1 mM lysine reduced lysP expression in both strains. Therefore, Lrp seems to be an activator of lysP transcription in the absence of lysine.
Fig. 8.
Effect of lrp deletion on lysP expression. E. coli strains MG-LR (PlysP::lacZ, ΔlysP) and MG-LR15 (PlysP::lacZ, ΔlysP, Δlrp::Kmr) were grown aerobically in glucose minimal medium with or without the addition of 0.1 mM lysine (A). To test the effect of leucine on lysP expression, the growth medium was supplemented with 0.6 mM valine and 0.4 mM isoleucine, with or without the addition of 10 mM leucine (B). At different times during growth samples were analyzed for β-galactosidase activity. (C) Complementation of the MG-LR15 (PlysP::lacZ, ΔlysP, Δlrp::Kmr) mutant with the pBADlrp plasmid. Strains MG-LR/pBAD24, MG-LR15/pBAD24 and MG-LR15/pBADlrp were grown in glucose minimal medium, and after 2.5 h 0.2% arabinose (wt/vol) was added to induce the expression of the lrp gene cloned in pBAD24. β-Galactosidase activities were determined in samples collected at different times, before and after induction. All experiments were performed in triplicates and mean values are presented. The standard deviations from the mean were less than 10%.
The regulatory effect of Lrp is sometimes modulated by the effector molecule l-leucine (6, 33). Because a high leucine concentration in the culture medium decreases the growth rate due to isoleucine restriction (39), the effect of leucine on lysP expression was analyzed by comparing the β-galactosidase activity of bacteria cultivated in glucose minimal medium supplemented with isoleucine and valine to that of cells grown under the same conditions but in the presence of isoleucine, valine, and leucine. We found that l-leucine had no effect on PlysP activity in the Δlrp::Kmr mutant or in the lrp+ strain (Fig. 8B). To confirm the activator role of Lrp in lysP transcription, the pBADlrp plasmid (in which the lrp gene was cloned under the control of the arabinose promoter) (Table 1) was introduced into strain MG-LR15, and lysP expression levels were monitored before and after the addition of l-arabinose to the culture medium. Figure 8C clearly shows that the induction of lrp expression stimulated lysP transcription. Together, these results suggest that Lrp potentiates the activator effect of ArgP on lysP transcription in the absence of lysine.
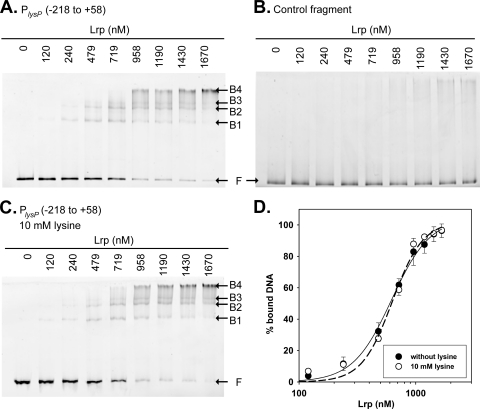
Among the regulatory targets of Lrp, there are many transcription factors that participate in the regulation of amino acid metabolism and molecule transport (9). Therefore, it was important to determine whether the activator role of Lrp in lysP expression was direct or indirect. Peeters et al. have recently demonstrated that argP expression was not regulated by Lrp (35). Thus, EMSAs were performed in the presence of an excess of nonspecific competitor to assess the binding of purified His6-Lrp to the lysP promoter/control region (Fig. 9). One to four different retarded complexes were observed when various concentrations of Lrp were incubated with the 276-bp fragment encompassing the lysP promoter/control region (PlysP) (Fig. 9A). The apparent KD obtained for the binding reaction was 610 ± 44 nM (Fig. 9D), and the Hill coefficient was 3.
Fig. 9.
Lrp binding to the lysP promoter region. (A and C) Electrophoretic mobility shift assays (EMSAs) of the fluorescently labeled PlysP fragment (positions −218/+58) with increasing concentrations of purified His6-Lrp in the absence (A) or presence (C) of lysine. (B) EMSA performed with a DNA fragment within the lysP coding sequence as a control for unspecific binding. The positions of free DNA (F) and Lrp-DNA complexes (B1 to B4) are marked. (D) Binding profiles obtained after the quantification of free DNA and Lrp-bound DNA in EMSA gels were fitted using the Hill equation.
As previously reported for several Lrp-regulated genes, the concentration-dependent binding of Lrp to lysP suggests cooperative binding of Lrp dimers to multiple binding sites resulting in complexes with different stoichiometries. Since the in vivo experiments demonstrated that the presence of lysine affected lysP expression levels (Fig. 8A), we evaluated whether lysine affects the binding of Lrp to the lysP control region in vitro. As shown in Fig. 9C and D, the addition of l-lysine to the binding assay neither changed the Lrp affinity nor changed the binding pattern. When His6-Lrp was incubated with a DNA fragment encompassing the lysP coding sequence (control fragment), no retarded bands were observed (Fig. 9B), confirming the specific binding of Lrp to the lysP control region.
Lrp modulates expression of the cadBA operon.
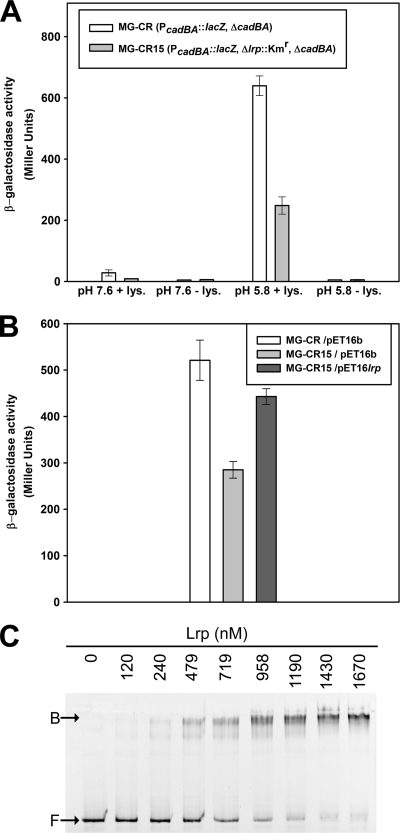
The Lrp regulon comprises several genes involved in amino acid synthesis and degradation. For example, expression of ldcC, coding for the constitutive lysine decarboxylase of E. coli, is downregulated by Lrp (53). Considering the important role of LysP in regulating the expression of cadA (55), which encodes the inducible lysine decarboxylase, we analyzed the effect of Lrp on transcription of the cadBA operon, which encodes the inducible lysine decarboxylase CadA and the lysine/cadaverine antiporter CadB. For this purpose, two strains that carry chromosomal promoter-lacZ fusions (PcadBA::lacZ) and are either lrp+ (MG-CR) or lrp null (Δlrp::Kmr, MG-CR15) were constructed. Cells were cultivated in glucose minimal medium at physiological (pH 7.6) or low (pH 5.8) pH, with or without the addition of 5 mM lysine, and β-galactosidase activities were determined. As expected, the PcadBA promoter was active only in cells that were exposed to low external pH in the presence of lysine (Fig. 10A), a condition that is known to induce the Cad system (27). However, under inducing conditions, the PcadBA activity in the Δlrp::Kmr mutant strain was >2-fold lower than that in the parent strain, suggesting that Lrp stimulates expression of cadBA.
Fig. 10.
Lrp stimulates cadBA expression. (A) Effect of lrp mutation on the expression of the cadBA operon under different growth conditions. Strains MG-CR (PcadBA::lacZ ΔcadBA) and MG-CR15 (PcadBA::lacZ Δlrp::Kmr ΔcadBA) were grown in glucose minimal medium at pH 7.6 or 5.8 with or without the addition of 5 mM lysine (lys.) under microaerobic conditions. After 7 h of incubation, samples were collected and β-galactosidase activities were determined. (B) Complementation of the Δlrp::Kmr mutant with the pET16lrp plasmid. Strains MG-CR/pET16b, MG-CR15/pET16b, and MG-CR15/pET16lrp were cultivated in glucose minimal medium (pH 5.8) with 5 mM lysine. After 7 h of incubation, β-galactosidase activities were determined. (C) Binding of His6-Lrp to the PcadBA promoter/control region. A fragment encompassing the PcadBA promoter/control region was incubated with increasing concentrations of purified His6-Lrp in the presence of salmon sperm DNA as a nonspecific competitor. The positions of free DNA (F) and the Lrp-DNA complex (B) are marked.
To corroborate these results, the pET16lrp plasmid (Table 1) was introduced into the Δlrp::Kmr mutant strain and the PcadBA activity in strains MG-CR/pET16b, MG-CR15/pET16b, and MG-CR15/pET16lrp was determined. The pET16b plasmid was chosen as an expression vector in this case, because the T7-driven promoter ensures very low expression levels in strain MG1655. The results presented in Fig. 10B reveal that the reintroduction of lrp into the Δlrp::Kmr mutant increases the PcadBA activity to levels similar to those for the parent strain. It has been demonstrated that Lrp does not alter intracellular levels of CadC, the transcriptional activator of the cadBA operon (42). Therefore, the possibility of an indirect effect of Lrp via CadC can be discarded.
To evaluate the capacity of Lrp to bind to the PcadBA promoter, EMSAs were performed with various concentrations of the purified His6-Lrp protein and a DNA fragment encompassing the PcadBA control region. Accordingly, His6-Lrp is able to bind to the promoter that drives the expression of cadBA with an apparent dissociation constant (KD) of 648 ± 66 nM and a Hill coefficient of 2.15 (Fig. 10C; see also Fig. S1 in the supplemental material). Together, these results indicate that Lrp upregulates expression of cadBA. With the identical functions of CadA and LdcC in amino acid catabolism taken into account, the opposite regulation of both genes by Lrp would be an efficient way for the cell to save energy.
DISCUSSION
ArgP is responsible for the lysine-dependent control of lysP transcription.
While studying the mechanisms involved in the regulation of the Cad system (lysine decarboxylase system) in E. coli, Neely and Olson had shown that lysP transcription is controlled by the exogenous lysine concentration (32). Here, we identified the LysR-type transcriptional regulator ArgP as the regulator responsible for the control of lysP transcription. Our results indicate that under lysine-limiting growth conditions, ArgP functions as a transcriptional activator of lysP expression by binding to a sequence located between positions −91 and −47 in the lysP promoter/control region. Specifically, a T-N11-A motif (nucleotides −65 to −53), characteristic for LTTR-dependent promoters (45), was identified. In vitro EMSAs as well as in vivo transcriptional studies indicated that this motif is crucial for ArgP binding. Deletion of the whole motif or substitution of the conserved nucleotides T and A prevented or reduced lysP induction in the absence of lysine. ArgP is a member of the LTTR protein family, which binds coeffectors. Arginine and lysine were found to bind to ArgP (24). In vitro assays indicated that ArgP bound to the lysP promoter/control region irrespective of the presence of lysine and arginine, but lysine increased the affinity of ArgP by a factor of 2.
ArgP of E. coli is also responsible for the lysine-dependent regulation of dapB, which encodes one of the enzymes of the diaminopimelate and lysine biosynthesis pathway (2), and argO, which encodes the arginine exporter ArgO (24, 30). It is important to note that the molecular mechanisms of lysine-dependent regulation by ArgP differ between argO and dapB (2, 24). In the case of dapB, lysine prevents binding of ArgP to its binding site, which is located in the position −118/−81 interval upstream of the transcriptional start site (2). In contrast, ArgP binds to a sequence between positions −85 and −20 of the argO operator/promoter and forms a stable binary complex in both its liganded and its unliganded forms. Arg-loaded ArgP binds to the argO promoter/control region and recruits the RNA polymerase, resulting in the induction of argO. Lys-loaded ArgP binds to the same sites, albeit with higher affinity, but restrains the polymerase in a molecular complex that is competent for neither productive nor abortive transcription.
Our results suggest that the mechanism for the ArgP-controlled lysP expression is similar to the one described for argO. Specifically, unloaded or Arg-loaded ArgP induces lysP expression, while the Lys-loaded form prevents expression. The lysine KD value reported for ArgP is 70 μM (24), which is in good agreement with the observed shutoff of the PlysP::lacZ activity at an external lysine concentration higher than 25 μM. In contrast to argO, external arginine seems not to be essential for transcription of lysP by ArgP. This difference might be related to the locations of the ArgP-binding sites within the promoter/control regions. The ArgP-binding site for argO extends up to nucleotide −20 and thereby overlaps the −35 promoter motif, whereas the ArgP-binding site for lysP extends up to position −47, which is upstream of the −35 promoter site (Fig. 2). Nonetheless, an arginine effect on lysP expression was detectable when lysine and arginine were simultaneously added to the cultures. Under this condition, arginine overrode the inhibitory effect of lysine by a factor of 3. Laishram and Gowrishankar (24) demonstrated that arginine and lysine compete for the binding to dimeric ArgP. Therefore, the levels of lysP expression measured in cells that were grown in the presence of both amino acids could be attributable to the simultaneous existence of Arg-ArgP and Lys-ArgP complexes, which are effective or ineffective, respectively, in transcriptional activation.
In general, the results obtained in this work underline the importance of ArgP in the transcriptional control of genes involved in basic amino acid transport. ArgP seems to be a versatile regulator, able to control gene expression of various basic amino acid transporters by responding to low-molecular-weight coeffectors in order to maintain a balance in the intracellular concentration of at least lysine and arginine.
Lrp-dependent regulation of lysP and cadBA.
The leucine-responsive protein (Lrp) was identified as another protein that specifically binds to a DNA sequence encompassing the lysP control region. Lrp has been designated a physiological barometer (14). Its main function is to control the expression of target genes and operons according to the nutritional status of the cell. Lrp upregulates genes during famine and downregulates genes during feast (6, 9). Most of the genes regulated by Lrp are involved in small-molecule transport and amino acid metabolism (9, 53). The amino acid l-leucine might act as a coeffector of Lrp and potentiates, overcomes, or has no effect on the function of Lrp upon its target genes (6, 33).
According to our results, Lrp potentiates the ArgP-mediated lysP expression when cells are cultivated in the absence of lysine, irrespective of l-leucine availability. Thus far, lysP has not been identified as a member of the Lrp regulon in the various genome-scale studies performed (9, 21, 53). While Lrp stimulated lysP expression in the absence of lysine, this global regulator did not alter the lysine-dependent repression of lysP. In addition, Lrp had a positive effect on the expression of the cadBA operon when cells were exposed to moderate acidic stress in the presence of lysine. A similar effect of Lrp has been reported for the cadBA operon of Vibrio vulnificus (42). Lrp binds cooperatively to the DNA at a degenerate consensus sequence (11), but also with high affinity in a nonspecific manner (36). This last characteristic, together with the high abundance of Lrp in the cell and the fact that binding of Lrp to the DNA causes major conformational changes (13, 57), classifies Lrp as a nucleoid-associated protein (NAP) (14).
The results presented here demonstrate that Lrp has a stimulating effect on the expression of both lysP and cadBA. As Lrp directly interacts with the lysP and cadBA control regions in EMSAs performed with a high concentration of nonspecific competitor DNA, the possibility of an indirect effect can be discarded. We have shown that ArgP is the main regulator for lysP, and CadC is the major regulator for the cadBA operon (12, 58). These regulators activate expression of the corresponding target genes in response to lysine availability (ArgP for lysP and CadC via interaction with LysP for cadBA) (55, 58) and low pH (CadC) (58). In this scenario, Lrp would impose another level of regulation, adjusting the expression levels of lysP and cadBA in response to the physiological status of the cell.
The mechanisms employed by Lrp to regulate transcription vary and often involve interaction with other regulator proteins and NAPs. Recently, the implication of Lrp in the regulation of the gene encoding the arginine exporter (argO) was reported. It was shown that Lrp and ArgP behave as competitive activators able to activate argO expression under different conditions (35). On the other hand, expression of the artPIQM operon, one of the systems responsible for arginine uptake, is downregulated by ArgR (5) and Lrp (21). Here, we found that Lrp binds to several sites at the lysP control region and is able to potentiate the transcriptional activation mediated by ArgP when lysine becomes limiting. Considering the in vivo and in vitro data presented in this work, the mechanism by which ArgP regulates argO transcription (24), and the known capacity of Lrp to alter the shape of the DNA by inducing bending and wrapping (57), a model for lysP regulation in which binding of Lrp to the lysP control region may favor and/or stabilize the ArgP-RNA polymerase-DNA complexes or introduce DNA conformational changes is conceivable.
Regarding cadBA transcriptional regulation, it is known that H-NS represses cadBA expression under noninducing conditions (46), and according to the current model, CadC binding dissolves the repressor complex formed by H-NS (22). The interplay between Lrp and other NAPs, in particular H-NS, in the regulation of several genes is well documented (10, 25, 38). According to the results described here, Lrp participates in the activation of cadBA under inducing conditions. It is conceivable that CadC and Lrp dissolve the repressor complex formed by H-NS in a joint action.
In conclusion, the three transporters for lysine (CadB, the LAO system, and LysP) in E. coli are produced under different conditions, and the corresponding genes are under the control of various regulators to meet diverse cellular needs. Expression of cadB, encoding the lysine/cadaverine antiporter CadB, is induced only at low pH and when external lysine is available to counteract acidic stress (27, 31). hisJQMP, encoding the histidine-binding protein and the ABC-type transporter of the LAO system, are repressed by Arg-loaded ArgR (4). In contrast, the arginine-ornithine-lysine binding protein which interacts with the same ABC transporter is induced under nitrogen-limiting conditions and controlled by NtrC (61), suggesting that the LAO system serves as a scavenging system for nitrogen-rich amino acids under conditions of nitrogen starvation. As demonstrated here, lysP expression is induced under lysine limitation and requires ArgP and Lrp. Therefore, the main role of LysP seems to be the uptake of lysine for biosynthetic purposes. When lysine is sufficiently available, expression of lysP is shut off.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft (JU270/5-3 and Exc114/1), the Humboldt Foundation (fellowship to J.R.), and the Elite Network of Bavaria (fellowship to I.H.). J.R. was a researcher on leave from CONICET.
We thank Tobias Kraxenberger and Britta Jungwirth for technical advice, Susanne Ude for constructing strains, and Luitpold Fried for MALDI-TOF measurements. We are grateful to Claude Gutierrez for generously providing E. coli strain JCP95.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 25 March 2011.
REFERENCES
- 1. Bachmann B. J. 1996. Derivations and genotypes of some mutant derivaties of Escherichia coli K-12, p. 2460–2488 In Neidhardt F. C., et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC [Google Scholar]
- 2. Bouvier J., Stragier P., Morales V., Remy E., Gutierrez C. 2008. Lysine represses transcription of the Escherichia coli dapB gene by preventing its activation by the ArgP activator. J. Bacteriol. 190:5224–5229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bradford M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 4. Caldara M., et al. 2008. Arginine biosynthesis in Escherichia coli: experimental perturbation and mathematical modeling. J. Biol. Chem. 283:6347–6358 [DOI] [PubMed] [Google Scholar]
- 5. Caldara M., Minh P. N., Bostoen S., Massant J., Charlier D. 2007. ArgR-dependent repression of arginine and histidine transport genes in Escherichia coli K-12. J. Mol. Biol. 373:251–267 [DOI] [PubMed] [Google Scholar]
- 6. Calvo J. M., Matthews R. G. 1994. The leucine-responsive regulatory protein, a global regulator of metabolism in Escherichia coli. Microbiol. Rev. 58:466–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Casadaban M. J. 1976. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J. Mol. Biol. 104:541–555 [DOI] [PubMed] [Google Scholar]
- 8. Celis T. F., Rosenfeld H. J., Maas W. K. 1973. Mutant of Escherichia coli K-12 defective in the transport of basic amino acids. J. Bacteriol. 116:619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cho B. K., Barrett C. L., Knight E. M., Park Y. S., Palsson B. O. 2008. Genome-scale reconstruction of the Lrp regulatory network in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 105:19462–19467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corcoran C. P., Dorman C. J. 2009. DNA relaxation-dependent phase biasing of the fim genetic switch in Escherichia coli depends on the interplay of H-NS, IHF and LRP. Mol. Microbiol. 74:1071–1082 [DOI] [PubMed] [Google Scholar]
- 11. Cui Y., Wang Q., Stormo G. D., Calvo J. M. 1995. A consensus sequence for binding of Lrp to DNA. J. Bacteriol. 177:4872–4880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dell C. L., Neely M. N., Olson E. R. 1994. Altered pH and lysine signalling mutants of cadC, a gene encoding a membrane-bound transcriptional activator of the Escherichia coli cadBA operon. Mol. Microbiol. 14:7–16 [DOI] [PubMed] [Google Scholar]
- 13. de los Rios S., Perona J. J. 2007. Structure of the Escherichia coli leucine-responsive regulatory protein Lrp reveals a novel octameric assembly. J. Mol. Biol. 366:1589–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dillon S. C., Dorman C. J. 2010. Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat. Rev. Microbiol. 8:185–195 [DOI] [PubMed] [Google Scholar]
- 15. Epstein W., Kim B. S. 1971. Potassium transport loci in Escherichia coli K-12. J. Bacteriol. 108:639–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fujii T., Aritoku Y., Agematu H., Tsunekawa H. 2002. Increase in the rate of L-pipecolic acid production using lat-expressing Escherichia coli by lysP and yeiE amplification. Biosci. Biotechnol. Biochem. 66:1981–1984 [DOI] [PubMed] [Google Scholar]
- 17. Gabrielsen O. S., Hornes E., Korsnes L., Ruet A., Oyen T. B. 1989. Magnetic DNA affinity purification of yeast transcription factor τ-a new purification principle for the ultrarapid isolation of near homogeneous factor. Nucleic Acids Res. 17:6253–6267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guzman L. M., Belin D., Carson M. J., Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heermann R., Zeppenfeld T., Jung K. 2008. Simple generation of site-directed point mutations in the Escherichia coli chromosome using Red(R)/ET(R) recombination. Microb. Cell Fact. 7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henzel W. J., et al. 1993. Identifying proteins from two-dimensional gels by molecular mass searching of peptide fragments in protein sequence databases. Proc. Natl. Acad. Sci. U. S. A. 90:5011–5015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hung S. P., Baldi P., Hatfield G. W. 2002. Global gene expression profiling in Escherichia coli K12. The effects of leucine-responsive regulatory protein. J. Biol. Chem. 277:40309–40323 [DOI] [PubMed] [Google Scholar]
- 22. Küper C., Jung K. 2005. CadC-mediated activation of the cadBA promoter in Escherichia coli. J. Mol. Microbiol. Biotechnol. 10:26–39 [DOI] [PubMed] [Google Scholar]
- 23. Laemmli U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 24. Laishram R. S., Gowrishankar J. 2007. Environmental regulation operating at the promoter clearance step of bacterial transcription. Genes Dev. 21:1258–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levinthal M., Lejeune P., Danchin A. 1994. The H-NS protein modulates the activation of the ilvIH operon of Escherichia coli K12 by Lrp, the leucine regulatory protein. Mol. Gen. Genet. 242:736–743 [DOI] [PubMed] [Google Scholar]
- 26. Meng S. Y., Bennett G. N. 1992. Nucleotide sequence of the Escherichia coli cad operon: a system for neutralization of low extracellular pH. J. Bacteriol. 174:2659–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meng S. Y., Bennett G. N. 1992. Regulation of the Escherichia coli cad operon: location of a site required for acid induction. J. Bacteriol. 174:2670–2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 29. Moore J. T., Uppal A., Maley F., Maley G. F. 1993. Overcoming inclusion body formation in a high-level expression system. Protein Expr. Purif. 4:160–163 [DOI] [PubMed] [Google Scholar]
- 30. Nandineni M. R., Gowrishankar J. 2004. Evidence for an arginine exporter encoded by yggA (argO) that is regulated by the LysR-type transcriptional regulator ArgP in Escherichia coli. J. Bacteriol. 186:3539–3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neely M. N., Dell C. L., Olson E. R. 1994. Roles of LysP and CadC in mediating the lysine requirement for acid induction of the Escherichia coli cad operon. J. Bacteriol. 176:3278–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Neely M. N., Olson E. R. 1996. Kinetics of expression of the Escherichia coli cad operon as a function of pH and lysine. J. Bacteriol. 178:5522–5528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Newman E. B., Lin R. 1995. Leucine-responsive regulatory protein: a global regulator of gene expression in E. coli. Annu. Rev. Microbiol. 49:747–775 [DOI] [PubMed] [Google Scholar]
- 34. Patte J. C., Akrim M., Mejean V. 1998. The leader sequence of the Escherichia coli lysC gene is involved in the regulation of LysC synthesis. FEMS Microbiol. Lett. 169:165–170 [DOI] [PubMed] [Google Scholar]
- 35. Peeters E., Nguyen Le Minh P., Foulquie-Moreno M., Charlier D. 2009. Competitive activation of the Escherichia coli argO gene coding for an arginine exporter by the transcriptional regulators Lrp and ArgP. Mol. Microbiol. 74:1513–1526 [DOI] [PubMed] [Google Scholar]
- 36. Peterson S. N., Dahlquist F. W., Reich N. O. 2007. The role of high affinity non-specific DNA binding by Lrp in transcriptional regulation and DNA organization. J. Mol. Biol. 369:1307–1317 [DOI] [PubMed] [Google Scholar]
- 37. Popkin P. S., Maas W. K. 1980. Escherichia coli regulatory mutation affecting lysine transport and lysine decarboxylase. J. Bacteriol. 141:485–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pul U., Wurm R., Wagner R. 2007. The role of LRP and H-NS in transcription regulation: involvement of synergism, allostery and macromolecular crowding. J. Mol. Biol. 366:900–915 [DOI] [PubMed] [Google Scholar]
- 39. Quay S. C., Dick T. E., Oxender D. L. 1977. Role of transport systems in amino acid metabolism: leucine toxicity and the branched-chain amino acid transport systems. J. Bacteriol. 129:1257–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reitzer L. 5 July 2005, posting date. Chapter 3.4.7, Catabolism of amino acids and related compounds. In Curtiss R., III (ed.), EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC: doi:10.1128/ecosal.3.6.1.3 [DOI] [PubMed] [Google Scholar]
- 41. Rey D. A., Puhler A., Kalinowski J. 2003. The putative transcriptional repressor McbR, member of the TetR-family, is involved in the regulation of the metabolic network directing the synthesis of sulfur containing amino acids in Corynebacterium glutamicum. J. Biotechnol. 103:51–65 [DOI] [PubMed] [Google Scholar]
- 42. Rhee J. E., Kim K. S., Choi S. H. 2008. Activation of the Vibrio vulnificus cadBA operon by leucine-responsive regulatory protein is mediated by CadC. J. Microbiol. Biotechnol. 18:1755–1761 [DOI] [PubMed] [Google Scholar]
- 43. Rodionov D. A., Vitreschak A. G., Mironov A. A., Gelfand M. S. 2003. Regulation of lysine biosynthesis and transport genes in bacteria: yet another RNA riboswitch? Nucleic Acids Res. 31:6748–6757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sandaltzopoulos R., Becker P. B. 1994. Solid phase DNase I footprinting: quick and versatile. Nucleic Acids Res. 22:1511–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schell M. A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47:597–626 [DOI] [PubMed] [Google Scholar]
- 46. Shi X., Waasdorp B. C., Bennett G. N. 1993. Modulation of acid-induced amino acid decarboxylase gene expression by hns in Escherichia coli. J. Bacteriol. 175:1182–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Simons R. W., Houman F., Kleckner N. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85–96 [DOI] [PubMed] [Google Scholar]
- 48. Soksawatmaekhin W., Kuraishi A., Sakata K., Kashiwagi K., Igarashi K. 2004. Excretion and uptake of cadaverine by CadB and its physiological functions in Escherichia coli. Mol. Microbiol. 51:1401–1412 [DOI] [PubMed] [Google Scholar]
- 49. Steffes C., Ellis J., Wu J., Rosen B. P. 1992. The lysP gene encodes the lysine-specific permease. J. Bacteriol. 174:3242–3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stragier P., Richaud F., Borne F., Patte J. C. 1983. Regulation of diaminopimelate decarboxylase synthesis in Escherichia coli. I. Identification of a lysR gene encoding an activator of the lysA gene. J. Mol. Biol. 168:307–320 [DOI] [PubMed] [Google Scholar]
- 51. Studier F. W., Moffatt B. A. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189:113–130 [DOI] [PubMed] [Google Scholar]
- 52. Sudarsan N., Wickiser J. K., Nakamura S., Ebert M. S., Breaker R. R. 2003. An mRNA structure in bacteria that controls gene expression by binding lysine. Genes Dev. 17:2688–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tani T. H., Khodursky A., Blumenthal R. M., Brown P. O., Matthews R. G. 2002. Adaptation to famine: a family of stationary-phase genes revealed by microarray analysis. Proc. Natl. Acad. Sci. U. S. A. 99:13471–13476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tetsch L., Jung K. 2009. The regulatory interplay between membrane-integrated sensors and transport proteins in bacteria. Mol. Microbiol. 73:982–991 [DOI] [PubMed] [Google Scholar]
- 55. Tetsch L., Koller C., Haneburger I., Jung K. 2008. The membrane-integrated transcriptional activator CadC of Escherichia coli senses lysine indirectly via the interaction with the lysine permease LysP. Mol. Microbiol. 67:570–583 [DOI] [PubMed] [Google Scholar]
- 56. Thomason L. C., Costantino N., Court D. L. 2007. E. coli genome manipulation by P1 transduction. Curr. Protoc. Mol. Biol. 79:1.17.1–1.17.8 [DOI] [PubMed] [Google Scholar]
- 57. Wang Q., Calvo J. M. 1993. Lrp, a major regulatory protein in Escherichia coli, bends DNA and can organize the assembly of a higher-order nucleoprotein structure. EMBO J. 12:2495–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Watson N., Dunyak D. S., Rosey E. L., Slonczewski J. L., Olson E. R. 1992. Identification of elements involved in transcriptional regulation of the Escherichia coli cad operon by external pH. J. Bacteriol. 174:530–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Weber A., Kogl S. A., Jung K. 2006. Time-dependent proteome alterations under osmotic stress during aerobic and anaerobic growth in Escherichia coli. J. Bacteriol. 188:7165–7175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yanisch-Perron C., Vieira J., Messing J. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119 [DOI] [PubMed] [Google Scholar]
- 61. Zimmer D. P., et al. 2000. Nitrogen regulatory protein C-controlled genes of Escherichia coli: scavenging as a defense against nitrogen limitation. Proc. Natl. Acad. Sci. U. S. A. 97:14674–14679 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.