Abstract
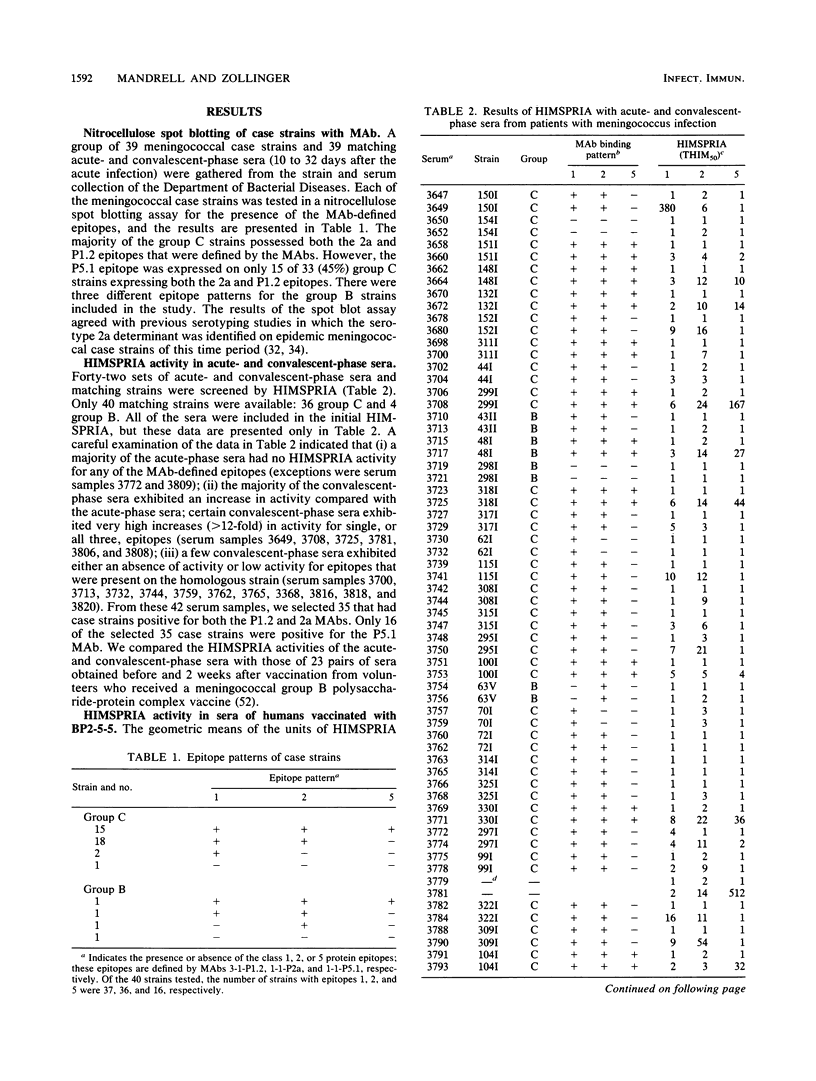
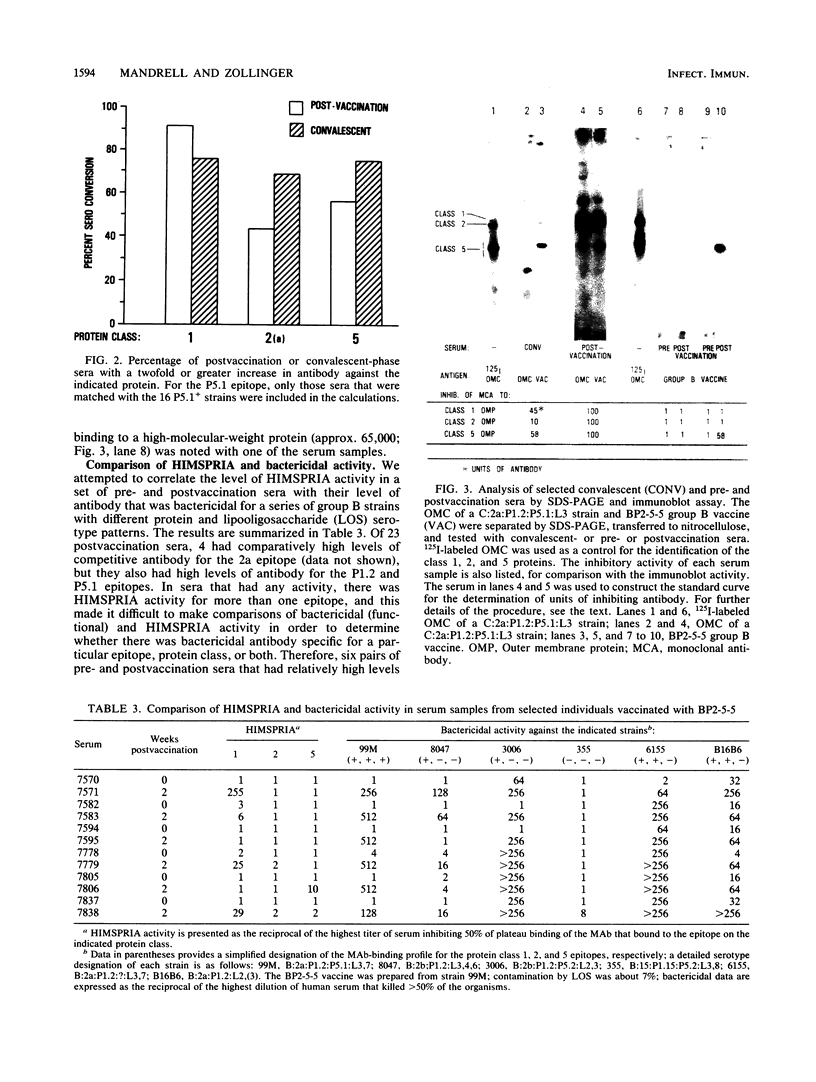
Antibody levels in 41 sets of human acute- and convalescent-phase meningococcal sera were compared with those in 23 sets of human prevaccination and 2-week postvaccination sera. We used a modification of a solid-phase radioimmunoassay (SPRIA) technique to test each of the human serum samples as inhibitors of monoclonal antibodies (MAbs) that bind (HIMSPRIA) to the outer membrane complex from a 2a:P1.2:P5.1 strain. We used three murine MAbs specific for the 2a, P1.2, and P5.1 epitopes on meningococcal class 1, 2, and 5 proteins, respectively, to detect antibodies with similar specificities in human sera. Each of 40 available matching strains from patients were also screened with the three MAbs in a nitrocellulose spot blot assay. A total of 37 (92%) were positive for the 2a epitope, 36 (90%) were positive for the P1.2 epitope, and 16 (40%) were positive for the P5.1 epitope. Of 38 available convalescent-phase sera, 27 (71%) matched with these strains and had detectable inhibiting antibody for each of the MAb-defined protein epitopes of the infecting strain. Three convalescent-phase sera had no HIMSPRIA activity for MAb-defined epitopes that were present on the infecting strain; others had activity for one or two of the epitopes. The results were similar for pre- and postvaccination sera. The average level of HIMSPRIA activity for the P1.2 epitope was greater than fivefold higher in postvaccination sera compared with that in convalescent-phase sera. Sera with distinct patterns of HIMSPRIA activity also were tested by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot analysis and showed a correlation between the HIMSPRIA activity for particular epitopes and the level of antibody binding to the immunoblotted proteins possessing those epitopes. A comparison of the HIMSPRIA and the bactericidal activity of selected postvaccination sera indicated a possible correlation between HIMSPRIA and bactericidal activity, but it also suggested the presence of bactericidal antibodies with specificities other than those defined by the MAbs.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoun L., Lavitola A., Aubert G., Prère M. F., Cremieux A. C., Martin P. M. Human antibody response to the 70-Kd common neisserial antigen in patients and carriers of meningococci or non-pathogenic Neisseria. Ann Inst Pasteur Microbiol. 1988 Mar-Apr;139(2):203–212. [PubMed] [Google Scholar]
- Ashton F. E., Ryan J. A., Jones C., Brodeur B. R., Diena B. B. Serotypes of Neisseria meningitidis associated with an increased incidence of meningitis cases in the Hamilton area, Ontario, during 1978 and 1979. Can J Microbiol. 1983 Jan;29(1):129–136. doi: 10.1139/m83-020. [DOI] [PubMed] [Google Scholar]
- Berger U., Sonntag H. G., Ulbrich C. Epidemiology of meningococcal infections in the Federal Republic of Germany, 1966-1984. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Mar;268(1):83–102. doi: 10.1016/s0176-6724(88)80118-4. [DOI] [PubMed] [Google Scholar]
- Beuvery E. C., Miedema F., van Delft R. W., Haverkamp J., Leussink A. B., te Pas B. J., Teppema K. S., Tiesjema R. H. Preparation and physicochemical and immunological characterization of polysaccharide-outer membrane protein complexes of Neisseria meningitidis. Infect Immun. 1983 Apr;40(1):369–380. doi: 10.1128/iai.40.1.369-380.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. R., Black W. J., Cannon J. G. Neisserial antigen H.8 is immunogenic in patients with disseminated gonococcal and meningococcal infections. J Infect Dis. 1985 Apr;151(4):650–657. doi: 10.1093/infdis/151.4.650. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Gotschlich E. C. Purification and partial characterization of the opacity-associated proteins of Neisseria gonorrhoeae. J Exp Med. 1984 Feb 1;159(2):452–462. doi: 10.1084/jem.159.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cochi S. L., Markowitz L. E., Joshi D. D., Owens R. C., Jr, Stenhouse D. H., Regmi D. N., Shrestha R. P., Lacharya I., Manandhar M., Gurubacharya V. L. Control of epidemic group A meningococcal meningitis in Nepal. Int J Epidemiol. 1987 Mar;16(1):91–97. doi: 10.1093/ije/16.1.91. [DOI] [PubMed] [Google Scholar]
- Craven D. E., Shen K. T., Frasch C. E. Natural bactericidal activity of human serum against Neisseria meningitidis isolates of different serogroups and serotypes. Infect Immun. 1982 Jul;37(1):132–137. doi: 10.1128/iai.37.1.132-137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finne J., Leinonen M., Mäkelä P. H. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983 Aug 13;2(8346):355–357. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- Frasch C. E., Mocca L. F. Heat-modifiable outer membrane proteins of Neisseria meningitidis and their organization within the membrane. J Bacteriol. 1978 Dec;136(3):1127–1134. doi: 10.1128/jb.136.3.1127-1134.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Zollinger W. D., Poolman J. T. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev Infect Dis. 1985 Jul-Aug;7(4):504–510. doi: 10.1093/clinids/7.4.504. [DOI] [PubMed] [Google Scholar]
- Greenwood B. M., Blakebrough I. S., Bradley A. K., Wali S., Whittle H. C. Meningococcal disease and season in sub-Saharan Africa. Lancet. 1984 Jun 16;1(8390):1339–1342. doi: 10.1016/s0140-6736(84)91830-0. [DOI] [PubMed] [Google Scholar]
- Grossman N., Schmetz M. A., Foulds J., Klima E. N., Jimenez-Lucho V. E., Leive L. L., Joiner K. A., Jiminez V. Lipopolysaccharide size and distribution determine serum resistance in Salmonella montevideo. J Bacteriol. 1987 Feb;169(2):856–863. doi: 10.1128/jb.169.2.856-863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J. Analyses of gonococcal lipopolysaccharide in whole-cell lysates by sodium dodecyl sulfate-polyacrylamide gel electrophoresis: stable association of lipopolysaccharide with the major outer membrane protein (protein I) of Neisseria gonorrhoeae. Infect Immun. 1984 Oct;46(1):202–212. doi: 10.1128/iai.46.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mandrell R. E., Zollinger W. D. Measurement of antibodies to meningococcal group B polysaccharide: low avidity binding and equilibrium binding constants. J Immunol. 1982 Nov;129(5):2172–2178. [PubMed] [Google Scholar]
- Mandrell R. E., Zollinger W. D. Use of a zwitterionic detergent for the restoration of the antibody-binding capacity of electroblotted meningococcal outer membrane proteins. J Immunol Methods. 1984 Feb 24;67(1):1–11. doi: 10.1016/0022-1759(84)90080-2. [DOI] [PubMed] [Google Scholar]
- Mandrell R., Schneider H., Apicella M., Zollinger W., Rice P. A., Griffiss J. M. Antigenic and physical diversity of Neisseria gonorrhoeae lipooligosaccharides. Infect Immun. 1986 Oct;54(1):63–69. doi: 10.1128/iai.54.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson L., Holbein B. E. Influence of nutrient limitation and low pH on serogroup B Neisseria meningitidis capsular polysaccharide levels: correlation with virulence for mice. Infect Immun. 1985 Feb;47(2):465–471. doi: 10.1128/iai.47.2.465-471.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade R. L., Jr, Johnston K. H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980 Mar;141(3):1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietzner T. A., Luginbuhl G. H., Sandstrom E., Morse S. A. Identification of an iron-regulated 37,000-dalton protein in the cell envelope of Neisseria gonorrhoeae. Infect Immun. 1984 Aug;45(2):410–416. doi: 10.1128/iai.45.2.410-416.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno C., Lifely M. R., Esdaile J. Immunity and protection of mice against Neisseria meningitidis group B by vaccination, using polysaccharide complexed with outer membrane proteins: a comparison with purified B polysaccharide. Infect Immun. 1985 Feb;47(2):527–533. doi: 10.1128/iai.47.2.527-533.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhall W. J., Sawyer W. D., Haak R. A. Cross-linking analysis of the outer membrane proteins of Neisseria gonorrhoeae. Infect Immun. 1980 Jun;28(3):785–791. doi: 10.1128/iai.28.3.785-791.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola H., Jónsdóttir K., Lystad A., Sievers C. J., Kallings I. Meningococcal disease in Scandinavia. Br Med J (Clin Res Ed) 1982 May 29;284(6329):1618–1621. doi: 10.1136/bmj.284.6329.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman J. T., Hopman C. T., Zanen H. C. Immunogenicity of meningococcal antigens as detected in patient sera. Infect Immun. 1983 Apr;40(1):398–406. doi: 10.1128/iai.40.1.398-406.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman J. T., Lind I., Jónsdóttir K., Frøholm L. O., Jones D. M., Zanen H. C. Meningococcal serotypes and serogroup B disease in north-west Europe. Lancet. 1986 Sep 6;2(8506):555–558. doi: 10.1016/s0140-6736(86)90123-6. [DOI] [PubMed] [Google Scholar]
- Poolman J. T., de Marie S., Zanen H. C. Variability of low-molecular-weight, heat-modifiable outer membrane proteins of Neisseria meningitidis. Infect Immun. 1980 Dec;30(3):642–648. doi: 10.1128/iai.30.3.642-648.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A., Vayo H. E., Tam M. R., Blake M. S. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J Exp Med. 1986 Nov 1;164(5):1735–1748. doi: 10.1084/jem.164.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougon G., Dubois C., Buckley N., Magnani J. L., Zollinger W. A monoclonal antibody against meningococcus group B polysaccharides distinguishes embryonic from adult N-CAM. J Cell Biol. 1986 Dec;103(6 Pt 1):2429–2437. doi: 10.1083/jcb.103.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansano M., Jr, Reynard A. M., Cunningham R. K. Inhibition of serum bactericidal reaction by lipopolysaccharide. Infect Immun. 1985 Jun;48(3):759–762. doi: 10.1128/iai.48.3.759-762.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafian S. K., Tam M. R., Morse S. A. Gonococcal protein I-specific opsonic IgG in normal human serum. J Infect Dis. 1983 Dec;148(6):1025–1032. doi: 10.1093/infdis/148.6.1025. [DOI] [PubMed] [Google Scholar]
- Saukkonen K., Abdillahi H., Poolman J. T., Leinonen M. Protective efficacy of monoclonal antibodies to class 1 and class 3 outer membrane proteins of Neisseria meningitidis B:15:P1.16 in infant rat infection model: new prospects for vaccine development. Microb Pathog. 1987 Oct;3(4):261–267. doi: 10.1016/0882-4010(87)90059-3. [DOI] [PubMed] [Google Scholar]
- Schneider H., Griffiss J. M., Mandrell R. E., Jarvis G. A. Elaboration of a 3.6-kilodalton lipooligosaccharide, antibody against which is absent from human sera, is associated with serum resistance of Neisseria gonorrhoeae. Infect Immun. 1985 Dec;50(3):672–677. doi: 10.1128/iai.50.3.672-677.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone M. R., Nowinski R. C. Topological mapping of murine leukemia virus proteins by competition-binding assays with monoclonal antibodies. Virology. 1980 Jan 30;100(2):370–381. doi: 10.1016/0042-6822(80)90528-0. [DOI] [PubMed] [Google Scholar]
- Swanson J., Mayer L. W., Tam M. R. Antigenicity of Neisseria gonorrhoeae outer membrane protein(s) III detected by immunoprecipitation and Western blot transfer with a monoclonal antibody. Infect Immun. 1982 Nov;38(2):668–672. doi: 10.1128/iai.38.2.668-672.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Surface-exposed protein antigens of the gonococcal outer membrane. Infect Immun. 1981 Dec;34(3):804–816. doi: 10.1128/iai.34.3.804-816.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Y., Frasch C. E. Development of a Neisseria meningitidis group B serotype 2b protein vaccine and evaluation in a mouse model. Infect Immun. 1984 Nov;46(2):408–414. doi: 10.1128/iai.46.2.408-414.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedege E., Frøholm L. O. Human antibody response to a group B serotype 2a meningococcal vaccine determined by immunoblotting. Infect Immun. 1986 Feb;51(2):571–578. doi: 10.1128/iai.51.2.571-578.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyle F. A., Artenstein M. S., Brandt B. L., Tramont E. C., Kasper D. L., Altieri P. L., Berman S. L., Lowenthal J. P. Immunologic response of man to group B meningococcal polysaccharide vaccines. J Infect Dis. 1972 Nov;126(5):514–521. doi: 10.1093/infdis/126.5.514. [DOI] [PubMed] [Google Scholar]
- Zak K., Diaz J. L., Jackson D., Heckels J. E. Antigenic variation during infection with Neisseria gonorrhoeae: detection of antibodies to surface proteins in sera of patients with gonorrhea. J Infect Dis. 1984 Feb;149(2):166–174. doi: 10.1093/infdis/149.2.166. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Dalrymple J. M., Artenstein M. S. Analysis of parameters affecting the solid phase radioimmunoassay quantitation of antibody to meningococcal antigens. J Immunol. 1976 Nov;117(5 PT2):1788–1798. [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E., Altieri P., Berman S., Lowenthal J., Artenstein M. S. Safety and immunogenicity of a Neisseria meningitidis type 2 protein vaccine in animals and humans. J Infect Dis. 1978 Jun;137(6):728–739. doi: 10.1093/infdis/137.6.728. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E., Griffiss J. M., Altieri P., Berman S. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J Clin Invest. 1979 May;63(5):836–848. doi: 10.1172/JCI109383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect Immun. 1983 Apr;40(1):257–264. doi: 10.1128/iai.40.1.257-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Type-specific antigens of group A Neisseria meningitidis: lipopolysaccharide and heat-modifiable outer membrane proteins. Infect Immun. 1980 May;28(2):451–458. doi: 10.1128/iai.28.2.451-458.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Moran E. E., Connelly H., Mandrell R. E., Brandt B. Monoclonal antibodies to serotype 2 and serotype 15 outer membrane proteins of Neisseria meningitidis and their use in serotyping. Infect Immun. 1984 Oct;46(1):260–266. doi: 10.1128/iai.46.1.260-266.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Marie S., Hoeijmakers J. H., Poolman J. T., Zanen H. C. Filter radioimmunoassay, a method for large-scale serotyping of Neisseria meningitidis. J Clin Microbiol. 1984 Aug;20(2):255–258. doi: 10.1128/jcm.20.2.255-258.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]