Abstract
The sucCD gene of Advenella mimigardefordensis strain DPN7T encodes a succinyl coenzyme A (succinyl-CoA) synthetase homologue (EC 6.2.1.4 or EC 6.2.1.5) that recognizes, in addition to succinate, the structural analogues 3-sulfinopropionate (3SP) and itaconate as substrates. Accumulation of 3SP during 3,3′-dithiodipropionic acid (DTDP) degradation was observed in Tn5::mob-induced mutants of A. mimigardefordensis strain DPN7T disrupted in sucCD and in the defined deletion mutant A. mimigardefordensis ΔsucCD. These mutants were impaired in growth with DTDP and 3SP as the sole carbon source. Hence, it was proposed that the succinyl-CoA synthetase homologue in A. mimigardefordensis strain DPN7T activates 3SP to the corresponding CoA-thioester (3SP-CoA). The putative genes coding for A. mimigardefordensis succinyl-CoA synthetase (SucCDAm) were cloned and heterologously expressed in Escherichia coli BL21(DE3)/pLysS. Purification and characterization of the enzyme confirmed its involvement during degradation of DTDP. 3SP, the cleavage product of DTDP, was converted into 3SP-CoA by the purified enzyme, as demonstrated by in vitro enzyme assays. The structure of 3SP-CoA was verified by using liquid chromatography-electrospray ionization-mass spectrometry. SucCDAm is Mg2+ or Mn2+ dependent and unspecific regarding ATP or GTP. In kinetic studies the enzyme showed highest enzyme activity and substrate affinity with succinate (Vmax = 9.85 ± 0.14 μmol min−1 mg−1, Km = 0.143 ± 0.001 mM). In comparison to succinate, activity with 3SP was only ca. 1.2% (Vmax = 0.12 ± 0.01 μmol min−1 mg−1) and the affinity was 6-fold lower (Km = 0.818 ± 0.046 mM). Based on the present results, we conclude that SucCDAm is physiologically associated with the citric acid cycle but is mandatory for the catabolic pathway of DTDP and its degradation intermediate 3SP.
INTRODUCTION
3,3′-Dithiodipropionic acid (DTDP) is an organic disulfide and a precursor for the production of polythioesters (PTEs) by bacteria (25). Further applications for DTDP are thermodynamic studies (40), development of secondary batteries (52), amino acid analysis (53), and the construction of self-assembling monolayers (10). Microbial production of PTEs from simple carbon sources and inorganic sulfur is currently not possible. Knowledge of the catabolism of organic sulfur compounds in bacteria could provide a reasonable strategy to engineer strains suitable for PTE production. A first step in this direction was the isolation of bacteria able to utilize DTDP as the sole source of carbon and energy. Advenella mimigardefordensis strain DPN7T, a betaproteobacterium, found in mature compost in a waste management facility was one of the isolates (15, 56).
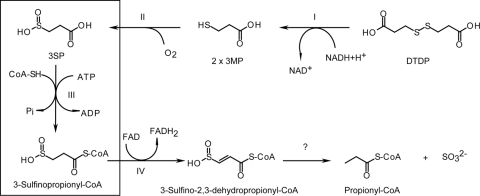
To elucidate the degradation pathway of DTDP and to identify the genes involved, transposon mutagenesis was applied to this bacterium (57). Two of the obtained Tn5::mob-induced mutants affected in growth on DTDP accumulated 3-sulfinopropionic acid (3SP). 3SP is a structural analogue of succinate, in which one carbon atom is substituted by a sulfur atom (see Fig. 2c and d). The Tn5::mob insertion in one mutant was mapped in a region 298 bp upstream of sucC, coding for a homologue of the β-chain of succinyl coenzyme A (succinyl-CoA) synthetases, and resulted in partially impaired growth on DTDP (see Fig. 4). Insertion of Tn5::mob into sucC completely impaired growth on DTDP in the other mutant. Thus, it was predicted that the succinyl-CoA synthetase homologue from A. mimigardefordensis strain DPN7T (SucCDAm) is involved in the catabolic pathway of DTDP (see Fig. 9) (57).
Fig. 2.
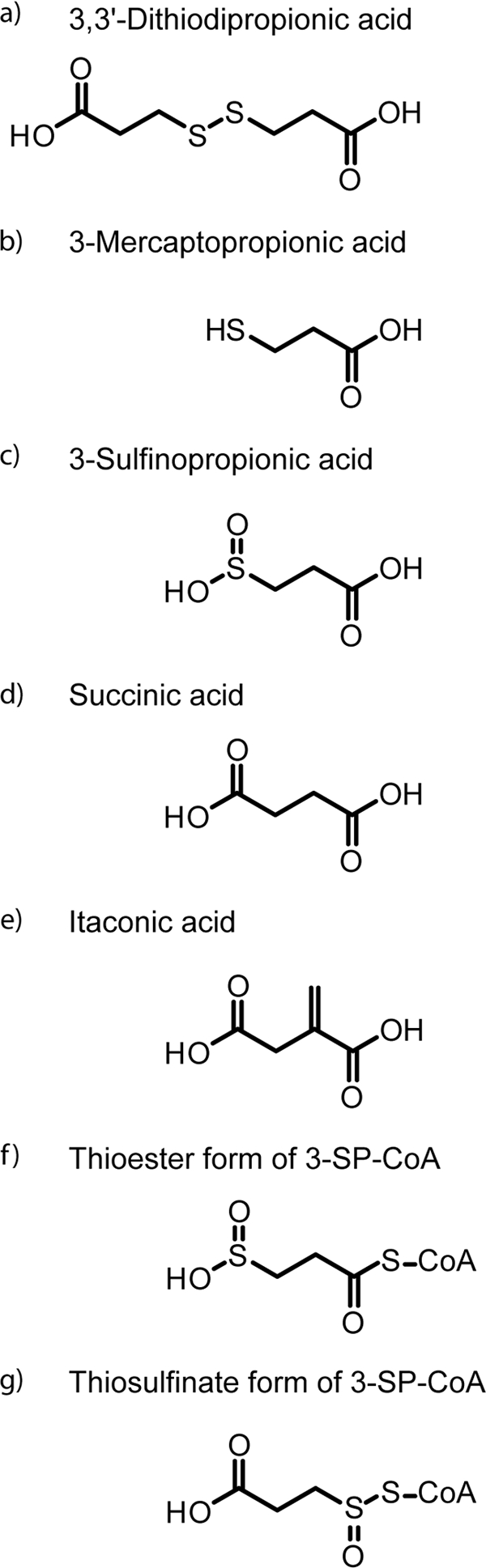
Structural formula of organic sulfur compounds in the present study. (a) 3,3′-Dithiodipropionic acid; (b) 3-mercaptopropionic acid; (c) 3-sulfinopropionic acid; (d) succinic acid; (e) itaconic acid. (f and g) Proposed thioester form (f) and thiosulfinate form (g) of 3SP-CoA, the reaction product of 3SP and CoA.
Fig. 4.
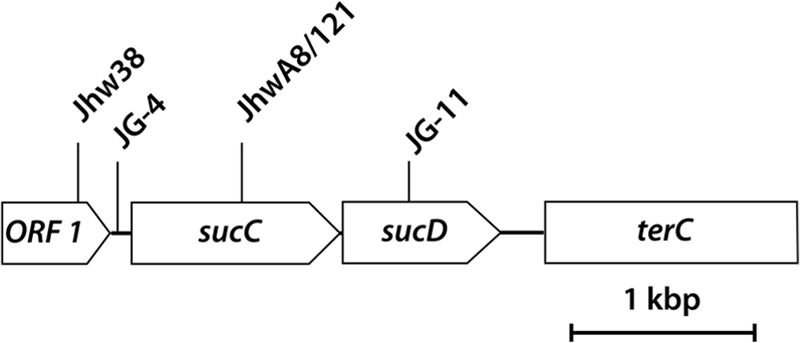
Localization of Tn5::mob insertions in the genomes of four independent Tn5-induced mutants of A. mimigardefordensis strain DPN7T. The positions of Tn5::mob insertions in the respective mutants are indicated as arrows. A region of 3,764 bp was sequenced. Abbreviations: ORF 1, open reading frame 1; sucC, β-chain of succinyl-CoA synthetase; sucD, α-chain of succinyl-CoA synthetase; terC, fragment of integral membrane protein that is putatively involved in tellurium resistance.
Fig. 9.
Proposed degradation pathway of DTDP. Initial cleavage by a dihydrolipoamide dehydrogenase (step I) yields two molecules of 3MP, which are further oxygenated by a dioxygenase (step II), yielding 3SP. The latter is activated to the corresponding CoA thioester by SucCD (step III), as shown in the present study. The next step is most probably the dehydrogenation of 3-sulfinopropionyl-CoA by an acyl-CoA-dehydrogenase homologue (step IV) in an FAD-dependent step. The sulfur moiety of the dehydrogenation product, 3-sulfino-2,3-dehydropropionyl-CoA, is putatively removed by an enzymatic or an autocatalytic reaction. Propionyl-CoA subsequently enters the central metabolism via the methyl citric acid cycle.
Succinyl-CoA synthetases (SucCD; EC 6.2.1.4 or EC 6.2.1.5) occur in prokaryotes and eukaryotes and are widely known for catalyzing the only substrate-level phosphorylation in the citric acid cycle (7, 31). Therein, the conversion of succinyl-CoA to succinate yields nucleoside triphosphates during aerobic metabolism. The reaction is completely reversible and supplies also succinyl-CoA for heme biosynthesis and ketone body activation, in particular during anaerobic growth (32). Several studies elucidating a variety of regulation systems, indicated the importance of SucCD as a control point of the citric acid cycle (5). Succinyl-CoA synthetases consist of α (SucD) and β (SucC) subunits, with mass ranges of 29 to 34 kDa and 41 to 45 kDa, respectively (49). In higher organisms and Gram-positive bacteria αβ-heterodimers are found, whereas in Gram-negative bacteria an α2β2-tetrameric structure usually occurs (6, 55).
The α-subunit comprises the active reaction site with a conserved histidine residue, which is phosphorylated during enzymatic catalysis. The phosphate moiety is subsequently transferred to a nucleoside diphosphate to yield the corresponding nucleoside triphosphate. Substitution of the conserved histidine residue by other amino acids upon mutagenesis yields an inactive enzyme (6, 26). The β-subunit confers the nucleotide binding site and determines the nucleotide specificity (18, 20).
SucCDs in Gram-negative bacteria such as Escherichia coli tend to be nonspecific with regard to the cofactor, and they use both coenzymes ATP and GTP. In Pseudomonas aeruginosa SucCD has a very broad nucleotide specificity and is able to use ADP, GDP, UDP, and CDP in combination with inorganic phosphate and succinyl-CoA to synthesize the corresponding nucleoside triphosphates (21). In eukaryotes, SucCDs with higher specificity are found. In mammals, the GTP-specific form is more highly expressed in anabolic tissues such as liver and kidney, whereas the ATP-specific form predominates in the testes and brain (23). The enzyme in the yeast Saccharomyces cerevisiae is specific for ATP (36).
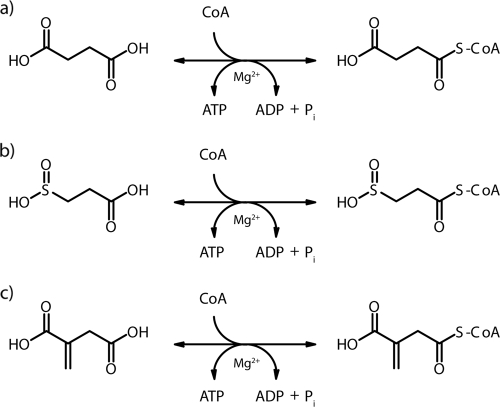
However, although many investigations concerning structure, regulation, and nucleotide specificity have been conducted, only a few studies have reported on the specificity of the organic acid or its CoA thioester as the other substrate of SucCDs. The conversion of succinyl-CoA to succinate and CoA, yielding ATP by SucCD, was first reported by Kaufman (22) (Fig. 1a). Adler et al. (1) reported also on the activation of itaconic acid (Fig. 1c), a structural analogue of succinate, to itaconyl-CoA by the succinyl-CoA synthetase of liver mitochondria as an initial step of itaconic acid dissimilation. The same reaction was described in Pseudomonas fluorescens and Pseudomonas sp. strain B2aba, respectively (12, 29, 30). Only recently, a SucCD from the hyperthermophilic archaeon Thermococcus kodakarensis, a succinyl-CoA synthetase with a nonclassical domain distribution that resembles the acetyl-CoA synthetases from Pyrococcus furiosus, was reported (42). This enzyme could use, in addition to succinate, isovalerate, 3-methyl thiopropionate, glutarate, adipate, and butyrate as substrates. Unfortunately, there are no reports about substrate utilization except for succinate and itaconate in SucCDs with classical domain distributions. In summary, the two substrates succinate and itaconate are activated to the corresponding CoA-thioester by succinyl-CoA synthetases with a classical domain distribution that can be compared to the SucCD investigated here.
Fig. 1.
Reactions of succinyl-CoA synthetases. (a to c) Activation of succinic acid to succinyl-CoA (a), 3SP to 3SP-CoA (b), and itaconic acid to itaconyl-CoA (c).
Only little is known about 3SP (Fig. 2c) and its metabolism. 3SP is a structural analogue of succinate and was first described as a degradation product of homohypotaurin, which is an inhibitor of nervous conduction (4). In the past, it was also considered as a promising antiradiation drug (46, 51). In A. mimigardefordensis strain DPN7T, as well as in Variovorax paradoxus strain TBEA6, 3SP was found to be an intermediate of 3-mercaptopropionic acid (3MP) degradation (8). There are no previous reports of succinyl-CoA synthetases catalyzing the activation of 3SP to 3SP-CoA (Fig. 1b). In the present study, we demonstrate this reaction and verify its essential involvement in the catabolism of DTDP in A. mimigardefordensis strain DPN7T.
MATERIALS AND METHODS
Bacterial strains and cultivation conditions.
All of the bacterial strains used in the present study are listed in Table 1. A. mimigardefordensis strain DPN7T and mutants were cultivated in nutrient broth (NB) medium (38) or mineral salt medium (MSM) (41) under aerobic conditions on a rotary shaker at an agitation of 130 rpm and at 30°C. Strains of E. coli were cultivated in Luria-Bertani (LB) medium (38) or ZYP-5052 complex medium for autoinduction according to the method of Studier (50). The latter was used for heterologous expression of genes in E. coli under the control of the lac promoter; cells were cultivated at an agitation of 130 rpm and at 30 or 37°C. Carbon sources were supplied as filter sterilized stock solutions as indicated in the text. For the maintenance of plasmids, antibiotics were prepared according to the method of Sambrook et al. (38) and added to the media at the following concentrations: ampicillin, 75 μg/ml; kanamycin, 50 μg/ml; and chloramphenicol, 34 μg/ml.
Table 1.
Strains, plasmids, and oligonucleotides used in this study
| Strain, plasmid, or oligonucleotide | Description or sequence (5′–3′)a | Source or reference |
|---|---|---|
| Strains | ||
| A. mimigardefordensis DPN7 | Wild type, DTDP-degrading bacterium | 1 (DSM 17166T, LMG 22922T) |
| A. mimigardefordensis ΔsucCD | Deletion of sucCD, no growth on DTDP and 3SP | This study |
| E. coli Top10 | F−mcrAΔ(mrr-hsdRMS-mcrBC) rpsL nupG φ80lacZΔM15 ΔlacX74 deoR recA1 araD139 Δ(ara-leu)7697 galU galK endA1 | Invitrogen, Carlsbad, CA |
| E. coli BL21(DE3)/pLysS | F−ompT hsdSB (rB− mB−) gal dcm (DE3), pLysS (Cmr) | Novagen, Madison, WI |
| E. coli BL21(DE3) | F−ompT hsdSB (rB− mB−) gal dcm (DE3) | Novagen, Madison, WI |
| Plasmids | ||
| pSUP5011 | Apr Cmr Kmr Tn5::mob | 27 |
| pBluescript SK(–) | AprlacPOZ′ | Stratagene, San Diego, CA |
| pBluescriptSK::sucCDrbs | Apr | This study |
| pGEM-T Easy | AprlacPOZ′ | Promega, Madison, WI |
| pGEM-T Easy::sucCDrbs | AprlacPOZ′ | This study |
| pJQ200mp18Tc | TcrsacB oriV oriT traJ | 35 |
| pJQ200mp18Tc::ΔsucCD | TcrsacB oriV oriT traJ | This study |
| Oligonucleotides | ||
| M13 forward | GTAAAACGACGGCCAGT | MWG Biotech AG, Ebersberg, Germany |
| M13 reverse | CAGGAAACAGCTATGAC | MWG Biotech AG, Ebersberg, Germany |
| IS50 walking | TCGGCCGCACGATGAAGAGC | MWG Biotech AG, Ebersberg, Germany |
| IS50 sequencing | CGTTACCATGTTAGGAGGTCACATGG | MWG Biotech AG, Ebersberg, Germany |
| sucCDforward_PstI | CTGCAGCAGTCTCAATTCGTGTGCTCGC | MWG Biotech AG, Ebersberg, Germany |
| sucCDreverse_XhoI_stop | CTCGAGTTACAGTACTGATTTGAGCAGTTTG | MWG Biotech AG, Ebersberg, Germany |
| sucCXbaI | TCTAGATGTCTCTGGGTTCTTCGGCAC | MWG Biotech AG, Ebersberg, Germany |
| sucCEcoRI | GAATTCGTATTACCTATTAACGTAGGATAAAAAAAC | MWG Biotech AG, Ebersberg, Germany |
| sucDXbaI | TCTAGACGCCTTCATCGTCGTCTGAC | MWG Biotech AG, Ebersberg, Germany |
| sucDEcoRI | AAAAGAATTCGTACCAGCGGTCATTTGTCG | MWG Biotech AG, Ebersberg, Germany |
For the abbreviations used in the E. coli genotypes, see reference 3. Cmr, chloramphenicol resistance; Apr, ampicillin resistance; Kmr, kanamycin resistance; Tcr, tetracycline resistance.
Chemicals.
Organic thiochemicals of high purity grade were purchased from Acros Organics (Geel, Belgium) or Sigma-Aldrich (Steinheim, Germany). CoA and ATP were purchased from Gerbu Biochemicals GmbH (Gaiberg, Germany). 3SP was synthesized according to the method of Jollès-Bergeret (19); the procedure was modified by one repetition of the step for alkaline cleavage of the intermediate bis-(2-carboxyethyl)sulfone (57). Synthesis and purity of the substance was confirmed by high-pressure liquid chromatography (HPLC), gas chromatography-mass spectrometry (GC/MS), and nuclear magnetic resonance (NMR) spectroscopy.
DNA isolation and recombinant DNA techniques.
Chromosomal DNA of A. mimigardefordensis strain DPN7T was isolated according to the method of Marmur (27). Plasmid DNA was isolated by using the GeneJET plasmid miniprep kit from Fermentas (St. Leon-Rot, Germany) according to the manufacturer's manual. DNA was digested with restriction endonucleases under conditions described by the manufacturer or according to the method of Sambrook et al. (38). PCRs were carried out in an Omnigene HBTR3CM DNA thermal cycler (Hybaid, Heidelberg Germany) using Pfx-DNA polymerase (Invitrogen, Karlsruhe, Germany) and Taq-DNA polymerase (Fermentas, St. Leon-Rot, Germany). PCR products were isolated from an agarose gel and purified by using a NucleoTrap kit (Macherey & Nagel, Düren, Germany) according to the manufacturer's instructions. T4 DNA ligase was purchased from Invitrogen (Karlsruhe, Germany). Primers were synthesized by MWG-Biotech AG (Ebersberg, Germany).
Transfer of DNA.
Competent cells of E. coli strains were prepared and transformed by the CaCl2 procedure (38).
DNA sequencing and sequence data analysis.
DNA sequences were determined according to the method of Sanger et al. (39). Sequencing was done by using an ABI Prism 3730 capillary sequencer at the Universitätsklinikum Münster with a BigDye Terminator v3.1 cycle sequencing kit according to the manufacturer's manual (Applied Biosystems, Darmstadt, Germany) or an LI-COR 4000L automatic sequencing apparatus (LI-COR, Inc., Biotechnology Division, Lincoln, NE) using a Thermo Long-Read cycle sequencing kit (Epicentre Technologies, Madison, WI) and IRD 800-labeled oligonucleotides (MWG-Biotech AG). The program BlastX (National Center for Biotechnology Information [http://www.ncbi.nml.nih.gov]) was used for the determination of nucleotide identity (2). The program BioEdit (16) was used for multiple sequence alignments.
Genome walking.
For sequencing of flanking genomic regions of known sequences, a PCR-based two-step genome walking method (34) was performed. IS50 walking and IS50 sequencing were used as primers after construction as described by Pilhofer et al. (34) (the primers are listed in Table 1).
Transposon mutagenesis.
For transposon mutagenesis of A. mimigardefordensis strain DPN7T, the suicide plasmid technique described previously (43, 44) was used. The vector pSUP5011 was transferred from E. coli S17-1 to the kanamycin (Km)-susceptible A. mimigardefordensis strain DPN7T by conjugation, using the spot agar mating technique (13). Tn5::mob-induced mutants were selected on MSM agar plates containing 50 μg of Km ml−1 (MSMKm) and 0.2% (wt/vol) sodium propionate or 0.2% (wt/vol) 3SP (master plates). Putative Tn5::mob-induced mutants were transferred in a coordinated pattern to MSMKm agar plates containing 0.4% (wt/vol) DTDP or 0.3% (wt/vol) 3SP (selection plates) and to corresponding master plates for further analysis.
Genotypic characterization of Tn5::mob-induced mutants.
Genomic DNA was isolated (27) and restricted with SalI or BamHI. The genomic DNA fragments were then ligated into pBluescript SK(−) DNA, which was linearized with the same restriction endonucleases; the ligation products were subsequently transformed into CaCl2-competent E. coli Top10 cells. Transformants were selected on LB medium containing Km, and hybrid plasmids were subsequently isolated and sequenced using the primers M13 forward, M13 reverse, and IS50 sequencing (see Table 1) (34).
Construction of sucCD precise deletion gene replacement plasmid.
The oligonucleotides used for PCR are listed in Table 1. The 663- and 529-bp fragments upstream and downstream of sucCD were amplified by using sucCXbaI/ sucCEcoRI or sucDEcoRI/sucDXbaI, respectively. The resulting fragments were EcoRI digested and ligated to yield a 1,192-bp fragment. This fragment was amplified using sucCXbaI and sucDXbaI, and the resulting PCR product was cloned into the XbaI site of pJQ200mp18Tc (35) to yield pJQ200mp18Tc::ΔsucCD.
Construction of sucCD gene replacement strain using the sacB system.
Gene replacement was accomplished by adaptation of standard protocols (37, 45). Plasmid pJQ200mp18Tc::ΔsucCD was used to generate the mutant A. mimigardefordensis ΔsucCD. The plasmid was mobilized from E. coli donor strain S17-1 to the A. mimigardefordensis strain DPN7T recipient strain by the spot agar mating technique (14). A successfully generated gene replacement strain was identified and confirmed by PCR analyses and DNA sequencing.
Analysis of 3SP by GC and GC/MS.
Lyophilized cells and cell-free supernatants were analyzed by gas chromatography (GC). Samples were subjected to methylation in the presence of 1 ml of chloroform, 0.850 ml of methanol, and 0.150 ml of sulfuric acid for 2 to 4 h at 100°C. Upon methylation, 2 ml of H2O was added, and the samples were vigorously shaken for 30 s. After phase separation, the organic layer containing the resulting methyl esters of the organic acids was analyzed in an HP6850 gas chromatograph equipped with a BP21 capillary column (50 m by 0.22 mm; film thickness, 250 nm; SGE, Darmstadt, Germany) and a flame ionization detector. Aliquots of synthesized 3SP and supernatants, which showed unknown substances during GC analysis, were analyzed by GC/MS. The samples were subjected to acid-catalyzed esterification in the presence of methanol as described previously. The resulting methyl esters were then characterized in an HP6890 gas chromatograph equipped with a model 5973 EI MSD mass selective detector (Hewlett-Packard, Waldbronn, Germany). Then, 3 μl of the organic phase was analyzed after split injection (split ratio, 20:1) using a BPX 35 capillary column (50 m by 0.22 mm; film thickness, 250 nm; SGE). Helium was used as carrier gas at a flow rate of 0.6 ml/min. The temperatures of the injector and detector were 250 and 240°C, respectively. The same temperature program as during GC analysis was applied. Identification of peaks was performed by using the AMDIS software in combination with the NIST database (47).
HPLC analysis.
HPLC analysis of chemically synthesized 3SP was carried out with a LaChrom Elite HPLC apparatus (VWR-Hitachi International GmbH, Darmstadt, Germany) applying a Metacarb 67H advanced C column (Varian, Palo Alto, CA; Bio-Rad Aminex equivalent). The column (300 mm by 6.5 mm) consisted of a sulfonated polystyrene resin in the protonated form. The column temperature was maintained at 30°C with a 2350 VWR-Hitachi column oven. An L-2490 VWR-Hitachi refractive index detector was used for detection. Aliquots of 20 μl were injected and eluted with 0.005 N sulfuric acid (H2SO4) in double-distilled water at a flow rate of 0.8 ml/min. Online integration and analysis was performed with EZ Chrome Elite Software (VWR-Hitachi International GmbH).
NMR spectroscopy.
3SP applied for screening of Tn5::mob-induced mutants and enzyme assays was chemically synthesized according to the method of Jollès-Bergeret (19). To confirm its identity, samples of 3SP were analyzed by NMR spectroscopy. For this, 10 mg of the disodium salt was dissolved in 800 μl of D2O and then transferred to NMR sample tubes (5 mm, thin wall, 9-in. length, 100 MHz, part no. 505-PS-9; Wilmad-LabGlass, Vineland, NJ). Measurement occurred on a Bruker AMX 400 (1H-NMR, 400.14 MHz; 13C-NMR, 100.61 MHz) device at 22°C. The software MestReNova (version 5.2.5-4119; Mestrelab Research S.L., Santiago de Compostela, Spain) was used for analysis of obtained data.
Analysis of CoA ester formation by LC/MS.
The formation of succinyl-CoA, itaconyl-CoA, and 3SP-CoA during enzyme assays was monitored by HPLC in combination with mass spectrometry (LC/MS). LC/MS analysis was carried out with an UltiMate 3000 HPLC apparatus (Dionex GmbH, Idstein, Germany) connected directly to an LXQ Finnigan (Thermo Scientific, Dreieich, Germany) mass spectrometer. A Nucleosil RP C18 (5 μm, 100-Å pores; Knauer GmbH, Berlin, Germany) reverse-phased column served to separate the CoA esters at 30°C. A 50 mM concentration of ammonium acetate (pH 5.0) adjusted with acetic acid (eluent A) and 100% (vol/vol) methanol (eluent B) served as eluents. Elution occurred at a flow rate of 0.3 ml/min. Ramping was performed as follows: equilibration with 90% eluent A for 2 min before injection and 90 to 45% eluent A for 20 min, followed by holding for 2 min, and then a return to 90% eluent A within 5 min after injection. Detection of CoA esters occurred at 259 nm with a photodiode array detector. The instrument was tuned by direct infusion of a solution of 0.4 mM CoA at a flow rate of 10 μl/min into the ion source of the mass spectrometer to optimize the ESI-MS system for maximum generation of protonated molecular ions (parents) of CoA derivatives. The following tuning parameters were retained for optimum detection of CoA esters: capillary temperature, 300°C; sheat gas flow, 12 liters/h; auxiliary gas flow, 6 liters/h; and sweep gas flow, 1 liter/h. The mass range was set to m/z 50 to 1,000 Da when run in the scan mode. The collision energy in the MS mode was set to 30 V and yielded fragmentation patterns that were in good accordance with those found in other publications (13).
Cloning of sucCD.
sucCD was amplified from total genomic DNA of A. mimigardefordensis strain DPN7T by PCR using Pfx-DNA polymerase (Invitrogen) and the following oligonucleotides: sucCDforward_PstI and sucCDreverse_XhoI_stop (Table 1). PCR products were isolated from agarose gels by using a NucleoTrap kit (Macherey & Nagel) and ligated with pGEM-T Easy DNA (Promega, Madison, WI), yielding pGEM-TEasy::sucCDrbs. Ligation products were transformed into CaCl2-competent cells, and transformants were selected on LB agar plates containing IPTG (isopropyl-β-d-thiogalactopyranoside) and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) plus ampicillin. For heterologous expression in the vector pBluescript SK(−) (Stratagene, San Diego, CA), sucCD was obtained by restriction of hybrid plasmid pGEM-Teasy::sucCDrbs with PstI and XhoI, purified from an agarose gel, and subsequently ligated into pBluescript SK(−), which was linearized with the same restriction endonucleases. The ligation product was used for transformation of CaCl2-competent cells of E. coli strain Top10. After selection of transformants using LB medium containing ampicillin, the hybrid plasmids were isolated, analyzed by sequencing, and transformed to CaCl2-competent cells of E. coli strain BL21(DE3)/pLysS (Novagen, Madison, WI).
Preparation of crude extracts.
Cells from 50- to 1,000-ml cultures were harvested by centrifugation (20 min, 4°C, 2,800 × g), washed twice, and stored at −20°C until usage. Cells were resuspended in 50 mM Tris-HCl buffer (pH 7.4) and subsequently disrupted by applying a French press (28) (Aminco, Silver Spring, MD) or a Sonoplus GM200 sonication apparatus (Bandelin, Berlin, Germany) equipped with a SH 213G boosterhorn and MS 72 or MS 73 microtip probes. The amplitude was 16 μm (1 min/ml), while cooling was performed in an NaCl-ice bath. Soluble protein fractions of crude extracts were obtained in the supernatants after 1.5 h of centrifugation at 100,000 × g and 4°C and were used for enzyme purifications.
Purification of SucCDAm.
After heterologous expression of SucCD from A. mimigardefordensis strain DPN7T (SucCDAm) and disruption of the cells by sonication, soluble protein fractions were applied to a Q-Sepharose Fast-Flow column (52 ml; GE Healthcare, Munich, Germany) at 4°C, which was equilibrated with 50 mM Tris-HCl (pH 7.4)–0 mM NaCl at a flow rate of 4 ml/min. The proteins were eluted by a step gradient with increasing sodium chloride concentrations at a flow rate of 4 ml/min as follows: 0 to 20 min, 0 mM NaCl; 20 to 65 min, 50 mM NaCl; 65 to 110 min, 75 mM NaCl; 110 to 155 min, 100 mM NaCl; and 155 to 210 min, 150 mM NaCl. SucCDAm eluted at 100 and 150 mM NaCl; that from the latter was used for enzyme assays.
Enzyme assays.
Standard in vitro activity of succinyl-CoA synthetase in direction of ADP formation was assayed by a continuous spectrophotometric assay according to the method of Cha (11). Measurements of succinate, itaconate, and 3SP were carried out at 30°C in the presence of 50 mM Tris-HCl (pH 7.4), 1 mM ATP, 0.1 mM CoA, 1 mM MgCl2, 2 mM phosphoenolpyruvate, and 0.1 mM NADH, together with 6 U of pyruvate kinase and 6 U of lactate dehydrogenase from rabbit muscle (Sigma) as coupling enzymes. The concentrations of the organic acids were assayed in the range of 0.1 to 10 mM (succinate) and 0.1 to 15 mM (itaconic acid and 3SP). ATP and CoA measurements were carried out as described above in the presence of 5 mM succinate. The formation of ADP accompanied by the formation of CoA thioester was measured as a decrease in NADH absorption at 340 nm. The auxiliary enzymes were tested to ensure that they were not rate limiting.
The utilization of Mn2+ instead of Mg2+ was measured under the same conditions as described above with 5 mM succinate as an organic acid. The concentration of MnCl2 was 1 mM. The utilization of GTP instead of ATP was also assayed by incubating 30 μg of purified enzyme in 1 ml of 100 mM Tris-HCl (pH 7.4) for 5 min to 2 h at 30°C under the following conditions: 1 mM CoA, 5 mM 3SP, and 10 mM MgCl2. The concentration of the GTP was 1 mM.
The formation of the expected CoA esters was verified by liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS). For this analysis, the reactions were stopped by the addition of 30 μl of 15% (wt/vol) trifluoroacetic acid. The samples were subsequently analyzed as described above.
Data deposition.
The complete nucleotide sequence and the deduced amino acid sequence for SucCDAm have been deposited in the GenBank database under accession number EU423870.
RESULTS
Analysis of synthesized 3SP by HPLC, GC/MS, and NMR spectroscopy.
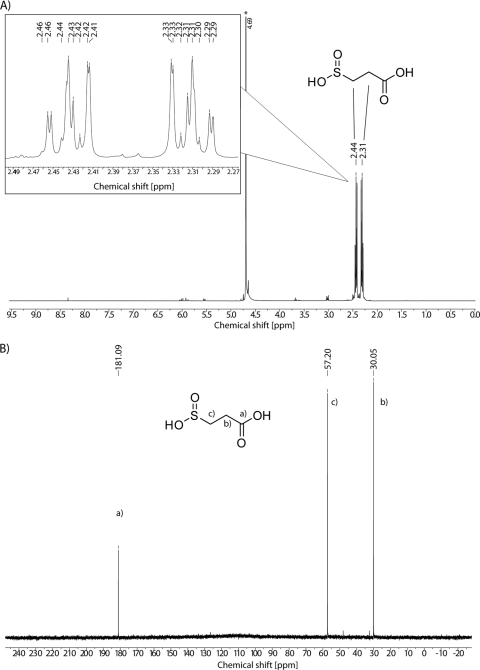
3SP applied for screening of Tn5::mob-induced mutants and for enzyme assays was chemically synthesized by using a modification of the method of Jollès-Bergeret (19). The reaction was started from 110 g of sodium formaldehyde sulfoxylate (purity, > 98%; 0.70 mol) plus 108 ml of acrylic acid (99.5% purity) and yielded 143 g (0.68 mol, 97% yield) of the intermediate bis-(2-carboxyethyl) sulfone. Then, 70 g (0.33 mol) of this was subjected to 200 ml of NaOH (5 N) for alkaline cleavage. After precipitation and washing, 64 g (0.35 mol, 106% yield) of the disodium salt of 3SP was obtained. Since the yield exceeded the expected 60 g (0.33 mol, 100% with respect to the starting material), which was attributed to incomplete cleavage, a second alkaline cleavage step was performed. Repeated alkaline cleavage and subsequent precipitation and washing of 58 g of the intermediate product yielded 9 g (0.05 mol) of 3SP, with a purity of ca. 98%. Samples of 3SP were analyzed by HPLC, GC/MS, and NMR spectroscopy to confirm its identity. HPLC analysis and fragmentation pattern results obtained by GC/MS were consistent with previous results and confirmed the identity of 3SP (8, 57; data not shown). Due to the high purity of ca. 98%, 1H- and 13C-NMR spectra of 3SP were recorded as depicted in Fig. 3.
Fig. 3.
NMR spectra of 3SP. (A) 1H-NMR spectrum at 400 MHz in D2O. Peaks are dedicated to the corresponding CH2-groups of 3SP. The inset depicts zoomed peaks. An asterisk indicates a solvent peak (HDO). (B) 13C-NMR spectrum at 100 MHz in D2O. Indices positions a, b, and c indicate the corresponding carbon atoms of 3SP.
Tn5::mob mutagenesis experiments.
In a previous study (57) a Tn5::mob transposon mutagenesis was conducted to identify the genes involved in the degradation of DTDP. Selection and identification of Tn5::mob-induced mutants was carried out on agar plates containing MSM and 0.4% (wt/vol) DTDP or 0.2% (wt/vol) disodium 3-sulfinopropionate, where the mutants should not grow, in comparison to master plates containing 0.5% (wt/vol) sodium gluconate or 0.2% (wt/vol) sodium propionate, where the mutants should grow like the wild type. In all, 20,000 transconjugants were obtained and screened for those not showing growth on DTDP or 3SP. Although the mutants JhwA8 and Jhw121 were fully impaired in growth, mutant Jhw38 showed slower growth on DTDP as the sole source of carbon and energy. Genotypic characterization of these mutants revealed that in mutants JhwA8 and Jhw121 the Tn5::mob transposon had inserted at an identical position in sucC (Fig. 4). Hence, only mutant Jhw121 was further investigated. In mutant Jhw38 the insertion was mapped 298 bp upstream of this gene (Fig. 4). Cultivations of Jhw121 in MSM containing succinate as a source of carbon and energy for growth in addition to DTDP led to the accumulation of 3SP. From this it was concluded that disruption of sucCD impaired the further catabolism of 3SP.
Due to the low number of mutants and the circumstance that a Tn5::mob insertion in only one of the two subunits of SucCD was thus far available, another transposon mutagenesis of A. mimigardefordensis strain DPN7T was conducted to identify further genes possibly required for the degradation of 3SP. In contrast to the previous study (57), selection and identification of Tn5::mob-induced mutants was now performed on selection plates containing 0.3% (wt/vol) 3SP and on master plates containing 0.2% (wt/vol) sodium propionate as described in Materials and Methods. As noted previously, 20,000 transconjugants were again screened, and four mutants occurred that were fully impaired in growth on 3SP. The Tn5::mob insertions in the genomes of two of these mutants (JG-11 and JG-4) were mapped directly in or in the proximity of sucCD. The growth of these mutants on MSM agar plates containing, as the sole source of carbon and energy, 0.2% (wt/vol) gluconate, propionate, taurine, or succinate was not affected compared to the wild type, thus indicating that in these mutants the genes specifically involved in the catabolism of 3SP were inactivated by the insertions of Tn5::mob.
Molecular characterization of Tn5::mob-induced mutants and construction of A. mimigardefordensis ΔsucCD.
Two methods were used to map the insertions of Tn5::mob in these mutants. A two-step genome walking method (34) was applied, and genomic libraries were also constructed. For the latter, genomic fragments of either mutant conferring Km resistance were cloned in E. coli Top10. Sequencing of these DNA fragments using oligonucleotides hybridizing to the terminal region of IS50L and the multiple cloning site of the used cloning vector pBluescript SK(−) revealed a continuous sequence of 3,764 bp. In mutant JG-4, which was fully impaired in growth with 3SP, Tn5::mob had been inserted into an intergenic region upstream of sucC. The insertion of Tn5::mob in mutant JG-11 disrupted an open reading frame (ORF) coding for the α-chain (sucD) of SucCDAm and also led to a 3SP-negative phenotype. The phenotypes of the mutants and the affected genes are summarized in Table 2, and the gene organization and insertion loci are shown in Fig. 4. An ORF putatively coding for tellurium resistance (terC) is located downstream of SucCD (57). The ORF upstream of SucCD was annotated to a conserved hypothetical protein. To verify the results obtained with the Tn5::mob-induced mutants, the defined deletion strain A. mimigardefordensis ΔsucCD was generated in addition.
Table 2.
Phenotypic and genotypic characterization of Tn5::mob-induced mutants of A. mimigardefordensis strain DPN7T relevant for this study
| Mutant | Phenotypea |
Insertion locus of Tn5::mob (gene product)b | Highest homology (% identical amino acids)c | Source or reference | |
|---|---|---|---|---|---|
| 3SP | DTDP | ||||
| JhwA8/121 | – | – | sucC (succinyl-CoA synthetase, beta-chain) | 93 (Bordetella pertussis Tohama I) | 3 |
| Jhw38 | – | +/− | 298 bp upstream of sucC* | 93 (Bordetella pertussis Tohama I) | 3 |
| JG-4 | – | – | 78 bp upstream of sucC* | 93 (Bordetella pertussis Tohama I) | This study |
| JG-11 | – | – | sucD (succinyl-CoA synthetase, beta-chain) | 89 (Bordetella avium 197N) | This study |
–, No growth; +/−, weak growth.
*, Closest specified gene identified adjacent to the Tn5::mob insertion locus.
The relevant strain is indicated in parentheses.
Degradation of DTDP and accumulation of 3SP.
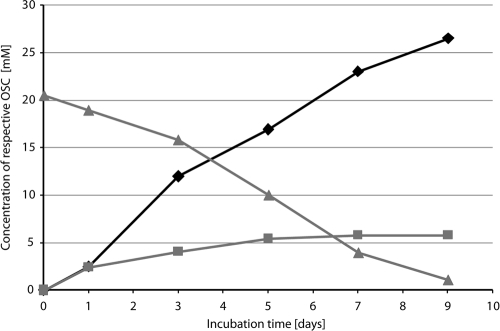
To identify the accumulation of putative intermediates, the wild type and mutants JG-4, JG-11, Jhw38, and Jhw121, which were obtained in this and a previous study (57), were precultivated in MSM containing 0.3% (wt/vol) sodium propionate. After 48 h, the cells were washed and transferred to Erlenmeyer flasks without baffles with MSM containing 0.3% (wt/vol) DTDP, which corresponds to about 14 mM as the sole carbon source. Samples were taken every 48 h, and aliquots of cell-free supernatants were analyzed by GC and GC/MS (data not shown). The wild type and any mutant were able to partially degrade DTDP. In the cultures of the wild type, as well as of the mutants Jhw38 and JG-4, a transient accumulation of 3SP was observed. Mutant Jhw121 accumulated 3SP at a comparably high concentration of up to 5 mM, whereas mutant JG-11 showed no accumulation of 3SP. The A. mimigardefordensis ΔsucCD strain accumulated even more 3SP in the supernatant if cultivated in MSM supplied with DTDP and another utilizable carbon source. The highest concentrations of 3SP were detected after preincubation of A. mimigardefordensis ΔsucCD in MSM containing 20 mM succinate for 48 h. The cells were then washed, transferred into fresh MSM containing 10 mM succinate and 20 mM DTDP, and cultivated at 30°C and 120 rpm for 9 days. Within this incubation time, almost all DTDP was consumed, and 26 mM 3SP was detectable in the supernatant (Fig. 5).
Fig. 5.
Degradation of DTDP and accumulation of 3MP and 3SP. A. mimigardefordensis ΔsucCD cells were preincubated for 48 h in mineral salt medium (MSM) containing 20 mM succinate. The cells were then washed, transferred into fresh MSM containing 10 mM succinate and 20 mM DTDP, and cultivated at 30°C and 120 rpm for 9 days. Within this incubation period, almost all of the DTDP was consumed, and 26 mM 3SP was detectable in the supernatant. Symbols: triangles, DTDP; squares, 3MP; diamonds, 3SP.
Analysis of the primary structure of SucCDAm.
In silico analyses of the amino acid sequences of SucC and SucD of A. mimigardefordensis strain DPN7T showed the highest similarities with the α-chain of succinyl-CoA synthetase from the Bordetella pertussis strain Tohama I (93% identity) and with the β-chain of succinyl-CoA synthetase from Bordetella avium strain 197N (89% identity), respectively (Table 2). The α-chain consists of 293 amino acids and comprises a Rossmann fold and a CoA-ligase domain. The β-chain consists of 386 amino acids and encloses a d-alanine–d-alanine ligase domain and an ATP binding site. A multiple sequence alignment of SucCD homologues is shown in Fig. S1 in the supplemental material.
Purification of SucCDAm.
The first attempts toward the heterologous expression of SucCDAm using several vectors of the pET system (Novagen) in various expression strains such as E. coli Tuner (DE3), E. coli Rosetta (DE3)/pLysS, or E. coli HMS174 (DE3) in combination with induction by IPTG failed. The α-subunit was expressed but formed inclusion bodies. Measures to prevent the formation of inclusion bodies, such as cultivation at lower temperature or the addition of small amounts of ethanol to the medium as described by Strandberg and Enfors (48), had no effect. Finally, construction of pBluescriptSK::sucCDrbs, transfer into E. coli BL21(DE3)/ pLysS, and subsequent induction using ZYP-5052 medium, an autoinduction medium according to Studier et al. (50), solved the problem. The soluble protein fraction, obtained after cell disruption and centrifugation, was applied to a Q-Sepharose Fast-Flow column. The column was equilibrated with 50 mM Tris-HCl (pH 7.4) containing no NaCl. Elution was then carried out by applying a step gradient of increasing sodium chloride concentrations. The purified enzyme was eluted in the 150 mM NaCl step and used for enzyme assays (Fig. 6). After centrifugation, the enzyme was shown to be soluble in the supernatant.
Fig. 6.
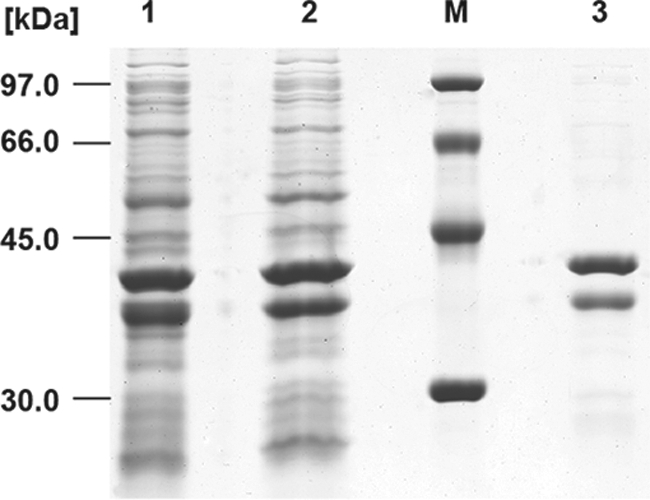
Purification of SucCDAm applying Q-Sepharose FF as revealed by SDS-PAGE (11.5% [wt/vol] acrylamide). Lane 1, crude extract of cells; lane 2, soluble protein fraction after 90 min of centrifugation at 100,000 × g and 4°C; lane M, low-molecular-weight calibration kit (GE Healthcare, Uppsala, Sweden); lane 3, purified SucCDAm eluted with 50 mM Tris-HCl (pH 7.4)–150 mM NaCl. The SDS-gel was stained with Coomassie brilliant blue R.
SucCDAm enzyme activity assay.
After expression and purification of soluble SucCD from A. mimigardefordensis strain DPN7T, the enzyme activity was determined by use of a continuous spectrophotometric assay as described in Materials and Methods. To verify the in vitro formation of the expected CoA esters, samples were withdrawn after finishing the spectrophotometric measurements and subjected to LC-ESI-MS. When succinate, itaconate, or 3SP was added to the assay, the formation of the respective CoA esters could be observed (Fig. 7). However, kinetic studies showed clear differences between the three substrates (Table 3). We found that, upon comparing the Vmax values, the highest activity of the enzyme occurred with succinate (Vmax = 9.85 ± 0.14 μmol min−1 mg−1), whereas the activity with itaconate was only ca. 15% and with 3SP only ca. 1.2% of the activity with succinate. With regard to the Km values, SucCDAm revealed the highest affinity to succinate (Km = 0.143 ± 0.001 mM) as a substrate. A 3-fold-higher Km value of the enzyme was obtained for itaconic acid and an ∼6-fold-higher Km value was obtained for 3SP, both leading to the assumption of a lower catalytic efficiency of SucCDAm for these substrates.
Fig. 7.
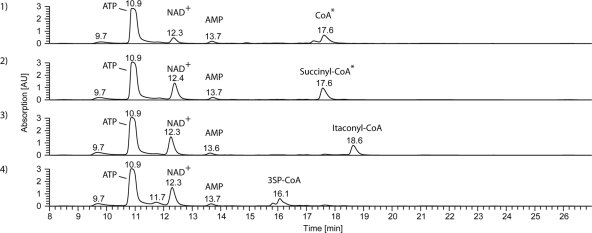
Typical HPLC chromatograms of enzyme assays. Enzyme assays were performed as described in Materials and Methods. Detection of compounds was carried out at 259 nm using a photo diode array detector. Identification of peaks was done using ESI-MS. Shown are the results obtained for a control omitting an organic acid as a substrate but containing SucCDAm (graph 1) and from assays performed in the presence of 5 mM succinate (graph 2), 5 mM itaconate (graph 3), or 5 mM 3SP (graph 4). *, CoA and succinyl-CoA were not separated by the applied HPLC methods. ESI-MS analysis revealed the complete transformation of CoA to succinyl-CoA.
Table 3.
Kinetic parameters of SucCD from A. mimigardefordensis strain DPN7T and E. colia
| Substrate | Mean ± SD |
||
|---|---|---|---|
| Vmax (μmol min−1 mg−1) |
Km (mM) |
||
| A. mimigardefordensis | E. coli | ||
| Succinate | 9.85 ± 0.14 | 0.143 ± 0.001 | 0.25 |
| Itaconate | 1.54 ± 0.15 | 0.448 ± 0.093 | ND |
| 3SP | 0.12 ± 0.01 | 0.818 ± 0.046 | ND |
| ATP | 15.84 ± 0.12 | 0.083 ± 0.002 | 0.004 |
| CoA | 12.67 ± 0.40 | 0.045 ± 0.007 | 0.070 |
For DPN7T, activity was measured by quantifying ADP generation rates using pyruvate kinase and lactate dehydrogenase in a coupled enzyme assay. Kinetic measurements for the organic acids were performed in the presence of 1 mM ATP and 0.1 mM CoA. ATP and CoA measurements were carried out in the presence of 5 mM succinate, 1 mM ATP (for CoA kinetics), and 0.1 mM CoA (for ATP kinetics). Vmax and Km values were determined by obtaining sets of velocity versus the concentration data. A minimum of six concentrations was used in each set. All values are expressed as means ± standard deviations of triplicates. Data for E. coli are from Joyce et al. (20).
As expected for a nucleotide triphosphate-dependent enzyme, no enzyme activity was observed in the absence of Mg2+. However, the formation of succinyl-CoA was also detected when 1 mM MgCl2 was exchanged by equimolar amounts of MnCl2 in the standard assay, applying 5 mM succinate as an organic acid.
Substitution of ATP by GTP was tested with a modified enzyme assay as described in Materials and Methods. The formation of 3SP-CoA in the presence of 3SP as an organic acid and GTP instead of ATP verified the recognition of GTP as a cofactor by SucCDAm.
Confirming the structure of 3SP-CoA.
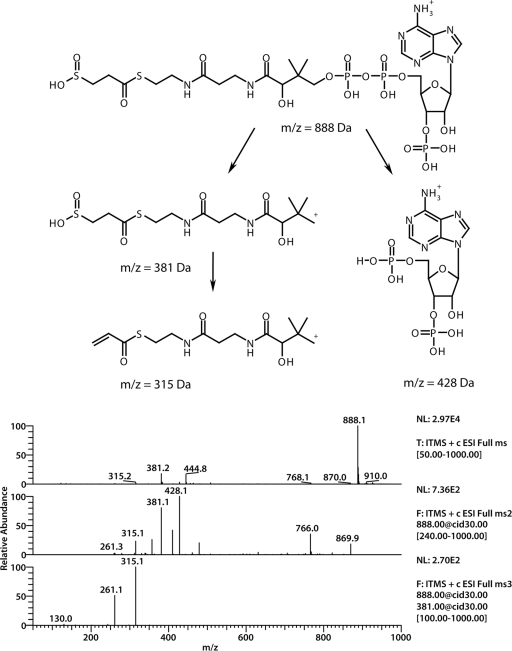
The formation of 3SP-CoA has, to our best knowledge, never been described before either enzymatically or chemically. Thus, 3SP-CoA could not also be purchased and could not be used as a reference for enzyme assays. To verify the formation of 3SP-CoA and its proposed chemical structure, LC-ESI-MS experiments were conducted. In full positive MS mode without collision-induced dissociation a parental ion (m/z = 888 Da) was observed (Fig. 8). The fragmentation of this ion led to two main fragments (m/z = 428 Da and m/z = 381 Da). Subsequent fragmentation of the daughter ion (m/z = 381 Da) yielded two additional fragments (m/z = 381 Da and m/z = 261 Da).
Fig. 8.
(Top) Structural formula of 3SP-CoA; (bottom) mass spectrometric data. The first is an ESI spectrum of 3SP-CoA in positive mode. In the middle is an MS spectrum of the parent ion (m/z = 888 Da). Two main fragments (m/z = 428 Da and m/z = 381 Da) were obtained. At the bottom, further fragmentation of the parent m/z 381 Da yielded daughter ions (m/z = 315 Da and m/z = 261 Da).
DISCUSSION
We report here that SucCD from A. mimigardefordensis strain DPN7T can catalyze the activation of 3SP, a DTDP degradation intermediate, to the corresponding 3SP-CoA thioester. Therefore, the enzyme is promiscuous, since it is involved in the citric acid cycle and is mandatory for the catabolism of DTDP and, in particular, its degradation intermediate 3SP. Succinyl-CoA synthetases catalyze the conversion of succinyl-CoA to succinate and CoA in the citric acid cycle. The energy of the thioester bond is conserved through the coupled phosphorylation of nucleoside diphosphates such as ADP or GDP. However, the reverse reaction is also important for the anabolism. In this case, SucCD activates succinate to the corresponding CoA thioester (24).
Two independent Tn5::mob transposon mutagenesis experiments from this and a previous study (57) yielded a total of 40,000 transconjugants; these were screened for the lack of growth on DTDP or 3SP. Four different Tn5::mob-induced mutants completely or almost completely impaired in DTDP and/or 3SP degradation or in growth on these compounds with Tn5::mob insertions directly in or in the proximity of SucCD (Fig. 4 and Table 2) indicated the involvement of SucCDAm in DTDP degradation. Due to the sequence similarities of the sucC- and sucD-encoded proteins to the two subunits of succinyl-CoA synthetases and a certain structural similarity of 3SP to succinate, it was predicted that 3SP is activated to the corresponding 3SP-CoA thioester by SucCDAm. This assumption was strengthened, since 3SP was accumulated in the supernatant (Fig. 5) during cultivation of the DTDP- and 3SP-negative mutant A. mimigardefordensis ΔsucCD in MSM containing DTDP and succinate as a carbon source for growth. A concentration of up to ∼26 mM 3SP in the supernatant indicates that this compound is neither toxic to the cells nor is it an inhibitor of any other enzyme critical for growth. Furthermore, 3SP seems not to be a substrate for any other enzyme in A. mimigardefordensis, except for SucCD. 3SP is formed in the cytoplasm of the cells from 3MP by a dioxygenase (Fig. 9) but was found in the supernatant. Since the compound carries two negative charges and would not diffuse out of the cells, it seems reasonable that a transporter is involved. 3SP exhibits structural similarities to succinate except for the exchange of a carbon atom in one of the carboxyl groups by a sulfur atom. For succinate, different transporters are known (17). Since succinate was utilized as a carbon source during accumulation experiments, A. mimigardefordensis must possess at least one succinate transporter. Other steps of DTDP catabolism are catalyzed by enzymes unspecific enough to accept intermediates of DTDP degradation. Therefore, this might also apply to the transport of 3SP. Consequently, it is not very likely that a special 3SP transporter has evolved, but it seems rather probable that the succinate transporter is also active with 3SP.
To confirm the proposed activation of 3SP to the corresponding 3SP-CoA thioester, sucCD was heterologously expressed in a recombinant E. coli strain BL21(DE3)/pLysS harboring pSK−::sucCDrbs, and the purified enzyme was subsequently applied in an enzyme assay. Analyses by LC-ESI-MS showed the formation of another compound with a similar absorption spectrum as CoA and succinyl-CoA, but with a significantly different retention time. In the concomitant total ion chromatogram a main peak of m/z = 888 Da was observed. This corresponded well to the value estimated for 3SP-CoA. To verify the formation of 3SP-CoA and to confirm its expected chemical structure, the main peak was further fragmented. The obtained daughter ion (m/z = 428 Da) is the typical ion for CoA derivatives (13) and can be assigned to the adenosine 3′,5′-diphosphate moiety. It results from a cleavage in the phosphate backbone region of the proposed 3SP-CoA ester. Since this fragment was derived from the nonvariable region of 3SP-CoA, the second observed daughter ion (m/z = 381 Da) was more interesting with respect to the structural analysis. It was obtained by the loss of a fragment (m/z = 507 Da) from the parent ion. Park et al. (33) used the constant decrease of m/z = 507 Da from parent ions of different CoA esters to ascertain the identity of the expected CoA derivative in their study. Therefore, these researchers calculated the expected mass of the second daughter by subtracting m/z = 507 Da from the respective parent ion. The ion at m/z = 381 Da (Fig. 8) corresponded to the expected mass of 3SP attached to the pantetheine moiety of CoA. Up to that point it was uncertain how 3SP was bound to CoA. Theoretically, 3SP could be bound as a thioester or as a thiosulfinate [(2carboxyethyl)sulfinic acid CoA ester] (Fig. 2f and g). Further fragmentation of m/z = 381 Da yielded m/z = 315 Da as a major fragment. This was assigned to the loss of H2SO2 from the m/z = 381 Da fragment, which is only possible if 3SP is attached to CoA as a thioester. The second fragment received during this fragmentation resulted from the total loss of the 3SP moiety and could be assigned to the dehydroxylated pantetheine residue (Fig. 8, m/z = 261 Da [structure not shown]).
Activation of itaconate by SucCD as an initial step of itaconate degradation in liver mitochondria and in P. fluorescens and Pseudomonas sp. strain B2aba, respectively, was reported earlier (1, 29, 30, 46). This transformation of itaconate to itaconyl-CoA was also verified by LC/MS for SucCD from A. mimigardefordensis strain DPN7T.
In all, succinate, itaconate, and the novel substrate 3SP are activated to the corresponding CoA thioesters (Fig. 1a to c). According to the obtained kinetic parameters, SucCDAm shows a different activity with regard to the three substrates. This might be due to the structural differences of succinate, 3SP, and itaconate. An additional methylene group in itaconate represents a rather minor structural difference in comparison to the substitution of a carbon atom by a sulfur atom in 3SP compared to the well-known substrate succinate (Fig. 2c to e). However, along with succinate and itaconate activation, the transformation of 3SP to the corresponding 3SP-CoA thioester, which is reported for the first time here, is the third reaction catalyzed by SucCDs with a classical domain distribution (Fig. 1a to c). In addition, proof of this reaction confirms the suggestion made by Jollès-Bergeret in 1974 that 3SP, as a close structural analogue of succinate, would undergo, at least in part, the same metabolic fate (19). Due to the possibility of substituting the cofactor Mg2+ by Mn2+ and the coenzyme ATP by GTP, SucCDAm shows behavior similar to that of other SucCDs from Gram-negative bacteria (21).
Another point to be discussed is whether the physiological function of SucCDAm is dedicated to the citric acid cycle (TCC) or to the catabolism of the DTDP degradation intermediate 3SP or to both. Tn5::mob insertions directly in or in the vicinity of the same sucCD resulted in a phenotype completely or partially impaired in growth on 3SP as well as on DTDP. For the A. mimigardefordensis ΔsucCD strain no growth on DTDP as the sole carbon source and accumulation of 3SP when succinate was applied as a carbon source in addition to DTDP were observed. Growth on any other tested carbon sources was not affected. The latter finding is in accordance with results reported recently for E. coli mutants carrying deletions of sucCD. E. coli MG1655 ΔsucCD mutants were generated in two independent studies (9, 54). In both studies, no growth retardation of the deletion mutants in LB medium or in minimal medium containing glucose as the sole carbon source was observed. Byung et al. (9) showed that in these mutants succinyl-CoA was supplied by α-ketoglutarate dehydrogenase encoded by sucAB, whereas a simultaneous deletion of both sucCD and sucAB was lethal. Thus, despite the lack of an active SucCD and hence a disrupted TCC in A. mimigardefordensis strain DPN7T mutants, growth on MSM agar plates containing 0.2% (wt/vol) gluconate, propionate, taurine, or succinate as the sole sources of carbon and energy was not detectably affected in all mutants compared to the wild type. The amino acid sequence of SucCDAm shows high similarity to other SucCDs, and the formation of 3SP-CoA was also observed in crude extracts from E. coli BL21(DE3)/pLysS not harboring the gene for SucCD from A. mimigardefordensis strain DPN7T. From this it can be concluded that the formation of 3SP-CoA is not a specific reaction of the SucCDAm but is also catalyzed by SucCD present in E. coli. Since disruption or deletion of the genes of the investigated SucCD impaired the growth of A. mimigardefordensis strain DPN7T on DTDP and 3SP, this bacterium seems to possess only one SucCD. Therefore, it is assumed that SucCDAm has both functions in A. mimigardefordensis strain DPN7T. It is physiologically dedicated to the TCC but can be functionally replaced by α-ketoglutarate dehydrogenase, whereas it is mandatory for the catabolic pathway of DTDP and its degradation intermediate 3SP.
We also confirmed another step in the previously proposed pathway of DTDP catabolism in A. mimigardefordensis strain DPN7T (57). The initial cleavage of DTDP into two molecules of 3-mercaptopropionate (3MP, Fig. 2b) by a dihydrolipoamide dehydrogenase in A. mimigardefordensis strain DPN7T (LpdAAm), a homodimeric flavoenzyme belonging to the family of pyridine nucleotide-disulfide oxidoreductases, was recently shown (58). The oxidation of 3MP to 3SP by a 3MP dioxygenase was first reported in Variovorax paradoxus strain TBEA6 (8). In four independent mutants of A. mimigardefordensis strain DPN7T that are impaired in growth on DTDP but not on 3SP, the Tn5::mob was mapped in a gene coding for a 3MP dioxygenase. Thus, the orthologue of A. mimigardefordensis strain DPN7T is most probably responsible for this reaction (57). It was also proposed that SucCDAm investigated here catalyzes the conversion of 3SP to 3SP-CoA. Based on the results of the present study, this proposed reaction was confirmed and ascribed to SucCDAm. Further studies concerning the fate of 3SP-CoA in the catabolism including the abstraction of sulfur by a desulfination reaction in A. mimigardefordensis strain DPN7T are currently in progress.
Supplementary Material
ACKNOWLEDGMENTS
The LC-ESI-MS device used in this study was provided by funds of the DFG (Deutsche Forschungsgemeinschaft, grant INST 211/415-1 FUGG), which we gratefully acknowledge.
We thank Kristina Kampmann, Marius Döring, Janine Richhardt, and Sarah Kohlwey for assistance in some of the experiments.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 22 April 2011.
REFERENCES
- 1. Adler J., Wang S.-F., Lardy H. A. 1957. The metabolism of itaconic acid by liver mitochondria. J. Biol. Chem. 229:865–879 [PubMed] [Google Scholar]
- 2. Altschul S. F., et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bachmann B. J. 1987. Linkage map of Escherichia coli K-12, p. 807–876 In Neidhardt F. C., et al. (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 7th ed., vol. 2. American Society for Microbiology, Washington, DC [Google Scholar]
- 4. Beart P. M., Johnston G. A. R. 1973. GABA uptake in rat brain slices: inhibition by GABA analogues and by various drugs. J. Neurochem. 20:319–324 [DOI] [PubMed] [Google Scholar]
- 5. Birney M., Um H. D., Klein C. 1996. Novel mechanisms of Escherichia coli succinyl-coenzyme A synthetase regulation. J. Bacteriol. 178:2883–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bridger W. A. 1971. Evidence for two types of subunits in succinyl coenzyme A synthetase. Biochem. Biophys. Res. Commun. 42:948–954 [DOI] [PubMed] [Google Scholar]
- 7. Bridger W. A. 1974. Succinyl-CoA synthetase, p. 581–606 In Boyer P. D. (ed.), The enzymes, vol. 10. Academic Press, Inc., New York, NY [Google Scholar]
- 8. Bruland N., Wübbeler J. H., Steinbüchel A. 2009. 3-Mercaptopropionate dioxygenase, a cysteine dioxygenase homologue, catalyzes the initial step of 3-mercaptopropionate catabolism in the 3,3-thiodipropionic acid-degrading bacterium Variovorax paradoxus. J. Biol. Chem. 284:660–672 [DOI] [PubMed] [Google Scholar]
- 9. Byung J. Y., et al. 2005. sucAB and sucCD are mutually essential genes in Escherichia coli. FEMS Microbiol. Lett. 254:245–250 [DOI] [PubMed] [Google Scholar]
- 10. Cha S. 1969. Succinate thiokinase from pig heart. Methods Enzymol. 13:62–69 [Google Scholar]
- 11. Codognoto L., et al. 2007. Electrochemical behavior of dopamine at 3,3′-dithiodipropionic acid self-assembled monolayers. Talanta 72:427–433 [DOI] [PubMed] [Google Scholar]
- 12. Cooper R. A., Itiaba K., Kornberg H. L. 1965. The utilization of aconate and itaconate by Micrococcus sp. Biochem. J. 94:25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dalluge J. J., et al. 2002. Separation and identification of organic acid-coenzyme A thioesters using liquid chromatography/electrospray ionization-mass spectrometry. Anal. Bioanal. Chem. 374:835–840 [DOI] [PubMed] [Google Scholar]
- 14. Friedrich B., Hogrefe C., Schlegel H. G. 1981. Naturally occurring genetic transfer of hydrogen-oxidizing ability between strains of Alcaligenes eutrophus. J. Bacteriol. 147:198–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gibello A., et al. 2009. Reclassification of the members of the genus Tetrathiobacter Ghosh et al. 2005 to the genus Advenella Coenye et al. Int. J. Syst. Evol. Microbiol. 59:1914–1918 [DOI] [PubMed] [Google Scholar]
- 16. Hall T. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acid Symp. Ser. 41:95–98 [Google Scholar]
- 17. Janausch I. G., Zientz E., Tran Q. H., Kröger A., Unden G. 2002. C4-dicarboxylate carriers and sensors in bacteria. Biochim. Biophys. Acta 1553:39–56 [DOI] [PubMed] [Google Scholar]
- 18. Johnson J. D., Mehus M. G., Tews K., Milabetz B. I., Lambeth D. O. 1998. Genetic evidence for the expression of ATP- and GTP-specific succinyl-CoA synthetases in multicellular eucaryotes. J. Biol. Chem. 273:27580–27586 [DOI] [PubMed] [Google Scholar]
- 19. Jollès-Bergeret B. 1974. Enzymatic and chemical synthesis of 3-sulfinopropionic acid, an analog of succinic acid. Eur. J. Biochem. 42:349–353 [DOI] [PubMed] [Google Scholar]
- 20. Joyce M. A., et al. 1999. Probing the nucleotide-binding site of Escherichia coli succinyl-CoA synthetase. Biochemistry 38:7273–7283 [DOI] [PubMed] [Google Scholar]
- 21. Kapatral V., Bina X., Chakrabarty A. M. 2000. Succinyl coenzyme A synthetase of Pseudomonas aeruginosa with a broad specificity for nucleoside triphosphate (NTP) synthesis modulates specificity for NTP synthesis by the 12-kilodalton form of nucleoside diphosphate kinase. J. Bacteriol. 182:1333–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaufman S. 1955. Studies on the mechanism of the reaction catalyzed by the phosphorylating enzyme. J. Biol. Chem. 216:153–164 [PubMed] [Google Scholar]
- 23. Lambeth D. O., Tews K. N., Adkins S., Frohlich D., Milavetz B. I. 2004. Expression of two succinyl-CoA synthetases with different nucleotide specificities in mammalian tissues. J. Biol. Chem. 279:36621–36624 [DOI] [PubMed] [Google Scholar]
- 24. Lambeth D. O. 2006. Reconsideration of the significance of substrate-level phosphorylation in the citric acid cycle. Biochem. Mol. Biol. Educ. 34:21–29 [DOI] [PubMed] [Google Scholar]
- 25. Lütke-Eversloh T., Steinbüchel A. 2003. Novel precursor substrates for polythioesters (PTE) and limits of PTE biosynthesis in Ralstonia eutropha. FEMS Microbiol. Lett. 221:191–196 [DOI] [PubMed] [Google Scholar]
- 26. Majumdar R., Guest J. R., Bridger W. A. 1991. Functional consequences of substitution of the active site (phospho)histidine residue of Escherichia coli succinyl-CoA synthetase. Biochim. Biophys. Acta 1076:86–90 [DOI] [PubMed] [Google Scholar]
- 27. Marmur J. 1961. A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J. Mol. Biol. 3:208–218 [Google Scholar]
- 28. Milner H. W., Lawrence N. S., French C. S. 1950. Colloidal dispersion of chloroplast material. Science 111:633–634 [DOI] [PubMed] [Google Scholar]
- 29. Nagai J. 1963. Studies on itaconate metabolism. I. Itaconyl-CoA synthesizing reaction in cell-free extracts of Pseudomonas fluorescens. J. Biochem. 53:181–187 [DOI] [PubMed] [Google Scholar]
- 30. Nagai J. 1963. Studies on itaconate metabolism. II. Citramalate metabolism in Pseudomonas fluorescens grown on itaconate. J. Biochem. 54:34–40 [DOI] [PubMed] [Google Scholar]
- 31. Nishimura J. S. 1986. Succinyl-CoA synthetase structure-function relationships and other considerations. Adv. Enzymol. 58:141–172 [DOI] [PubMed] [Google Scholar]
- 32. Park S.-J., Chao G., Gunsalus R. P. 1997. Aerobic regulation of the sucABCD genes of Escherichia coli, which encode alpha-ketoglutarate dehydrogenase and succinyl coenzyme A synthetase: roles of ArcA, Fnr, and the upstream sdhCDAB promoter. J. Bacteriol. 179:4138–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park J. W., Jung W. S., Park S. R., Park B. C., Yoon Y. J. 2007. Analysis of intracellular short organic acid-coenzyme A esters from actinomycetes using liquid chromatography-electrospray ionization-mass spectrometry. J. Mass Spectrom. 42:1136–1147 [DOI] [PubMed] [Google Scholar]
- 34. Pilhofer M., et al. 2007. Characterization of bacterial operons consisting of two tubulins and a kinesin-like gene by the novel two-step gene walking method. Nucleic Acids Res. 35:e135 doi: 10.1093/nar/gkm836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pötter M., Müller H., Steinbüchel A. 2005. Influence of homologous phasins (PhaP) on PHA accumulation and regulation of their expression by the transcriptional repressor PhaR in Ralstonia eutropha H16. Microbiology 151:825–833 [DOI] [PubMed] [Google Scholar]
- 36. Przybyla-Zawislak B., Dennis R. A., Zakharkin S. O., McCammon M. T. 1998. Genes of succinyl-CoA ligase from Saccharomyces cerevisiae. Eur. J. Biochem. 258:736–743 [DOI] [PubMed] [Google Scholar]
- 37. Quandt J., Hynes M. F. 1993. Versatile suicide vectors which allow direct selection for gene replacement in Gram-negative bacteria. Gene 127:15–21 [DOI] [PubMed] [Google Scholar]
- 38. Sambrook J., Fritsch E. F., Maniatis T. 1998. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 39. Sanger F., Nicklen S., Coulson A. R. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. U. S. A. 74:5463–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saxena R. S., Gupta A. 1984. Electrochemical studies on the composition, stability constants, and thermodynamics of Ti(I) complexes with dithiodipropionic acid. Monatsschr. Chem. 115:1293–1298 [Google Scholar]
- 41. Schlegel H. G., Kaltwasser G. H., Gottschalk G. 1961. A submersion method for culture of hydrogen-oxidizing bacteria: growth physiological studies. Arch. Mikrobiol. 38:209–222 [PubMed] [Google Scholar]
- 42. Shikata K., Fukui T., Atomi H., Imanaka T. 2007. A novel ADP-forming succinyl-CoA synthetase in Thermococcus kodakaraensis structurally related to the archaeal nucleoside diphosphate-forming acetyl-CoA synthetases. J. Biol. Chem. 282:26963–26970 [DOI] [PubMed] [Google Scholar]
- 43. Simon R., Priefer U., Pühler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Biotechnology 1:784–791 [Google Scholar]
- 44. Simon R. 1984. High-frequency mobilization of Gram-negative bacterial replicons by the in vitro constructed Tn5-mob transposon. Mol. Gen. Genet. 196:413–420 [DOI] [PubMed] [Google Scholar]
- 45. Slater S., et al. 1998. Multiple beta-ketothiolases mediate poly(beta-hydroxyalkanoate) copolymer synthesis in Ralstonia eutropha. J. Bacteriol. 180:1979–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Srivastava P. K., Field L. 1975. Organic disulfides and related substances. 38. Some disulfide and trisulfide sulfinate salts as antiradiation drugs. J. Med. Chem. 18:798–802 [DOI] [PubMed] [Google Scholar]
- 47. Stein S., Levitsky A., Fateev O., Mallard G. 1998. The NIST Mass Spectral Search Program, Windows software version 1.6d. National Institute of Standards and Technology, Gaithersburg, MD [Google Scholar]
- 48. Strandberg L., Enfors S. O. 1991. Factors influencing inclusion body formation in the production of a fused protein in Escherichia coli. Appl. Environ. Microbiol. 57:1669–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Studart-Guimarães C., et al. 2005. Identification and characterisation of the α and β subunits of succinyl CoA ligase of tomato. Plant Mol. Biol. 59:781–791 [DOI] [PubMed] [Google Scholar]
- 50. Studier F. W. 2005. Protein production by auto-induction in high-density shaking cultures. Prot. Expr. Purif. 41:207–235 [DOI] [PubMed] [Google Scholar]
- 51. Sweeney T. R. 1979. Survey of compounds from the Antiradiation Drug Development Program of the U.S. Army Medical Research and Development Command, p. 5, 672, 688, 689, 769, and 770 Walter Reed Army Institute of Research, Washington, DC [Google Scholar]
- 52. Tsutsumi H., Okada S., Oishi T. 1998. A potentially biodegradable polyamide containing disulfide bonds as a positive material for secondary batteries. Electrochim. Acta 43:427–429 [Google Scholar]
- 53. Tuan Y.-H., Phillips R. D. 1997. Optimized determination of cystine/cysteine and acid-stable amino acids from a single hydrolysate of casein- and sorghum-based diet and digesta samples. J. Agric. Food Chem. 45:3535–3540 [Google Scholar]
- 54. Veit A., Polen T., Wendisch V. F. 2007. Global gene expression analysis of glucose overflow metabolism in Escherichia coli and reduction of aerobic acetate formation. Appl. Microbiol. Biotechnol. 74:406–421 [DOI] [PubMed] [Google Scholar]
- 55. Wolodko W. T., Kay C. M., Bridger W. A. 1986. Active enzyme sedimentation, sedimentation velocity, and sedimentation equilibrium studies of succinyl-CoA synthetases of porcine heart and Escherichia coli. Biochemistry 25:5420–5425 [DOI] [PubMed] [Google Scholar]
- 56. Wübbeler J. H., Lütke-Eversloh T., Vandamme P., Van Trappen S., Steinbüchel A. 2006. Tetrathiobacter mimigardefordensis sp. nov., isolated from compost, a betaproteobacterium capable of utilizing the organic disulfide 3,3′-dithiodipropionic acid. Int. J. Syst. Evol. Microbiol. 56:1306–1310 [DOI] [PubMed] [Google Scholar]
- 57. Wübbeler J. H., Bruland N., Kretschmer K., Steinbüchel A. 2008. Novel pathway for catabolism of the organic sulfur compound 3,3′-dithiodipropionic acid via 3-mercaptopropionic acid and 3-sulfinopropionic acid to propionyl-coenzyme A by the aerobic bacterium Tetrathiobacter mimigardefordensis strain DPN7. Appl. Environ. Microbiol. 74:4028–4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wübbeler J. H., Raberg M., Brandt U., Steinbüchel A. 2010. Dihydrolipoamide dehydrogenases of Advenella mimigardefordensis and Ralstonia eutropha catalyze cleavage of 3,3′-dithiodipropionic acid into 3-mercaptopropionic acid. Appl. Environ. Microbiol. 76:7023–7028 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.