Abstract
In contrast to direct activation of platelet-derived growth factor (PDGF) receptor α (PDGFRα) via PDGF, indirect activation via growth factors outside the PDGF family failed to induce dimerization, internalization, and degradation of PDGFRα. Chronically activated, monomeric PDGFRα induced prolonged activation of Akt and suppressed the level of p53. These events were sufficient to promote both cellular responses (proliferation, survival, and contraction) that are intrinsic to proliferative vitreoretinopathy (PVR) and induce the disease itself. This signature signaling pathway appeared to extend beyond PVR since deregulating PDGFRα in ways that promote solid tumors also resulted in chronic activation of Akt and a decline in the level of p53.
INTRODUCTION
Proliferative vitreoretinopathy (PVR) is an example of a human disease for which current therapies are unsatisfactory. PVR occurs in 5 to 11% of patients that undergo retinal surgery to correct a detached retina (24). Repeat surgery is the only effective treatment option (11); however, the risk of a recurrent episode of PVR is substantial, and anatomical success is attained in only 60 to 80% of individuals who undergo this procedure (10, 46). A variety of pharmacological approaches have been tested, yet they have not proven effective (4, 57, 63). Thus, there is an acute need for new therapy options for individuals who are afflicted by this blinding disease. In the United States alone, between 1,687 and 3,712 patients succumb to PVR every year (23, 64).
A proven approach for developing new therapies is to identify molecular mediators of key events driving pathology. For instance, identification of proangiogenic factors such as vascular endothelial growth factor (VEGF) has led to development of anti-VEGF-based therapies, which have revolutionized treatment of patients with the neovascular form of age-related macular degeneration (21, 26, 53, 62). Similarly, inroads into our appreciation for how PVR develops have led to both gene therapy- and pharmacology-based approaches for preventing experimental PVR (30, 40, 66).
Mislocalization of cells into the vitreous, an event that occurs when the retina tears and/or detaches, is one of the events that contribute to the development of PVR (38). The vitreous contains many growth factors and cytokines (38), which promote cellular responses (survival, proliferation, extracellular matrix production, and contraction) that are intrinsic to the formation of an epiretinal membrane, its contraction, and its resultant retinal redetachment, i.e., PVR (38).
Platelet-derived growth factor receptor α (PDGFRα) is strongly implicated in both clinical and experimental PVR (38). It is present and activated in human PVR membranes (12, 13, 51). Expression of a functional PDGFRα profoundly increases the ability of cells to induce experimental PVR (3, 32). In light of the fact that vitreous from humans or animals that are experiencing PVR have high levels of PDGF isoforms that activate PDGFRα (36), we were surprised to find that neutralizing them did not effectively protect rabbits from developing PVR (41). Rather, PVR was being driven by indirect activation of PDGFRα, which occurred in response to vitreal growth factors outside the PDGF family (non-PDGFs) (41).
Direct activation of PDGFRα is a well-studied sequence of events in which PDGF assembles PDGFR monomers into a dimer and thereby robustly activates the receptor's kinase. PDGFRα activated in this way engages internalization and degradation of the receptor, which limits its output. Indirect activation of PDGFRα proceeds intracellularly. Non-PDGFs increase the level of reactive oxygen species (ROS), which activate Src family kinases (SFKs) that facilitate indirect activation of PDGFRα (37).
While indirect activation of growth factor receptors (also called transactivation) has been reported, and the mechanism by which indirect activation proceeds has been studied (14, 25, 28, 37, 44, 45, 50, 58, 60), little is known regarding how an indirectly activated receptor propagates intracellular signaling events (7). Since such signaling is tightly associated with pathology (41), identification of the key players and pathways was an opportunity to build the conceptual foundation necessary to develop effective therapeutic options for individuals afflicted by PVR.
MATERIALS AND METHODS
Major reagents.
Recombinant human PDGF-A was purchased from Peprotech, Inc. (Rocky Hill, NJ). Antibodies against PDGFRα, phospho-Akt (p-Akt) (S473), Akt, phospho-Erk, Erk, phospho-Akt substrate, and p53 (catalogue no. 2524) were purchased from Cell Signaling (Danvers, MA). Antibodies against phospho-Mdm2 (S166) and Mdm2 were from SAB Signalway (Pearland, TX) and ABGENT (San Diego, CA), respectively. The two antiphosphotyrosine antibodies, 4G10 and PY20, were purchased from Upstate (Lake Placid, NY) and BD Transduction Laboratories (Madison, WI), respectively. Phospho-PDGFRα (Y742) was described previously (40). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG and goat anti-mouse IgG secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Enhanced chemiluminescence substrate for detection of HRP was from Pierce Protein Research Products (Rockford, IL). The cell surface protein isolation kit (EZ-link sulfo-NHS-SS-biotin) and BS3 (C16H18N2Na2O14S2) cross-linker were purchased from Pierce. Cycloheximide was purchased from Sigma (Saint Louis, Missouri).
Cell culture.
Primary mouse embryo fibroblasts (MEF) (wild-type [WT] p53+/+ or mutant p53−/−) were generous gifts from Tyler Jacks (David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA).
F, Fα, FαΔx, Fβ, and R627 cells were previously described (3, 37, 54). Briefly, they are mouse embryo fibroblasts derived from mice null for both pdgfr genes. They were immortalized with simian virus 40 (SV40) T antigen. Fα, R627, or FαΔx cells are F cells in which we reexpressed human PDGFRα that was the full-length receptor, the full-length kinase-inactive mutant, or the mutant lacking the majority of the extracellular domain, respectively.
Primary rabbit conjunctiva fibroblasts (RCF) were isolated from rabbit conjunctiva as previously described (47). MEF, F, Fα, FαΔx, and RCF cells were maintained in Dulbecco's modified Eagle's medium (DMEM; high glucose; Gibco-BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; Gemini Bio Products, Calabasas, CA), 500 U/ml of penicillin, and 500 μg/ml of streptomycin. 293GPG cells (49) were cultured in DMEM supplemented with 10% FBS, 2 mM l-glutamine, 1 μg/ml tetracycline (Sigma), 2 μg/ml puromycin (Sigma), 0.3 mg/ml G418 (Sigma), 16.7 mM HEPES (Invitrogen). The medium used during virus collection from 293GPG cells was DMEM supplemented with 10% FBS, 2 mM l-glutamine, 16.7 mM HEPES. All cells were cultured at 37°C in a humidified 5% CO2 atmosphere.
Overexpression of PDGFRα in MEF.
Retroviral constructs (pLHDCX3 and pLHDCX3-PDGFRα) were transfected into 293GPG cells by use of Lipofectamine plus (Invitrogen). Virus-containing medium was collected for 5 days and then concentrated (25, 000 × g for 90 min at 4°C). MEF cells were infected by incubating cells with the concentrated retrovirus in DMEM supplemented with 10% FBS and 8 μg/ml Polybrene (hexadimethrine bromide) (Sigma) for 24 h. Successfully infected cells were selected in histidine-free DMEM supplemented with 5 mM histidinol (Sigma). The level of PDGFRα in the resulting cells was determined by Western blotting using an anti-PDGFRα antibody.
Mutagenesis of PDGFRα and expression in cells.
PDGFRαΔxM was PDGFRαΔx (41) in which both Tyr 731 and Tyr 742 were changed to Phe in accordance with instructions provided with the QuikChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). The mutagenic primers (positions 2329 to 2384) were 5′-CAATGGTGACTTCATGGACATGAAGCAGGCTGATACTACACAGTTTGTCCCCATGC-3′ and its complement. The V842 mutant (Asp at 842 changed to Val) was made using the same strategy, and the mutagenic primers (positions 2660 to 2689) were 5′-GCCTGGCCAGTCATCATGCATGATTCG-3′ and its complement. The sequencing primers to confirm the point mutations were (for positions 2120 to 2139) 5′-GTAAACTTGCTGGGAGCCTG-3′ and (for positions 2491 to 2515) 5′-CCTTTCAGATGATAACTCAGAAGGC-3′, respectively. The primers were synthesized by the DNA core facility of Massachusetts General Hospital (MGH) (Boston, MA). The PDGFRα mutants were subcloned into the pLHDCX3 retroviral vector. This resulting constructs were transfected into 293GPG cells (49), and the resulting virus was used to infect either F or MEF cells. The successfully infected cells were selected for their ability to proliferate in histidine-free medium containing histidinol (5 mM). Expression of the PDGFRα mutants was determined by Western blotting using an anti-PDGFRα antibody.
Suppression of PDGFRα expression in MEF.
An oligonucleotide (CCTGGAGAAGTGAGAAACAAA) corresponding to NM_011058.1-937 in a hairpin-pLKO.1 lentiviral vector, the packaging plasmid (pCMV-dR8.91), the envelope plasmid (VSV-G/pMD2.G), and 293T packaging cells were from Dana-Farber Cancer Institute/Harvard Medical School (Boston, MA).
To prepare PDGFRα short hairpin RNA (shRNA) lentivirus, a mixture of packaging plasmid (900 ng), envelope plasmid (100 ng), hairpin-pLKO.1 vector (1,000 ng) (or a hairpin-pLKO.1 vector containing the PDGFRα shRNA oligonucleotide), and TransIT-LT1 were mixed and incubated at room temperature for 30 min. The transfection mixture was transferred to 293T cells that were approximately 70% confluent. After 18 h, the medium was replaced with growth medium modified to contain 30% serum, and virus was harvested at 40 h posttransfection. The viral harvest was repeated at 24-h intervals 3 times. The virus-containing medium was pooled and centrifuged at 800 × g for 5 min, and the supernatant was used to infect MEF. The successfully infected cells were selected for the ability to proliferate in medium containing puromycin (1 μg/ml). The resulting cells were characterized by Western blot analysis using an antibody against PDGFRα.
Immunoprecipitation and Western blot analysis.
Cells (90% confluence) were cultured in DMEM without serum for 24 h and then treated with PDGF-A (50 ng/ml) or normal rabbit vitreous (RV) (in which PDGF is undetectable by enzyme-linked immunosorbent assay [ELISA] [41]) for 10 min. The cells were washed twice with ice-cold phosphate-buffered saline (PBS) and then lysed in extraction buffer (EB; 10 mM Tris-HCl, pH 7.4, 5 mM EDTA, 50 mM NaCl, 50 mM NaF, 1% Triton X-100, 20 μg/ml aprotinin, 2 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride). Lysates were clarified by centrifugation at 13,000 × g at 4°C for 15 min, and PDGFRα was immunoprecipitated from the lysates as previously described (37). The antibodies for immunoprecipitation and Western blot analysis were a crude rabbit polyclonal anti-PDGFRα antibody (27P) and a 1:1 mixture of antiphosphotyrosine antibodies (4G10/PY20), respectively. The primary blotted membrane was stripped and reprobed with an anti-PDGFRα antibody.
Collagen gel contraction assay.
The gel contraction assay was performed as previously described (22, 31, 41). Briefly, cells were suspended in 1.5 mg/ml of neutralized collagen I (Inamed, Fremont, CA) (pH 7.2) at a density of 106 cells/ml and transferred into a 24-well plate (Falcon, Franklin Lakes, NJ) that had been preincubated with PBS-5 mg/ml bovine serum albumin (BSA) overnight. The gel was solidified by incubation at 37°C for 90 min and then overlaid with 0.5 ml DMEM or 1:1 DMEM-RV. The gel diameter was measured on days 1, 2, 3 and 4 and was initially 15 mm. The area was calculated using the formula 3.14 × (diameter/2)2. Each experimental condition was assayed in duplicate.
Cell proliferation assay.
Cells were seeded into 24-well plates at a density of 30,000 cells/well in DMEM-10% FBS. After the cells had attached (approximately 8 h), the medium was aspirated and the cells were rinsed twice with PBS and cultured in serum-free DMEM or DMEM-RV (1:1). The cells were counted in a hemocytometer on days 1, 2, 3 and 4; at least three independent experiments were performed.
Apoptosis assay.
Cells were seeded into 60 mm-dishes at a density of 1 ×105 cells per dish in DMEM-10% FBS. After the cells had attached, they were treated as described for the proliferation assay. On day 3, the cells were harvested and stained with fluorescein isothiocyanate (FITC)-conjugated annexin V and propidium iodide according to the instructions provided with the apoptosis kit (BD Biosciences, Palo Alto, CA). The cells were analyzed by flow cytometry in a Beckman Coulter XL instrument (39). At least three independent experiments were performed.
p53 reporter assay.
Oligonucleotides containing the p53 binding element (reporter) (34) 5′-CACGTTTGCCTTGCCTGGACTTGCCTGGCCTTGCCTTGGACATGCCCGGGCTGTCAGATCTGGGTATATAATGGA-3′ and its mutant 5′-CACGTTTTAATTTAATTTAATTTAATTTAATTTAATTGGACATTAACGGGCTGTCAGATCTGGGTATATAATGGA-3′ were synthesized by the DNA core facility of MGH and subcloned into the pGL3-basic vector encoding firefly luciferase (Promega, Madison, WI) by restriction endonucleases KpnI/HindIII. The resultant vectors pGL3-p53 wt and pGL3-p53 mt were confirmed by DNA. MEF and MEF p53−/− (negative controls) were cultured in 96-well plates to 90% confluence, and transfected with pGL3-p53 wt or pGL3-p53 mt plus pRL-RK encoding Renilla luciferase in accordance with the manufacturer's instructions (dual-luciferase reporter assay system; Promega).
At 24 h posttransfection, the cells were treated with PDGF-A (50 ng/ml) or RV (1:1 dilution in PBS). Two hours later, the cells were washed in PBS and lysed in passive lysis buffer (Promega) by gentle rocking for 15 min. The lysates were mixed with luciferase assay reagent II (LAR II; Promega) in a luminometer tube, and firefly luciferase activity was recorded. Stop & Glo reagent (Promega) was subsequently added, and Renilla luciferase activity was determined.
Half-life-of-PDGFRα assay.
Fα cells that reached 80% confluence in 24-well plates were starved for 24 h, pretreated with cycloheximide (1 mM) for 30 min, and then treated with PDGF-A (50 ng/ml) or RV (1:1 in DMEM) for 5, 10, 30, and 120 min. The cells were washed twice with PBS and lysed in SDS-PAGE sample buffer. Equal volumes of the lysates were subjected to SDS-PAGE and Western blot analysis using antibodies against PDGFRα. To independently assess the level of loading among samples, the membrane was subsequently stripped and reprobed with an anti-RasGAP antibody.
Internalization assay.
Fα cells were grown to 90% confluence, starved for 24 h, pretreated with cycloheximide (1 mM) for 30 min, and then treated with either PDGF-A (50 ng/ml) for 10, 30, and 60 min or RV (1:1 in DMEM) for 30 and 60 min. Cell surface proteins were isolated in accordance with the instructions provided with the Pierce cell surface protein isolation kit (Pierce). Briefly, cells were washed twice with PBS, and sulfo-NH-SS-biotin (0.25 mg/ml in PBS) was added. After the dishes were rocked for 30 min at 4°C, quenching solution was added, the cells were washed twice with PBS, extraction buffer (EB) was added, and the dishes were rocked at 4°C for 30 min. The lysates were centrifuged for 15 min at 13, 000 × g at 4°C, and the supernatants were added to EB-washed NeutrAvidin agarose and incubated for 60 min at room temperature with end-over-end mixing. The NeutrAvidin agarose was washed 5 times with radioimmunoprecipitation assay (RIPA) buffer, and the proteins that remained bound were eluted in SDS sample buffer and subjected to Western blot analysis with antibodies against PDGFRα and Axl (as a loading control).
Dimerization assay.
Fα cells that reached 90% confluence in 6-well plates were starved for 24 h and treated with PDGF-A for 1 and 3 min or RV for 3 and 60 min. Subsequently, the cells were washed with ice-cold PBS and incubated with BS3 solution (2 mM) for 30 min on ice with gentle shaking. The cross-linking reaction was terminated by adding Tris (20 mM) for 5 min at room temperature. The cell lysates were harvested in EB buffer, and the clarified lysates were subjected to Western blot analysis using an anti-PDGFRα antibody.
Rabbit model for PVR and preparation of rabbit vitreous.
Pigmented rabbits were purchased from Covance (Denver, PA). PVR was induced in the right rabbit eye as previously described (3, 47). Briefly, a gas vitrectomy was performed by injecting 0.1 ml of perfluoropropane (C3F8) (Alcon, Fort Worth, TX) into the vitreous cavity 4 mm posterior to the corneal limbus. One week later, all rabbits received two injections: (i) 0.1 ml of platelet-rich plasma (PRP) and (ii) 0.1 ml DMEM containing 2 × 105 of the desired cells. The retinal status was evaluated with an indirect ophthalmoscope fitted with a +30 D fundus lens on days 1, 3, 5, 7, 14, 21, and 28 after surgery. PVR was graded according to the Fastenberg classification from 0 through 5 (15). On day 28, the animals were sacrificed, and the eyes were enucleated and frozen at −80°C. All surgeries were performed under aseptic conditions and pursuant to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The protocol for the use of animals was approved by the Schepens Animal Care and Use Committee.
To prepare the rabbit vitreous, the vitreous was dissected from the eyeball while it was still frozen, permitted to thaw, and then centrifuged at 4°C for 5 min at 10,000 × g. The resulting supernatant was used for all analyses.
Statistics.
The results from the rabbit studies were subjected to Mann-Whitney analysis, whereas all other data were analyzed using the unpaired t test. P values of less than 0.05 were considered statistically significant.
RESULTS
Unlike direct activation of PDGFRα via PDGF, indirect activation via vitreous did not promote dimerization, internalization, and degradation of PDGFRα.
In addition to PDGFs, non-PDGFs activate PDGFRs. Unlike PDGFs, which activate PDGFRs directly, non-PDGFs do so indirectly. Our previously published studies indicate that these events proceed intracellularly and require elevation of reactive oxygen species (ROS) and activation of Src family kinases (SFKs) (37). Indirect activation of PDGFRα is sufficient to trigger diseases such as PVR (41), and therefore, investigating how indirectly activated PDGFRs signal represents an opportunity to understand the basis of diseases that afflict humans and are difficult to treat effectively (11). More specifically, the goal of this project was to identify signaling events that were induced by indirectly activated PDGFRα and required for experimental PVR.
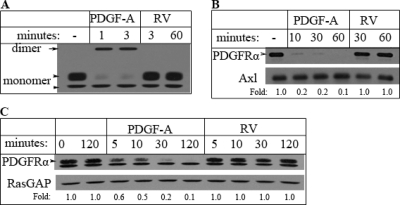
Direct activation of PDGFRα (via PDGF-A) induced rapid dimerization (Fig. 1 A), internalization (Fig. 1B), and subsequent degradation of PDGFRα (Fig. 1C). Direct activation of PDGFRα reduced its half-life from more than 120 min in unstimulated cells to approximately 10 min (Fig. 1C). These observations are consistent with previous reports (6, 55, 59). In contrast, indirect activation of PDGFRα (via rabbit vitreous [RV]) did not induce detectable dimerization (Fig. 1A), internalization (Fig. 1B), or degradation (Fig. 1C) of PDGFRα. Within the 120-min time course of these experiments, there was no difference in the amount of PDGFRα present in resting versus the amount in RV-stimulated cells. RV is vitreous harvested from healthy rabbits; it contains numerous non-PDGFs but no detectable PDGFs (36). One may be inclined to posit that RV failed to engage PDGFRα trafficking because it did not activate PDGFRα; however, the additional experiments that are discussed below reveal that this was not the case.
Fig. 1.
Indirect activation of PDGFRα failed to promote its dimerization, internalization, or degradation. (A) Receptor dimerization. Fα cells (immortalized MEF derived from embryos null for both pdgfr genes that were modified to reexpress PDGFRα [3]) were plated and cultured until they reached approximately 80% confluence, whereupon they were starved in serum-free DMEM for 24 h and pretreated with cycloheximide (1 mM) for 30 min. Subsequently, these cells were treated with PDGF-A or vitreous from healthy rabbits (RV) for the indicated times; while RV contains many growth factors outside the PDGF family, there are no detectable PDGFs (36). As expected, PDGF-A facilitated dimerization of PDGFRs. In contrast, RV did not. The arrow points to the dimeric form of PDGFRα, whereas the arrowheads indicate the positions of mature (170 kDa; top arrowhead) and immature (160 kDa; bottom arrowhead) PDGFRα that is monomeric. (B) Receptor internalization. Fα cells were treated as described for panel A, then washed twice with ice-cold PBS, and incubated with sulfo-NHS-SS-biotin for 1 h. The biotinylation reaction was quenched, and cells were washed twice with ice-cold PBS and lysed. NeutrAvidin agarose was added to the clarified lysates, and the mixture was gently rotated for 1 h at room temperature. The NeutrAvidin agarose beads were washed 5 times with RIPA buffer, and proteins that were retained were eluted with sample buffer and subjected to Western blot analysis using the indicated antibodies. The level of Axl was monitored as a loading control. PDGF-A promoted the disappearance of PDGFRα from the cell surface, whereas RV did not. The arrowhead points to the mature (170-kDa) form of PDGFRα. (C) Receptor degradation. Fα cells were treated as described for panel A for the indicated times. Total cell lysates were subjected to Western blot analysis using the indicated antibodies. The RasGAP blot served as a loading control. PDGF-A promoted degradation of PDGFRα, whereas RV did not. The arrowhead points to the mature (170-kDa) form of PDGFRα. The band below the mature form of PDGFRα is the immature species that is insensitive to PDGF-induced degradation since it is not on the cell surface. In all 3 panels of this figure, the data presented are representative of at least three independent experiments.
We conclude that the mechanism by which PDGFRα is activated profoundly influences its fate. Unlike direct activation of PDGFRα, indirect activation did not trigger clearing of the receptor from the cell surface and subsequent degradation. Since location and duration are important determinants of signaling outcome, we proceeded by comparing downstream signaling events triggered by directly and indirectly activated PDGFRα.
Indirect activation of PDGFRα selectively prolonged activation of Akt.
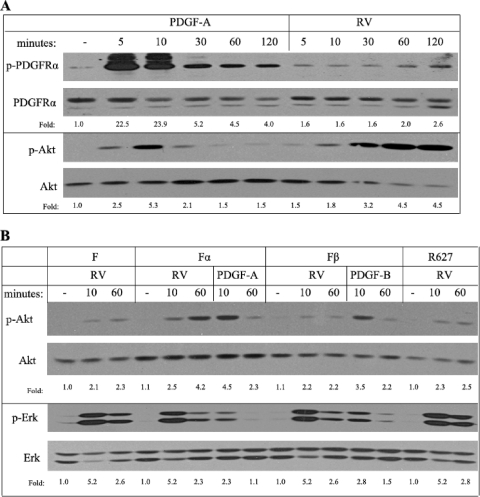
PDGF triggered robust tyrosine phosphorylation of PDGFRα that peaked within 10 min and then declined substantially (Fig. 2 A). In contrast, this response was mild and sustained in RV-treated cells (Fig. 2A). The results were comparable when PDGFRα phosphorylation was monitored in total cell lysates with a phospho-PDGFRα antibody (as was done for Fig. 2A) or with an anti-phosphotyrosine antibody on PDGFRα immunoprecipitates (data not shown). Despite the roughly 10-fold difference in PDGFR tyrosine phosphorylation, both PDGF and RV provoked comparable magnitudes of Akt phosphorylation, yet this event occurred at different times and for unequal durations (Fig. 2A). In response to PDGF-A, Akt phosphorylation peaked at 10 min and subsequently declined to near-basal levels, whereas in RV-treated cells, Akt activation peaked at 60 min and persisted to the 120-min time point. Thus, while PDGF-A and RV both activated PDGFRα and Akt, there were profound differences in the amplitude and/or kinetics.
Fig. 2.
Akt activation was prolonged in response to indirect activation of PDGFRα. (A) Time course of RV- and PDGF-induced signaling events. Fα cells were treated as described for Fig. 1A for the indicated times, the cells were lysed, and total lysates were subjected to immunoblotting using the indicated antibodies; p-PDGFRα recognized pY742, and p-Akt recognized pS473. Both types of activators promoted phosphorylation of PDGFRα and Akt, yet with dissimilar amplitudes and kinetics. (B) Comparison of PDGFRs for their ability to drive sustained Akt activation. F (immortalized MEF from embryos null for both PDGFR genes), Fα, Fβ (F cells modified to reexpress PDGFRβ [3]), and R627 (same as Fα, except that the receptor is a kinase-inactive mutant [54]) were treated as described for Fig. 1A for the indicated times. Clarified lysates were subjected to Western blot analysis using the indicated antibodies; p-Erk recognized pT202/Y204. Comparison of F and Fα cells indicates that expression of PDGFRα selectively enhanced prolonged activation of Akt in response to RV. Unlike WT PDGFRα, neither kinase-inactive PDGFRα (R627) nor WT PDGFRβ was able to boost Akt activity at the 60-min time point. PDGFRβ, WT PDGFRα, and R627 PDGFRα were expressed to the level routinely observed in fibroblasts (approximately 1 × 105 receptors/cell) (3, 9, 54). In both panels of this figure, the data presented are representative of at least three independent experiments.
Since RV contains many growth factors and cytokines for which fibroblasts typically express receptors, it is likely that at least some of the RV-induced signaling events were independent of PDGFRα. To address this issue, we compared RV-triggered signaling levels in a matched set of cell lines that differed only for expression of PDGFRα. F cells are immortalized mouse embryo fibroblasts (MEF) derived from embryos null for both pdgfr genes, whereas Fα cells were generated by reexpressing PDGFRα in F cells (3). Consistent with a previous report that expression of PDGFRα potentiated the ability of non-PDGFs to acutely activate Akt (65), RV-dependent activation of Akt was greater in Fα cells than in F cells. This difference was more pronounced at the 60-min time point (Fig. 2B). The RV-induced Erk responses were comparable in both cell lines (Fig. 2B), which indicated that the presence of PDGFRα did not augment Erk activation as it did with Akt. We conclude that the presence of PDGFRα selectively altered signaling events induced by RV; while there was no effect on Erk, expression of PDGFRα increased Akt activation, especially at the latter time points.
Phosphatidylinositol 3-kinase (PI3K)/Akt were essential effectors of indirectly activated PDGFRα.
Since engaging PDGFRα indirectly activated Akt activation (Fig. 2) and induced experimental PVR (41), we considered whether these two events were causally related. As a first step to evaluate this possibility, we assessed Akt activation in cells expressing PDGFRβ or kinase-inactive PDGFRα (R627), which induces PVR poorly (3, 33). RV-stimulated Akt activation in these two cell lines was not substantially greater than that in F cells (Fig. 2B). These observations indicate the only PDGFRα augmented responsiveness to RV and established a good correlation between prolonged activation of Akt and induction of PVR.
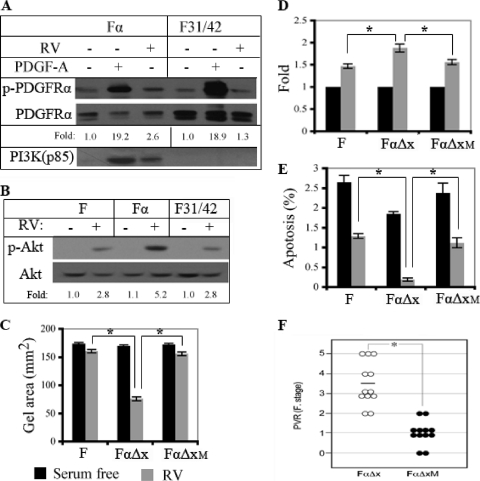
To test whether PI3K/Akt activation was an essential signaling event for cellular responses induced by indirectly activated PDGFRα, we generated a PDGFRα mutant that was unable to activate PI3K/Akt in response to indirect activation of PDGFRα. Mutating tyrosine phosphorylation sites that are required for PI3K/Akt activation in response to direct activation (Tyr 731 and 742) (54) prevented vitreous-dependent association of PI3K with PDGFRα (Fig. 3 A) and reduced Akt activation to the level observed in cells that express no PDGFRs (Fig. 3B).
Fig. 3.
PI3K/Akt was an essential effector of indirectly activated PDGFRα. (A, B) Tyr 731 and 742 were required for PI3K/Akt activation in response to indirect activation of PDGFRα. For panel A, Fα and F31/42 cells (same as Fα except that the PDGFRα has a Tyr-to-Phe substitution at residues 731 and 742 [54]) were cultured to approximately 80% confluence, serum starved for 24 h, and treated with PDGF-A or RV for 10 min, and the clarified lysates were immunoprecipitated with an antibody against PDGFRα. The resulting samples were subjected to Western blot analysis using antibodies against phosphotyrosine (top panel) or PI3K (p85) (bottom panel). The membrane was stripped and reprobed using an antibody against PDGFRα (middle panel). Three independent experiments showed that RV stimulated PI3K binding to WT PDGFRα but not the F31/42 mutant. For panel B, the indicated cells were serum starved for 24 h, treated with RV for 1 h, and lysed. Clarified lysates were subjected to Western blot analysis using the indicated antibodies. In three independent experiments, the levels of RV-induced activation of Akt were 2.53- ± 0.25-fold, 4.73- ± 0.50-fold, and 2.57- ± 0.25-fold for F, Fα, and F31/42 cells, respectively. There were statistically significant differences in extent of Akt activation between F and Fα (P = 0.0025) and between Fα and F31/42 (P = 0.03) but not between F and F31/42 (P = 0.875). Stated a different way, we consistently observed the same level of Akt activation in F and F31/42 cells, which was significantly lower than that observed in Fα cells. (C) Cell contraction assay. F, FαΔx (same as Fα, except that the extracellular domain is absent), or FαΔxM (same as FαΔx except that Tyr 731 and 742 are mutated to Phe) cells were embedded in a collagen matrix at a density of 106 cells/ml and cultured in serum-free medium that was (gray bars) or was not (black bars) supplemented with RV. The diameter of the collagen gel was measured on day 3. The means ± standard deviations of results from three independent experiments are shown; an asterisk indicates P values of <0.05 as determined using an unpaired t test. While FαΔx cells responded to RV robustly, the other two cell types did not. (D) Cell proliferation assay. The indicated cell types were seeded at a low cell density in serum-free medium that was (gray bars) or was not (black bars) supplemented with RV. After 3 days, the cells were counted and the data from 3 independent experiments are presented as means ± standard deviations for the ratio of cell proliferation in RV/cell proliferation in serum-free medium (fold increase); an asterisk denotes P values of <0.05 as determined using an unpaired t test. Compared with the response to RV, PDGF-A induced a 2.2- ± 0.3-fold increase in the cell number (37). While RV promoted proliferation of all cell lines, the response was enhanced in FαΔx but not in the FαΔxM cells. (E) Apoptosis assay. Same as for panel D, except on day 3 apoptosis was measured by fluorescence-activated cell sorting (FACS) analysis of cells that were stained with FITC-conjugated annexin V and propidium iodide. Cells in early apoptosis were defined as the population that was positive for annexin V but negative for propidium iodide. The bars indicate the percentages of apoptotic cells. The means ± standard deviations of results from three independent experiments are shown; an asterisk denotes P values of <0.05 as determined using an unpaired t test. A representative set of raw data is available on request. While expression of the αΔx PDGFRα reduced the number of apoptotic cells, the mutant receptor was unable to protect cells. (F) Assessment of the PVR potential in a rabbit model of PVR. PVR was induced in rabbits by injecting FαΔx or FαΔxM cells along with platelet-rich plasma as described in Materials and Methods. Each symbol represents the clinical score of an individual rabbit; the data presented are for the day 28 time point; the data for the earlier points are available on request. The Fastenberg grading system was used, with the stages defined as follows: stage 0, no disease; stage 1, membrane formation; stage 2, vitreoretinal traction, with no retinal detachment; stage 3, retinal detachment, up to 2 quadrants; stage 4, retinal detachment, with more than 2 quadrants but not complete detachment; stage 5, complete retinal detachment (15). Mann-Whitney analysis of the data indicated a statistically significant difference between the two groups. The results indicate that PI3K activation was essential for induction of PVR via PDGFRα, which can be activated only by the indirect route.
Since fibroblasts synthesize and secrete PDGF-C (17, 19, 36, 43), it was important to modify PDGFRα to prevent it from being activated directly by endogenously produced PDGF-C in these cell-based assays. To this end, we employed a PDGFRα mutant that was missing the extracellular domain (αΔX); while αΔX cannot be activated directly, it is still activatable by the indirect route (41). We also included cells that expressed no PDGFRs (to gauge the extent of RV-induced responses that were PDGFRα independent) and cells expressing an αΔX mutant (FαΔXM) that was unable to activate PI3K/Akt. Only in cells expressing PDGFRα capable of activating PI3K/Akt did we observe an RV-induced boost in 3 cellular responses that are relevant to PVR (contraction, proliferation, and protection from apoptosis) (Fig. 3C to E).
Finally, we assessed whether PI3K/Akt was an essential effector of indirectly activated PDGFRα in the context of experimental PVR. The most commonly used model of experimental PVR involves injection of cells into the vitreous of rabbits (1). The rabbits are monitored for the formation of a membrane, which contracts and thereby causes a retinal detachment. As shown in Fig. 3F, the PVR potential of FαΔXM was significantly reduced compared with that of FαΔX. A statistically significant difference between the two groups first appeared on day 3 and persisted to the end of the experiment (day 28) (data available on request). None of the rabbits injected with the FαΔXM cells progressed to stage 3 or higher (Fig. 3F), i.e., none of them developed retinal detachment, which is the most sight-threatening and clinically relevant phase of the disease.
Taken together, these findings indicate that PI3K/Akt was a key effector of indirectly activated PDGFRα. This observation is consistent with our previous finding that PI3K was essential for experimental PVR (33). The present findings extend our previous ones by addressing the question of PI3K's importance when the receptor is activated indirectly; the previous studies were done with cells expressing full-length PDGFRα, and as a result, it was not possible to distinguish whether PDGFRα was being activated directly or indirectly.
Indirectly activated PDGFRα suppressed p53 expression and function.
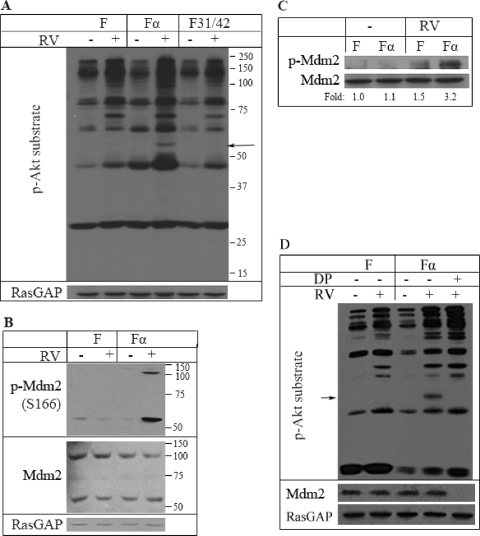
To identify downstream effectors of Akt in cells that underwent indirect activation of PDGFRα, we probed total cell lysates of RV-treated F, Fα, or F31/42 cells with a phospho-Akt substrate antibody. The 55-kDa band was one of the RV-induced species that increased in Fα cells more than in the two control cell lines (Fig. 4 A). Scan site analysis indicated that Mdm2 (murine double minute) was one of the “hits” that had both a consensus Akt phosphorylation site (boldface) (RKRRRSLSFDPSLGL) and a predicted molecular mass of approximately 55 kDa. Additional support for the possibility that Mdm2 was the 55-kDa species was provided by a previous report that Akt phosphorylates Mdm2 (at S166) (67). To consider if RV promoted phosphorylation of Mdm2, we probed cell lysates prepared from resting or RV-treated F and Fα cells with phospho- and pan-Mdm2 antibodies. In Fα but not F cells, RV strongly increased phosphorylation of the 55-kDa species and the more commonly reported 110-kDa form of Mdm2 (Fig. 4B). Furthermore, similar results were observed when the experiment was repeated with Mdm2 immunoprecipitates instead of total cell lysates (Fig. 4C). Finally, the 55-kDa species was not detected in a phospho-Akt substrate Western blot analysis of lysates that had been immunodepleted of Mdm2 (Fig. 4D). These experiments indicate that indirect activation of PDGFRα increased phosphorylation of Mdm2.
Fig. 4.
Mdm2 was a key target of indirectly activated PDGFRα. (A) Phospho-Akt substrate blotting. The indicated cell types were treated with RV for 1 h and lysed, and the clarified lysates were subjected to Western blot analysis using an antibody that recognized proteins phosphorylated at the consensus Akt site ([R/K]X[R/K]XX[T/S]). The membrane was stripped and reprobed with an anti-RasGAP antibody. The 55-kDa species is an RV-inducible species that was consistently stronger in Fα cells. (B) Phospho-Mdm2 blotting. Same as for panel A except that a phospho-Mdm2 (pS166) antibody was used. The blot was then stripped and reprobed with an anti-Mdm2 antibody and then an anti-RasGAP antibody. RV induced Mdm2 phosphorylation in Fα cells but not in F cells. (C) Mdm2 immunoprecipitates. The lysates in panel A were immunoprecipitated with an anti-Mdm2 antibody, and the resulting samples were probed with the phospho-Akt substrate antibody. The membrane was subsequently stripped and reprobed with an anti-Mdm2 antibody (bottom panel). In three independent experiments, RV induced a 2.0- ± 0.2-fold-greater increase in phosphorylation of Mdm2 in Fα cells than in F cells. (D) The 55-kDa species was not detectable in lysates immunodepleted for Mdm2. Same as for panel A, except that some of the lysates were subjected to Mdm2 immunodepletion (DP), which involved 3 successive rounds of immunoprecipitation with an anti-Mdm2 antibody prior to analysis.
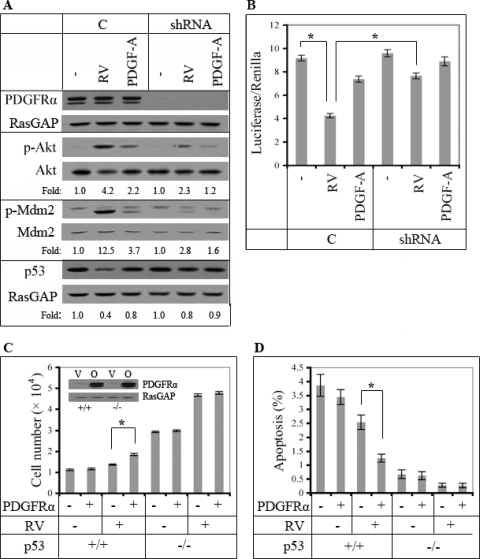
Mdm2 suppresses the level of p53, and Akt-dependent phosphorylation of Mdm2 potentiates this phenomenon (5, 16, 20, 48, 67). Consequently, we considered whether suppression of p53 was a key step in the mechanism by which RV promoted PDGFRα-dependent proliferation and survival (Fig. 3). To study p53, we switched from SV40-immortalized MEF (in which p53 function may be compromised) to primary MEF isolated from wild-type or p53-null embryos. RV increased Mdm2 phosphorylation and decreased the level of p53 in primary MEF (Fig. 5 A), and this phenomenon was dependent on expression of PDGFRα (Fig. 5A). The change in the level of p53 was associated with the expected changes in p53 function (Fig. 5B). Additional experiments with p53 promoter mutants indicated that the transcriptional activity monitored in these experiments was dependent on a classical p53 binding element in the promoter region (data not shown).
Fig. 5.
Indirectly activated PDGFRα enhanced proliferation and survival by reducing p53 expression and function. (A) Importance of PDGFRα expression for RV-dependent reduction of p53 in primary MEF. Lentiviruses were used to stably express shRNAs directed against green fluorescent protein (GFP) (C) or PDGFRα (shRNA) in primary MEF. The resulting cells were either left untreated or exposed to PDGF-A or RV. Duplicate plates were harvested at the 1- or 2-h time point, and the clarified lysates were subjected to Western blot analysis using the indicated antibodies. All of the data presented are from the 1-h time point, except those in the bottom panel, since a greater change in p53 is typically observed at the 2-h time point. The data indicate that RV was better than PDGF in activating Akt, inducing phosphorylation of Mdm2 and suppressing the level of p53. The stark advantage of RV over PDGF was much less apparent (especially for reducing p53) in cells in which PDGFRα expression was suppressed. The data in this panel are representative of three independent experiments. In this series of experiments, RV and PDGF-A induced the following changes in Akt activation: 4.50- ± 0.30-fold and 2.40- ± 0.17-fold in “C” cells and 2.43- ± 0.15-fold and 1.27- ± 0.06-fold in “shRNA” cells. The difference in levels of RV-induced activation of Akt in the “C” and “shRNA” cells was statistically significant (P = 0.004). For p53, the RV- and PDGF-A-induced declines were 0.40- ± 0.10-fold and 0.83- ± 0.06-fold in “C” cells and 0.77- ± 0.06-fold and 0.83- ± 0.10-fold in “shRNA” cells. The difference in levels of RV-induced suppression of p53 in the “C” and “shRNA” cells was statistically significant (P = 0.005). (B) Assay of p53-dependent transcriptional activity. The cells described for panel A were cotransfected with plasmids pGL3-p53 (a p53 activity reporter) and pRL-TK (to control for the efficiency of transfection). After 24 h, the cells were stimulated with PDGF-A or RV for 2 h, and then the activity of the reporter constructs was monitored using a dual-luciferase reporter assay system. In three independent experiments, reduced expression of PDGFRα compromised the ability of RV to attenuate p53-dependent transcription. (C) RV enhanced proliferation only in p53-expressing cells. PDGFRα was stably overexpressed in primary MEF derived from either WT (+/+) or p53-null (−/−) embryos. The resulting cells were monitored for RV-dependent cell proliferation as described for Fig. 3D. The means ± standard deviations of results from three independent experiments are shown; an asterisk denotes P values of <0.05 as determined using an unpaired t test. The proliferative advantage of PDGFRα-overexpressing cells observed in the presence of RV was dependent on expression of p53. (D) RV protected against apoptosis only in p53-expressing cells. The cells described for panel C were subjected to the same type of apoptosis assay used for Fig. 3E. The means ± standard deviations of results from three independent experiments are shown; an asterisk denotes P values of <0.05 as determined using an unpaired t test. The greater ability of RV to protect PDGFRα-overexpressing cells from apoptosis was observed only in p53-expressing cells.
We also assessed the importance of p53 in several cellular responses. Increasing expression of PDGFRα promoted RV-dependent proliferation and reduced apoptosis in cells that expressed p53, whereas in p53-null cells, the increased expression of PDGFRα was no longer advantageous (Fig. 5C and D). Taken together, these studies reveal that indirect activation of PDGFRα reduced the amount of p53 and that this event was required for the RV-dependent boost of cellular events intrinsic to PVR.
Prolonged activation of Akt, a signature signaling event of indirectly activated PDGFRα, was associated with a decline in the level of p53.
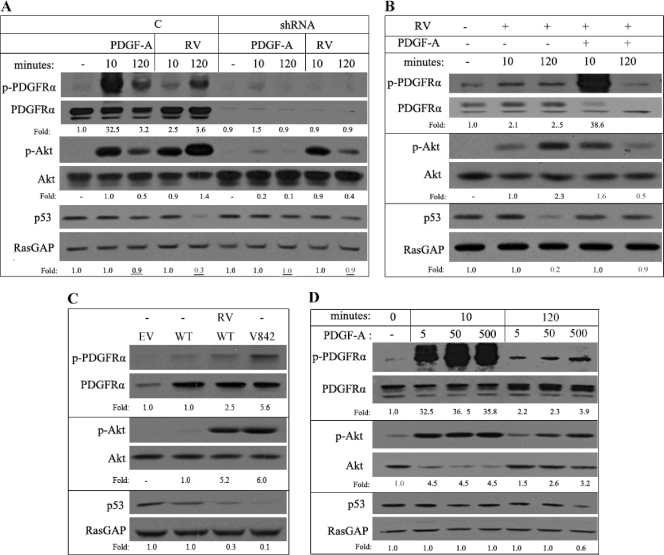
Compared with PDGF, RV triggered a 3-fold-greater decline in the level of p53 (Fig. 6 A). The enhanced ability of RV to suppress the level of p53 was dependent on PDGFRα: in primary MEF that expressed a reduced level of PDGFRα, RV ineffectively reduced the level of p53 (Fig. 6A). We conclude that indirect activation of PDGFRα more effectively reduced the level of p53 than direct activation of PDGFRα.
Fig. 6.
Sustained activation of Akt was associated with a decline in the level of p53. (A) Comparison of PDGF and RV for their ability to reduce the level of p53. The cells described for Fig. 5A were serum starved for 24 h, treated with PDGF-A or RV for the indicated times, and lysed, and clarified lysates were subjected to Western blot analysis using the indicated antibodies. The data presented are representative of 3 independent experiments, which all showed that RV was better than PDGF at reducing the level of p53 and that this phenomenon largely disappeared when expression of PDGFRα was reduced. (B) Comparison of RV versus RV plus PDGF. Serum-starved MEF were pretreated with cycloheximide (1 mM) for 30 min, RV or RV plus PDGF-A was added for the indicated times, the cells were lysed, and clarified lysates were subjected to Western blot analysis using the indicated antibodies. In three independent experiments, we observed that while the combination of RV plus PDGF-A activated Akt more robustly than RV alone at the 10-min time point, this high level of Akt activation did not persist, and the level of p53 was only modestly altered. (C) The V842 PDGFRα mutant chronically activated Akt and reduced the level of p53. MEF cells expressing an empty vector (EV), wild-type PDGFRα (WT), or mutant PDGFRα D842V (V842) were treated with or without RV for 2 h. Cell lysates were prepared and subjected to Western blot analysis using the indicated antibodies. We consistently observed that V842 was activated and promoted chronic activation of Akt and suppression of p53. (D) Intense, direct activation of PDGFRα induced sustained Akt activation and a decline in the level of p53. Serum-starved MEF were treated with PDGF-A (5, 50, and 500 ng/ml) for the indicated times and lysed, and the clarified lysates were subjected to Western blot analysis with the indicated antibodies. We observed that the highest dose of PDGF was able to induce both prolonged activation of Akt and a decline in the level of p53. These results in all panels of this figure are representative of at least three independent experiments.
The decline in the level of p53 was associated with a prolonged half-life of activated PDGFRα (Fig. 1) and persistent activation of Akt (Fig. 2 and 6A). If this relationship is causal, then reducing the half-life of activated PDGFRα should shorten the duration for which Akt is active and prevent the decline in p53. To test this idea, we simultaneously stimulated cells with indirect agonists (that lead to prolonged activation of Akt and a reduction in p53) and direct agonists (that shorten the half-life of PDGFRα). The half-life of PDGFRα, the duration of Akt activation, and the decline in p53 were all attenuated in cells stimulated in this way compared with what was observed in cells stimulated with only indirect agonists (i.e., RV) (Fig. 6B). We conclude that acute and even robust activation of Akt did not result in a substantial decline in the level of p53. Rather, prolonged activation was necessary, and this occurred when PDGFRα was activated indirectly and thereby avoided the endocytosis/degradation pathway that cuts short PDGFRα signaling.
Chronic activation of Akt and a low level of p53 are a common theme in PDGFRα-driven pathologies.
We considered whether the signaling pathway associated with an epigenetic disease such as PVR was relevant to other pathologies that are believed to be driven by deregulation of PDGFRα. Gastrointestinal stromal tumors (GIST) in human patients harbor a mutant version of either c-kit or PDGFRα (27, 29). The most commonly occurring mutation in PDGFRα is a point mutation in the activation loop (Asp to Val at 842) that constitutively activates the receptor's kinase (27). Expression of the mutant carrying this substitution, but not WT PDGFRα, sharply increased Akt phosphorylation and suppressed the level of p53 (Fig. 6C). The idea that a decline in p53 is related to pathology is supported by the observation that the level of p53 is reduced in 50% of GIST (52).
In the case of cancers such as glioblastomas, PDGFRα can be deregulated when it is coexpressed with PDGFs, a setting that results in constitutive, direct activation of PDGFRα (2). We mimicked this setting by administering high doses of PDGF. While even a subsaturating dose (5 ng/ml) of PDGF acutely activated Akt, saturating (50 ng/ml) and supersaturating doses were required for prolonged activation of Akt (Fig. 6D). A decline in p53 was observed only at the supersaturating dose of PDGF (Fig. 6D). Thus, constitutive, direct activation of PDGFRα also induced chronic activation of Akt and a fall in the level of p53.
We conclude that deregulation of PDGFRα by three different mechanisms engages the same signaling pathway that is characterized by chronic activation of Akt and decline in p53.
DISCUSSION
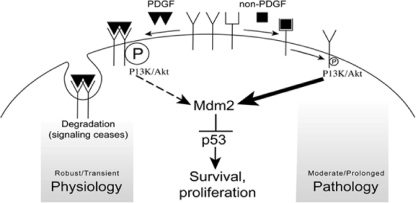
We report that indirectly activated, monomeric PDGFRα evaded the intrinsic mechanism to limit the duration of PDGFRα signaling and thereby chronically activated Akt and suppressed the level of p53 (Fig. 7). This sequence of events was associated with enhanced proliferation and survival of cells and manifestation of experimental PVR. Finally, the relevance of this signal pathway appears to extend beyond PVR since deregulating PDGFRα in a way that promotes two types of cancer also engaged it.
Fig. 7.
Two routes for activating PDGFRα lead to nonidentical signaling events that are associated with either physiology or pathology. PDGF activates its receptor directly, and this involves ligand-driven dimerization, robust autophosphorylation, and activation of intracellular signaling pathways such as PI3K/Akt. Because directly activated PDGFRα is rapidly internalized and degraded, activation of Akt is transient, which is insufficient to reduce the level of p53. We speculate that this sequence of events prevails as PDGF/PDGFR contribute to establishing and maintaining physiology. The indirect route for activating PDGFRα proceeds intracellularly and results in only modest phosphorylation of PDGFRα, which is sufficient to activate PI3K/Akt. In this setting Akt activity persists, at least in part because indirectly activated PDGFRα is not internalized or degraded. Prolonged activation of Akt is associated with a reduction in the level of p53, which sets the stage for pathology. Note that the direct route for activating PDGFRα can induce prolonged Akt activation and a decline in p53, provided that a sufficiently large dose of PDGF is used. It is plausible that such a scenario occurs when PDGF and PDGFR are cooverexpressed, a situation that exists in a subset of human cancers (2).
The identification of p53 as a potential contributor to PVR is intriguing because this disease develops quickly (days to weeks) and may not include the acquisition of multiple genetic lesions to incapacitate the p53 pathway as is the case for the majority of human cancers (42). These observations indicate that genetic and epigenetic disease mechanisms may engage the same signaling pathways and hence be responsive to therapies targeting the common elements. We are currently testing whether p53-related therapies that have been developed to fight cancer would be suitable for patients afflicted by PVR.
Following direct activation of PDGFRα via PDGF, phosphorylation of PDGFRα increased sharply and then declined and remained low (Fig. 6D). While the acute drop in the level of PDGFRα phosphorylation is traditionally explained by degradation of PDGFRα, it does not explain why receptor phosphorylation remains suppressed, since the quantity of PDGFRα recovered to near-prestimulation level within 30 min (data not shown) (probably due to resynthesis). Nevertheless, the extent of PDGFRα phosphorylation remained substantially lower than the initial peak, even in the presence of a high concentration of PDGF (Fig. 6D). These observations indicate that direct activation of PDGFRα triggers at least two mechanisms to limit the amount of phosphorylated PDGFRα; one involves degradation of the activated receptor, whereas the second appears to be degradation independent; we speculate that it involves phosphotyrosine phosphatases (37, 61).
Given the existence of at least several mechanisms for reducing the level of activated/phosphorylated PDGFRα, the persistence of indirectly activated PDGFRα is remarkable. Even a mechanism in which indirectly activated PDGFRα circumvents all pathways that reduce PDGFRα phosphorylation would probably be an incomplete explanation of this phenomenon. There may also be a positive-feedback loop driving persistent phosphorylation of PDGFRα. The initial increase in phosphorylation of PDGFRα is dependent on a ROS/Src-driven mechanism (37). These events are triggered by non-PDGFs that engage their own receptors (e.g., binding of epidermal growth factor [EGF] or basic fibroblast growth factor [bFGF] to EGFR or FGF receptor [FGFR], respectively) and thereby induce ligand-induced receptor degradation that suppresses their output as observed for PDGF/PDGFRα. If this was the only means by which PDGFRα was phosphorylated in response to non-PDGFs, then the kinetics of phosphorylation of PDGFRα under these conditions should roughly reflect what happens in response to direct agonists such as PDGF. Figure 2 shows that this is not the case; phosphorylation of PDGFRα increased instead of decreasing at the later time points. This suggests that the initial boost in ROS/Src activity that was triggered by non-PDGFs may have been followed by a second, persistent mechanism for elevating ROS/Src. For instance, early activation of PI3K/Akt is expected to activate mTOR and thereby reduce the autophagy-dependent clearance of dysfunctional mitochondria that are generating ROS (68). This would establish a positive-feedback loop in which elevated ROS would activate Src, which would promote PDGFRα phosphorylation and thereby sustain PI3K/Akt/mTOR activity, leading to constitutive ROS generation. Our ongoing studies are focused on elucidating the events that permit constitutive activation of PDGFRα following its indirect activation via non-PDGFs.
The observation that less than 10% of the maximal level of PDGFRα phosphorylation was sufficient to drive signaling events, cellular responses, and disease indicates that even a low level of PDGFRα phosphorylation can be very potent. Note that the stark difference in the amplitude of PDGFRα phosphorylation observed in response to direct versus indirect agonists was not maintained at the level of downstream signaling events. This may be because the receptor can be phosphorylated at many tyrosine residues, but only a subset of these sites are required to engage a specific signaling event (56). For instance, Akt activation is dependent on phosphorylation of PDGFRα at Y731 and Y742. Mutating these sites only modestly decreased overall phosphorylation of PDGFRα when it was activated directly (because the other sites are still phosphorylated), whereas the effect was much greater when the receptor was activated indirectly (because these sites are the major phosphorylation sites under these conditions) (Fig. 3A). So receptor phosphorylation is not necessarily a good measure of the magnitude of downstream signaling events. These observations have recalibrated our appreciation of how much activation is relevant, i.e., even 10% of the maximal phosphorylation is sufficient to drive cellular responses and even disease, provided that it occurs on the appropriate residues.
In light of the fact that a relatively small amount of receptor phosphorylation is sufficient for disease progression, it is possible that only a small number of receptors are necessary to drive such a response, if they are activated appropriately. Indeed, we found that very low levels of PDGFRα (well below the amount observed in primary MEF) were sufficient to promote experimental PVR and that substantially increasing PDGFRα expression only modestly augmented the PVR potential of such cells (our unpublished results). We conclude that when activated appropriately (i.e., indirectly to circumvent the intrinsic negative feedback), only a small amount of receptor (approximately 10-fold less than is routinely observed on a fibroblast [1 × 105] [8]) is necessary to drive disease.
Perhaps more surprising than the fact that PDGFRs are expressed in excess of what is necessary to trigger cellular responses (a concept that was introduced approximately 40 years ago [18, 35]) is the finding that PDGF is not required to drive the event (41). Furthermore, since PDGF limits the duration of PDGFR signaling, PDGF may serve to mitigate pathological signaling emanating from indirectly activated PDGFRα. It is only when PDGFs are present at a very high level that we begin to observe signaling events associated with pathology (Fig. 6D), a situation that has been associated with certain cancers (2). Thus, physiological levels of PDGFs may function not only to trigger physiology-related events but also to suppress pathology-related signaling by limiting the duration of signaling by PDGFRs when activated by non-PDGFs.
ACKNOWLEDGMENTS
We thank Tyler Jacks for providing the primary MEF from WT and p53-null mice. We appreciate the advice and input from the following members of the Kazlauskas laboratory: Sarah Jacobo, Ruta Motiejunaite, Eun Young Park, Steven Pennock, Guo-Xiang Ruan, Maryada Sharma, and Magdalena Staniszewska.
Funding for this research was provided by an NIH grant (EY012509) to A.K.
Footnotes
Published ahead of print on 28 February 2011.
REFERENCES
- 1. Agrawal R. N., et al. 2007. In vivo models of proliferative vitreoretinopathy. Nat. Protoc. 2:67–77 [DOI] [PubMed] [Google Scholar]
- 2. Andrae J., Gallini R., Betsholtz C. 2008. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 22:1276–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andrews A., et al. 1999. Platelet-derived growth factor plays a key role in proliferative vitreoretinopathy. Invest. Ophthalmol. Vis. Sci. 40:2683–2689 [PubMed] [Google Scholar]
- 4. Asaria R. H., et al. 2001. Adjuvant 5-fluorouracil and heparin prevents proliferative vitreoretinopathy: Results from a randomized, double-blind, controlled clinical trial. Ophthalmology 108:1179–1183 [DOI] [PubMed] [Google Scholar]
- 5. Ashcroft M., et al. 2002. Phosphorylation of HDM2 by Akt. Oncogene 21:1955–1962 [DOI] [PubMed] [Google Scholar]
- 6. Avrov K., Kazlauskas A. 2003. The role of c-Src in platelet-derived growth factor alpha receptor internalization. Exp. Cell Res. 291:426–434 [DOI] [PubMed] [Google Scholar]
- 7. Baroni S. S., et al. 2006. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N. Engl. J. Med. 354:2667–2676 [DOI] [PubMed] [Google Scholar]
- 8. Bowen-Pope D. F., Ross R. 1982. Platelet-derived growth factor. II. Specific binding to cultured cells. J. Biol. Chem. 257:5161–5171 [PubMed] [Google Scholar]
- 9. Bowen-Pope D. F., Ross R. 1984. The platelet-derived growth factor receptor, vol. 109. Academic Press, Inc., New York, NY [Google Scholar]
- 10. Cassidy L., Barry P., Shaw C., Duffy J., Kennedy S. 1998. Platelet derived growth factor and fibroblast growth factor basic levels in the vitreous of patients with vitreoretinal disorders. Br. J. Ophthalmol. 82:181–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Charteris D. G. 1998. Growth factors in proliferative vitreoretinopathy. Br. J. Ophthalmol. 82:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cui J., et al. 2009. PDGF receptors are activated in human epiretinal membranes. Exp. Eye Res. 88:438–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cui J. Z., et al. 2007. Stage specificity of novel growth factor expression during development of proliferative vitreoretinopathy. Eye 21:200–208 [DOI] [PubMed] [Google Scholar]
- 14. Dolloff N. G., Russell M. R., Loizos N., Fatatis A. 2007. Human bone marrow activates the Akt pathway in metastatic prostate cells through transactivation of the alpha-platelet-derived growth factor receptor. Cancer Res. 67:555–562 [DOI] [PubMed] [Google Scholar]
- 15. Fastenberg D. M., Diddie K. R., Sorgente N., Ryan S. J. 1982. A comparison of different cellular inocula in an experimental model of massive periretinal proliferation. Am. J. Ophthalmol. 93:559–564 [DOI] [PubMed] [Google Scholar]
- 16. Feng J., et al. 2004. Stabilization of Mdm2 via decreased ubiquitination is mediated by protein kinase B/Akt-dependent phosphorylation. J. Biol. Chem. 279:35510–35517 [DOI] [PubMed] [Google Scholar]
- 17. Fredriksson L., Li H., Fieber C., Li X., Eriksson U. 2004. Tissue plasminogen activator is a potent activator of PDGF-CC. EMBO J. 23:3793–3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Freychet P., Roth J., Neville D. M., Jr 1971. Insulin receptors in the liver: specific binding of (125 I)insulin to the plasma membrane and its relation to insulin bioactivity. Proc. Natl. Acad. Sci. U. S. A. 68:1833–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gilbertson D. G., et al. 2001. Platelet-derived growth factor C (PDGF-C), a novel growth factor that binds to PDGF alpha and beta receptor. J. Biol. Chem. 276:27406–27414 [DOI] [PubMed] [Google Scholar]
- 20. Gottlieb T. M., Leal J. F., Seger R., Taya Y., Oren M. 2002. Cross-talk between Akt, p53 and Mdm2: possible implications for the regulation of apoptosis. Oncogene 21:1299–1303 [DOI] [PubMed] [Google Scholar]
- 21. Gragoudas E. S., Adamis A. P., Cunningham E. T., Jr., Feinsod M., Guyer D. R. 2004. Pegaptanib for neovascular age-related macular degeneration. N. Engl. J. Med. 351:2805–2816 [DOI] [PubMed] [Google Scholar]
- 22. Grinnell F., Ho C. H., Lin Y. C., Skuta G. 1999. Differences in the regulation of fibroblast contraction of floating versus stressed collagen matrices. J. Biol. Chem. 274:918–923 [DOI] [PubMed] [Google Scholar]
- 23. Haimann M. H., Burton T. C., Brown C. K. 1982. Epidemiology of retinal detachment. Arch. Ophthalmol. 100:289–292 [DOI] [PubMed] [Google Scholar]
- 24. Han D. 2008. Proliferative vitreoretinopathy, vol. 183. Elsevier Saunders, Philadelphia, PA [Google Scholar]
- 25. Heeneman S., Haendeler J., Saito Y., Ishida M., Berk B. C. 2000. Angiotensin II induces transactivation of two different populations of the platelet-derived growth factor beta receptor. Key role for the p66 adaptor protein Shc. J. Biol. Chem. 275:15926–15932 [DOI] [PubMed] [Google Scholar]
- 26. Heier J. S., et al. 2006. Ranibizumab for treatment of neovascular age-related macular degeneration: a phase I/II multicenter, controlled, multidose study. Ophthalmology 113:633–642.e4 [DOI] [PubMed] [Google Scholar]
- 27. Heinrich M. C., et al. 2003. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 299:708–710 [DOI] [PubMed] [Google Scholar]
- 28. Herrlich A., et al. 1998. Ligand-independent activation of platelet-derived growth factor receptor is a necessary intermediate in lysophosphatidic, acid-stimulated mitogenic activity in L cells. Proc. Natl. Acad. Sci. U. S. A. 95:8985–8990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hirota S., et al. 1998. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279:577–580 [DOI] [PubMed] [Google Scholar]
- 30. Ikuno Y., Kazlauskas A. 2002. An in vivo gene therapy approach for experimental proliferative vitreoretinopathy using the truncated platelet-derived growth factor alpha receptor. Invest. Ophthalmol. Vis. Sci. 43:2406–2411 [PubMed] [Google Scholar]
- 31. Ikuno Y., Kazlauskas A. 2002. TGFbeta1-dependent contraction of fibroblasts is mediated by the PDGFalpha receptor. Invest. Ophthalmol. Vis. Sci. 43:41–46 [PubMed] [Google Scholar]
- 32. Ikuno Y., Leong F. L., Kazlauskas A. 2000. Attenuation of experimental proliferative vitreoretinopathy by inhibiting the platelet-derived growth factor receptor. Invest. Ophthalmol. Vis. Sci. 41:3107–3116 [PubMed] [Google Scholar]
- 33. Ikuno Y., Leong F. L., Kazlauskas A. 2002. PI3K and PLCgamma play a central role in experimental PVR. Invest. Ophthalmol. Vis. Sci. 43:483–489 [PubMed] [Google Scholar]
- 34. Kern S. E., et al. 1991. Identification of p53 as a sequence-specific DNA-binding protein. Science 252:1708–1711 [DOI] [PubMed] [Google Scholar]
- 35. Kono T., Barham F. W. 1971. The relationship between the insulin-binding capacity of fat cells and the cellular response to insulin. Studies with intact and trypsin-treated fat cells. J. Biol. Chem. 246:6210–6216 [PubMed] [Google Scholar]
- 36. Lei H., et al. 2007. A potential role for PDGF-C in experimental and clinical proliferative vitreoretinopathy. Invest. Ophthalmol. Vis. Sci. 48:2335–2342 [DOI] [PubMed] [Google Scholar]
- 37. Lei H., Kazlauskas A. 2009. Growth factors outside of the platelet-derived growth factor (PDGF) family employ reactive oxygen species/Src family kinases to activate PDGF receptor alpha and thereby promote proliferation and survival of cells. J. Biol. Chem. 284:6329–6336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lei H., Rheaume M. A., Kazlauskas A. 2010. Recent developments in our understanding of how platelet-derived growth factor (PDGF) and its receptors contribute to proliferative vitreoretinopathy. Exp. Eye Res. 90:376–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lei H., Romeo G., Kazlauskas A. 2004. Heat shock protein 90alpha-dependent translocation of annexin II to the surface of endothelial cells modulates plasmin activity in the diabetic rat aorta. Circ. Res. 94:902–909 [DOI] [PubMed] [Google Scholar]
- 40. Lei H., et al. 2010. N-Acetylcysteine suppresses retinal detachment in an experimental model of proliferative vitreoretinopathy. Am. J. Pathol. 177:132–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lei H., et al. 2009. Growth factors outside the PDGF family drive experimental PVR. Invest. Ophthalmol. Vis. Sci. 50:3394–3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Levine A. J. 1997. p53, the cellular gatekeeper for growth and division. Cell 88:323–331 [DOI] [PubMed] [Google Scholar]
- 43. Li X., et al. 2000. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat. Cell Biol. 2:302–309 [DOI] [PubMed] [Google Scholar]
- 44. Linseman D. A., Benjamin C. W., Jones D. A. 1995. Convergence of angiotensin II and platelet-derived growth factor receptor signaling cascades in vascular smooth muscle cells. J. Biol. Chem. 270:12563–12568 [DOI] [PubMed] [Google Scholar]
- 45. Liu Y., Li M., Warburton R. R., Hill N. S., Fanburg B. L. 2007. The 5-HT transporter transactivates the PDGFbeta receptor in pulmonary artery smooth muscle cells. FASEB J. 21:2725–2734 [DOI] [PubMed] [Google Scholar]
- 46. Michels R. G., Wilkinson C. P., Rice T. A. 1990. Proliferative retinopathy. Mosby, St. Louis, MO [Google Scholar]
- 47. Nakagawa M., Refojo M. F., Marin J. F., Doi M., Tolentino F. I. 1995. Retinoic acid in silicone and silicone-fluorosilicone copolymer oils in a rabbit model of proliferative vitreoretinopathy. Invest. Ophthalmol. Vis. Sci. 36:2388–2395 [PubMed] [Google Scholar]
- 48. Ogawara Y., et al. 2002. Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J. Biol. Chem. 277:21843–21850 [DOI] [PubMed] [Google Scholar]
- 49. Ory D. S., Neugeboren B. A., Mulligan R. C. 1996. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc. Natl. Acad. Sci. U. S. A. 93:11400–11406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Prenzel N., et al. 1999. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402:884–888 [DOI] [PubMed] [Google Scholar]
- 51. Robbins S. G., et al. 1994. Platelet-derived growth factor ligands and receptors immunolocalized in proliferative retinal diseases. Invest. Ophthalmol. Vis. Sci. 35:3649–3663 [PubMed] [Google Scholar]
- 52. Romeo S., et al. 2009. Cell cycle/apoptosis molecule expression correlates with imatinib response in patients with advanced gastrointestinal stromal tumors. Clin. Cancer Res. 15:4191–4198 [DOI] [PubMed] [Google Scholar]
- 53. Rosenfeld P. J., et al. 2005. Maximum tolerated dose of a humanized anti-vascular endothelial growth factor antibody fragment for treating neovascular age-related macular degeneration. Ophthalmology 112:1048–1053 [DOI] [PubMed] [Google Scholar]
- 54. Rosenkranz S., DeMali K. A., Gelderloos J. A., Bazenet C., Kazlauskas A. 1999. Identification of the receptor-associated signaling enzymes that are required for platelet-derived growth factor-AA-dependent chemotaxis and DNA Synthesis. J. Biol. Chem. 274:28335–28343 [DOI] [PubMed] [Google Scholar]
- 55. Rosenkranz S., et al. 2000. Src family kinases negatively regulate platelet-derived growth factor alpha receptor-dependent signaling and disease progression. J. Biol. Chem. 275:9620–9627 [DOI] [PubMed] [Google Scholar]
- 56. Rosenkranz S., Kazlauskas A. 1999. Evidence for distinct signaling properties and biological responses induced by the PDGF receptor alpha and beta subtypes. Growth Factors 16:201–216 [DOI] [PubMed] [Google Scholar]
- 57. Schiff W. M., et al. 2007. Safety and efficacy assessment of chimeric ribozyme to proliferating cell nuclear antigen to prevent recurrence of proliferative vitreoretinopathy. Arch. Ophthalmol. 125:1161–1167 [DOI] [PubMed] [Google Scholar]
- 58. Siegbahn A., Johnell M., Nordin A., Aberg M., Velling T. 2008. TF/FVIIa transactivate PDGFRbeta to regulate PDGF-BB-induced chemotaxis in different cell types: involvement of Src and PLC. Arterioscler Thromb. Vasc. Biol. 28:135–141 [DOI] [PubMed] [Google Scholar]
- 59. Sorkin A., Westermark B., Heldin C. H., Claesson-Welsh L. 1991. Effect of receptor kinase inactivation on the rate of internalization and degradation of PDGF and the PDGF beta-receptor. J. Cell Biol. 112:469–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tanimoto T., Lungu A. O., Berk B. C. 2004. Sphingosine 1-phosphate transactivates the platelet-derived growth factor beta receptor and epidermal growth factor receptor in vascular smooth muscle cells. Circ. Res. 94:1050–1058 [DOI] [PubMed] [Google Scholar]
- 61. Tonks N. K. 2006. Protein tyrosine phosphatases: from genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 7:833–846 [DOI] [PubMed] [Google Scholar]
- 62. van Wijngaarden P., Coster D. J., Williams K. A. 2005. Inhibitors of ocular neovascularization: promises and potential problems. JAMA 293:1509–1513 [DOI] [PubMed] [Google Scholar]
- 63. Wiedemann P., Hilgers R. D., Bauer P., Heimann K. 1998. Adjunctive daunorubicin in the treatment of proliferative vitreoretinopathy: results of a multicenter clinical trial. Daunomycin Study Group. Am. J. Ophthalmol. 126:550–559 [DOI] [PubMed] [Google Scholar]
- 64. Wilkes S. R., Beard C. M., Kurland L. T., Robertson D. M., O'Fallon W. M. 1982. The incidence of retinal detachment in Rochester, Minnesota, 1970-1978. Am. J. Ophthalmol. 94:670–673 [DOI] [PubMed] [Google Scholar]
- 65. Zhang H., et al. 2007. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J. Clin. Invest. 117:730–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zheng Y., et al. 2003. Platelet-derived growth factor receptor kinase inhibitor AG1295 and inhibition of experimental proliferative vitreoretinopathy. Jpn. J. Ophthalmol. 47:158–165 [DOI] [PubMed] [Google Scholar]
- 67. Zhou B. P., et al. 2001. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat. Cell Biol. 3:973–982 [DOI] [PubMed] [Google Scholar]
- 68. Yen W. L., Klionsky D. J. 2008. How to live long and prosper: autophagy, mitochondria, and aging. Physiology (Bethesda) 23:248–262 [DOI] [PubMed] [Google Scholar]