Abstract
Background: Resonance Raman spectroscopy (RRS) has been suggested as a feasible method for noninvasive carotenoid measurement of human skin. However, before RRS measures of dermal carotenoids can be used as a biomarker, data on intra- and intersubject variability and validity are needed.
Objective: The purpose of this study was to evaluate the reproducibility and validity of RRS measures of dermal total carotenoids and lycopene in humans.
Design: In study 1, 74 men and women with diverse skin pigmentation were recruited. RRS measures of the palm, inner arm, and outer arm were obtained at baseline, 1 wk, 2 wk, 1 mo, 3 mo, and 6 mo (to maximize seasonal variation). The RRS device used visible light at 488 nm to estimate total carotenoids and at 514 nm to estimate lycopene. Reproducibility was assessed by intraclass correlation coefficients (ICCs). In study 2, we recruited 28 subjects and assessed dietary carotenoid intake, obtained blood for HPLC analyses, performed RRS measures of dermal carotenoid status, and performed dermal biopsies (3-mm punch biopsy) with dermal carotenoids assessed by HPLC.
Results: ICCs for total carotenoids across time were 0.97 (palm), 0.95 (inner arm), and 0.93 (outer arm). Total dermal carotenoids assessed by RRS were significantly correlated with total dermal carotenoids assessed by HPLC of dermal biopsies (r = 0.66, P = 0.0001). Similarly, lycopene assessed by RRS was significantly correlated with lycopene assessed by HPLC of dermal biopsies (r = 0.74, P < 0.0001).
Conclusion: RRS is a feasible and valid method for noninvasively assessing dermal carotenoids as a biomarker for studies of nutrition and health.
INTRODUCTION
Carotenoids are plant pigments that humans routinely ingest on a daily basis. Particularly high concentrations are found in fruit and vegetables. The most prevalent carotenoids consumed in North American diets include the following: α-carotene, β-carotene, lycopene, lutein, zeaxanthin, and β-cryptoxanthin (1). These common dietary carotenoids can be measured by biochemical methods in blood and in other tissues following extraction, and it is known that blood/tissue carotenoid concentrations correlate with dietary intake (2). Also, plasma concentrations of carotenoids significantly increase in response to fruit/vegetable behavioral interventions (3, 4). Given their widespread distribution in fruit and vegetables, carotenoids have been of great interest as an objective biomarker of fruit and vegetable intake. The National Academy of Sciences concluded, “Blood concentrations of carotenoids are the best biological markers for consumption of fruits and vegetables” (1).
Self-reported fruit and vegetable intake has been inversely associated with the risk of a number of chronic diseases, most notably with various cancers (5) and cardiovascular disease (6), and also with other diseases of aging (7). However, recent research has highlighted concerns about the validity of subjective, self-reported dietary intake data (8). This has motivated the development of reliable and objective biomarkers as indicators of dietary intake for studies on human health. Similarly, dietary intervention trials typically assess self-reported changes in diet, but it is well recognized that reporting can be biased, particularly in the setting of randomized trials where participants may want to please researchers with regard to their adherence to intervention (social desirability bias). Again, objective validation that dietary behavior change has been successful is critical to the interpretation of diet intervention trials.
To date, studies of fruit and vegetable intake have relied on carotenoid analyses in plasma or serum by the use of HPLC. This approach is proven and has been linked with important health outcomes, including total mortality (9), but has some key disadvantages, especially cost of phlebotomy, sample processing/storage, sample analysis, and the necessity of venipuncture, which may introduce participation bias as some people are unwilling to give blood. As a further disadvantage, carotenoid concentrations in blood fluctuate in response to recent dietary intake, with an estimated half-life of <12 d for β-carotene (10).
Resonance Raman light scattering spectroscopy (RRS) is a form of laser spectroscopy that detects the characteristic vibrational/rotational energy levels of a molecule. Carotenoids are particularly well suited to RRS, as all have a strongly absorbing conjugated carbon backbone molecule structure, providing the basis for efficient resonant laser excitation of the molecules. The backbone consists of alternating carbon double- and single-bonds, with the conjugation length differing between particular carotenoid species. The stretch vibration frequencies of the carbon double and single bonds can be detected with RRS. In homogeneous solvent systems, the intensity of the resonance Raman scattered light is linearly related to the carotenoid concentration, thus serving as an optical measure for carotenoid content.
These light scattering properties have led us to explore the use of RRS for the noninvasive quantitative optical measurement of carotenoids and their spatial distributions in living human tissue, initially in the human macula (retina) (11–14). In healthy subjects, carotenoids are typically very highly concentrated in this retinal area (11) and are thought to protect this tissue region via optical filtering and antioxidant action. RRS-based detection of carotenoids in human macula is facilitated by their relatively high level, their location just below an optically transparent region of the retinal layer system, and absence of non-Raman scatterers in the retinal light excitation path. Furthermore, fluorescence contributions to the overall light response originate only from distal retinal layers and therefore can be easily subtracted.
We subsequently proposed that RRS could be a feasible method for noninvasive carotenoid measurement for human skin (15–18), recognizing that skin is much more challenging for RRS assessment. Difficulties arise here because carotenoid levels are ≈2 orders of magnitude lower relative to the human macula, and skin contains other chromophores in the optical light excitation and detection paths besides carotenoids (including collagen, melanin, porphyrins, hemoglobin, etc). Furthermore, the tissue morphology of the optically accessible outer skin tissue layers is heterogeneous, causing strong, diffusive, non-Raman light scattering effects in the total spectral response. Some of the confounding influences should be minimized by choosing skin tissue sites with a relatively thick stratum corneum layer, such as the palm of the hand or the sole of the foot.
Following our lead, other groups are now starting to use RRS in human skin (19–21). These studies suggest that dermal carotenoid levels as measured by RRS are correlated with fruit and vegetable intake and increase in response to carotenoid supplementation, and that there is variability in this biomarker across subjects. This supports that dermal carotenoids as assessed by RRS are a promising biomarker of fruit and vegetable intake for human research. However, before RRS can be accepted as a reliable biomarker for human research, the method needs to be scrutinized more thoroughly in view of the challenges noted above. Some of the dermal chromophores and scatterers existing in the optical light paths may vary between subjects in their concentration and also between tissue sites and therefore will vary in their relative contributions to the overall light response. Data are therefore critically needed on intrasubject variability, intersubject variability, and, most important, validity (as compared with chemical analysis of excised tissue) in humans.
In contrast to all other major dietary carotenoids that are widely distributed in fruits and vegetables, lycopene is found in relatively few foods, with most dietary lycopene coming from tomatoes and tomato products (22). Top food sources of lycopene include foods such as pizza, spaghetti, lasagna, and ketchup (23). Thus, lycopene is not considered a biomarker of total fruit and vegetable intake but rather tomato product intake. Lycopene is also of considerable interest as a possible chemopreventive agent for prostate and other cancers (24). Due to its shorter conjugation length in comparison to other skin carotenoids, the absorption spectrum for lycopene is shifted ≈20 nm toward longer wavelengths in comparison to other carotenoids. This allows for the potential to assess lycopene separately with RRS by using a different excitation wavelength with selectively enhanced Raman scattering cross-section (15).
Here, we 1) assessed the intra- and interperson variability in total carotenoid status and lycopene status as assessed by RRS, at the same body site, at different body sites, and over time; 2) determined the correlation between total carotenoid status of human skin as assessed by RRS and other measures of carotenoid status, including plasma carotenoid levels and carotenoid levels in dermal biopsies; and 3) determined the correlation between lycopene status of human skin as assessed by RRS and other measures of lycopene status, including plasma lycopene levels and lycopene levels in dermal biopsies.
SUBJECTS AND METHODS
Reproducibility study
Subjects
Our goal for the reproducibility study was to recruit a convenience sample of 75 normal healthy adults between the ages of 21 and 65 y. We attempted to recruit men and women, smokers and nonsmokers [plasma carotenoid concentrations of smokers are known to be lower than those of nonsmokers (25)], and subjects with a wide spectrum of skin pigmentation. Some study participants were recruited from persons affiliated with Yale University (New Haven, CT), including graduate students, faculty, clerical and technical workers, physical plant workers, and custodial workers. We also posted flyers at other locations adjacent to Yale to recruit more broadly. Trained research assistants explained the purpose of the study and then obtained written informed consent. The research assistant then interviewed participants in-person to obtain basic demographic information (age, sex, height, weight, current smoking status, use of nutritional supplements, etc). Participants were asked to self-identify race, and, under the guidance of the research assistant, skin color (inner arm) with use of a color wheel of skin tone samples used for prosthetic devices. All procedures were approved by the Institutional Review Board at the Yale University School of Medicine, with this first human study opened for recruitment in November 2004.
For the biomarker measurements, we developed and used an instrument suitable for RRS measurements on human tissue in vivo (see methods described below for details). We assessed dermal carotenoid status in the palm of the hand, the inner forearm, and outer forearm. It is known that carotenoids concentrate in the palm (vegetarians often have yellow-orange palms), so the palm was an obvious choice for measurements. The other 2 sites were selected for convenience, with one being more likely sun-exposed (outer arm) than the other (inner arm). Participants returned to the study office for reassessment at the following time points: 1 wk, 2 wk, 1 mo, 3 mo, and 6 mo. The latter time points, in particular, were chosen to examine possible seasonal dietary influences on dermal carotenoid levels (this study was done in a climate with marked seasonal variation).
Raman spectroscopy
Details of RRS instrumentation have been reported elsewhere (15, 17). Briefly, we developed and used an instrument suitable for RRS measurements on human tissue in vivo, which consists of a small air-cooled, multiline argon laser to produce simultaneously blue light at 488 nm (for total carotenoids) and green light at 514 nm (for lycopene; also see the Supplemental Figure under “Supplemental data” in the online issue). The laser output was split into 2 separate, monochromatic beams with use of a dichroic beam splitter. Each beam was filtered with a line pass dielectric filter, sent through a variable optical density component, coupled into an optical fiber, and routed into a light delivery and collection probe module. In each beam a computer-controlled shutter was used to sequentially expose the skin tissue site with each excitation wavelength. For each tissue measurement, the attenuators were adjusted to provide identical laser excitation intensities for each channel, and the probe was held in continuous contact with the selected tissue site to ensure measurements of exactly the same tissue probe volume under blue and green light excitation. Light backscattered from the skin was routed via fiber bundle to a spectrograph, spectrally dispersed, recorded with a charge-coupled device camera, digitized, analyzed for carotenoid resonance Raman response, and displayed, in near real-time on a computer monitor, with use of home-developed software routines.
The strongest feature of the optical response of the skin is Rayleigh scattering, which is particularly dominant due to the rough tissue morphology of the outer skin layer (stratum corneum). This component is filtered out with use of 2 holographic notch filters in the detection path. Furthermore, a strong, spectrally broad fluorescence background response occurs that is due to skin autofluorescence. Under the above excitation conditions, it originates mainly from collagen-related chromophores. This fluorescence contribution (and its corresponding absorption) cannot be avoided. Superimposed on the autofluorescence background, with ≈2 orders of magnitude lower intensity, are typically the 2 carotenoid resonance Raman intensity peaks, which are characterized by both their distinct spectral positions and their narrow bandwidths. The 2 peaks are retrieved with optimized resolution and signal-to-noise ratio by using a light detector with high dynamic range by fitting the fluorescence background with a fourth-order polynomial and subtracting it from the original spectrum. The intensity of the carbon-carbon double bond (C=C) stretch Raman intensity, occurring with a frequency shift of 1524 cm−1, is strongest and therefore used as a quantitative measure (score) of the dermal carotenoid level.
We used a laser power of <10 mW and an exposure time of 30 s with an elliptical spot size of 2 mm by 3 mm. Some subject scans had high autofluorescence (although uncommon in the palm) such that the detector was saturated and unable to obtain a clear Raman signal; for these scans, we reduced the exposure time to 15 s and doubled the Raman score accordingly. This approach (reducing exposure time, doubling response) was verified by using subjects with normal autofluorescence (26). Measures were taken in triplicate, and we used the average of the measures for data analysis. An external diamond sample with Raman response in the spectral vicinity of the C=C stretch frequency was used daily to correct for any possible drifts in detector sensitivity and laser output over the months required for recruitment and data collection (6-mo follow-up).
The RRS uses a class 2-B laser (National Laser Inc, Salt Lake City, UT) and with the laser power and spot size used in these studies, it is safe to expose skin for ≤30,000 s. However, as is the case with laser pointers, direct eye contact should be avoided. Thus, both the laser (RRS) operator and the participant wore protective eyewear (Kentek Corporation, Pittsfield, NH) during the RRS procedures.
Dietary intake data
Participants were asked to complete a food-frequency questionnaire (FFQ) at baseline and at 6 mo. The questionnaire was developed and validated at the Fred Hutchinson Cancer Research Center. This FFQ has been used on several studies where carotenoids were a primary exposure of interest, such as the Olestra Post-Marketing Surveillance Study (27). A validity study was undertaken to compare nutrient intake as estimated from this FFQ compared with that of 8 d of food records and recalls. The correlation coefficient for β-carotene from the validity study was 0.57 (28). The frequency data were linked with the University of Minnesota Nutrition Coding Center Nutrient Data System for estimation of nutrient intake. This database contains the current carotenoid content of foods, as evaluated and updated by the US Department of Agriculture. Participants were asked to report their usual frequency of consumption of selected fruit and vegetables over the past 2 mo. Although the half-life of carotenoids in skin has not been studied and needs further evaluation, it is plausible that it is longer than the half-life of carotenoids in blood, which is ≈2 wk (10), suggesting that Raman counts in skin respond to changes in carotenoid intake within the past 2 mo.
Participants were asked to bring in bottles of any nutritional supplements they were consuming, because some nutritional supplements, such as certain multivitamins, contain β-carotene as a source of vitamin A, or possibly other carotenoids. If participants were using supplements at entry, they were asked to continue taking the same supplement for the 6-mo study period. If participants were not taking supplements at entry, they were asked to continue abstaining from nutritional supplements for the study period.
Validity study
Subjects
For this portion of the research, we recruited a total of 30 normal healthy adults between the ages of 21 and 65 y (2 subjects were used to pilot and revise procedures; 28 were test subjects and used in this analysis) with recruitment beginning in November 2005. Participants had to be willing to undergo phlebotomy and dermal biopsy. After obtaining signed informed consent, participants were interviewed to obtain demographic data and were asked to complete the FFQ described previously. RRS was then used to evaluate dermal carotenoid levels (as described above) but this time in the posterior hip area (an inconspicuous site where a small biopsy scar would not be noticeable even when wearing a bathing suit). The area of assessment was marked on the skin so that the dermatologic surgeon could biopsy from that region. Subjects were given injected anesthetic (Lidocaine; Hospira Inc, Lake Forest, IL) at the biopsy site. Once the skin was numb, a dermatologic surgeon removed a 3-mm punch biopsy of skin. Residual adipose tissue was removed from the sample before placing it into a cryovial. Biopsy samples were snap-frozen immediately with use of liquid nitrogen. Biopsy sites were sutured to facilitate rapid healing.
Blood samples (10 mL) were obtained by venipuncture by a trained phlebotomist and collected into heparinized tubes. Tubes were protected from light, chilled but not frozen, and then centrifuged to obtain plasma. Plasma aliquots were obtained and stored at –70°C before HPLC analysis.
Extraction and HPLC analyses
Extractions were carried out as described elsewhere (29). First, 280 μL of phosphate buffered saline was added to the skin punch tissue. Then, 35 μL collagenase solution (50 mg/mL; Sigma catalog no. E −1644) was added, vortex-mixed, and incubated at 37°C for 1 h. Tissues were homogenized on ice, and 35 μL of protease solution (20 mg/mL; Sigma catalog no. 11360) were added, vortex-mixed, and incubated at 37°C for 0.5 h. Four milliliters of sodium dodecyl sulfate-ethanol-butylated hydroxytoluene solution were added and vortexed for 60 s. Samples were then extracted twice with hexane (2 × 500 μL) and dried down before HPLC injection.
Plasma samples (100 μL) were treated with ethanol and hexane containing 0.1% (wt:vol) butylated hydroxytoluene and centrifuged to remove the proteins. The proteins were reextracted with hexane (3 × 300 μL), and the combined extract was evaporated to dryness under reduced pressure at <40°C. After evaporation of the solvent, the residue was reconstituted in 200 μL of HPLC mobile phase and centrifuged for 5 min at 2000 × g before analysis.
The chromatographic conditions for carotenoid separation and quantitation were similar to those reported earlier (30). The mobile phase was an isocratic mixture of acetonitrile:isopropanol:ethyl acetate (50:40:10 vol:vol) at a flow rate of 0.7 mL/min. The analysis was performed on a reversed-phase Luna C18[30] analytic column, [250-mm length × 4.6-mm id (Phenomenex, Torrance, CA); particle size = 5 μm; pore size = 100 Angstroms]. The column was maintained at room temperature, and the HPLC detector was operated at 450 nm. Peak identities were confirmed by photodiode-array spectra, mass spectra, and coelution with authentic standards as necessary. To avoid overloading the mass spectrometer with eluted molecules, 50% of the eluant was directed to waste with the help of a diverter valve. Lutein and zeaxanthin coeluted but were quantitated based on a single ion monitoring method (31).
Mass spectrometry equipment and analysis
Mass spectrometry analysis was performed by using a Thermo Electron MSQ single quadrupole mass spectrometer (San Jose, CA) equipped with an atmospheric pressure chemical ionization source. The protonated precursor molecular ions were initially acquired in 2 full scan modes (A: 200–500 Da, B: 500–800 Da) with 0.2 step size and 2-ms dwell time. Selected ion monitoring was performed by using a dwell time of 200 ms for each channel. In selected ion monitoring mode the channel mass-to-charge ratio used was 551 ± 2 for lutein (dehydrated form), 569 ± 2 for zeaxanthin, 552 ± 2 for β-cryptoxanthin, and 537 ± 2 for β-carotene and lycopene. Typical conditions were corona discharge current = 5 μA, RF lens bias voltage = 0.1 V, cone voltage = 80 V, and heater temperature = 500°C. The ion source and tuning lens parameters were optimized automatically by infusing carotenoid standards via the built-in injector operated manually. For routine quantitative analysis, the ThemoElectron MSQ QUAN BROWSER (Xcaliber) was used. The software provides a peak integration of the ion intensity for each of the programmed mass ranges. Automated analysis was followed by manual confirmation of in-source fragmentation patterns. For calibration, standard solutions were injected in different volumes to achieve final injected concentrations ranging from 1 to 1000 pg and 1 to 8 ng. Accuracy and precision of the method were determined by generating intra- and interday variability data from a series of samples in the range of 1–1000 pg injected 5 times on a single day.
Statistical analyses
Descriptive statistics were used to describe the distribution of Raman carotenoid scores in the study population and to compare various subgroups in their mean dermal carotenoid status. Intra-class correlation coefficients (ICCs) were used to evaluate reproducibility of the measures over time and over the 3 body sites. To assess validity, pairwise Pearson's correlation coefficients were calculated from the data. All statistical computations were conducted by using SAS version 9.1 (SAS Institute Inc, Cary, NC) with a P value of 0.05 considered statistically significant. One subject was identified as an outlier (Raman measure outside 3 SD units) so was not included in the Pearson's correlation analysis of total carotenoids.
RESULTS
To examine the reproducibility of the RRS biomarker measures of dermal total carotenoid and dermal lycopene levels, we recruited a total of 75 healthy adults. One participant came in for a baseline scan but did not complete the baseline questionnaire or any other scan so was excluded from all analyses. Of the remaining 74 subjects, the mean age was 37 y (median: 33 y), 62% were female, 84% were white, and 15% were current smokers (Table 1).
TABLE 1.
Resonance Raman spectroscopy (RRS) for total carotenoids by baseline characteristics of the study population (n = 74)
| n (%) | RRS score1 | P value2 | |
| Age | 0.10 | ||
| ≤33 y | 37 (50.0) | 1.68 ± 0.53 | |
| >33 y | 37 (50.0) | 1.47 ± 0.54 | |
| Sex | 0.10 | ||
| Male | 28 (37.8) | 1.44 ± 0.55 | |
| Female | 46 (62.2) | 1.66 ± 0.52 | |
| Race-ethnicity | 0.72 | ||
| White | 62 (83.8) | 1.57 ± 0.51 | |
| Nonwhite | 12 (16.2) | 1.63 ± 0.70 | |
| Education | 0.11 | ||
| >College graduate | 30 (40.5) | 1.70 ± 0.46 | |
| ≤College graduate | 44 (59.5) | 1.50 ± 0.58 | |
| Current smoking status | 0.70 | ||
| Yes | 11 (14.9) | 1.31 ± 0.72 | |
| No | 63 (85.1) | 1.63 ± 0.50 | |
| Cigarettes smoked per day | 0.14 | ||
| None | 63 (85.1) | 1.63 ± 0.50 | |
| 1–9 | 7 (9.5) | 1.49 ± 0.82 | |
| 10–20 | 3 (4.0) | 1.01 ± 0.50 | |
| >20 | 1 (1.4) | 0.903 | |
| Current alcohol drinker | 0.65 | ||
| Yes | 59 (79.7) | 1.56 ± 0.50 | |
| No | 15 (20.3) | 1.64 ± 0.70 | |
| Weight status | 0.80 | ||
| Underweight (BMI <18.5 kg/m2) | 4 (5.4) | 1.67 ± 0.40 | |
| Healthy weight (BMI 18.5 to <25.0 kg/m2) | 45 (60.8) | 1.69 ± 0.54 | |
| Overweight (BMI 25.0 to <30.0 kg/m2) | 20 (27.0) | 1.32 ± 0.43 | |
| Obese (BMI ≥30.0 kg/m2) | 5 (6.8) | 1.57 ± 0.84 | |
| Skin tone | 0.06 | ||
| Light | 62 (83.8) | 1.63 ± 0.53 | |
| Medium | 9 (12.2) | 1.47 ± 0.58 | |
| Dark | 3 (4.0) | 0.89 ± 0.35 |
Mean ± SD RRS palm measures of dermal carotenoid status at 6 time points.
P for difference in means across groups derived by Student's t test (binary variables) or ANOVA (multilevel variables).
SD not applicable (n = 1).
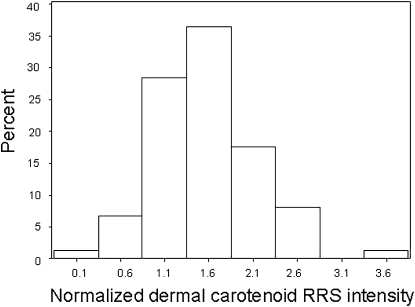
The distribution of Raman intensities for total carotenoids in the palm at the baseline visit is shown in Figure 1.. The data appear to be approximately normally distributed, as confirmed by the Kolmogorov-Smirnov test. Median Raman intensities for total carotenoids (normalized Raman units) were consistently highest in the palm, then inner forearm, and lowest in the outer forearm (ratios of 3.7 and 1.8 compared with 1.0 in normalized Raman units, respectively; data not shown). Similarly, median lycopene intensities were highest in the palm, then inner forearm, and lowest in the outer forearm (ratios of 2.6 and 1.6 compared with 1.0 in normalized Raman units, respectively; data not shown). Lycopene accounted for, on average, 32% of total Raman intensities in the palm.
FIGURE 1.
Histogram showing distribution of total carotenoids in the palm as assessed at baseline by using resonance Raman spectroscopy (RRS) (reproducibility study; n = 74). Note bell-shaped appearance of histogram indicating a normal distribution.
We assessed the reproducibility of the dermal carotenoid biomarker over time with ICCs across the 3 body sites (palm, inner forearm, and outer forearm) and over each of the 6 time points for total carotenoids and lycopene. ICCs for total carotenoids for each of the 6 time points ranged from 0.85 to 0.89, indicating that participants who had high Raman counts for one body site were similarly high in the other body sites assessed. ICCs for total carotenoids within each body site over time were as follows: palm = 0.97, inner forearm = 0.95, and outer forearm = 0.93, showing that biomarker levels were consistent over time within each body site, with the highest reproducibility shown for the palm. ICCs for lycopene for each of the 6 time points ranged from 0.83 to 0.92, indicating that participants who had high lycopene Raman counts in one body site were similarly high in the other body sites assessed. ICCs for lycopene within each body site over time were as follows: palm = 0.93, inner forearm = 0.94, and outer forearm = 0.93.
We examined stratum-specific ICCs for total carotenoids and for lycopene by sex, age, race, skin tone, and smoking status; there was no evidence that ICCs varied consistently across any of these strata (data not shown).
Food-frequency dietary data were available for all but one participant; total carotenoid intake was significantly (r = 0.52, P < 0.001) predictive of dermal carotenoids, as was total fruit and vegetable intake (r = 0.39, P = 0.008; correlation coefficients are energy-adjusted).
We averaged the 6 total dermal carotenoid measurements for each subject taken over time and then examined how average dermal carotenoid status varied as a function of demographic/lifestyle variables (Table 1). None of the comparisons were significantly different, although we observed a tendency for higher carotenoid status in younger subjects, women, more educated subjects, those who smoked less or not at all, lean subjects (body mass index, in kg/m2: <25), and subjects with lighter skin tone.
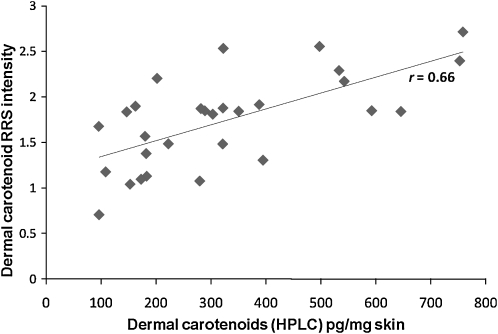
To verify that the RRS measures of total carotenoids and lycopene were valid, we recruited a sample of 28 healthy adults who agreed to provide dietary data and undergo phlebotomy and dermal posterior hip biopsy (3-mm punch biopsy) and be scanned at the site of the biopsy with use of RRS. We then compared correlations between the RRS biomarkers (total carotenoids and lycopene) and their relative levels in the dermal skin biopsies and in blood by using HPLC. Total carotenoid level in skin by RRS was significantly correlated with total carotenoid level in skin by HPLC (r = 0.66, P = 0.0001; Figure 2). Total carotenoid level in skin (palm RRS) was also significantly correlated with total carotenoid level in plasma (HPLC; r = 0.62, P = 0.006).
FIGURE 2.
Dermal total carotenoids in skin as assessed by HPLC analysis of dermal biopsy compared with dermal total carotenoids in skin as assessed by resonance Raman spectroscopy (RRS) (validity study; n = 28). Pearson's correlation coefficient r = 0.66 (P = 0.0001).
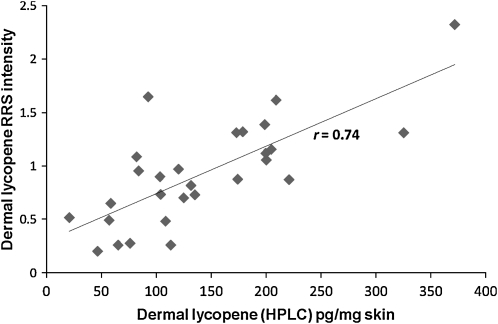
The lycopene concentration estimates can be approached in 2 ways. One way is to measure the lycopene RRS signal with 514-nm excitation and assume that 100% of the signal is due to lycopene. An alternative approach is correct the signal obtained with 514-nm excitation for the small amount of signal that would be expected to come from other carotenoids, as described elsewhere (15), which we refer to as the adjusted lycopene Raman score. We compared these 2 estimates of dermal RRS lycopene against the HPLC measures; both were strongly and significantly correlated with HPLC measures [r = 0.74 for adjusted lycopene by RRS compared with HPLC, P < 0.0001 (see Figure 3), and r = 0.68 for unadjusted lycopene by RRS compared with HPLC, P < 0.0001]. Lycopene level in skin (RRS) was also significantly correlated with lycopene level in plasma (HPLC; r = 0.42, P = 0.03 for adjusted lycopene; r = 0.41, P = 0.04 for unadjusted lycopene).
FIGURE 3.
Dermal lycopene in skin as assessed by HPLC analysis of dermal biopsy compared with dermal lycopene in skin as assessed by resonance Raman spectroscopy (RRS) (validity study; n = 28). Pearson's correlation coefficient r = 0.74 (P < 0.0001).
Our 2 human studies were not designed to directly measure and assess the effect of melanin on skin carotenoid RRS measures; however, in the reproducibility study, we examined how RRS carotenoid scores varied by race and skin tone. As shown in Table 1, RRS values were higher in nonwhites (which included Asians and African Americans) compared with whites but lower in the palm of the 3 subjects with the darkest skin. If melanin were having a filtering effect on the light that led to an underestimation of dermal carotenoid RRS counts, then the ratio of arm to palm total RRS carotenoid scores should be lower in dark-skinned participants (eg, higher ratio of melanin in outer arm compared with palm). We examined arm-to-palm ratios of total carotenoids in participants with dark compared with light skin pigmentation and saw no significant differences, although we had limited power to see such differences given our sample size.
DISCUSSION
Our findings indicate that the RRS dermal carotenoid biomarker is reproducible and valid and correlated with dietary intake of fruit and vegetables, and thus is promising for use in human studies. Carotenoid levels in skin (total carotenoids at baseline) were approximately normally distributed in contrast to blood carotenoid concentrations, which are known to be quite skewed with mean values exceeding medians for all of the major carotenoids [eg, see National Health and Nutrition Examination Survey data (1)] due to the relatively short half-life of carotenoids in blood, and therefore influence of recent carotenoid-containing meals.
In terms of feasibility of use, subjects responded well to the scanner, with each skin measurement taking only 1 min to complete, including data acquisition and processing time. The RRS device used in these studies was not easily portable; we have since developed a more portable device to overcome this limitation. We observed some laser drift with the device used in these studies over the months of data collection, supporting the need for concurrent use of an external standard as was done in these studies.
Our results indicate that the palm is preferred for RRS, with the highest carotenoid levels found in the palm, as well as the highest ICCs (presumably related in part to the greater carotenoid levels found in the palm).
The data from our study indicate that RRS measures of lycopene are valid and that RRS can effectively separate lycopene from the other carotenoids. Because there remains strong interest in health effects of lycopene (24), future studies can consider adding dermal lycopene as a noninvasive biomarker of lycopene status.
These studies had strengths and limitations. Most important, this is, to our knowledge, the first study to directly compare RRS measures against HPLC analyses of skin biopsies ex vivo in humans. Although it would have been desirable to observe correlation coefficients better than 0.7, it must be recognized that chemical analysis of 3-mm skin samples will also have some measurement error associated with it. Another important strength is that we evaluated variation in these biomarkers at multiple time points over 6 mo in a climate with strong seasonal influences. A limitation to our study is that our study population had only a small number of participants with deeply pigmented skin, precluding us from being able to better assess the impact of melanin on dermal carotenoid measurements. Our study population was limited to persons under the age of 65 y; skin quality may change with age, although this should be less of a concern in the palm. Future studies should ensure adequate representation of participants with a full range of skin pigmentation, with direct measures of melanin content of skin, and a greater age range.
It should be noted that the device we used for this RRS study was built for our purposes, and our results (eg, reproducibility) may not be applicable to results obtained by others with use of different devices with low sensitivities and/or different laser excitation wavelengths. It also should be noted that there are no data at this time linking dermal carotenoid levels with health outcomes; incorporation of RRS measures into epidemiologic studies is needed before making inferences about the health implications of dermal carotenoid levels.
Although more work is needed before dermal carotenoids as assessed by RRS becomes a widely used biomarker for human studies, our results to date are very promising and support further investigation. Having an objective indicator of nutritional status that can be noninvasively measured in 1 min is highly attractive. Also, it is quite clear that self-reported diet has substantial measurement error; having an objective biomarker with excellent measurement characteristics should greatly assist in research evaluating diet/nutritional interventions and diet/nutrition with regard to numerous health outcomes.
Of note, RRS may be a particularly useful biomarker of nutritional status in children, because children's dietary assessment is exceptionally difficult to do, and alternatives to venipuncture are particularly attractive for studies in children. We have recently obtained RRS measures of dermal carotenoids in a large sample of preschool children and have had excellent acceptance of the technique in this population (32).
In summary, we hereby report that RRS as used in this study is reproducible and valid as a biomarker of nutritional status for human studies, supporting its future utilization as a biomarker for translational research studies.
Supplementary Material
Acknowledgments
We thank the participants in these 2 human studies, the interviewer/phlebotomists, the students who assisted with data collection, and the staff at the Yale Dermatologic Surgery Suite.
The authors’ responsibilities were as follows—STM: experimental design, funding, manuscript drafting, and review; BC: experimental design, supervision of human studies, and manuscript review; SS: recruitment of study subjects, data collection, and manuscript review; HL: experimental design, statistical analysis, and manuscript review; DJL: experimental design, coordination of biopsy clinical procedures, and manuscript review; EW: clinical biopsies and manuscript review; IE: spectroscopy support, training, data interpretation, and manuscript review; PB: biochemical analyses of blood and tissues and manuscript review; PSB: experimental design, supervision of biochemical analyses, and manuscript review; WG: design and construction of laser system, oversight of training for data collection, and manuscript review. WG and PSB hold patents for the methods described in this article. The authors reported no other competing interests.
REFERENCES
- 1.Institute of Medicine, National Academy of Sciences, Food and Nutrition Board, Panel on Dietary Antioxidants and Related Compounds Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academies Press, 2000 [PubMed] [Google Scholar]
- 2.Peng YM, Peng YS, Lin Y, Moon T, Roe DJ, Ritenbaugh C. Concentrations and plasma-tissue-diet relationships of carotenoids, retinoids, and tocopherols in humans. Nutr Cancer 1995;23:233–46 [DOI] [PubMed] [Google Scholar]
- 3.McEligot AJ, Rock CL, Flatt SW, Newman V, Faerber S, Pierce JP. Plasma carotenoids are biomarkers of long-term high vegetable intake in women with breast cancer. J Nutr 1999;129:2258–63 [DOI] [PubMed] [Google Scholar]
- 4.Lanza E, Schatzkin A, Daston C, et al. Implementation of a 4-y, high-fiber, high-fruit-and-vegetable, low-fat dietary intervention: results of dietary changes in the Polyp Prevention Trial. Am J Clin Nutr 2001;74:387–401 [DOI] [PubMed] [Google Scholar]
- 5.World Cancer Research Fund, American Institution for Cancer Research Food, nutrition, physical activity, and the prevention of cancer: a global perspective. The Second Expert Report. Washington, DC: AICR, 2007 [Google Scholar]
- 6.Liu S, Manson JE, Lee IM, et al. Fruit and vegetable intake and risk of cardiovascular disease: the Women's Health Study. Am J Clin Nutr 2000;72:922–8 [DOI] [PubMed] [Google Scholar]
- 7.Krinsky NI, Mayne ST, Sies H. Carotenoids in health and disease. New York, NY: Marcel Dekker, 2004 [Google Scholar]
- 8.Kristal AR, Peters U, Potter JD. Is it time to abandon the food frequency questionnaire? Cancer Epidemiol Biomarkers Prev 2005;14:2826–8 [DOI] [PubMed] [Google Scholar]
- 9.Ray AL, Semba RD, Walston J, et al. Low serum selenium and total carotenoids predict mortality among older women living in the community: the women's health and aging studies. J Nutr 2006;136:172–6 [DOI] [PubMed] [Google Scholar]
- 10.Rock CL, Swendseid ME, Jacob RA, McKee RW. Plasma carotenoid levels in human subjects fed a low carotenoid diet. J Nutr 1992;122:96–100 [DOI] [PubMed] [Google Scholar]
- 11.Bernstein PS, Yoshida MD, Katz NB, McClane RW, Gellermann W. Raman detection of macular carotenoid pigments in intact human retina. Invest Ophthalmol Vis Sci 1998;39:2003–11 [PubMed] [Google Scholar]
- 12.Ermakov IV, McClane RW, Gellermann W, Bernstein PS. Resonant Raman detection of macular pigment levels in the living human retina. Opt Lett 2001;26:202–4 [DOI] [PubMed] [Google Scholar]
- 13.Gellermann W, Ermakov IV, Ermakova MR, McClane RW, Zhao DY, Bernstein PS. In vivo resonant Raman measurement of macular carotenoid pigments in the young and the aging human retina. J Opt Soc Am A Opt Image Sci Vis 2002;19:1172–86 [DOI] [PubMed] [Google Scholar]
- 14.Sharifzadeh M, Bernstein PS, Gellermann W. Nonmydriatic fluorescence-based quantitative imaging of human macular pigment distributions. J Opt Soc Am A Opt Image Sci Vis 2006;23:2373–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ermakov IV, Ermakova MR, Gellermann W, Lademann J. Noninvasive selective detection of lycopene and beta-carotene in human skin using Raman spectroscopy. J Biomed Opt 2004;9:332–8 [DOI] [PubMed] [Google Scholar]
- 16.Hata TR, Scholz TA, Ermakov IV, et al. Non-invasive Raman spectroscopic detection of carotenoids in human skin. [see comment] J Invest Dermatol 2000;115:441–8 [DOI] [PubMed] [Google Scholar]
- 17.Ermakov IV, Ermakova MR, McClane RW, Gellermann W. Resonance Raman detection of carotenoid antioxidants in living human tissues. Opt Lett 2001;26:1179–81 [DOI] [PubMed] [Google Scholar]
- 18.Ermakov IV, Sharifzadeh M, Bernstein PS, Gellermann W. Application of resonance Raman spectroscopy to the detection of carotenoids in vivo : Landrum JT. Carotenoids–physical, chemical and biological functions and properties. Atlanta, GA: CRC Press, 2009:87–109 [Google Scholar]
- 19.Darvin ME, Patzelt A, Knorr F, Blume-Peytavi U, Sterry W, Lademann J. One-year study on the variation of carotenoid antioxidant substances in living human skin: influence of dietary supplementation and stress factors. J Biomed Opt 2008;13:044028-1–044028-9 [DOI] [PubMed] [Google Scholar]
- 20.Rerksuppaphol S, Rerksuppaphol L. Effect of fruit and vegetable intake on skin carotenoid detected by non-invasive Raman spectroscopy. J Med Assoc Thai 2006;89:1206–12 [PubMed] [Google Scholar]
- 21.Blume-Peytavi U, Rolland A, Darvin ME, et al. Cutaneous lycopene and beta-carotene levels measured by resonance Raman spectroscopy: high reliability and sensitivity to oral lactolycopene deprivation and supplementation. Eur J Pharm Biopharm 2009;73:187–94 [DOI] [PubMed] [Google Scholar]
- 22.Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med 2005;26:459–516 [DOI] [PubMed] [Google Scholar]
- 23.Mayne ST, Cartmel B, Silva F, et al. Plasma lycopene concentrations in humans are determined by lycopene intake, plasma cholesterol concentrations and selected demographic factors. J Nutr 1999;129:849–54 [DOI] [PubMed] [Google Scholar]
- 24.Davis CD, Clevidence B, Swanson CA, Ziegler RG, Dwyer JT, Milner JA. A research agenda for lycopene/tomato supplementation and cancer prevention. J Nutr 2005;135:S2074. [DOI] [PubMed] [Google Scholar]
- 25.Faure H, Preziosi P, Roussel AM, et al. Factors influencing blood concentration of retinol, alpha-tocopherol, vitamin C, and beta-carotene in the French participants of the SU.VI.MAX trial. Eur J Clin Nutr 2006;60:706–17 [DOI] [PubMed] [Google Scholar]
- 26.Ermakov IV, Gellerman W. Validation study of Raman-based skin carotenoid detection. Arch Biochem Biophys (i n press). [DOI] [PubMed] [Google Scholar]
- 27.Rock CL, Thornquist MD, Kristal AR, et al. Demographic, dietary and lifestyle factors differentially explain variability in serum carotenoids and fat-soluble vitamins: baseline results from the sentinel site of the Olestra Post-Marketing Surveillance Study. J Nutr 1999;129:855–64 [DOI] [PubMed] [Google Scholar]
- 28.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol 1999;9:178–87 [DOI] [PubMed] [Google Scholar]
- 29.Peng YM, Peng YS, Lin Y. A nonsaponification method for the determination of carotenoids, retinoids, and tocopherols in solid human tissues. Cancer Epidemiol Biomarkers Prev 1993;2:139–44 [PubMed] [Google Scholar]
- 30.Weissenberg M, Schaeffler I, Menagem E, Barzilai M, Levy A. Isocratic non-aqueous reversed-phase high-performance liquid chromatographic separation of capsanthin and capsorubin in red peppers (Capsicum annuum L.), paprika and oleoresin. J Chromatogr A 1997;757:89–95 [DOI] [PubMed] [Google Scholar]
- 31.Bhosale P, Serban B. Zhao da Y, Bernstein PS. Identification and metabolic transformations of carotenoids in ocular tissues of the Japanese quail Coturnix japonica. Biochemistry 2007;46:9050–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scarmo S. Noninvasive measurement of carotenoids in human skin as a biomarker of fruit and vegetable intake. PhD dissertation Yale University, New Haven, CT, 2009 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.