Abstract
ΔNp73α, a dominant-negative inhibitor of p53 and p73, exhibits antiapoptotic and transforming activity in in vitro models and is often found to be upregulated in human cancers. The mechanisms involved in the regulation of ΔNp73α protein levels in normal and cancer cells are poorly characterized. Here, we show that that IκB kinase beta (IKKβ) increases ΔNp73α protein stability independently of its ability to activate NF-κB. IKKβ associates with and phosphorylates ΔNp73α at serine 422 (S422), leading to its accumulation in the nucleus, where it binds and represses several p53-regulated genes. S422A mutation in ΔNp73α abolished IKKβ-mediated stabilization and inhibition of p53-regulated gene expression. Inhibition of IKKβ activity by chemical inhibitors, overexpression of dominant-negative mutants, or gene silencing by siRNA also resulted in ΔNp73α destabilization, which under these conditions was rapidly translocated into the cytoplasm and degraded by a calpain-mediated mechanism. We also present evidence for the IKKβ and ΔNp73α cross talk in cancer-derived cell lines and primary cancers. Our data unveil a new mechanism involved in the regulation of the p73 and p53 network.
INTRODUCTION
p53 and its family members, p63 and p73, are transcription factors that play an important role in the regulation of the cell cycle, apoptosis, and cancer development (4, 23). All three proteins show similarity in the amino acid sequences of their N-terminal transcription activation (TA), DNA binding, and oligomerization domains. p73 and p53 are also functionally related, since they have the ability to bind a similar set of p53 regulatory elements (REs) (16). Both proteins are functionally regulated by posttranslational modifications, and p73 appears to be subject to more complex regulatory mechanisms than p53 at transcriptional level. The p73 gene is expressed as multiple isoforms that differ in their N and/or C terminus. The generation of different transcripts of p73 involves the use of two distinct promoters (P1 and P2) and/or alternative splicing. The mRNA of the full-length p73 isoform (TAp73) is transcribed by the P1 promoter located upstream of exon 1, while an isoform called ΔNp73 is generated by using the P2 promoter in intron 3 (P2). Three additional Δ isoforms, ΔN′p73, ΔEx2p73, and ΔEx2/3p73, arise from alternative splicing of the transcripts originating from the first exons. All ΔN isoforms lack the TA domain located at the N terminus (exons 2 and 3). Multiple splicing of exons 10 to 14 generate additional TA and ΔN p73 isoforms (α, β, γ, δ, ε, ζ, θ, η, and η1) that differ at the C terminus, affecting the biological properties of p73 isoforms (19, 30). For instance, ΔNp73β induces cell cycle arrest and apoptosis, while ΔNp73α exerts antiapoptotic functions and promotes cellular transformation (21).
The antiapoptotic function of ΔNp73α can be explained by at least two mechanisms. In the first, ΔNp73α competes with p53 for binding to p53 REs and prevents the activation of p53- or p73-regulated genes. In the second, ΔNp73α associates with TAp73 to form transcriptionally inactive heterodimer complexes (4, 23). Thus, ΔNp73α acts as a dominant-negative inhibitor of p53 and p73 transcriptional functions.
High ΔNp73 levels have been found in a number of human malignancies, including cancers of the breast, prostate, liver, lung, and thyroid (4). Overexpression of ΔNp73α in cancer cell lines inhibits the expression of p53/p73-regulated genes and increases proliferation (13, 15, 34). In addition, high levels of ΔNp73α in cancer cells with wild-type p53 and/or p73 functions correlate with increased drug resistance (4, 23). Accordingly, an unfavorable prognosis of some cancers is correlated with high ΔNp73 expression levels (8, 22).
Several mechanisms that influence TAp73 protein levels have been elucidated. Similar to the case with p53, p73 half-life and activity are regulated by posttranslational modifications, such as phosphorylation and acetylation (2, 7, 11, 12, 14, 27, 33). Upon induction of DNA damage by cisplatin, p73 is phosphorylated at three distinct sites by Chk1, c-Abl, and PKCδ (2, 11, 12, 27, 33). In addition, a more recent study showed that the same DNA damaging agent induces the translocation of I kappa B kinase α (IKKα) in the nucleus, which in turn phosphorylates TAp73 at the N terminus, increasing its stability (10). In contrast to p73, very little is known about the events involved in controlling ΔNp73 levels.
Here we describe a novel mechanism that regulates the protein levels and activity of ΔNp73α via phosphorylation by IKKβ, which leads to stabilization of ΔNp73α and stimulation of its prosurvival activity.
MATERIALS AND METHODS
Expression vectors.
Cellular and viral genes were expressed using the retroviral vector pBabe (24) or pLXSN (Clontech, Palo Alto, CA) and the expression vector pcDNA-3 (Invitrogen). The pLXSN-HPV38 E6/E7 construct has been previously described (5). The following constructs were generated during this study, using standard molecular biology techniques: pBabe-puro-FlagDN-IKK, pBabe-puro ΔN-IκBα lacking the first N-terminal 36 amino acids (kindly provided by Thomas Gilmore, Boston University), pcDNA3 HA-ΔNp73α mutants (A159 A163, A418 A422, A418, A422, and A521 A525 mutants), and pcDNA3 HA-ΔNp73α deletion mutants (amino acids 1 to 300, 1 to 450, or 350 to 587). pcDNA-Flag-IκBα wild type was provided by Thomas Gilmore (Boston University). pcDNA3 wild-type HA-ΔNp73α and -β were kindly provided by Takashi Tanaka (Columbia University, New York), while pcRK5-Flag-IKKα, pcRK5-Flag-IKKβ, and pcRK5-Myc-tagged IKKβ deletion mutants (amino acids 1 to 303 or 304 to 756) were kindly provided by David Goeddel (Tulirak, San Francisco, CA).
Cell culture procedures.
Keratinocyte cultures and generation of high-titer retroviral supernatants were carried out as previously described (5). Saos-2, HNC-136, human embryonic kidney (HEK293), HCC1937, and Cal-51 cells and mouse embryonic fibroblasts (MEFs) were cultured in fetal calf serum (FCS) and Dulbecco's modified Eagle medium (DMEM) (Gibco) using standard culturing conditions. Wild-type and IKK null MEFs were kindly provided by Inder Verma (Salk Institute, San Diego, CA). Cells were transiently transfected with the different expression vectors by using FuGENE6 reagent (Roche).
Gene silencing of IKKα and -β was obtained using synthetic small interfering RNA (siRNA) (Table 1). siRNA or scrambled RNA at a concentration of 50 nM was transfected using Oligofectamine according to the standard protocol (Invitrogen).
Table 1.
Sequences of different siRNAs used for gene silencing
| Target | siRNA sequence or description (source) |
|---|---|
| IKKα gene | 5′-GCAGGCUCUUUCAGGGACA-3′ |
| IKKβ gene | 5′-CGUACGCGGAAUACUUCGA-3′ |
| p65 gene | siGenome SMART pool M-003533-02-0005, human RELA, NM_021975 (Thermo Scientific) |
| Scrambled (negative control) | 5′-GGUGGAAGAGGUGGUGAGC-3′ |
Downregulation of ΔNp73α was achieved by transfecting the antisense (AS) oligonucleotide as previously described (1).
Pulse-chase labeling.
For metabolic labeling, 24 h after transfection, HEK293 cells were grown for 1 h at 37°C in DMEM lacking l-methionine (Gibco) and pulsed for 1 h with 50 μCi/ml of EasyTag l-[S35]methionine (Perkin Elmer). Afterwards, cells were washed with phosphate-buffered saline and chased with complete culture medium for 4, 8, and 12 h. Then, cells were washed in cold phosphate-buffered saline and collected. Cellular pellets were stored at −80°C until further analysis.
In vitro cell treatments.
Cells were treated with the IκBα kinase inhibitor Bay11-7082 (20 μM) (Calbiochem) for 2 h. The proteosome inhibitors MG132 (CBZ-Leu-Leu-Leu-al) (50 μM) (Sigma) and lactacystin (10 μM) (Calbiochem), calpain inhibitor PD150606 (50 μM) (Calbiochem), calpain inhibitor VI (150 μM) (Calbiochem), and cysteine protease inhibitor E-64 (30 μM) (Calbiochem) were added to the culture medium for 8 h. Tumor necrosis factor alpha (TNF-α) (B&D) was used at the final concentration of 10 ng/ml at different time points, as indicated in each specific experiment. For the determination of ΔNp73α, half-life cells were treated with cycloheximide as previously described (1). An in vitro calpain cleavage assay was performed as previously described (25).
For in vitro protein dephosphorylation, samples were treated with 100 U of λ phosphatase (BioLabs) for 30 min at 30°C.
For ionizing radiation treatment, cells were trypsinized and resuspended in DMEM containing 10% FCS. Afterwards, cells were irradiated with a dose of 30 Gy.
RT-PCR.
Total cellular RNA was extracted from cells or tissues using the Absolutely RNA Miniprep kit (Stratagene). Reverse transcriptase (RT)-PCR analyses were carried out as described previously (5). The primer sequences used for RT-PCR are indicated in Table 2.
Table 2.
Sequences of primers used for RT-PCR analyses, for ChIP, and for cloninga
| Promoter or gene for: | Primer sequence |
|---|---|
| Mouse ΔNp73 | F: 5′-GTGACCCCATGAGACACCTC-3′ |
| R: 5′-GTATGTCCAGGTGGCCGAC-3′ | |
| Mouse GAPDH | F: 5′-GCCAAAAGGGTCATCATC-3′ |
| R: 5′-TGCCAGTGAGCTTCCCGTTC-3′ | |
| ΔNp73 | F: 5′-AACCATGCTGTACGTCGGTGACCCC-3′ |
| R: 5′-GCGACATGGTGTCGAAGGTGG-3′ | |
| GAPDH | F: 5′-AAGGTGGTGAAGCAGGCGT-3′ |
| R: 5′-GAGGAGTGGGTGTCGCTGTT-3′ | |
| Pig3 | F: 5′-GCTTCAAATGGCAGAAAAGC-3′ |
| R: 5′-AACCCATCGACCATCAAGAG-3′ | |
| Pig3b | F. 5′-CCCAGGACTGCGTTTTGCCT-3′ |
| R: 5′-GGTCCATTTTCCAGGCATGG-3′ | |
| p21b | F: 5′-CGAGGCAGGCCAAGGG-3′ |
| R: 5′-GCAGAGGATGGATTGTTCA-3′ | |
| HA-ΔNp73 (1–300) | F: 5′-AAAAAAGCTTATGTATCCATACGATGTCCCTGATTACGCTATGCTGTACGTCGGTGACCC-3′ |
| R: 5′-AAAAGCGGCCGCTTAATGCCGCCGCTTCTTCACA-3′ | |
| HA-ΔNp73 (11–450) | F: 5′-AAAAAAGCTTATGTATCCATACGATGTCCCT GATTACGCTATGCTGTACGTCGGTGACCC-3′ |
| R: 5′-AAAAGCGGCCGCTTATCCTGTTAAAAAACTGACGA-3′ | |
| HA-ΔNp73 (3001–587) | F: 5′-AAAAAAGCTTATGTATCCATACGATGTCCCTGATTACGCTCATGGAGACGAGGACACGTA-3′ |
| R: 5′-AAAAGCGGCCGCTCAGTGGATCTCGGCCTCCGT-3′ |
F, forward; R, reverse.
Promoter.
Immunoblotting and antibodies.
Total protein extracts, sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), and immunoblotting were prepared as described by Accardi et al. (1). The following antibodies were used: β-actin (C4; MP Biomedicals), human p53 (NCL-CM1; Novocastra Laboratories Ltd.), p73 (anti-p73 Ab-1; Calbiochem), IKKβ and IKKα (Upstate), hemagglutinin (HA)-peroxidase-high affinity (3F10; Roche), Flag antibody (M5;Sigma), c-Myc (Sigma), mouse p73 (Ab-2; NeoMarkers), phospho-IκBα Ser32/36 (9246), Iκbα (9242), and p21WAF1/CIP1 (DCS60 2946) from Cell Signaling, and anti-IgG (Diagenode).
The anti-phosphoserine 422 ΔNp73 antibody was generated by Biogenes. A synthetic peptide, C-Nle-SSSH-pS-AQS-Nle-V-amide, conjugated with the carrier protein lactase-phlorizin hydrolase, was synthesized. Six boosts were administered to the rabbits over a period of 3 months. Final bleedings were collected, and monospecific IgG was purified over affinity columns.
Immunoprecipitation.
For immunoprecipitations of overexpressed proteins, total cell extracts (250 μg) were precleared with Sepharose CL-6B for 1 h at 4°C. After preclearing, total protein extracts were mixed with 40 μl Flag M2-conjugated beads (Sigma), 50 μl of anti-c-Myc agarose-conjugate (Sigma), or 1 μg of anti-HA antibody mixed with 30 μl of protein A/G Sepharose (Roche) and incubated overnight at 4°C. Beads were extensively washed in lysis buffer and analyzed by immunoblotting. Anti-IKKβ antibody (Upstate) and anti-ΔNp73 (IMG-313A; Imgenex) were used to immunoprecipitate endogeneous IKKβ and ΔNp73 using 1 to 2 mg of total extracts of 38E6E7HFK, HNC-136, HCC1937, and Cal-51 cells.
Chromatin immunoprecipitation (ChIP) was performed with Diagenode Shearing ChIP and OneDay ChIP kits according to the manufacturers' protocols.
Immunofluorescence.
Immunofluorescence staining of monolayer cultured keratinocytes was performed on cells grown on cover slides and fixed with 4% formaldehyde, permeabilized with PBS-0.1% Triton X, and stained using the following primary antibodies: anti-human ΔNp73 (IMG-313A; Imgenex), anti-rabbit IKKβ (Millipore), and anti-p65 (Santa Cruz). Alexa Fluor 488 goat anti-rabbit IgG(H+L) and Alexa Fluor 532 goat anti-mouse IgG(H+L) were used as secondary antibodies. The slides were mounted using mounting medium containing DAPI (4′,6-diamidino-2-phenylindole) (H-1200; Vectashield) and analyzed with an immunofluorescence Axioplan2 microscope from Zeiss or by confocal laser scan microscopy (Leica). For fluorescence resonance energy transfer (FRET), cells were fixed, permeabilized, and stained as explained above. As secondary antibodies, the combination of Alexa 488 (donor) and Alexa 555 (acceptor) was used. After staining, cells were analyzed using the Leica TCS SP5 II spectral confocal system. To measure FRET, three images were acquired sequentially in the same order through the following: (i) an Alexa 488 filter set (excitation, 488 nm; emission, 520 to 580 nm; filter at 488 nm), (ii) an Alexa 555 filter set (excitation, 543 nm; emission, 590 to 700 nm; filter at 540 nm), and (iii) a FRET filter set (excitation, 488 nm; emission, 580 to 700 nm; filter at 540 nm) according to methods in previous publications (6, 26).
Immunohistochemistry.
Normal (n = 2) and invasive breast cancers (n = 1 grade I specimen; n = 1 grade II specimen; and n = 3 grade III specimens) were provided and histologically examined by L. Frappart (Pathology Department, Hospital E. Herriot, Lyon, France). Processing of the specimens was performed in accordance with the ethical guidelines for handling of human material. Immunohistochemical stainings were carried out on 3-μm sections and performed on serial sections, using the antibody anti-human ΔNp73 (1:500; Imgenex), anti-mouse IKKβ (1:300; Upstate), or ΔNp73α-P-422S antibody. Preincubation of ΔNp73α-P-422S antibody with phosphorylated or unphosphorylated ΔNp73α peptide was performed at room temperature for 30 min. Immunohistochemical signal was revealed with the Vectastain EliteABC kit (PK 6102; Vector Laboratories) according to the manufacturer's protocol. Images (magnification, ×40) were taken with a Nikon Eclipse E600 camera. The intensity of nuclear staining was quantified by the color deconvolution technique with the Common Centre of Quantimetry (CCQ) (UBCL, Lyon, France).
In vitro kinase assay.
In vitro phosphorylation of ΔNp73 by IKKβ was performed by combining 2 μg of the glutathione S-transferase (GST) fusion proteins with the IKKβ immunoprecipitated from HPV38 expressing keratinocytes pretreated or not with Bay11. The reaction was performed in 30 μl of kinase buffer (20 mM HEPES, 10 mM MgCl2, 1 mM dithiothreitol [DTT], 10 mM p-nitrophenyl phosphate (PNPP), 100 mM β-glycerol-3-phosphate, 25 μM NaV, and 40 μM cold ATP) and 20 μCi of [γ-32P]ATP (Perkin Elmer) at 30°C for 30 min. The reaction was terminated by the addition of SDS-PAGE sample buffer. Lysates were separated by SDS-PAGE (12%), transferred to nitrocellulose, and visualized on X-ray films.
In vivo 32P protein labeling.
HEK293 cells were transfected with the different constructs as appropriate. After 24 h, cells were grown for 3 h in serum-free and phosphate-free medium and then incubated with 0.3 mCi/ml of [32P]orthophosphate for three additional hours. Cells were lysed in lysis buffer (20 mM Tris-HCl [pH 8], 200 mM NaCl, 0.5% Nonident P-40, 1 mM EDTA, 10 mM KCl, 1 mM DTT, 50 mM NaF, 50 mM β-glycerophosphate, 1 mM Na orthovanadate, and 0.1 μM okadaic acid) and immunoprecipitated with 1 μg of anti-HA-tag antibody (Roche). The immunopellets were separated on a 10% SDS gel. The gel was dried and exposed to an X-ray film. The same experiment was performed in parallel in the absence of [32P]orthophosphate and analyzed by immunoblotting. The amount of radioactivity incorporated on the HA-ΔNp73 protein was measured by using a PhosphorImager (445-SI) and quantified by the ImageQuant software program (Molecular Dynamics). The protein levels were quantified as described by Accardi et al. (1).
RESULTS
IKK pathway influences ΔNp73α protein levels.
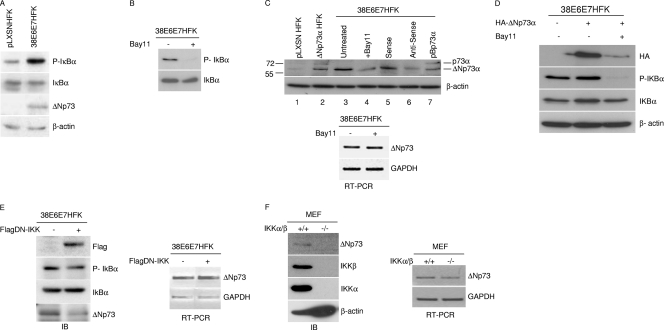
Based on previous findings on the ability of IKKα to stabilize TAp73 (10), we evaluated whether ΔNp73α stability could be regulated by the NF-κB pathway. We used human foreskin keratinocytes expressing the E6 and E7 oncoproteins of human papillomavirus (HPV) type 38 (38E6E7HFK) that, as previously shown, express high ΔNp73α levels and not other p73 isoforms (1). As shown in Fig. 1A, IκBα was found to be hyperphosphorylated in 38E6E7HFK cells in comparison to primary keratinocytes (pLXSNHFK). The phosphorylation and degradation of IκBα is mediated by activated IKKα and IKKβ in the canonical pathway of NF-κB; thus, our findings indicate that IKK complex is constitutively activated in these cells. Inhibition of IKK by the chemical compound Bay11-7082 (Bay11) led to a reduction in IκBα phosphorylation (Fig. 1B) and in the levels of 60- to 65-kDa protein, which is recognized by an anti-p73 antibody and most likely corresponds to ΔNp73α (Fig. 1C, top panel, compare lanes 3 and 4). Indeed, this protein comigrated with the ectopically expressed ΔNp73α in HFKs (Fig. 1C, lane 2). In addition, its expression was significantly decreased by antisense oligonucleotides specific for the ΔNp73 isoforms and showed a migration different from that of p73 (Fig. 1C, top panel, lanes 5 to 7). In contrast to protein levels, Bay11 treatment did not significantly alter ΔNp73α transcript levels (Fig. 1C, bottom panel). Similar results were obtained when a hemagglutinin (HA) tag-ΔNp73α fusion protein (HA-ΔNp73α) was ectopically expressed in 38E6E7HFK cells. In fact, immunoblotting using HA tag antibody showed that ΔNp73α protein levels were strongly affected by Bay11 treatment (Fig. 1D). Similar to Bay11 treatment, blocking IKK signaling pathways by overexpressing a dominant-negative mutant of IKKβ (DN-IKK) in 38E6E7HFK cells also resulted in reduced levels of phospho-IκBα and a decrease in ΔNp73α protein levels (Fig. 1E, left panel), while no significant changes in ΔNp73α mRNA levels were observed (Fig. 1E, right panel). Finally, immunoblotting with an antibody that recognizes exclusively the ΔNp73 form in mouse embryo fibroblasts (MEFs) (28) showed that ΔNp73α is expressed in MEFs but not in IKKα/β knockout MEFs (Fig. 1F, left panel). Also, in this case, no significant difference was observed in ΔNp73 mRNA levels in wild-type and IKKα/β null MEFs (Fig. 1F, right panel).
Fig. 1.
The IKK complex regulates the stability of the ΔNp73 protein. (A) Protein extracts of primary keratinocytes transduced with empty retrovirus vector (pLXSN HFK) or immortalized keratinocytes expressing HPV type 38 E6 and E7 (38E6E7HFK) were analyzed by immunoblotting with the indicated antibodies. (B) 38E6E7HFK cells were treated with Bay11 or dimethyl sulfoxide (DMSO) for 2 h. Protein extracts were prepared and analyzed by immunoblotting with the indicated antibodies. (C) Protein extracts of pLXSN HFKs or HFKs stably expressing ΔNp73α or p73α, 38E6E7HFK cells, or 38E6E7HFK cells cultured in the presence of Bay11 or transfected with ΔNp73 sense or antisense oligonucleotide were analyzed by immunoblotting with the indicated antibodies (upper panel). Total RNA was also extracted from Bay11-treated 38E6E7HFK cells, and ΔNp73 or GAPDH mRNA levels were measured by RT-PCR (bottom panel). (D) 38E6E7HFK cells were transfected with a HA-ΔNp73α construct. Cells were treated with Bay11 or DMSO for 2 h and processed as for panel A. (E) 38E6E7HFK cells were transduced with pBabe-puro (pBp) or pBp-flagged dominant-negative kinase-dead IKK (Flag-DN-IKK) retrovirus. Protein extracts were analyzed by immunoblotting with the indicated antibodies (left panel). Total RNA was also extracted from both cell lines, and ΔNp73 or GAPDH mRNA levels were measured by RT-PCR (right panel). (F) Protein extracts from wild-type MEFs (IKKα/β+/+) and IKKα/β−/− MEFs were prepared and analyzed by immunoblotting using the indicated antibodies (left panel). Total RNA was prepared from wild-type or IKKα/β knockout MEFs, and the levels of ΔNp73 transcript were determined by RT-PCR (right panel).
Thus, an active IKK complex is correlated with the accumulation of the ΔNp73α protein without increasing its gene transcription.
ΔNp73α stabilization is dependent on IKKβ activity and does not require NF-κB activation.
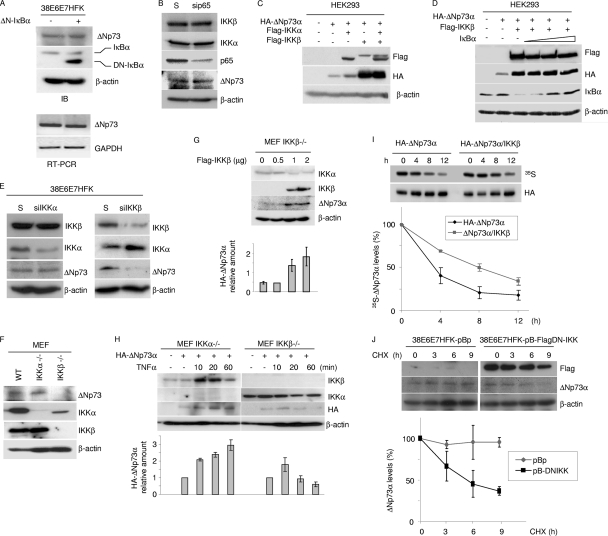
IKK activates the transcription factor NF-κB by promoting degradation of IκB and subsequent translocation of NF-κB into the nucleus, where it activates the transcription of target genes (17, 18). ΔNp73α accumulation in 38E6E7HFK cells may be directly regulated by IKK complex independently of NF-κB activation, or it requires the transcription of specific NF-κB-regulated genes. To discriminate between these two possibilities, we generated a stable cell line of 38E6E7HFK that expressed a nondegradable deletion mutant of IκBα (ΔN-IκBα), which lacks the first 36 amino acids at the N terminus and is not regulated by IKK. Accordingly, activation of IKK by TNF-α in ΔN-IκBα-expressing 38E6E7HFK cells did not result in p65 translocation to the nucleus (data not shown). In these cells, no decrease in ΔNp73α mRNA or protein levels occurred (Fig. 2A) in the presence of ectopic levels of ΔN-IκBα. Similarly, downregulation of NF-κB p65 by siRNA did not alter ΔNp73α protein levels (Fig. 2B). Thus, the accumulation of ΔNp73α mediated by IKK appears to be independent of NF-κB. To further evaluate a direct role for IKK in ΔNp73α accumulation, we expressed IKKα and/or IKKβ in HEK293 cells together with ΔNp73α. IKKα only marginally affected ΔNp73α protein levels, while IKKβ induced strong ΔNp73α accumulation (Fig. 2C). In the same experimental model (HEK293 cells), increasing the concentration of IκBα did not affect the ability of IKKβ to stabilize ΔNp73α (Fig. 2D). Gene silencing by siRNA of IKKα or IKKβ in 38E6E7HFK cells confirmed that IKKβ is mainly responsible for the increased protein levels of ΔNp73α (Fig. 2E). Accordingly, ΔNp73α could be detected in IKKα−/− MEFs, but it is markedly lower in IKKβ−/− MEFs (Fig. 2F). Reintroduction of IKKβ into IKKβ−/− MEFs led to an increase in endogenous ΔNp73α protein levels (Fig. 2G). In addition, exposure of cells to TNF-α, a potent activator of IKK, led to sustained accumulation of HA-ΔNp73α in IKKα−/− MEFs but not in IKKβ−/− cells (Fig. 2H). Finally, overexpression of IKKβ increased the stability of ΔNp73α in HEK293 cells, while inhibition of IKKβ by overexpression of DN-IKK resulted in a decrease in the half-life of endogenous ΔNp73α in 38E6E7HFK cells (Fig. 2I and J). As a whole, these data provide further evidence for a direct role of IKKβ but not ΙKKα in ΔNp73α protein stability.
Fig. 2.
IKKβ increases ΔNp73 protein levels. (A) 38E6E7HFK cells were transduced with pBp or with pBp-ΔN-IκBα superrepressor (ΔN-IκBα). Protein extracts were analyzed by immunoblotting using the indicated antibodies (top panel). Total RNA was also extracted from both cell lines, and ΔNp73 or GAPDH mRNA levels were measured by RT-PCR (bottom panel). (B) Scrambled (S) RNA and siRNA for p65 (sip65) was transfected in 38E6E7HFK cells. Thirty-six hours after transfection, protein extracts were analyzed by immunoblotting with the indicated antibodies. (C and D) HEK293 cells were transfected with different expression constructs as indicated. After 24 h, protein extracts were analyzed by immunoblotting with the indicated antibodies. (E) Scrambled (S) RNA and siRNA for IKKα (siIKKα) or IKKβ (siIKKβ) was transfected in 38E6E7HFK cells. Thirty-six hours after transfection, protein extracts were analyzed by immunoblotting with the indicated antibodies. (F) IKKα/β+/+ (WT), IKKα−/−, and IKKβ−/− MEF cellular protein extracts were analyzed by immunoblotting with the indicated antibodies. (G) IKKβ−/− MEF cells were transfected with increasing concentrations of pcDNA3-Flag-IKKβ, and 24 h after transfection, protein extracts were analyzed by immunoblotting with the indicated antibodies (top panel). The ΔNp73 protein signal was quantified by the Quantity One software program (Bio-Rad), normalized on the levels of β-actin, and the values obtained were reported in the histogram (bottom panel). The data are the means of results from two independent experiments. (H) IKKα−/− MEF and IKKβ−/− MEF cells were transfected with pcDNA3-HA-ΔNp73α and treated with TNF-α at the indicated time points. Protein extracts were analyzed by immunoblotting with the indicated antibodies (top panel). The amounts of HA-ΔNp73 signal in the Western blot were quantified as explained for panel G and are reported in the histogram (bottom panel). The data are the means of results from two independent experiments. (I) HEK293 cells were transfected with HA-tagged ΔNp73α in the absence or presence of overexpressed Flag-tagged IKKβ. Twenty-four hours after transfection, cells were labeled for 1 h with l- [35S]methionine, chased for the indicated times, and collected. Following anti-HA immunoprecipitation, the immunocomplexes were loaded on an SDS-PAGE gel and transferred onto a polyvinylidene difluoride (PVDF) membrane. Autoradiography (top panel) and then anti-HA Western blotting (middle panel) were performed; 35S-HA-ΔNp73 bands were quantified by Image Lab (Bio-Rad) and normalized on the total levels of immunoprecipitated HA-ΔNp73 protein. The percentage of ΔNp73 at time zero was referred to as 100%, and the percentages of protein at the different time points were calculated relative to that at time zero and reported in the histogram (lower panel). The data are the means of results for two independent experiments. (J) 38E6E7HFK cells stably expressing a dominant-negative inhibitor of IKKβ (pB-FlagDN-IKK) were generated and cultured in the presence of CHX for the indicated number of hours. At each time point, cells were collected. Protein extracts were prepared and analyzed by immunoblotting with the indicated antibodies (upper panel). The levels of ΔNp73 were quantified by Quantity One (Bio-Rad) and normalized on the levels of β-actin. The percentages of ΔNp73 at the different time points were calculated as for Fig. 1G and are reported in the histogram (lower panel). The data are the means of results for two independent experiments.
IKKβ interacts with ΔNp73α.
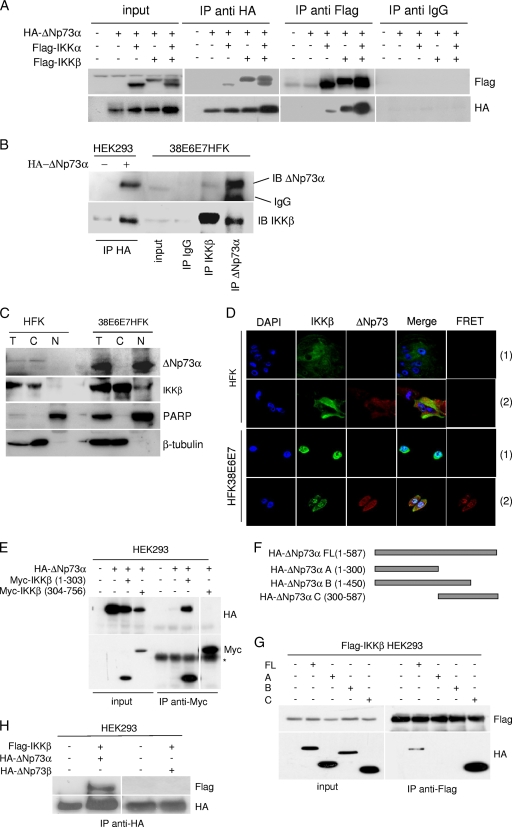
Since we found that IKKβ influences ΔNp73α protein levels, we next evaluated whether it interacts physically with ΔNp73α. Tagged proteins, Flag-IKKα and/or -β and HA-ΔNp73α, were coexpressed in HEK293 cells, and immunoprecipitations were performed with a HA- or Flag-specific antibody. As shown in Fig. 3A, an IKKβ/ΔNp73α complex was immunoprecipitated by the HA and Flag antibodies. A weak interaction was also observed between ΔNp73α and IKKα that was increased when IKKβ was also overexpressed (Fig. 3A). ΔNp73α/IKKα interaction may be mediated by IKKβ, which has the ability to bind both proteins, or alternatively by the IKKα binding site, previously characterized in TAp73, which is also present in ΔNp73α (10). The interaction between IKKβ and ΔNp73α was also confirmed in 38E6E7HFK cells by immunoprecipitating endogenous IKKβ or ΔNp73α (Fig. 3B). We next evaluated the colocalization of the two cellular proteins by cellular fractionation and immunofluorescent staining. IKKβ was found in the cytoplasm of primary HFK cells and in the cytoplasm and nucleus in 38E6E7HFK cells by both assays (Fig. 3C). Regarding ΔNp73α, immunofluorescence staining showed that it is localized exclusively in the cytoplasm in normal keratinocytes, while it was detected in the cytoplasm and in the nucleus in 38E6E7HFK cells (Fig. 3D). In contrast, ΔNp73α appeared to be localized only in the nucleus in cellular fractionation experiments (Fig. 3C). The difference in ΔNp73α localization determined with the two assays may be due to the use of two different antibodies. However, FRET experiments showed that IKKβ and ΔNp73α interact in the nuclei of 38E6E7HFK cells (Fig. 3D), providing additional evidence for the interaction of the two cellular proteins. No FRET signal was detected when cells were stained only with the anti-IKKβ antibody (Fig. 3D). As an additional control, we also determined the IKKα cellular localization in primary or 38E6E7HFK cells, which appeared to be mainly cytoplasmic and perinuclear in both types of cells (data not shown).
Fig. 3.
IKKβ directly interacts with ΔNp73. (A) HEK293 cells were transfected with the indicated expression plasmids, and 24 h after transfection, protein extracts were subjected to immunoprecipitation followed by immunoblotting. “Input” represents 1/10 of total extracts used for the immunoprecipitation. (B) One-milligram protein extracts of 38E6E7HFK cells were immunoprecipitated with the indicated antibodies, followed by immunoblotting. “Input” represents 1/10 of total extracts used for the immunoprecipitation. As a control, immunocomplexes obtained from HEK293 cells transfected with pcDNA or pcDNA3-HA-ΔNp73α were included in the experiment. (C) Primary HFK and 38E6E7HFK cells were collected and fractionated by using a nuclear extraction kit (Panomics). After fractionation, total extract (T), cytoplasm (C), and nucleus (N) were analyzed by immunoblotting with the indicated antibodies. (D) Primary HFK cells transduced with empty retrovirus (pLXSN) or 38E6E7HFK cells were seeded on coverslips. After immunofluorescent staining for ΔNp73 and IKKβ with specific antibodies, fluorescent signal was visualized using confocal microscopy. FRET analysis was performed as explained in Materials and Methods. A representative image of a FRET positive signal for each cell line is shown in the right panels (2). As a negative control, FRET analyses were performed with cells stained only with the fluorochrome donor (Alexa 488) (1). (E) HEK293 cells were transfected with the indicated expression plasmids; 24 h posttransfection, protein extracts were immunoprecipitated and analyzed by immunoblotting. The last lane was obtained in the same experiment and was taken from a different area of the same SDS-PAGE gel. The asterisk indicates the IgG heavy chain. (F) Schematic representation of ΔNp73 deletion mutants. (G) HEK293 cells were transfected with pcDNA3 constructs expressing wild-type or deleted HA-ΔNp73 mutants together with pcDNA3-Flag-IKKβ as indicated. After 24 h, cells were collected and protein extracts were prepared. Immunoprecipitations were performed using an anti-Flag antibody, and pellets were analyzed by immunoblotting. Input represents 1/10 of total extracts used for the immunoprecipitation. (H) HEK293 cells were transfected with pcDNA3 constructs as indicated. After 24 h, protein extracts were immunoprecipitated with an HA-tag antibody, and pellets were analyzed by immunoblotting.
To identify the region of IKKβ responsible for its interaction with ΔNp73α, two IKKβ deletion mutants, at the N and C terminuses, were expressed in HEK293 cells. The truncated IKKβ molecule containing the first 303 amino acids was able to interact with ΔNp73α, while no association was detected with the C-terminal IKKβ region (amino acids 304 to 756) (Fig. 3E). Next, we sought to identify the region of ΔNp73α involved in the interaction with IKKβ. Three ΔNp73α deletion mutants were generated and expressed in HEK293 cells (Fig. 3F). Immunoprecipitation experiments showed that only mutant C, containing the last 287 amino acids, retained the ability to bind IKKβ (Fig. 3G). Thus, IKKβ and ΔNp73α interact via their respective N and C terminuses.
Several isoforms of ΔNp73 that differ in their C terminus and properties have been described (23). For instance, ΔNp73β is a shorter form that lacks the last 137 amino acids of ΔNp73α and exerts proapoptotic functions similarly to p53, in contrast to ΔNp73α (30). Coexpression of HA-ΔNp73β and Flag-IKKβ in HEK293 cells followed by immunoprecipitation with an anti-HA-tag antibody showed no interaction between the two proteins (Fig. 3H). Thus, the ability to complex with IKKβ is an exclusive property of the ΔNp73α isoform and requires the C-terminal 137 amino acids.
IKKβ phosphorylates ΔNp73α at serine 422.
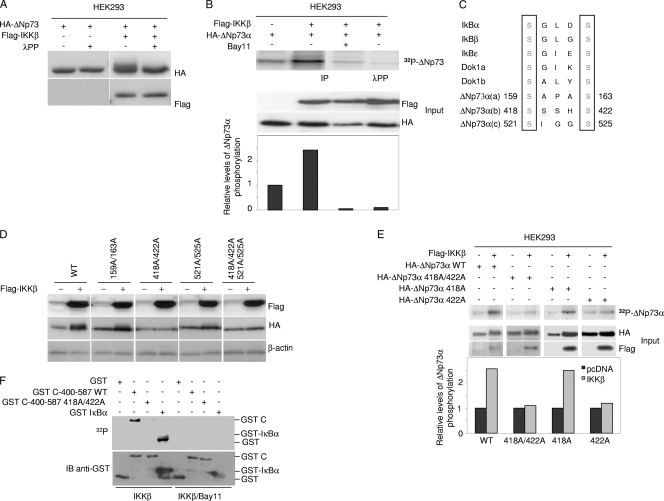
We next determined whether ΔNp73α is a substrate of IKKβ. When cotransfected with IKKβ in ΗΕK293, ΔNp73α migrated as a diffuse band in SDS-PAGE, while treatment of the total cellular extract with λ serine/threonine protein phosphatase (λPP) resulted in the appearance of a sharp protein band (Fig. 4A). In vivo 32P labeling experiments with HEK293 cells expressing HA-ΔNp73α showed that expression of Flag-IKKβ resulted in elevated ΔNp73α phosphorylation, which was inhibited in Bay11-treated cells (Fig. 4B). In addition, incubation of the HA-ΔNp73α immunopellet from IKKβ-expressing HEK293 cells with λPP led to a decrease in levels of phosphorylated ΔNp73α (Fig. 4B). Collectively, these data indicate that IKKβ phosphorylates ΔNp73α at serine and/or threonine residues.
Fig. 4.
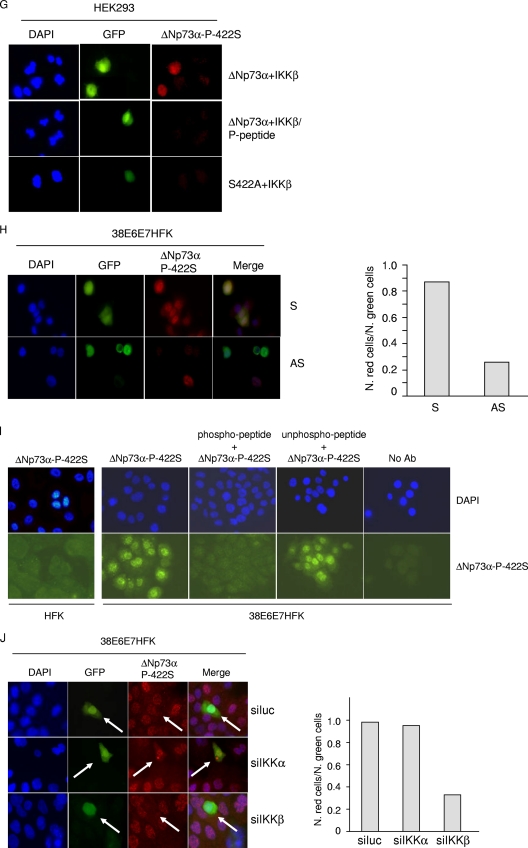
IKKβ phosphorylates ΔNp73. (A) HEK293 cells were transfected with expression plasmids as indicated. After 24 h, protein extracts were treated or not with λPP and analyzed by immunoblotting. (B) HEK293 cells were transfected with the indicated pcDNA3 constructs and cultured in the presence of [32P]orthophosphate with or without Bay11. Immunoprecipitations were performed using anti-HA-tag antibody. One immunopellet was treated with λ-phosphatase (top panel, fourth lane). Proteins were analyzed by autoradiography and by immunoblotting (top and lower panels, respectively). The histogram (bottom panel) shows the phosphorylation levels of the different ΔNp73α protein bands. For each band, radioactive signal was normalized to the immunoblotting signal of the input. (C) Amino acid sequence alignments of IKK phosphorylation sites of two cellular proteins that are targeted by IKK complex (IκB and Dok1) with potential IKK phosphorylation sites of ΔNp73α. Numbers indicate the positions of serines in ΔNp73α that are potentially phosphorylated by IKK. (D) HEK293 cells were transfected with wild-type ΔNp73α (WT) or various alanine substitution mutants with or without Flag-IKKβ. Protein extracts were analyzed by immunoblotting with the indicated antibodies. (E) HEK293 cells were transfected with the indicated constructs, and 32P labeling was performed as described in the legend for panel B. Immunoprecipitation was performed using anti-HA-tag antibody, followed by immunoblotting or autoradiography (top panels). The histogram (bottom panel) was obtained as explained in the legend for panel B. (F) Kinetic reactions were carried out as described in Materials and Methods using GST fusion proteins with the C-terminal domain of wild-type or 422A mutant ΔNp73α or IκBα. Radioactive and cold proteins were detected by autoradiography and immunoblotting, respectively (top and lower panels). (G) HEK293 cells were cotransfected with IKKβ and wild-type ΔNp73α or the 442A ΔNp73α mutant together with pE-GFPCI. Cells were seeded on coverslips and subjected to immunofluorescence analysis as described in Materials and Methods with the indicated antibodies. (H) 38E6E7HFK cells were transfected with S or AS against ΔNp73α together with the pE-GFPCI construct. Thirty hours posttransfection, cells were stained with anti-ΔNp73α P-422S antibody and analyzed by immunofluorescence. Representative images are shown in the left panel. Quantification of ΔNp73α P-422S and GFP-positive cells or GFP-positive cells was determined by counting at least 100 transfected cells in more than 10 different fields (right panel). (I) HFK or 38E6E7HFK cells were stained with a specific antibody against the 422S-phosphorylated form of ΔNp73α. As controls, immunofluorescence was also performed without the primary antibody (no Ab) or with primary antibody preincubated with the phosphorylated or nonphosphorylated 422S ΔNp73α peptide. (J) 38E6E7HFK cells were cotransfected with pE-GFPCI and siRNAs against IKKα (siIKKα), IKKβ (siIKKβ), or siLuc as a negative control. Thirty hours posttransfection, cells were stained with anti-ΔNp73α P-422S antibody and subjected to immunofluorescence analysis. Representative imagines are shown in the left panel; arrows indicate transfected cells. Quantification of ΔNp73α P-422S and GFP-positive cells or GFP-positive cells was determined by counting at least 100 transfected cells in more than 10 different fields (right panel).
A comparison of ΔNp73α amino acid sequence with known IKK substrates (17, 20) revealed the presence of three potential IKKβ phosphorylation sites (Fig. 4C). To determine whether one or more of these sites are indeed phosphorylated by IKKβ, we generated several ΔNp73α mutants, in which the serines within the hypothetical IKKβ phosphorylation sites were mutated to alanines. The protein levels of the ΔNp73α 159A 163A and 521A 525A mutants were increased in the presence of IKKβ in HEK293 cells similarly to the wild-type protein (Fig. 4D). In contrast, mutations of serines 418 and 422 decreased ΔNp73α accumulation induced by IKKβ (Fig. 4D), suggesting that phosphorylation of one or both serine residues by IKKβ plays a role in ΔNp73α accumulation. In vivo 32P labeling experiments showed that ectopic expression of IKKβ induced phosphorylation of wild-type and S418A mutant ΔNp73α in HEK293 cells, while no difference was observed with the 418A 422A and 422A mutants (Fig. 4E), indicating that S422 is the major IKKβ-induced phosphorylation site of ΔNp73α. Immunoprecipitated IKKβ also induced phosphorylation of a GST-ΔNp73α C-terminus fusion protein in vitro, while the same fusion protein harboring the 422A mutation was not targeted by IKKβ (Fig. 4F).
In order to determine whether the phosphorylation of ΔNp73α by IKKβ at S422 occurs in vivo and to study the biological relevance of this modification, we next generated a rabbit polyclonal antibody against the ΔNp73α peptide that contained phosphorylated 422S. This antibody showed a weak antigen affinity in immunoblotting (data not shown), while it appeared to detect the phosphorylated form of ΔNp73α in immunofluorescence experiments. Cells expressing wild-type ΔNp73α and IKKβ showed a clear immunofluorescent signal when stained with the anti-ΔNp73α-P-442S antibody, while cells expressing the ΔNp73α-422A mutant together with IKKβ were not stained (Fig. 4G). In addition, ΔNp73α downregulation decreased the staining with anti-ΔNp73α-P-442S antibody, further confirming the specificity of this antibody for ΔNp73α (Fig. 4H). Immunofluorescent staining of 38E6E7HFK cells but not of primary keratinocytes revealed a strong nuclear staining that was efficiently decreased by preincubation of the antibody with the ΔNp73α phosphorylated peptide (Fig. 4I). In contrast, preincubation of the antibody with nonphosphorylated peptide only marginally affected ΔNp73α nuclear staining in 38E6E7HFK cells (Fig. 4I). Finally, downregulation of IKKβ but not IKKα in 38E6E7HFK cells decreased ΔNp73α staining with ΔNp73α-P-422S antibody (Fig. 4J).
Together, these data show that IKKβ phosphorylates ΔNp73α at serine 422.
ΔNp73α phosphorylation is important for nuclear accumulation and prevention of calpain-mediated degradation.
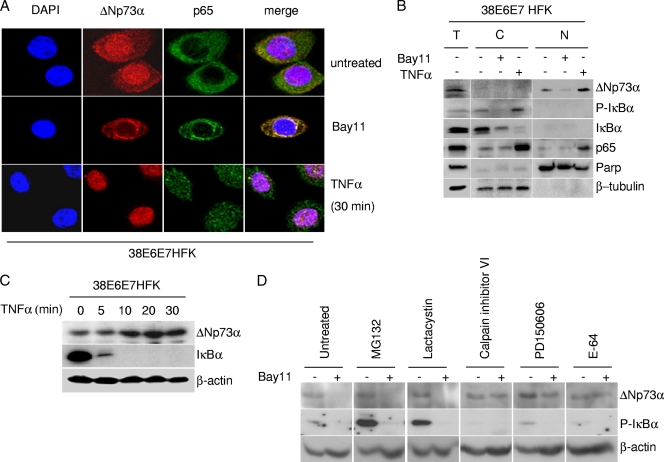
Next, we further investigated the biological significance of IKK-mediated ΔNp73α phosphorylation. Bay11 treatment of 38E6E7HFK cells resulted in a significant reduction of nuclear ΔNp73α levels (Fig. 5A). In contrast, TNF-α-exposed cells showed an increase in nuclear ΔNp73α levels over those of the untreated cells (Fig. 5A). Similar results were obtained with cellular fractionation experiments (Fig. 5B). ΔNp73α nuclear accumulation mediated by TNF-α was also evident in immunoblotting (Fig. 5C). Thus, IKKβ phosphorylation appears to favor nuclear ΔNp73α accumulation.
Fig. 5.
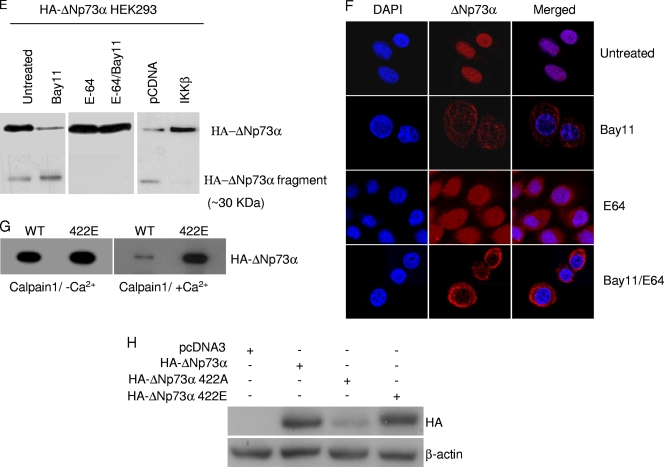
IKKβ affects stability and intracellular localization of ΔNp73α. (A) 38E6E7HFK cells were treated with TNF-α or Bay11 and ΔNp73, and p65 cellular localization was visualized by immunofluorescence with indicated antibodies. (B) 38E6E7HFK cells treated with TNF-α or Bay11 were collected and fractionated by using a nuclear extraction kit (Panomics). After fractionation, total extract (T), cytoplasm (C), and nucleus (N) were analyzed by immunoblotting with the indicated antibodies. (C) 38E6E7HFK cells were treated with TNF-α at the indicated time points. Protein extracts were prepared and analyzed by immunoblotting using the indicated antibodies. (D) 38E6E7HFK cells were treated with the indicated protease inhibitors for 8 h and Bay11 (+) or DMSO (−) for 2 h. Protein extracts were analyzed by immunoblotting. (E) HEK293 cells were transfected with the pcDNA3 HA-ΔNp73 construct and cultured under specified conditions (left and right panels) or cotransfected with different pcDNA3 constructs in the indicated combinations (right panel). Cells were then treated with indicated inhibitors followed by immunoprecipitation of whole-cell lysates and immunoblotting with the indicated antibodies. (F) 38E6E7HFK cells were seeded on coverslips and treated for 8 h with E-64 and/or for 2 h with Bay11. After the cells were fixed, ΔNp73 was visualized by immunofluorescence with specified antibodies. (G) In vitro-translated HA-tagged WT and mutant 422E ΔNp73 were incubated with recombinant calpain I in the presence or absence of Ca2+. After 30 min, the reaction was stopped by the addition of SDS loading buffer, and samples were analyzed by immunoblotting with an anti-HA-tag antibody. (H) 38E6E7HFK cells were transfected with the pcDNA3-expressing wild type or 422A or 422E HA-ΔNp73α. After 24 h, protein extracts were analyzed by immunoblotting with the indicated antibodies.
The decrease in ΔNp73α levels upon inhibition of IKKβ is not mediated by the proteasome pathway, since treatment with the proteasome inhibitor MG132 or lactacystin did not restore ΔNp73α levels in HPV38 E6/E7 keratinocytes cultured in the presence of Bay11, while it efficiently stabilized the phosphorylated form of IκBα that is known to be degraded by the proteasomes (Fig. 5D). However, inhibitors of the calcium-dependent proteases calpain μ and m (calpain inhibitor VI and PD150606) were able to efficiently stabilize ΔNp73α in Bay11-treated 38E6E7HFK cells (Fig. 5D). Similar results were obtained with a general inhibitor of the cysteine proteases, E-64 (Fig. 5D). In agreement with these data, we observed that immunoblotting of cellular extracts from HA-ΔNp73α-transfected HEK293 cells revealed the presence of full-length HA-ΔNp73α and smaller fragments, i.e., a 30-kDa protein band (Fig. 5E, left panel). The levels of the latter protein band were increased upon Bay11 treatment (Fig. 5E, left panel), while the addition of the inhibitor of the cysteine proteases E-64 or expression of IKKβ prevented the formation of the 30-kDa HA-ΔNp73α fragment (Fig. 5E, central and right panels). Simultaneous inhibition of IKKβ by Bay11 and of calpains by E-64 resulted in a cytoplasmic accumulation of ΔNp73α (Fig. 5F), indicating that the nonphosphorylated form of ΔNp73α is subject to degradation in the cytoplasm. The ability of calpain to target ΔNp73α was also observed in an in vitro assay. In vitro-translated ΔNp73α was degraded by calpain 1 but only in the presence of Ca2+ (Fig. 5G). In contrast, the 422E ΔNp73α mutant mimicking the phosphorylated form of ΔNp73α at S422 was not efficiently targeted by calpain in the same assay (Fig. 5G), further confirming the involvement of 422S phosphorylation in ΔNp73α stability. A similar conclusion was reached by comparing the levels of ΔNp73α 422Α and 422E mutants in 38E6E7HFK cells (Fig. 5H).
Together, these data indicate that ΔNp73α is cleaved by calpain, leading to its degradation, and that this event is inhibited by IKKβ-mediated phosphorylation.
IKKβ enhances the ability of ΔNp73α to inhibit p53 transcriptional functions.
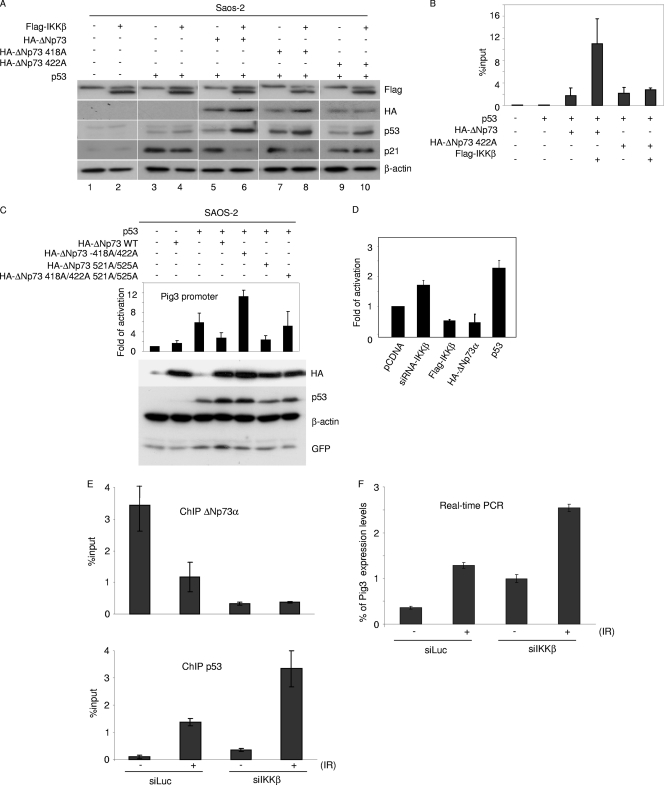
To evaluate whether IKKβ-mediated nuclear accumulation of ΔNp73α results in downregulation of p53-regulated genes, we transfected the p53-null Saos-2 cells with p53 alone or together with IKKβ and/or ΔNp73α. As expected, p53 overexpression resulted in an increase in the endogenous protein levels of p21WAF1, by which the gene is p53-positively regulated (Fig. 6A). ΔNp73α expression reduced p53-mediated p21WAF1 upregulation, a result that was further enhanced by coexpression of IKKβ (Fig. 6A). In addition, coexpression of IKKβ with the IKKβ phosphorylation mutant ΔNp73α 422A did not have any effect on endogenous p21 levels, in contrast to results for the ΔNp73α 418A mutant (Fig. 6A). Chromatin immunoprecipitation followed by real-time PCR revealed that IKKβ increased the binding to the p21WAF1 promoter of the ΔNp73α wild type but not to that of the ΔNp73 S422A mutant (Fig. 6B). Similar results were obtained with two additional p53-regulated promoters, i.e., Pig3 and Fas (data not shown). Transient-transfection experiments with Saos-2 cells with a construct containing the Pig3 promoter cloned in front of the luciferase reporter gene showed that only the 422A mutation affected the ΔNp73α property of counteracting p53's ability to activate the Pig3 promoter, while the other ΔNp73α mutants, the 418A, 521A, and 525A mutants, displayed an efficiency similar to that of the wild-type protein (Fig. 6C). Transient-transfection experiments with the Pig3 promoter-luciferase construct in 38E6E7HFK cells showed that silencing of IKKβ expression by siRNA induced activation of the Pig3 promoter, while ectopic expression of IKKβ resulted in an opposite effect (Fig. 6D).
Fig. 6.
IKK-mediated phosphorylation of ΔNp73α increases its p53 inhibitory activity. (A) Saos-2 cells were transfected with different pcDNA3 constructs in the indicated combinations. After 24 h, protein extracts were analyzed by immunoblotting with the indicated antibodies. (B) Saos-2 cells were transfected with different pcDNA3 constructs in the indicated combinations. After 36 h, ChIP was performed using an anti-HA-tag antibody and followed by real-time PCR, using primers flanking the p53 RE within the p21 promoter. Simultaneously, 1/10 of the total chromatin was processed. The values in the histogram were obtained by dividing for each sample the amount of p21 promoter which is bound by ΔNp73-HA by the total amount of p21 promoter present in the input. (C) Saos-2 cells were transfected with the following constructs: Pig3prom-firefly luciferase reporter construct, a constitutively expressing Renilla construct, pcDNA3-HA-ΔNp73α (wild type or 418A/422A, 521A/525A, or 418A/422A/521A/525A mutant), pcDNA-p53, and pE-GFPCI. After 24 h, cells were collected and processed for the luciferase assay as described in Materials and Methods (upper panel). In parallel, protein extracts were prepared and analyzed by immunoblotting with the indicated antibodies (lower panel). (D) 38E6E7HFK cells were transfected with the following constructs: Pig3prom-firefly luciferase reporter construct and a constitutively expressing Renilla construct, in combination with siRNA-IKKβ, Flag-IKKβ, pCDNA HA-ΔNp73α, or pcDNA-p53. Thirty-six hours posttransfection, cells were collected and lysed. Luciferase activity was measured and expressed as fold activation in comparison to that of the control (pCDNA). The variation in fold activities between the different conditions was significant (P < 0.05). (E and F) 38E6E7HFK cells were transfected with siIKKβ or siLuc as a negative control; 24 h posttransfection, cells were treated with ionizing radiations (IR) (30 Gy). After irradiation, cells were allowed to grow for 8 h and processed for ChIP (E) or gene expression analysis (F). ChIP followed by real-time PCR was performed using an anti-ΔNp73 antibody (upper panel) or anti-p53 antibody (lower panel) and with primers flanking the p53-RE within the pig3 promoter. Simultaneously, 1/10 of the total chromatin was processed. The values reported in the histogram were obtained as for panel B (E). Pig3 mRNA levels in cells subjected to the indicated treatments were determined by real-time RT-PCR (F).
Finally, to further demonstrate that IKKβ can counteract the activation of p53-regulated transcription, promoting ΔNp73α accumulation, we activated p53 in 38E6E7HFK cells by ionizing radiations (IR) in the presence or absence of IKKβ. We observed by ChIP experiments followed by real-time PCR that upon induction of DNA damage, IKKβ significantly alleviated the binding of p53 to its RE in the Pig3 promoter, due the occupancy by ΔNp73α (Fig. 6E). In the same experiment, we determined Pig3 expression levels by real-time PCR, which correlated with the amount of p53 recruited to the Pig3 promoter in the presence or absence of IKKβ and IR (Fig. 6F).
Together, these data show that IKKβ-mediated ΔNp73α phosphorylation and accumulation result in a reduction of the transcriptional functions of p53.
ΔNp73α and IKKβ cross talk in cancer cell lines and primary cancers.
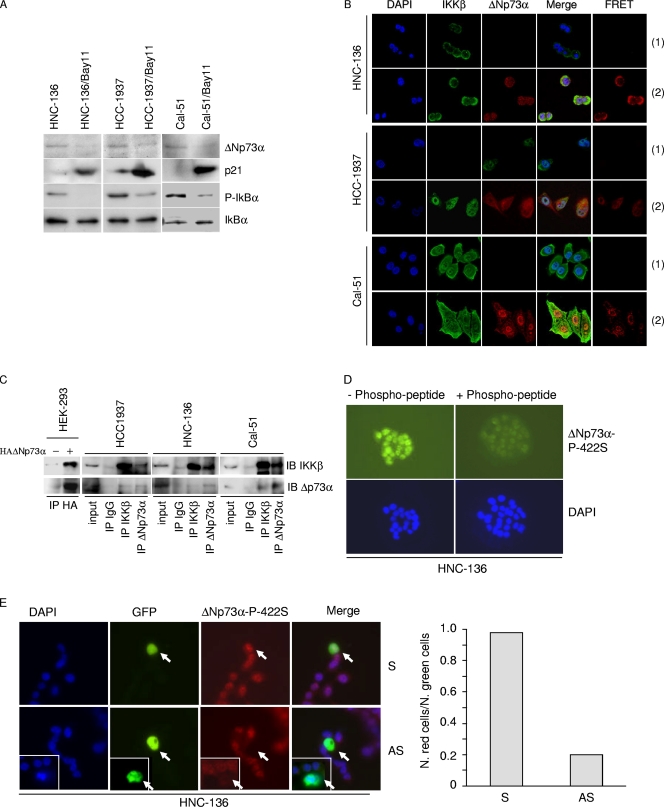
NF-κB is constitutively present in the nucleus of a number of human cancer cells as a result of constitutive activation of IKK (17, 18). This sustained activation of NF-κB contributes to the proliferation and survival of cancer cells. Therefore, we examined whether the stabilization of ΔNp73α via phosphorylation by activated IKKβ also occurs in cancer cells. We identified one head-and-neck cell line (HNC-136) and two breast cancer cell lines (HCC-1937 and Cal-51) that harbored the wild-type p53 gene and expressed ΔNp73α (Fig. 7A). Inhibition of IKKβ by Bay11 led to a decrease in ΔNp73α (Fig. 7A), indicating that IKKβ influences ΔNp73α stability in these cancer cell lines. As shown above with the other experimental models, the decreased levels of ΔNp73α upon Bay11 treatment were associated with a significant upregulation of p21WAF1 (Fig. 7A). Thus, as observed in the above-described experimental models, IKKβ appears to regulate ΔNp73α in these cancer cell lines. In support of this conclusion, IKKβ and ΔNp73α were detected in the nucleus of these cell lines (Fig. 7B), while IKKα localized mainly in the cytoplasm (data not shown). Most importantly, FRET staining showed interaction between the two proteins (7B), which was also observed in immunoprecipitation experiments (Fig. 7C).
Fig. 7.
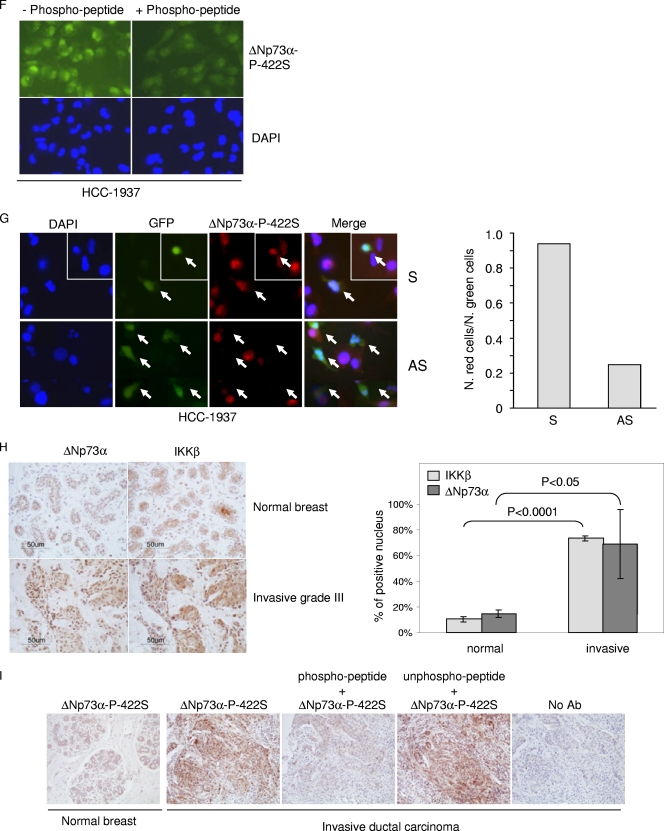
IKKβ and ΔNp73α cross talk in cancer-derived cell lines and primary cancers. (A) HNC-136, HCC-1937, and Cal-51 cell lines were treated with Bay11 for 2 h, and total protein extracts were analyzed by immunoblotting with the indicated antibodies. (B) HCC-1937, HNC-136, and Cal-51 cells were stained with the indicated antibodies. Fluorescent staining was visualized by confocal microscopy. FRET positive signals (2) and FRET negative controls (1) are shown in the right panels. (C) One-milligram protein extracts of HCC-1937, HNC-136, and Cal-51 were immunoprecipitated with an anti-IKKβ or -ΔNp73α antibody. As negative controls, the same amounts of total extracts from each cell line were immunoprecipitated with anti-IgG. Immunocomplexes were analyzed by immunoblotting. One-tenth of total extracts used for the immunoprecipitation was loaded as input. As a control, immunocomplexes obtained from HEK293 cells transfected with pcDNA or pcDNA3-HA-ΔNp73α were included in the experiment. (D) HNC-136 cells were stained with the indicated antibodies. Fluorescent staining was visualized by confocal microscopy. As a control, primary antibody was preincubated with an excess of 422S-phosphorylated peptide. (E) HNC-136 cells were cotransfected with pE-GFPCI and S or AS against ΔNp73α. Thirty hours posttransfection, cells were stained with anti-ΔNp73α P-422S antibody and analyzed for immunofluorescence. Representative images are shown in the left panel; arrows indicate transfected cells. The white frame indicates a different field. Quantification of ΔNp73α P-422S and GFP-positive cells or GFP-positive cells was determined by counting at least 100 transfected cells in more than 10 different fields (right panel). (F) HCC-1937 cells were stained as explained in the legend for panel D. (G) HCC-1937 cells were transfected and processed as explained in the legend for panel E. Representative images are shown in the left panel; arrows indicate transfected cells. The white frame indicates a different field. Quantification of ΔNp73α P-422S and GFP-positive cells or GFP-positive cells was determined by counting at least 100 transfected cells in more than 10 different fields (right panel). (H) ΔNp73 and IKKβ cellular localization was analyzed in normal and cancer breast tissues (left panel, representative staining). The histogram (right panel) shows the quantification of the percentage of cells in normal (n = 1) or cancer tissues (n = 3) with nuclear staining for IKKβ and ΔNp73α. (I) Normal and breast cancer tissues were stained with a specific antibody against the 422S-phosphorylated form of ΔNp73α. As controls, immunostaining was also performed without the primary antibody (no Ab) or with primary antibody preincubated with a phosphorylated or nonphosphorylated 422S ΔNp73α peptide.
We next determined whether ΔNp73α is phosphorylated at serine 422 in HNC-136 and HCC-1937. Immunofluorescence stainings with ΔNp73α-P-422S antibody revealed nuclear staining in both cancer cell lines that was strongly affected by preincubation of the ΔNp73α-P-422S antibody with the phosphor peptide (Fig. 7D and F). Downregulation of ΔNp73α significantly decreased staining with the ΔNp73α-P-422S antibody, further confirming the specificity of this antibody (Fig. 7E and G).
To further evaluate the cross talk between ΔNp73α and IKKβ in cancer cells, we have analyzed normal (n = 2) and breast cancer (n = 5) tissues by immunohistochemistry. In four cancer tissues, we observed that IKKβ and ΔNp73α colocalized in the nucleus. In contrast, in normal tissues the two proteins were seldom detected in the nucleus (Fig. 7H). In addition, immunohistochemical stainings of normal (n = 2) or breast cancers (n = 3) with ΔNp73α-P-422S antibody revealed a nuclear signal only in cancer cells. Representative staining is shown in Fig. 7I.
Together, these data provide additional lines of evidence for the cross talk between IKKβ and ΔNp73α.
DISCUSSION
Several data support the role of ΔNp73α in human carcinogenesis. Its levels have been found to be elevated in different types of human cancers, and importantly, ΔNp73α-positive cancers showed a less favorable prognosis than ΔNp73α-negative cancers (8, 22). In addition, several independent studies have demonstrated that ΔNp73α acts as a TAp73/p53 dominant-negative inhibitor, altering the cell cycle and apoptosis regulation (4, 23).
Although several mechanisms of ΔNp73 mRNA generation have been characterized, it is still not well understood how ΔNp73 protein stability is regulated. Here we show that ΔNp73α is a substrate of IKKβ, a key regulatory kinase of NF-κB activation. IKKβ phosphorylated ΔNp73α at serine 422, leading to its stabilization in the nucleus. This event is associated with the inhibition of p53/TAp73-regulated promoters, such as p21 and Pig3.
The regulation of ΔNp73α by IKKβ is unique, since the domain required for its interaction and stability is missing in the ΔNp73β isoform. Interestingly, this β isoform, in contrast to ΔNp73α, exerts a proapoptotic function, and its levels are not affected by IKKβ. These data further highlight the role of the IKKβ/ΔNp73α interaction in promoting cellular proliferation. Inhibition of IKKβ by different means induced rapid degradation of ΔNp73α via a calpain-mediated mechanism. A previous study has reported that several p73 forms, including ΔNp73α, are targeted by calpain (25). Our findings demonstrate that ΔNp73α stability is regulated by posttranslational modification. In fact, simultaneous inhibition of IKKβ and calpain resulted in ΔNp73α accumulation in the cytoplasm, suggesting that the hypophosphorylated form of ΔNp73α is processed in this cellular compartment. However, our data do not exclude that in addition to calpain, other cellular pathways may be involved in the regulation of protein levels of the unphosphorylated form of ΔNp73α.
Although our data demonstrate a link between IKKβ-induced phosphorylation and nuclear accumulation of ΔNp73α, its precise mechanisms of translocation into the nucleus remain to be elucidated. Two possible scenarios can be envisaged. IKKβ could interact and phosphorylate ΔNp73α in the cytoplasm and be transported together into the nucleus. Alternatively, IKKβ is transported into the nucleus by an independent mechanism, where it phosphorylates ΔNp73α. While the unphosphorylated ΔNp73α could freely shuttle between the nucleus and cytoplasm, it is possible that after phosphorylation it is retained in the nuclear compartment. Studies are in progress to address these issues. The observation that viral oncoproteins such as E6 and/or E7 from HPV38 can trigger IKKβ nuclear localization may facilitate our understanding of the mechanisms controlling IKKβ subcellular localization.
The ability to influence ΔNp73α levels appears to be a specific property of IKKβ, since IKKα interacted weakly with ΔNp73α, and inhibition of IKKα expression in human and rodent cells did not affect ΔNp73α stability. Interestingly, a recent study has described that IKKα but not IKKβ regulates stability, nuclear accumulation, and functions of TAp73 (10). Similarly to what we have observed for IKKβ and ΔNp73α, these events correlate with translocation of IKKα into the nucleus. A model can be proposed in which the activation of the mechanisms responsible for IKKα or IKKβ nuclear accumulation favor apoptosis or proliferation via stabilization of TAp73 or ΔNp73α, respectively. Interestingly, an additional study has recently shown that IKKβ phosphorylates p53 at two specific serines, promoting its ubiquitination and degradation by β-TrCP1 and independently of MDM2 and NF-κB (31). Taking into consideration our data and those of Xia's study (31), IKKβ appears to modulate p53 transcriptional activity through two independent mechanisms, the first promoting its phosphorylation and destabilization and the second inducing phosphorylation and accumulation of the p53 antagonist ΔNp73α. This model underlines the key role of IKKβ in carcinogenesis, which indeed could be a potential target for novel anticancer therapeutic strategies. Several independent studies have identified novel nuclear functions of IKKα and IKKββ in the regulation of cellular gene expression and proliferation (3, 9, 32), further supporting the key role of these kinases in the nucleus independently of their ability to promote NF-κB nuclear translocation. During carcinogenesis, mechanisms that regulate the nuclear localization of IKKα or IKKβ may be altered, with consequent accumulation of ΔNp73α and loss of TAp73 functions. Our initial screening of cancer-derived cell lines and primary cancers indicates that IKKβ can be found in the nuclei of cancer cells. In addition, the IKKβ nuclear localization showed a tight correlation with ΔNp73α accumulation. Interestingly, inhibition of IKK activity in these cancer cell lines corresponded with a rescue of p53 transcriptional functions and an increase in their sensitivity to chemotherapeutic drugs (R. Accardi, unpublished data), consistent with the downregulation of the ΔNp73α levels.
We have previously shown that the E6 and E7 oncoproteins from cutaneous HPV38 activate ΔNp73α transcription (1). In this study, we show that in addition to upregulation of the ΔNp73α mRNA levels, HPV38 E6 and E7 are able to increase ΔNp73α stability. Our preliminary data on Epstein-Barr virus show that its major oncoprotein LMP1, a strong activator of IKK and the NF-κB pathway (29), promotes ΔNp73α accumulation (Accardi, unpublished), suggesting that this mechanism is shared with other viruses.
In summary, our data describe a novel mechanism linking two cellular proteins, IKKβ and ΔNp73α, that play a key role in human carcinogenesis.
ACKNOWLEDGMENTS
We are grateful to all members of the Infections and Cancer Biology group for their support, to Lucien Frappart, Thomas Gilmore, David Goeddel, Elliot Kieff, Alain Puisieux, and Inder Verma for kindly providing reagents and/or specimens, to the Common Centre of Quantimetry CCQ (UBCL, Lyon, France) for immunohistological analyses, and to John Daniel and Uzma Hasan for critical reading of the manuscript.
This study was supported partially by grants to B.S.S. from La Ligue Contre le Cancer (Comité du Rhône and Drôme) and by grants to M.T. from La Ligue Contre le Cancer (Comités du Rhône, Drôme and Savoie), Association pour la Recherche sur le Cancer, European Union (LSHC-2005-018704), and Association for International Cancer Research, as well as grants to M.T. and L.G. from DKFZ-Cancéropôle Grand-Est, German-French cooperation program.
Footnotes
Published ahead of print on 11 April 2011.
REFERENCES
- 1. Accardi R., et al. 2006. Skin human papillomavirus type 38 alters p53 functions by accumulation of deltaNp73. EMBO Rep. 7:334–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agami R., Blandino G., Oren M., Shaul Y. 1999. Interaction of c-Abl and p73alpha and their collaboration to induce apoptosis. Nature 399:809–813 [DOI] [PubMed] [Google Scholar]
- 3. Anest V., et al. 2003. A nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression. Nature 423:659–663 [DOI] [PubMed] [Google Scholar]
- 4. Buhlmann S., Putzer B. M. 2008. DNp73 a matter of cancer: mechanisms and clinical implications. Biochim. Biophys. Acta 1785:207–216 [DOI] [PubMed] [Google Scholar]
- 5. Caldeira S., et al. 2003. The E6 and E7 proteins of cutaneous human papillomavirus type 38 display transforming properties. J. Virol. 77:2195–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clegg R. M. 1992. Fluorescence resonance energy transfer and nucleic acids. Methods Enzymol. 211:353–388 [DOI] [PubMed] [Google Scholar]
- 7. Costanzo A., et al. 2002. DNA damage-dependent acetylation of p73 dictates the selective activation of apoptotic target genes. Mol. Cell 9:175–186 [DOI] [PubMed] [Google Scholar]
- 8. Dominguez G., et al. 2006. DeltaTAp73 upregulation correlates with poor prognosis in human tumors: putative in vivo network involving p73 isoforms, p53, and E2F-1. J. Clin. Oncol. 24:805–815 [DOI] [PubMed] [Google Scholar]
- 9. Fu L., et al. 2009. BAFF-R promotes cell proliferation and survival through interaction with IKKbeta and NF-kappaB/c-Rel in the nucleus of normal and neoplastic B-lymphoid cells. Blood 113:4627–4636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Furuya K., et al. 2007. Stabilization of p73 by nuclear IkappaB kinase-alpha mediates cisplatin-induced apoptosis. J. Biol. Chem. 282:18365–18378 [DOI] [PubMed] [Google Scholar]
- 11. Gong J. G., et al. 1999. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature 399:806–809 [DOI] [PubMed] [Google Scholar]
- 12. Gonzalez S., Prives C., Cordon-Cardo C. 2003. p73alpha regulation by Chk1 in response to DNA damage. Mol. Cell. Biol. 23:8161–8171 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Grob T. J., et al. 2001. Human delta Np73 regulates a dominant negative feedback loop for TAp73 and p53. Cell Death Differ. 8:1213–1223 [DOI] [PubMed] [Google Scholar]
- 14. Haupt Y., Maya R., Kazaz A., Oren M. 1997. Mdm2 promotes the rapid degradation of p53. Nature 387:296–299 [DOI] [PubMed] [Google Scholar]
- 15. Ishimoto O., et al. 2002. Possible oncogenic potential of DeltaNp73: a newly identified isoform of human p73. Cancer Res. 62:636–641 [PubMed] [Google Scholar]
- 16. Jost C. A., Marin M. C., Kaelin W. G., Jr 1997. p73 is a simian [correction of human] p53-related protein that can induce apoptosis. Nature 389:191–194 (Erratum, 399:817, 1999.) [DOI] [PubMed] [Google Scholar]
- 17. Karin M. 2006. Nuclear factor-kappaB in cancer development and progression. Nature 441:431–436 [DOI] [PubMed] [Google Scholar]
- 18. Karin M. 2008. The IkappaB kinase—a bridge between inflammation and cancer. Cell Res. 18:334–342 [DOI] [PubMed] [Google Scholar]
- 19. Lee C. W., La Thangue N. B. 1999. Promoter specificity and stability control of the p53-related protein p73. Oncogene 18:4171–4181 [DOI] [PubMed] [Google Scholar]
- 20. Lee S., et al. 2004. IkappaB kinase beta phosphorylates Dok1 serines in response to TNF, IL-1, or gamma radiation. Proc. Natl. Acad. Sci. U. S. A. 101:17416–17421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu G., Nozell S., Xiao H., Chen X. 2004. DeltaNp73beta is active in transactivation and growth suppression. Mol. Cell. Biol. 24:487–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lunghi P., et al. 2006. MEK1 inhibition sensitizes primary acute myelogenous leukemia to arsenic trioxide-induced apoptosis. Blood 107:4549–4553 [DOI] [PubMed] [Google Scholar]
- 23. Melino G., De Laurenzi V., Vousden K. H. 2002. p73: friend or foe in tumorigenesis. Nat. Rev. Cancer 2:605–615 [DOI] [PubMed] [Google Scholar]
- 24. Morgenstern J. P., Land H. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Munarriz E., et al. 2005. Calpain cleavage regulates the protein stability of p73. Biochem. Biophys. Res. Commun. 333:954–960 [DOI] [PubMed] [Google Scholar]
- 26. Nieminen J., Kuno A., Hirabayashi J., Sato S. 2007. Visualization of galectin-3 oligomerization on the surface of neutrophils and endothelial cells using fluorescence resonance energy transfer. J. Biol. Chem. 282:1374–1383 [DOI] [PubMed] [Google Scholar]
- 27. Ren J., et al. 2002. p73beta is regulated by protein kinase Cdelta catalytic fragment generated in the apoptotic response to DNA damage. J. Biol. Chem. 277:33758–33765 [DOI] [PubMed] [Google Scholar]
- 28. Rossi M., et al. 2005. The ubiquitin-protein ligase Itch regulates p73 stability. EMBO J. 24:836–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sylla B. S., et al. 1998. Epstein-Barr virus-transforming protein latent infection membrane protein 1 activates transcription factor NF-kappaB through a pathway that includes the NF-kappaB-inducing kinase and the IkappaB kinases IKKalpha and IKKbeta. Proc. Natl. Acad. Sci. U. S. A. 95:10106–10111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ueda Y., Hijikata M., Takagi S., Chiba T., Shimotohno K. 1999. New p73 variants with altered C-terminal structures have varied transcriptional activities. Oncogene 18:4993–4998 [DOI] [PubMed] [Google Scholar]
- 31. Xia Y., et al. 2009. Phosphorylation of p53 by IkappaB kinase 2 promotes its degradation by beta-TrCP. Proc. Natl. Acad. Sci. U. S. A. 106:2629–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yamamoto M., et al. 1994. Effect of tumor suppressors on cell cycle-regulatory genes—Rb suppresses P34(Cdc2) expression and normal p53 suppresses cyclin A expression. Exp. Cell Res. 210:94–101 [DOI] [PubMed] [Google Scholar]
- 33. Yuan Z. M., et al. 1999. p73 is regulated by tyrosine kinase c-Abl in the apoptotic response to DNA damage. Nature 399:814–817 [DOI] [PubMed] [Google Scholar]
- 34. Zaika A. I., et al. 2002. DeltaNp73, a dominant-negative inhibitor of wild-type p53 and TAp73, is up-regulated in human tumors. J. Exp. Med. 196:765–780 [DOI] [PMC free article] [PubMed] [Google Scholar]