Abstract
We document a biphasic effect of Rac2 on the activation and inhibition of PLD2. Cells overexpressing Rac2 and PLD2 simultaneously show a robust initial (<10 min) response toward a chemoattractant that is later (>30 min) greatly diminished over PLD2-only controls. The first phase is due to the presence of a Rac2-PLD2 positive-feedback loop. To explain the mechanism for the Rac2-led PLD2 inhibition (the second phase), we used leukocytes from wild-type (WT) and Rac2−/− knockout mice. Rac2−/− cells displayed an enhanced PLD2 (but not PLD1) enzymatic activity, confirming the inhibitory role of Rac2. Late inhibitory responses on PLD2 due to Rac2 were reversed in the presence of phosphatidylinositol 4,5-bisphosphate (PIP2) both in vitro (purified GST-PH-PLD2, where GST is glutathione S-transferase and PH is pleckstrin homology) and in vivo. Coimmunoprecipitation and immunofluorescence microscopy indicated that PLD2 and Rac2 remain together. The presence of an “arc” of Rac2 at the leading edge of leukocyte pseudopodia and PLD2 physically posterior to this wave of Rac2 was observed in late chemotaxis. We propose Rac-led inhibition of PLD2 function is due to sterical interference of Rac with PLD2's PH binding site to the membrane and deprivation of the PIP2. This work supports the importance of functional interactions between PLD and Rac in the biological response of cell migration.
INTRODUCTION
Cell migration is vital to wound healing, angiogenesis, embryonic development, and immune function (17). Local activation and amplification of signaling events on the side of the cells directly facing chemoattractants facilitates localized actin polymerization. It also leads to morphological polarity and the establishment of a dominant-leading pseudopodium and rear cell body compartment (17, 30). The actin-dependent processes driving neutrophil chemotaxis are downstream of the Rac members of the Rho family of small GTPases (5, 6, 9, 15, 20, 37, 45). Moreover, regulation of the actin cytoskeleton that is required for normal cell polarization and chemotaxis is tightly regulated by Rac GTPases in discrete cytoplasmic domains both within the leading edge lamella and within the tail of chemotaxing neutrophils (40, 47). Rac1 is required for the detection of a chemoattractant gradient and generation of a single dominant lamella, while Rac2 is the isoform responsible for regulating the actin cytoskeletal machinery required for efficient neutrophil translocation (41).
The leukocyte activation cascade is a sequence of adhesion and activation events that end with extravasation of the leukocyte to the inflamed site. Rac1 is recruited to cell-cell contact sites together with E-cadherin, and the beginning of adhesion induces activation of Rac1. Rac1 is also responsible for controlling membrane ruffling and the formation of lamellipodia, and in migrating cells, Rac1 is generally required at the leading edge for lamellipodium extension and formation of new adhesions (35). Much less is known about the role of the other Rac isoform, Rac2, in leukocyte (macrophage) function.
Phospholipase D (PLD), an enzyme which is located in the plasma membrane and catalyzes the hydrolysis of phosphatidylcholine to form phosphatidic acid (PA), is also involved in the regulation of cytoskeleton dynamics (28). A role for PLD and its product PA has been presumed in the regulation of actin (4, 16, 31, 32). The formation of lamellipodium structures and membrane ruffles is blocked by PLD inhibition (26, 38); thus, PLD activity is critical for leukocyte cell migration (19).
It has been reported previously that Rac1 activates PLD1 (11, 24, 34, 43, 44). When the single residue Ser-124, which is solvent exposed on the insert helix of Cdc42 and other similar small GTPases, is mutated to alanine, it does not interfere with Arf- or protein kinase C (PKC)-mediated PLD activity (43). This information suggests an essential contact with human PLD, which activates the lipase. However, insert helices of other small GTPases like RhoA and Rac1 do not have the same specificity/affinity for PLD as does Cdc42.
It has been hypothesized that positive regulation of PLD by small GTPases occurs via specific upstream signaling pathways in addition to crucial charge-charge interactions between the large charged residues on the surface of a specific small GTPase insert helix and PLD that are requisite for proper positioning of key activation residues. With all these facts in hand, it could be possible that Rac1 and Rac2 during the course of cell migration sequentially activate PLD1 (but probably not PLD2, the subject of the present study).
However, little is known about whether the other mammalian lipase, PLD2, is affected by a similar GTPase-mediated mechanism, nor it is known if the Rac2 isoform (more prevalent in blood cells than Rac1) will be involved in PLD2-mediated functionalities (such as PLD2-induced chemotaxis) (19). This study elucidates the effect of Rac on PLD2, with the surprising discovery of a dual effect of the GTPase on the lipase, whereby Rac affects PLD2 enzymatic activity both positively and negatively, and this explains the progression through chemotaxis in leukocytes. Further, we provide the detailed molecular mechanism underlying this effect.
MATERIALS AND METHODS
Animals.
The Rac2−/− mice used in this study were previously generated by targeted disruption of the rac2 gene (37), which had been backcrossed into C57BL/6 mice for more than 11 generations. Wild-type (WT) C57BL/6 control mice were from the Jackson Laboratory. Animals were housed under specific pathogen-free conditions and fed autoclaved food and water as needed. Mice used in this study were between 8 and 20 weeks of age.
Harvesting of murine BM cells.
Bone marrow (BM) was flushed from mouse bones (femurs, tibiae, and hips) from WT and Rac2−/− mice in harvesting medium (RPMI medium with l-glutamine, 1% penicillin-streptomycin [Pen-Strep] and 1% Glutamax) using a 22-gauge needle (Becton Dickinson, San Jose, CA) under sterile conditions. The initial yield was approximately 7 × 107 total BM cells per mouse. These BM cells were plated in 10-cm tissue culture dishes for 2 h to eliminate adherent stromal cells. Nonadherent BM cells (at a usual yield of 5.5 × 107 cells per mouse) were resuspended in harvesting medium supplemented with 10% fetal calf serum (FCS) at a cell density of 5 × 107 cells/ml. Some cells were resuspended in freezing medium (50% alpha-minimal essential medium [α-MEM], 40% FCS, 10% dimethyl sulfoxide [DMSO] and 1% Pen-Strep), frozen slowly (1°C/min) until ready for liquid N2, and kept for later use. BM cells were used to generate macrophages or neutrophils (BM-derived macrophages [BMDM] or BM-derived neutrophils [BMDN], respectively) after induction of differentiation in vitro.
BMDM cells.
Bone marrow cells were plated for differentiation into macrophages in a 5-day protocol as previously described (45). Briefly, cells (2 ml of cells at 5 × 106 cells/ml per 10 cm-dish) were diluted in BMDM medium (RPMI medium with l-glutamine, 10% FCS, 1% Pen-Strep, and 1% Glutamax) supplemented with the cytokines interleukin-3 ([IL-3] 10 ng/ml final concentration) and macrophage colony-stimulating factor ([M-CSF] 20 ng/ml final concentration). Cells were cultured at 37°C in 5% CO2 for 3 days in nontreated tissue culture plasticware or in 10-cm petri dishes. On day 3, the medium was replaced with fresh BMDM supplemented with M-CSF only, and cells were cultured for two additional days. On day 5, nonadherent cells were removed with vigorous washing in phosphate-buffered saline (PBS). The adherent macrophage monolayer was incubated in culture medium for 2 more hours. Differentiation of cells into macrophages was verified by cytospinning (Thermo Electron Corp., Waltham, MA), followed by staining (Diff-Quik; Fisher Scientific) and observation under the microscope, which showed expected macrophage anatomy.
In vitro differentiation of granulocytes from murine bone marrow.
Bone marrow cells were induced to differentiate into neutrophils with IL-3 and granulocyte-CSF (G-CSF), as described previously (45). Briefly, 2 ml of bone marrow cells at 2 × 106 cells/ml per experimental condition was diluted in bone marrow-derived neutrophil medium (α-MEM, 20% FCS, 1% Pen-Strep, and 1% Glutamax) supplemented with the differentiating cytokines IL-3 (10 ng/ml final concentration) and G-CSF (20 ng/ml final concentration). Cells were cultured at 37°C in 5% CO2 for 3 days in T-75 flasks. On day 3, the medium was replaced with fresh BMDM medium (with 10% FCS instead of 20%) and supplemented with G-CSF only, and cells were cultured for three additional days. On day 6, differentiation of cells into neutrophils was verified by cytospinning (Thermo Electron Corp., Waltham, MA), followed by staining (Diff-Quik) and observation under the microscope; samples showed the expected neutrophil anatomy, with 80 to 90% mature neutrophils. Neutrophils were resuspended in PBG buffer (1× PBS, 0.1% bovine serum albumin [BSA], 1% glucose, pH 7.35; sterile filtered) at 4 × 106 cells/ml.
Nucleofection of macrophages and predifferentiated neutrophils.
An Amaxa-derived nucleofection protocol was used to transfect plasmid DNAs into macrophages (BMDM). When transfection was completed, cells were immediately plated in six-well plates (non-tissue culture-treated plastic) in prewarmed RPMI medium-based medium supplemented with 20% FCS. Cells were cultured at 37°C in 5% CO2 for 36 h to allow for maximum protein expression. At the end of this period, transfection efficiencies for a control enhanced green fluorescent protein (eGFP) plasmid was at least 65% for BMDM with >85% viability at 36 h posttransfection and 70% at 48 h, but viability dropped down to ∼60%, so we often used them at 36 h only; for BMDN efficiency was ∼50%. Cells were used for the experiments at a density of 1 × 106 cells/experimental condition. Optimal protein expression was observed for 36 to 48 h posttransfection, as verified using Western blot analyses of stimulated lysates.
Morphology of chemotaxing BMDM.
BMDM that were used for chemotaxis as described above were fixed onto coverslips using 4% paraformaldehyde for 10 min at room temperature, permeabilized with 0.5% Triton X-100 in PBS for 10 min at room temperature, and then incubated in 10% fetal calf serum (FCS)–0.1% Triton X-100 in PBS for up to 4 h at room temperature. Endogenous PLD2 was detected in these cells using a goat anti-PLD2 (N-20 antibody) IgG primary antibody overnight at 4°C, followed by incubation with a donkey anti-goat fluorescein isothiocyanate (FITC)-conjugated IgG secondary antibody for 1 h at room temperature. Nuclei were stained using at a 1:2,000 dilution of 4′,6′-diamidino-2-phenylindole (DAPI) in PBS for 5 min at room temperature. Coverslips were mounted onto glass microscope slides using VectaShield mounting medium, and cells were visualized using a Nikon 50 Eclipse epifluorescence microscope.
Cell culture.
Murine RAW264.7 macrophage or COS-7 cells were maintained in either low- or high-bicarbonate Dulbecco's modified Eagle's medium (DMEM) in a humidified, 5% CO2 incubator, respectively. Viability assays were routinely conducted with 0.4% trypan blue stain in cell preparations prior to all analyses, and >95% were viable. Cells were ultimately resuspended in DMEM–0.1% BSA at a concentration 1.5 × 106 cells/ml for use in chemotaxis assays.
Cell migration assay.
BMDN, BMDM, or RAW264.7 cells were resuspended at a density of 5 × 105 cells/ml in chemotaxis buffer (Hanks balanced salt solution supplemented with 0.5% BSA for BMDN or BMDM cells or DMEM with 0.5% BSA for RAW264.7 cells). A total of 200 μl was placed in the upper chambers (or inserts) of transwell inserts that were separated from the lower wells by a 6.5-mm diameter, 5- (BMDN cells) or 8-μm-pore-size (BMDM or RAW264.7 cells) polycarbonate membrane. For the study of chemotaxis, IL-8 (BMD neutrophils) or M-CSF (BMD macrophages and RAW264.7 cells) was prepared fresh the day of the experiment in 1× PBS–0.5% BSA, pH, 7.2, at a stock concentration of 1 mM. When ready for chemotaxis, IL-8 or M-CSF was diluted to a 3 nM working concentration in 500 μl of chemotaxis buffer and placed into the lower wells of 24-well plates. Cell migration inserts were incubated for 1 h at 37°C under a 5% CO2 atmosphere. The number of cells that migrated to the lower wells was calculated by placing 10-μl aliquots on a hemocytometer and counting four fields in duplicate.
PLD activity assay.
Immunocomplex samples were processed for PLD2 activity in PC8 liposomes and n-[3H]butanol beginning with the addition of the following reagents, as previously described (21): 3.5 mM PC8 phospholipid, 45 mM HEPES (pH 7.8), and 1.0 mCi n-[3H]butanol in a liposome form (final concentrations are given). Samples were incubated for 20 min at 30°C with continuous shaking. Addition of 0.3 ml of ice-cold chloroform-methanol (1:2) stopped the reactions. Lipids were then isolated and resolved by thin-layer chromatography. The amount of [3H]phosphatidyl butanol (PBut) that comigrated with PBut standards was measured by scintillation spectrometry.
Statistical analysis.
Data are presented as the means ± standard errors of the means (SEM). The difference between means was assessed by a single-factor analysis of variance (ANOVA) test. A P value of <0.05 was considered to indicate a significant difference.
RESULTS
Rac2 has a dual (enhancing and inhibitory) effect on PLD2-induced chemotaxis.
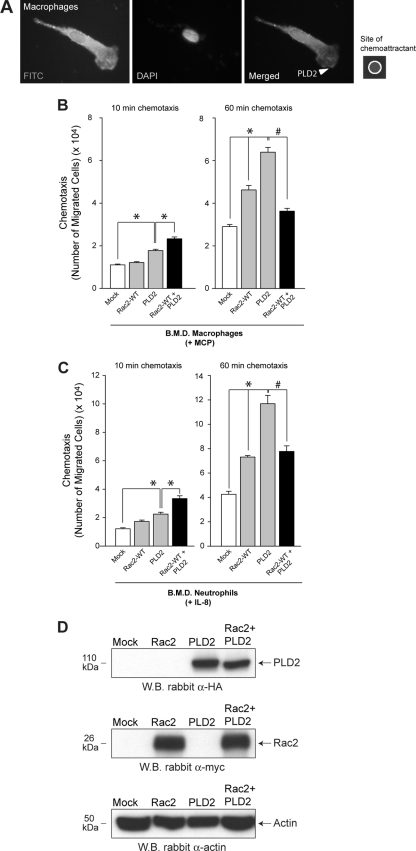
Primary cells, i.e., neutrophils and macrophages (derived from normal, WT mice), respond strongly to chemoattractants IL-8 and monocyte chemoattractant protein (MCP), respectively. We have included immunofluorescence microscopy of chemotaxing cells to verify the morphology of the migrating BMD macrophages. As shown in Fig. 1A, examination of one macrophage representative of the chemotaxing morphology indicated that recombinant PLD2 localizes to both the cytoplasm and the leading edge of the extending pseudopodia following chemoattractant stimulation.
Fig. 1.
(A) Morphological analysis of chemotaxing BMDM in response to 3 nM M-CSF using immunofluorescence microscopy. Visualization of recombinant PLD2 was detected following incubation of previously stimulated, fixed cells with goat anti-myc FITC-conjugated IgG antibodies. (B and C) Primary cells (BMD macrophages or neutrophils) were transfected with the indicated construct for 2 days, after which chemotaxis was analyzed in transwell assays using the indicated chemoattractants for 10 or 60 minutes. (D) Western blot (WB) analyses of lysates from BMDM were performed for the same samples as in panel B to establish the importance of the ratio of PLD2 to Rac2 compared to the absolute protein expression level of each enzyme. HA-tagged PLD2 was detected using rabbit polyclonal anti-HA IgG antibodies (upper panel). Myc-tagged Rac2 was detected using rabbit polyclonal anti-myc IgG antibodies (middle panel). An actin loading control was detected using rabbit polyclonal anti-actin IgG antibodies (lower panel). α, anti.
It has previously been demonstrated that PLD and its enzymatic activity toward chemokines are physiologically relevant to the process of leukocyte migration (19). This chemotactic response is enhanced in bone-marrow derived macrophages transfected with a PLD2 construct at early (10 min) and late (60 min) (Fig. 1B) times of chemotaxis. Transfection of cells with WT Rac2 WT had no effect on PLD-mediated chemotaxis. However, when both WT Rac2 WT and PLD2 constructs were cotransfected into cells, the resulting chemotactic capability of these cells was enhanced at 10 min compared to the mock negative controls, in which chemotaxis slightly less robust at 60 min (Fig. 1B). Chemotaxis of cells at 60 min that were doubly transfected was still significantly higher than that of mock-transfected cells at this time point. Similar results were found when BMD neutrophils were used instead of BMD macrophages (Fig. 1C).
As shown in Fig. 1D, Western blot analyses of the absolute protein expression levels of both Rac2 and PLD2 did not deviate following double transfection of both proteins in the cells regardless of time (either 10 min or 60 min) compared to the singly transfected control samples, which indicates that relative protein expression levels of either protein did not change during coexpression. Having demonstrated that the absolute expression levels of the proteins are the same, we believe that it is the ratio of PLD2 to Rac2 that is the most important piece of information.
The ratio of PLD2 to Rac2 affects the duality of Rac2 on PLD2-mediated chemotaxis.
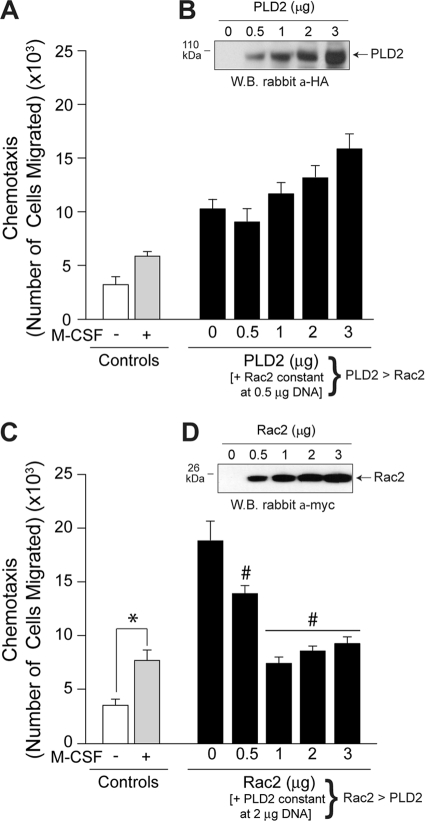
The first scenario in which Rac2 has a positive effect on a PLD2-mediated process is when cells are simultaneously transfected with a constant amount of Rac2 and increasing concentrations of PLD2 (Fig. 2A) (PLD2 is in excess in the reaction mixture/cell), which results in cell migration being concomitantly stimulated. Figure 2B is a Western blot of the cotransfected lysates that was probed with anti-hemagglutinin (HA) antibodies specific for the HA-tagged PLD2 to indicate increasing PLD2 protein expression. These data indicated that Rac2 had a positive effect on PLD2 when there was an overwhelming amount of PLD2 in the reaction mixture/cell. The second scenario whereby Rac2 has a negative effect on a PLD2-mediated process occurred when cells were transfected with a constant amount of PLD2 and increasing concentrations of Rac2 in vivo (Fig. 2C) (Rac2 is in excess in the reaction mixture or cell), which resulted in concomitantly reduced cell migration. Figure 2D is a Western blot of these cotransfected lysates that was probed with anti-myc antibodies specific for the myc-tagged Rac2 to indicate increasing Rac2 protein expression. These data indicated that Rac2 had a negative effect on PLD2 when there was an overwhelming amount of Rac2 in the cell.
Fig. 2.
The PLD2-to-Rac2 ratio determines the outcome of chemotaxis. (A) Cells were transfected with a constant amount of Rac2 DNA (0.5 μg) and variable amounts of a PLD2 construct. PLD2 is in excess in the reaction mixtures, compared to Rac2. Cells were used for chemotaxis analysis in transwell assays with M-CSF. (B) A Western blot of the cotransfected lysates was probed with anti-HA (a-HA) antibodies specific for the HA-tagged PLD2 to indicate increasing PLD2 protein expression. (C) Cells were transfected with a constant amount of PLD2 DNA (2 μg) and variable amounts of an Rac2 construct. Rac2 is in excess in the reaction mixtures compared to PLD2. (D) A Western blot of the cotransfected lysates was probed with anti-myc (a-myc) antibodies specific for the myc-tagged Rac2 to indicate increasing Rac2 protein expression. Data are means ± SEM from at least three independent experiments performed in duplicate. Differences between means, as determined by ANOVA, are indicated as above (*) or below (#) the negative-control levels.
Rac2 has a dual effect on PLD2 activity.
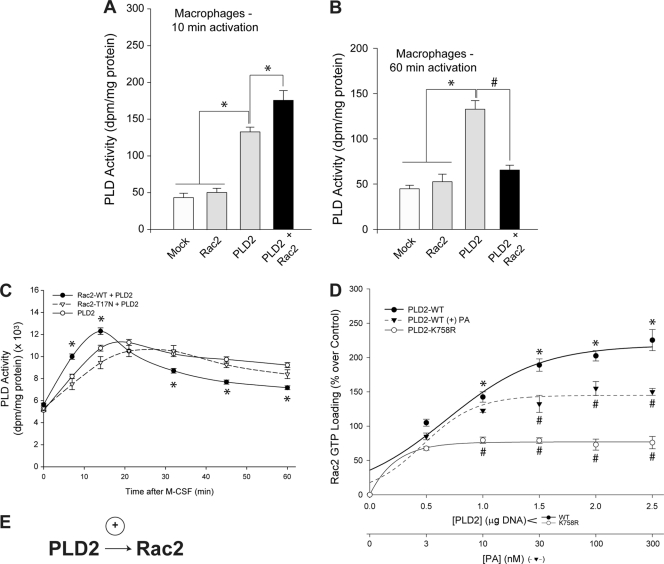
We also present scenarios under which Rac has dual effects on PLD2 activity. Following agonist stimulation, the first scenario occurred when cells were incubated for short periods of time with the chemoattractant, which resulted in enhanced PLD enzymatic activity (Fig. 3A) and could be correlated to enhanced chemotaxis (Fig. 1B and C). These two sets of data indicate that Rac2 has a positive effect on PLD2 at initial (early) times of stimulation and when there is an overwhelming amount of PLD2 in the reaction mixture/cell. The second scenario occurred when BMDM cells were incubated for long periods of time with the chemoattractant (60 min), which resulted in not only reduced PLD enzymatic activity (Fig. 3B) but also reduced chemotaxis (Fig. 1B and C). These two sets of data indicate that Rac2 has a negative effect on PLD2 at late times of stimulation and when there is an overwhelming amount of Rac2 in the cell.
Fig. 3.
A dual effect of Rac2 on PLD2 lipase activity. Primary cells (BMDM) were transfected with the indicated construct for 2 days, at which time lysates were prepared for in vitro enzymatic assays. (A) BMDM overexpressing Rac2, PLD2, or both were stimulated for 10 min with 3 nM M-CSF, and lysates were prepared from which PLD activity was measured. (B) BMDM overexpressing Rac2, PLD2, or both were stimulated for 60 min with M-CSF, and lysates were prepared from which PLD activity was measured. (C) Same experiment as described for panels A and B except that the lysates of BMDM were immunoprecipitated with antibodies specific for PLD prior to measurement of the PLD activity in liposomes with [3H]butanol. PLD activity values for controls were 761 ± 93 dpm/mg of protein. Data are means ± SEM from at least three independent experiments performed in duplicate. Differences between means, as determined by ANOVA, above the negative-control levels are indicated (*). (D) Effect of PLD2 on Rac2 GTP loading activity. Increasing concentrations of PLD2 plasmid DNA (0, 0.5, 1, 1.5, 2, and 2.5 μg) and a constant amount of Rac2 plasmid DNA were transfected into macrophages; 48 h posttransfection, cells were stimulated with 3 nM M-CSF for 10 min, and lysates were prepared and used for binding to PAK-1 PBD-agarose to measure the effect of WT PLD2 or a lipase-dead mutant on GTP loading of Rac2 in vivo. Samples were electrophoresed onto 4 to 20% gradient gels using SDS-PAGE and transferred onto polyvinylidene difluoride membrane. Rac2 GTP loading was measured by probing polyvinylidene difluoride membranes with rabbit polyclonal anti-myc antibodies specific for the myc-tagged Rac2 protein; enhanced chemiluminescence was performed according to the manufacturer's instructions. The lower x axis shows the measurement of the effect of increasing PA concentrations (0, 3, 10, 30, 100, and 300 nM) on GTP loading of Rac2 in vivo. Data are means ± SEM from at least three independent experiments performed in duplicate. Differences between means, as determined by ANOVA, above (*) or below (#) the negative-control levels are indicated.
The dual effect of Rac2 on PLD2 chemotaxis was also correlated to a similar effect on PLD2 activity (Fig. 3C). BMD macrophages were transfected with both Rac2 and PLD2, and lysates were utilized for a range of time to measure lipase activity in response to chemoattractant. In controls (cells transfected with PLD2 alone), the activity remained elevated even 60 min after addition of M-CSF to the cell suspension. In cotransfected samples, PLD activity followed a biphasic pattern, with an initial (10 to 15 min) increase followed by a later (>30 min) decrease in activity that returned the lipase activity to near basal levels. Thus, as first reported here, Rac2 has a dual (enhancing and inhibitory) effect on PLD2-mediated chemotaxis.
PLD2 positively affects Rac2 GTP loading activity.
PLD2 positively affects Rac2 GTP loading activity of macrophages transfected with constant WT Rac2 and increasing amounts of PLD2 plasmid DNA (Fig. 3D, filled circles), which is dependent on an intact lipase activity as transfection with the PLD2 lipase-dead mutant PLD2 K758R (Fig. 3D, open circles) had a significantly smaller effect on Rac2 GTP loading (Fig. 3D). Alternatively, a similar positive effect of PLD2 on GTP loading of Rac2 occurred in the presence of increasing PA (filled triangles). Both of these pieces of data strongly indicate the existence of a positive interaction between PLD2 and Rac2 that is dependent on both an intact Rac2 GTP loading activity and an intact PLD2 lipase activity during this first phase of the PLD2 and Rac2 interaction at early stages of stimulation, as indicated in the cartoon model (Fig. 3E).
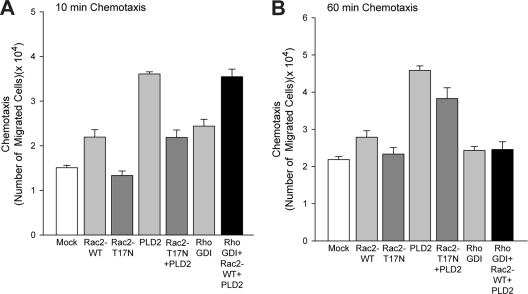
It has been shown in another study that Rac2 localizes predominantly to endomembranes through the use of Rho-guanine nucleotide dissociation inhibitor (RhoGDI), which prevents the release of GDP from Rho proteins and specifically targets many small GTPases to certain compartments within the cell (23). It was reported that overexpression of Rho proteins in general was able to overwhelm the capacity of endogenous RhoGDI to confer and properly modulate proper targeting of the small GTPase. Taking this report into consideration when we performed our chemotaxis experiments, we have also included data from additional transfections using Rac2 and RhoGDI (Fig. 4).
Fig. 4.
Effect of dominant negative Rac2 or RhoGDI on PLD2 lipase activity. Primary cells (BMDM) were transfected with the indicated construct for 2 days, after which chemotaxis was analyzed in transwell assays using the indicated chemoattractants for 10 (A) or 60 (B) minutes. Data are means ± SEM from at least three independent experiments performed in duplicate.
As shown in Fig. 4A, the effect of RhoGDI in combination with Rac2 and PLD2 at early times of chemotaxis is similar to that of PLD2 alone, which is definitely increased over that of RhoGDI alone. At later times of chemotaxis (Fig. 4B), the effects of both RhoGDI alone and RhoGDI in combination with Rac2 and PLD2 are decreased compared to the effect of PLD2 alone. This indicates that the effect originally seen with Rac2 was not simply due to overexpression of Rac2 only. We believe that GDI targets Rac2 to the membrane, where it inhibits both PLD2 and chemotaxis.
As we documented a similar effect of Rac2 and PLD2 coexpression on late chemotaxis (60 min) compared with coexpression of Rac2, PLD2, and RhoGDI (Fig. 1B and C and 4B), we believe that RhoGDI is targeting Rac2 to the plasma membrane in our cells. Additionally, we also found that Rac2 T17N abrogates all previous effects seen with WT Rac2 WT, indicating that the GTP loading activity of Rac2 must be important to exert its action on PLD2 (Fig. 4).
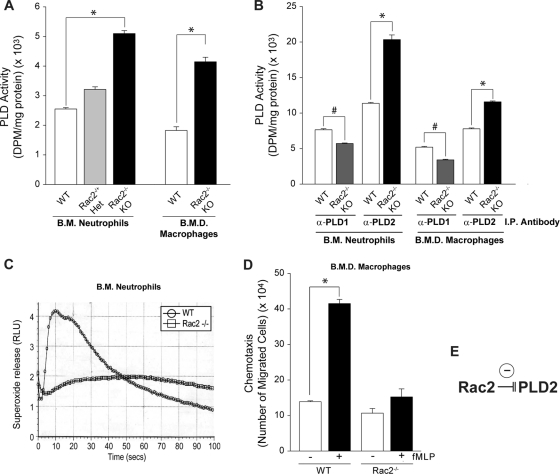
Endogenous PLD activity from bone marrow neutrophils and bone marrow-derived macrophages is high in Rac2−/− KO mice. The negative effect of Rac2 on PLD2 activity was also confirmed in primary cells from Rac2−/− KO mice (Fig. 5). Both bone marrow neutrophils and bone marrow-derived macrophages were deficient in Rac2. Rac2−/− cells had more PLD activity than their WT counterparts (90% increase) (Fig. 5A), suggesting that the absence of Rac2 has an activator effect on PLD activity.
Fig. 5.
Endogenous PLD activity from BMDN and BMDM is high in Rac2−/− KO mice. (A) BMDN and BMDM were isolated under sterile conditions, and lysates were prepared from which total PLD activity was measured. The figure also shows results of lysates from neutrophils from heterozygous mice (Rac2−/− Het). (B) The experiment is the same as that described for the previous panel except that the lysates were immunoprecipitated with antibodies specific for either PLD1 or PLD2 prior to the measurement of PLD activity. (C) Control of cell functionality as BMDN from WT mice show an increase in superoxide release, whereas BMDN from Rac2−/− KO mice show lack of response. (D) Chemotaxis of BMDM comparing cells from WT and Rac2−/− KO mice in the absence or presence of 20 nM fMLP. (E) Model representing the negative effect of Rac2 on PLD2 processes. Data are means ± SEM from at least three independent experiments performed in duplicate. Differences between means, as determined by ANOVA, above (*) or below (#) the negative-control levels are indicated. RLU, relative light units.
The converse of this effect whereby Rac2 inhibits PLD2 lipase activity and PLD2-mediated chemotaxis has already been shown in Fig. 1B and C, 2C, and 3B. To further establish the validity of Rac2 acting negatively on PLD2, we next transfected primary cells from Rac2−/− KO mice (Fig. 5B) with either PLD1 or PLD2 for use in lipase measurements following immunoprecipitation of cell lysates with either anti-PLD1 or anti-PLD2 antibodies. Here, we further implicate a robust dependence of PLD1 on Rac as overexpression of PLD1 in primary cells from Rac2−/− knockout (KO) mice resulted in decreased lipase activity (also observed by other authors at least for Rac1). As a technical note, we should point out that we had immunoprecipitated either PLD1 or PLD2 to separate the two isoforms and then normalized for total protein, which resulted in an obvious and significant positive effect with PLD2 but not for PLD1. Additionally, our PLD2 activity assays are not supplemented with Arf6 and RhoA, which are requisite for detection of the low basal level of PLD1 in the cell. We are utilizing cofactors in our PLD2 activity assays that are more specific for PLD2 and not PLD1.
Conversely, transfection of PLD2 into primary cells from these Rac2 knockout mice resulted in increased lipase activity and further recapitulates the fact that Rac2 negatively regulates PLD2-mediated processes (Fig. 5E). Figure 5C is a control of cell functionality as BMDN from WT mice show an increase in superoxide release, whereas BMDN from Rac2−/− KO mice show lack of response. Similar to PLD activity, superoxide release increases in WT cells in response to stimulating neutrophil agonists, whereas superoxide release in Rac2−/− cells is not elevated in response to the same agonist. Second, looking at chemotactic ability of these same cells, Fig. 5D shows that WT macrophages underwent chemotaxis more in response to formyl-methionyl-leucyl-phenylalanine (fMLP) than the Rac2−/− counterparts, suggesting that the absence of Rac2 has an inhibitory effect on chemotaxis.
On the mechanism of Rac2 inhibition of PLD2: rescuing effect of PIP2.
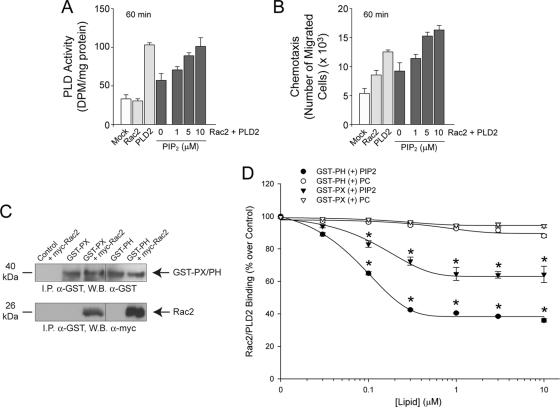
Next, we decided to explore further the mechanism that Rac2 at late stages of cell migration could inhibit PLD2. We reasoned that this could be reversed somehow and found that a key cofactor for the enzymatic reaction (phosphatidylinositol 4,5-bisphosphate [PIP2]) would just do that when used in large enough concentrations. Using PIP2 in the enzymatic reactions or in vitro with cells, Fig. 6 shows the rescuing effect of additional PIP2 on Rac2-inhibited PLD activity (Fig. 6A) and PLD2-mediated chemotaxis (Fig. 6B). We believe one explanation for this effect is that the potential exits for excess PIP2 to translocate PLD2 and Rac2 from vesicles to the plasma membrane, which would result in increased PLD2 activity and chemotaxis of affected cells, while decreased PIP2 would reverse this process and sequester PLD2 and Rac2 in the cytoplasmic vesicles. The exact mechanism for this to occur will become evident in subsequent experiments in this study, as the interaction of PLD2-Rac2 and PIP2 is further dissected.
Fig. 6.
Rescuing effect of PIP2 on Rac2-inhibited PLD2 activity. (A) Macrophages were transfected with the indicated constructs, and prior to the assay of PLD2 activity, the mixtures were supplemented with increasing concentrations of PIP2 in the reaction mixture following 60 min of agonist stimulation. (B) Macrophages were transfected with the indicated constructs, and prior to chemotaxis, the mixtures were supplemented with increasing concentrations of PIP2 (beyond what is normally used for in vitro enzymatic assays) in the cell culture well and then underwent chemotaxis for 60 min in response to agonist stimulation. (C) Pulldown assay. GST-tagged PX and PH proteins purified from pGEX-4T-1-PX- or pGEX-4T-1-PH-overexpressing cells were bound to GST-Sepharose beads. In parallel experiments, cells were transfected with myc-Rac2 constructs, and lysates were obtained. These lysates were mixed with the beads, and bound fractions of GST-PX and GST-PX were eluted. Bound fractions were analyzed by Western blotting for the presence of proteins. The blot was first developed using anti-GST antibody to ensure that the PX and PH proteins were present. The same blot was stripped and probed with anti-myc antibody to check for the presence of associated Rac2. (D) Rac2-PLD2 disruption by PIP2. The pulled down complex Sepharose-GST-PX(or PH)-Rac2 was incubated with increasing amounts of PIP2 (beyond what is normally used for in vitro enzymatic assays) or PC and analyzed for the presence of myc-Rac2 bound to PLD2. Data are means ± SEM from at least three independent experiments performed in duplicate. Differences between means, as determined by ANOVA, above the negative-control levels are indicated (*).
PIP2 competes with Rac2 in its binding to GST-PLD2.
The results throughout this study on the modulation of PLD2 by Rac2 suggested a close, agonist-dependent interaction in the cell. Given the results of the experiments shown in Fig. 6A and B, we suspected that a certain PLD2 domain involved in lipid binding was at play. We hypothesized that Rac2 binds to PLD2 at the pleckstrin homology (PH) domain or possibly even the phox homology (PX) domain. Therefore, we separately subcloned the PH or PH domain of PLD2 into the pGEX-4T-1 vector, which would allow us to express two different glutathione S-transferase (GST)-tagged proteins. Figure 6C is an immunoprecipitation assay with the purified, GST-tagged PH and PX proteins, which are both able to be bound by myc-tagged Rac2 from RAW264.7 macrophages and can be pulled down with α-myc antibodies specific for Rac2. The PH domain of PLD2 is preferentially bound by Rac2 by an approximate factor of 2-fold more than the PX domain. This result demonstrates that the protein-protein interaction of Rac2-PLD2 involves at least the PH domain of PLD2 and, to a much lesser extent, possibly the PX domain.
The Rac2-PLD2 interaction was further analyzed by incubation of Sepharose-GST-PH or -PX-Rac2 complexes with increasing amounts of PIP2. As indicated in Fig. 6D, PIP2 caused an ∼60% reduction in GST-PH-PLD2 (filled circles) bound to myc-Rac2 and an ∼30% reduction in GST-PX-PLD2 (filled triangles) bound to myc-Rac2, whereas incubation of either truncated PLD2 with the negative-control lipid, phosphatidylcholine (PC), had no effect on Rac2 binding to PLD2 (open circles and triangles). In conclusion, Rac2 specifically interacts with greater preference to the PH domain of PLD2 than with the PX domain, and both of these interactions can be disrupted by excess amounts of PIP2 in vitro. We posit that as Rac2 binds to PLD2, this interaction deprives the availability of PLD for its key factor and function.
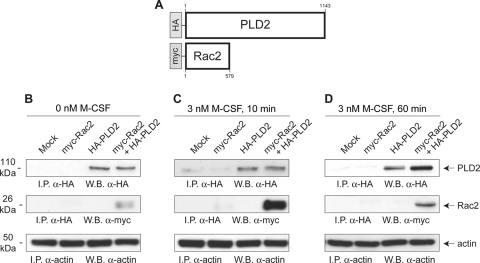
Rac2 and PLD2 form a protein-protein complex in the cell.
The results throughout this study of biochemical data on the modulation of PLD2 by Rac2 suggested that these two proteins may be in close spatial proximity in the cell. We generated a eukaryotic PLD2 construct tagged with HA and a Rac2 construct tagged with myc to be used in RAW264.7 macrophages to measure the protein-protein interaction of Rac2-PLD2 (Fig. 7A). Figure 7B to D show the binding interaction of these two proteins via coimmunoprecipitation as a function of time. Rac2 could be immunoprecipitated with antibodies directed against PLD2's HA tag (middle sections). Further, we observed that this immunoprecipitation was enhanced if the cells had been allowed to be stimulated with 3 nM M-CSF for a full 10 min (Fig. 7C) compared to 60 min (Fig. 7D) or unstimulated cells (Fig. 7B). These data strongly support the existence of a protein-protein interaction between Rac2 and PLD2 in the cell that is dependent upon the stimulant at relatively short periods of time.
Fig. 7.
Demonstration of protein-protein interaction between PLD2 and Rac2. Macrophages were transfected with tagged expression plasmids, with myc for Rac2 and HA for PLD2 alone or in combination (A). After 2 days, cells were harvested and incubated with 0 nM M-CSF (B), 3 nM M-CSF for 10 min (C), or 3 nM M-CSF for 60 min (D). Cells were then lysed, immunoprecipitated with anti-HA antibodies to pull down PLD2, and electrophoresed in SDS gels, and resulting blots were probed with anti-HA antibodies to detect PLD2 or anti-myc antibodies to detect Rac2. The region of the gel where PLD2 is expected (molecular mass of ∼110 kDa) is shown (top panels). The region of the gel where Rac2 is expected following interaction with PLD2 (molecular mass of ∼26 kDa) is also shown. The third panel is a control that shows equal loading in all lanes as ascertained with anti-actin antibodies.
During early cell chemotaxis, PLD2 translocates to the cell membrane and facilitates lamellipodium extension, whereas in late chemotaxis/immobilization, Rac2 facilitates.
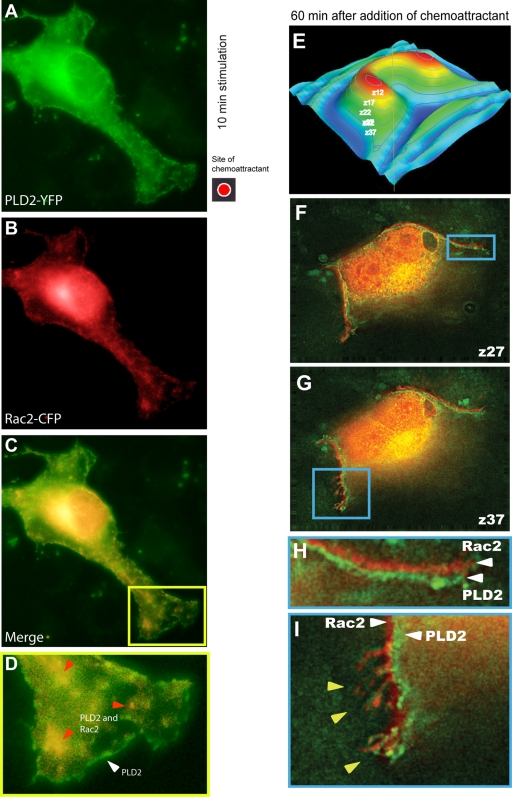
We wanted to ascertain if Rac2 and PLD2 would colocalize simultaneously along the plasma membrane. Using macrophages that were transfected with fluorescently tagged PLD2-yellow fluorescent protein (YFP) and Rac2-cyan fluorescent protein (CFP), cells were directionally stimulated with chemoattractant that was applied to a particular location on the microscope slide (Fig. 8, red spot) to begin the chemotactic process. Figure 8A to C show that PLD2 and Rac2 colocalize to the advancing lamellipodia and specifically to vesicle-like formations close to the button end of the advancing pseudopodia at early stages (10 min) of chemotaxis (Fig. 8D). This colocalization of PLD2 and Rac2 in vesicles in the cytoplasm appears to be very robust.
Fig. 8.
PLD2 is located in the cell membrane of advancing pseudopodia in early chemotaxis and behind Rac2 in late chemotaxis. YFP-PLD2 and CFP-Rac2 were transfected into cells for 24 h (the last 2 h of which the cells were serum starved). When ready for microscopy, cells were treated with M-CSF for 10 min (A to D) that was added to a localized region on the coverslip (red dot) with a micropipette, which forms a temporary chemoattractant gradient. To increase the contrast between the YFP- and CFP-tagged proteins, YFP-PLD2 is depicted as green fluorescence, while CFP-Rac2 is depicted as red fluorescence. (D) Enlargement of a section of panel C, with fluorescence imaging showing that some PLD2 localizes in the leading edge of the plasma membrane (white triangle), whereas some PLD2 and Rac2 colocalize in vesicles inside the lamellipodia (red triangles). (E to I) Cells were transfected with YFP-PLD2 and CFP-Rac2 for 2 days. After this period of time, cells were exposed to the chemoattractant for 60 min. Cells were then taken for fluorescence microscopy. Panels F and G represent two-step sweeps of the z-stack corresponding to sections, as indicated in the cartoon in panel E, and show finger-like projections with Rac2 and PLD2 juxtaposed. Panel H is a close-up of the image in panel F; panel I is a close-up of the image in panel G. A fraction of the fluorescence signals localizes in the plasma membrane for both CFP-Rac2 (shown in red here for better clarity to distinguish it from the green of the other fluorophore) and YFP-PLD2 (shown in green).
Additionally, there is also a distinct pool of PLD2 that localizes only to the leading edge of the moving macrophage, whereas Rac2 does not, indicating that PLD2 drives chemotaxis at these early stages of agonist stimulation. Indeed, the leading edge appears to be completely devoid of Rac2, consistent with its preferred targeting to endomembranes (42). In contrast, there may be trace colocalization of both PLD2 via its PH domain and Rac2 via a positive charge-independent mechanism to cellular lateral membranes via signaling of phosphoinositide-3,4,5-triphosphate (PIP3), which accumulates at the leading edge of chemotactic leukocytes and Dictyostelium (22, 39, 42).
Next, we concentrated on macrophages that had been exposed to the chemoattractant for a much longer period of time (60 min). At this length of time, cells normally cease to emit pseudopodia and become immobilized to the substrate, thus being an ideal system that emulates the late conditions of chemotaxis that would be equivalent to cell immobilization at the site of infection. Figure 8E to I represent serial cross-sections of a cell that indicate different planes between the adhesion points on the z-axis. Figure 8F and G represent five-step sweeps of the z-stack corresponding to the sections indicated in the cartoon model (Fig. 8E) and show the relevant optical sections that correspond to Fig. 8F and G.
Most of the colocalization between Rac2 and PLD2 takes place at the Golgi region around the nucleus. However, there is a fraction of the fluorescence signals that localized in the plasma membrane for both CFP-Rac2 (red) and YFP-PLD2 (green) (Fig. 8F and G). Most interestingly, these two signals do not overlap, but, rather, they are extremely adjacent to each other (at about 0.5 mm apart), such that Rac2 is closer to the plasma membrane than PLD2. Figure 8H and I are further magnifications of these two areas of colocalization. The white arrows mark the position of Rac2 and PLD2 within the membrane or their close association with it. To our knowledge, this is the first time subcellular localization of PLD2 is documented in particular reference to another signaling molecule and with Rac2 geographically in front of it.
A movie that expands the data of Fig. 8E to I and shows the colocalization of PLD2 and Rac2 in two “waves” in the cell's plasma membrane can be found at http://www.med.wright.edu/video/jgc/. This movie shows the composite of all images acquired through the z-axis of a cell, presenting consistent localization of YFP-PLD2 and CFP-Rac2 at the plasma membrane throughout the z-stack.
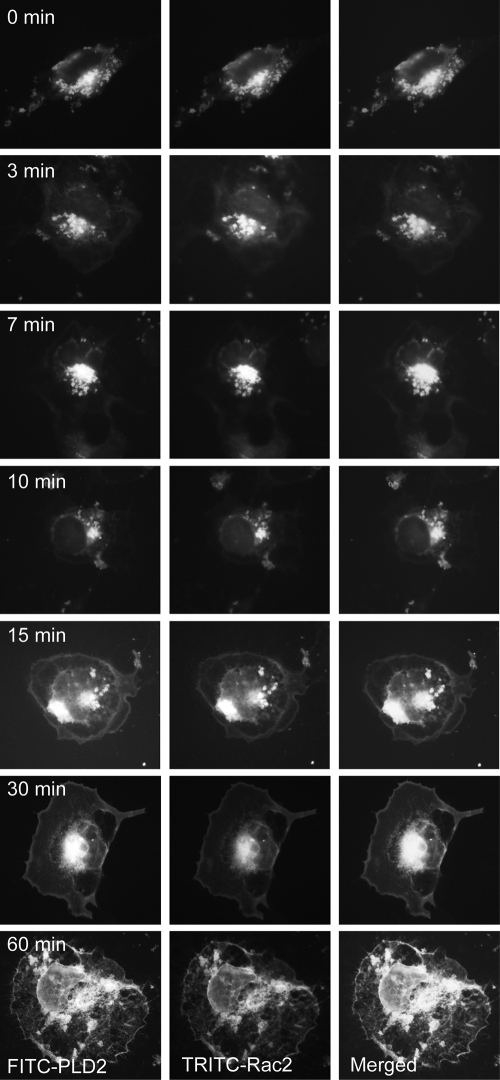
We have included additional static microscopic images of our cells to show that PLD2 and Rac2 migrate from intracellular vesicles to the leading edge as a function of time (during exposure to chemoattractant) (Fig. 9). As shown, both Rac2 and PLD2 are localized to vesicles at early times, while both proteins are localized to the leading edge following 30 to 60 min of incubation with epidermal growth factor (EGF).
Fig. 9.
PLD2 and Rac2 migrate from intracellular vesicles to the leading edge as a function of time. YFP-PLD2 and CFP-Rac2 were transfected into cells for 24 hours (for the last 2 h of which the cells were serum starved). When ready for microscopy, cells were treated for 10 min with M-CSF that was added to a localized region on the cover slip with a micropipette, which forms a temporary chemoattractant gradient. Fluorescence imaging showing that PLD2 and Rac2 migrate from intracellular vesicles to the leading edge as a factor of time of incubation with EGF.
A model that explains the dual function of Rac2 on PLD2.
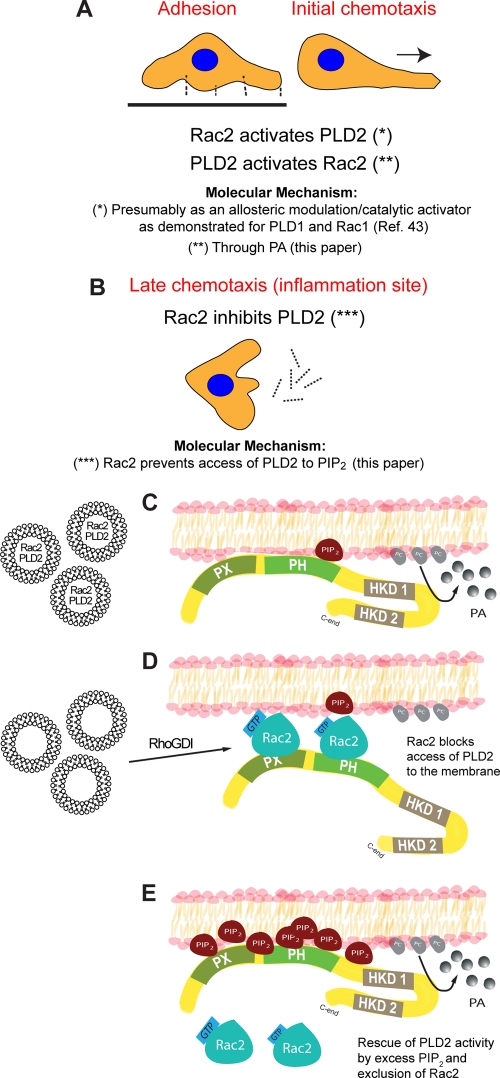
This study has shown the functional consequences of an interaction between Rac2 and PLD2 as it relates the time line of chemotaxis to the ability of Rac2 to function as a dual regulator of the lipase. We propose that PLD2 is needed for early chemotaxis (∼10 min), which is enhanced by the presence of Rac2 (Fig. 10A). We further propose that Rac2 provides a strong negative signal to inactivate PLD2 during late chemotaxis (∼60 min) (Fig. 10B). The mechanism for the former could be related to what is already known for the other PLD isoform, PLD1, whereby PLD2 via the PH domain binds to PIP2 and is a necessary cofactor for PLD function (Fig. 10C). On the other hand, Rac2 exerts a negative effect on PLD2, and this is accomplished when Rac2 occupies the PH domain, pulling PLD2 apart from the normal environment and out of the reach of PIP2 and other essential cofactors (Fig. 10D), which serves to terminate chemotaxis. Additionally, PLD2 activity can be rescued and restored in cells by the presence of excess PIP2, which simultaneously excludes Rac2 from the membrane (Fig. 10E). As all this was observed in primary cells (neutrophils and macrophages) as well as in cell lines (macrophages), we believe this mechanism could be extended to a large array of migratory cells. Further, this represents an example of simplicity and economy of cell resources as just two molecules can be sufficient for accomplishing a well-defined biological function.
Fig. 10.
Model that explains the mechanism of the duality of Rac2 on PLD2. (A) During early chemotaxis, Rac2 has a positive (enhancing) effect on PLD2 activity. Both Rac2 and PLD2 cooperate to ensure that the cell starts moving toward the inflammation site. (B) At later times, and particularly when the leukocyte confronts the invading pathogen at the infection site, Rac2 inactivates PLD2, and the cell becomes immobilized. (C) At the molecular level, the explanation of a negative feedback of Rac2 on PLD2 could be explained by the esteric effect of key molecules around the membrane of the advancing leukocyte lamellipodia. During early chemotaxis, PLD2 is anchored to the membrane via its PX and PH domains, where it finds the enzyme cofactor PIP2 and its substrate, PC. At later times (D), Rac2 interferes with the binding of PLD to the membrane and positions itself in front of PLD2. As this study has shown, Rac is able to bind to PLD2 at PH domain (on the other hand, Rac2 is able to bind to PIP2 [35]). This sterical positioning of Rac2 deprives PLD2 of its natural environment, contributing to its inactivation. (E) The negative effect of Rac2 on PLD2 can be negated by overwhelming concentrations of PIP2 that displace Rac2 from its binding to the membrane, thus restoring full PLD activity. HKD, histidine kinase domain.
DISCUSSION
This study presents evidence for the first time that a monomeric GTPase can both promote and inhibit a phospholipase activity, which serves to explain the timeline of chemotactic response. Further, the geographical location of fluorescently tagged PLD2 and Rac2 in cells is the key to defining the mechanism that Rac2 at the molecular level is sterically impeding the access of PLD2 to the membrane and its subsequent activation. We have shown the functional consequences of an interaction between Rac2 and PLD2, whereby Rac2 functions as a master switch that dually affects PLD2 activity, both positively and negatively, given the proper circumstances. It was important to define how PLD2 is regulated since its mechanism of regulation is less well characterized than that of PLD1. PLD is involved in the regulation of fundamental cellular functions, such as calcium mobilization, secretion, superoxide production, endocytosis, exocytosis, vesicle trafficking, glucose transport, rearrangement of the actin cytoskeleton, mitogenesis, and survival (27, 28). Regulation of PIP2 synthesis takes a central role in signaling by Rho GTPases and, as this study shows, a connection between Rac2-PIP2-PLD is evident.
PLD1 was originally generated by PLD hydrolyzing membranous phosphatidylcholine (PC) (3) and regulated through direct binding of PLD1 to Rac1 and/or Rho with its C terminus (7, 33). We found that leukocytes from Rac2−/− mice had a lower basal PLD1 activity than their wild-type counterparts, which was consistent with the report of Powner and Wakelam (34). We should note that previous studies (3, 7, 33) have implicated small GTPases (Arf-1, RhoA, Rac1, and Cdc42) on the PLD1 isoform, but the other isoform, PLD2 (subject of this study) has remained understudied as it was thought that PLD2 was regulated more directly through phosphatidylinositol-4,5-kinase (PI4,5K) and the concomitant generation of PIP2 than by GTPases. Our study shows direct evidence that PLD2 is regulated by the monomeric GTPase Rac2, which is in agreement with Powner and Wakelam (34) in that PLD2 is also regulated by PIP2 (we, in fact, show a new role for PIP2 as the rescuing molecule for a negative Rac2-PLD2 interaction).
It has been reported previously that Rac1 activates PLD1 (11, 24, 34, 43, 44). Concerted effects of Rac1 and Rac2 on PLD2 are not known. Other biological phenomena have assigned a role for each isoform of Rac in cell signaling. Such is the case of phagocytosis; it has been shown that Rac1 and Rac2 are activated sequentially and regulate phagocytosis (14). In this case, activation of Rac1 is biphasic; initially Rac1 is activated, and then this activation is shortly followed by a stronger phase of activation. The second stronger phase of Rac1 overlaps with the activation of Rac2. At any rate, both Rac1 and Rac2 are involved in cell migration. Knockout mouse studies with Rac1 and Rac2 have revealed that both Rac1 and Rac2 isoforms have a role in cell spreading and migration. Zhang et al. showed that neutrophils depend on Rac1 for initial cell spreading and, later, that Rac2-regulates Arp2/3- and cofilin-mediated actin polymerization for continuous expansion of the leading lamellipodium (46). Given all of these facts, it could be possible that Rac1 and Rac2 during the course of cell migration sequentially activate PLD1 (but not likely PLD2, the subject of the present study).
Spatial and temporal control of Rac GTPase activation is required for accurate directional cell movement. We have shown here using macrophages at early times of chemotaxis that both Rac2 and PLD2 were robustly colocalized to cytoplasmic vesicles. Rac2 has been shown to interact with and bind to PA-containing vesicles in the neutrophil cytosol, implicating a role for PLD2 in this process (8). Rac2 preferentially localizes in a positive-charge-independent mechanism at early stages of chemotaxis to endomembranes, which then fuse to the phagosomes later during cell migration (42). Overexpression of PLD2 in ionomycin-stimulated RBL-2H3 cells triggered translocation of PLD2 from the plasma membrane to intracellular compartments and concomitantly increased PLD activity of exosomes, which correlated directly with the amount of exosome released (18).
All this information implies a dual role for vesicles in both the delivery and retrieval steps of PLD2 to the plasma membrane during agonist stimulation following the subsequent delivery of small GTPases to the plasma membrane. We believe that excess PIP2 could result in both translocation of PLD2 and Rac2 from vesicles to the plasma membrane and in increased PLD2 activity and chemotaxis of affected cells, while decreased PIP2 could reverse this process and sequester PLD2 and Rac2 in the cytoplasmic vesicles. This conclusion is supported by the fact that PIP2 acts as a second messenger in Ras/Rac-induced disruption of the actin cytoskeleton (10), which implies a role for PIP2 in the regulation of Rac2 movement to the cell surface.
Increases in PIP2 are required for actin filament uncapping prior to membrane ruffling, which is mediated by Rac GTPases and results in lamellipodium formation (12). Also, as PLD-derived PA stimulates PIP2 production during clathrin coat assembly on lysosomes in vitro and is responsible for increased PA synthesis (1), overexpression of PLD2 coincides with increased PIP2 concentrations at membrane ruffles (13).
Rac's intracellular location cycles between the cytosol and the cell membrane, and its translocation to the membrane allows it to interact with its downstream effectors. We have also shown that at late times of chemotaxis, both Rac2 and PLD2 translocated to the cell membrane of macrophages after agonist activation, with Rac2 localized closer to the membrane and PLD2 directly behind Rac2. With Rac-GTP loading (Rac activity) being an exception, membrane localization is also required for the initiation of downstream effector signaling (2, 3). Chemoattractants cause the localization of PH domain-containing proteins to the stimulated face of chemotactic cells (29, 30). Dictyostelium cells overexpressing a GFP-tagged PH domain (also found in PLD enzymes) translocated toward the membrane and colocalized with phosphatidylinositol-(3,4,5)-trisphosphate (PIP3) following agonist stimulation (22). Additionally, both PLD2 and PA have been implicated in the insulin-induced translocation of the small GTPase Raf-1 from the cytoplasm to the plasma membrane (36).
Although PLD2 aids in lamellipodium formation during chemotaxis, Rac2 provides a strong negative signal during the process, which terminates chemotaxis or pseudo-podium retraction. In migrating cells, Rac is generally required at the leading edge for lamellipodium extension and formation of new adhesions (35). We propose the existence of a termination mechanism that signals to disassemble the lamellipodia when the physiological need arises following a change in localization of chemoattractant. This process is mediated by Rac2, which negatively feeds back on PLD2 and terminates the originating chemotactic signal.
We think the lipase activity is a necessary upstream signal during the initial steps of lamellipodium formation. Nishikimi et al. (25) have shown that the interaction of PLD2 and PA signals to DOCK2, which mediates Rac activation and actin remodeling. PA secured DOCK2 at the leading edge of a migrating neutrophil, and PA and PIP3 were coparticipants in the process. One can argue that PA was produced by PLD elsewhere in the cell, but we believe PLD has to be localized to the membrane throughout this entire process. Nishikimi et al. (25) make an excellent case for the sequential need of the two lipids for translocation and stabilization.
We also show that Rac2 drives the physiological process of late chemotaxis, while PLD2 drives early chemotaxis. We have shown here that a dual effect of Rac2 on PLD2 exists, which regulates the phagocyte function of chemotaxis (during full chemotaxis Rac2 synergizes with PLD2 allowing full cell movement; when the cell stops at the site of infection, then Rac2 starts driving the process and provides negative-feedback signals). Rac2 exerts a previously unknown negative effect on PLD2 and signals the cell to stop at the infection site, which is beneficial to the host in mounting an inflammatory response against an invading pathogen.
ACKNOWLEDGMENTS
Grants HL056653 (J.G.-C.) and R15GM084407 (P.B.) from the National Institutes of Health have supported this work.
Footnotes
Published ahead of print on 28 March 2011.
REFERENCES
- 1. Arneson L. S., Kunz J., Anderson R. A., Traub L. M. 1999. Coupled inositide phosphorylation and phospholipase D activation initiates clathrin-coat assembly on lysosomes. J. Biol. Chem. 274:17794–17805 [DOI] [PubMed] [Google Scholar]
- 2. Beemiller P., et al. 2010. A Cdc42 activation cycle coordinated by PI 3-kinase during Fc receptor-mediated phagocytosis. Mol. Biol. Cell 21:470–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chae Y. C., et al. 2008. Phospholipase D activity regulates integrin-mediated cell spreading and migration by inducing GTP-Rac translocation to the plasma membrane. Mol. Biol. Cell 19:3111–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cross M. J., et al. 1996. Stimulation of actin stress fibre formation mediated by activation of phospholipase D. Curr. Biol. 6:588–597 [DOI] [PubMed] [Google Scholar]
- 5. Dinauer M. C. 2007. Disorders of neutrophil function: an overview. Methods Mol. Biol. 412:489–504 [DOI] [PubMed] [Google Scholar]
- 6. Dinauer M. C. 2003. Regulation of neutrophil function by Rac GTPases. Curr. Opin. Hematol. 10:8–15 [DOI] [PubMed] [Google Scholar]
- 7. Exton J. H. 2002. Regulation of phospholipase D. FEBS Lett. 531:58–61 [DOI] [PubMed] [Google Scholar]
- 8. Faugaret D., Chouinard F. C., Harbour D., El Azreq M. A., Bourgoin S. G. 2011. An essential role for phospholipase D in the recruitment of vesicle amine transport protein-1 to membranes in human neutrophils. Biochem. Pharmacol. 81:144–156 [DOI] [PubMed] [Google Scholar]
- 9. Glogauer M., et al. 2003. Rac1 deletion in mouse neutrophils has selective effects on neutrophil functions. J. Immunol. 170:5652–5657 [DOI] [PubMed] [Google Scholar]
- 10. He H., et al. 1998. Role of phosphatidylinositol 4,5-bisphosphate in Ras/Rac-induced disruption of the cortactin-actomyosin II complex and malignant transformation. Mol. Cell. Biol. 18:3829–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Henage L. G., Exton J. H., Brown H. A. 2006. Kinetic analysis of a mammalian phospholipase D: allosteric modulation by monomeric GTPases, protein kinase C, and polyphosphoinositides. J. Biol. Chem. 281:3408–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hinchliffe K. 2000. Intracellular signalling: is PIP(2) a messenger too? Curr. Biol. 10:R104–R105 [DOI] [PubMed] [Google Scholar]
- 13. Honda A., et al. 1999. Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell 99:521–532 [DOI] [PubMed] [Google Scholar]
- 14. Hoppe A. D., Swanson J. A. 2004. Cdc42, Rac1, and Rac2 display distinct patterns of activation during phagocytosis. Mol. Biol. Cell 15:3509–3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ishizaki H., et al. 2006. Defective chemokine-directed lymphocyte migration and development in the absence of Rho guanosine diphosphate-dissociation inhibitors alpha and beta. J. Immunol. 177:8512–8521 [DOI] [PubMed] [Google Scholar]
- 16. Kam Y., Exton J. H. 2001. Phospholipase D activity is required for actin stress fiber formation in fibroblasts. Mol. Cell. Biol. 21:4055–4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lauffenburger D. A., Horwitz A. F. 1996. Cell migration: a physically integrated molecular process. Cell 84:359–369 [DOI] [PubMed] [Google Scholar]
- 18. Laulagnier K., et al. 2004. PLD2 is enriched on exosomes and its activity is correlated to the release of exosomes. FEBS Lett. 572:11–14 [DOI] [PubMed] [Google Scholar]
- 19. Lehman N., et al. 2006. Phagocyte cell migration is mediated by phospholipases PLD1 and PLD2. Blood 108:3564–3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li S., et al. 2002. Chemoattractant-stimulated Rac activation in wild-type and Rac2-deficient murine neutrophils: preferential activation of Rac2 and Rac2 gene dosage effect on neutrophil functions. J. Immunol. 169:5043–5051 [DOI] [PubMed] [Google Scholar]
- 21. Liscovitch M., Czarny M., Fiucci G., Tang X. 2000. Phospholipase D: molecular and cell biology of a novel gene family. Biochem. J. 345:401–415 [PMC free article] [PubMed] [Google Scholar]
- 22. Ma L., Janetopoulos C., Yang L., Devreotes P. N., Iglesias P. A. 2004. Two complementary, local excitation, global inhibition mechanisms acting in parallel can explain the chemoattractant-induced regulation of PI(3,4,5)P3 response in dictyostelium cells. Biophys. J. 87:3764–3774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Michaelson D., et al. 2001. Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding. J. Cell Biol. 152:111–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Momboisse F., et al. 2009. βPIX-activated Rac1 stimulates the activation of phospholipase D, which is associated with exocytosis in neuroendocrine cells. J. Cell Sci. 122:798–806 [DOI] [PubMed] [Google Scholar]
- 25. Nishikimi A., et al. 2009. Sequential regulation of DOCK2 dynamics by two phospholipids during neutrophil chemotaxis. Science 324:384–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O'Luanaigh N., et al. 2002. Continual production of phosphatidic acid by phospholipase D is essential for antigen-stimulated membrane ruffling in cultured mast cells. Mol. Biol. Cell 13:3730–3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oude Weernink P. A., Han L., Jakobs K. H., Schmidt M. 2007. Dynamic phospholipid signaling by G protein-coupled receptors. Biochim. Biophys. Acta 1768:888–900 [DOI] [PubMed] [Google Scholar]
- 28. Oude Weernink P. A., Lopez de Jesus M., Schmidt M. 2007. Phospholipase D signaling: orchestration by PIP2 and small GTPases. Naunyn Schmiedebergs Arch. Pharmacol. 374:399–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parent C. A., Blacklock B. J., Froehlich W. M., Murphy D. B., Devreotes P. N. 1998. G protein signaling events are activated at the leading edge of chemotactic cells. Cell 95:81–91 [DOI] [PubMed] [Google Scholar]
- 30. Parent C. A., Devreotes P. N. 1999. A cell's sense of direction. Science 284:765–770 [DOI] [PubMed] [Google Scholar]
- 31. Pleskot R., et al. 2010. Mutual regulation of plant phospholipase D and the actin cytoskeleton. Plant J. 62:494–507 [DOI] [PubMed] [Google Scholar]
- 32. Porcelli A. M., Ghelli A., Hrelia S., Rugolo M. 2002. Phospholipase D stimulation is required for sphingosine-1-phosphate activation of actin stress fibre assembly in human airway epithelial cells. Cell Signal. 14:75–81 [DOI] [PubMed] [Google Scholar]
- 33. Powner D. J., Hodgkin M. N., Wakelam M. J. 2002. Antigen-stimulated activation of phospholipase D1b by Rac1, ARF6, and PKCα in RBL-2H3 cells. Mol. Biol. Cell 13:1252–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Powner D. J., Wakelam M. J. 2002. The regulation of phospholipase D by inositol phospholipids and small GTPases. FEBS Lett. 531:62–64 [DOI] [PubMed] [Google Scholar]
- 35. Ridley A. J. 2001. Rho proteins: linking signaling with membrane trafficking. Traffic 2:303–310 [DOI] [PubMed] [Google Scholar]
- 36. Rizzo M. A., et al. 1999. Phospholipase D and its product, phosphatidic acid, mediate agonist-dependent raf-1 translocation to the plasma membrane and the activation of the mitogen-activated protein kinase pathway. J. Biol. Chem. 274:1131–1139 [DOI] [PubMed] [Google Scholar]
- 37. Roberts A. W., et al. 1999. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity 10:183–196 [DOI] [PubMed] [Google Scholar]
- 38. Santy L. C., Casanova J. E. 2001. Activation of ARF6 by ARNO stimulates epithelial cell migration through downstream activation of both Rac1 and phospholipase D. J. Cell Biol. 154:599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sasaki A. T., Firtel R. A. 2005. Finding the way: directional sensing and cell polarization through Ras signalling. Novartis Found. Symp. 269:73–91, 223–230 [PubMed] [Google Scholar]
- 40. Schafer D. A., et al. 1998. Visualization and molecular analysis of actin assembly in living cells. J. Cell Biol. 143:1919–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sun C. X., et al. 2004. Rac1 is the small GTPase responsible for regulating the neutrophil chemotaxis compass. Blood 104:3758–3765 [DOI] [PubMed] [Google Scholar]
- 42. Ueyama T., et al. 2005. Isoform-specific membrane targeting mechanism of Rac during Fc gamma R-mediated phagocytosis: positive charge-dependent and independent targeting mechanism of Rac to the phagosome. J. Immunol. 175:2381–2390 [DOI] [PubMed] [Google Scholar]
- 43. Walker S. J., Brown H. A. 2002. Specificity of Rho insert-mediated activation of phospholipase D1. J. Biol. Chem. 277:26260–26267 [DOI] [PubMed] [Google Scholar]
- 44. Wennerberg K., et al. 2002. RhoG signals in parallel with Rac1 and Cdc42. J. Biol. Chem. 277:47810–47817 [DOI] [PubMed] [Google Scholar]
- 45. Yamauchi A., et al. 2004. Rac2-deficient murine macrophages have selective defects in superoxide production and phagocytosis of opsonized particles. J. Immunol. 173:5971–5979 [DOI] [PubMed] [Google Scholar]
- 46. Zhang H., Sun C., Glogauer M., Bokoch G. M. 2009. Human neutrophils coordinate chemotaxis by differential activation of Rac1 and Rac2. J. Immunol. 183:2718–2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhelev D. V., Alteraifi A. 2002. Signaling in the motility responses of the human neutrophil. Ann. Biomed. Eng. 30:356–370 [DOI] [PubMed] [Google Scholar]