Abstract
Here we investigate the mechanisms regulating Greatwall (Gwl), a serine/threonine kinase essential for promoting the correct timing of mitosis. We identify Gwl as a unique AGC kinase that, unlike most AGC members, appears to be devoid of a hydrophobic motif despite the presence of a functional hydrophobic pocket. Our results suggest that Gwl activation could be mediated by the binding of its hydrophobic pocket to the hydrophobic motif of another AGC kinase. Our molecular modeling and mutagenic analysis also indicate that Gwl displays a conserved tail/linker site whose phosphorylation mediates kinase activation by promoting the interaction of this phosphorylated residue with two lysines at the N terminus. This interaction could stabilize the αC-helix and maintain kinase activity. Finally, the different phosphorylation sites on Gwl are identified, and the role of each one in the regulation of Gwl kinase activity is determined. Our data suggest that only the phosphorylation of the tail/linker site, located outside the putative T loop, appears to be essential for Gwl activation. In summary, our results identify Gwl as a member of the AGC family of kinases that appears to be regulated by unique mechanisms and that differs from the other members of this family.
INTRODUCTION
The Greatwall (Gwl) serine/threonine kinase was first identified in Drosophila, in which mutations of this gene disrupted cell cycle progression (2, 38). Mutant cells were delayed in G2 phase and also failed to correctly perform chromosome condensation. A further study of Xenopus egg extracts demonstrated that Gwl was required to maintain the mitotic state in metaphase II-arrested oocytes (cytostatic factor [CSF] extracts) as well as in cycling extracts (39). This study showed that depletion of Gwl from CSF extracts induced a rapid inactivation of cyclin B-Cdc2 due to the accumulation of inhibitory phosphorylations on the catalytic subunit of this complex, leading to the hypothesis that this kinase was required to maintain the cyclin B-Cdc2 amplification loop (39, 40).
However, recent data from our and another laboratory demonstrated that the main role of Gwl is not the regulation of the autoamplification loop of cyclin B-Cdc2, but rather the inhibition of PP2A (8, 13, 24, 34), the phosphatase responsible for dephosphorylation of cyclin B-Cdc2 substrates (27). Thus, two different kinases, cyclin B-Cdc2 and Gwl, are essential to maintain the mitotic state, the former to phosphorylate mitotic substrates and the latter to prevent a massive dephosphorylation of these substrates. Consequently, an accurate activation and inactivation of Gwl is crucial for promoting the correct timing of mitosis. However, currently, little is known about the mechanisms regulating the activity of this kinase.
By sequence homology Gwl has been classified as a member of the AGC family of kinases (26); however, no data about the mechanisms regulating this kinase have been reported (28). Moreover, Gwl is a peculiar kinase, as it contains a very long insert of about 500 amino acids between kinase subdomains VII and VIII, the typical location of the activation or T loop (38).
The AGC kinase subfamily contains at least 60 members, including PKA, PKG, PKC, RSK, and PDK1 (31, 32). These serine/threonine kinases regulate cellular division, growth, survival, metabolism, motility, and differentiation (17, 23, 30). Similar to all known serine/threonine kinases, active AGC kinases have a bilobal structure, with a smaller amino-terminal lobe (known as the N lobe) and a large carboxy-terminal lobe (known as the C lobe). These two lobes sandwich one molecule of ATP that serves as the phosphate donor during phosphorylation (19). The N lobe contains important catalytic elements common to all serine/threonine kinases, such as the glycine-rich motif or the αC-helix, and some other motifs specific to AGC kinases, such as the hydrophobic pocket (12) and the tail/linker phosphate-binding site (16). Similarly, the C lobe contains motifs highly conserved among all the serine/threonine kinases, including the catalytic loop, the activation loop, the P + 1 loop, and the DFG motif (19), and some specific motifs present exclusively in the AGC kinases, such as the tail/linker site, the turn motif (16), and the hydrophobic motif (12). Activation of AGC kinases involves an initial phosphorylation of the activation loop mediated by upstream kinases (typically PDK1) which anchor the activation loop to the catalytic loop, promoting conformational changes in the αC-helix of the N lobe. These changes coordinate the formation of a network of hydrogen bonds between a glutamic acid in αC-helix, a lysine in β3, and the phosphates of ATP that is required for the catalytic activity of the kinase (22, 37). Finally, to fully activate AGC kinases, the αC-helix must be stabilized by the interaction of the C tail with the N tail. Three different regions of the C tail are important for N-tail interaction: the N-lobe tether (NLT), active site tether (AST), and the C-lobe tether (CLT) (20). The NLT contains the hydrophobic motif [consensus sequence FxxF(S/T)(Y/F)] that, once phosphorylated on the last serine or threonine, wraps around and anchors the two aromatic residues onto the hydrophobic pocket of the N lobe and the phosphate of the serine or threonine onto an arginine residue in the αC-helix, stabilizing the repositioning of this helix (12). Finally, two serines of the AST named the turn motif and the tail/linker are also phosphorylated. One of these serines in the tail/linker site participates in the binding of the hydrophobic motif to the hydrophobic pocket. Hence, once this serine is phosphorylated, its phosphate interacts with a phosphate-binding site in the glycine-rich loop of the N lobe. This binding provides an anchoring point for the tail, which increases the local concentration of the hydrophobic motif in the vicinity of the hydrophobic pocket, stimulating their binding (16).
MATERIALS AND METHODS
Immunization procedures and antibodies.
Anti-Xenopus and anti-human Greatwall (xGwl and hGwl, respectively) antibodies were obtained as previously described (6, 34). Anti-phospho-S875 antibodies of human Greatwall were generated against a peptide (H2N-CAQHLTV[phosphoS]GFSL-COOH) corresponding to the C-terminal sequence of the hGwl protein. Peptides were coupled to thyroglobulin for immunization and to immobilized bovine serum albumin for affinity purification as previously described (25).
Polyclonal anti-phospho-Ser Cdk substrates were obtained from Cell Signaling Technology, Inc. Affinity-purified antibodies against Cdc27 were previously described (9, 24). Mouse monoclonal antibodies against hemagglutinin (HA) (clone 12CA5) were obtained from Roche Diagnostics (Germany).
Preparation of Xenopus egg extracts, mRNA translation, and immunodepletion.
CSF egg extracts were prepared from unfertilized Xenopus eggs that were arrested at the metaphase of the second meiotic division as previously described (14).
Interphase egg extracts were prepared from dejellied unfertilized eggs transferred in MMR/4 (25 mM NaCl, 0.1 mM CaCl2, 0.5 mM KCl, 0.25 MgCl2, 0.025 mM Na EGTA, 1.25 mM HEPES-NaOH, pH 7.7). Extracts were prepared 50 min after ionophore addition by the same procedure as that described for CSF extracts.
mRNAs encoding different wild-type (Wt) and mutant forms of Gwl were transcribed in vitro with SP6 RNA polymerase. For mRNA translation, CSF egg extracts were supplemented with dithiothreitol (DTT; 1 mM), RNAguard (0.4 U/μl extract; Amersham Biosciences), tRNA (0.1 μg/μl), and the corresponding mRNA (0.05 μg/μl extract) and incubated for 2 h at 20°C (35).
Immunoprecipitations/immunodepletions were performed using 10 μl of extracts, 10 μl of magnetic protein G-Dynabeads (Dynal), and 2 μg of each antibody. Antibody-linked beads were washed 2 times with RIPA (NaH2PO4 [10 mM], NaCl [100 mM], EDTA [5 mM], Triton X-100 [1%], deoxycholate [0.5%], β-glycerophosphate [80 μM], NaF [50 mM], DTT [1 mM]), 2 times with Tris, 50 mM, pH 7.5, and incubated for 15 min at room temperature (RT) with 10 μl of Xenopus egg extracts. For immunodepletion, the supernatant was recovered and used for subsequent experiments.
Greatwall kinase assays.
An HA-tagged form of the Wt or the different Gwl mutants were overexpressed in mitotic egg extracts by the addition of its mRNA. Once the mRNA was translated, 10 μl of the overexpressed mitotic extracts were mixed with 10 μl of magnetic protein G-Dynabeads (Dynal) prelinked with 1.5 μg of anti-HA antibodies and incubated for 20 min at room temperature. Beads were washed three times with RIPA plus 300 mM NaCl and three times with Tris, 50 mM, pH 7.5. Twenty percent of this immunoprecipitate was used for Western blot analysis using fluorescent secondary antibodies Dylight 800, conjugated (Thermo Scientific). The levels of each hGwl protein recovered in the immunoprecipitate were measured by using an Odyssey infrared imaging system (Li-Cor Biosciences). The rest of the immunoprecipitate was mixed with 10 μl of MBP mix (HEPES [20 mM], MgCl2 [10 mM], ATP [100 μM], and myelin basic protein [MBP; 1 mg/ml]) plus 2 μCi of [γ-33P]ATP. Twenty minutes later, reactions were stopped by adding Laemmli sample buffer and analyzed by SDS-PAGE. The phosphorylation of the myelin basic protein induced by Gwl was subsequently determined by measuring by densitometry the autoradiography with ImageJ software. Finally, the activity levels were corrected by the amount of protein present at each immunoprecipitate and represented as a percentage of the phosphorylation compared to that of the Wt form.
Site-directed mutagenesis.
Deletions and point or double mutations were obtained by using the QuikChange site-directed mutagenesis kit according to the manufacturer's protocol (Stratagene, Amsterdam, Netherlands).
Identification of phosphorylation sites in the Greatwall protein.
Overexpressed Xenopus Greatwall from interphase and CSF extracts were recovered by immunoprecipitation and submitted to SDS-PAGE. Human glutathione S-transferase (GST)-Gwl was purified from Sf9 cells and either prephosphorylated in vitro with purified Plx1 (Fig. 6D) or directly submitted to SDS-PAGE (Fig. 6C). Bands were excised, cut into two pieces, rinsed, and subjected to in-gel reduction and S-carbamidomethylation. Overnight in-gel digestion was performed by using modified porcine trypsin (Promega, Lyon, France) or endoproteinase Glu-C from Staphylococcus aureus V8 (Sigma-Aldrich, St. Louis, MO) at 20 ng/μl in a 25 mM ammonium bicarbonate solution at 37°C. Peptide extraction from the gel was performed as previously described (36).
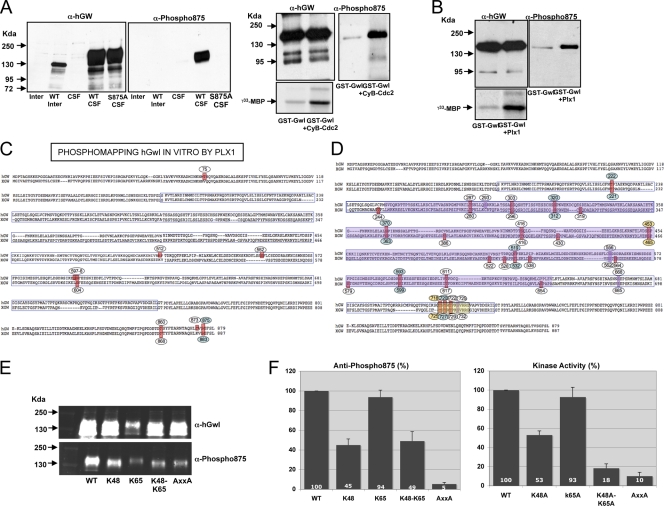
Fig. 6.
The interaction of the tail/linker phosphate with the tail/linker binding sites and the presence of the PxxP motif are essential to maintain Gwl kinase activity. (A) A total of 1.5 μl of nontranslated interphase or mitotic extracts and of mitotic overexpressed Wt HA-hGwl and the S875A mutant were submitted to Western blotting by using anti-hGwl antibodies or anti-phospho serine 875 antibodies. GST-hGwl was incubated or not with 40 units of purified cyclin B-Cdc2 (Ozyme). The mix was divided into two equal fractions. In one fraction, the levels of GST-hGwl protein and the phosphorylation of S875 were analyzed by Western blotting. In the other fraction, GST-hGwl was recovered by immunoprecipitation, and the kinase activity was measured ([γ-33P]MBP). (B) Represented is the kinase activity of a GST-hGwl purified from Sf9 insect cells and a GST-hGwl kinase phosphorylated in vitro by a purified His-Plx1. The levels of GST-hGwl and the phosphorylation of S875 were also analyzed by Western blot. (C) GST-hGwl protein was phosphorylated in vitro by His-Plx1 protein and the phospho-amino acids were identified by mass spectrometry. (D) Represented are the phosphorylation sites found in the central insert in an active xGwl obtained from mitotic egg extracts. Violet square represents sequence from positions 254 to 640 that is not required for Gwl activity. Yellow square indicates deletions performed to eliminate phosphorylation sites T718, Y720, T722, and S725. (E) A total of 1.5 μl of mitotic egg extracts overexpressed with the indicated mutants were analyzed by Western blotting by the use of anti-hGwl antibodies and anti-phospho-S875 antibodies and fluorescent secondary antibodies. (F) The levels of phosphorylation of S875 described in the legend to panel E were measured by using an Odyssey infrared imaging system, corrected by the amount of expression of each ectopic protein obtained by the same system, and represented as a percentage of the phosphorylation compared to that of the Wt form. The kinase activities of these mutants are also represented.
Dried peptide extracts were dissolved in 14 μl of 0.05% trifluoroacetic acid in 2% acetonitrile before nano-liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis. Online nano-LC-MS/MS analyses were performed using an Ultimate 3000 system (Dionex, Amsterdam, Netherlands) coupled to a nanospray LTQ Orbitrap mass spectrometer (Thermo Fisher Scientific, Bremen, Germany).
RESULTS
Gwl conserves the basic structural features of a serine/threonine kinase despite the presence of a long insertion in the kinase domain.
Gwl has an atypical amino acid sequence for an AGC kinase with a large insertion of 550 amino acids between the catalytic subdomains VII and VIII. This sequence could correspond to the activation loop; however, its large length raises the question of whether this insert really corresponds to an activation loop and even whether this kinase behaves as a conventional serine/threonine kinase. In order to analyze these issues we performed a series of mutations of the residues that are highly conserved across eukaryotic serine/threonine kinases and that are involved in basic catalytic functions (21). We first mutated one of the glycines of the glycine-rich G loop of the human Gwl (hGwl) kinase into serine (G44S) (Fig. 1A). This domain covers and anchors the nontransferable ATP α/β-phosphates and leaves the γ-phosphate solvent exposed, positioning the ATP appropriately for γ-phosphate transfer to the substrate (1, 18). In order to analyze the activity of this mutant we performed an in vitro kinase assay using myelin basic protein (MBP) as a substrate. We also investigated the physiological functionality of this mutant by performing rescue experiments with hGwl mutants in CSF extracts (mitotic extracts). We have previously shown that depletion of Xenopus Gwl (xGwl) from mitotic extracts induces mitotic exit and that this phenotype can be rescued by the addition of the wild-type (Wt) human form of this kinase (6). In vitro-transcribed mRNA coding for various HA-hGwl mutants were added to mitotic egg extracts, and after translation, endogenous xGwl was completely immunodepleted (Fig. 1B). The mitotic state was then analyzed by determining the phosphorylation state of the cyclin B-Cdc2 substrate Cdc27 and the general phosphorylation signal of cyclin B-Cdc2 substrates using a phospho-serine Cdc2 substrate-specific antibody. Mutation of glycine 44 to serine in hGwl decreased its activity by 95% (Fig. 1C). Moreover, this mutant was incapable of maintaining the mitotic state in egg extracts, shown by loss of phosphorylation on Cdc27 and cyclin B-Cdc2 substrates (Fig. 1D). These results are in agreement with previous results obtained with xGwl (39) and indicate that the glycine-rich loop is essential for Gwl kinase activity and functionality.
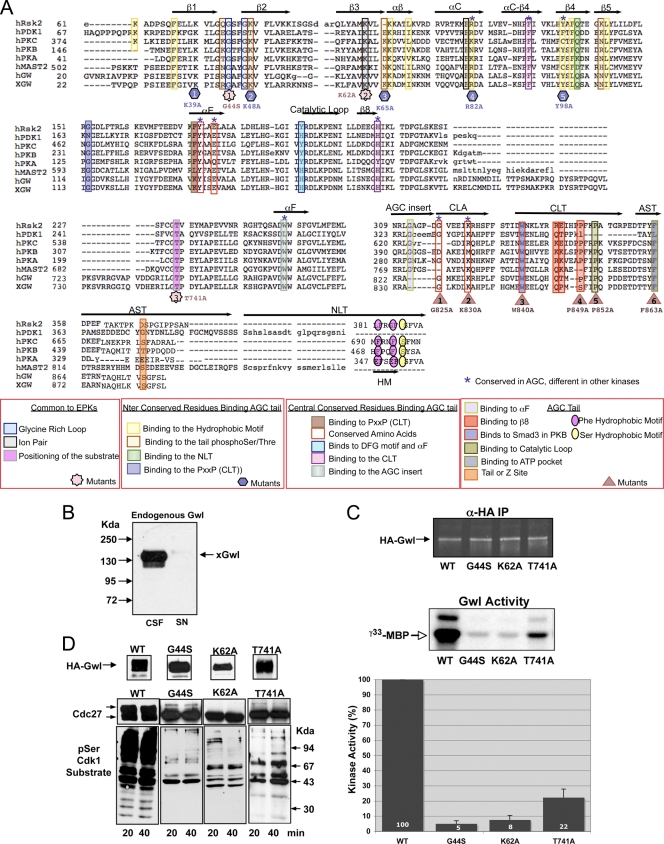
Fig. 1.
Greatwall conserves most of the residues distinguishing AGC from other eukaryotic kinases. (A) Alignment showing representative AGC kinases as well as human and Xenopus Gwl (hGw and xGW, respectively). Key regions of the C terminus of AGC kinases are indicated above the alignment. The patterns of conserved residues observed with AGC kinases and with human and xenopus Greatwall are indicated by colored squares. Colors represent different intramolecular interactions and are indicated at the bottom of the figure. Mutants of Gwl are indicated by shapes. (B) A total of 10 μl of CSF extracts was immunodepleted with 2 μg of anti-xGwl antibodies. A total of 1.5 μl of CSF extracts and of the supernatant (SN) was loaded and treated for Western blot analysis to detect endogenous xGwl. (C) Wt and the G44S, K62A, and T741A HA-tagged forms of hGwl were expressed in mitotic egg extracts and subsequently immunoprecipitated with anti (α)-HA antibodies. A total of 20% of this immunoprecipitate (IP) was used to measure the levels of each protein by Western blotting with fluorescent secondary antibodies (HA-Gwl); the remaining 80% was used to measure kinase activity as described in Material and Methods ([γ-33P]MBP). The levels of phosphorylation of MBP in the autoradiography were quantified by densitometry using Image J and corrected by the amount of each ectopic protein present in the immunoprecipitate. The activities are represented as the percentage of kinase activity measured in the Wt hGwl form. Experiments were performed in triplicate. Values are expressed as the mean ± standard deviation. (D) Mitotic extracts overexpressing the HA-Wt and G44S, K62A, and T741A mutants of hGwl were depleted of endogenous xGwl. Supernatants were used to analyze the level of overexpression with anti-HA antibodies by Western blotting. A total of 20 and 40 min after depletion, two aliquots of this supernatant were also analyzed for their capacity to maintain mitosis by measuring the phosphorylation of Cdc27 and of the different cyclin B-Cdc2 substrates by Western blot analysis.
We next performed a second mutagenesis of hGwl in which lysine 62 was mutated to alanine. This mutation induced similar effects on the G44S mutant. The K62A mutant had only 8% of the total Wt activity and was incapable of rescuing mitotic exit in Xenopus egg extracts after endogenous xGwl depletion (Fig. 1C and D).
Finally, mutation of the threonine to an alanine residue within the GTP domain of the P + 1 pocket (T741A mutant, star no. 3, Fig. 1A), an important substrate interaction site (21), decreased kinase activity by 78% compared to the Wt, yet, despite the fact that it was still partially active, it was incapable of rescuing the mitotic exit induced by xGwl depletion (Fig. 1C and D).
These results indicate that hGwl conserves the basic structural features shared by most eukaryotic serine/threonine protein kinases.
The CLA, CLT, and AST regions of the C tail specific to AGC kinases are functional in Gwl.
The conserved residues and structural features that define the AGC family of kinases (CLT, AST, or NLT) are primarily localized in the C tail (20) (Fig. 1A). However, in addition to the C tail, these kinases also contain a conserved sequence at subdomain XI of the catalytic core. This domain is named the C-lobe anchor (CLA) (Fig. 1A) (20). The Gwl amino acid sequence has significant homology with other AGC kinases in its N and C terminus. Moreover when analyzed in detail, most of the residues of the CLA and the C tail that are specific to the AGC kinases are conserved.
In particular, the following residues are conserved between Gwl of different species and other AGC kinases: G287PKA/G825Gwl and K293PKA/K830Gwl in the CLA region, W303PKA/W840Gwl and PxxP motif in the CLT region, and F328PKA/F863Gwl in the AST region. We analyzed whether these conserved residues were required for Gwl activity by mutating to alanine residues G825, K830, W840, and F863. The mutations G825A and K830A did not change either kinase activity or the capacity to maintain mitosis (Fig. 2A and B), indicating that they do not participate in the activity and/or activation of Gwl. The W840A mutant triggered a partial decrease of kinase activity (57%), although this mutant was still capable of maintaining mitosis, indicating that this amino acid participates in but is not essential for kinase activity. Finally, a mutation of F863 in the AST segment induced a 71% reduction in kinase activity and a complete loss of the mitotic state, suggesting that this phenylalanine is essential for Gwl activity and functionality. These results are in agreement with the modeling analysis in which the conserved phenylalanine of the AST segment plays an essential role in ATP pocket binding, whereas the mostly conserved glycine and lysine of the CLA motif and tryptophan of the CLT segment do not form clear intramolecular interactions (20).
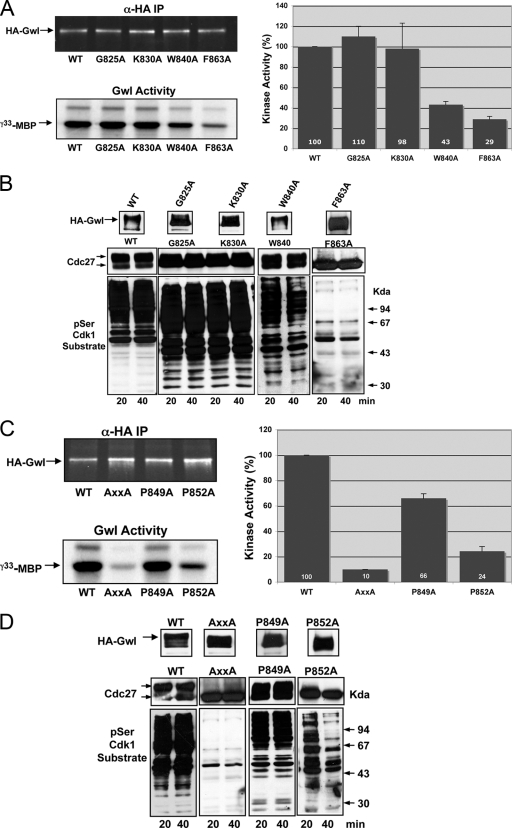
Fig. 2.
Gwl belongs to the AGC family of kinases. (A) HA-Wt and G825A, K830A, W840A and F863A mutants were used to measure kinase activity as described in the legend to Fig. 1B. (B) Similar to Fig. 1C except for the overexpression of the G825A, K830A, W840A, and F863A mutants. (C) Kinase activity of the HA-Wt, the P849A P852A double mutant (AxxA), and the P849A and P852A simple mutants of hGwl was measured as described in the legend to Fig. 1B. (D) The levels of expression and the phosphorylation of Cdc27 and cyclin B-Cdc2 substrates in these mutants were also analyzed as described in the legend to Fig. 1C.
It has already been shown that the PxxP motif of the CLT segment plays an important role in the activity of PKC (20). Thus, we studied whether this motif is also functional in the Gwl kinase by mutating each proline to alanine. This substitution produced a significant decrease in the kinase activity (90%) and the loss of the mitotic state (Fig. 2C and D). In order to analyze the participation of each proline of the PxxP, we mutated each one to alanine separately. The results of this experiment indicate that although P849A mutation induces a partial (34%) decrease of kinase activity it is not essential to maintain the mitotic state, whereas P852A induced a significant reduction in the activity of Gwl (76%), and although at 20 min after endogenous Gwl depletion cyclin B-Cdc2 substrates displayed a partial phosphorylation, this mutant was incapable of maintaining the mitotic state at later time points, indicating that this residue is critical for the functionality of this motif in Gwl kinase (Fig. 2C and D).
These results demonstrate that Gwl contains the conserved residues of the CLA, CLT, and AST regions of the C tail that define AGC kinases and that they are functional.
Gwl possess a functional hydrophobic pocket, but it is devoid of a conventional hydrophobic motif.
In addition to the C tail, most members of the AGC family have conserved amino acids in the N terminus that are required to stabilize the active kinase. These include K93PKA (R59Rsk2, R131PDK1, and R82Gwl) and F109PKA (Y75Rsk2, Y115PKB, and Y98Gwl). These two amino acids form part of the hydrophobic pocket (Fig. 1A). The first one binds to the phosphorylated residue of the hydrophobic motif, whereas the second one binds to the last phenylalanine of this motif, promoting the stabilization of the active kinase.
We mutated these two amino acids of Gwl to alanine. These mutations promoted a significant decrease in the kinase activity (87% decrease with R82A and 93% decrease with Y98A) and prevented the two mutants from maintaining mitosis in Gwl-depleted mitotic extracts, indicating that Gwl has a functional hydrophobic pocket that is essential for its kinase activity and functionality (Fig. 3A and B). However, despite the presence of a functional hydrophobic pocket, the amino acid sequence homology of Gwl with other AGC kinases indicates that this kinase does not have an NLT segment and consequently is devoid of a conventional hydrophobic motif (Fig. 1A).
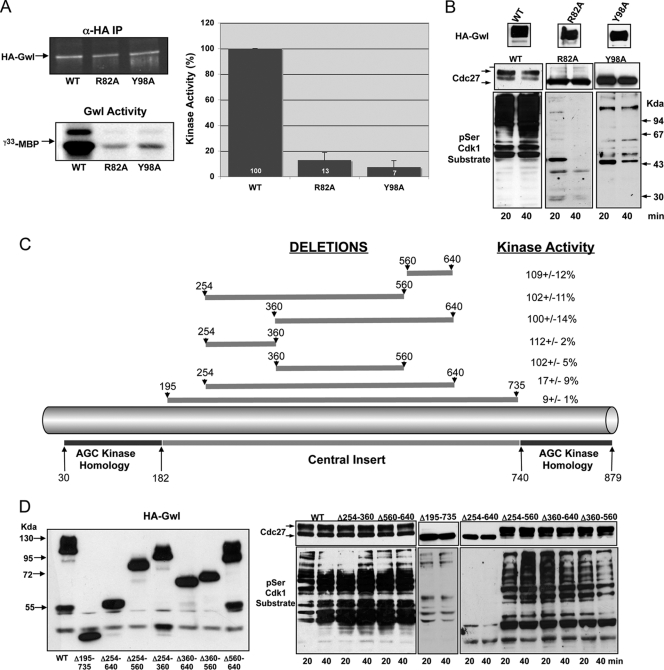
Fig. 3.
Gwl contains a functional hydrophobic pocket and a long insert between catalytic domains VII and VIII. (A) The activity of the R82A and Y98A mutants of the hydrophobic pocket were analyzed and compared to the Wt form. (B) The functionality of the different mutants was studied by measuring the phosphorylation of Cdc27 and the different cyclin B-Cdc2 substrates in xGwl-depleted CSF extracts. (C) Depiction of the hGwl sequence in which the position of the central insert is shown. Different deletions and percentage of kinase activity of each mutant are indicated. (D) The expression and the capacity of these mutants to maintain mitosis are shown.
Gwl does not require a specific sequence of the central insert to be active.
For most AGC kinases, activation involves at least two highly conserved regulatory motifs: the activation loop, located in the catalytic domain, and the hydrophobic motif in the NLT segment of the C tail. Although Gwl belongs by sequence homology to the AGC kinases, it appears to be a unique member of this family, as these two major motifs are not conserved. First, as reported above, Gwl is devoid of a conventional hydrophobic motif. Second, Gwl contains a long amino acid sequence inserted between the catalytic subdomains VII and VIII that could act as an activation loop (or T loop). However, although this insert is correctly located to be a T loop, the length of the Gwl insert is much larger (557 amino acids) than the standard T-loop (20 to 30 amino acids) of other kinases (19).
To investigate the role of this insert in Gwl kinase activity we first analyzed its sequence similarity with orthologs and close paralogs. No significant sequence similarities are detected using PSI-BLAST or HHsearch (33). Moreover, predictions performed using JPRED3 (10) indicates that little or no stable secondary structures are formed within the insert. According to SAPS analysis (5), the insert is enriched in serine (13.7%). A similar trend is observed with the human ortholog, with both serine (14.3%) and asparagine (7.0%) being more frequent than they are in usual human proteins. As a whole, this sequence analysis suggests that the long insert in hGwl is rather unique and is likely natively unfolded.
We next proceeded by performing a series of deletions covering most of the amino acids in this zone and analyzed the effect of each deletion on the activity and the capacity of each mutant to maintain mitosis. We first deleted a sequence from position 195 to 735 (a deletion of 540 amino acids) (Fig. 3C). This deletion induced a 91% decrease of the kinase activity and a loss in the capacity to maintain mitosis (Fig. 3D). Subsequently, we performed a shorter deletion between residues 254 and 640 (386 amino acids). Similar to the previous mutation, this deletion significantly decreased kinase activity and the capacity to maintain the mitotic state. To analyze if there was a specific sequence required for kinase activity between positions 254 and 640, we performed shorter deletions. The first one, from residue 360 to residue 560 (200 amino acids), maintained 100% of kinase activity and completely rescued the phenotype induced by Gwl depletion in mitotic extracts. The second one, from position 360 to 640 (280 amino acids), was also functional. The third one, between residues 254 to 560 (306 amino acids), displayed 100% of activity and was able to maintain mitosis. Finally, we analyzed kinase activity and functionality of the mutants in which sequences from 254 to 360 and from 560 to 640 were deleted. Neither of these sequences appears to be required. Together these data suggest that, if this insert in the Gwl kinase domain corresponds to an activation loop, this activation loop appears not to require a specific sequence between positions 254 and 640 to be active.
Molecular modeling suggests a binding of the tail/linker phosphate of the AST segment with the tail/linker-binding site of the N terminus.
Apart from the phosphorylation of the hydrophobic motif and the activation loop, several AGC kinases also contain another phosphorylation site that promotes their integrity, termed the tail/linker phosphorylation site. In PKBβ the phosphorylation of this residue promotes the interaction with the tail/linker phosphate-binding site at the N terminus and facilitates the interaction of the hydrophobic motif with the hydrophobic pocket, maintaining kinase activity (16).
The N-terminal amino acids described as the tail/linker phosphate-binding sites in PKBβ (16) are conserved in Gwl. These include residues R43, K48, K65, and N105 of hGwl. Moreover, this kinase also contains a conserved tail/linker site (E34PKA, S in other AGC kinases, S875Gwl). Since Gwl does not have a conventional hydrophobic motif, we hypothesized that the tail/linker phosphate binding to the N-terminal tail/linker phosphate binding sites could participate in the stabilization of the active Gwl kinase.
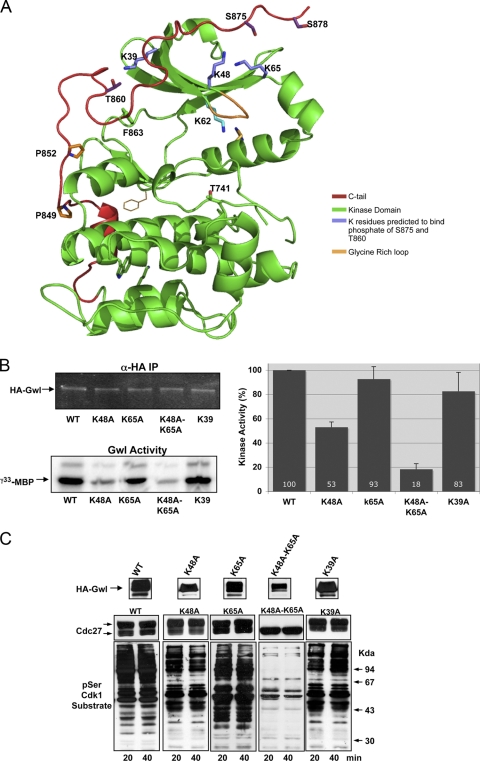
To analyze this hypothesis we first performed molecular modeling of the structure of the kinase domain of Gwl excluding the long central insertion, based on that of the PKA structure (Fig. 4A). Molecular modeling supports the possibility of a binding of phospho-S875 with K48 and K65 at the N terminus, two lysines that have already been shown to interact with the tail/linker phosphate in PKBβ (16). Moreover, a second putative interaction could be present between phospho-T860 and K39.
Fig. 4.
The binding of the tail/linker phosphate to the tail/linker binding sites at the N terminus is essential for Gwl activity. (A) Model of active Gwl is shown as a ribbon representation with the side chains of selected residues. The kinase domain is shown in green, the C tail is shown in red, the K residues predicted to bind phosphates of S875 and T860 are shown in violet, and the glycine-rich domain is shown in orange. (B) Kinase activity of the K48A, K65A, K48A K65A, and K39A mutants. (C) The levels of expression and the capacity to maintain mitosis of the Wt and each mutant are measured.
We first mutated either K48 or K65 or both residues to alanine (Fig. 4B and C). The kinase activity of the K48A mutant decreased by 47%, whereas a decrease of 7% was observed in the K65A mutant. However, the K48A K65A double mutant lost 82% of kinase activity and was incapable of maintaining mitosis. Finally, we also mutated lysine 39 to alanine, which maintained 83% of kinase activity and was capable of maintaining mitosis (Fig. 4B and C). These results suggest that K48 and K65, but not K39, are essential for the stabilization of the active kinase.
However, the binding of K48 and K65 to S875 is dependent on the phosphorylation of S875, and consequently, to finally demonstrate that these two residues maintain Gwl activity through its association to S875, we must first show that the active Gwl kinase is phosphorylated on S875.
Mass spectrometry identification of multiple sites phosphorylated on Gwl.
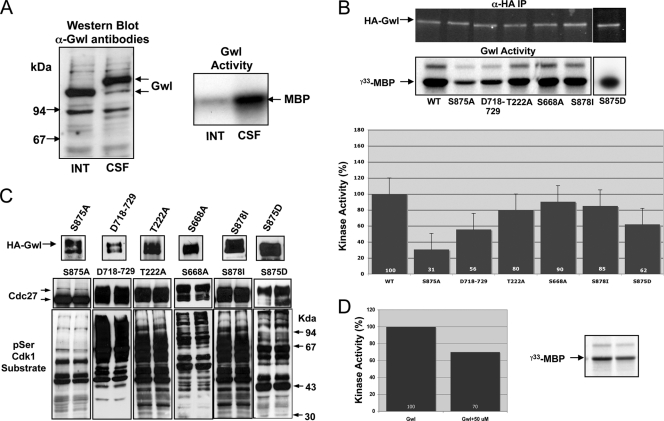
Gwl is phosphorylated during mitosis, and this phosphorylation correlates with the activation of the kinase (Fig. 5A). To determine the roles that these phosphorylations have on the activation of Gwl, we first examined the different residues that were phosphorylated in vivo when Gwl was overexpressed by the addition of mRNA encoding xGwl in interphase and in mitotic egg extracts. After translation, xGwl was recovered by immunoprecipitation and used for mass spectrometry analysis to identify the different phosphorylation sites. Two different phosphorylated sites were clearly identified in xGwl obtained from interphase egg extracts, S465 (S453 in hGwl) and T725 (T718 in hGwl). Twenty-five different phosphorylation sites, including the two sites obtained in inactive Gwl, were identified in xGwl from mitotic extracts. We detected, among the different phosphorylation sites, S883 (S875 in hGwl), corresponding to the tail/linker phosphorylation site. The facts that this residue is phosphorylated in active Gwl kinase and that the K48A K65A double mutant induced a loss of kinase activity suggest that in this kinase the phosphate of phospho-S875 could be involved in active kinase stabilization by interacting with K48 and K65 (Fig. 4A). In fact, this seems to be the case, since the mutation of S875 to a nonphosphorylatable alanine dramatically decreased kinase activity (69% decrease) and promoted the complete loss of its functionality (Fig. 5B and C), whereas the phosphomimetic mutant S875D, although it presented a partial decrease of kinase activity (38%), completely rescued the mitotic exit phenotype. Moreover, the addition of a peptide containing the N-terminal binding site of S875 (residues from G44 to M66 of hGwl) that would compete for the intramolecular binding of K48 and K65 to S875 decreased Gwl kinase activity by 30% (Fig. 5D), suggesting that in hGwl the interaction of phospho-S875 with K48 and K65 participates in the stabilization of the active kinase. Consequently, the phosphorylation of this residue likely plays an essential role in Gwl activation and in mitotic entry.
Fig. 5.
Phosphorylation of S875 is essential to maintain Gwl activity. (A) The electrophoretic mobility of xGwl was analyzed in interphase (INT) and mitotic (CSF) egg extracts by Western blotting. xGwl was immunoprecipitated from interphase and mitotic egg extracts, and the kinase activities of the immunoprecipitates were analyzed. (B) Activity of the indicated phosphorylation mutants. (C) The levels of overexpression of the different HA-hGwl forms and their capacity to maintain mitosis were analyzed. (D) Inactive GST-hGwl was incubated for 15 min with a synthetic peptide containing 50 μM of an N-terminal sequence of hGwl (residues G44 to M66). Subsequently, 40 units of purified cyclin B-Cdc2 (Ozyme) was added to the mix to activate hGwl. A total of 15 min later, active hGwl was recovered by immunoprecipitation with anti-GST antibodies, and the immunoprecipitate was used to measure hGwl activity. For Gwl assays, Gwl was incubated with cyclin B-Cdc2 with or without the N-terminal binding peptide (50 μM), with MBP used as the substrate. Kinase activity of hGwl is represented as the percentage of the activity of the nontreated kinase.
The identity of the kinase(s) responsible for Gwl phosphorylation is not known; however, previous results indicate that Gwl can be phosphorylated in vitro by cyclin B/Cdc2 and by Plx1. In this regard, it has been reported that cyclin B-Cdc2-dependent phosphorylation of Gwl promotes kinase activation, whereas phosphorylation of Gwl by Plx1 has no effect on kinase activity (39).
Six different sites phosphorylated in vitro by cyclin B-Cdc2 have already been described in xGwl (xT244, xS654, xS677/hS660, xT221/hT222, xS363/hS370, and xS465/hS453) (39). However, surprisingly, despite the fact that cyclin B-Cdc2 seems to promote Gwl activation, phosphorylation of the tail/linker residue xS883/hS875, which is critical for Gwl activity (Fig. 5B and C), was not detected as a phospho-site. To determine whether cyclin B-Cdc2 can phosphorylate S875 on Gwl, we used an antibody that specifically recognizes this phospho-site. This antibody does not recognize xGwl either in interphase or in mitotic extracts (Fig. 6A). However, it recognizes a band in Xenopus mitotic extracts in which a Wt form of hGwl has been overexpressed. Finally, it does not recognize any band when either the Wt or the S875A mutant of hGwl is translated in interphase or mitotic extracts, respectively. The addition of purified cyclin B-Cdc2 to inactive GST-hGwl induced the activation of this kinase (Fig. 6A, [γ-33P]MBP). Moreover, when the antibody against phospho-S875 was used to determine the phosphorylation of this residue, we observed a clear band at the molecular weight of GST-hGwl that was almost completely absent in nonphosphorylated GST-hGwl, indicating that despite the fact that it was not previously detected as a phosphorylated site, cyclin B-Cdc2 can phosphorylate S875 of Gwl in vitro (Fig. 6A).
We next tested whether in vitro phosphorylation of hGwl by Plx1 can also phosphorylate S875 residue. To do that, an inactive recombinant GST-hGwl protein was phosphorylated in vitro with a purified His-Plx1 protein. As shown above, GST-hGwl did not have kinase activity (Fig. 6B); however, contrary to what has previously been shown (39), the in vitro phosphorylation of this protein by Plx1 promoted a significant increase in the kinase activity as well as a clear phosphorylation of S875 (Fig. 6B). Thus, Plx1, as cyclin B-Cdc2, can phosphorylate S875 of hGwl and activate this kinase.
To further investigate the role of Plx1 phosphorylation in Gwl activation, we proceeded by performing phospho-mapping in both nontreated and in vitro Plx1-phosphorylated GST-hGwl. Sixteen different phosphorylation sites were observed with nontreated GST-hGwl protein. One was present in inactive Gwl from interphase extracts, and nine were present in xGwl obtained from mitotic extracts. Since this purified protein is inactive, these results suggest that the phosphorylation of these sites does not result in kinase activation. Twenty-four different residues were detected in GST-hGwl after Plx1 phosphorylation (Fig. 6C). Sixteen of them corresponded to those detected in the nontreated GST-hGwl kinase. Five of them were not conserved in xGwl. From the remaining three residues specific to Plx1 phosphorylation only one of them, S875, was also detected in active Gwl obtained from mitotic extracts.
We could hypothesize that S875 is the sole phosphorylation that is required for hGwl activation; however, we cannot exclude the possibility that the other detected phospho-sites, although they do not confer kinase activity, could participate in Gwl activation. To test this hypothesis, we first studied the role of phospho-residues other than S875 by mutagenesis. Most of phospho-residues detected in Gwl obtained from mitotic extracts were localized in the central insert (sequence from positions 182 to 740). Our results shown above suggest that no specific sequences within residues 254 to 640 of this insert are critical for kinase activity (Fig. 3C and D). Thus, only phospho-amino acids T222, S668 (found in the database at www.phosphosite.org but absent in our phosphomapping analysis), T718, Y720, T722, and S725 of hGwl are likely to be responsible for kinase activation (Fig. 6D). We first performed a deletion of the sequence from T718 to G729, and we analyzed the activity and the functionality of this deleted kinase. This deletion includes four of the six residues phosphorylated outside the sequence 254 to 640. The data shown in Fig. 5B and C indicate that this deletion induced a moderate decrease of kinase activity (44%) but did not affect its functionality, suggesting that these phosphorylations are not essential for Gwl activation. We next performed a point mutation to alanine of the residue T222. This mutation induced a decrease of only 20% of kinase activity but maintained the mitotic state, indicating that it is also not essential for the activation of this kinase. Finally we performed a mutation to alanine of S668, a residue that we did not detect in our phosphorylation analysis but that is annotated as a phosphosite in databases (www.phosphosite.org) (11, 29). This mutant showed normal kinase activity and completely rescued the Gwl phenotype in mitotic extracts. Thus, Gwl phosphorylation in the central insert does not seem to regulate kinase activation.
Finally, we analyzed whether the phosphorylation of residue S878 absent from our analysis but reported in databases could be required for kinase activity. This residue, located outside the central insert, appears to be dispensable for kinase activation since its mutation did not affect kinase activity or functionality (Fig. 5B and C).
All these data suggest that S875 is the sole residue whose phosphorylation is essential for kinase activation.
Disruption of the interaction of the tail/linker phosphate with the tail/linker phosphate-binding site as well as mutation of the PxxP motif prevent normal phosphorylation of hS875.
Previous data in PKBα and in PRK2 have shown that the interaction of the phosphate of the tail/linker site to the tail/linker binding sites protects this amino acid from being dephosphorylated (16).
To test whether, as for PKBα and in PRK2, the binding of the tail/linker binding site to the tail/linker of Gwl protects the phosphorylation of this residue, we used the anti-phospho-human S875 antibody (Fig. 6E and F). Mutation of K48 and the K48 K65 double mutant to alanine induced a significant reduction in the phosphorylation of S875 (55% and 51% decrease, respectively), a decrease that was concomitant with a drop in kinase activity (47%, and 82% decrease, respectively) (Fig. 6F). These results indicate that the interaction of K48 and K65 with S875 prevents S875 dephosphorylation.
Besides the N-terminal–C-tail-interaction effect on C-tail phosphorylation, a role for the PxxP motif in the activation of PKC by promoting phosphorylation of the turn motif has also been described (15). We therefore tested whether PxxP could participate in the phosphorylation of S875 and in the activation of Gwl. To do that, we analyzed the effect of the double mutation of the PxxP motif to alanine. This mutation induced a dramatic decrease of the phosphorylation of S875 (95% decrease) that was concomitant with a significant drop in kinase activity (90% decrease). These results suggest that, as for PKC, the PxxP motif of Gwl regulates phosphorylation of the tail/linker motif in this kinase, participating in its activation. Moreover, this decrease in kinase activity correlates with dephosphorylation of S875, confirming the hypothesis that this phosphorylation site is essential for the maintenance of the active kinase.
Activation of Gwl requires a two-step mechanism: first, a phosphorylation of S875 and subsequently, a binding of the hydrophobic motif of another AGC kinase to the hydrophobic pocket of Gwl.
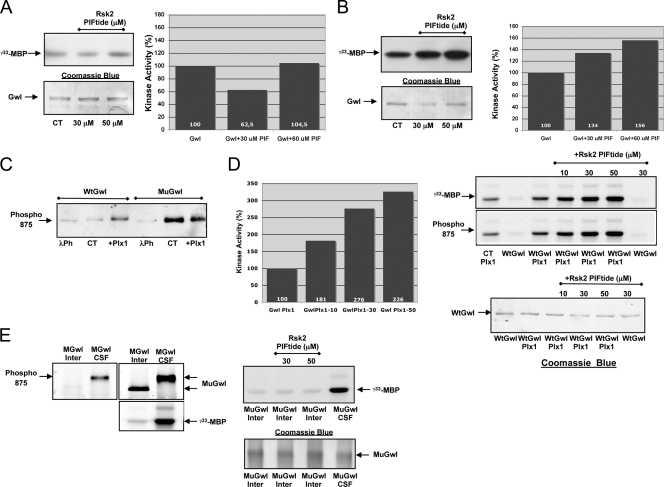
As described above, Gwl is devoid of a conventional hydrophobic motif but presents a functional hydrophobic pocket. The AGC kinase PDK1 contains, similarly to Gwl, a functional hydrophobic pocket but is devoid of a hydrophobic motif. The hydrophobic pocket of PDK1 binds to the hydrophobic motif of other AGC kinases, a docking interaction that activates PDK1 and induces the phosphorylation of the T loop of the docked substrate by this kinase (3, 4). Since Gwl has a functional hydrophobic pocket, it is possible that, as for PDK1, it could be activated by binding to the hydrophobic motif of another AGC kinase. We tested this hypothesis by incubating a wild-type GST-hGwl protein purified from Sf9 insect cells with a synthetic peptide containing the phosphorylated hydrophobic motif of Rsk2 (PIFtide). Purified wild-type GST-hGwl did not present kinase activity, and the addition of the PIFtide did not promote kinase activation (Fig. 7A). However, we tested the effect of the PIFtide on the activation of a mutant form of Gwl that possesses a partial activity. As depicted in Fig. 7B, the incubation of the hydrophobic motif of Rsk2 increased kinase activity to 156%. As expected, only the Gwl mutant displaying a partial activity, but not the inactive wild-type form, presented phosphorylation of residue S875. Moreover, only the wild type but not the Gwl mutant displayed further phosphorylation of S875 when submitted to an in vitro phosphorylation with purified Plx1, suggesting that all the pool of this mutant is phosphorylated in the S875 residue (Fig. 7C). These results suggest that the activation of Gwl by the binding of the hydrophobic motif of another AGC kinase to the hydrophobic pocket of Gwl will take place only once the S875 is phosphorylated. If this is the case, the incubation of PIFtide with a wild-type Gwl kinase in which residue S875 is prephosphorylated in vitro by purified Plx1 should induce a significant increase of the kinase activity. Conversely, if the mutant Gwl form is dephosphorylated by its incubation in interphase egg extracts, the addition of the PIFtide should not promote kinase activation. As hypothesized, the phosphorylation of wild-type Gwl with Plx1 promotes a partial activation of this kinase, and this activity increases 3-fold after incubation with the highest dose of PIFtide (Fig. 7D). The addition of the GST-Gwl mutant to interphase egg extracts promotes S875 dephosphorylation and kinase inactivation, and the further addition of the different doses of the PIFtide did not promote the activation of this dephosphorylated kinase (Fig. 7E). Together these results indicate that the complete activation of Gwl is mediated by the first phosphorylation of residue S875 followed by the binding of the hydrophobic pocket of Gwl to the phosphorylated hydrophobic motif of another AGC kinase.
Fig. 7.
Gwl activation first requires phosphorylation of S875 and a subsequent binding of the hydrophobic motif of another AGC kinase with the hydrophobic pocket of Gwl. (A) Wild-type GST-hGwl was obtained from Sf9 insect cells and incubated or not with 30 and 50 μM of a synthetic peptide containing the phosphorylated hydrophobic motif of Rsk2 (PIFtide, sequence PGIPPSANLFRGFpSFVA). After a 15-min incubation, kinase activity was directly measured by adding MBP mix and [γ-33P]ATP and represented as the percentage of the nonincubated Wt Gwl. The levels of GST-hGwl in each point are shown by Coomassie blue staining. (B) Similar to experiment depicted in panel A, except that a mutant form of hGwl with a partial activity (K72M) instead of a wild-type Gwl protein was used. (C) Purified Wt or mutant hGwl (MuGwl) was either treated with λ-phosphatase (λPh), nontreated (CT) or prephosphorylated in vitro with purified His-Plx1 (+Plx1) and subsequently submitted to SDS-PAGE and Western blotting with anti-phospho 875 antibodies. (D) Wild-type GST-hGwl purified from Sf9 cells was phosphorylated in vitro with His-Plx1 (WtGwl+Plx1), incubated (+Rsk2 PIFtide) or not with a dose of 10, 30, and 50 μM PIFtide, and used to measure the kinase activity as well as the phosphorylation of S875. Under one condition, WtGwl was directly incubated with 30 μM of PIFtide, and the kinase activity was measured. Control (CT Plx1) corresponds to an in vitro phosphorylation assay in which GST-hGwl was not added and corresponds to the [γ-33P]MBP background promoted by Plx1. The amount of GST-hGwl at each point is shown by Coomassie blue staining. The levels of phosphorylation of MBP in the autoradiography were quantified by densitometry using Image J and corrected by the amount of GST-hGwl at each point. The activities are represented as the percentage of kinase activity measured in Wt hGwl form and the [γ-33P]MBP background levels promoted by Plx1 were deducted. (E) The mutant GST-hGwl form was incubated into interphase (Inter) or mitotic egg extracts (CSF) for 15 min and subsequently recovered by immunoprecipitation. Immunoprecipitates were then used to measure kinase activity or to analyze hGwl levels and phosphorylation of S875 by Western blotting with anti-hGwl and anti-phospho 875 antibodies. Two of these immunoprecipitates were supplemented with 30 or 50 μM PIFtide, and the kinase activity was measured. In one condition, the GST-hGwl mutant was incubated in CSF extracts, immunoprecipitated, and used to measure kinase activity (MuGwl CSF).
DISCUSSION
Greatwall is a novel serine/threonine kinase essential for promoting a correct timing of mitosis (6, 8, 34). The activation of this kinase is required at mitotic entry to promote the inhibition of PP2A, the phosphatase responsible for cyclin B-Cdc2 substrate dephosphorylation, whereas the inactivation of Gwl is crucial to allow a subsequent reactivation of this phosphatase and the dephosphorylation of mitotic substrates promoting mitotic exit (27, 34). Consequently, the correct timing of Gwl activation and inactivation is fundamental for promoting correct cell division.
Little is known about the mechanisms promoting Gwl activation: only that Gwl is phosphorylated during mitosis and that this phosphorylation correlates with the activation of this kinase.
In this study we analyzed the mechanisms responsible for Gwl activation. We show that although this is a unique kinase containing a very long insertion between catalytic domains VII and VIII, it behaves as a classical serine/threonine kinase, with functional conserved basic structural residues.
We also demonstrate that most of the AGC conserved residues of the CLA, CLT, and AST domains are functional in Gwl. However, for most AGC kinases, two different phosphorylations are required for their activation, phosphorylation of the activation loop and phosphorylation of the hydrophobic motif (12, 22, 37), and neither of these two phosphorylations appears to be required for Gwl activation.
In this regard, Gwl contains a very long insert (557 amino acids) between catalytic domains VII and VIII, the classical location of the T loop in other AGC kinases. This insert could correspond to a T loop; however, two different findings call this fact into question. First, its length does not correspond to the conventional T loop of 20 to 60 amino acids. Second, the phosphorylation of a particular residue of this insert does not appear to be essential for Gwl activity. Accordingly, the point mutation or deletion of all the phospho-residues in this insert did not perturb kinase functionality. Thus, it is unlikely that Gwl could be regulated by a phosphorylation of the T loop.
Apart from the phosphorylation of the T-loop and the hydrophobic motif, most AGC kinases are phosphorylated on the tail/linker site in the AST region. This phosphorylation has been proposed in PKBβ to promote the binding of the AST site with the tail/linker binding sites in the N-terminal part of the sequence, increasing the local concentration of the hydrophobic motif in the vicinity of the hydrophobic pocket and stimulating their binding, a binding that is essential for maintaining kinase activation (16). Gwl has a conserved tail/linker site (S875) and conserved tail/linker binding sites at the N terminus (K48 and K65). Moreover, our molecular modeling suggests an interaction of the phosphate of S875 with K48 and K65. Our phosphomapping results indicate that S875 is phosphorylated, and our mutagenic results clearly show that these interactions take place and are essential for kinase activity. It is likely that these interactions could stabilize the αC-helix to promote correct kinase activation. In addition, the presented data demonstrate that the interaction of the tail/linker site with the tail/linker binding site protects phosphorylation of the tail/linker amino acid, since when this interaction is disrupted by mutagenesis, S875 phosphorylation decreases. Moreover, dephosphorylation of S875 correlates with the decrease of kinase activity, a fact that confirms the hypothesis that phosphorylation of S875 is essential for the stabilization of the active kinase.
Another particular feature of Gwl is that, unlike most AGC kinases, it does not display a conventional hydrophobic motif, although, as it has been demonstrated for PDK1, it has a functional hydrophobic pocket. PDK1 displays the intrinsic ability to phosphorylate its own T-loop residue and thus possesses a basal kinase activity (7). It has also been shown that the binding of the hydrophobic pocket of PDK1 to the phosphorylated hydrophobic motif of their substrates promotes conformational changes in the PDK1 catalytic core. These changes promote a severalfold increase of activity of this kinase and the phosphorylation of their substrates in their T loop (3). Our results show that, similarly to PDK1, a synthetic peptide encompassing the carboxy-terminal hydrophobic motif of Rsk2 stimulates Gwl activity, suggesting that the binding of the hydrophobic pocket of Gwl to the phosphorylated hydrophobic motif of other AGC kinases participates in the activation of this kinase, likely by promoting conformational changes in its catalytic core. However, this activation takes place only if the tail/linker residue of Gwl (S875) has previously been phosphorylated.
Thus, although Gwl belongs to the AGC kinases, it appears to be regulated differently from the rest of the members of this family. Our results give key insights into the mechanisms that regulate Gwl activation. We propose that two different steps are required to promote this activation. The first step engages the phosphorylation of the tail/linker residue (residue S875). This phosphorylation promotes the binding of the phosphate of this residue with the tail/linker-binding site at the N terminus of Gwl, stabilizing the αC-helix in a partially active form. The second step involves the association of the hydrophobic pocket of Gwl to the phosphorylated hydrophobic motif of another AGC kinase, resulting in the complete activation of Gwl. In this model, the kinase responsible for the phosphorylation of the tail/linker site would be critical for promoting the correct timing of mitosis. We do not know which kinase plays this role in vivo; however, we know that the tail/linker site can be phosphorylated in vitro by both Plx1 and by cyclin B-Cdc2. It is possible that cyclin B-Cdc2 phosphorylates Gwl at mitotic entry. This phosphorylation could then participate in a positive feedback in which the accumulation of the cyclin B-Cdc2 complex could results in a partial activation of Gwl that through the inhibition of PP2A could participate in Cdc25 activation and Myt1/Wee1 inhibition, triggering the cyclin B-Cdc2 amplification loop. However, we cannot exclude the possibility that Plx1, already active in prior G2 cyclin B-Cdc2, could also be the primary kinase that activates Gwl by S875 phosphorylation. Once Gwl is activated, cyclin B-Cdc2 could maintain the tail/linker phosphorylation and thereby ensure and maintain the activation of Gwl until cyclin B degradation at mitotic exit.
ACKNOWLEDGMENTS
We thank C. Jessus for helpful discussion. We thank Ariane Abrieu for the generous gift of recombinant His-Plx1 protein and Yvan Boublik for the recombinant GST-hGwl.
This work was supported by the Association pour la Recherche sur le Cancer (contract number JR/AD/MDV-A09/2/5004) and by Université Montpellier 2. A.B. is a Fondation pour la Recherche Medicale fellow. B.M. is supported by ANR PFTV and Cancéropole Grand Sud Ouest.
Footnotes
Published ahead of print on 28 March 2011.
REFERENCES
- 1. Aimes R. T., Hemmer W., Taylor S. S. 2000. Serine-53 at the tip of the glycine-rich loop of cAMP-dependent protein kinase: role in catalysis, P-site specificity, and interaction with inhibitors. Biochemistry 39:8325–8332 [DOI] [PubMed] [Google Scholar]
- 2. Archambault V., Zhao X., White-Cooper H., Carpenter A. T., Glover D. M. 2007. Mutations in Drosophila Greatwall/Scant reveal its roles in mitosis and meiosis and interdependence with Polo kinase. PLoS Genet. 3:e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biondi R. M., et al. 2000. Identification of a pocket in the PDK1 kinase domain that interacts with PIF and the C-terminal residues of PKA. EMBO J. 19:979–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biondi R. M., Kieloch A., Currie R. A., Deak M., Alessi D. R. 2001. The PIF-binding pocket in PDK1 is essential for activation of S6K and SGK, but not PKB. EMBO J. 20:4380–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brendel V., Bucher P., Nourbakhsh I. R., Blaisdell B. E., Karlin S. 1992. Methods and algorithms for statistical analysis of protein sequences. Proc. Natl. Acad. Sci. U. S. A. 89:2002–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burgess A. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc. Natl. Acad. Sci. U. S. A. 107:125564–125569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Casamayor A., Morrice N. A., Alessi D. R. 1999. Phosphorylation of Ser-241 is essential for the activity of 3-phosphoinositide-dependent protein kinase-1: identification of five sites of phosphorylation in vivo. Biochem. J. 342(2):287–292 [PMC free article] [PubMed] [Google Scholar]
- 8. Castilho P. V., Williams B. C., Mochida S., Zhao Y., Goldberg M. L. 2009. The M phase kinase Greatwall (Gwl) promotes inactivation of PP2A/B55delta, a phosphatase directed against CDK phosphosites. Mol. Biol. Cell 20:4777–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Castro A., et al. 2001. Cyclin B/cdc2 induces c-Mos stability by direct phosphorylation in Xenopus oocytes. Mol. Biol. Cell 12:2660–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cole C., Barber J. D., Barton G. J. 2008. The Jpred 3 secondary structure prediction server. Nucleic Acids Res. 36:W197–W201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dephoure N., et al. 2008. A quantitative atlas of mitotic phosphorylation. Proc. Natl. Acad. Sci. U. S. A. 105:10762–10767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frödin M., et al. 2002. A phosphoserine/threonine-binding pocket in AGC kinases and PDK1 mediates activation by hydrophobic motif phosphorylation. EMBO J. 21:5396–5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gharbi-Ayachi A., et al. 2010. The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science 330:1673–1677 [DOI] [PubMed] [Google Scholar]
- 14. Glotzer M., Murray A. W., Kirschner M. W. 1991. Cyclin is degraded by the ubiquitin pathway. Nature 349:132–138 [DOI] [PubMed] [Google Scholar]
- 15. Gould C. M., Kannan N., Taylor S. S., Newton A. C. 2009. The chaperones Hsp90 and Cdc37 mediate the maturation and stabilization of protein kinase C through a conserved PXXP motif in the C-terminal tail. J. Biol. Chem. 284:4921–4935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hauge C., et al. 2007. Mechanism for activation of the growth factor-activated AGC kinases by turn motif phosphorylation. EMBO J. 26:2251–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hauge C., Frodin M. 2006. RSK and MSK in MAP kinase signalling. J. Cell Sci. 119:3021–3023 [DOI] [PubMed] [Google Scholar]
- 18. Hirai T. J., Tsigelny I., Adams J. A. 2000. Catalytic assessment of the glycine-rich loop of the v-Fps oncoprotein using site-directed mutagenesis. Biochemistry 39:13276–13284 [DOI] [PubMed] [Google Scholar]
- 19. Huse M., Kuriyan J. 2002. The conformational plasticity of protein kinases. Cell 109:275–282 [DOI] [PubMed] [Google Scholar]
- 20. Kannan N., Haste N., Taylor S. S., Neuwald A. F. 2007. The hallmark of AGC kinase functional divergence is its C-terminal tail, a cis-acting regulatory module. Proc. Natl. Acad. Sci. U. S. A. 104:1272–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kannan N., Neuwald A. F. 2005. Did protein kinase regulatory mechanisms evolve through elaboration of a simple structural component? J. Mol. Biol. 351:956–972 [DOI] [PubMed] [Google Scholar]
- 22. Komander D., Kular G., Deak M., Alessi D. R., van Aalten D. M. 2005. Role of T-loop phosphorylation in PDK1 activation, stability, and substrate binding. J. Biol. Chem. 280:18797–18802 [DOI] [PubMed] [Google Scholar]
- 23. Kozma S. C., Thomas G. 2002. Regulation of cell size in growth, development and human disease: PI3K, PKB and S6K. Bioessays 24:65–71 [DOI] [PubMed] [Google Scholar]
- 24. Lorca T. C., et al. 2010. Constant regulation of both the MPF amplification loop and the Greatwall-PP2A pathway is required for metaphase II arrest and correct entry into the first embryonic cell cycle. J. Cell Sci. 123:2281–2291 [DOI] [PubMed] [Google Scholar]
- 25. Lorca T., et al. 1998. Fizzy is required for activation of the APC/cyclosome in Xenopus egg extracts. EMBO J. 17:3565–3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Manning G., Whyte D. B., Martinez R., Hunter T., Sudarsanam S. 2002. The protein kinase complement of the human genome. Science 298:1912–1934 [DOI] [PubMed] [Google Scholar]
- 27. Mochida S., Ikeo S., Gannon J., Hunt T. 2009. Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J. 28:2777–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mora A., Komander D., van Aalten D. M., Alessi D. R. 2004. PDK1, the master regulator of AGC kinase signal transduction. Semin. Cell Dev. Biol. 15:161–170 [DOI] [PubMed] [Google Scholar]
- 29. Olsen J. V., et al. 2010. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci. Signal 3:ra3. [DOI] [PubMed] [Google Scholar]
- 30. Parekh D. B., Ziegler W., Parker P. J. 2000. Multiple pathways control protein kinase C phosphorylation. EMBO J. 19:496–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pearce L. R., Komander D., Alessi D. R. 2010. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 11:9–22 [DOI] [PubMed] [Google Scholar]
- 32. Peterson R. T., Schreiber S. L. 1999. Kinase phosphorylation: keeping it all in the family. Curr. Biol. 9:R521–R524 [DOI] [PubMed] [Google Scholar]
- 33. Söding J. 2005. Protein homology detection by HMM-HMM comparison. Bioinformatics 21:951–960 [DOI] [PubMed] [Google Scholar]
- 34. Vigneron S., et al. 2009. Greatwall maintains mitosis through regulation of PP2A. EMBO J. 28:2786–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vigneron S., et al. 2004. Kinetochore localization of spindle checkpoint proteins: who controls whom? Mol. Biol. Cell 15:4584–4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wilm M., et al. 1996. Femtomole sequencing of proteins from polyacrylamide gels by nano-electrospray mass spectrometry. Nature 379:466–469 [DOI] [PubMed] [Google Scholar]
- 37. Yang J., et al. 2002. Molecular mechanism for the regulation of protein kinase B/Akt by hydrophobic motif phosphorylation. Mol. Cell 9:1227–1240 [DOI] [PubMed] [Google Scholar]
- 38. Yu J., et al. 2004. Greatwall kinase: a nuclear protein required for proper chromosome condensation and mitotic progression in Drosophila. J. Cell Biol. 164:487–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yu J., Zhao Y., Li Z., Galas S., Goldberg M. L. 2006. Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol. Cell 22:83–91 [DOI] [PubMed] [Google Scholar]
- 40. Zhao Y., et al. 2008. Roles of Greatwall kinase in the regulation of Cdc25 phosphatase. Mol. Biol. Cell 19:1317–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]